Abstract
BACKGROUND:
Occipital lobe epilepsy (OLE) is an uncommon but debilitating focal epilepsy syndrome with seizures often refractory to medical management. While surgical resection has proven a viable treatment, previous studies examining postoperative seizure freedom rates are limited by small sample size and patient heterogeneity, thus exhibiting significant variability in their results.
OBJECTIVE
To review the medical literature on OLE so as to investigate rates and predictors of both seizure freedom and visual outcomes following surgery.
METHODS
We reviewed manuscripts exploring surgical resection for drug-resistant OLE published between January 1990 and June 2015 on PubMed. Seizure freedom rates were analyzed and potential predictors were evaluated with separate meta-analyses. Postoperative visual outcomes were also examined.
RESULTS
We identified 27 case series comprising 584 patients with greater than 1 yr of follow-up. Postoperative seizure freedom (Engel class I outcome) was observed in 65% of patients, and was significantly predicted by age less than 18 yr (odds ratio [OR] 1.54, 95% confidence interval [CI] 1.13-2.18), focal lesion on pathological analysis (OR 2.08, 95% CI 1.58-2.89), and abnormal preoperative magnetic resonance imaging (OR 3.24, 95% 2.03-6.55). Of these patients, 175 also had visual outcomes reported with 57% demonstrating some degree of visual decline following surgery. We did not find any relationship between postoperative visual and seizure outcomes.
CONCLUSION
Surgical resection for OLE is associated with favorable outcomes with nearly two-thirds of patients achieving postoperative seizure freedom. However, patients must be counseled regarding the risk of visual decline following surgery.
Keywords: Epilepsy, Extra-temporal, Occipital lobe, Outcome, Resection, Surgery
ABBREVIATIONS
- CI
confidence interval
- EEG
electroencephalography
- ETLE
extra-temporal lobe epilepsy
- MCD
malformation of cortical dysplasia
- MRI
magnetic resonance imaging
- OLE
occipital lobe epilepsy
- OR
odds ratio
- TLE
temporal lobe epilepsy
Occipital lobe epilepsy (OLE) is relatively uncommon, accounting for 5% to 10% of focal epilepsy cases.1-3 Causes are wide-ranging and include metabolic, structural, neoplastic, traumatic, infective, and idiopathic etiologies.1 OLE semiology often involves visual symptoms including hallucinations, blindness, eye blinking, and nystagmus.4-6 However, OLE can also manifest with altered mental status and generalized tonic-clonic activity, suggesting electrical spread to neighboring cortical regions, and complicating accurate diagnosis and seizure localization.7 Overall, OLE is often a severely debilitating disorder with significant impact on patient quality of life.
For OLE patients with medically refractory seizures, focal surgical resection has proven a viable therapeutic option, with many studies reporting postoperative seizure freedom in the majority of patients.3,4,8-11 Despite this reported success, OLE surgery is not without its challenges. While OLE can arise from an identifiable focal lesion, such as a malformation of cortical dysplasia (MCD) or tumor, identifying a seizure focus in many other cases can be challenging, as magnetic resonance imaging (MRI) and ictal electroencephalography (EEG) are not always localizing.1,3,12,13 Additionally, because of anatomic proximity to the visual cortex, OLE surgery is associated with the risk of postoperative visual decline.8,13-15
In previous reports of resective epilepsy surgery, seizure freedom is achieved in approximately 60% to 80% of individuals with temporal lobe epilepsy (TLE) and 40% to 60% of patients with extra-temporal lobe epilepsy (ETLE).16-18 Compared to other focal epilepsies, OLE is less common and thus relatively understudied. The case studies that are available show significant variability in their results due to their generally small sample size and patient heterogeneity. No randomized-controlled trials or systematic reviews of OLE surgical outcomes have yet been reported. Consequently, specific rates and predictors of both seizure freedom and visual decline following surgery are not known. Such information is critical to improve patient selection for OLE surgery. In this paper, we provide first the systematic review and meta-analysis of outcomes following surgical resection for drug-resistant OLE.
METHODS
Article Selection and Data Extraction
A PubMed search was performed for unique entries between January 1990 and June 2015 using the following query guidelines: ((occipital AND (seizure* OR epilepsy)) AND (surgery OR surgical OR resection)). We selected 1990 as a starting date because prior to this few papers were published examining OLE surgery outcomes and of those available, many used surgical techniques different from modern therapeutic approaches. Inclusion criteria required that each manuscript be a peer-reviewed clinical investigative study or case series of seizure outcome in surgical treatment of occipital, temporo-occipital, and/or parieto-occipital epilepsy. As such, case reports, review articles, and conference abstracts or papers were not included. Exclusion criteria included (1) <5 patients with epilepsy involving the occipital lobe (5 being the minimum number of patients needed to limit bias in analysis as we have established in previous systematic reviews17,19), (2) <12 mo mean or median follow-up so as to ensure stability of Engel outcomes as previously demonstrated,20 (3) data redundant with another manuscript, (4) outcomes for epilepsy involving the occipital lobe unable to be separated from nonoccipital cases, (5) seizure outcome not reported using the Engel et al21 or a directly translatable scale, (6) patients treated with disconnective surgery, such as corpus callostomy or multiple subpial transections, and (7) body of manuscript not in English. Studies including resections for epilepsies not involving the occipital lobe were included in part where data could be disaggregated. This article selection process is summarized in Figure 1, with included studies listed in Table 1. Two separate reviewers applied the inclusion criteria to the PubMed search result; there were no disagreements. Three separate reviewers applied the exclusion criteria to the remaining articles. There were 2 instances of disagreement, and in each case, the opinion of the 2 agreeing authors was used. This study was planned and executed in accordance with published Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.22
FIGURE 1.
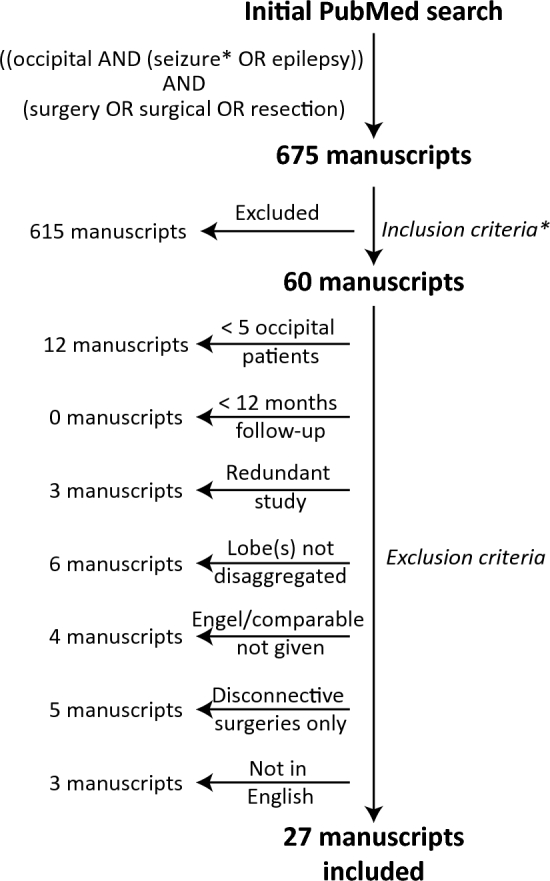
Flow chart summarizing the manuscript selection process. Overall, 675 manuscripts were examined, 60 met inclusion criteria, and 27 met both inclusion and exclusion criteria. *Inclusion criteria are listed in the Methods.
TABLE 1.
Included Studies
| First author | Year | No. | Age group | Invasive monitoring (no.)a | % Seizure freedom |
|---|---|---|---|---|---|
| Appel25 | 2015 | 19 | Adults | Yes (16) | 42.1 |
| Aykut-Bingol5 | 1998 | 35 | Mixed | Yes (19) | 45.7 |
| Barba26 | 2005 | 9 | Mixed | Yes (2) | 66.7 |
| Battaglia14 | 2012 | 7 | Pediatric | No | 57.1 |
| Bautista27 | 1999 | 7 | Mixed | Yes (13) | 28.6 |
| Bidziński11 | 1992 | 11 | Mixed | No | 91.0 |
| Binder13 | 2008 | 52 | Mixed | Yes (22) | 69.2 |
| Blume28 | 1991 | 16 | Mixed | Yes (5) | 25.0 |
| Caicoya29 | 2007 | 7 | Adult | Yes (6) | 71.4 |
| Daniel10 | 2007 | 13 | Mixed | No | 92.3 |
| Davis30 | 2012 | 43 | Mixed | No | 51.2 |
| Hong31 | 2002 | 7 | Mixed | No | 57.1 |
| Ibrahim32 | 2012 | 40 | Pediatric | Yes (23) | 65.0 |
| Jehi9 | 2009 | 25 | Mixed | Yes (24) | 84.0 |
| Jobst33 | 2010 | 12 | Mixed | Yes (14) | 50.0 |
| Kivelev15 | 2012 | 9 | Mixed | No | 66.7 |
| Kuzniecky34 | 1997 | 6 | Pediatric | Yes (5) | 50.0 |
| Lee12 | 2005 | 26 | Mixed | Yes (26) | 61.5 |
| Liava8 | 2014 | 51 | Pediatric | Yes (24) | 86.3 |
| Salanova35 | 1992 | 36 | Mixed | Yes (6) | 47.2 |
| Sarkis36 | 2012 | 36 | Mixed | Yes (34) | 69.4 |
| Sinclair37 | 2005 | 6 | Pediatric | Yes (6) | 33.3 |
| Tandon3 | 2009 | 21 | Mixed | Yes (8) | 81.0 |
| Williamson4 | 1992 | 20 | Mixed | Yes (17) | 90.0 |
| Yang38 | 2015 | 35 | Mixed | Yes (30) | 71.4 |
| Yun40 | 2006 | 22 | Mixed | No | 68.2 |
| Zentner39 | 1996 | 13 | Mixed | Yes (30) | 61.5 |
| Total | 584 | 330 |
aSubdural strip, subdural grid, or intracranial depth electrodes or a combination of the 3.
In extracting data from these studies, postoperative seizure outcomes were classified as freedom from disabling seizures (Engel class IA-D outcome) or persistent disabling seizures (Engel II-VI) at ≥1 yr after surgery. Additionally, postoperative visual outcomes were defined as either no change in vision following surgery or some decline in hemispheric vision compared to a preoperative baseline, as reported in individual studies. Notably, visual decline did not necessarily equate to visual loss.
Statistical Analysis
Data were analyzed as previously described.23 Specifically, seizure freedom rates and visual outcomes following surgery were determined for all patients. Data were then stratified by potential variables of interest. Preliminary intergroup comparisons were made using Pearson's chi-square tests. Factors identified as associated with seizure freedom were dichotomized and then evaluated using meta-analyses. Meta-analyses were not completed for visual outcomes since no factor met statistical significance following initial analysis. To minimize selection bias, variables selected for meta-analysis had to include at least 80 patients across 5 or more different investigations, with each study comparing at least 2 separation conditions for the variable analyzed. For each meta-analysis performed, the appropriateness of a mixed-effects model was determined with the Cochran Q statistic evaluating heterogeneity between studies. Given limitations of the Q statistic in meta-analyses with a relatively small number of source studies, the I2 index was also calculated for each meta-analysis to estimate true between-study variance.24 Individual studies were appropriately aggregated using inverse-variance weighting. Effect size for individual variables across studies was expressed using forest plots with odds ratios (OR) determined using a 95% confidence interval (CI). Differences between groups were explored using the ORs of the pooled proportions. Funnel plots were used to judge publication bias with none being found. All statistical analysis was performed with SPSS version 20 (IBM Inc, Armonk, New York) with statistical significance defined as P < .05.
RESULTS
Twenty-seven prospective or retrospective case series reporting postoperative seizure outcomes and associated factors in patients undergoing surgery for drug-resistant OLE were analyzed.3-5,8-15,25-38 The PubMed search did not identify any usable prospective controlled trials. In total, these studies included 584 patients, with 6 to 52 individuals per report (Table 1). Overall, 65% of OLE patients achieved postoperative seizure freedom (Engel Class I outcome), with rates ranging from 25% to 100% within individual studies and no trend observed across time (Figure 2).
FIGURE 2.
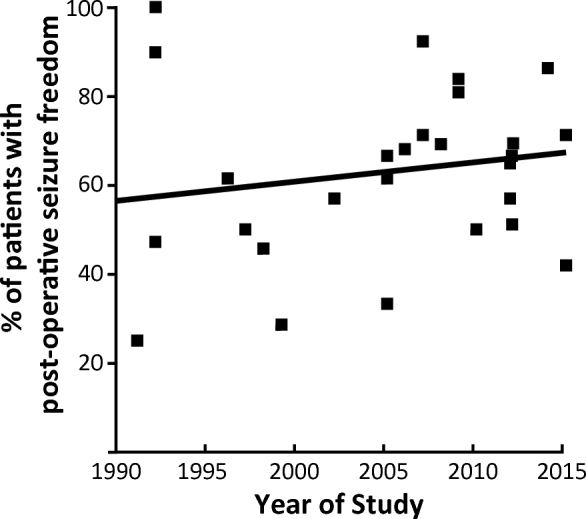
Postoperative seizure freedom rate across all studies by publication date. Each data point represents a single study, with rate of postoperative seizure freedom (Engel class I outcome) plotted against year of study publication. A line of best fit is provided. No significant trend is observed (r = 0.16, P = .43).
Several factors were examined for potential relationship to postoperative seizure outcomes (Table 2). Among demographic factors, seizure freedom was achieved more often in children and adolescents (age less than 18 yr of age) compared to adults (Table 2, A). Examining epilepsy characteristics, seizure freedom was more often achieved in patients with MCD, tumor, or vascular malformation compared to those with gliosis, normal tissue, and other pathology subtypes (Table 2, B). Additionally, patients with abnormal diagnostic MRIs more often had Engel I outcomes after surgery (Table 2, C). With regard to resection type, quadrantectomy (defined as a multilobe resection of the posterior quadrant including occipital, parietal, and posterior temporal regions) or lesionectomy achieved higher rates of favorable seizure outcomes than corticectomy or lobectomy (Table 3, D). No difference in seizure outcome was noted amongst other factors examined.
TABLE 2.
Postoperative Seizure Outcomes Stratified by Patient Characteristics
| Engel I | Engel II-IV | P value | Studies | |||
|---|---|---|---|---|---|---|
| (A) Demographics | Age | <18 yr | 81 (74) | 28 (26) | <.001* | 3, 8, 14, 15, 24, 26, 28, 34, 36, 55 |
| ≥18 yr | 46 (50) | 46 (50) | ||||
| Gender | Female | 21 (58) | 15 (42) | .25 | 3, 14, 15, 25, 26, 28, 33, 36 | |
| Male | 36 (72) | 14 (28) | ||||
| Side | Left | 62 (62) | 38 (38) | .54 | 3, 8, 10, 14, 15, 25-28, 33, 34, 36 | |
| Right | 57 (67) | 28 (33) | ||||
| (B) Epilepsy characteristics | Pathology | MCD | 61 (71) | 25 (29) | <.001* | 3-5, 8, 10, 13-15, 25-28, 33, 34, 36 |
| Tumor | 44 (82) | 10 (18) | ||||
| Vascular malformation | 18 (72) | 7 (28) | ||||
| Gliosis only or normal | 25 (53) | 22 (47) | ||||
| Other | 5 (25) | 15 (75) | ||||
| Seizure type | Partial only | 31 (59) | 22 (41) | .26 | 3, 5, 14, 25-28, 33, 36 | |
| Generalized | 27 (47) | 31 (53) | ||||
| Preoperative | Yes | 304 (64) | 174 (36) | .62 | 3, 8, 14, 15, 25, 28, 29, 34 | |
| visual deficit | No | 57 (67) | 28 (33) | |||
| Visual aura | Yes | 32 (46) | 38 (54) | .06 | 3, 26-28, 32-34 | |
| No | 17 (71) | 7 (29) | ||||
| Duration of epilepsy | <5 yr | 29 (67) | 14 (33) | .10 | 8, 27, 28, 34 | |
| 5-10 yr | 24 (62) | 15 (38) | ||||
| 10-20 yr | 11 (48) | 12 (52) | ||||
| > 20 yr | 6 (35) | 11 (65) | ||||
| (C) Diagnostics | Ictal EEG | Localized | 72 (62) | 45 (38) | .99 | 3, 5, 8, 12, 25-28, 34, 36 |
| Not localized | 49 (62) | 30 (38) | ||||
| Interictal EEG | Localized | 55 (58) | 40 (42) | .64 | 3, 5, 8, 12, 25-28, 34, 36 | |
| Not localized | 49 (62) | 30 (38) | ||||
| MRI | Abnormal | 86 (74) | 30 (26) | <.001* | 3, 8, 15, 25-28, 34, 36 | |
| Normal | 14 (33) | 29 (67) | ||||
| (D) Surgical factors | Resection extent | Occipital only | 78 (67) | 38 (33) | .75 | 3, 4, 8, 13, 14, 25-27, 32-34, 36 |
| Temporo-occipital | 32 (68) | 15 (32) | ||||
| Parieto-occipital | 18 (62) | 11 (38) | ||||
| Temporo-parieto-occipital | 19 (76) | 6 (24) | ||||
| Resection type | Lesionectomy | 56 (70) | 24 (30) | .02 | 3-5, 14, 15, 25, 32, 34 | |
| Corticectomy | 5 (50) | 5 (50) | ||||
| Lobectomy | 21 (51) | 20 (49) | ||||
| Quandrantectomy | 12 (92) | 1 (8) | ||||
| Total | 379 (65) | 205 (35) |
EEG = electroencephalography; MRI = magnetic resonance imaging.
*Statistically significant value (P ≤ .05).
TABLE 3.
Postoperative Visual Outcomes Stratified by Patient Characteristics
| No change | Decline | P value | Studies | |||
|---|---|---|---|---|---|---|
| (A) Demographics | Age | <18 yr | 22 (56) | 17 (44) | .47 | 3, 8, 14 |
| ≥18 yr | 14 (47) | 16 (53) | ||||
| Gender | Female | 10 (53) | 9 (47) | .99 | 3, 14, 15, 25, 28 | |
| Male | 15 (56) | 12 (44) | ||||
| Side | Left | 18 (53) | 16 (47) | |||
| Right | 17 (52) | 16 (48) | .99 | 3, 14, 15, 25, 28 | ||
| (B) Epilepsy characteristics | Pathology | MCD | 10 (39) | 16 (61) | .06 | 3, 14, 15, 25, 28 |
| Tumor | 13 (77) | 4 (23) | ||||
| Vascular malformation | 4 (36) | 7 (64) | ||||
| Gliosis only or normal | 5 (50) | 5 (50) | ||||
| Other | 2 (100) | 0 (0) | ||||
| Seizure type | Partial only | 13 (59) | 9 (41) | .99 | 3, 14, 25, 28 | |
| Generalized | 8 (62) | 5 (38) | ||||
| Preoperative | Yes | 19 (51) | 18 (49) | .07 | 3, 8, 12-15, 25, 28 | |
| visual deficit | No | 31 (33) | 64 (67) | |||
| Visual aura | Yes | 6 (55) | 5 (45) | .99 | 3, 28 | |
| No | 3 (43) | 4 (56) | ||||
| Duration of epilepsy | <5 yr | 5 (36) | 9 (64) | .21 | 8, 25, 28 | |
| 5-10 yr | 10 (71) | 4 (29) | ||||
| 10-20 yr | 3 (60) | 2 (40) | ||||
| >20 yr | 2 (33) | 4 (67) | ||||
| (C) Diagnostics | Ictal EEG | Localized | 17 (47) | 19 (53) | .54 | 3, 8, 25, 28 |
| Not localized | 9 (60) | 6 (40) | ||||
| Interictal EEG | Localized | 14 (47) | 16 (53) | .57 | 3, 8, 25, 28 | |
| Not localized | 12 (57) | 9 (43) | ||||
| MRI | Abnormal | 30 (50) | 30 (50) | .16 | 3, 8, 15, 25, 28 | |
| Normal | 0 (0) | 2 (100) | ||||
| (D) Surgical factors | Resection extent | Occipital only | 11 (52) | 10 (48) | .23 | 3, 8, 14, 25 |
| Temporo-occipital | 4 (36) | 7 (64) | ||||
| Parieto-occipital | 6 (86) | 1 (14) | ||||
| Temporo-parieto-occipital | 3 (60) | 2 (40) | ||||
| Resection type | Lesionectomy | 16 (55) | 13 (45) | .64 | 3, 14, 15, 25 | |
| Corticectomy | 4 (67) | 2 (33) | ||||
| Lobectomy | 1 (33) | 2 (67) | ||||
| Quandrantectomy | 0 (0) | 0 (0) | ||||
| Total | 76 (43) | 99 (57) |
EEG = electroencephalography; MRI = magnetic resonance imaging.
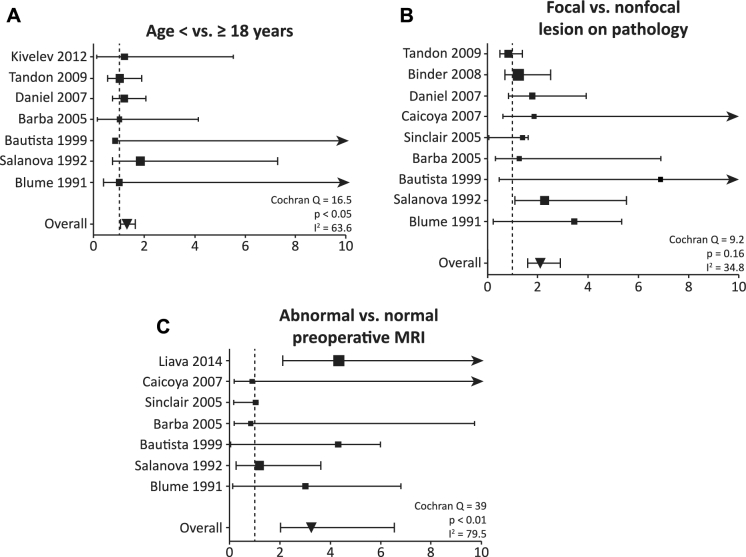
Potential predictors of postoperative seizure freedom identified on preliminary analysis were then evaluated with formal meta-analysis (with the exception of resection type given that fewer than 5 manuscripts compared at least 2 different resection types in the same study). As summarized in Figure 3, seizure freedom was achieved significantly more often in patients less than 18 yr of age compared to older individuals (OR 1.54, 95% CI 1.13-2.18), in patients with a focal lesion (MCD, tumor, or vascular malformation) on pathological examination compared to those with nonfocal pathology (OR 2.08, 95% CI 1.58-2.89), and in individuals with abnormal vs normal preoperative MRI (OR 3.24, 95% 2.03-6.55).
FIGURE 3.
Meta-analyses examining factors associated with seizure freedom after occipital lobe and posterior quadrant epilepsy surgery. Statistically significant predictors of postoperative seizure freedom included A age < 18 (vs ≥18) yr (Cochran Q = 16.5, P < .05, I2 = 63.6), B focal (vs nonfocal) lesion on pathological examination (Q = 9.2, P = .16, I2 = 34.8), and C abnormal (vs normal) preoperative MRI (Q = 39, P < .01, I2 = 79.5). The effect size for each study is represented as OR of factors associated with seizure freedom (larger OR indicates greater likelihood of seizure freedom), with proportional study weight estimated by the size of each point. Error bars represent 95% CI, with arrowheads indicating an upper limit off of the scale. The size of each point estimates proportional study weight, and the vertical dashed line represents OR = 1.
Next, rates of change in hemispheric vision following OLE surgery compared to a preoperative baseline, as reported in individual studies, were evaluated. Such data were available from 8 studies totaling 175 patients with 7 to 52 patients per study.3,8,12-15,26,29 Overall, 57% of patients experienced some degree of hemispheric visual decline after surgery. Several factors were examined for potential association with postoperative visuals outcomes, although no significant relationships were observed (Table 3). Of note, patients with normal vision before surgery were more likely to experience visual decline after surgery (67%) compared to those with preoperative visual deficits (49%) with a trend nearing significance (Table 3B). Rates of visual decline were similar between patients with Engel I (47%) and Engel II to IV (50%) seizure outcomes.
DISCUSSION
In this study, we provide a systematic review and meta-analysis of seizure outcomes following surgery for drug-resistant OLE. Compiling data from 27 unique investigations with 584 patients, we found that 65% of these patients achieved postoperative seizure freedom which in turn was predicted by younger patient age, an abnormal preoperative MRI, and focal lesion on pathological examination. These findings may aid in both patient selection and outcome prediction in the surgical treatment of OLE.
Historically, successful surgical management of OLE has been hindered by numerous diagnostic and technical challenges.4,39 One of the foremost difficulties for OLE surgery is precise identification of the epileptic focus. Unlike TLE, scalp EEG has proven to have limited diagnostic utility in both identifying OLE and creating a surgical plan.25 Several studies have demonstrated that in OLE, the most common EEG finding is not spike activity observed over the occipital lobe, but rather sharp and spike waves over the temporal cortex.32 One study included here demonstrated that only 17% of known OLE patients examined had scalp EEG showing occipital onset of seizure activity.35 As such, it is not surprising that OLE patients can be misdiagnosed as having TLE based on “pseudo-occipital” seizures and spikes observed with extracranial EEG data alone.25 These limitations with scalp EEG for OLE seem to be associated with the anatomic position of the occipital lobe at the base of the cerebrum as well as its multiple connections to adjacent brain regions, which enable exceptionally rapid spread of seizure activity, thereby muddying precise localization of activity onset.4,28,38
Due to these limitations associated with scalp EEG, more invasive techniques—including subdural and depth electrodes—are frequently utilized to diagnose and anatomically characterize OLE as evident by the more than 50% of patients reported here receiving invasive monitoring (Table 1).4,5,25 In one study directly comparing OLE with TLE, 84% of OLE surgical cases utilized invasive recording for precise epileptic focus localization compared to only 14% of TLE cases.25 Despite their proven utility, intracranial electrodes do not solve all the diagnostic challenges for OLE. Unlike TLE and frontal lobe epilepsy which are more often focal in nature, OLE can commonly be multifocal, crossing not only lobar but also hemispheric boundaries, making not only diagnosis but surgical management very difficult.5
For the OLE cases that do seem to arise from a single radiographic lesion, some have asserted that removing this tissue alone may prove inadequate. Specifically, it has been suggested that OLE is not a focal but rather a regional disease in which achieving true seizure freedom requires removing the entire epileptogenic zone—an area that often extends beyond abnormalities seen on imaging.39 Some have argued that defining this zone requires identifying areas of seizure initiation, spread, and termination5,35—information that may require intracranial recordings.
Adding to these diagnostic challenges is the technical challenge of resecting epileptic tissue abutting multiple areas of eloquent cortex. Vision loss is a known risk with surgery in the posterior quadrant due to proximity of the visual cortex—as evident by 57% of patients reported here who experienced postoperative visual decline (Table 3). However, language deficits, alexia, and neglect are also known risks of OLE surgery due to the proximity of key language centers and the parietal lobe to OLE foci.25 Attempts to preserve these areas of eloquent cortex while removing epileptic tissue often lead to suboptimal seizure outcomes—a point highlighted by several of the studies included here.28,29,39,40
Despite these known obstacles associated with achieving seizure freedom with OLE surgery, our analysis revealed nearly two-thirds of OLE patients undergoing surgery achieving seizure freedom—outcomes more favorable than those typically reported for frontal lobe epilepsy surgery, the most common ETLE syndrome.21,41-44 Additionally, this seizure freedom rate for OLE surgery lies within the lower range of rates usually observed (60%-80%) in studies of anterior temporal lobectomy for TLE.16,45-47 Nevertheless, while surgical outcomes for medically refractory TLE have been validated by randomized-controlled trials,20,48 no such trial has yet been performed for OLE. A randomized-controlled trial comparing seizure outcomes following surgical vs continued medical therapy for intractable ETLE is still needed.
In OLE patients with an abnormal preoperative MRI, we observed a rate of postoperative seizure freedom more than double that seen in individuals without remarkable neuroimaging. This finding is consistent with several previous investigations of OLE surgery.8,13,35,37,40 As has been shown in other focal epilepsy syndromes, abnormalities on neuroimaging help target surgical resection and often suggest a focal pathological lesion.41,44,49 With respect to pathology, OLE patients with focal lesions on pathological analysis—including MCD, tumor, or vascular malformation—achieved higher rates of Engel class I outcomes than those with nonfocal findings—such as gliosis or normal appearing tissue. Improved postsurgical outcomes with focal lesions has similarly been described in OLE5,9,30,35,50 as well as TLE51,52 and other forms of ETLE.41,47,53 Furthermore, in patients with focal lesions, gross-total lesionectomy has previously been associated with dramatically increased rates of postoperative seizure freedom compared to subtotal resection.4,8,9,14,35 Notably, while there is significant overlap between neuroimaging and pathological findings as outcome predictors, not all resections involving an abnormal MRI uncover a focal lesion on pathological analysis, and not all focal pathological lesions are detected on preoperative MRI. Therefore, neither the lack of MRI abnormality nor focal lesion necessarily precludes surgery in patients with otherwise clear and strongly concordant electrographic and semiological data.
With regard to patient demographics, postoperative seizure freedom rates were nearly 50% higher in patients younger than 18 yr of age. This may suggest earlier intervention is warranted in children with clearly refractory OLE who are otherwise favorable surgical candidates.
Finally, visual outcome data were available in a minority of studies examined, and suggested that greater than 50% of individuals experience some visual decline after surgery compared to preoperative baseline. However, given the paucity of data available, and the lack of standardization with regard to visual outcome reporting across studies, this finding should be interpreted with caution. In addition, insufficient data were available to examine potential predictors of visual outcome. Because of the impact of vision on quality of life, further studies including detailed, formal visual field examination both before and after OLE surgery are critically needed.54
Limitations
There are several limitations to consider in this investigation, including the innate shortcomings of meta-analysis technique in neurosurgical studies previously outlined by Sampson and Barker.55 Specifically, in this meta-analysis, data were aggregated from multiple studies to generate a larger patient study group. While this does enhance detection of statistically significant correlations between various variables, the validity of the findings depends on the data quality collected by others and is thus susceptible to selection bias. Additionally, the power of the analysis may be limited by the relatively few studies contributing to each meta-analysis. However, the use of both Cochran Q statistic and I2 index to evaluate study heterogeneity suggests that our applied statistical model was appropriate.24 Next, the methods used to characterize visual loss following surgery were not always defined or differed between individual investigations, which may contribute to further bias with regard to visual outcome data. Data regarding the use and type of electrographic monitoring were also limited in the source studies, which limits our ability to ascertain the potential effect of invasive recordings on seizure outcome. Furthermore, in a systematic review, variables of interest cannot be disaggregated and as such, it is impossible to perform a multivariate analysis looking for interactions across variables. Finally, even though measures to ensure selection of dependable sources were utilized, the systematic review approach largely depends on appropriately aggregating high volumes of patients (over 500) across time. Replicating such numbers with a prospective case series, even if multi-institutional, would be quite challenging. Additionally, PRISMA guidelines were utilized to improve the quality of the present analysis and findings reported.22
CONCLUSION
Drug-resistant OLE is a relatively uncommon but debilitating focal epilepsy syndrome. Nearly two-thirds of patients achieved seizure freedom after surgery with pediatric age group, focal lesion on pathological analysis, and abnormal preoperative MRI predicting favorable seizure outcomes. These findings may help guide outcome prediction and patient counseling in the surgical treatment of OLE. While some visual decline was reported in greater than half of individuals after surgery, further study of visual outcomes in OLE surgery will be critical, given the paucity of data currently available and the influence of visual outcome on quality of life. A randomized-controlled trial of surgical therapy for ETLE, including OLE, has not yet been performed and remains an important goal.
Disclosures
This work was supported in part by the NIH K99 NS097618 (DJE).The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
COMMENTS
The availability of “higher evidence” studies in the epilepsy surgery field is greatly sparse or not present at all. Consequently, not all medical questions are amenable to be clarified through randomized trials. In the era of evidence-based medicine, meta-analysis can help organize and evaluate the nature of the available information. The issue of using summary data from the literature for meta-analysis is the variability in what is reported, making the transition to clinical practice more challenging and uncertain. In this manuscript, the authors report the first systematic review and meta-analysis of surgical resection for drug-resistant occipital lobe epilepsy. Nearly 70% of patients achieved seizure freedom after surgery in specific groups (mainly children and lesional MRI cases). These results are not surprising and confirm previous impressions related to the favorable outcome associated with posterior quadrant medically intractable epilepsy, mainly in children with lesional MRIs. As the authors stated, a randomized-controlled is required to confirm the meta-analysis conclusions. I commend the authors for their excellent work.
Jorge Gonzalez-Martinez
Cleveland, Ohio
The authors have provided a useful systematic review of surgery for occipital and posterior quadrant epilepsy according to PRISMA recommendations. The methodology is sound.
The results are not quite what I expected. Although there is probably a significant reporting bias, the seizure freedom rate is comparable to that from temporal lobe epilepsy surgery. However, the rate of visual deficits after surgery is high at >50%. It would have been helpful to clinicians had the authors analyzed how invasive monitoring influences outcome, including techniques used. However, this study provides useful data for counseling patients considering surgery for occipital lobe epilepsy.
Jason M. Schwalb
West Bloomfield, Michigan
REFERENCES
- 1. Taylor I, Scheffer IE, Berkovic SF. Occipital epilepsies: identification of specific and newly recognized syndromes. Brain. 2003;126(pt 4):753-769. [DOI] [PubMed] [Google Scholar]
- 2. Manford M, Hart YM, Sander JW, Shorvon SD. National general practice study of epilepsy (NGPSE): partial seizure patterns in a general population. Neurology. 1992;42(10):1911-1917. [DOI] [PubMed] [Google Scholar]
- 3. Tandon N, Alexopoulos AV, Warbel A, Najm IM, Bingaman WE. Occipital epilepsy: spatial categorization and surgical management. J Neurosurg. 2009;110(2):306-318. [DOI] [PubMed] [Google Scholar]
- 4. Williamson PD, Thadani VM, Darcey TM, Spencer DD, Spencer SS, Mattson RH. Occipital lobe epilepsy: clinical characteristics, seizure spread patterns, and results of surgery. Ann Neurol. 1992;31(1):3-13. [DOI] [PubMed] [Google Scholar]
- 5. Aykut-Bingol C, Bronen RA, Kim JH, Spencer DD, Spencer SS. Surgical outcome in occipital lobe epilepsy: implications for pathophysiology. Ann Neurol. 1998;44(1):60-69. [DOI] [PubMed] [Google Scholar]
- 6. Panayiotopoulos CP. Visual phenomena and headache in occipital epilepsy: a review, a systematic study and differentiation from migraine. Epileptic Disord. 1999;1(4):205-216. [PubMed] [Google Scholar]
- 7. Yilmaz K, Karatoprak E. Epilepsy classification and additional definitions in occipital lobe epilepsy. Epileptic Disord. 2015;17(3):299-307. [DOI] [PubMed] [Google Scholar]
- 8. Liava A, Mai R, Tassi L et al. Paediatric epilepsy surgery in the posterior cortex: a study of 62 cases. Epileptic Disord. 2014;16(2):141-164. [DOI] [PubMed] [Google Scholar]
- 9. Jehi LE, O’Dwyer R, Najm I, Alexopoulos A, Bingaman W. A longitudinal study of surgical outcome and its determinants following posterior cortex epilepsy surgery. Epilepsia. 2009;50(9):2040-2052. [DOI] [PubMed] [Google Scholar]
- 10. Daniel RT, Meagher-Villemure K, Farmer J-PP, Andermann F, Villemure J-GG. Posterior quadrantic epilepsy surgery: technical variants, surgical anatomy, and case series. Epilepsia. 2007;48(8):1429-1437. [DOI] [PubMed] [Google Scholar]
- 11. Bidziński J, Bacia T, Ruzikowski E. The results of the surgical treatment of occipital lobe epilepsy. Acta Neurochir. 1992;114(3-4):128-130. [DOI] [PubMed] [Google Scholar]
- 12. Kun Lee S, Young Lee S, Kim D-WW, Soo Lee D, Chung C-KK. Occipital lobe epilepsy: clinical characteristics, surgical outcome, and role of diagnostic modalities. Epilepsia. 2005;46(5):688-695. [DOI] [PubMed] [Google Scholar]
- 13. Binder DK, Von Lehe M, Kral T et al. Surgical treatment of occipital lobe epilepsy. J Neurosurg. 2008;109(1):57-69. [DOI] [PubMed] [Google Scholar]
- 14. Battaglia D, Chieffo D, Tamburrini G et al. Posterior resection for childhood lesional epilepsy: neuropsychological evolution. Epilepsy Behav. 2012;23(2):131-137. [DOI] [PubMed] [Google Scholar]
- 15. Kivelev J, Koskela E, Setälä K, Niemelä M, Hernesniemi J. Long-term visual outcome after microsurgical removal of occipital lobe cavernomas. J Neurosurg. 2012;117(2):295-301. [DOI] [PubMed] [Google Scholar]
- 16. Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7(6):525-537. [DOI] [PubMed] [Google Scholar]
- 17. Englot DJ, Lee AT, Tsai C et al. Seizure types and frequency in patients who "fail" temporal lobectomy for intractable epilepsy. Neurosurgery. 2013;73(5):838-844. [DOI] [PubMed] [Google Scholar]
- 18. Englot DJ, Raygor KP, Molinaro AM et al. Factors associated with failed focal neocortical epilepsy surgery. Neurosurgery. 2014;75(6):648-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Englot DJ, Chang EF, Vecht CJ. Epilepsy and brain tumors. Handbook Clin Neurol. 2016;134:267-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318. [DOI] [PubMed] [Google Scholar]
- 21. Engel J, Van Ness P, Rasmussen T. Outcome with respect to epileptic seizures. In: Engel J, ed. Surgical Treatment of the Epilepsies. 2nd ed New York: Raven Press; 1993:609-621. [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;338:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Englot DJ, Magill ST, Han SJ, Chang EF, Berger MS, McDermott MW. Seizures in supratentorial meningioma: a systematic review and meta-analysis. J Neurosurg. 2016;124(6):1552-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Meth. 2006;11(2):193-206. [DOI] [PubMed] [Google Scholar]
- 25. Appel S, Sharan AD, Tracy JI, Evans J, Sperling MR. A comparison of occipital and temporal lobe epilepsies. Acta Neurol Scand. 2015;132(4):284-290. [DOI] [PubMed] [Google Scholar]
- 26. Barba C, Doglietto F, De Luca L et al. Retrospective analysis of variables favouring good surgical outcome in posterior epilepsies. J Neurol. 2005;252(4):465-472. [DOI] [PubMed] [Google Scholar]
- 27. Bautista RE, Cobbs MA, Spencer DD, Spencer SS. Prediction of surgical outcome by interictal epileptiform abnormalities during intracranial EEG monitoring in patients with extrahippocampal seizures. Epilepsia. 1999;40(7):880-890. [DOI] [PubMed] [Google Scholar]
- 28. Blume WT, Whiting SE, Girvin JP. Epilepsy surgery in the posterior cortex. Ann Neurol. 1991;29(6):638-645. [DOI] [PubMed] [Google Scholar]
- 29. Caicoya AG, Macarrón J, Albísua J, Serratosa JMM. Tailored resections in occipital lobe epilepsy surgery guided by monitoring with subdural electrodes: characteristics and outcome. Epilepsy Res. 2007;77(1):1-10. [DOI] [PubMed] [Google Scholar]
- 30. Davis KL, Murro AM, Park YD, Lee GP, Cohen MJ, Smith JR. Posterior quadrant epilepsy surgery: predictors of outcome. Seizure. 2012;21(9):722-728. [DOI] [PubMed] [Google Scholar]
- 31. Hong K-SS, Lee SK, Kim J-YY, Lee D-SS, Chung C-KK. Pre-surgical evaluation and surgical outcome of 41 patients with non-lesional neocortical epilepsy. Seizure. 2002;11(3):184-192. [DOI] [PubMed] [Google Scholar]
- 32. Ibrahim GM, Fallah A, Albert GW et al. Occipital lobe epilepsy in children: characterization, evaluation and surgical outcomes. Epilepsy Res. 2012;99(3):335-345. [DOI] [PubMed] [Google Scholar]
- 33. Jobst BC, Williamson PD, Thadani VM et al. Intractable occipital lobe epilepsy: clinical characteristics and surgical treatment. Epilepsia. 2010;51(11):2334-2337. [DOI] [PubMed] [Google Scholar]
- 34. Kuzniecky R, Gilliam F, Morawetz R, Faught E, Palmer C, Black L. Occipital lobe developmental malformations and epilepsy: clinical spectrum, treatment, and outcome. Epilepsia. 1997;38(2):175-181. [DOI] [PubMed] [Google Scholar]
- 35. Salanova V, Andermann F, Olivier A, Rasmussen T, Quesney LF. Occipital lobe epilepsy: electroclinical manifestations, electrocorticography, cortical stimulation and outcome in 42 patients treated between 1930 and 1991. Surgery of occipital lobe epilepsy. Brain. 1992;115(pt 6):1655-1680. [DOI] [PubMed] [Google Scholar]
- 36. Sarkis RA, Jehi L, Najm IM, Kotagal P, Bingaman WE. Seizure outcomes following multilobar epilepsy surgery. Epilepsia. 2012;53(1):44-50. [DOI] [PubMed] [Google Scholar]
- 37. Sinclair DB, Wheatley M, Snyder T, Gross D, Ahmed N. Posterior resection for childhood epilepsy. Pediat Neurol. 2005;32(4):257-263. [DOI] [PubMed] [Google Scholar]
- 38. Yang P-FF, Jia Y-ZZ, Lin Q et al. Intractable occipital lobe epilepsy: clinical characteristics, surgical treatment, and a systematic review of the literature. Acta Neurochir. 2015;157(1):63-75. [DOI] [PubMed] [Google Scholar]
- 39. Zentner J, Hufnagel A, Ostertun B et al. Surgical treatment of extratemporal epilepsy: clinical, radiologic, and histopathologic findings in 60 patients. Epilepsia. 1996;37(11):1072-1080. [DOI] [PubMed] [Google Scholar]
- 40. Yun C-HH, Lee SK, Lee SY, Kim KK, Jeong SW, Chung C-KK. Prognostic factors in neocortical epilepsy surgery: multivariate analysis. Epilepsia. 2006;47(3):574-579. [DOI] [PubMed] [Google Scholar]
- 41. Englot DJ, Wang DD, Rolston JD, Shih TT, Chang EF. Rates and predictors of long-term seizure freedom after frontal lobe epilepsy surgery: a systematic review and meta-analysis. J Neurosurg. 2012;116(5):1042-1048. [DOI] [PubMed] [Google Scholar]
- 42. Elsharkawy AE, Alabbasi AH, Pannek H et al. Outcome of frontal lobe epilepsy surgery in adults. Epilepsy Res. 2008;81(2-3):97-106. [DOI] [PubMed] [Google Scholar]
- 43. Hosking PG. Surgery for frontal lobe epilepsy. Seizure. 2003;12(3):160-166. [DOI] [PubMed] [Google Scholar]
- 44. Lee JJ, Lee SK, Lee S-YY et al. Frontal lobe epilepsy: clinical characteristics, surgical outcomes and diagnostic modalities. Seizure. 2008;17(6):514-523. [DOI] [PubMed] [Google Scholar]
- 45. McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127(pt 9):2018-2030. [DOI] [PubMed] [Google Scholar]
- 46. Foldvary N, Nashold B, Mascha E et al. Seizure outcome after temporal lobectomy for temporal lobe epilepsy: a Kaplan-Meier survival analysis. Neurology. 2000;54(3):630-634. [DOI] [PubMed] [Google Scholar]
- 47. Téllez-Zenteno JFF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128(pt 5):1188-1198. [DOI] [PubMed] [Google Scholar]
- 48. Engel J, McDermott MP, Wiebe S et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307(9):922-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ferrier CH, Engelsman J, Alarcón G, Binnie CD, Polkey CE. Prognostic factors in presurgical assessment of frontal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1999;66(3):350-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dalmagro CL, Bianchin MM, Velasco TR et al. Clinical features of patients with posterior cortex epilepsies and predictors of surgical outcome. Epilepsia. 2005;46(9):1442-1449. [DOI] [PubMed] [Google Scholar]
- 51. Jutila L, Immonen A, Mervaala E et al. Long term outcome of temporal lobe epilepsy surgery: analyses of 140 consecutive patients. J Neurol Neurosurg Psychiatry. 2002;73(5):486-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bell ML, Rao S, So EL et al. Epilepsy surgery outcomes in temporal lobe epilepsy with a normal MRI. Epilepsia. 2009;50(9):2053-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cascino GD, Jack CR, Parisi JE et al. MRI in the presurgical evaluation of patients with frontal lobe epilepsy and children with temporal lobe epilepsy: pathologic correlation and prognostic importance. Epilepsy Res. 1992;11(1):51-59. [DOI] [PubMed] [Google Scholar]
- 54. Brown GC. Vision and quality-of-life. Trans Am Ophthalmol Soc. 1999;97:473-511. [DOI] [PubMed] [Google Scholar]
- 55. Sampson JH, Barker FG. Methodology and reporting of meta-analyses in the neurosurgical literature. J Neurosurg. 2014;120(4):791-794. [DOI] [PubMed] [Google Scholar]