Abstract
Gap junctions are specialized membrane domains containing tens to thousands of intercellular channels. These channels permit exchange of small molecules (<1000 Da) including ions, amino acids, nucleotides, metabolites and secondary messengers (e.g., calcium, glucose, cAMP, cGMP, IP3) between cells. The common reductionist view of these structures is that they are composed entirely of integral membrane proteins encoded by the 21 member connexin human gene family. However, it is clear that the normal physiological function of this structure requires interaction and regulation by a variety of proteins, especially kinases. Phosphorylation is capable of directly modulating connexin channel function but the most dramatic effects on gap junction activity occur via the organization of the gap junction structures themselves. This is a direct result of the short half-life of the primary gap junction protein, connexin, which requires them to be constantly assembled, remodeled and turned over. The biological consequences of this remodeling are well illustrated during cardiac ischemia, a process wherein gap junctions are disassembled and remodeled resulting in arrhythmia and ultimately heart failure.
Keywords: Phosphorylation, Connexin43, kinase, gap junction
1. Introduction
1.1. Gap junctions are dynamic structures made up of rapidly turned over proteins
In both cells and tissue, gap junctions (also referred to as gap junction plaques) appear as distinct, discernable, 0.1–5μm long intermembrane structures via electron and light microscopy. Freeze fracture electron microscopy [1] and atomic force microscopy [2] clearly shows these structures consist of a tightly packed array of intercellular channels. Biochemical evidence shows that these channels are composed of proteins from the connexin gene family (21 members in humans) [3–6], with connexin43 (Cx43) being the most ubiquitously expressed and the most studied. Cx43 is characterized by its 1–3 hour half-life [7–11] – which is much shorter than average turnover times for other integral membrane proteins (t1/2 =17–100 h in hepatocytes [12], t1/2 >75 h in 3T3 cells [13]). Live cell imaging of Cx43 tagged with green fluorescent protein highlights the consequences of this rapid turnover showing that these proteins generate subcellular structures that are in a constant state of flux [14–16]. Connexins present in the gap junction appears to be segregated by age with the newest or “youngest” channels moving into the periphery of gap junction plaques through lateral accretion of Cx43 hexamers from the plasma membrane while the “oldest” protein resides in the center of the plaque. The size and longevity of individual gap junctions can be balanced through accretion of “new” protein and internalization of the oldest protein [15, 17]. Finally, rapid gap junction internalization can be triggered through a variety of stimuli including ischemia, wounding, oncogene activation and growth factor treatment [18]. Figure 1 shows two potential mechanisms for gap junction internalization: one via formation of double membrane structure containing connexins from both cells termed an annular junction (AJ) [19–26] and the other an example of gap junction disruption and channel undocking followed by endocytosis. Regulation of different internalization mechanisms are not well understood but rapid turnover of the gap junction structure in response to a variety of specific signals has clearly shown that cells can rapidly alter signaling and communication with their neighbors, a process that seems especially important under situations involving cell injury or stress, such as cardiac ischemia and epidermal wounding.
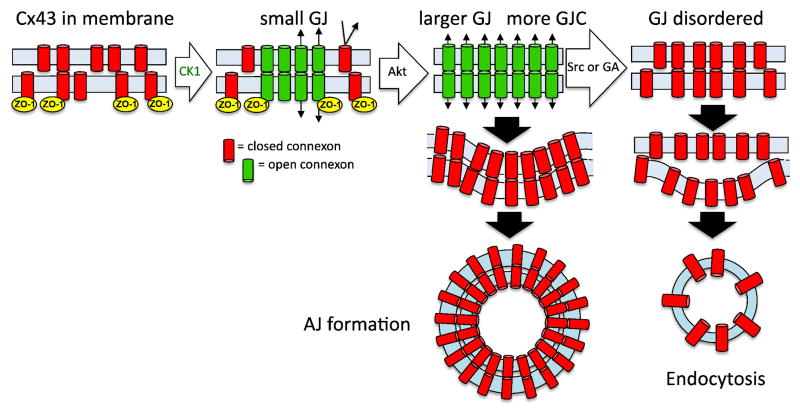
Figure 1.
A model of gap junction assembly and disassembly. Under homeostatic conditions, connexons aggregate into gap junctions via interaction with ZO-1 and CK1. Acute disassembly can be induced by a variety of stimuli that initially lead to Akt-mediated phosphorylation on Cx43, which inhibits ZO-1 interaction, and permits gap junction growth. Depending on stimuli and phosphorylation status of Cx43, internalization can occur via annular junction (AJ) formation or through disruption of gap junction organization and channel coupling then subsequent endocytosis.
1.2. Gap junctions and kinases-overview
Activation of different kinase systems has been shown to regulate connexin biology. Connexins are integral membrane proteins with 4 transmembrane domains and cytoplasmic N- and C-termini. Many connexins (e.g., Cx31, 32, 37, 40, 43, 45, 46, and 50) have multiple phosphorylation sites – with Cx43 being the most clearly mapped and analyzed. At least nineteen of the twenty-six serines and 2 of the 6 tyrosines in the C-terminal region of Cx43 have been identified as phosphorylation sites in cells or tissue and represent targets of a variety of kinases which serve to coordinate gap junction stability and function [16, 27].
Cx43 phosphorylation events can occur within 15 min of synthesis [28] and several kinases appear to affect the assembly of gap junctions. Cx43 migrates as multiple bands in SDS-PAGE with many cell types displaying prominent bands often labeled P0, P1 and P2 which represent sequential stages of Cx43 processing from the plasma membrane to the gap junction in untreated cells [29]. Various stimuli that induce rapid channel closure and gap junction internalization are also able to induce phosphorylation-dependent, SDS stable conformational changes visible as migration shifts shown in Figure 2. As a result, there is a rich history of utilizing SDS-PAGE to understand Cx43 phosphorylation and gap junction function. However, development of phosphospecific antibodies, kinase sensors and new imaging modalities provides an opportunity to more clearly define how the activation and coordination of kinase systems are integrated with gap junction biology.
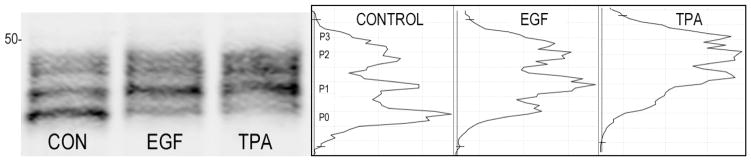
Figure 2.
Rapid changes in Cx43 phosphorylation occur in response to compounds that induce gap junction disassembly. Immunoblot analysis of NRK lysates with antibody to total Cx43 shows loss of the P0 form of Cx43 within 5 minutes of treatment with EGF 20ng/ml or TPA 50nM (Sigma). Linescans of the band densities are shown on the right. Antibodies and methods used are as previously described [104].
2. Gap junction assembly
2.1. Phosphorylation events involved in gap junction assembly
As a typical integral membrane protein, Cx43 is synthesized in the endoplasmic reticulum and traffics to the plasma membrane through the Golgi apparatus. Less conventionally, oligomerization of Cx43 into a hexamer, termed a connexon or “hemichannel”, occurs in the trans-Golgi network [30]. Transition of the connexon to the plasma membrane seems to involve phosphorylation on S364/365, an event which causes a conformational change visible as P1 by SDS-PAGE [31]. Casein kinase 1 (CK1) then phosphorylates Cx43 on S325/328/330 during the transition of Cx43 from the plasma membrane into the gap – junction staining with an antibody to these phosphorylated residues exclusively recognizes the P2 form of Cx43 in SDS-PAGE and only Cx43 present in the gap junction [32]. Inhibition of gap junction assembly has been shown to occur when cells are treated 12-O-tetradeconoylphorbol-13-acetate (TPA) [8], but due to the pleiotropic effects of TPA on cells and Cx43 phosphorylation (discussed in Section 3) it is not entirely clear what drives this inhibition.
2.2. Dynamics of gap junction assembly, channel aggregation and function
Freeze fracture electron microscopy indicates that nascent gap junctions form when connexons accumulate in a distinct region of the plasma membrane termed a formation plaque [33, 34]. Connexons initially form small clusters or aggregates in this region where the intermembrane distance between cells is already similar to that seen in mature gap junctions. This area presumably provides an opportunity for docking and formation of the intercellular channels and is potentially distinct from the “perinexus” defined as the region around an existing gap junction that includes unincorporated connexons and other connexin-associated proteins [35]. Over time these small aggregates of channels grow and fuse resulting in densely packed functional junctions. Bukauskas, et al., determined that gap junction size is a critical determinant of channel opening [36, 37]. They estimated that a minimum plaque size of 200–400 channels was necessary for GJ channels opening, and that more channels were likely to be open with increasing plaque size. However, even in plaques of ~2000 channels only 10–20% of channels were open. Treatment of cells with glycyrrhetinic acid has been shown to disrupt channel organization and function [38]. Thus, the arrangement of channels appears to be a critical parameter regulating function.
Live cell imaging using fluorescently-tagged Cx43 has clearly shown that connexins enter existing gap junctions via lateral diffusion through the plasma membrane to the peripheral edges of the gap junction followed by movement towards the interior of the gap junctions [15, 17]. Imaging of fixed cells and tissue shows that Cx43 connexons are also found at high concentration in areas surrounding gap junctions, a region that has been termed the perinexus [35, 39]. There is increasing consideration of these connexin rich areas as a scaffold or hub where intercellular communication intersects with intracellular signaling via kinases, the cytoskeleton and other junctional proteins [39–41]. This idea is discussed further in the context of cardiac tissue in Section 6.
2.3. ZO-1 regulates gap junction assembly
The concept of the perinexus originated, at least in part, with the finding that interaction of Cx43 with the MAGUK protein, ZO-1, occurs outside the periphery of gap junctions and plays a role in gap junction assembly. Results from Gourdie and colleagues have shown that ZO-1 interaction with the C-terminal region of Cx43 causes a reduction in gap junction size and conversely, elimination of the ZO-1 interaction leads to larger gap junctions. Furthermore, they showed that ZO-1 exerts its effect through interaction with Cx43 in the plasma membrane, limiting their movement into the gap junction [39, 42]. This has resulted in a working model where ZO-1 is involved in somehow sensing and regulating gap junction size.
3. Induced disassembly of gap junctions
The regulation of connexin and gap junction turnover are still not well understood. This is, in part, due to the fact that while the overall turnover of connexin molecules is fairly consistent and rapid across most cell types and conditions, the stability of individual gap junction plaques can be highly variable [43]. This is presumably due to altered kinetics of connexin assembly into and removal from a given gap junction. However, rapid restructuring followed by internalization of gap junctions occurs in most cell types in response to a variety of stimuli, including treatment with phorbol esters or growth factors [44, 45], oncogene activation [46, 47], ischemia [48–51] and epidermal wounding [52]. These events are coincident with increased phosphorylation on specific serines representing a kinase program which coordinates induced gap junction disassembly [16]. Figures 3 and 4 show examples of a time course of acute disassembly, where waves of phosphorylation occur within minutes. This program appears to be initiated by phosphorylation of Cx43 on S373 [53]. Figure 3 shows immunofluorescence of NRK cells that have been treated with TPA and immunolabeled with antibodies to total Cx43 (green) and Cx43 phosphorylated on S373 (red); after 5 minutes of treatment (middle panel), we see a dramatic increase in S373 phosphorylation visible as yellow. Under control conditions we see a variety of sizes of gap junctions, while after 5 minutes of TPA treatment most have taken on a larger more elongated appearance. By 30 minutes, gap junctions are diminished and in various states of disassembly and internalization. This is coincident with rapid gap junction growth providing a temporary increase in channel open probability. Over this time course, the western blot in Figure 4 shows sustained phosphorylation on S262 and S279/282 as channels close and gap junctions are broken down in response to EGF or TPA (see Figure 3). However, there are differences in phosphorylation patterns where TPA mediated activation of PKC also results in phosphorylation at S368. We discuss these events in a stepwise (i.e., waves) fashion below.
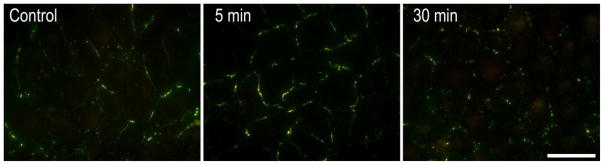
Figure 3.
Spatiotemporal changes in gap junction size and phosphorylation during induced disassembly. NRK cells were fixed and stained with a mouse antibody to total Cx43 (green) and a rabbit antibody to Cx43 phosphorylated on S373 (red). Gap junctions in control cells range in size and show little signal for pS373. After 5 minutes of TPA treatment (100nM) gap junction size is increased and Cx43 is phosphorylated on S373 (yellow). After 30 minutes of treatment gap junctions are diminished, disorganized and in various states of internalization. Antibodies and methods used were as described in [53]. (bar=25μm).
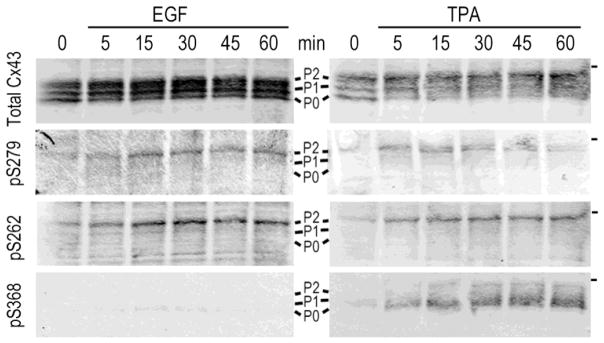
Figure 4.
Phosphorylation of Cx43 on S262, S279/282 accumulates upon treatment of NRK cells with EGF or TPA, while S368 is only phosphorylated in response to TPA. NRK cell lysates taken at various times after treatment were analyzed by immunoblot with antibodies to total Cx43 and rabbit antibodies to S262 (Santa Cruz Biotechnology), 279/282 [76] and S368 (R&D systems). Antibodies and methods used were as described in [76]. The position of the 50kDa marker is indicated on the right side.
3.1 Wave 1: Akt phosphorylation and gap junction growth
It has been shown that Akt phosphorylates Cx43 on S373 [53, 54]. Phosphorylation on S373 by Akt occurs not only in response to induced disassembly but in response to proteasome inhibition. Both of these conditions also result in increased junction size [53]. Increased gap junction size in response to proteasome inhibition initially led to the idea that ubiquitination of Cx43 is a signal for internalization and degradation, an idea that continues to be presented. However, we showed that it is not ubiquitination of Cx43, but rather ubiquitination of Akt, and its subsequent stabilization by proteasome inhibitors and increased phosphorylation of Cx43 at S373 that leads to increased gap junction size and stabilization [55]. We also found that the phosphorylation status of S373 controls the ability of Cx43 to interact with ZO-1 [53]. As discussed in section 2.3. ZO-1 is a MAGUK protein often associated with tight junctions but also an important regulator of gap junctions. Our lab has shown that conditions which stimulate phosphorylation on S373 or exogenous expression of a S373D phosphomimetic mutant of Cx43 led to larger gap junctions and a lack of ZO-1 interaction, by both immunofluorescence and co-immunoprecipitation [53]. Thus, ZO-1 appears to be involved in both constitutive assembly of gap junctions and during acute disassembly. Interestingly, these data support a model where gap junction growth is actually the first step in disassembly. While this seems a bit counterintuitive as part of a disassembly program, in fact, this increased assembly of connexons into gap junctions may be a way to more fully clear non-junctional connexin from the plasma membrane yielding larger gap junctions that are more suitable for internalization. In fact, a temporal sequence of Cx43 phosphorylation events occurs after wounding of human skin. First, Cx43 phosphorylation at S373 occurs [53] followed by phosphorylation at S279/282 and then S368 which correlates with loss of total Cx43 [56]. Figure 1 shows a model where Akt induced inhibition of Cx43:ZO-1 interaction results in connexons being rapidly shuttled into gap junctions, thus, depleting the plasma membrane of incoming gap junction channels. New gap junctions must then be formed de novo from anterograde trafficking of newly synthesized Cx43 molecules providing a delay in resumption of gap junction communication.
3.2 Wave 2: ERK phosphorylation on S279/282 and S262, channel closure and internalization
Work from several labs has shown that activation of ERK1/2 by a variety of stimuli results in direct phosphorylation of S279/282 [57–62]. This is also shown in Figure 3 where both TPA and EGF result in increased phosphorylation on S279/282. This review focuses primarily on the effects of Cx43 phosphorylation on the gap junction life cycle, however, phosphorylation can also affect gap junction channel properties as has been previously reviewed [63, 64]. Electrophysiology experiments utilizing S279/282 mutants show that these events are able to decrease gap junction channel “open probability” [57]. Thus, while induced disassembly results in a rapid increase in GJC in response to phosphorylation by Akt, there is a subsequent rapid shutdown of intercellular communication in response to phosphorylation by ERK. We hypothesize that this is necessary to keep gap junctions closed during internalization. In addition, we see a temporally similar increase in S262 phosphorylation. This phosphorylation event has also been shown by several labs but there seems to be some controversy in the field as to whether S262 represents an ERK or PKC site (discussed below). In any case, phosphorylation on S279/282 and S262 do appear to have functionally distinct consequences. While mutation of S279/282 have shown clear effects on channel closure, mutation of S262 has been shown to have more broad effects on cell behaviors including cell cycle progression and cell survival in cardiomyocytes. These experiments showed that a lack of S262 phosphorylation led to decreased ability of cells to enter S phase [65] and increased cell death in response to ischemia [66]. If these sites are, indeed, all targeted by ERK family members it is intriguing that there are distinct results of Cx43 phosphorylation that are so tightly coupled.
3.3 Wave 3: PKC phosphorylation, channel gating (and inhibition of GJ assembly?)
Numerous labs have shown that treatment with phorbol esters results in S368 phosphorylation. In NRK cells as shown here (Figure 4), EGF alone did not induce S368 phosphorylation but this site is phosphorylated during physiologically relevant examples of acute disassembly including keratinocyte scratch wounds [56] and cardiac ischemia [59]. This may indicate that different signaling inputs can activate distinct pathways for gap junction internalization. For example, Cone, et. al. [67] utilized fluorescent kinase biosensors to show that stimulation with phorbol esters led to recruitment of PKC to the gap junction, and they visualized the highest concentration of S368 phosphorylation in the center of gap junctions. They were also able to see phosphorylated subregions of a gap junction become internalized. Though visualization of individual gap junctions is beyond the resolution limits of fluorescent microscopy, these data do indicate that subregions of gap junctions may be internalized; we speculate that this occurs through a stepwise disassembly process where intercellular channels ‘undock’ from one another allowing for endocytosis to occur and that this process is distinct from annular junction formation. Interestingly, a role for ZO-1 in gap junction remodeling is again seen as ZO-1 binding inhibits phosphorylation on S368 and conversely S368 phosphorylation inhibits ZO-1 binding [68]. While mutagenesis studies show that phosphorylation on S368 clearly alters channel permeability [69], it is still not clear how it may cooperate with other phosphorylation events to induce internalization or inhibit gap junction assembly.
3.4. S262: ERK or PKC site?
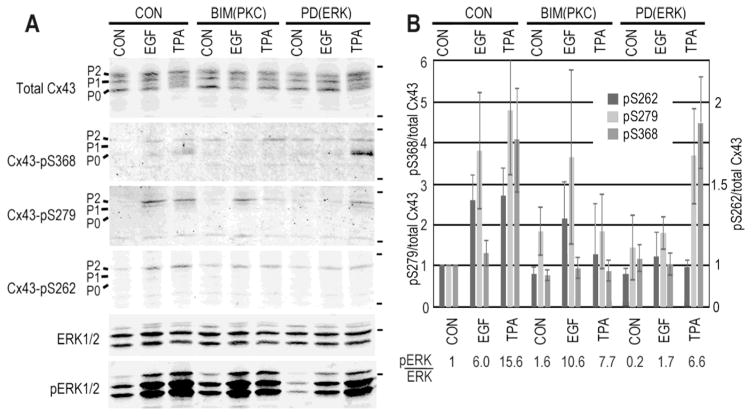
The gap junction literature often refers to S262 as a PKC target, in part, due to the fact that is often increased under conditions where PKC is activated (e.g., [65]). However, with the advent of phospho-antibodies to S262 and S368 most cell biological evidence seems consistent with 262 being primarily an ERK target. Figure 5 shows an example of cell lysates where either ERK (EGF treatment) or ERK and PKC (TPA treatment) are activated. While S368, a bona fide PKC site, is only phosphorylated in response to TPA, S279/282 and S262 are phosphorylated in response to both; it is notable that the increase in S262 phosphorylation was less dramatic than S279/282 and S368 but the 40% increase was significant (p<0.012 for EGF and p<0.014 for TPA). Probing with a phospho-ERK antibody shows that ERK is activated in response to both stimuli. Pretreatment of these cells with the PKC inhibitor, bisindomaleimide (BIM), led to inhibition of TPA induced S262 phosphorylation (p=0.6 comparing BIM control to TPA treated) while EGF treatment still induced S262 phosphorylation (p<0.05 comparing BIM control to EGF treated). In addition, inhibition of ERK activation with PD98059 was capable of diminishing S262 phosphorylation by both EGF and TPA. Finally, linear regression analysis of ERK phosphorylation and activation (measured as the ratio of phosphorylated ERK over total ERK, indicated below graph in Figure 5) compared to the ratio of S262 phosphorylation to total Cx43 showed an R2=0.810 whereas the S368 phosphorylation ratio had a R2=0.236. Similar data has been shown by other labs in other cell lines [70, 71]. While we can’t firmly state that S262 is a direct ERK1/2 site, the site does fit a consensus sequence for a proline-directed kinase [72] and biochemical and cell biology data argue that this event is typically correlated with ERK activation.
Figure 5.
Phosphorylation on S262 correlates with activation of ERK. NRK cells were treated with EGF and TPA for 30 minutes in the presence of the PKC inhibitor bisindomaleimide (BIM) or the ERK inhibitor PD98059 (PD) (Calbiochem). A. Cell lysates were analyzed by immunoblot with antibodies to S262, S279/282, S368 and ERK phosphorylated on Thr202/Tyr204 (Cell Signaling Technologies). The positions of the 50kDa and 36kDa (for the 4-Cx43 blots) are shown on the right. B. Graph shows quantification of Cx43 phosphorylation levels (n=3) and the ratio of pERK/total ERK is shown below. Note the scale on the left refers to S279/282 and S368 relative phosphorylation levels and the one on the right, which is non-linear, to S262. Linear regression analysis shows the phosphorylation on S262 is correlated to ERK phosphorylation with an R2=0.812.
4. Src and Gap Junction Disassembly
Activation of Src has long been shown to result in downregulation of gap junction communication and decreased Cx43 in gap junction plaques [46, 47]. Gap junction channel closure can occur relatively rapidly and there is still debate as to whether this is due to a direct effect of Src phosphorylation on Cx43 [73, 74] or due to Src activation of ERK1/2 and subsequent phosphorylation at ERK sites [75]. While Src is able to directly phosphorylate Cx43 on Y247 and Y265 to help mediate these effects there appears to be both cooperativity and redundancy via activation of other kinases by Src including ERK1/2 and PKC that could effect channel gating and gap junction stability [76]. During chronic expression of v-src, we find that while S262, S279/282 and S368 are phosphorylated on Cx43, Akt activity and consequent S373 phosphorylation are diminished [16]. While this seems distinct from the pathways described above where disassembly is initiated by phosphorylation on S373 and inhibition of Cx43:ZO-1 interaction, Src itself can displace ZO-1 binding from Cx43 [77, 78] perhaps bypassing the need for S373 phosphorylation under these conditions. Additionally, while Src activity is clearly associated with diminished gap junctions, a population of (closed) gap junctions often remains [75, 76]. Interestingly, we have shown by immunostaining that these gap junctions contain variable levels of pY247 [16] and these data suggest that pY247 may serve to mark specific areas, or subdomains, of a gap junction for disassembly, similar to what was observed with phosphorylated S368 and the PKC kinase sensor [67] (discussed in Section 3.3). The combination of diminished and non-functional gap junctions with a Cx43 phosphorylation pattern consistent with disassembly may indicate that Src activation destabilizes gap junctions through increased disassembly kinetics.
5. Mechanisms of gap junction internalization: disaggregation, endocytosis and annular gap junctions
It has been clearly shown that gap junction internalization can occur by a distinctive mechanism where one cell internalizes an entire gap junction via formation of double membrane structure termed an annular junction [19–26] (see Fig. 1) or “connexisome” [79]. This process requires that the plasma membranes of both cells undergo repair by a mechanism which is not presently well understood or investigated. However, there is some controversy as to whether this is the primary or sole means of disassembling and regulating gap junctions. The appearance of annular junctions, easily visualized by electron microscopy, varies widely across cell and tissue types and conditions. As discussed above, live cell imaging shows gap junctions to be fluid, dynamic structures. Thus, it seems likely that connexins can also be internalized through less severe means, such as endocytosis. In one study, treatment of cells with glycyrrhetinic acid, a compound which inhibits gap junction communication, led to disruption of the arrangement of connexin channels in the gap junction as shown by freeze fracture microscopy [38]. In this case, while channel size and morphology appeared normal, the channels were found in a disordered arrangement with irregular spacing, rather than being in a densely packed array. In a separate study, increased phosphorylation and interaction with both Src [80] and PKC [81] was observed in response to treatment with glycyrrhetinic acid. These data together have led us to speculate that phosphorylation and channel packing/organization cooperate to direct the timing and route of gap junction internalization either through an endocytic process or formation of annular junctions. Attempts to clearly map these functions have proven challenging due to the number of kinases involved in Cx43 regulation. What does seem clear is that gap junction disassembly can be signaled through multiple pathways; these redundancies argue for the importance of gap junction regulation, especially under conditions involving tissue or cellular remodeling.
6. Importance of connexin 43 in cardiac tissue
Gap junctions play a critical role in impulse propagation in the heart and are localized to a distinct and specialized structure referred to as the intercalated disc (ID) [1, 82]. In fact, dysregulation of gap junctions is a signature of many cardiac pathologies [83]. Cx43, Cx40, and Cx45 can all be found in heart and are expressed in a spatially distinct manner with Cx43 being the dominant gap junction component in the ventricle or working myocardium (reviewed in [84]). Cardiomyocytes are coupled from end to end through the collection of proteins and subcellular structures which form the ID [1, 82]. The ID is a highly organized hub containing gap junctions, mechanical junctions and ion channels that coordinate and regulate the synchronous beating of the heart. Recent developments in high resolution and 3D imaging are beginning to elucidate just how important and complex these interactions are and highlight the importance of connexins in generating and maintaining function at the ID [40].
6.1. Cardiac tissue
Across species, Cx43 in the ventricle is highly phosphorylated as evidenced by its shifted migration by SDS-PAGE that can be eliminated by phosphatase treatment [48–51]. Furthermore, analysis with phosphospecific antibodies show that this migratory isoform of Cx43 is heavily phosphorylated on S325/S328/S330 by casein kinase 1 and S365 by a still unidentified kinase, sites associated with gap junction formation [59, 85]. Immunohistochemical analysis of mouse heart confirms that phosphorylation of Cx43 at these sites occurs at the intercalated disc [59, 85]. Cx43 has been shown to interact with a variety of proteins at the ID including ZO-1 [86] and plakophilin [87] and to be involved in supporting sodium channel function [88, 89]. These findings show that Cx43 can affect cardiac conduction, not only via gap junctions, but through protein interactions leading to the idea of a cardiac “connexome” [40] that could, in the future, be a target for intervention in cardiac pathology.
6.2 Cardiac ischemia
As noted above, gap junction remodeling is a common feature of cardiac pathologies and can be rapidly induced by ischemia. Typically, there is a loss of Cx43 at the intercalated disc and often a shift to the lateral edges of the myocyte, a process referred to as lateralization (e.g. [48]). SDS-PAGE analysis of Cx43 from compromised hearts of many etiologies show a shift to the faster migrating forms of the protein, indicating a change in its phosphorylation state [90]. These events can occur within minutes during ischemic insult [31]. Within 30 minutes, Cx43 is dephosphorylated on 365 and S325/328/330 while phosphorylation at S373 and S368 are increased, similar to what is seen with phorbol ester treatment in cell culture (Figures 2–4 and [53, 60]).
Conversely, the presence of Cx43 and gap junctions in the face of ischemia has been shown to be protective. For example, Cx43 plays a critical role in the phenomenon of preconditioning wherein short bouts of ischemia preceding a longer ischemic interval can actually protect the heart from damage [91]. Under these conditions, Cx43 localization at the ID is preserved and it is maintained as the highly phosphorylated slow migrating isoform via SDS-PAGE [49, 92]. Phosphorylation on S262 has been shown to increase in response to ischemic preconditioning and contribute to protection, though it is notable that in this case, PKCε expression correlated with S262 phosphorylation [66]. Similarly, work utilizing Cx43 knock-in mice where S325/S328/S330 have been mutated to glutamic acid, mimicking phosphorylation, or alanine, eliminating phosphorylation, have shown the phosphorylation on S325/328/330 promotes gap junction stability and resistance to arrhythmia induced by programmed electrical stimulation [93]. Compared to wild type, knock-in mice expressing Cx43 with a S368 mutation were insensitive to sphingosine-1-phosphate-induced cardioprotection during ischemia-reperfusion injury [94]. Furthermore, although this review is focused in Cx43 found in gap junctions, there is also a significant body of work showing a role for mitochondrial Cx43 in providing protection to cardiac tissue [95–97].
Intriguingly, the three kinases targeting Cx43 for disassembly discussed in Section 3, Akt, ERK1/2 and PKC, are all critical pathways whose modulation can alter the extent of myocardial infarct in response to ischemia/reperfusion. Both Akt and ERK1/2 are part of what has been termed the reperfusion injury salvage kinase (RISK) pathway. Spatiotemporal regulation of these kinases is able to determine the extent of damage to cardiac tissue [98]. Furthermore, PKC has been shown to play an essential role in preconditioning as a PKCε knock-out mouse does not respond to preconditioning [99]. In the case of the PKCε knock-out mouse, gap junction stability did appear to be critical to the preconditioned phenotype. Cx43 is thus, poised at a crossroads between kinase activation, gap junction stability and cellular damage. A better understanding of the role that Cx43 and gap junctions play in directing and maintaining function at the ID could provide new therapeutic opportunities.
7. Conclusions and future research
Gap junctions are increasingly being understood to be a dynamic membrane domain providing for interaction of a variety of kinases, channels and structural proteins. The assembly and disassembly of gap junctions is highly regulated by a sequence of protein kinase activation and Cx43 phosphorylation events. We know that disassembly of gap junctions occurs in response to a variety of stimuli but depending on the cell type or tissue, the phosphorylation events and kinetics driving disassembly can be variable. This is a likely a result of the proteins interacting at and around the gap junction. While annular junction or “connexisome” formation is well documented in specific cell types and during autophagy, it is not clear that it can account for all types of gap junction turnover. In Figure 1 we present a model whereby an alternate disassembly process occurs in subregions of the gap junction where channels undock and assume less dense channel packing then undergo an endocytic process (i.e., upon src activation or glycyrrhetinic acid treatment). The fact that there is so much complexity and redundancy in regulating gap junctions certainly argues for their importance in allowing rapid responses both at the intracellular and intercellular levels.
While the apparent complexity of gap junction regulation presents challenges, new technologies continue to propel the field forward. Advances in microscopy are increasingly able to capture rapid dynamics at the plasma membrane (e.g., biosensors [67] and lattice light sheet microscopy [100]) and to discern 3 dimensional structures at extremely high resolution (e.g., super-resolution imaging [53, 87] and EM tomography [40, 101]). This rendering of 3-dimensional gap junctions and connexisomes and the spatiotemporal orientation of interacting proteins highlight the interplay of gap junction structure and function. Work involving the manipulation of connexins, especially Cx43, in tissue and organisms show that gap junction biology can affect health and outcomes as shown by work utilizing Cx43 mutant mice [93, 94, 102] or Cx43-targeted reagents [68, 103]. Non-junctional roles for Cx43, particularly its role in the cardiac mitochondria, are expanding our view of this protein. New frameworks and technologies will continue to pave the way to understanding the mechanisms by which gap junctions both sense and shape cellular responses to injury and ultimately, provide new avenues to improve outcomes.
Highlights.
Gap junctions, composed of connexin proteins, allow for intercellular communication
The life cycle of connexin43 (Cx43), the major gap junction protein, is highly regulated
Through direct phosphorylation of Cx43, kinases regulate gap junctional communication
Cx43 phosphorylation is critical during epidermal wounding and cardiac ischemia
Acknowledgments
A grant from the National Institutes of Health supported the work presented from the Lampe laboratory (R01GM055632). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- Cx43
Connexin 43
- AJ
annular junction
- SDS-PAGE
sodium dodecylsulfate-polyacrylamide gel electrophoresis
- TPA
12-O-tetradeconoylphorbol-13-acetate
- ZO-1
zonula occludens-1
- EGF
epidermal growth factor
- PKC
protein kinase C
- ERK1/2
extracellular signal-related kinase
- ID
intercalated disc
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Severs NJ. The cardiac gap junction and intercalated disc. Int J Cardiol. 1990;26(2):137–73. doi: 10.1016/0167-5273(90)90030-9. [DOI] [PubMed] [Google Scholar]
- 2.Muller DJ, et al. Conformational changes in surface structures of isolated connexin 26 gap junctions. EMBO J. 2002;21(14):3598–607. doi: 10.1093/emboj/cdf365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Ann Rev Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- 4.Bruzzone R, White TW, Paul DL. Connections with connexins: The molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 5.Willecke K, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 6.Saez JC, et al. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83(4):1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 7.Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J. 1991;273:67–72. doi: 10.1042/bj2730067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lampe PD. Analyzing phorbol ester effects on gap junction communication: A dramatic inhibition of assembly. J Cell Biol. 1994;127:1895–1905. doi: 10.1083/jcb.127.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musil LS, Beyer EC, Goodenough DA. Expression of the gap junction protein connexin43 in embryonic chick lens: Molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol. 1990;116:163–175. doi: 10.1007/BF01868674. [DOI] [PubMed] [Google Scholar]
- 10.Beardslee M, et al. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- 11.Darrow BJ, et al. Expression of multiple connexins in cultured neonatal rat ventricular myocytes. Circ Res. 1995;76:381–387. doi: 10.1161/01.res.76.3.381. [DOI] [PubMed] [Google Scholar]
- 12.Chu F-F, Doyle D. Turnover of plasma membrane proteins in rat hepatoma cells and primary cultures of rat hepatocytes. J Biol Chem. 1985;260:3097–3107. [PubMed] [Google Scholar]
- 13.Hare JF, Taylor K. Mechanisms of plasma membrane protein degradation: Recycling proteins are degraded more rapidly than those confined to the cell surface. Proc Nat Acad Sci USA. 1991;88:5902–5906. doi: 10.1073/pnas.88.13.5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan K, et al. Trafficking, assembly and function of a connexin43-green fluorescent protein chimera in live mammalian cells. Mol Biol Cell. 1999;10:2033–2050. doi: 10.1091/mbc.10.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauf U, et al. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A. 2002;99(16):10446–51. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solan JL, Lampe PD. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett. 2014;588(8):1423–9. doi: 10.1016/j.febslet.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaietta G, et al. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296(5567):503–7. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 18.Marquez-Rosado L, et al. Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim Biophys Acta. 2012;1818(8):1985–92. doi: 10.1016/j.bbamem.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Archard HO, Denys FR. Development of annular gap junctions in guinea pig epithelia. J Oral Pathol. 1979;8(4):187–97. doi: 10.1111/j.1600-0714.1979.tb01885.x. [DOI] [PubMed] [Google Scholar]
- 20.Fong JT, et al. Internalized gap junctions are degraded by autophagy. Autophagy. 2012;8(5):794–811. doi: 10.4161/auto.19390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson KE, et al. Phosphorylation on Ser-279 and Ser-282 of connexin43 regulates endocytosis and gap junction assembly in pancreatic cancer cells. Mol Biol Cell. 2013;24(6):715–33. doi: 10.1091/mbc.E12-07-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan K, et al. The origin of annular junctions: a mechanism of gap junction internalization. J Cell Sci. 2001;114(Pt 4):763–73. doi: 10.1242/jcs.114.4.763. [DOI] [PubMed] [Google Scholar]
- 23.Leithe E, Brech A, Rivedal E. Endocytic processing of connexin43 gap junctions: a morphological study. Biochem J. 2006;393(Pt 1):59–67. doi: 10.1042/BJ20050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickel B, et al. Visualizing the effect of dynamin inhibition on annular gap vesicle formation and fission. J Cell Sci. 2013;126(Pt 12):2607–16. doi: 10.1242/jcs.116269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piehl M, et al. Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol Biol Cell. 2007;18(2):337–47. doi: 10.1091/mbc.E06-06-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Severs NJ, et al. Fate of gap junctions in isolated adult mammalian cardiomyocytes. Circ Res. 1989;65(1):22–42. doi: 10.1161/01.res.65.1.22. [DOI] [PubMed] [Google Scholar]
- 27.Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc Res. 2004;62(2):233–45. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Crow DS, et al. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol Cell Biol. 1990;10:1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- 31.Sosinsky GE, et al. The C-terminus of Connexin43 adopts different conformations in the golgi and gap junction as detected with structure specific antibodies. Biochem J. 2007;408:375–385. doi: 10.1042/BJ20070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper CD, Lampe PD. Casein kinase 1 regulates connexin43 gap junction assembly. J Biol Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- 33.Johnson R, et al. Gap junction formation between reaggregating Novikoff hepatoma cells. Proc Natl Acad Sci USA. 1974;71:4536–4540. doi: 10.1073/pnas.71.11.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson RG, et al. Gap junction assembly: roles for the formation plaque and regulation by the C-terminus of connexin43. Mol Biol Cell. 2012;23(1):71–86. doi: 10.1091/mbc.E11-02-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhett JM, et al. Cx43 Associates with Na(v)1.5 in the Cardiomyocyte Perinexus. J Membr Biol. 2012;245(7):411–22. doi: 10.1007/s00232-012-9465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alcala J, Katar M, Maisel H. Lipid composition of chick lens fiber cell gap junctions. Curr Eye Res. 1982;2(9):569–78. doi: 10.3109/02713688208996357. [DOI] [PubMed] [Google Scholar]
- 37.Bukauskas FF, et al. Clustering of Connexin 43-EGFP Gap Junction Channels and Functional Coupling in Living Cells. Proc Natl Acad Sci, USA. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberg GS, et al. Evidence that disruption of connexon particle arrangements in gap junction plaques is associated with inhibition of gap junctional communication by a glycyrrhetinic acid derivative. Exp Cell Res. 1996;222(1):48–53. doi: 10.1006/excr.1996.0006. [DOI] [PubMed] [Google Scholar]
- 39.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011;22(9):1516–28. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leo-Macias A, Agullo-Pascual E, Delmar M. The cardiac connexome: Non-canonical functions of connexin43 and their role in cardiac arrhythmias. Semin Cell Dev Biol. 2016;50:13–21. doi: 10.1016/j.semcdb.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang SS, Shaw RM. Trafficking highways to the intercalated disc: new insights unlocking the specificity of connexin 43 localization. Cell Commun Adhes. 2014;21(1):43–54. doi: 10.3109/15419061.2013.876014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter AW, et al. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16(12):5686–98. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falk MM. Connexin-specific distribution within gap junctions revealed in living cells. J Cell Sci. 2000;113(Pt 22):4109–20. doi: 10.1242/jcs.113.22.4109. [DOI] [PubMed] [Google Scholar]
- 44.Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- 45.Lau AF, et al. Epidermal growth factor disrupts gap-junctional communication and induces phosphorylation of connexin43 on serine. Mol Biol Cell. 1992;3(8):865–874. doi: 10.1091/mbc.3.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atkinson MM, Sheridan JD. Altered junctional permeability between cells transformed by v-ras, v-mos or v-src. Am J Physiol. 1988;255:C674–C683. doi: 10.1152/ajpcell.1988.255.5.C674. [DOI] [PubMed] [Google Scholar]
- 47.Azarnia R, Loewenstein WR. Intercellular communication and control of cell growth. X. Alteration of junctional permeability by the src gene. J Membr Biol. 1984;82:191–205. doi: 10.1007/BF01871629. [DOI] [PubMed] [Google Scholar]
- 48.Beardslee MA, et al. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87(8):656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 49.Jain SK, Schuessler RB, Saffitz JE. Mechanisms of delayed electrical uncoupling induced by ischemic preconditioning. Circ Res. 2003;92(10):1138–1144. doi: 10.1161/01.RES.0000074883.66422.C5. [DOI] [PubMed] [Google Scholar]
- 50.Miura T, et al. Protective role of gap junctions in preconditioning against myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;286(1):H214–21. doi: 10.1152/ajpheart.00441.2003. [DOI] [PubMed] [Google Scholar]
- 51.Schulz R, et al. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J. 2003;17(10):1355–1357. doi: 10.1096/fj.02-0975fje. [DOI] [PubMed] [Google Scholar]
- 52.Lampe PD, et al. Cellular interaction of integrin a3b1 with laminin 5 promotes gap junctional communication. J Cell Biol. 1998;143(6):1735–1747. doi: 10.1083/jcb.143.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunn CA, Lampe PD. Injury-triggered Akt phosphorylation of Cx43: a ZO-1-driven molecular switch that regulates gap junction size. J Cell Sci. 2014;127(Pt 2):455–64. doi: 10.1242/jcs.142497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park DJ, et al. Akt phosphorylates Connexin43 on Ser373, a “mode-1” binding site for 14-3-3. Cell Commun Adhes. 2007;14(5):211–226. doi: 10.1080/15419060701755958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunn CA, et al. Activation of Akt, not connexin 43 protein ubiquitination, regulates gap junction stability. J Biol Chem. 2012;287(4):2600–7. doi: 10.1074/jbc.M111.276261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richards TS, et al. Protein kinase C spatially and temporally regulates gap junctional communication during human wound repair via phosphorylation of connexin43 on serine368. J Cell Biol. 2004;167(3):555–562. doi: 10.1083/jcb.200404142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cottrell GT, et al. Mechanism of v-Src- and mitogen-activated protein kinase-induced reduction of gap junction communication. Am J Physiol Cell Physiol. 2003;284(2):C511–20. doi: 10.1152/ajpcell.00214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou L, Kasperek EM, Nicholson BJ. Dissection of the molecular basis of pp60(v-src) induced gating of connexin 43 gap junction channels. J Cell Biol. 1999;144(5):1033–1045. doi: 10.1083/jcb.144.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lampe PD, et al. Analysis of Connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. J Cell Sci. 2006;119(Pt 16):3435–3442. doi: 10.1242/jcs.03089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ek-Vitorin JF, et al. Selectivity of Connexin 43 Channels Is Regulated Through Protein Kinase C-Dependent Phosphorylation. Circ Res. 2006;98(12):1498–1505. doi: 10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalcheva N, et al. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc Natl Acad Sci U S A. 2007;104(51):20512–6. doi: 10.1073/pnas.0705472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qu J, et al. Gap Junction Remodeling and Spironolactone-Dependent Reverse Remodeling in the Hypertrophied Heart. Circ Res. 2008;104(3):365–71. doi: 10.1161/CIRCRESAHA.108.184044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ek-Vitorin JF, Burt JM. Structural basis for the selective permeability of channels made of communicating junction proteins. Biochim Biophys Acta. 2013;1828(1):51–68. doi: 10.1016/j.bbamem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moreno AP. Connexin phosphorylation as a regulatory event linked to channel gating. Biochim Biophys Acta. 2005;1711(2):164–171. doi: 10.1016/j.bbamem.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 65.Doble BW, et al. Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocytes. J Cell Sci. 2004;117(Pt 3):507–14. doi: 10.1242/jcs.00889. [DOI] [PubMed] [Google Scholar]
- 66.Srisakuldee W, et al. Phosphorylation of connexin-43 at serine 262 promotes a cardiac injury-resistant state. Cardiovasc Res. 2009;83(4):672–81. doi: 10.1093/cvr/cvp142. [DOI] [PubMed] [Google Scholar]
- 67.Cone AC, et al. Protein kinase Cdelta-mediated phosphorylation of Connexin43 gap junction channels causes movement within gap junctions followed by vesicle internalization and protein degradation. J Biol Chem. 2014;289(13):8781–98. doi: 10.1074/jbc.M113.533265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Quinn MP, et al. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ Res. 2011;108(6):704–15. doi: 10.1161/CIRCRESAHA.110.235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lampe PD, et al. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;126:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sirnes S, et al. Interplay between PKC and the MAP kinase pathway in Connexin43 phosphorylation and inhibition of gap junction intercellular communication. Biochem Biophys Res Commun. 2009;382(1):41–5. doi: 10.1016/j.bbrc.2009.02.141. [DOI] [PubMed] [Google Scholar]
- 71.Nimlamool W, Andrews RM, Falk MM. Connexin43 phosphorylation by PKC and MAPK signals VEGF-mediated gap junction internalization. Mol Biol Cell. 2015;26(15):2755–68. doi: 10.1091/mbc.E14-06-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noren NK, et al. p120 catenin regulates the actin cytoskeleton via rho family GTPases. J Cell Biol. 2000;150:567–579. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin R, et al. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J Cell Biol. 2001;154(4):815–27. doi: 10.1083/jcb.200102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swenson KI, et al. Tyrosine phosphorylation of the gap junction protein connexin43 is required for pp60src-induced inhibition of communication. Cell Regul. 1990;1:989–1002. doi: 10.1091/mbc.1.13.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitra SS, Xu J, Nicholson BJ. Coregulation of multiple signaling mechanisms in pp60v-Src-induced closure of Cx43 gap junction channels. J Membr Biol. 2012;245(8):495–506. doi: 10.1007/s00232-012-9500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solan JL, Lampe PD. Connexin 43 in LA-25 cells with active v-src is phosphorylated on Y247, Y265, S262, S279/282, and S368 via multiple signaling pathways. Cell Commun Adhes. 2008;15(1):75–84. doi: 10.1080/15419060802014016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Toyofuku T, et al. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J Biol Chem. 2001;276(3):1780–8. doi: 10.1074/jbc.M005826200. [DOI] [PubMed] [Google Scholar]
- 78.Sorgen PL, et al. Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1. J Biol Chem. 2004;279(52):54695–54701. doi: 10.1074/jbc.M409552200. [DOI] [PubMed] [Google Scholar]
- 79.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394(Pt 3):527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chung TH, et al. 18beta-glycyrrhetinic acid promotes src interaction with connexin43 in rat cardiomyocytes. J Cell Biochem. 2007;100(3):653–64. doi: 10.1002/jcb.21018. [DOI] [PubMed] [Google Scholar]
- 81.Liang JY, et al. Effects of 18-glycyrrhetinic acid on serine 368 phosphorylation of connexin43 in rat neonatal cardiomyocytes. Cell Biol Int. 2008;32(11):1371–9. doi: 10.1016/j.cellbi.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 82.Revel JP, Karnovsky MJ. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hesketh GG, Van Eyk JE, Tomaselli GF. Mechanisms of gap junction traffic in health and disease. J Cardiovasc Pharmacol. 2009;54(4):263–72. doi: 10.1097/FJC.0b013e3181ba0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Severs NJ. Connexins in the Heart. In: Harris A, Locke D, editors. Connexins: A Guide. Humana Press; New York: 2009. pp. 435–456. [Google Scholar]
- 85.Solan JL, et al. Phosphorylation of Cx43 at S365 is a gatekeeper event that changes the structure of Cx43 and prevents downregulation by PKC. J Cell Biol. 2007;179:1301–1309. doi: 10.1083/jcb.200707060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palatinus JA, et al. ZO-1 determines adherens and gap junction localization at intercalated disks. Am J Physiol Heart Circ Physiol. 2011;300(2):H583–94. doi: 10.1152/ajpheart.00999.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agullo-Pascual E, et al. Super-resolution fluorescence microscopy of the cardiac connexome reveals plakophilin-2 inside the connexin43 plaque. Cardiovasc Res. 2013;100(2):231–40. doi: 10.1093/cvr/cvt191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malhotra JD, et al. Tyrosine-phosphorylated and nonphosphorylated sodium channel beta1 subunits are differentially localized in cardiac myocytes. J Biol Chem. 2004;279(39):40748–54. doi: 10.1074/jbc.M407243200. [DOI] [PubMed] [Google Scholar]
- 89.Jansen JA, et al. Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm. 2012;9(4):600–7. doi: 10.1016/j.hrthm.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saffitz JE, Hames KY, Kanno S. Remodeling of gap junctions in ischemic and nonischemic forms of heart disease. J Membr Biol. 2007;218(1–3):65–71. doi: 10.1007/s00232-007-9031-2. [DOI] [PubMed] [Google Scholar]
- 91.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 92.Schulz R, Heusch G. Connexin 43 and ischemic preconditioning. Cardiovasc Res. 2004;62(2):335–44. doi: 10.1016/j.cardiores.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 93.Remo BF, et al. Phosphatase-Resistant Gap Junctions Inhibit Pathological Remodeling and Prevent Arrhythmias. Circ Res. 2011;108(12):1459–1466. doi: 10.1161/CIRCRESAHA.111.244046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morel S, et al. Sphingosine-1-phosphate reduces ischaemia-reperfusion injury by phosphorylating the gap junction protein Connexin43. Cardiovasc Res. 2016;109(3):385–96. doi: 10.1093/cvr/cvw004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruiz-Meana M, et al. Mitochondrial connexin43 as a new player in the pathophysiology of myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2008;77(2):325–33. doi: 10.1093/cvr/cvm062. [DOI] [PubMed] [Google Scholar]
- 96.Schulz R, et al. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol Ther. 2015;153:90–106. doi: 10.1016/j.pharmthera.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schulz R, et al. Connexin 43 in ischemic pre- and postconditioning. Heart Fail Rev. 2007;12(3–4):261–6. doi: 10.1007/s10741-007-9032-3. [DOI] [PubMed] [Google Scholar]
- 98.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61(3):448–60. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 99.Hund TJ, et al. Protein kinase Cepsilon mediates salutary effects on electrical coupling induced by ischemic preconditioning. Heart Rhythm. 2007;4(9):1183–93. doi: 10.1016/j.hrthm.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Majoul IV, et al. Fast structural responses of gap junction membrane domains to AB5 toxins. Proc Natl Acad Sci U S A. 2013;110(44):E4125–33. doi: 10.1073/pnas.1315850110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Norris RP, Baena V, Terasaki M. Localization of phosphorylated connexin 43 by serial section immunogold electron microscopy. J Cell Sci. 2017 doi: 10.1242/jcs.198408. [DOI] [PubMed] [Google Scholar]
- 102.Johnstone SR, et al. MAPK phosphorylation of connexin 43 promotes binding of cycline and smooth muscle cell proliferation. Circ Res. 2012;111(2):201–11. doi: 10.1161/CIRCRESAHA.112.272302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moore K, et al. A synthetic connexin 43 mimetic peptide augments corneal wound healing. Exp Eye Res. 2013 doi: 10.1016/j.exer.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Solan JL, et al. Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci. 2003;116(11):2203–2211. doi: 10.1242/jcs.00428. [DOI] [PubMed] [Google Scholar]