Abstract
NMDA receptors are preeminent neurotransmitter-gated channels in the central nervous system, which respond to glutamate in a manner that integrates multiple external and internal cues. They belong to the ionotropic glutamate receptor family and fulfill unique and critical roles in neuronal development and function. These roles depend on characteristic response kinetics, which reflect the receptor’s operation. Here, we review biologically salient features of the NMDA receptor signal and their mechanistic origins. Knowledge of distinctive NMDA receptor biophysical properties, their structural determinants, and physiological roles is necessary to understand the physiologic and neurotoxic actions of glutamate, and to design effective therapeutics.
Introduction
Glutamate is the main chemical transmitter in the central nervous system (CNS) where it initiates and regulates a wide array of physiological events. Ongoing glutamatergic signaling is essential for neuronal viability, the normal functioning of the nervous system circuitry, and for many vital behavioral responses to environmental challenges and experiences1. The ubiquity of glutamate as neurotransmitter in brain and spinal cord poses an important processing challenge to neurons: how to derive information that regulates widely different molecular events from a single chemical signal? Among the molecular transducers that have evolved to sense glutamate transients and to interpret their informational content, the ionotropic glutamate receptor (iGluR) family encompasses structurally related glutamate-gated excitatory channels that differ in their sensitivities and responses to this ubiquitous chemical signal2,3. Regulated expression of receptor subunits endows neurons with specific complements of iGluR family members that transduce external glutamate transients into distinct cellular signals. How receptors with related structures support biologically diverse signals is unknown.
Within the iGluR family, NMDA, AMPA, and kainate receptors have largely similar structures4–9 (Fig. 1A); yet, from each, glutamate elicits excitatory currents with class-specific kinetic characteristics. Most excitatory synapses have a mix of AMPA and NMDA receptors and the excitatory postsynaptic current (EPSC) reflects this heterogeneity. Following a synaptic stimulus, AMPA receptor currents rise and subside fastest; they determine the onset and maximal amplitude of the EPSC. In contrast, NMDA receptor currents rise and decline more slowly; thus, they set the decay of the EPSC and strongly influence the total positive charge entering the cell (Fig. 1B). Given the overall similar architectures of AMPA and NMDA receptors, it is likely that the biologically relevant attributes of their output originate from subtle differences in atomic arrangements.
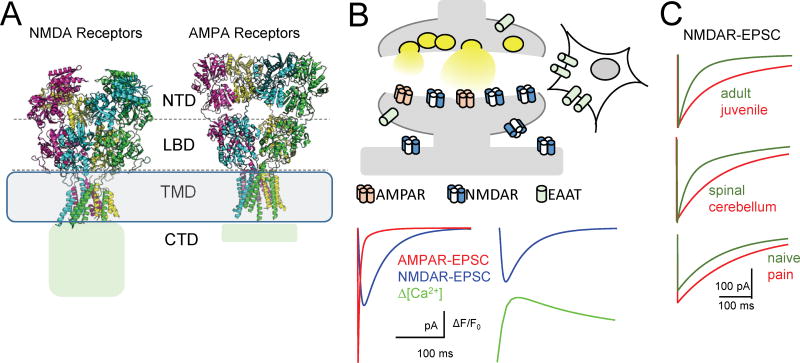
Figure 1. iGluR family members have similar structures, but distinctive output.
(A) Structural models of two iGluR representatives reveal overall similarity between NMDA and AMPA receptor architectures; both have large ectodomains composed of N-terminal (NTD) and ligand binding (LBD) domains, a short transmembrane domain (TMD), and cytoplasmic C-terminal domains (CTD), the latter being structurally unresolved.
(B) Stereotypical map and electrical responses of central excitatory synapses. Top, glutamate molecules released from presynaptic vesicles diffuse across the synaptic cleft, bind to post-synaptic iGluRs to produce the EPSC, and to extrasynaptic glutamate receptors and transporters (EAAT). Bottom left, the typical EPSC has two components, which can be separated pharmacologically into AMPA143 (red) and NMDA88 (blue) currents, each with characteristic time-dependent amplitudes. The NMDA receptor-mediated component is visibly longer; its decay kinetics set the EPSC decay timecourse. Bottom right, simultaneous recording of NMDAR-EPSC and corresponding rise in Ca2+ fluorescence in a single synaptic spine144,145.
(C) NMDAR-EPSCs differ in maximal amplitude and kinetics across synapse development: hippocampal synapse at P10 compared with P30 (top)102,103; cellular type: cerebellar compared with spinal synapse (middle)19,146; and synaptic state: spinal synapse before and after induced neuropathic pain (bottom)147.
All traces represent simulations based on values from the cited reports.
Among the most recognizable features of synaptic NMDA receptor currents (NMDAR-EPSC) are their slow rise, extended durations, high Ca2+ content, and acute sensitivity to voltage-dependent blockage2. However, even these signature traits vary substantially with neuronal type and developmental stage (Fig. 1C). A major obstacle to understanding how functional variations arise from structure and how they impact biology is that the molecular composition of native NMDA receptors is yet undefined. Here, we summarize current knowledge of the physiologic output and biophysical operation of molecularly defined NMDA receptors in vitro, and how these relate to signals produced in situ by synaptic and non-synaptic receptors.
Observing the NMDA receptor output
NMDA receptors are heterotetramers of seven genetically encoded, differentially expressed subunits: GluN1, which is processed into eight molecularly distinct splice variants, four GluN2 (A-D), and two GluN3 (A-B)10–13. Therefore, the functional differences observed across native preparations reflect, at least in part, the distinct composition of their subunit complement, as well as cell-specific and synapse-specific modulatory factors. However, the exact composition of native receptors is unknown, because the expression patterns of individual subunits overlap substantially, and subunits can combine in different ways to produce a broad diversity of tetrameric receptors14. This experimental roadblock was addressed by examining the functional properties of molecularly defined recombinant receptors in heterologous cells.
These approaches established that functional glutamatergic NMDA receptors contain at least one GluN1 subunit, providing a functionally-required glycine-binding site and at least one GluN2 subunit, providing the neurotransmitter-binding site. Further, molecular composition strongly influences the class-specific signatures of the NMDA receptor current: kinetics, Ca2+ content, voltage-dependency of block15–20. Therefore, if considering together diheteromeric GluN1/GluN2 and triheteromeric GluN1/GluN2/GluN3 combinations, the known subunits and splice variants can assemble in as many as 756 distinct molecular entities. Presently, functional characteristics are known for a small number of NMDA receptor subtypes, and the atomic structure of only one (GluN1/GluN2B) (Fig. 1A) has been reported4,5,21. These facts highlight the considerable gap in understanding how distinctive properties of NMDA receptor currents arise, are controlled, and influence cellular responses. Given the many essential functions of NMDA receptors in physiology and neuropathology these barriers must be surmounted. When attempting to characterize responses from distinct subunit combinations in vitro, it is instructive to distill from the available literature the features of the NMDA receptor response that are most salient for the receptor’s biological roles and how these relate to receptor structure.
Abundant research indicates that the biological purpose of NMDA receptors is to sense the simultaneous presence of glutamate and a variety of other (chemical, metabolic, and physical) cues, and to respond by gating a Ca2+-rich cationic current that integrates and reflects this information22. Their sensory function is accomplished by external epitopes that recognize diffusible ligands such as glutamate, glycine, H+, and Zn2+, etc. Their ionotropic function is accomplished by the controlled gating of a transmembrane pore that allows substantial flow of Na+, K+, and Ca2+. Both binding and gating reactions originate from structures specific to each NMDA receptor protein and influence important properties of the current10. These reactions can be modified by environmental and cellular factors including membrane tension, structural and signaling proteins, and enzymatic modifications23. To understand how molecular structure and the environment control NMDA receptor output, it is necessary to know how the receptive and ionotropic functions of NMDA receptors are coupled.
Efforts to understand how NMDA receptor currents arise and are modulated were stimulated by the observation that the EPSC has a substantial NMDA-responsive constituent, which controls EPSC decay kinetics24. The EPSC decay is a strong modulator of synaptic summation and plasticity25, therefore, research was immediately focused on examining the time course of the NMDAR-EPSC decay at a variety of central synapses (Fig. 1C). It was soon appreciated, however, that a large fraction of NMDA receptors are also present at non-synaptic locations where they serve separate cellular functions26–28. Because non-synaptic receptors experience markedly different waveforms of glutamate exposure, subcellular location strongly influences the time course of receptor response, the total charge transferred, and how modulators affect receptor current and cellular outcome.
Measuring synaptic output
Synaptic receptors experience brief pulses of high glutamate concentrations. Vesicle fusion at presynaptic terminals release glutamate and elevate the synaptic cleft glutamate concentrations into the mM range, with microsecond timecourse. Dense expression of glutamate transporters on nearby cells, combined with passive diffusion of glutamate away from the cleft restore glutamate concentrations to very low resting levels within 1 ms (Fig. 1B)29. The timecourse and amplitudes of synaptic glutamate concentrations will, therefore, vary with the type of stimulation (number of vesicles released, release frequency), cleft geometry, type and number of glutamate transporters expressed, and other synapse-specific attributes; however, it is generally accepted that postsynaptic AMPA and NMDA receptors at synapses experience brief periods of exposure to high glutamate concentrations29.
Currents mediated by synaptic NMDA receptors can be isolated pharmacologically and have been recorded from interconnected neurons in dissociated cultures, tissue slices, and in whole animals (Fig. 1B, C; Box 1). Relative to the AMPA-gated currents, the NMDA-gated currents rise more slowly and have substantially longer bi-exponential deactivations, which dominate and control the decay phase of the EPSC (Fig. 2A). The EPSC decay time sets the interval over which incoming stimuli summate to influence synaptic transmission, plasticity, and the computational properties of the dendritic network. Importantly, NMDA receptor kinetics also control the high Ca2+ content fluxed by NMDAR-EPSCs. To address the experimental limitations imposed by the anatomy of synapses, synaptic-like responses are elicited in vitro with fast-perfusion techniques.
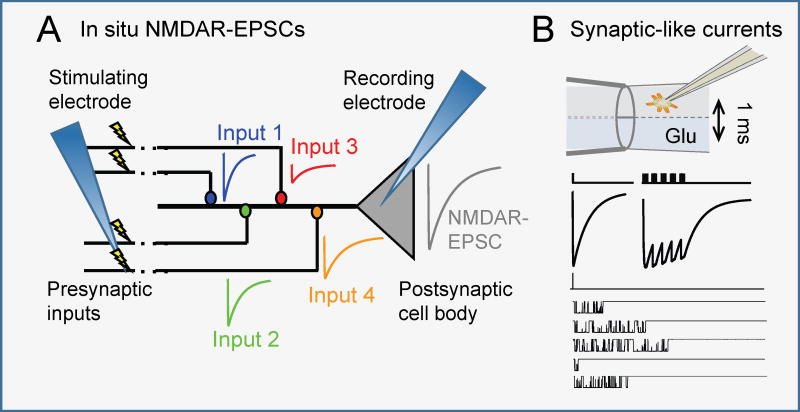
Box 1. Recording NMDA receptor synaptic output.
NMDA receptor-mediated synaptic currents (NMDAR-EPSC) recorded with the whole-cell patch-clamp technique138 reveal synapse-specific kinetics139. A polished glass pipette filled with intracellular-like solution (recording electrode) is pushed onto the somatic membrane of a neuron to form a high-resistance seal. Next, light suction or a brief electric shock ruptures the membrane and connects the intracellular milieu with the recording electrode solution. This approach can register spontaneous as well as EPSCs evoked with electrical stimulation of afferent pathways (stimulating electrode). The NMDA receptor component recorded at the soma represents the sum of responses across all stimulated synapses (Inputs 1 – 4), which can differ in kinetics and amplitude. The molecular composition of responding receptors is unknown, and is presently inferred with pharmacological or genetic methods.
Synaptic-like macroscopic or microscopic currents can be elicited from somatic (or recombinant) receptors by exposing these to brief (~1 ms) pulses of glutamate-containing solution (with glycine present) by rapid piezo-driven movement of theta-shaped double-barreled flow pipes43,88,95. A small cell or excised membrane patch is first attached to the recording electrode and then lifted and positioned into the glutamate-free stream of the theta tube; next, a piezo-driven actuator moves the perfusion pipette rapidly (0.2 µs) back and forth to expose receptors to the glutamate-containing stream. Glutamate concentration, the residence time in each stream, and the stimulus frequency can all be controlled experimentally thus mimicking synaptic-like exposures while recording time-dependent current amplitudes; in addition, this method is amenable to recombinant preparations where receptor identity is also controlled. However, patch excision isolates the channel from intracellular factors that may be important in gating, and imposes physical deformations onto the cellular cortex.
Box 1.
Recording synaptic NMDA receptor signals
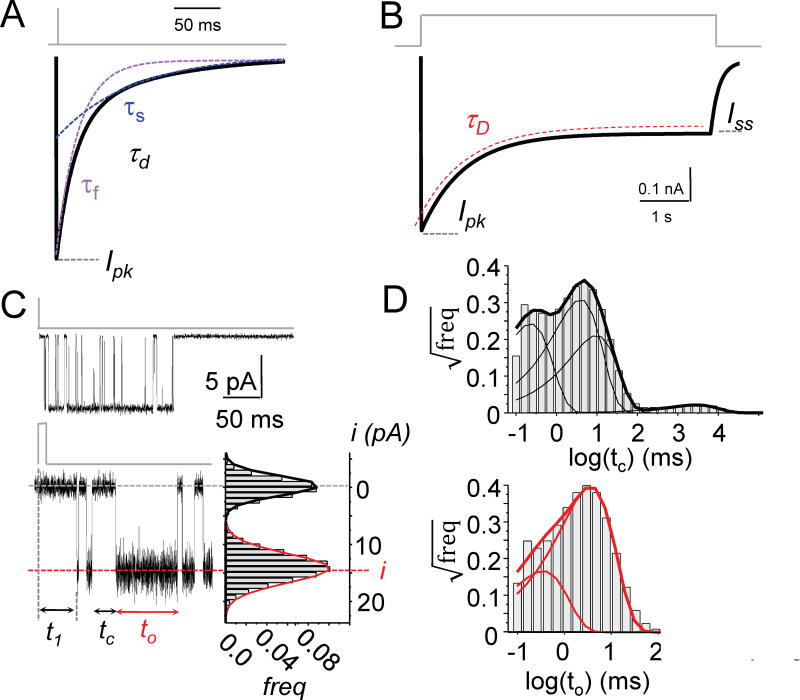
Figure 2. Observable features of the NMDA receptor output.
(A) Macroscopic currents elicited with brief synaptic-like stimuli (1 ms, 1 mM Glu) are described by their peak amplitude (Ipk) and decay kinetics (τd). Decay kinetics are quantified by fitting declining mono-exponential (τd) or bi-exponential functions (τf, τs) to the declining phase of the current.
(B) Macroscopic currents elicited with long nonsynaptic-like stimuli (>2 s, 1 mM Glu) are described by peak (Ipk) and steady-state (Iss) current amplitudes measured directly from the record; desensitization kinetics, determined by fitting declining mono-exponential functions (τD) to the response between Ipk and Iss; and desensitization extent, calculated as the Iss/Ipk ratio.
(C) Microscopic currents are recorded as downward deflections from a zero current baseline (top) and expanded (below) to allow direct measurement of current amplitude distributions (i) (bottom right) and duration for each opening (to) and closure (tc); responses elicited with brief synaptic-like stimuli (1 ms, 1 mM Glu) also reveal the receptor’s latency to the first opening (t1).
(D) Complex probability distributions of open (to) and closed (tc) durations (thick lines) observed in each record yield quantitative information about channel output, including the number and lifetime of kinetic components determined by fitting multiple exponential functions to the data (thin lines).
Outside of synapses, synaptic-like responses are reproduced by applying exogenous glutamate in pulses of controlled duration and concentration (1 ms, 1 mM) onto membrane patches excised from cell bodies expressing endogenous or recombinant NMDA receptors (Box 1). The macroscopic currents recorded with this approach have decay kinetics similar to those measured for NMDAR-EPSCs. Thus, even if cell-specific factors such as auxiliary proteins that modulate the NMDA receptor response may exist30–33, they are not necessary to reproduce the natural features of the NMDA receptor synaptic response. With this methodology it was established that the kinetics of the synaptic-like current decay is subtype dependent and is modulated by endogenous34 and pharmacologic ligands35–37 and by intracellular effectors38–40. Therefore, the NMDAR-EPSC decay is determined by the receptor subtype expressed and on a faster time scale by external diffusible modulators and intracellular signaling.
Measuring non-synaptic output
In contrast to synaptic NMDA receptors, non-synaptic NMDA receptors experience shallower and more prolonged glutamate transients. Thus, instead of producing discrete, rapidly fluctuating signals, they generate long-lasting sustained currents. Much less is known about the kinetics and modulatory mechanisms of native non-synaptic receptor currents, largely because the origins, levels, and kinetics of non-synaptic glutamate transients are poorly defined. Non-synaptic receptor currents can be isolated and observed in synaptically connected neurons by first blocking synaptic receptors with a slowly dissociating pore blocker (MK-801) (Box 2). These experiments have revealed unique cellular outcomes of non-synaptic NMDA receptor currents41,42. However, this approach lacks the recording resolution necessary to register kinetic information about the response. For this reason, non-synaptic NMDA receptor behaviors are typically investigated in isolated cells or excised patches exposed to prolonged (>2 s) applications of exogenous glutamate.
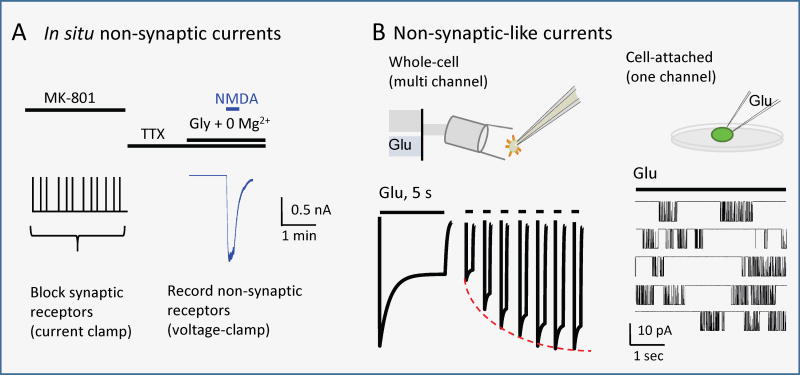
Box 2. Recording NMDA receptor non-synaptic output.
Non-synaptic receptors are, by definition, distant from synaptic release sites; they can be peri-synaptic (surrounding the post synaptic density on the spine head), extrasynaptic (on spine necks or dendrite shafts), somatic (on the cell body), or axonal (on axons)10. Non-synaptic receptors are engaged during high-frequency firing when the buffering capacity of glutamate transporters is exceeded. Diffusion laws dictate that with distance, the nonsynaptic glutamate signal decreases in amplitude and increases in duration relative to the synaptic transient140. Non-synaptic NMDA receptors may differentially serve as mediators of excitotoxicity26.
The activity of nonsynaptic NMDA receptors is probed in connected neurons (cultures or slices) with chemical or electrical stimuli delivered after synaptic receptors had been blocked by light synaptic stimulation in the presence of a long-lasting open channel blocker (MK-801). The residual NMDA receptor response after washing out the blocker (blue) presumably originates from non-synaptic receptors (Modified with permission from REF. 141)141. This approach lacks the temporal and spatial resolutions necessary for mechanistic investigations of non-synaptic receptors.
Non-synaptic-like macroscopic responses are examined by recording whole-cell or excised-patch currents during controlled exposure to agonist to elicit a sustained current (1 s – 5 s). The flow of perfusate can be switched within 100 – 400 ms with solenoid-valves; for receptors with desensitization times in the ms-range, this technique affords sufficient temporal resolution to study desensitization and resensitization kinetics. However, given the long recording times, the electrode solution “replaces” the intracellular cytosol and may affect channel activity. Alternatively, non-synaptic-like currents can be examined in intact cell-attached membranes. This method offers observations with the highest time resolution and duration (up to hours), and can be minimally invasive. However, switching external solutions is impracticable, and the initial phase of current is lost during seal formation; also, mechanical deformations imposed onto the membrane and extracellular matrix may affect channel activity.
Box 2.
Recording non-synaptic NMDA receptor signals
In native preparations, extrasynaptic AMPA receptors desensitize rapidly and fully (>99%) such that their contribution to the observed sustained current is negligible. In contrast, studies with recombinant NMDA receptors showed that NMDA receptor desensitization is incomplete, varies among the recombinant NMDA receptor subtypes examined so far, and is controlled by diffusible ligands and cellular factors (Box 2, Fig. 2B)43–45. Based on these observations, it is believed that tonic activation of non-synaptic NMDA receptors represents the main mechanism of excitation by extrasynaptic glutamate. In contrast, it remains unclear to what extent synaptic receptors desensitize during synaptic transmission and whether regulated changes in desensitization kinetics modulate the amplitude and decay phase of the NMDAR-EPSC in response to a single pulse. Notably, desensitization attenuates NMDAR-EPSCs in response to trains of synaptic stimuli.
Whether produced by synaptic or non-synaptic receptors, the cellular excitatory potential is always the summation of microscopic currents gated by individual receptors. The specialized architecture of synapses has prevented the direct observation of unitary currents from synaptic receptors; however, both synaptic-like and non-synaptic-like unitary currents have been recorded from somatic and recombinant NMDA receptors (Box 1, 2)46–51. When observing single-molecule currents in the absence of blockers and allosteric modulators, GluN2A- and GluN2B-containing receptors produce unitary currents with relatively large, uniform amplitudes (Fig. 2C), have high signal-to-noise ratios, and provide direct, accurate, and precise information about the receptor’s conductance over a range of experimental conditions46,52. Under similar conditions, GluN2C- and GluN2D-containing receptors produce currents with multiple smaller conductance levels45,53–57. In addition, because these currents mark the real-time opening and closing of the observed channel, the record allows direct measurement of open channel durations. These approaches (Fig. 2C, D) established that unitary NMDA receptor currents vary in both amplitude and kinetic patterns across native preparations and specific recombinant subtypes46,50,51,58. However, until relatively recently, it had been unclear how these microscopic parameters relate to the characteristic features describing macroscopic responses.
Modeling the operation of NMDA receptors
Because the decay of the NMDA receptor current sets the decay of the EPSC and is developmentally and regionally regulated, it is important to understand the molecular transformations that produce the observed current fluctuations. The activation reaction of each NMDA receptor can be imagined as a sequence of step-wise elementary changes in function; still, because the underlying transformations cannot be observed directly with the available experimental methods, they must be deduced with modeling approaches (Box 3). The goal of kinetic modeling is to delineate the elementary steps that produce the observed signal and the timing with which these steps occur. For each transition, mass action defines the time-dependent occupancies of the initial and final receptor states and the composition of the system at equilibrium; thus, once established by extensive testing against experimental observations, a kinetic mechanism can be tremendously useful in characterizing the unitary steps responsible for salient features of the macroscopic signal, and can predict new behaviors that are either inaccessible experimentally or have escaped detection. A comprehensive kinetic mechanism describing the entire spectrum of native conformations is far too complex for practical value. Thus, the most useful models are those that accurately represent the underlying mechanism with the minimal level of detail that still accounts for the observed signal.
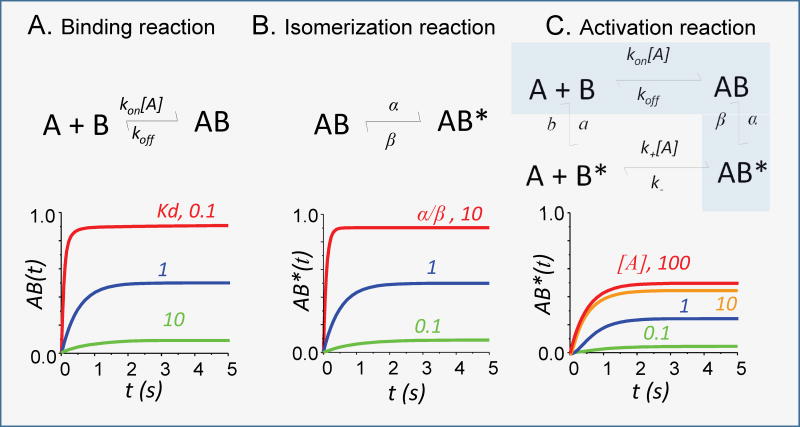
Box 3. Modelling the operation of ligand-gated ion channels.
The first reaction mechanism proposed and tested for a natural process describes a bimolecular binding reaction as a reversible process governed by the law of mass action. In this system, a simple set of mathematical relations describes the time-dependent and steady-state distribution of molecular species in solution relative to initial concentrations and the rate constants for the binding (kon) and dissociation (koff) reactions.
The first reaction mechanism proposed for the gating of an ion channel describes the process as a reversible isomerization between an inactive (non-conducting) species (AB) and an active (ion-conductive) species (AB*)142. In this model, the rate constants for the channel-opening (β) and closing (α) reactions fully determine the time-dependent and steady-state occupancies.
Minimal reaction mechanism to describe the binding-dependent activation of a protein connects an initial binding step with an isomerization step. In a fully reversible system, this mechanism must include four reactions, describing ligand binding/dissociation to/from the resting and active conformations, and conversely, activation/deactivation of the apo and liganded states. The time-dependent and equilibrium occupancy of the bound active conformation AB* is governed by a complex set of equations. The occupancy of any one state depends on all eight rate constants and can be determined experimentally only in limiting conditions. For example, the shaded coupled reactions (A+B ↔ AB ↔ AB*) represent the classic Michaelis-Menten model for enzyme-catalyzed bimolecular reactions, for which mathematical relations have been derived and tested successfully.
Box 3.
Conceptual models of ligand-gated ion channels
Modeling with conceptual models
As with all ligand-gated channels, the NMDA receptor activation reaction must include an agonist-binding reaction and a pore-opening reaction, which are linked (Box 3). For each elementary reaction, relatively simple mathematical relationships define how the postulated states are occupied in time. With a mechanism in hand, experiments can be designed to measure system-specific parameters such as ligand affinity and response kinetics. However, when linking two such elementary reactions, thermodynamic arguments require full reaction cycles (Box 3) such that both the number of parameters that define state occupancies in time and the complexity of their mathematical relationships increase rapidly, rendering the system opaque to experimental observation59. In practice, mechanisms of ligand-gated channels are rarely represented with full thermodynamic models; instead, empiric simplifications are sought.
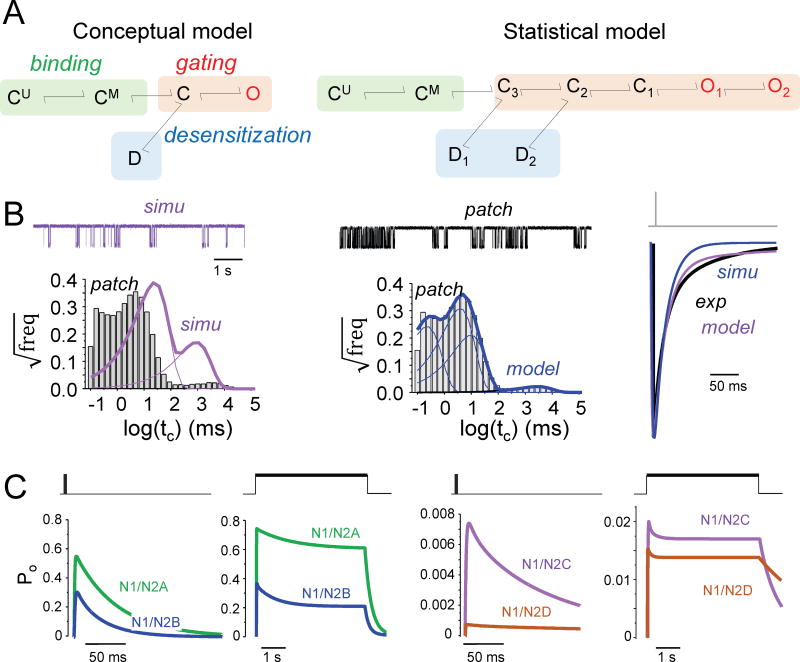
For NMDA receptors, a feasible mechanism must account for three critical observations. First, activation requires two agonist molecules60 and agonist affinity impacts the decay kinetics61; second, competitive antagonists prevent glutamate-elicited currents if applied prior to the glutamate stimulus, but are ineffective if applied after the agonist is removed61; and third, during sustained glutamate exposure, NMDA receptor currents decline substantially from an initial peak level (Ipk) to a steady-state level (Iss)62. With these considerations, two glutamate-binding steps must precede channel openings; channels must close before glutamate can dissociate; and glutamate-bound receptors can desensitize. To account for these observation, Lester and Jahr63 proposed a kinetic model where receptors (assumed saturated with glycine) bind sequentially two molecules of glutamate and the resulting doubly-liganded, closed receptors (C) can isomerize into either active (O) or desensitized (D) conformations, from which glutamate cannot dissociate (Fig. 3A, left). By fitting this model to synaptic-like macroscopic responses, one can estimate rate constants for the three postulated equilibria: binding, gating, and desensitization63–65. Over the past 20 years, this and versions expanded to include glycine binding/dissociation steps60,66 have been used to characterize and differentiate native and recombinant NMDA receptors, to identify the rate constants sensitive to drugs and mutations, and will continue to be useful for comparison and classification purposes60,63,67–72. However, if used to simulate single-channel currents, this conceptual model predicts open and closed duration distributions with no obvious relationship to experimental observations37,43,73–78 (Fig. 3B).
Figure 3. Models of NMDA receptor operation.
(A) Minimal model (left) required to describe macroscopic currents includes two sequential glutamate binding steps to resting (CU) and monoliganded (CM) receptors, followed by one-step isomerization reactions: gating (C—O) and desensitization (C—D)63. Minimal model (right) required to describe one-channel currents also includes two sequential glutamate binding steps, followed by a complex gating sequence (C-C-C-O-O)83–85, and two desensitization steps (C—D)44,89.
(B) The conceptual model fits well (simu, purple) the rise and decay of recorded synaptic-like macroscopic current (patch, black); but not the distribution of closures observed in one-channel currents (patch, black). The statistical model (model, blue) estimates well both the synaptic-like current and the single-channel event distributions. Both models predict mono-exponential decay for the synaptic-like current and are therefore too simple to fully account for NMDAR-EPSC.
(C) Traces were simulated in response with the ‘average’ statistical models reported for GluN1/GluN2A88, GluN1/GluN2B44, GluN1/GluN2C45, and GluN1/GluN2D receptors99; they predict substantial differences in peak open probabilities, overall kinetics, and charge transferred in response to synaptic-like (left) and non-synaptic-like (right) glutamate transients (thick black lines).
Modeling with statistical models
At the single molecule level, NMDA receptor currents are relatively easy to detect; in contrast, their opening patterns are complex and pose steep modeling challenges. When classified by their lifetimes, each of the open and the closed classes of events have multiple kinetic constituents, indicating that the reaction mechanism must include several open and closed states51,79–81. To manage this complexity, select experimental conditions help to isolate portions of the activation mechanism. For example, when stimulated with brief synaptic-like pulses, or when recording equilibrium activity in very low agonist concentrations, receptors cycle primarily among states that directly connect the resting and open states, thus, providing clues about the activation/deactivation sequence. Alternatively, recording equilibrium activity in very high agonist concentrations, binding/dissociation events are experimentally invisible and desensitized events are separated statistically, to focus on the gating reaction.
In synaptic-like recordings, data show two principal closed durations, strongly indicating that the activation/deactivation pathway must include at least two closed and one open states82. In equilibrium recordings, where many more events can be captured, data show three closed and two open states, thus, expanding the activation/deactivation portion of the mechanism to a minimum of five states83,84. The order in which these states are accessed is not immediately obvious, however, several experimental observations limit the many possible arrangements. First, NMDA receptors have relatively long latency to opening, thus favoring a mechanism where (at least) two slow transitions through closed states precede opening82. Second, statistical arguments indicate that the two open states are most likely connected83. However, these requirements are fulfilled by both cyclic45,76,85,86 and linear83,84 arrangements, and cannot be discriminated on statistical grounds. Therefore, the order in which kinetic transitions occur and their physical correlates remain to be established. For simplicity, we will use here the linear activation sequence.
This linear model has been expanded to include glutamate-binding steps44,84,87, thus providing a more accurate tool to measure microscopic binding kinetics in separation from gating transitions; and with glycine binding steps88. Last, equilibrium activity recorded with an allosteric modulator that selectively stabilized desensitized states provided evidence for two separate desensitization steps, which are most likely accessed from separate pre-open states89. Even when ignoring glycine binding, the resulting model with 9 states (7 closed and 2 open) and 14 independent rate constants (Fig. 3A, right) is too complex to be fully determined by measurements under a single experimental paradigm. Instead, the full model is assembled in steps, by first deriving topologies and rates for specific portions of the mechanism and then testing its predictions against a battery of macroscopic behaviors, which includes dose- and frequency-response relationships, kinetics of synaptic-like response, desensitization and resensitization kinetics, etc.
Both the macro- and microscopic models of NMDA receptor activation predict with reasonable accuracy the overall duration of the synaptic-like current (Fig 3A, B). However, neither accounts for its biphasic kinetics. This is mostly because in both models the rate constants are relatively close in value and, thus, predict smooth monophasic decay. What are the processes that cause the population response to decay with two kinetic phases?
Modeling microscopic heterogeneity
Since the earliest observations of unitary currents in biological preparations, it was apparent that they were kinetically heterogeneous, containing distinct and randomly recurring activity patterns. Because the change in kinetic pattern is abrupt and reversible, it most likely reflects a low-probability conformational change, referred to as modal gating. Little is known about NMDA receptor modal gating because: modes can only be discerned and separated in microscopic current records; long observation windows are necessary to observe modes; and their number and duration varies from one record to the next. Further, for heterogeneous portions in the record to reflect a true mode shift, the recorded signal must originate from the same channel thus requiring recordings from one-channel patches. Despite these impediments, evidence is accumulating that modal gating is a universal property of ion channels that shapes their functional output and is biologically regulated90–92.
For NMDA receptors three modes have been reported to date, which can be classified by open probabilities into low (L), medium (M), and high (H)93. Modes were first noted in NMDA receptor records as relatively short periods of high open probability48. Because the kinetics of the NMDA receptor single-channel record is complex even within one mode, in practice, modes are generally ignored. One approach is to exclude from analyses portions of the record that stand out as different and focus on the most prevalent mode76; this approach provides kinetic information for dominant kinetic mode. Alternatively, the entire data set is included and analyzed regardless of possible heterogeneity; in this case, the calculated values for kinetic parameters represent weighted averages across the specific modes that happen to be captured in these records46,76,82,88,94–98. As long as these limitations are recognized and acknowledged, and if the perturbations investigated do not affect the channel’s modal transition(s), both approaches are valid and useful. However, neither ‘main-mode’ nor ‘average’ models predict the biphasic decay of the NMDAR-EPSC (Fig. 2A).
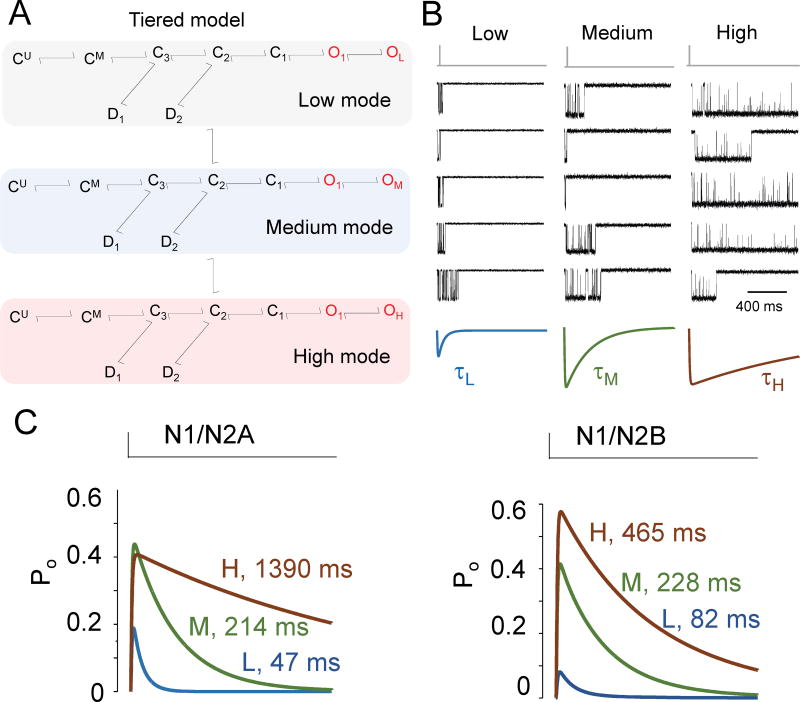
During an individual mode the current is well described by schemes having the same number and arrangement of states as the ‘average’ model (Fig. 3B) but with distinct sets of rate constants44,84,99. Thus, a tiered model was proposed (Fig. 4A) where the channel always operates with the same basic mechanism: is initiated by two sequential and identical glutamate-binding steps followed by a gating sequence of three closed and two open states, and two desensitized states; in addition, two modal transitions can cause a subset of rate constants in this core mechanism to change for a while. With 27 states and 46 independent rates, this model is too complex to have practical value. Instead, stimulation protocols and recording conditions are selected to drive receptors into certain regions of the tiered model, thus warranting contraction into simpler models. For example, binding reactions may be omitted when recording with supra-saturating concentrations of agonists83,86,100, and models with only one aggregated open state may be used if the perturbation studied does not affect open channel durations85,101. It is important to keep in mind that the mechanism that produces modal behavior is unknown. Nonetheless, evidence supports a role for modal transitions in setting the NMDA receptor response decay kinetics (Fig. 4B).
Figure 4. Insights from statistical models.
(A) Tiered model accounts for modal gating, defined as spontaneous transitions between patterns of activity with distinguishable rates. Periods of low, medium, and high gating have been identified statistically and separated in one-channel records to estimate mode-specific rate constants44,83,84,99,101;
(B) Synaptic-like unitary responses (top, black) recorded successively from the same GluN1/GluN2A receptor can be grouped by their pattern of opening consistent with modal gating. The sum currents for each kinetic group have distinct peak amplitudes and decay kinetics101.
(C) Statistical models derived from modes observed in GluN1/GluN2A84 and GluN1/GluN2B44 single-receptor recordings predict distinct peak open probabilities, and decay times that span several orders of magnitude. Therefore, the complex decay kinetics observed for NMDAR-EPSCs and for synaptic-like NMDA receptor currents may reflect molecular (subtype) as well as kinetic (modes) heterogeneities of the activated receptors.
Glutamate dissociation and receptor desensitization have been the only factors demonstrated to control the kinetics of NMDA receptor current decay61,63. However, using single-channel recordings obtained at several equilibrium subsaturating concentrations of glutamate, and after separating activity by mode, it was ascertained that although each mode predicted responses with distinct decay times(Fig. 4C), neither glutamate binding/dissociation rate constants, nor desensitization/resensitization rate constants changed with gating mode44,84. Notably, the decay time constants predicted by the low and medium modes (τL, τM), were close in value to the fast and slow components of the biphasic synaptic decay (τf, τs), respectively. These observations ushered the hypothesis that the biphasic nature of the NMDAR-EPSC decay reflects receptor populations that gate in separate modes and, therefore, may be controlled by changing the relative fraction of channels occupying each mode.
This premise was tested by recording one-channel currents in response to brief glutamate pulses. If indeed, the population response originates from a mixture of kinetically distinct receptors, the summation of synaptic-like unitary currents would display bi-exponential decay only when the recorded sequence of activations contains more than one modal shift. Long recordings from one-channel patches stimulated continuously with synaptic-like pulses clearly showed activations with distinct open probabilities indicative of modes. Consistent with the rare occurrence of modal shifts, activations with similar kinetics tended to occur in runs; importantly, when segregated by open probability (mode), the average current of unitary activations decayed with mode-specific mono-exponential timecourse101 (Fig. 4B). Based on these results the present view tis that the characteristically biphasic decay of synaptic-like NMDA receptor currents reflects an underlying heterogeneity in receptor gating mode at the time of stimulation. Native receptors display modal gating46,48,101; however, whether synaptic receptors truly exist in distinct kinetics modes remains to be tested. In this respect, it will be critical to discover pharmacologic agents or genetic modifications that can shift the modal composition of NMDA receptors.
Novel insights afforded by statistical models
The decay of the NMDAR-EPSC has been widely investigated due to its biological significance, but also because it can be readily measured experimentally and compared across preparations and conditions. From a biological standpoint the total charge transferred by an NMDAR-EPSC, which also defines the amount of Ca2+ entering the cell, is critical for physiology; however, it is rarely reported because it is more challenging to measure. The charge injected depends not only on the channels’ response timecourse but also on their open probability, which is difficult to estimate from macroscopic measurements. In this arena, statistical models are powerful instruments because they estimate absolute values for a range of metrics, including channel open probabilities, time course, and charge transfer.
For example, it is well established that the decay time of the NMDAR-EPSC becomes much shorter as a synapse matures, and this functional observation has been correlated with decreased GluN2B and increased GluN2A subunit expression102,103. However, it remains unclear whether and how changes in GluN2 subunit expression affect the amount of charge transferred by the NMDAR-EPSC. Statistical models suggest that for a constant number of GluN1 subunits, a change in receptor type from GluN2B to GluN2A will not only shorten the decay time course but will almost double the current amplitude, while maintaining a similar charge influx (Fig. 3C); these predicted differences may contribute to the reported subtype-specific cellular consequences, and therefore are worthwhile testing. Further, statistical models can help to better understand how non-synaptic NMDA receptors contribute to cellular physiology. For these receptors, differences in equilibrium open probability and their sensitivity to drugs and mutations that affect the extent of desensitization are critically important. Statistical models can help estimate differences in the levels of sustained current passed by different receptor types (Fig. 3C).
Results from simulations with statistical models were first to suggest that NMDA receptor current amplitudes may depend on stimulation frequency. For GluN1/GluN2A receptors (Fig. 3A, right), the statistical model predicts that once receptors become fully liganded the probability that they will continue along the activation reaction and eventually open (C3➞C2-C1-O1-O2) is almost equal with the probability that at least one glutamate molecule will dissociate to abort a response (C3➞GM-GU). This quantitative relationship ensures that after a brief (1 ms) glutamate pulse, only half of the receptors stimulated will contribute to the peak current, whereas the other half can only be engaged with longer pulses (>10 ms) or high frequency bursts (>50 Hz). This was validated experimentally thus confirming the veracity of the statistical model and revealing a new feature of NMDA receptor output84. This novel property may be important in defining the relationship between stimulation frequency, synaptic state (defined by the types and kinetic modes of the NMDA receptors expressed), and the ensuing synaptic plasticity. It is important to keep in mind that endogenous modulators, cellular factors, and mutation may differentially affect individual transitions within a reaction scheme changing not only parameters that have been classically measured, such as decay time, but also previously unsuspected properties, such as modal gating and frequency discrimination93.
Physiological application of models
Kinetic models are valuable tools to relate biologically-salient features of the NMDA receptor output with elementary transitions implied by the kinetic mechanism and, further, with the atomic details of the structural mechanism. Notably, they help anticipate how mutations and pharmacologic agents affect the output, and to delineate the mechanism by which changes occur. Two examples illustrate this point.
Glycine and zinc are critical endogenous modulators of brain activity and have been implicated in neuropathologies104,105. Both modulate excitatory transmission106 in part by modulating NMDA receptor currents62,107. Macroscopic recordings showed that zinc is an allosteric modulator of NMDA receptors108 and helped to locate residues responsible for high-affinity binding on the NTD of the GluN2A subunit109,110. Genetic disruption of this binding site produced animals with altered synaptic transmission and heightened pain response, thus demonstrating a physiological action of zinc mediated by the high-affinity binding-site on GluN2A on NMDAR-EPSC and animal behaviors34,111. Mechanistic investigations including kinetic modeling of macroscopic and single-channel currents concluded that ambient zinc reduces channel open probability by slowing the activation reaction to produce smaller synaptic-like currents that decay faster98,112,113. Structural investigations confirm the location of the zinc binding site and are consistent with a mechanism where zinc binding to the GluN2 NTD induces cleft closure that is transmitted to the glutamate binding domain and presumably to the gate21,114–116. A future goal is to complete molecular dynamics simulations in tetrameric receptors to fully link changes in domain structures, with specific kinetic steps within the activation reaction, and with macroscopic features of the synaptic-like response.
Glycine is required for the activation of NMDA receptors62,117 and, although difficult to measure in vivo, is believed to be widely present in the CNS interstitial fluid118 where fluctuations in its concentrations influence the activity of NMDA receptors119. Macroscopic recordings show that in subsaturating concentrations of glycine, glutamate-elicited currents are smaller and desensitize faster. Conceptual models fitted to these data are consistent with a mechanism where glutamate-binding decreases the receptor’s glycine affinity120, an example of negative cooperativity possibly explained by intersubunit LBD interactions. However, kinetic analyses and modeling of single-channel currents show no evidence of agonist cooperativity; instead, they suggest that the preopen states from which glutamate and glycine dissociate to terminate the response are kinetically and topologically distinct (C3 and C2, respectively, in Fig. 3A, right). The resulting model, predicts correctly that in subsaturating glycine concentrations synaptic-like currents decay faster, and display deeper desensitization during high frequency stimulation88. The glycine binding site is located on the LBD of GluN1 subunits117,121, where it may serve to stabilize cleft-closed conformation, as illustrated by functional measurements and atomic simulations with isolated GluN1 LBDs122–124. However, similar investigations on tetrameric receptors will be necessary to elucidate whether glycine occupancy affects receptor activity by changing the glutamate-binding site or by influencing the gate independently.
Magnesium, a ubiquitous endogenous ion, critically regulate NMDA receptor currents. Notably, Mg2+ binding in the channel pore directly blocks currents in a voltage-dependent manner52. Evidence suggests an asymmetric trapping block mechanism125, which is further complicated by biphasic, fast and slow dissociation rates that are subunit-specific126. For practical reasons the majority of modeling studies have been done in low or Mg2+-free media44,76,84,85. For this reason, it remains unclear how, in addition to blocking current, Mg2+-binding influences channel gating. Given the critical significance of Mg2+ block and modulation of NMDA receptor activity in brain function, it will be important to describe these phenomena with detailed kinetic models. Similarly, there is a strong clinical interest in the actions and mechanisms of other NMDA receptor blockers, such as ketamine and memantine, as effective, fast-acting antidepressant77 and as corrective of cognitive deficits associated with mild Alzheimer’s disease127, respectively. Detailed characterization of their mechanism will help better understand their therapeutic effects.
Structural basis of kinetic models
Perhaps the most baffling aspect of statistical models of NMDA receptors is the multiplicity of their kinetic states. Structural models show that functional NMDA receptors consist of at least nine defined modules that maintain function in isolation: four N-terminal domains, four ligand-binding domains, and a pore domain. Even if we imagine each module in only two conformations, resting and active, the resulting possible combinations predict a large array of discrete structures, which may differ in their stabilities and functional properties. This diversity is supported by cryo-EM data; they revealed an assortment of closed and open liganded conformations differing primarily in the relative positioning of the extracellular modules, of which the resolved structures represent only 60% of observations21. However, these static data offer no information about the sequence in which these structures occur. Molecular dynamics simulations are also unlikely to reveal how these structures interconvert partly due to an inability to simulate the relatively slow (ms to s) transition rates predicted by the model with current computation power128. For now, evidence is lacking on how the available structures relate to kinetic states.
In the statistical model, states are defined only by their most basic functional properties. C and O states differ in their electrical properties, and simply denote receptors with closed (non-conducting) or open (conducting) pores, respectively. From this definition, one can infer that C and O states differ at least in the position of residues that form the channel gate. Further, states differ in their ability to bind or dissociate ligand. For example, CU and CM can both bind glutamate, and CM and C3 can dissociate glutamate. Thus, these four states may represent receptors whose glutamate-binding pockets on GluN2 subunits are in extended conformations. Similarly, CU and CM can both bind glycine, and CM and C2 can dissociate glycine; therefore, they may represent receptors whose glycine-binding clefts on GluN1 subunits are extended. Conversely, neither glutamate nor glycine can dissociate from D1, D2, C1, or O states; therefore, these states may represent receptors with all ligand-binding pockets closed. Mutagenesis identified structural elements critical in receptor activation. Decoupling ligand binding from pore movements by inserting glycine residues in the connecting linkers selectively perturbed activation as revealed by single-channel analysis of these constructs97. Similarly, constraining the movement of transmembrane helices relative to pore axis severely perturbed gating illustrating the importance of these elements in the activation pathway100. However, it remains unclear how to integrate these additional conformational changes in the kinetic model.
Conformational changes in functional NMDA receptors are detected in functional receptors by measuring the relative distance between fluorescent probes. These studies were successful in identifying specific structural differences between NMDA receptor subtypes129, caused by allosteric modulators114,115, and agonist binding130–132. Presently, the sampling intervals required to capture sub-millisecond transition rates limit the reach of these optical approaches; similarly, limited spatial resolution precluded imaging transitions in single glutamate receptors; however, the success of single-molecule FRET in other channels133 may indicate feasibility for NMDA receptors as well.
Combining optical measurements with current output helps to develop and test hypotheses that assign specific conformational changes to individual transitions postulated by the statistical model. Furthermore, computational dynamics modelling based on the available NMDA receptor structures will provide more detailed structural models of activation128,134. As longer time scales become accessible this approach promises to bridge the present gap between structural and kinetic models of gating.
Nonionic outputs of NMDA receptors
Historically, NMDA receptor signals have been measured electrophysiologically as excitatory currents, or optically as changes in intracellular Ca2+ (Fig 1B). These approaches reflect the prominent NMDA receptors function as excitatory Ca2+-permeable receptors. Recently, two groups have reported evidence that agonist binding to NMDA receptors can launch intracellular signals that are independent of current flow. Glycine binding to the GluN1 subunits triggers reactions that prime NMDA receptors for clathrin-mediated endocytosis, a form of synaptic plasticity135. Conversely, agonist binding to GluN2 subunits causes conformational movements in the cytoplasmic domain, and initiates downstream cellular processes that result in long-term synaptic depression, even when GluN1 binding-sites and/or channel pores are blocked130,131.
The statistical model predicts that glycine and glutamate dissociate from receptor states with distinct lifetimes (C2 and C3, respectively)88. Although, how these states differ structurally is unknown, it is plausible that resting, liganded receptors differ not only in the atomic arrangement of extracellular domains, as demonstrated by crystallography and functional imaging but also in the arrangement of cytoplasmic domains, which presently remain unresolved. If this is indeed correct, the converse may be true as well: intracellular interactions that change the position of cytoplasmic domains will affect the receptors’ sensitivities to agonists. Therefore, the output of synaptic and non-synaptic receptors may be distinct not only by differences in agonist exposure but also by the atomic arrangements of the resting states, due to interactions with cellular factors.
A role for NMDA receptor conformational changes as the sole mediator of signal transduction adds a new level of interest to the electrically silent portion of the NMDA receptor activation reaction. Integrated electrophysiological and optical approaches promise to offer valuable information about electrically invisible portions of the model.
Conclusions and future perspectives
NMDA receptors mediate a substantial proportion of neural signaling. More than 50 trillion brain synapses are glutamatergic, and NMDA receptor currents consume 25% of the energy required by cortical neurons at rest136. To understand how NMDA receptor signals arise it is important to delineate not only general mechanisms of NMDA receptor activation and modulation, but also the details that allow NMDA receptors to produce its rich repertoire of electrical and conformational signals. The activation mechanisms of several diheteromeric NMDA receptor representatives have been investigated in detail. To date, little to no information is available about the functional output and operation of triheteromeric receptors. Given that they may represent a substantial fraction of native NMDA receptors, moving forward it will be important to investigate the output and operational mechanisms of these receptors as well137.
A large body of work demonstrates that although NMDA receptors share substantial sequence homology with AMPA and kainate receptors their output and operation are remarkably distinct. To delineate the basis for their separate mechanisms it will be important to focus more on the structural differences between receptor classes. NMDA receptor mechanisms represent valuable tools in delineating the structural correlates of function. To date, structural information exists for several atomic arrangements of GluN2B-containing NMDA receptors. Assembling these conformers into classes corresponding to each of the functional states identified by kinetic modeling, will remain a major line of investigation. Understanding how subtle differences in structure produce changes in specific rate constants holds enormous promise in rationally controlling NMDA receptor signals and may herald a new era of precision pharmacology.
Key Points.
NMDA receptor isoforms respond to glutamate with distinct kinetics and have dynamic, complex and incompletely delineated expression profiles; precise mechanistic information for specific receptor isoforms is derived from recombinant preparations.
Functional attributes of recombinant receptor current match well those of the NMDA receptor-mediated response recorded from synaptic and nonsynaptic native receptors.
Kinetic models derived from one-channel recordings reproduce all known features of the macroscopic response and reveal novel biophysical properties that underlie physiologically salient features of the synaptic current.
The NMDA receptor response amplitude and ionic charge transfer, which initiate synaptic plasticity, depend on stimulation frequency as predicted by the kinetic model.
The biphasic decay time of the NMDA receptor synaptic response, which sets the window for coincident depolarization, reflects the proportion of receptors gating in distinct kinetic modes. This insight was afforded by statistical evaluation of single-channel behavior.
Assigning molecular structures to the kinetic states postulated by statistically derived models of NMDA receptor activation is an active area of research.
Acknowledgments
We would like to thank members of the Popescu lab, and our editor and reviewers for insights and helpful suggestions.
Glossary
- Excitatory postsynaptic current
The net flow of positively charged ions into a postsynaptic neuron observed in response to spontaneously occurring or experimentally evoked neurotransmitter release. In the mammalian central nervous system this current is the glutamate-gated electrical output of multiple synaptic receptors (synaptic iGluRs)
- Single-channel record
Document that represents a digital sampling of electrical currents produced by the opening of individual channel proteins; it can register the activity of one or several simultaneously active channels.
- Open probability
Parameter used to express quantitatively the activity of ion channels; it expresses the fraction of time during which the channel is open and allows ionic flow.
- Open time
The mean duration of openings defined in the single-channel record as events with non-zero amplitude; metric that correlates with the stability of conducting channel conformations.
- Closed time
The mean duration of closures defined in the single-channel record as periods of zero-current amplitude; metric that correlates with the stability of non-conducting conformations.
- Bursts
Sequence of openings and brief closures; for ion channels whose single-channel record contains closures of n distinct durations, n − 1 types of bursts can be defined to contain from 1 to n − 1 types of closures.
- Modes
Distinct patterns of activity that can be discerned in the single-channel record of almost all ion channels; each kinetic pattern or mode reflects a unique reaction mechanism, characterized by different numbers or arrangements of states or/and different values for particular rate constants.
- Reaction mechanism
The pathway of energy changes experienced by a molecule during a conformational or chemical transformation; it postulates a number of elementary states in which the system can be found, how these states can interconvert, and the rates with which these steps occur.
- Binding reaction
The physical association between two initially separate entities; it describes the transition from apo (unbound) to liganded receptor states.
- Gating reactions
Molecular isomerizations between inactive and active states; specifically, for ion channels, gating refers to the transitions that connect closed non-conducting to open ion-conducting conformations.
- Rate constants
Numbers that define the frequency with which transitions occur. They are expressed in s−1 for isomerization reactions.
- Resting states
Families of conformations defined functionally by their ability to recognize and bind agonist and inability to generate an electrical signal; for glutamate receptors, qualifying structures must have the glutamate-binding cleft inan extended binding-compatible conformation and the pore closed (non-conducting).
- Open states
Families of kinetic states defined functionally by their ability to pass current; for glutamate receptors, qualifying structures must have the glutamate-binding cleft in a contracted (‘closed’) conformation that is incompatible with agonist binding or dissociation, and the pore open (conducting).
- Desensitized states
Families of conformations defined functionally by their inability to bind or dissociate agonist and inability to pass current; for glutamate receptors, qualifying structures must have the glutamate-binding cleft in a tight binding-incompatible conformation and the pore closed (non-conducting).
- Simulations
In silico calculations used to predict time-dependent occupancies for kinetic states given a reaction mechanism (model), initial state occupancies, and a stimulus defined by duration and amplitude.
Biographies
Gary J Iacobucci
Gary Iacobucci holds B.S. in Biological Sciences and B.A. in Psychology degrees from the University at Buffalo, SUNY while pursuing a PhD in Biochemistry at the Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, SUNY. He uses electrophysiological and computational approaches to investigate Ca2+-dependent regulation of NMDA receptors.
Gabriela K Popescu
Gabriela Popescu is Professor of Biochemistry and Anesthesiology in the Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, SUNY. Her laboratory studies mechanisms of excitatory transmission in the mammalian central nervous system. Current projects aim to match conformational dynamics of synaptic excitatory channels with neuronal function and dysfunction.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutrition. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- 2.Traynelis SF, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nat. Rev. Neurosci. 2005;6:841–849. doi: 10.1038/nrn1784. [DOI] [PubMed] [Google Scholar]
- 4.Karakas E, Furukawa H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science. 2014;344:992–997. doi: 10.1126/science.1251915. One of a two of labs to report the first full tetrameric receptor of GluN1/GluN2B was reported revealing unique architecture that distinguishes NMDA receptors from other ionotropic glutamate receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CH, et al. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature. 2014;511:191–197. doi: 10.1038/nature13548. One of a two of labs to report the first full tetrameric receptor of GluN1/GluN2B was reported revealing unique architecture that distinguishes NMDA receptors from other ionotropic glutamate receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. The first crystal structure of a full tetrameric ionotropic glutamate receptor allowed researchers to both directly explore structure-function relationships in AMPA receptors and infer structural interpretations of NMDA receptor function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durr KL, et al. Structure and dynamics of AMPA receptor GluA2 in resting, pre-open, and desensitized states. Cell. 2014;158:778–792. doi: 10.1016/j.cell.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyerson JR, et al. Structural basis of kainate subtype glutamate receptor desensitization. Nature. 2016;537:567–571. doi: 10.1038/nature19352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyerson JR, et al. Structural mechanism of glutamate receptor activation and desensitization. Nature. 2014;514:328–334. doi: 10.1038/nature13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 11.Pachernegg S, Strutz-Seebohm N, Hollmann M. GluN3 subunit-containing NMDA receptors: not just one-trick ponies. Trends Neurosci. 2012;35:240–249. doi: 10.1016/j.tins.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Zukin RS, Bennett MV. Alternatively spliced isoforms of the NMDARI receptor subunit. Trends Neurosci. 1995;18:306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]
- 13.Seeburg PH, et al. The NMDA receptor channel: molecular design of a coincidence detector. Rec. Prog. Hormone Res. 1995;50:19–34. doi: 10.1016/b978-0-12-571150-0.50006-8. [DOI] [PubMed] [Google Scholar]
- 14.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 15.Glasgow NG, Siegler Retchless B, Johnson JW. Molecular bases of NMDA receptor subtype-dependent properties. J Physiol. 2015;593:83–95. doi: 10.1113/jphysiol.2014.273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci. STKE. 2004:re16. doi: 10.1126/stke.2552004re16. 2004. [DOI] [PubMed] [Google Scholar]
- 17.Hansen KB, Ogden KK, Yuan H, Traynelis SF. Distinct functional and pharmacological properties of Triheteromeric GluN1/GluN2A/GluN2B NMDA receptors. Neuron. 2014;81:1084–1096. doi: 10.1016/j.neuron.2014.01.035. Because recombinant systems restricted functional studies to diheteromeric receptors, the authors develop a method to overcome this limitation and characterized for the first time unique pharmacological properties of triheteromeric NMDA receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ceccon M, Rumbaugh G, Vicini S. Distinct effect of pregnenolone sulfate on NMDA receptor subtypes. Neuropharmacol. 2001;40:491–500. doi: 10.1016/s0028-3908(00)00197-0. [DOI] [PubMed] [Google Scholar]
- 19.Rumbaugh G, Vicini S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci. 1999;19:10603–10610. doi: 10.1523/JNEUROSCI.19-24-10603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vicini S, et al. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. A complete functional characterization of molecularly pure NMDA receptor subtypes in a recombinant system definitively revealed subtype-specific kinetic and pharmacological properties. [DOI] [PubMed] [Google Scholar]
- 21.Tajima N, et al. Activation of NMDA receptors and the mechanism of inhibition by ifenprodil. Nature. 2016 doi: 10.1038/nature17679. Using cryo-EM, several unique closed-state molecular structures are observed in the presence of agonist, thus, substantiating electrophysiological observations of complex close time distributions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy MB, Beale HC, Carlisle HJ, Washburn LR. Integration of biochemical signalling in spines. Nat. Rev. Neurosci. 2005;6:423–434. doi: 10.1038/nrn1685. [DOI] [PubMed] [Google Scholar]
- 23.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr. Op. Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Forsythe ID, Westbrook GL. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collingridge GL, Herron CE, Lester RA. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J Physiol. 1988;399:301–312. doi: 10.1113/jphysiol.1988.sp017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogan-Cann AD, Anderson CM. Physiological Roles of Non-Neuronal NMDA Receptors. Trends Pharmacol. Sci. 2016 doi: 10.1016/j.tips.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Bouvier G, Bidoret C, Casado M, Paoletti P. Presynaptic NMDA receptors: Roles and rules. Neuroscience. 2015;311:322–340. doi: 10.1016/j.neuroscience.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 29.Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- 30.Kalia LV, Pitcher GM, Pelkey KA, Salter MW. PSD-95 is a negative regulator of the tyrosine kinase Src in the NMDA receptor complex. EMBO J. 2006;25:4971–4982. doi: 10.1038/sj.emboj.7601342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng D, et al. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol. 2009;7:e41. doi: 10.1371/journal.pbio.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rycroft BK, Gibb AJ. Regulation of single NMDA receptor channel activity by alpha-actinin and calmodulin in rat hippocampal granule cells. J Physiol. 2004;557:795–808. doi: 10.1113/jphysiol.2003.059212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bard L, et al. Dynamic and specific interaction between synaptic NR2-NMDA receptor and PDZ proteins. Proc Natl Acad Sci U S A. 2010;107:19561–19566. doi: 10.1073/pnas.1002690107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vergnano AM, et al. Zinc dynamics and action at excitatory synapses. Neuron. 2014;82:1101–1114. doi: 10.1016/j.neuron.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 35.Amico-Ruvio SA, Paganelli MA, Myers JM, Popescu GK. Ifenprodil effects on GluN2B-containing glutamate receptors. Mol. Pharmacol. 2012;82:1074–1081. doi: 10.1124/mol.112.078998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Legendre P, Westbrook GL. Ifenprodil blocks N-methyl-D-aspartate receptors by a two-component mechanism. Mol. Pharmacol. 1991;40:289–298. [PubMed] [Google Scholar]
- 37.Blanpied TA, Clarke RJ, Johnson JW. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J Neurosci. 2005;25:3312–3322. doi: 10.1523/JNEUROSCI.4262-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang S, Ehlers MD, Bernhardt JP, Su CT, Huganir RL. Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors. Neuron. 1998;21:443–453. doi: 10.1016/s0896-6273(00)80553-x. [DOI] [PubMed] [Google Scholar]
- 39.Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995;267:1510–1512. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- 40.Tingley WG, et al. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol. Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman AM, et al. Opposing roles of synaptic and extrasynaptic NMDA receptor signaling in cocultured striatal and cortical neurons. J Neurosci. 2012;32:3992–4003. doi: 10.1523/JNEUROSCI.4129-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 43.Wyllie DJ, Behe P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol. 1998;510(Pt 1):1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amico-Ruvio SA, Popescu GK. Stationary gating of GluN1/GluN2B receptors in intact membrane patches. Biophys. J. 2010;98:1160–1169. doi: 10.1016/j.bpj.2009.12.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dravid SM, Prakash A, Traynelis SF. Activation of recombinant NR1/NR2C NMDA receptors. J Physiol. 2008;586:4425–4439. doi: 10.1113/jphysiol.2008.158634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borschel WF, et al. Gating reaction mechanism of neuronal NMDA receptors. J Neurophysiol. 2012;108:3105–3115. doi: 10.1152/jn.00551.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark BA, Farrant M, Cull-Candy SG. A direct comparison of the single-channel properties of synaptic and extrasynaptic NMDA receptors. J Neurosci. 1997;17:107–116. doi: 10.1523/JNEUROSCI.17-01-00107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jahr CE, Stevens CF. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987;325:522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- 49.Ascher P, Bregestovski P, Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stern P, Behe P, Schoepfer R, Colquhoun D. Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proc. Biol. Sci. 1992;250:271–277. doi: 10.1098/rspb.1992.0159. [DOI] [PubMed] [Google Scholar]
- 51.Howe JR, Colquhoun D, Cull-Candy SG. On the kinetics of large-conductance glutamate-receptor ion channels in rat cerebellar granule neurons. Proc. R. Soc. Lond. B. Biol. Sci. 1988;233:407–422. doi: 10.1098/rspb.1988.0030. [DOI] [PubMed] [Google Scholar]
- 52.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 53.Vance KM, Hansen KB, Traynelis SF. GluN1 splice variant control of GluN1/GluN2D NMDA receptors. J Physiol. 2012;590:3857–3875. doi: 10.1113/jphysiol.2012.234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siegler Retchless B, Gao W, Johnson JW. A single GluN2 subunit residue controls NMDA receptor channel properties via intersubunit interaction. Nat. Neurosci. 2012;15:406–413. S401–402. doi: 10.1038/nn.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green GM, Gibb AJ. Characterization of the single-channel properties of NMDA receptors in laminae I and II of the dorsal horn of neonatal rat spinal cord. Eur. J Neurosci. 2001;14:1590–1602. doi: 10.1046/j.0953-816x.2001.01790.x. [DOI] [PubMed] [Google Scholar]
- 56.Palecek JI, Abdrachmanova G, Vlachova V, Vyklick L., Jr Properties of NMDA receptors in rat spinal cord motoneurons. Eur. J Neurosci. 1999;11:827–836. doi: 10.1046/j.1460-9568.1999.00489.x. [DOI] [PubMed] [Google Scholar]
- 57.Cull-Candy SG, et al. NMDA receptor diversity in the cerebellum: identification of subunits contributing to functional receptors. Neuropharmacol. 1998;37:1369–1380. doi: 10.1016/s0028-3908(98)00119-1. [DOI] [PubMed] [Google Scholar]
- 58.Gibb AJ, Kojima H, Carr JA, Colquhoun D. Expression of cloned receptor subunits produces multiple receptors. Proc. Biol. Sci. 1990;242:108–112. doi: 10.1098/rspb.1990.0112. [DOI] [PubMed] [Google Scholar]
- 59.Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Brit. J Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benveniste M, Clements J, Vyklicky L, Jr, Mayer ML. A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol. 1990;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lester RA, Clements JD, Westbrook GL, Jahr CE. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990;346:565–567. doi: 10.1038/346565a0. By using precisely timed application of competative antagonists before and after application of agonist, the authors reveal that the intrinsic gating kinetics of the channel set the time-course of synaptic decay rather than agonist affinity. [DOI] [PubMed] [Google Scholar]
- 62.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 63.Lester RA, Jahr CE. NMDA channel behavior depends on agonist affinity. J Neurosci. 1992;12:635–643. doi: 10.1523/JNEUROSCI.12-02-00635.1992. The authors develop the first minimal conceptual model needed to describe the macroscopic current in response to brief pulses of agonist with separate kinetic states for glutamate binding, desensitization, and gating. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clements JD, Westbrook GL. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron. 1991;7:605–613. doi: 10.1016/0896-6273(91)90373-8. [DOI] [PubMed] [Google Scholar]
- 65.Rosenmund C, Feltz A, Westbrook GL. Synaptic NMDA receptor channels have a low open probability. J Neurosci. 1995;15:2788–2795. doi: 10.1523/JNEUROSCI.15-04-02788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benveniste M, Mayer ML. Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. Biophys. J. 1991;59:560–573. doi: 10.1016/S0006-3495(91)82272-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kampa BM, Clements J, Jonas P, Stuart GJ. Kinetics of Mg2+ unblock of NMDA receptors: implications for spike-timing dependent synaptic plasticity. J Physiol. 2004;556:337–345. doi: 10.1113/jphysiol.2003.058842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nahum-Levy R, Lipinski D, Shavit S, Benveniste M. Desensitization of NMDA receptor channels is modulated by glutamate agonists. Biophys. J. 2001;80:2152–2166. doi: 10.1016/S0006-3495(01)76188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dilmore JG, Johnson JW. Open channel block and alteration of N-methyl-D-aspartic acid receptor gating by an analog of phencyclidine. Biophys. J. 1998;75:1801–1816. doi: 10.1016/S0006-3495(98)77622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen N, Li B, Murphy TH, Raymond LA. Site within N-Methyl-D-aspartate receptor pore modulates channel gating. Mol. Pharmacol. 2004;65:157–164. doi: 10.1124/mol.65.1.157. [DOI] [PubMed] [Google Scholar]
- 71.Kloda A, Clements JD, Lewis RJ, Adams DJ. Adenosine triphosphate acts as both a competitive antagonist and a positive allosteric modulator at recombinant N-methyl-D-aspartate receptors. Mol. Pharmacol. 2004;65:1386–1396. doi: 10.1124/mol.65.6.1386. [DOI] [PubMed] [Google Scholar]
- 72.Horak M, Vlcek K, Petrovic M, Chodounska H, Vyklicky L., Jr Molecular mechanism of pregnenolone sulfate action at NR1/NR2B receptors. J Neurosci. 2004;24:10318–10325. doi: 10.1523/JNEUROSCI.2099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan H, Hansen KB, Vance KM, Ogden KK, Traynelis SF. Control of NMDA receptor function by the NR2 subunit amino-terminal domain. J Neurosci. 2009;29:12045–12058. doi: 10.1523/JNEUROSCI.1365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qian A, Buller AL, Johnson JW. NR2 subunit-dependence of NMDA receptor channel block by external Mg2+ J Physiol. 2005;562:319–331. doi: 10.1113/jphysiol.2004.076737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kleckner NW, Pallotta BS. Burst kinetics of single NMDA receptor currents in cell-attached patches from rat brain cortical neurons in culture. J Physiol. 1995;486(Pt 2):411–426. doi: 10.1113/jphysiol.1995.sp020822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schorge S, Elenes S, Colquhoun D. Maximum likelihood fitting of single channel NMDA activity with a mechanism composed of independent dimers of subunits. J Physiol. 2005;569:395–418. doi: 10.1113/jphysiol.2005.095349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niciu MJ, Henter ID, Luckenbaugh DA, Zarate CA, Jr, Charney DS. Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu. Rev. Pharmacol. Toxicol. 2014;54:119–139. doi: 10.1146/annurev-pharmtox-011613-135950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O'Leary T, Wyllie DJ. Single-channel properties of N-methyl-D-aspartate receptors containing chimaeric GluN2A/GluN2D subunits. Biochem. Soc. Trans. 2009;37:1347–1354. doi: 10.1042/BST0371347. [DOI] [PubMed] [Google Scholar]
- 79.Gibb AJ, Colquhoun D. Glutamate activation of a single NMDA receptor-channel produces a cluster of channel openings. Proc. Biol. Sci. 1991;243:39–45. doi: 10.1098/rspb.1991.0007. [DOI] [PubMed] [Google Scholar]
- 80.Howe JR, Cull-Candy SG, Colquhoun D. Currents through single glutamate receptor channels in outside-out patches from rat cerebellar granule cells. J Physiol. 1991;432:143–202. doi: 10.1113/jphysiol.1991.sp018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stern P, Cik M, Colquhoun D, Stephenson FA. Single channel properties of cloned NMDA receptors in a human cell line: comparison with results from Xenopus oocytes. J Physiol. 1994;476:391–397. doi: 10.1113/jphysiol.1994.sp020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Auerbach A, Zhou Y. Gating reaction mechanisms for NMDA receptor channels. J Neurosci. 2005;25:7914–7923. doi: 10.1523/JNEUROSCI.1471-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Popescu G, Auerbach A. Modal gating of NMDA receptors and the shape of their synaptic response. Nat. Neurosci. 2003;6:476–483. doi: 10.1038/nn1044. Analysis of stationary single-channel gating records reveals robust heterogeneity in the kinetics, known as modal gating, over time allowing the first complete kinetic mechanism of channel activation to be derived. [DOI] [PubMed] [Google Scholar]
- 84.Popescu G, Robert A, Howe JR, Auerbach A. Reaction mechanism determines NMDA receptor response to repetitive stimulation. Nature. 2004;430:790–793. doi: 10.1038/nature02775. [DOI] [PubMed] [Google Scholar]
- 85.Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat. Neurosci. 2003;6:144–152. doi: 10.1038/nn1000. Using stationary single-channel recordings, the first complete kientic mechanism was derived for GluN1/GluN2B diheteromeric receptors. [DOI] [PubMed] [Google Scholar]
- 86.Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kussius CL, Popescu GK. NMDA receptors with locked glutamate-binding clefts open with high efficacy. J Neurosci. 2010;30:12474–12479. doi: 10.1523/JNEUROSCI.3337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cummings KA, Popescu GK. Glycine-dependent activation of NMDA receptors. J Gen. Physiol. 2015;145:513–527. doi: 10.1085/jgp.201411302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kussius CL, Popescu GK. Kinetic basis of partial agonism at NMDA receptors. Nat. Neurosci. 2009;12:1114–1120. doi: 10.1038/nn.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bicknell BA, Goodhill GJ. Emergence of ion channel modal gating from independent subunit kinetics. Proc. Natl. Acad. Sci. USA. 2016;113:E5288–5297. doi: 10.1073/pnas.1604090113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ji S, Sun W, George AL, Jr, Horn R, Barchi RL. Voltage-dependent regulation of modal gating in the rat SkM1 sodium channel expressed in Xenopus oocytes. J Gen. Physiol. 1994;104:625–643. doi: 10.1085/jgp.104.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alzheimer C, Schwindt PC, Crill WE. Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J Neurosci. 1993;13:660–673. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Popescu G. Mechanism-based targeting of NMDA receptor functions. Cell. Mol. Life Sci. 2005;62:2100–2111. doi: 10.1007/s00018-005-5227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aman TK, Maki BA, Ruffino TJ, Kasperek EM, Popescu GK. Separate intramolecular targets for protein kinase A control N-methyl-D-aspartate receptor gating and Ca2+ permeability. J Biol. Chem. 2014;289:18805–18817. doi: 10.1074/jbc.M113.537282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Borschel WF, Cummings KA, Tindell LK, Popescu GK. Kinetic contributions to gating by interactions unique to N-methyl-D-aspartate (NMDA) receptors. J Biol. Chem. 2015;290:26846–26855. doi: 10.1074/jbc.M115.678656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borschel WF, Murthy SE, Kasperek EM, Popescu GK. NMDA receptor activation requires remodelling of intersubunit contacts within ligand-binding heterodimers. Nat. Comm. 2011;2:498. doi: 10.1038/ncomms1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kazi R, Dai J, Sweeney C, Zhou HX, Wollmuth LP. Mechanical coupling maintains the fidelity of NMDA receptor-mediated currents. Nat. Neurosci. 2014;17:914–922. doi: 10.1038/nn.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Erreger K, Traynelis SF. Zinc inhibition of rat NR1/NR2A N-methyl-D-aspartate receptors. J Physiol. 2008;586:763–778. doi: 10.1113/jphysiol.2007.143941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vance KM, Hansen KB, Traynelis SF. Modal gating of GluN1/GluN2D NMDA receptors. Neuropharmacol. 2013;71:184–190. doi: 10.1016/j.neuropharm.2013.03.018. The authors use single-channel gating records to derived the first single-channel mechanism of GluN1/GluN2D diheteromeric receptors including identifying heterogenous modal gating behaviors for this subtype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kazi R, et al. Asynchronous movements prior to pore opening in NMDA receptors. J Neurosci. 2013;33:12052–12066. doi: 10.1523/JNEUROSCI.5780-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang W, Howe JR, Popescu GK. Distinct gating modes determine the biphasic relaxation of NMDA receptor currents. Nat. Neurosci. 2008;11:1373–1375. doi: 10.1038/nn.2214. The first tiered model of NMDA receptor modal gating was proposed and experimentally validated as a mechanism for the multi-component synaptic response in neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kirson ED, Yaari Y. Synaptic NMDA receptors in developing mouse hippocampal neurones: functional properties and sensitivity to ifenprodil. J Physiol. 1996;497(Pt 2):437–455. doi: 10.1113/jphysiol.1996.sp021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lindlbauer R, Mohrmann R, Hatt H, Gottmann K. Regulation of kinetic and pharmacological properties of synaptic NMDA receptors depends on presynaptic exocytosis in rat hippocampal neurones. J Physiol. 1998;508(Pt 2):495–502. doi: 10.1111/j.1469-7793.1998.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Balu DT, Coyle JT. The NMDA receptor ‘glycine modulatory site’ in schizophrenia: d-serine, glycine, and beyond. Curr. Opin. Pharmacol. 2015;20:109–115. doi: 10.1016/j.coph.2014.12.004. doi: http://dx.doi.org/10.1016/j.coph.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- 106.Forsythe ID, Westbrook GL, Mayer ML. Modulation of excitatory synaptic transmission by glycine and zinc in cultures of mouse hippocampal neurons. J Neurosci. 1988;8:3733–3741. doi: 10.1523/JNEUROSCI.08-10-03733.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peters S, Koh J, Choi DW. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987;236:589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- 108.Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fayyazuddin A, Villarroel A, Le Goff A, Lerma J, Neyton J. Four residues of the extracellular N-terminal domain of the NR2A subunit control high-affinity Zn2+ binding to NMDA receptors. Neuron. 2000;25:683–694. doi: 10.1016/s0896-6273(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 110.Paoletti P, et al. Molecular organization of a zinc binding N-terminal modulatory domain in a NMDA receptor subunit. Neuron. 2000;28:911–925. doi: 10.1016/s0896-6273(00)00163-x. [DOI] [PubMed] [Google Scholar]
- 111.Nozaki C, et al. Zinc alleviates pain through high-affinity binding to the NMDA receptor NR2A subunit. Nat. Neurosci. 2011;14:1017–1022. doi: 10.1038/nn.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Amico-Ruvio SA, Murthy SE, Smith TP, Popescu GK. Zinc effects on NMDA receptor gating kinetics. Biophys. J. 2011;100:1910–1918. doi: 10.1016/j.bpj.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zheng F, et al. Allosteric interaction between the amino terminal domain and the ligand binding domain of NR2A. Nat. Neurosci. 2001;4:894–901. doi: 10.1038/nn0901-894nn0901-894. [pii] [DOI] [PubMed] [Google Scholar]
- 114.Sirrieh RE, MacLean DM, Jayaraman V. Amino-terminal domain tetramer organization and structural effects of zinc binding in the N-methyl-D-aspartate (NMDA) receptor. J Biol. Chem. 2013;288:22555–22564. doi: 10.1074/jbc.M113.482356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sirrieh RE, MacLean DM, Jayaraman V. A conserved structural mechanism of NMDA receptor inhibition: A comparison of ifenprodil and zinc. J Gen. Physiol. 2015;146:173–181. doi: 10.1085/jgp.201511422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karakas E, Simorowski N, Furukawa H. Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J. 2009;28:3910–3920. doi: 10.1038/emboj.2009.338. doi:emboj2009338 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- 118.Harsing LG, Jr, Matyus P. Mechanisms of glycine release, which build up synaptic and extrasynaptic glycine levels: the role of synaptic and non-synaptic glycine transporters. Brain Res. Bull. 2013;93:110–119. doi: 10.1016/j.brainresbull.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 119.Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc. Natl Acad. Sci. USA. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Benveniste M, Clements J, Vyklicky L, Jr, Mayer ML. A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol. (Lond) 1990;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Furukawa H, Gouaux E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J. 2003;22:2873–2885. doi: 10.1093/emboj/cdg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cooper DR, et al. Conformational transitions in the glycine-bound GluN1 NMDA receptor LBD via single-molecule FRET. Biophys. J. 2015;109:66–75. doi: 10.1016/j.bpj.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dolino DM, et al. Structural dynamics of the glycine-binding domain of the N-methyl-D-aspartate receptor. J Biol. Chem. 2015;290:797–804. doi: 10.1074/jbc.M114.605436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yao Y, Belcher J, Berger Anthony J, Mayer Mark L, Lau Albert Y. Conformational Analysis of NMDA Receptor GluN1, GluN2, and GluN3 Ligand-Binding Domains Reveals Subtype-Specific Characteristics. Structure. 2013 doi: 10.1016/j.str.2013.07.011. doi: http://dx.doi.org/10.1016/j.str.2013.07.011. [DOI] [PMC free article] [PubMed]
- 125.Vargas-Caballero M, Robinson HP. Fast and slow voltage-dependent dynamics of magnesium block in the NMDA receptor: the asymmetric trapping block model. J Neurosci. 2004;24:6171–6180. doi: 10.1523/JNEUROSCI.1380-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Clarke RJ, Glasgow NG, Johnson JW. Mechanistic and structural determinants of NMDA receptor voltage-dependent gating and slow Mg2+ unblock. J Neurosci. 2013;33:4140–4150. doi: 10.1523/JNEUROSCI.3712-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004;1:101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dai J, Zhou HX. An NMDA receptor gating mechanism developed from MD simulations reveals molecular details underlying subunit-specific contributions. Biophys J. 2013;104:2170–2181. doi: 10.1016/j.bpj.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sirrieh RE, MacLean DM, Jayaraman V. Subtype-dependent N-methyl-D-aspartate receptor amino-terminal domain conformations and modulation by spermine. J Biol. Chem. 2015;290:12812–12820. doi: 10.1074/jbc.M115.649723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aow J, Dore K, Malinow R. Conformational signaling required for synaptic plasticity by the NMDA receptor complex. Proc. Natl. Acad. Sci. USA. 2015;112:14711–14716. doi: 10.1073/pnas.1520029112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dore K, Aow J, Malinow R. Agonist binding to the NMDA receptor drives movement of its cytoplasmic domain without ion flow. Proc. Natl. Acad. Sci. USA. 2015;112:14705–14710. doi: 10.1073/pnas.1520023112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rambhadran A, Gonzalez J, Jayaraman V. Conformational changes at the agonist binding domain of the N-methyl-D-aspartic acid receptor. J Biol. Chem. 2011;286:16953–16957. doi: 10.1074/jbc.M111.224576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang S, Vafabakhsh R, Borschel WF, Ha T, Nichols CG. Structural dynamics of potassium-channel gating revealed by single-molecule FRET. Nat. Struct. Mol. Biol. 2016;23:31–36. doi: 10.1038/nsmb.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dai J, Zhou HX. Semiclosed Conformations of the Ligand-Binding Domains of NMDA Receptors during Stationary Gating. Biophys. J. 2016;111:1418–1428. doi: 10.1016/j.bpj.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nong Y, et al. Glycine binding primes NMDA receptor internalization. Nature. 2003;422:302–307. doi: 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- 136.Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 137.Tovar KR, McGinley MJ, Westbrook GL. Triheteromeric NMDA receptors at hippocampal synapses. J Neurosci. 2013;33:9150–9160. doi: 10.1523/JNEUROSCI.0829-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 139.Kumar SS, Huguenard JR. Pathway-Specific Differences in Subunit Composition of Synaptic NMDA Receptors on Pyramidal Neurons in Neocortex. J Neurosci. 2003;23:10074–10083. doi: 10.1523/JNEUROSCI.23-31-10074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dzubay JA, Jahr CE. The concentration of synaptically released glutamate outside of the climbing fiber-Purkinje cell synaptic cleft. J Neurosci. 1999;19:5265–5274. doi: 10.1523/JNEUROSCI.19-13-05265.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bengtson CP, Dick O, Bading H. A quantitative method to assess extrasynaptic NMDA receptor function in the protective effect of synaptic activity against neurotoxicity. BMC Neurosci. 2008;9:11. doi: 10.1186/1471-2202-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cho CH, St-Gelais F, Zhang W, Tomita S, Howe JR. Two families of TARP isoforms that have distinct effects on the kinetic properties of AMPA receptors and synaptic currents. Neuron. 2007;55:890–904. doi: 10.1016/j.neuron.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 144.Sobczyk A, Svoboda K. Activity-dependent plasticity of the NMDA-receptor fractional Ca2+ current. Neuron. 2007;53:17–24. doi: 10.1016/j.neuron.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 145.Zito K, Scheuss V, Knott G, Hill T, Svoboda K. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61:247–258. doi: 10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bardoni R, Magherini PC, MacDermott AB. NMDA EPSCs at glutamatergic synapses in the spinal cord dorsal horn of the postnatal rat. J Neurosci. 1998;18:6558–6567. doi: 10.1523/JNEUROSCI.18-16-06558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nie H, Weng HR. Impaired glial glutamate uptake induces extrasynaptic glutamate spillover in the spinal sensory synapses of neuropathic rats. J Neurophysiol. 2010;103:2570–2580. doi: 10.1152/jn.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]