Abstract
Repetitive mild traumatic brain injury (rmTBI; e.g., sports concussions) is common and results in significant cognitive impairment. Targeted therapies for rmTBI are lacking, though evidence from other injury models indicates that targeting N-methyl-d-aspartate (NMDA) receptor (NMDAR)-mediated glutamatergic toxicity might mitigate rmTBI-induced neurologic deficits. However, there is a paucity of preclinical or clinical data regarding NMDAR antagonist efficacy in the rmTBI setting. To test whether NMDAR antagonist therapy improves outcomes after rmTBI, mice were subjected to rmTBI injury (4 injuries in 4 days) and randomized to treatment with the NMDA antagonist memantine or with vehicle. Functional outcomes were assessed by motor, anxiety/impulsivity and mnemonic behavioral tests. At the synaptic level, NMDAR-dependent long-term potentiation (LTP) was assessed in isolated neocortical slices. At the molecular level, the magnitude of gliosis and tau hyper-phosphorylation was tested by Western blot and immunostaining, and NMDAR subunit expression was evaluated by Western blot and polymerase chain reaction (PCR). Compared to vehicle-treated mice, memantine-treated mice had reduced tau phosphorylation at acute time points after injury, and less glial activation and LTP deficit 1 month after injury. Treatment with memantine also corresponded to normal NMDAR expression after rmTBI. No corresponding protection in behavior outcomes was observed. Here we found NMDAR antagonist therapy may improve histopathological and functional outcomes after rmTBI, though without consistent corresponding improvement in behavioral outcomes. These data raise prospects for therapeutic post-concussive NMDAR antagonism, particularly in athletes and warriors, who suffer functional impairment and neurodegenerative sequelae after multiple concussions.
Keywords: Traumatic Brain Injury, Concussion, Repetitive Concussion, NMDAR
1. Introduction
Scientific attention into the sequelae of repetitive mild traumatic brain injury (rmTBI) has increased in recent years. Clinically, rmTBI is associated with long-term neurological impairment including memory disturbances, Parkinsonism, behavioral changes, speech irregularities, and gait abnormalities.1, 2 Preclinical TBI models indicate that glutamate-mediated excitotoxicity plays an early and pivotal role in the cascade of secondary injury events that follow a single instance of TBI.3, 4 However, despite encouraging preclinical data, clinical trials targeting glutamatergic toxicity, specifically mediated by activation of the N-methyl-d-aspartate (NMDA) receptor (NMDAR), have not been successful.5
NMDARs are glutamate- and voltage-gated cation (largely calcium) channels composed of individual protein subunits. Seven subunits are identified. Heterotetrameric assemblies of NMDARs typically include NR1 subunits with NR2 subunits or a mixture of NR2 and NR3 subunits.6 While calcium influx via NMDAR is critical for synaptic plasticity, excess intracellular Ca2+ is detrimental and is the proximal signal for excitotoxicity in the post-TBI setting of excess glutamate and NMDAR hyperactivation. Excitotoxicity in the setting of severe TBI triggers a host of cellular responses resulting in unmet metabolic demand, oxidative stress, inhibition of the mitochondrial electron transport chain, inflammation and cell death, all of which have been the target for therpaeutic interventions.7–9 NMDAR activation thus, is a rational target to prevent the secondary injury cascade after brain injury, but has not been evaluated in the setting of rmTBI.
NMDAR subtypes have distinct gating and permeation properties, resulting in distinct patterns of susceptibility to excitotoxcity. In fact, alterations in NMDAR assembly, function and distribution are well-described in the post-injury excitotoxic cascade after severe TBI.9–11 In contrast, detailed understanding of NMDAR pathophysiology, structure and function after mild TBI, particularly rmTBI, is lacking, and the effect of repetitive mild injury on NMDAR expression is unknown. Yet, in the absence of either preclinical or clinical data, clinicians nevertheless routinely employ NMDAR-targeted therapies to mild TBI patients suffering the most severe cognitive symptoms, 12 many of whom have suffered rmTBI.13–15 However, the possibility that routine NMDAR antagonist use may have no effect, or even a detrimental effect, has not been adequately explored in the rmTBI setting.
Small clinical case series and a retrospective case study of the NMDAR antagonist amantadine suggest that NMDAR blockade improves cognitive outcomes after a single instance of mild TBI and clinical trials of the NMDAR antagonist memantine for single mild TBI instances are ongoing.16 Yet whether therapies targeting NMDAR are effective after successive injuries in the rmTBI setting has not been tested, even though clinically, patients with rmTBI are most at risk for adverse neurologic outcomes.
Results from preclinical models of single-instance severe TBI indicate that targeting glutamate mediated toxicity may be efficacious only at the earliest time points after injury, during the transient and short-lived posttraumatic NMDAR hyperactivation.11 Yet such physiologic evidence from isolated concussive injuries may not be relevant in the rmTBI setting where repetitive injury may occur over weeks to months, and NMDAR function after the last of a series of injuries may be very different than after the first injury. Indeed, some data indicate that NMDAR expression is depressed at long intervals after TBI, and NMDAR blockade at these late timepoints may interfere with recovery.11 Thus, whether NMDAR blockade is an appropriate target after a series of injuries, as in the setting of rmTBI, is unknown.
We recently developed a mouse rmTBI model that results in persistent deficits in exploratory behavior, balance, and spatial memory, and is associated with the early accumulation of phosphorylated tau and chronic gliosis.17–19 We now test whether memantine treatment after the last of a series of injuries in rmTBI improves posttraumatic functional and histopathological outcomes.
2. Methods
All experiments were approved by the Boston Children’s Hospital institutional animal care and use committee and complied with the NIH Guide for the Care and Use of Laboratory Animals. 95 adult (age 8 weeks) male C57BL/6 mice were obtained from the Jackson Laboratories (Bar Harbor, ME) for these experiments.
2.1 Repetitive Mild TBI
Mice were randomized to either rmTBI or sham injury. The rmTBI was performed as previously described.18, 20 Briefly, mice were anesthetized for 45 seconds using 4% isoflurane in oxygen. Anesthetized mice were placed on a delicate tissue (Kimwipe, Irving, TX) and the head was placed directly under a plastic hollow guide tube centered over the bregma. Mice were held by the tail as an impact was delivered to the dorsal skull. The impact was delivered by dropping a 54 g metal bolt from a 71 cm height, resulting in a rotational acceleration of the head through the Kimwipe. Mice underwent a single closed head injury daily for 4 consecutive days, a modification of our prior reported injury regimen of 7 injuries in 9 days.18 Within 1 hour after the last injury, mice received either intraperitoneal injection of memantine (10mg/kg)21 or vehicle (saline), each a volume of 0.2 ml. A separate cohort underwent sham injury only, which consisted of 4 daily anesthesia exposures only. Mice were therefore randomized to the following groups: 4 injuries in 4 days and memantine treatment (n = 29); 4 injuries in 4 days and vehicle treatment (n = 37); 4 sham injuries in 4 days (n = 30). All mice recovered in room air after verum or sham injury. All behavioral and histopathological testing were conducted by investigators blinded to injury status, using color coding stored in a password protected computer.
2.2 Immunoblotting
Three days after the last injury, a subset of animals were sacrificed for immunoblotting to determine the expression of phosphorylated tau, total tau, amyloid precursor protein (APP) and β-actin in injured memantine treated, injured vehicle treated and sham injured mice; another group was sacrificed 1 month after the last injury to determine expression of phosphorylated tau, total tau, APP, NR1 and NR2B after injury (n = 6–8/group). The brain tissues (cortex and hippocampus) were lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM sodium chloride, 1 mM ethylenediaminetetraacetic acid, 1% NP-40, 1% Sodium Deoxycholic acid, 0.1% sodium dodecylsulfate, 1 mM phenylmethylsulfonyl fluoride and protease inhibitor cocktail) with phosphatase inhibitor cocktail (Santa Cruz Biotechnology, Dallas, TX). Proteins (30–40μg) were separated by electrophoresis on 4–15% SDS polyacrylamide gels (Bio-Rad, CA), then transferred to a polyvinylidene fluoride (PVDF) membrane. The membranes were blocked with 5% fat free milk for 1 h and then incubated with primary antibodies NR2B (1:1000), NR1 (1:1000), phospho tau (T231, 1:2500), tau-5 (1:1000), β-actin (1:5000) (Abcam, Cambridge, MA, USA, 1:1000) or APP (1: 1000, Millipore, Billerica, MA) overnight at 4°C. The blots were further incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies. Immunoreactivity was detected using a chemiluminescence system and ImageQuant LAS 4000 (GE, PA) according to the manufacturer’s protocol. Band signal intensity was quantified using Image J software. The density of each sample was normalized to the density of β-actin.
2.3 Assessments of motor function, spatial memory, locomotor activity, anxiety and impulsivity-like phenotypes
Motor function was assessed on day 4, after the last of the rmTBI injuries or sham injury, by rotarod. Briefly, the rotarod consists of a 4 cm diameter rotating drum, on which a test mouse is placed. The time (s) between placement on the rotarod and fall off of the rotarod is recorded as a measure of motor function. Rotarod testing was conducted over 3 days with one day of habituation followed by two testing days. On testing days, mice were placed on the rod at 4 rpm for 10 seconds to acclimate to the rod speed after which the rod was accelerated at 0.1 rpm/sec. Each mouse completed 4 trials/day on the testing days, with a minimum of 10 minutes rest between trials.
Spatial learning and memory were assessed using a Morris water maze paradigm (MWM) on days 11–14 after the last injury. MWM testing was conducted as previously described.18, 22 A white pool (83 cm diameter, 60 cm deep) was filled with water to 29 cm depth. Water temperature was maintained at approximately 24°C and a target platform (a round, clear, plastic platform 10 cm in diameter) was positioned 1 cm below the surface of the water. Several highly visible intra- and extra-maze cues were located in and around the pool. During hidden and visible platform trials, mice were randomized to one of four starting quadrants. Mice were placed in the tank facing the wall and given 90 seconds to find the platform, mount the platform, and remain on it for 5 seconds. Mice were then placed under a heat lamp to dry before their next run. Time until the mouse mounted the platform (escape latency) was measured and recorded. Mice that failed to mount the platform within the allotted time (90 seconds) were guided to the platform by the experimenter and allowed 10 seconds to become acquainted with its location. Each mouse was subjected to a maximum of two trials per day, each consisting of four runs, with a 45-minute break between trials. For visible platform trials, a red reflector was used to mark the top of the target platform. For probe trials, mice were placed in the tank with the platform removed and given 60 seconds to explore the tank. Noldus Ethovision 9 software tracked swim speed, total distance moved, and time spent in the target quadrant where the platform was previously located.
The open field test, an established test for studying locomotor activity and anxiety in mice confined to a novel arena, was applied on day 16 after the last injury. The arena consisted of a 45 cm diameter opaque, plastic circle with walls 20 cm high. The arena was placed inside a plastic transparent box with an Ethovision video tracking system (Wageningen, the Netherlands) mounted to the top and placed in an enclosed chamber to prevent distraction. Each mouse was placed in the same part of the edge of the arena, facing the wall to begin its trial. The arena was virtually divided into three concentric circular sections: an “inner” circle 20 cm in diameter (area of 314 cm2); a surrounding “neutral” ring, inner diameter 20 cm wide, outer diameter, 40 cm (area of 932 cm2); and the “outer” ring, inner diameter 40 cm, outer diameter 60 cm (area of 1570 cm2). Mice were given 10 minutes to explore the arena. Time spent in each of the three regions was recorded and assessed as an anxiety metric. Time spent in the “inner” ring constituted least anxious behavior, while time spent in the “outer” ring, by the perimeter of the arena, constituted anxious behavior.
Impulsivity behaviors were assessed in the elevated plus maze on day 20 after injury. The elevated plus-maze apparatus (Lafayette Instruments, Lafayette, IN) consisted of two open and two closed arms (30 × 5 cm) extending out opposite from each other from a central platform (decision zone). Mice were placed on the center platform of the maze, facing a closed arm, and allowed to explore the apparatus for 5 minutes. A computer-assisted video-tracking system (Noldus Ethovision) recorded the total time spent in the open center (decision zone), and closed compartments. The percent time spent in the open arms was used as a surrogate measure of impulsivity behaviors; mice with lower levels of impulsivity behaviors spend less time in the open arms. The maze was cleaned between tests with a weak ethanol solution and dried.
2.4 In vitro synaptic plasticity measures
Four weeks after injury, mice were anesthetized with isoflurane (NDC 10019-360-40, Baxter Healthcare Corporation Deerfield, IL, USA) and decapitated (n=7–9/group). The brains were quickly removed and placed for sectioning in ice-cold treatment artificial cerebrospinal fluid (tACSF) containing (in mM) NaCl 124, KCl 3, NaH2PO4 1.25, NaHCO3 26, CaCl2 2, MgSO4 2, and glucose 10 (pH 7.4, and bubbled with 95% O2 and 5% CO2 gas mixture). Coronal slices (thickness: 350 μm) that contained primary motor cortex (M1) were cut with a Vibratome 1000P (LeicaVT1000P, Leica Microsystems Inc., Buffalo Grove, IL, USA) and transferred to a chamber with oxygenated tACSF for 90 min at 30°C before recording. Slices were finally transferred to the chamber of MED64 probe (MED-P5155, AutoMate Scientific, Inc., Berkeley, CA, USA) with oxygenated recording ACSF (rACSF) containing (in mM) NaCl 124, KCl 3, NaH2PO4 1.25, NaHCO3 26, CaCl2 2, MgSO4 1, and glucose 10 (pH 7.4) at 30°C.
We focused on primary motor cortex for electrophysiology studies given our prior published results demonstrating reversible deficits in neocortical but not hippocampal LTP after mTBI.17 In this study, fEPSPs were recorded by a multi-electrode array recording system (MED64 system) with MED-P5155 probe (AutoMate Scientific, Inc., Berkeley, CA, USA). The size of each electrode in the array was 50 μm × 50 μm, and all 64 electrodes were arranged in an 8 × 8 square pattern with an inter-electrode distance of 150 μm to cover 1.1 mm2. After incubation, one M1 slice was positioned in the center of the MED64 probe to be fully covered by the 8 × 8 electrode square. A fine mesh and a mesh anchor were placed on top of the slice to immobilize the slice during recording. The probe, with the immobilized slice, was connected to two MED64 amplifiers [MED64 Head Amplifier (MED-A64HE1) and Main Amplifier (MED-A64MD1), AutoMate Scientific, Inc., Berkeley, CA, USA]. The slice was continuously perfused with oxygenated, fresh rACSF at the rate of 2 ml/min using a peristaltic pump (Minipuls 3, Gilson, Inc., Middleton, WI).
Data were collected using Mobius software (Mobius 0.4.2). Field potentials were induced in mouse M1 slices by single pulses (0.2 ms) delivered at 0.05 Hz through one planar microelectrode. The fEPSP was recorded from layer II/III by stimulating the vertical pathway (layer V to II/III). The stimulus intensity was sufficient to induce a fEPSP slope approximating 50% of the maximum slope in all electrophysiology experiments. The fEPSP slope was chosen to monitor synaptic responses because fEPSP amplitude is frequently contaminated by the population spike.23 A stable fEPSP slope for 20 minutes was required and recorded as baseline before LTP induction. We used a high-frequency stimulation (HFS: 200 Hz for 1 sec) to induce LTP. The data were filtered with a low-cut of 1Hz and high-cut of 10 kHz, and digitized at a 20 kHz sampling rate.
2.5 Preparation of brain homogenates and subcellular fractions
Four weeks after injury, brain homogenates and subcellular fractionations were prepared as previously described with slight modifications.24 Briefly, mice cortical and hippocampal tissues were lysed in 6 volumes (volume/tissue weight) of buffer I containing 320 mM sucrose, 10 mM Tris, pH 7.4, 1 mM Na3VO4, 5 mM NaF, 0.5mM of phenylmethanesulfonylfluoride (PMSF), 1 mM EDTA, and 1 mM EGTA with a tissue grinder (Thermo Fisher Scientific, MA). Homogenates were centrifuged at 800g at 4°C for 10 minutes to obtain P1 pellets and supernatants (S1). The S1 was centrifuged at 10,000g at 4°C for 10 minutes to obtain P2 pellets and supernatants (S2). The P2 fractions were suspended in buffer II containing 0.5% Triton X-100, 10 mM Tris, pH 7.4, 1 mM Na3VO4, 5 mM NaF, 1 mM EDTA, and 1 mM EGTA, and further centrifuged at 100,000g at 4°C for 1 hour to obtain pellets (P3) and supernatants (S3). The P3 were then resuspended in buffer I with 0.5% SDS. Protein concentration was determined using a Bio-Rad Protein Assay Dye solution (Bio-Rad, Hercules, CA). Equal amounts of protein were loaded into 4–15% gradient gel for further analysis.
2.6 mRNA extraction and quantitative real-time polymerase chain reaction analysis
Four weeks after injury, total RNAs were extracted from the hippocampus or cortex using Illustra RNAspin Mini Kit (GE healthcare life science, Pittsburgh, PA). Complementary DNA (cDNA) was synthesized from one microgram of total RNA using iScriptTM RT-qPCR Kit (BIO-RAD, Hercules, CA). Quantitative real-time polymerase chain reaction analysis was performed on StepOne™ from Applied Biosystems. The primers used in the study were as follows: NR2B, forward: GCCAAACTGGAAGAACATGG; reverse: TCTGCTCAGACTCTCACCCC. NR1, forward: GGAGAGCTAGGGGCAAGC; reverse: GTTGCTCAGCTCGGACCAG. GAPDH (used as housekeeping gene), forward: GGAGAGCTAGGGGCAAGC; reverse: TCGTCCCGTAGACAAAATGG. Power SYBR Green PCR Master Mix was purchased from Life Technologies. The thermal cycler conditions were as follows: 10 minutes at 95°C, followed by 45 cycles of a 2-step PCR consisting of a 95°C step for 15 seconds followed by a 60°C step for 25 seconds. Amplifications were carried out in triplicate and the relative expression of target genes was determined by the ΔΔCT method.
2.7 Immunohistochemistry
Mice were perfused transcardially 1 month after injury and brains were collected for histopathological outcomes. Serial 20 μm coronal frozen sections from sham (n=6) and injured (memantine treated n= 6, vehicle treated n=6) brains were cut on a cryostat (Leica, Leitz-Park, Germany) from the anterior frontal lobes through the posterior extent of the dorsal hippocampus. Every 10th section was collected and mounted on slides. After hydrogen peroxide treatment and incubation in a blocking solution containing 3% normal donkey serum, sections were incubated overnight at 4°C with anti-IBA-1 (WAKO, 1:250) antibody. The following day, sections were washed and incubated sequentially with appropriate secondary antibody, Vectastain Elite ABC kit (Vector, Burlington, CA), and diaminobenzadine (DAB), and mounted with Permount (Thermo-Fisher Scientific, Waltham, Massachusetts).
2.8 Quantification of microglia
For quantifying the number of microglial cells in the brains, twenty-micrometer thick bain sections were prepared. Three sections (bregma −1.64, −1.84 and −2.04) from each brain were selected. The IBA1 positive cells in the left cortex (counting area defined as from interhemispheric fissure to 2.5 cm straight away from fissure) and left hippocampus were counted under microscope (100X objective). The number reflected the amount of microglia in each side of the hippocampus. The operator was blinded to the groups.
2.9 Statistical Analyses
Data are presented as mean ± standard error of the mean or median and interquartile range (IQR) as appropriate. Continuous variables were compared between injured and sham injured mice and memantine treated versus vehicle treated mice at single time points using analysis of variance (ANOVA) or Kruskal-Wallis for univariate testing as appropriate. To account for repeated measures over time, MWM and rotorod latencies were analyzed by linear regression with clustered, robust standard errors. Statistical significance was considered p < 0.05. These analyses were performed using Stata 11.2 (StataCorp, College Station, TX).
fEPSP data were analyzed off line by the MED64 Mobius software. To improve the signal-to-noise ratio, 3 successive responses were averaged. To quantify the magnitude of LTP, fEPSP slope values 30 min (45–55min) after HFS application were normalized and expressed as fold changes of the averaged baseline (0–10min). Statistics were performed using the number of mice as the ‘n’ value (1–3 slices per each mouse). Statistical significance between more than 2 groups was determined by one-way ANOVA and post hoc test (Bonferroni’s Multiple Comparison Test) using Prism (GraphPad, La Jolla, CA) software. Paired t-test was used to compare fEPSP slope changes of each group to the baseline. Differences with p < 0.05 were considered statistically significant. Experimental data in the figure and text are presented as means ± SE.
3. Results
3.1 Memantine attenuates beta-amyloid precursor protein (APP) expression after rmTBI at acute but not chronic time points
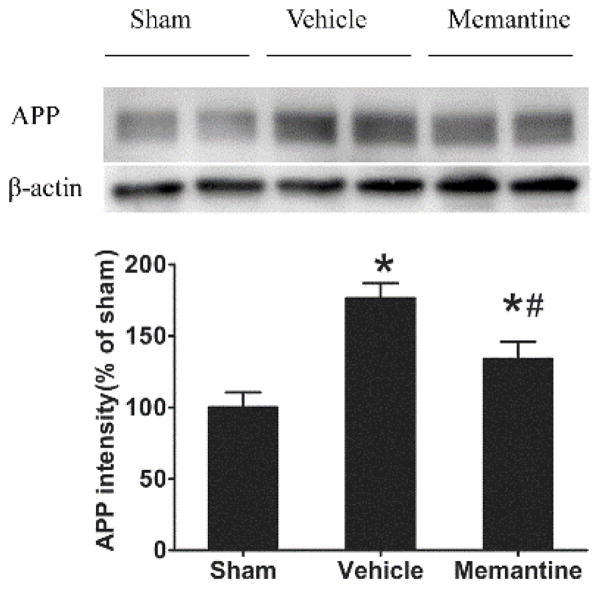
APP upregulation is a hallmark of axonal injury and has been found in TBI patients.25–27 Here we examined cortical APP expression in the brain 3 days and 1 month after the last rmTBI injury. APP expression was increased after injury in both injured vehicle (1.7-fold) and injured memantine treated mice (1.3-fold). However, memantine treatment significantly attenuated APP over-expression in injured mice (p<0.05, Figure 1). One month after the last injury, there was no significant difference of APP expression among sham, vehicle treated and memantine treated mice (relative expression of APP in vehicle treated mice was 120% of that in sham, p=0.14; expression of APP in memantine treated mice was 111% of that in sham, p=0.37).
Figure 1. Acute memantine treatment suppresses APP increase after rmTBI.
Memantine was given acutely after each injury and APP expression was examined 3 days after last injury. (A) a representative image of APP and β-actin Western blot image. (B). Densitometry was used for semi-quantifying intensity of protein expression. The expression of APP was normalized by βactin expression and further compared to sham group. *compared to sham group, p<0.05; # compared to vehicle treated injury group, p<0.05; n = 8/group.
3.2 Treatment with memantine after rmTBI mitigates the accumulation of phosphorylated tau at acute time points
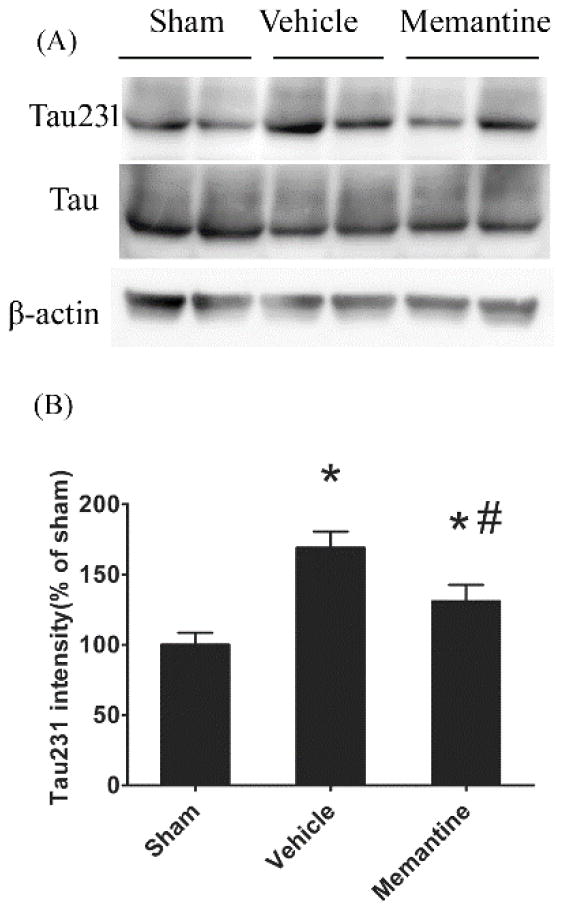
We have previously shown that phosphorylation at the T231 site of tau is an early pathogenic event in the development of tauopathy which appears early in the cortex but not hippocampus after rmTBI.17 Here, we examined cortical phosphorylated tau 3 days and 1 month after rmTBI. Cortical phosphorylated tau (T231) was increased 70% compared to sham controls, though no significant changes were observed in the hippocampus. The early, post-injury increase in phosphorylated tau was attenuated in injured memantine treated mice (24% reduction in injured memantine treated mice compared to injured vehicle treated mice, p<0.05, Figure 2). One month after the last rmTBI injury, there was no significant difference of phosphorylated tau (T231) expression between groups (relative expression of phosphorylated tau in vehicle treated mice was 117.5% of that in sham, p=0.13; expression of phosphorylated tau in memantine treated mice as 104% of that in sham, p=0.63). Total tau expression between groups was almost identical.
Figure 2. Acute increase of tau phosphorylation after rmTBI.
The cortices were collected from sham, injured vehicle or injured memantine treated mice 3 days after last injury. Phosphorylated tau (T231) and total tau expressions were examined by Western blot. (A) representative Images of Western blots. (B) semi-quantitative results using densitometry. * injury vs. sham, p<0.05; # memantine vs. vehicle, p<0.05. Data are presented as mean ± SEM, n = 8/group
3.3 Treatment with memantine rescues NMDAR subunit loss after injury and partially restores LTP
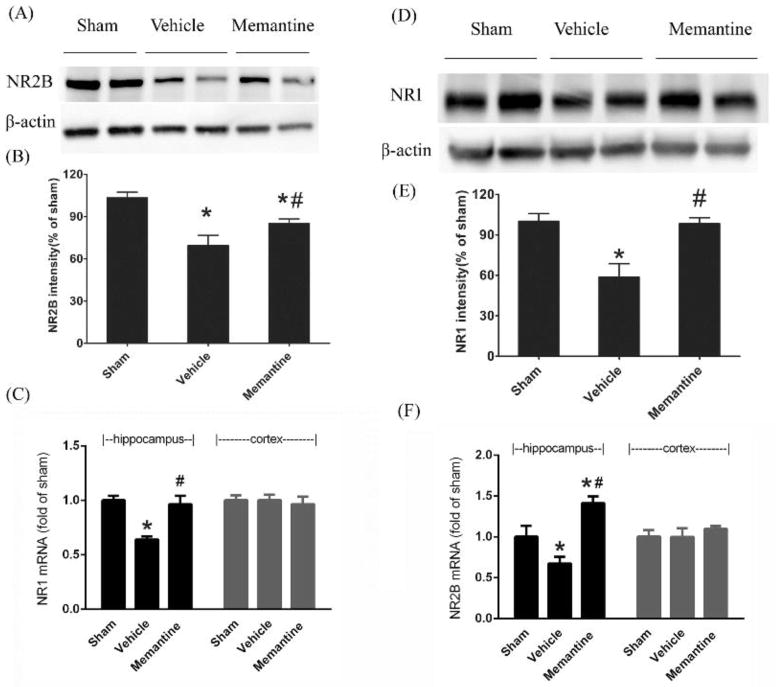
One month after injury, using densitometry measurement of immunoblots, NR2B subunit expression dropped 31% (p<0.05) and NR1 subunit expression was reduced 37% (p<0.05) in injured vehicle treated mice compared to sham controls. mRNA levels of both units also decreased significantly after injury (p<0.05). Treatment with memantine mitigated the post-injury decline in NR1 and NR2B subunit mRNA expression (Figure 3).
Figure 3. Memantine treatment restores NR1 and NR2B expression 1 month after rmTBI are rescued in injured memantine treated mice compared to vehicle treated mice.
(A) representative image of NR2B immunoblotting. (B) quantitatively analyed NR2B protein expression.(C) mRNA of NR2B expression. (D) representative image of NR1 immunoblotting. (E) quantitative expression of NR1 protein and (F) mRNA expression of NR1 analyzed by real time PCR.*p<0.05 compared to sham, #p<0.05 compared to vehicle. Data are presented as means ± SE, n = 8/group.
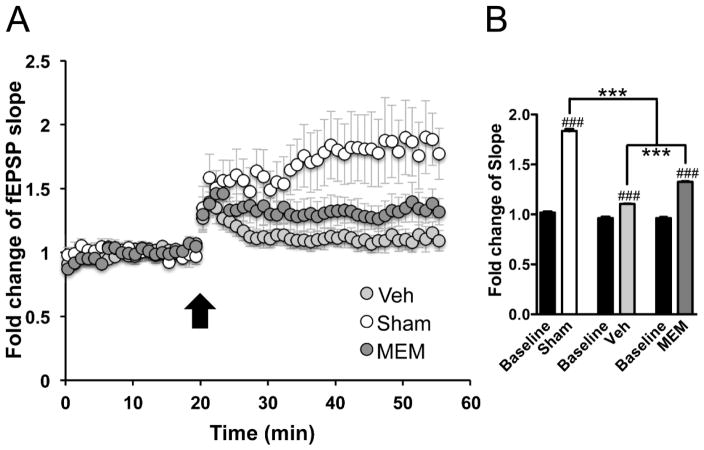
One month after rmTBI, neocortical slice recordings revealed attenuated LTP in the vehicle treated group, where potentiation was 10% above baseline (110.4 ± 0.8% of baseline, n=9, p<0.001 as compared to baseline by paired-t test), in contrast to 84% fEPSP slope increase (183.6 ± 1.7% of the baseline, n=8, p<0.001 as compared to baseline by paired-t test) in the sham-injured group. LTP in the memantine-treated group, was incompletely preserved with fEPSP slope potentiation to 33% above baseline (132.6 ± 2.4% of the baseline, n=7, p<0.001 as compared to baseline by paired-t test), which was significantly (p<0.001 by one-way ANOVA post hoc test) greater than the LTP magnitude in the vehicle-treated group (Figure 3). Significant difference of fEPSP slopes at 30 min (45–55min) after HFS application was found among the sham-injured, vehicle- and memantine-treated group [F(2,30)=786.3, p<0.001 by one-way ANOVA, Figure 4].
Figure 4. Treatment with memantine partially restores the LTP deficit after rmTBI.
(A) The LTP responses induced by HFS in M1 slices. LTP magnitude (fEPSP slope change 30min after HFS relative to baseline) was attenuated in the vehicle treated mice (Veh: 110.4 ± 0.8% of baseline; n=9, p<0.001) as compared to sham (183.6 ± 1.7% of baseline; n=8, p<0.001). In the memantine (MEM) treated group, this LTP deficit was partially recovered (132.6 ± 2.4% of baseline; n=7, p<0.001). (B) Statistic analysis of the fEPSP slopes changes averaged from the last 10-min’s (45–55min) recording of each group [F(2,30)=786.3, p<0.001 by one-way ANOVA]: ***p<0.001 indicates post hoc test between two means as indicated in the graph. ###p<0.001 indicates paired t-test between fESPS slope changes and individual baseline (initial 10-min’s recording). n = 8/group
3.4 Memantine suppresses microglial activation after rmTBI
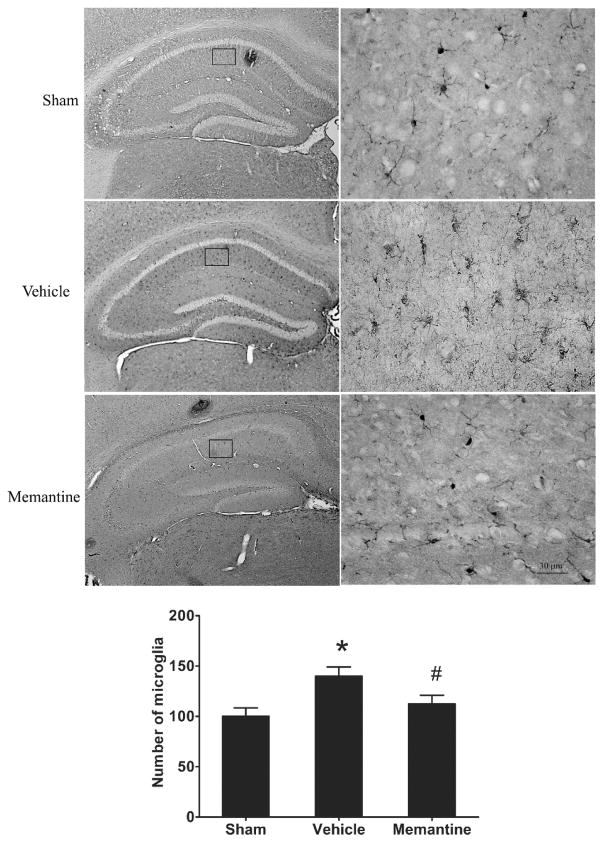
One month after rmTBI, the number of IBA1 positive cells in the hippocampus of injured vehicle treated mice was increased by 50% compared to sham, while injured memantine treated mice had no significant difference in IBA1 positive cells compared to sham (Figure 5). However, we did not detect a significant difference of IBA1 positive cells in the cortex between shams, injured vehicle treated and injured memantine treated groups (data not shown).
Figure 5. Treatment with memantine attenuates increase of microglial cell after rmTBI.
(A) IBA1 staining images in hippocampus with lower (left panel) and higher (right panel) magnifications. (B) IBA1 positive cells in left hippocampus from 3 coronal sections (bragma −1.62, −1.86 and −2.1mm) each mouse were counted under microscope.* p<0.05, vehicle vs. sham, #p<0.05, memantine vs. vehicle. The data are presented as mean/section ± SEM, n= 6/group.
3.5 Treatment with memantine after rmTBI does not improve behavioral outcomes
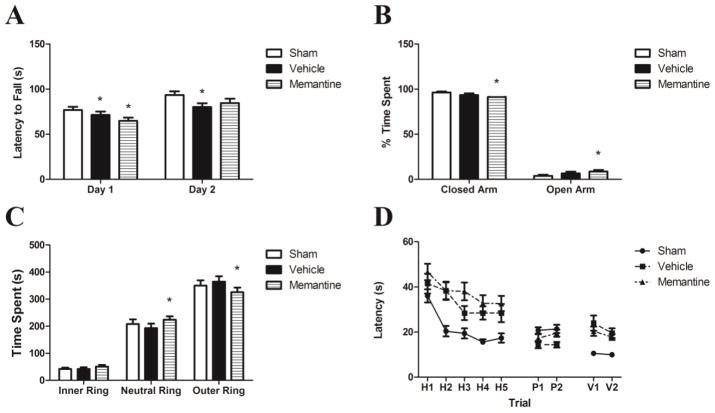
On days 4–6 after the last injury, injured vehicle-treated mice had decreased latency to fall on rotarod compared to sham mice on days 1 and 2, while injured memantine treated mice had similar rotarod performance compared to sham on day 2 (Figure 6A). 20 days after the last injury, injured memantine treated mice spent less time in the closed arm of the elevated plus maze compared to vehicle treated injured and sham injured mice (85% vs 95% and 96% respectively, p<0.001, Figure 6B). Injured memantine treated mice also demonstrated decreased time in the outer ring on open field testing compared to sham and vehicle treated mice (p<0.001, Figure 6C). Compared to sham injured mice, injured mice demonstrated impaired performance on MWM (p<0.0001, Figure 6D). The injury effect was worse in memantine vs. vehicle treated mice (p<0.001).
Figure 6. Behavior outcomes.
(A) Rotarod task demonstrated deficits of motor balance in injured mice compared compared to sham, n = 28 injured memantine-treated, n= 28 injured vehicle-treated, n = 23 sham,* p<0.05 compared to sham (data are mean± SEM). (B) During elevated plus maze test, injured memantine-treated mice spent less time in closed arm and more time in open arm compared to sham and injured vehicle treated mice, n = 28 injured memantine-treated, n= 28 injured vehicle-treated, n = 24 sham, *p<0.05 compared to sham (data are mean± SEM). (C) In open field test, the injured memantine-treated mice spent less time in outer ring and more time in neutral ring compared to sham and injued vehicle-treated groups, n = 28 injured memantine-treated, n = 28 injured vehicle-treated, n = 24 sham,*p<0.05 compared to sham. No difference of time spent in inner ring (data are mean± SEM). (D) In Morris water maze test, both vehicle and memantine treated injured groups mice had significant deficit of spatial memory, though the effect of injury was worse in injured memantine treated mice, n = 21 injured memantine-treated, n = 21 injured vehicle-treated, n = 18 sham. Continuous variables were compared between injured and sham injured mice and memantine treated versus vehicle treated mice at single time points using analysis of variance (ANOVA) or Kruskal-Wallis for univariate testing as appropriate. To account for repeated measures over time, MWM and rotorod latencies were analyzed by linear regression with clustered, robust standard errors.
4. Discussion
We found that administration of memantine after rmTBI improves histopathological outcomes, restores NMDAR subunit loss and partially mitigates loss of neocortical synaptic plasticity, though without a corresponding beneficial effect in behavioral outcomes. The histopathological data are encouraging, and considering that NMDAR antagonists are routinely used in the mild TBI setting, indicate a potential utility of NMDAR blockade even if administered after a series of concussive injuries, though caution is warranted given behavior outcomes in this study.
To our knowledge, this is the first study to evaluate the potential protective effects of NMDAR blockade in the setting of rmTBI in vivo. Prior studies in lateral fluid percussion TBI have suggested that early glutamate release after injury is a proximal event in the cascade of post-injury intracellular Ca2+ accumulation, axonopathy and metabolic crisis that characterizes secondary injury.4, 10 NMDAR antagonists have, in this setting, been shown to inhibit APP increase and axonal injury after an isolated, severe TBI episode.28, 29 Another recent study demonstrated treatment with memantine significantly protected against cell death, LTP loss and astrogliosis after repetitive stretch injury.30 Our results indicate that this therapeutic approach may also be useful after repetitive mild TBI.
The mechanisms by which rmTBI causes cognitive dysfunction remain unknown. However, absent cell death and gross structural injury in preclinical 19 and clinical rmTBI suggest synaptic dysfunction is a likely candidate to explain rmTBI-associated functional deficits. Prior studies suggest that key mediators of synaptic function, including NMDAR, tau and glial cells, are perturbed after TBI and rmTBI.17, 18, 31, 32 Here we evaluated whether NMDAR-directed therapy can restore post-injury changes in these key mediators with concomitant improvement in functional outcomes.
First, we found that early treatment with memantine after rmTBI mitigated early accumulation of hyperphosphorylated tau after rmTBI. Both preclinical and clinical studies have suggested that tau phosphorylation is implicated in the causal pathway leading from rmTBI to tauopathy, particularly as described in chronic traumatic encephalopathy (CTE).17, 33, 34 We examined phosphorylation at the T231 residue, which has been previously shown to be a critical, early phosphorylation site associated with the first stages of detectable tauopathy.17 We confirmed results from our prior studies detailing early phosphorylation of T231 after rmTBI, but interestingly also found that early treatment with memantine attenuates tau phosphorylation at the acute time point. The mechanism of this protective effect is unclear. Published data indicate that the NR2A subunit may be important in limiting tau phosphorylation via a PKC/GSK3β pathway.35 It is possible that the early reduction in tau phosphorylation, seen with early memantine treatment in our model, is resultant from preservation (or restoration) of physiologic NMDAR subunit composition. NMDAR antagonist treatment may also mitigate the toxicity of hyperphosphorylated tau in this setting.36
Next, we found that treatment with memantine prevented pathologic changes in NMDAR subunit expression. Prior studies in more severe TBI models have demonstrated NMDAR subunit loss at subacute time points after injury.9–11, 37 In our rmTBI model, we found a similar pattern of NMDAR loss after rmTBI. Characterizing the full time course of recovery of NMDAR expression after rmTBI could have significant implications for therapies targeting NMDAR and is an important gap in knowledge. However, we found that administration of memantine at acute time points after injury preserved NMDAR expression 1 month after rmTBI. Notably, the decrement of NR1 and NR2B after injury were of similar magnitiude, suggesting that NMDAR composition may not significantly change after rmTBI, but further charcterization of NMDAR subunit expression in this setting could further guide therapeutic interventions.
The protection against NMDAR subunit loss after injury in memantine-treated injured mice was associated with improved cortical LTP 1 month after the last injury compared to vehicle-treated injured mice. While therapies targeting hippocampal LTP deficits have previously been described in multiple brain injury models, no studies have demonstrated changes in neocortical LTP after TBI or directly addressed therapeutic interventions targeting neocortical LTP. Neocortical LTP may be particularly relevant to the cognitive symptoms of mild TBI where we and others find mnemonic and performance deficits referable to aberrant neocortical plasticity.17 We found that LTP was partially restored when memantine treatment was adminsistered early after rmTBI, correlating with preservation of NMDAR expression after injury. These data could have immediate translatable impact, in that changes in neocortical synaptic plasticity after closed head injury could potentially be diagnosed and monitored in the clinical setting using transcranial magnetic stimulation (TMS).38
Treatment with memantine also attenuated microgliosis after rmTBI. Several direct and indirect mechanisms may explain the reduction in Iba1 positive cells in injured memantine treated mice. Early NMDAR blockade after injury may act to dampen the microglial response to glutamate. By decreasing tau phosphorylation after injury, NMDAR blockade may also inhibit NMDAR in microglia and decrease the stimulus for microglial activation.39 Whether or not early treatment with NMDAR antagonists persistently attenuates or merely delays the glial response to injury was beyond the scope of the current study, but will need to be addressed in future efforts.
It is notable that despite the beneficial effects on NMDAR expression, tau phosphorylation and gliosis, we did not find a corresponding effect of NMDAR blockade on behavioral outcomes. This is also consistent with incomplete preservation of neocortical LTP. In contrast, prior studies of memantine in healthy rats and mice showed memantine improved outcomes in spatial, pain and social recognition memory tasks,40, 41 though the effects of memantine on LTP in healthy brains have sometimes been contradictory.21, 42 As with any intervention, the issues that future experiments will have to address are those of dose and timing. Nevertheless, the favorable histologic and electrophysiologic outcomes in our experiment raise prospects for a therapeutic role of NMDA blockade in rmTBI.
The treatment window for NMDAR antagonist therapy after rmTBI is likely complex, and may require a personalized approach based on timing and severity of injuries. Biegon found that hyperactivation of glutamate NMDAR after injury is short-lived following severe TBI, suggesting a brief window where NMDAR antagonists might confer a beneficial effect.11 Furthermore, stimulation of NMDAR by NMDA 24 and 48 h postinjury produced a significant attenuation of neurological deficits (blocked by coadministration of MK801) and restored cognitive performance 14 days postinjury. However, preclinical and clinical studies have suggested strong benefits of NMDAR antagonist therapy in the setting of Alzheimer’s pathology43 suggesting that NMDAR targets may also be relevant to the long term neurodegenerative changes associated with rmTBI. Further studies are needed to better characterize the temporal expression and function of NMDAR after rmTBI.
This study has several important limitations. First, we evaluated the effect of a single NMDAR antagonist, memantine on outcomes after rmTBI. Memantine is a noncompetitive NMDAR antagonist whose action is theoretically contingent upon prior activation of the receptor (thus blocking higher concentrations of agonist better than lower concentrations) and may affect extrasynaptic NMDAR activity more than synaptic NMDAR.44 Other NMDAR anatogonists, including amantadine16 (which is also commonly clinically used, but has dopaminergic properties in addition to NMDAR antagonism), may have different effects on post-injury outcomes and each should be evaluated for its individual therapeutic profile. Second, we utilized a repetitive injury model, relevant to athletes and veterans, which may limit the clinical translation to the majority of mild TBI. Third, we evaluated treatment at acute, but not subacute or chronic time points after injury. As the time course of postinjury NMDAR expression and activation is dynamic, it is possible that treatment at various time points after rmTBI may have markedly different effect profiles. Fourth, we did not address NR2A expression but focused on NR1 and NR2B expression as these subunits have been implicated in the response to stretch injuries.45 Fiftth, we ordered our behavioral testing based on the availability of the apparati in our neurobehavioral core and the order of testing may have affected the results of the tests. Despite this, the order was consistent throughout the experiments, the potential effects of the order of behavior testing should be similar between groups. Sixth, we did not include a single mTBI injury as an additional control group. Prior preclinical studies of memantine have suggested a beneficial effect of memantine after single moderate or severe injuries46–48 but the effects of memantine have not been well-studied in a single mTBI injury and will ne important to assess in future studies.
This study suggests that NMDAR antagonist therapy after rmTBI may be beneficial in treating post-injury synaptic dysfunction in the neocortex, partially reversing deficits in LTP, mitigating pathologic NMDAR loss, and reducing tau phosphorylation and APP expression. To our knowledge, this is the first study to directly address targeting NMDAR after rmTBI, which is relevant to athletes and veterans who have prolonged cognitive sequelae of repetitive concussion. While NMDAR antagonists are frequently employed in the setting of post-TBI cognitive dysfunction, further preclinical and clinical data are needed to guide clinicians as to the optimal timing and duration of this common therapeutic intervention after concussion.
Highlights.
Repetitive mild TBI results in tau phosphorylation, NMDAR loss, impaired function
NMDAR-mediated excitotoxicity is proposed to mediate these post-injury sequelae
Treatment with an NMDAR antagonist partially prevented these sequelae injury
Acknowledgments
Funding Sources and Potential Conflicts of Interest
CHB IDDRC supported all the behavior studies reported. Rebekah Mannix is supported by T32 HD40128-11A1 and by a grant from Harvard Catalyst (National Football League Players Association) and by philanthropic support from the National Hockey League Alumni Association through the Corey C. Griffin Pro-Am Tournament. Zhongrong Mei is supported by Guangzhou Medical College Research Grants(1201421151). Dr. Meehan receives royalties from ABC-Clio publishing for the sale of his book, Kids, Sports, and Concussion: A guide for coaches and parents, and royalties from Wolters Kluwer for working as an author for UpToDate. He is under contract with ABC-Clio publishing for a future book entitled, Concussions, and with Springer International publishing for a future book entitled, Head and Neck Injuries in Young Athletes. His research is funded, in part, by a grant from Harvard Catalyst (National Football League Players Association) and by philanthropic support from the National Hockey League Alumni Association through the Corey C. Griffin Pro-Am Tournament.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychological medicine. 1973;3:270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- 2.Martland H. Punch drunk. Journal of the American medical Association. 1928;91:1103–1107. [Google Scholar]
- 3.Takahashi H, Manaka S, Sano K. Changes in extracellular potassium concentration in cortex and brain stem during the acute phase of experimental closed head injury. Journal of neurosurgery. 1981;55:708–717. doi: 10.3171/jns.1981.55.5.0708. [DOI] [PubMed] [Google Scholar]
- 4.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. Journal of neurosurgery. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 5.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? The Lancet. Neurology. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 6.Schuler T, Mesic I, Madry C, Bartholomaus I, Laube B. Formation of NR1/NR2 and NR1/NR3 heterodimers constitutes the initial step in N-methyl-D-aspartate receptor assembly. The Journal of biological chemistry. 2008;283:37–46. doi: 10.1074/jbc.M703539200. [DOI] [PubMed] [Google Scholar]
- 7.Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- 8.Olney JW, Sharpe LG. Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science. 1969;166:386–388. doi: 10.1126/science.166.3903.386. [DOI] [PubMed] [Google Scholar]
- 9.Osteen CL, Giza CC, Hovda DA. Injury-induced alterations in N-methyl-D-aspartate receptor subunit composition contribute to prolonged 45calcium accumulation following lateral fluid percussion. Neuroscience. 2004;128:305–322. doi: 10.1016/j.neuroscience.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 10.Giza CC, Maria NS, Hovda DA. N-methyl-D-aspartate receptor subunit changes after traumatic injury to the developing brain. Journal of neurotrauma. 2006;23:950–961. doi: 10.1089/neu.2006.23.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biegon A, Fry PA, Paden CM, Alexandrovich A, Tsenter J, Shohami E. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: Implications for treatment of neurological and cognitive deficits. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5117–5122. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meehan WP., 3rd Medical therapies for concussion. Clinics in sports medicine. 2011;30:115–124. ix. doi: 10.1016/j.csm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabadi MH, Jordan BD. The cumulative effect of repetitive concussion in sports. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine. 2001;11:194–198. doi: 10.1097/00042752-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP, Jr, Matthews A, Mihalik JR, Cantu RC. Recurrent concussion and risk of depression in retired professional football players. Medicine and science in sports and exercise. 2007;39:903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- 15.Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, Onate JA, Kelly JP. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA: the journal of the American Medical Association. 2003;290:2549–2555. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- 16.Reddy CC, Collins M, Lovell M, Kontos AP. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. The Journal of head trauma rehabilitation. 2013;28:260–265. doi: 10.1097/HTR.0b013e318257fbc6. [DOI] [PubMed] [Google Scholar]
- 17.Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen CH, Yao Y, Lin YM, Driver JA, Sun Y, Wei S, Luo ML, Albayram O, Huang P, Rotenberg A, Ryo A, Goldstein LE, Pascual-Leone A, McKee AC, Meehan W, Zhou XZ, Lu KP. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature. 2015;523:431–436. doi: 10.1038/nature14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannix R, Berglass J, Berkner J, Moleus P, Qiu J, Andrews N, Gunner G, Berglass L, Jantzie LL, Robinson S, Meehan WP., 3rd Chronic gliosis and behavioral deficits in mice following repetitive mild traumatic brain injury. Journal of neurosurgery. 2014;121:1342–1350. doi: 10.3171/2014.7.JNS14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mannix R, Meehan WP, Mandeville J, Grant PE, Gray T, Berglass J, Zhang J, Bryant J, Rezaie S, Chung JY, Peters NV, Lee C, Tien LW, Kaplan DL, Feany M, Whalen M. Clinical correlates in an experimental model of repetitive mild brain injury. Annals of neurology. 2013;74:65–75. doi: 10.1002/ana.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meehan WP, 3rd, Zhang J, Mannix R, Whalen MJ. Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery. 2012;71:885–891. doi: 10.1227/NEU.0b013e318265a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J, Mufti A, Stan Leung L. Effects of memantine on hippocampal long-term potentiation, gamma activity, and sensorimotor gating in freely moving rats. Neurobiology of aging. 2015;36:2544–2554. doi: 10.1016/j.neurobiolaging.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of neuroscience methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 23.Bortolotto ZA, Anderson WW, Isaac JT, Collingridge GL. Synaptic plasticity in the hippocampal slice preparation. Curr Protoc Neurosci. 2001;Chapter 6(Unit 6):13. doi: 10.1002/0471142301.ns0613s16. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Xin X, Dong Y, Zhang Y, Yu B, Mao J, Xie Z. Surgical incision-induced nociception causes cognitive impairment and reduction in synaptic NMDA receptor 2B in mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:17737–17748. doi: 10.1523/JNEUROSCI.2049-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci Lett. 1993;160:139–144. doi: 10.1016/0304-3940(93)90398-5. [DOI] [PubMed] [Google Scholar]
- 26.Sherriff FE, Bridges LR, Sivaloganathan S. Early detection of axonal injury after human head trauma using immunocytochemistry for beta-amyloid precursor protein. Acta Neuropathol. 1994;87:55–62. doi: 10.1007/BF00386254. [DOI] [PubMed] [Google Scholar]
- 27.Geddes JF, Vowles GH, Beer TW, Ellison DW. The diagnosis of diffuse axonal injury: implications for forensic practice. Neuropathol Appl Neurobiol. 1997;23:339–347. [PubMed] [Google Scholar]
- 28.Goda M, Isono M, Fujiki M, Kobayashi H. Both MK801 and NBQX reduce the neuronal damage after impact-acceleration brain injury. J Neurotrauma. 2002;19:1445–1456. doi: 10.1089/089771502320914679. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Liu J, Fox HS, Xiong H. N-methyl-D-aspartate receptor-mediated axonal injury in adult rat corpus callosum. J Neurosci Res. 2013;91:240–248. doi: 10.1002/jnr.23150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Effgen GB, Morrison B., 3rd Memantine Reduced Cell Death, Astrogliosis, and Functional Deficits in an in vitro Model of Repetitive Mild Traumatic Brain Injury. Journal of neurotrauma. 2016 doi: 10.1089/neu.2016.4528. [DOI] [PubMed] [Google Scholar]
- 31.Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewen A, Fredriksson A, Li GL, Olsson Y, Hillered L. Behavioural and morphological outcome of mild cortical contusion trauma of the rat brain: influence of NMDA-receptor blockade. Acta neurochirurgica. 1999;141:193–202. doi: 10.1007/s007010050286. [DOI] [PubMed] [Google Scholar]
- 33.Turner RC, Lucke-Wold BP, Logsdon AF, Robson MJ, Dashnaw ML, Huang JH, Smith KE, Huber JD, Rosen CL, Petraglia AL. The Quest to Model Chronic Traumatic Encephalopathy: A Multiple Model and Injury Paradigm Experience. Frontiers in neurology. 2015;6:222. doi: 10.3389/fneur.2015.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanaan NM, Cox K, Alvarez VE, Stein TD, Poncil S, McKee AC. Characterization of Early Pathological Tau Conformations and Phosphorylation in Chronic Traumatic Encephalopathy. Journal of neuropathology and experimental neurology. 2015 doi: 10.1093/jnen/nlv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Montigny A, Elhiri I, Allyson J, Cyr M, Massicotte G. NMDA reduces Tau phosphorylation in rat hippocampal slices by targeting NR2A receptors, GSK3beta, and PKC activities. Neural plasticity. 2013;2013:261593. doi: 10.1155/2013/261593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamat PK, Rai S, Swarnkar S, Shukla R, Ali S, Najmi AK, Nath C. Okadaic acid-induced Tau phosphorylation in rat brain: role of NMDA receptor. Neuroscience. 2013;238:97–113. doi: 10.1016/j.neuroscience.2013.01.075. [DOI] [PubMed] [Google Scholar]
- 37.Kumar A, Zou L, Yuan X, Long Y, Yang K. N-methyl-D-aspartate receptors: transient loss of NR1/NR2A/NR2B subunits after traumatic brain injury in a rodent model. Journal of neuroscience research. 2002;67:781–786. doi: 10.1002/jnr.10181. [DOI] [PubMed] [Google Scholar]
- 38.Bashir S, Vernet M, Yoo WK, Mizrahi I, Theoret H, Pascual-Leone A. Changes in cortical plasticity after mild traumatic brain injury. Restorative neurology and neuroscience. 2012;30:277–282. doi: 10.3233/RNN-2012-110207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaindl AM, Degos V, Peineau S, Gouadon E, Chhor V, Loron G, Le Charpentier T, Josserand J, Ali C, Vivien D, Collingridge GL, Lombet A, Issa L, Rene F, Loeffler JP, Kavelaars A, Verney C, Mantz J, Gressens P. Activation of microglial N-methyl-D-aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann Neurol. 2012;72:536–549. doi: 10.1002/ana.23626. [DOI] [PubMed] [Google Scholar]
- 40.Wesierska MJ, Duda W, Dockery CA. Low-dose memantine-induced working memory improvement in the allothetic place avoidance alternation task (APAAT) in young adult male rats. Frontiers in behavioral neuroscience. 2013;7:203. doi: 10.3389/fnbeh.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishikawa R, Kim R, Namba T, Kohsaka S, Uchino S, Kida S. Time-dependent enhancement of hippocampus-dependent memory after treatment with memantine: Implications for enhanced hippocampal adult neurogenesis. Hippocampus. 2014;24:784–793. doi: 10.1002/hipo.22270. [DOI] [PubMed] [Google Scholar]
- 42.Mancini M, Ghiglieri V, Bagetta V, Pendolino V, Vannelli A, Cacace F, Mineo D, Calabresi P, Picconi B. Memantine alters striatal plasticity inducing a shift of synaptic responses toward long-term depression. Neuropharmacology. 2016;101:341–350. doi: 10.1016/j.neuropharm.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Coria H, Green KN, Billings LM, Kitazawa M, Albrecht M, Rammes G, Parsons CG, Gupta S, Banerjee P, LaFerla FM. Memantine improves cognition and reduces Alzheimer’s-like neuropathology in transgenic mice. The American journal of pathology. 2010;176:870–880. doi: 10.2353/ajpath.2010.090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia P, Chen HS, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh P, Doshi S, Spaethling JM, Hockenberry AJ, Patel TP, Geddes-Klein DM, Lynch DR, Meaney DF. N-methyl-D-aspartate receptor mechanosensitivity is governed by C terminus of NR2B subunit. The Journal of biological chemistry. 2012;287:4348–4359. doi: 10.1074/jbc.M111.253740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamprecht MR, Morrison B., 3rd A Combination Therapy of 17beta-Estradiol and Memantine Is More Neuroprotective Than Monotherapies in an Organotypic Brain Slice Culture Model of Traumatic Brain Injury. Journal of neurotrauma. 2015;32:1361–1368. doi: 10.1089/neu.2015.3912. [DOI] [PubMed] [Google Scholar]
- 47.Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 48.Rao VL, Dogan A, Todd KG, Bowen KK, Dempsey RJ. Neuroprotection by memantine, a non-competitive NMDA receptor antagonist after traumatic brain injury in rats. Brain research. 2001;911:96–100. doi: 10.1016/s0006-8993(01)02617-8. [DOI] [PubMed] [Google Scholar]