1. Introduction
Chronic pain after surgery is a major health problem [9; 17], yet research on its causes and predictors is hindered by our lack of knowledge of how pain usually resolves. Many rules-of-thumb are commonly communicated to patients (e.g., “the pain will start to subside after 3 days”), but little is known about a typical pain experience after surgery and the variability around this typical experience. To examine the pain experience after surgery, we utilized a statistical technique called growth curve modeling.
Growth curve modeling has been previously applied to describe patterns of recovery over weeks in patients with acute low back pain [5] and over days in hospitalized medical and surgical patients [10]. Patterns of pain resolution in the first 5-6 5-6 postoperative days have been described and correlated with persistent pain months later [1; 4; 14], yet the few studies which have modeled these patterns have been restricted to short time periods (two weeks) [3] or included a paucity (≤ 6) measures [11; 13; 15; 16]. The lone exception is a study of 32 subjects who contributed 16 observations over 6 weeks after surgery, but modeling was not applied [7]. In the present study, we examined a large group of patients recovering after surgery for an extended period of time.
To more precisely measure pain resolution after surgery, pain must be measured frequently immediately following surgery and continue for at least the time when most individuals report complete resolution. These assessments can be quite burdensome given that many patients report high levels of pain immediately after surgery, a distracting state that might reduce adherence to data collection procedures. If a common pain resolution model could be found, individuals whose pain resolves at the upper or lower bounds of this model could be identified as either being at high risk for chronic pain or alternatively among those whose pain resolves far quicker than normal; in either case, such identification could better target interventions. It is unclear, however, whether pain after surgery resolves according to a single typical trajectory (with some variability around this trajectory), or is better characterized as consisting of several different patterns of trajectories (i.e., subpopulations), each with their own unique variability. Thus, understanding the best approach to model change in pain after surgery is an important step from both research and translational standpoints.
Our primary aim was to examine these three methodological issues. As such, we tested the feasibility of measuring pain intensity in patients after major surgery on a daily basis for prolonged periods following discharge, examined several different statistical models in the search for a common pain resolution model, and examined subtle deviations in the calibrations of the best fitting pain resolution model as initial evidence for different subpopulations of individuals' resolution of pain after surgery. We hypothesized that collecting this data is feasible with only modest levels of missing data, and that a common model form could be found to characterize the time course in reduction of pain after surgery.
2. Methods
To address the study's aims, we examine individual differences in the day-to-day experience during the resolution of pain after two major surgeries: lower limb total joint arthroplasty (TJA) and cesarean delivery (CD). These two groups of participants were collected sequentially as part of our ongoing research program examining pain resolution after surgery, but none of the data reported in this manuscript have been published elsewhere. Each surgical population is described, below.
2.1. Lower limb Total Joint Arthroplasty (TJA)
Following Institutional Review Board approval, adult patients (American Society of Anesthesiologists physical status 1, 2, or 3) undergoing elective TJA (unicompartmental or total knee replacement or hip replacement) were recruited from a single center (Wake Forest University Health Sciences) into this observational study. We excluded patients who did not understand English, and pregnant women or those within 1 year of childbirth. The trial was registered prior to enrollment of the first subject (NCT01390298). The purpose of the study was explained, questions were answered, and all patients gave written informed consent. Demographics, medical history, indication for surgery, and current joint function were recorded. Many questionnaires were completed 1-6 weeks before surgery but are not considered here (please see the trial registration for a complete list).
All surgical procedures occurred at Wake Forest University Health Sciences, and all patients were followed postoperatively by the members of the Department of Orthopaedics and the Regional Anesthesia and Acute Pain Service of the Department of Anesthesiology. Surgical, anesthetic, and postoperative analgesic care was routine and not used in any of the statistical models for this observational study.
Beginning on the day of discharge, patients completed the Short Form McGill Pain Questionnaire [12], a visual analog scale (VAS) for pain intensity (10 cm line anchored on the left with not painful at all and on the right with most pain imaginable; separate scores for current, with movement, and most intense in the last 24 hr), and Stress Inventory [2] twice a day for 14 days following hospital discharge and daily at the end of the day for the following two weeks. The SF-MPQ and VAS were then collected weekly until 12 weeks after discharge, and then monthly until 6 months after discharge. The SF-MPQ, VAS and Stress Inventory were completed in approximately 10-15 minutes.
Patients were allowed to choose between paper and pencil diaries or an electronic diary, using a small hand-held device that had been programmed to query these outcomes using Pendragon Forms VI (Pendragon Software, Corp., Chicago, IL). Patients were introduced prior to surgery to the diary and the touch screen, and then allowed time to make several practice entries. They were also asked to make a diary entry with a pre-determined set of responses to ensure they understood the diary system.
2.2 Cesarean Section (CD)
Following Institutional Review Board approval, adult patients (American Society of Anesthesiologists physical status 1, 2, or 3) undergoing non-emergent CD were recruited from a single center (Maya Angelou Women's Center) into this observational study. We excluded patients who did not understand English. The trial was registered prior to enrollment of the first subject (NCT01996592). The purpose of the study was explained, questions were answered, and all patients gave written informed consent. Demographics, medical history, and indication for surgery were recorded. Many questionnaires were completed 1-5 days before surgery but are not considered here (please see the trial registration for a complete list).
All surgical procedures occurred at the Maya Angelou Women's Health Center, and all patients were followed postoperatively by the members of the Department of Obstetrics and Gynecology and the Section on Obstetric Anesthesia of the Department of Anesthesiology, Wake Forest Health Sciences. Surgical, anesthetic, and postoperative analgesic care was routine and not used in any of the statistical models for this observational study. Unless contraindicated or refused by the parturient, patients typically receive subarachnoid anesthesia with intrathecal hyperbaric bupivacaine 10-12 mg, 15-20 mcg fentanyl, and preservative free morphine 150-200 mcg.
Beginning on the day of discharge, patients completed a series of 7 questions via an online survey, paper and pencil forms, or SMS texting daily until 60 days after surgery. These questions were: What is today's date? What is your pain intensity right now? What is your pain unpleasantness right now? What was your worst pain in the past 24 hours? What was your worst pain unpleasantness in the past 24 hours? What was your average pain in the past 24 hours? What was your average pain unpleasantness in the past 24 hours? Scores were numerical between 0 (not painful or unpleasant at all) to 10 (worst pain or unpleasantness imaginable).
2.3 Primary Outcome
The TJA and CD studies were not coincident in time and utilized minimalist (CD) or extensive (TJA) daily reporting. For the purpose of this analysis, we utilized a common metric in both studies, the worst pain in the previous 24 hr, as the measure to examine trajectory of recovery from pain after surgery.
2.4 Sample Size
Given the lack of prior knowledge regarding the daily time course of recovery from pain following discharge after these procedures, we requested a time period of up to 12 months to recruit the convenience sample for each of these two surgical populations. Since the goals were to explore the nature of the time course of recovery and the effect size of predictors of this time course, formal hypothesis testing was not used to guide sample size estimation.
2.5 Statistical Methods
Statistical analyses were conducted using R version 3.2 (R Foundation for Statistical Computing, Vienna, Austria) and RStudio version 0.98.501 (RStudio, Inc.). Descriptive statistics were calculated for all variables such that mean (standard deviation) were used for normally distributed variables; median [range] for non-parametric data; and frequency (percentage) for count data. For all analyses, two-tailed hypothesis testing was used with p < 0.05 interpreted for statistical significance.
A data-driven approach was used to explore the resolution of pain that could be attributed to recovery in both the CD and TJA cohorts. The daily worst VAS score was chosen as the primary outcome given that it was used in both groups for 60 days after surgery. Examination of the raw data suggested a curvilinear decline in pain for most individuals. Linear, quadratic, cubic, and logarithmic curve fitting was explored based on the intuitive fit of these forms to the data. Additionally, a Gompertz polynomial model was also fit based on the models used to describe the wound recovery rates of pressure ulcers [19]. Linear mixed models were constructed with lme4 (Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version. 2014;1). To fit the models, pain following surgery for each cohort was modeled separately with correlated random effects used for intercept and change parameters (i.e., slopes) based on individual subjects.
To examine patterns in the residuals that could be indicative of neglected change processes, the autocorrelation function of the model residuals was examined for each subject during the process of determining best model fit. The first-order autocorrelation was plotted to characterize the pattern of errors. Because a high degree of autocorrelation remained in the residuals even for models that fit the data well, an additional model form was applied to characterize a multi-phased change process. For these models, we applied Bayesian change-point analysis using MCMCpack (Martin AD, Quinn KM, Park JH. MCMCpack, Version 1.0-1. 2011). Full data and code are available upon request.
3. Results
3.1. Participant Characteristics and Daily Diaries
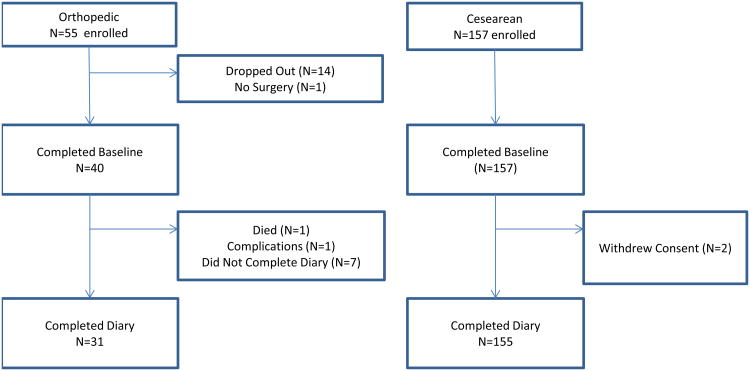
During December, 2011 through July, 2012, N = 31 participants who underwent TJA completed a diary during at least one measurement occasion of the post-operative assessment period. During May, 2013 through June, 2014, N = 155 CD participants completed a diary during at least one measurement occasion of the post-operative assessment period. Figure 1 displays the disposition of participants who began each study, dropped out for any reason, and completed the assessment period. Table 1 displays the demographic and surgical characteristics of both samples. Orthopedic surgery participants were female (51.6%), white (83.9%), African-American (16.1%), had an average age of 61.1 (10.6) and mean CESD total score of 14.5 (10.9). CD participants were all female, white (63%), African-American (31%), had an average age of 30.2 (7.0) and mean Promis Depression total score of 11.2 (4.1).
Figure 1.
Flow chart of participant disposition for both orthopedic and cesarean section cohorts.
Table 1. Demographic Characteristics.
| Lower Extremity Total Joint Arthroplasty (TJS) Cohort (n=31) | Cesarean Delivery (CD) Cohort (n=155) | |
|---|---|---|
| Age (years) (Mean (SD)) | 61.1 (10.6) | 30.2 (7.0) |
| Gender (n (%)) | ||
| Male | 15 (48.4%) | |
| Female | 16 (51.6%) | 155 (100%) |
| Surgery Type (n (%)) | ||
| Knee | 21 (67.7%) | |
| Hip | 10 (32.3%) | |
| Race (n (%)) | ||
| African-American | 5 (16.1%) | 47 (31%) |
| White | 26 (83.9%) | 98 (63%) |
| Hispanic | 0 (0%) | 8 (5%) |
| Other | 0 (0%) | 2 (1%) |
| CESD Total (Mean (SD)) | 14.5 (10.9) | |
| Promis Depression Total (Mean (SD)) | 11.22 (4.1) | |
Promis=Promise Health Organization Emotional Distress-Depression Short Form 8b (Range [8, 40])
CESD=Center for Epidemiological Studies Scale of Depression Scale (Range [0, 60])
There were missing data from the daily diaries. TJA participants each provided a median [range] of 49 [18, 51] diary entries (N = 1477), of which there were two entries daily from date of discharge through day 14 post-surgery, then once daily for the next 14 days, while the remaining were weekly and monthly assessments. CD participants each provided a median [range] of 57 [1, 60] daily diary entries (N = 7612) once daily from date of discharge through day 60 post-surgery. The majority of participants in each surgical population (97% of TJA and 75% of CD patients) had 7 or fewer days with missing entries over the 2 month period after surgery (Figure 2).
Figure 2.
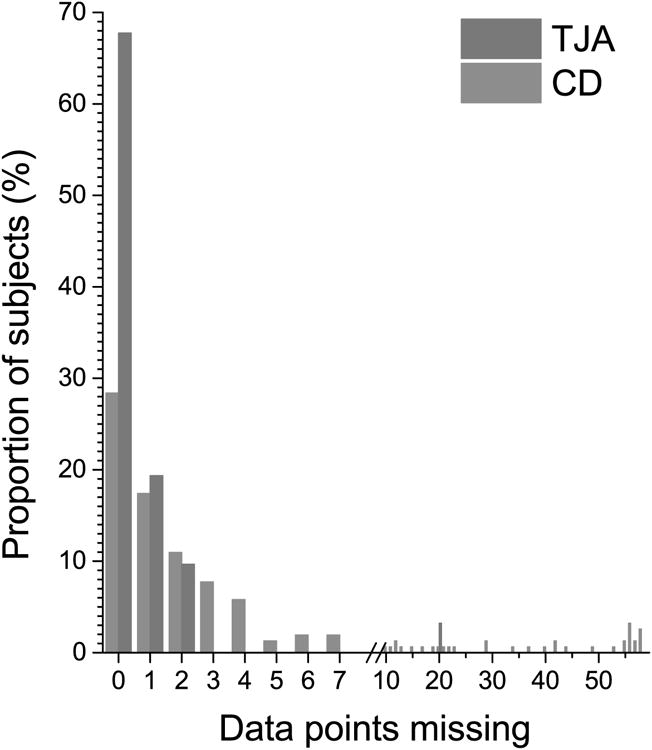
Distribution of days with missing data over the 60 day period after hospital discharge in the two surgical populations (TJA in red = lower extremity total major joint arthroplasty; CD in blue = cesarean delivery). Of note that the frequency of diary entry differed between the groups, with 60 days with data entry for the CD population and 32 days for the TJA population.
The burden on support personnel and method of diary entry differed significantly by surgical population. For the CD group, over 90% or participants entered daily diary information via SMS texting to study personnel. Up to 50 subjects were providing daily diary information per day at the time of peak enrollment, requiring a minimum of 2 hr per day of study personnel time in receiving and responding to text messages. A few subjects elected to use paper and pencil, but none returned the forms. The remainder used a daily online survey with email reminders generated by REDCap electronic data capture tool hosted at Wake Forest School of Medicine through the Wake Forest Clinical and Translational Science Institute (UL1 TR001420; PI: McClain) [8] with more missing data than those using SMS texting.
For the TJA group, all participants recorded daily diary information via paper and pencil. Up to 19 subjects were providing daily diary information per day at the time of peak enrollment.
3.2 Model Forms
Examination of the candidate model forms yielded two models that best described the systematic changes in pain over time that occurs after surgery. The first model predicted that pain reports decreased as a function of the log of time (log(time)) model. When estimated in these samples, the predictions from this model were monotonic in that the individual model predictions steadily decreased over time in the vast majority of individuals (94% TJA, 100% CD) but in a few cases increased over time (6% TJA, 0% CD). The second model used a cubic polynomial function that predicted that pain reports decreased as a function of the time, time2, and time3 (i.e., cubic model). When estimated in these samples, the predictions from this model reflected two inflection points in the rate of recovery in direction of pain reporting (i.e., changes in the rate of change). Despite the fact that the cubic model uses more parameters (i.e., more information) to describe pain reports, it was superior to the log(time) model in fitting the data from the CD (22278 (log(time)) versus 21415 (cubic)) patients, but not those undergoing TJA (BIC: 3417 (log(time)) versus 4022 (cubic)). The residuals (i.e., the lack of fit) from both models were scrutinized and compared. We chose the log(time) form to model change in pain based on: its simplicity (1 change parameter versus 3), nearly identical fit with the more complicated cubic model, better expected performance at the extremes of the observation period, and ease of interpretation for evaluating external influences on change in pain over time.
The individual model fits are presented in Figure 3a with the residuals from these fits in Figure 3b. The model parameters for the two samples were:
Figure 3.
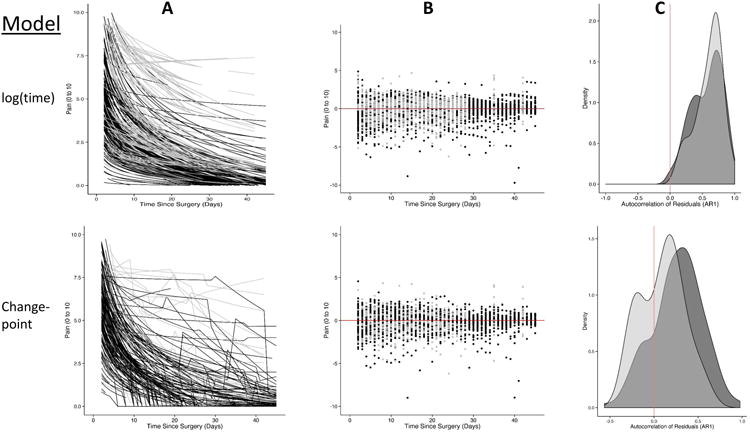
The modeled pain trajectories over time (A), the residuals (i.e., error) around these predictions (B) and the first-order autocorrelation of the residuals (i.e., how related errors are over subsequent time points) (C). The grey represents the total joint arthroplasty participants and the black the cesarean delivery participants. The top row represents the log(time) models that appear to represent the change in pain over time, but the use of a change-point model (bottom row) fit the data even better while reducing the correlation in the errors.
The random effects components (variance components) revealed that there is substantial variation [standard error] in intercepts (i.e., discharge pain scores) across participants in both samples: TJA (σ2=13.5 [3.7]), p<.001 and CD (σ2=8.3 [1.0]), p<.001. These estimates reflect that participants report a range of pain experiences following surgery. Furthermore, there is substantial variation in slopes (i.e., change in pain) across participants indicating that apparent change in pain rates vary widely across participants. Finally, there is a substantial association between intercepts and slopes in both samples: TJA (-3.2[1.0]), p=.002 and CD (-1.9[.3]), p<.001, indicating that higher pain scores at discharge are associated with more rapid reductions in pain.
3.3 Day-to-day pain experience during recovery
The log(time) model was the most parsimonious representation of the recovery process. However, further examination of how this model fit the observed data provides a wealth of information about the nature of the recovery process. We examined each of the residual series, the variance in pain scores after applying the log(time) recovery model, for patterns that would reflect either a lack of fit of the model or suggest some other underlying process not encapsulated by the model. In so doing, we were able to identify several issues with the model and to identify several change patterns that are not well accounted for by the model.
The residuals from the log(time) model contained substantial amounts of autocorrelation (Figure 3C). When the current error (i.e., residual) of a prediction is correlated with past values or past predictions, a pattern exists in the residuals that can bias the estimates of change. For example, Figure 4 displays individual examples of several different patterns of change that all introduce patterns (autocorrelation) into the residuals. For ease of communication we named the patterns, and close examination can identify where the errors become correlated. In the “fast” reduction in pain the recovery model underestimates the reduction while attempting to accommodate the many “0” pain reports later in the diary. In this case, the intercepts of the model (i.e., pain at day 0) are biased low making the model predictions at first systematically low and then systematically high for the rest of the recovery period. In the pain “flare” pattern, for a short duration, pain intensity is strikingly enhanced, but then returns to its previous trajectory. In such cases, the model describes the reduction in pain very well for most of the recovery period, but substantially underestimates pain during the pain flare-up. In pain “state” change pattern the recovery model describes the overall reduction in pain quite well, but close examination indicates that the pain reports are consistent from day-to-day but exhibit a change in state from higher to lower only after several days within the state (e.g., a pain of ‘4’ is experienced for five days, but then resolves to a ‘3’ for four days). In the final example, a “delayed” reduction in pain is observed when the pain remains stubbornly high but quickly resolves at a later time. The log(time) model again primarily underestimates the reduction in pain (especially mid-way through recovery) such that pain reports are predicted to be lower than actual for much of the recovery process, but then substantially over-estimates the pain after it has resolved. In each of these examples, the pattern of change deviates from the relationship specified by a log(time) model. Thus, to properly accommodate the nature of change, we explored an additional strategy to improve fit.
Figure 4.
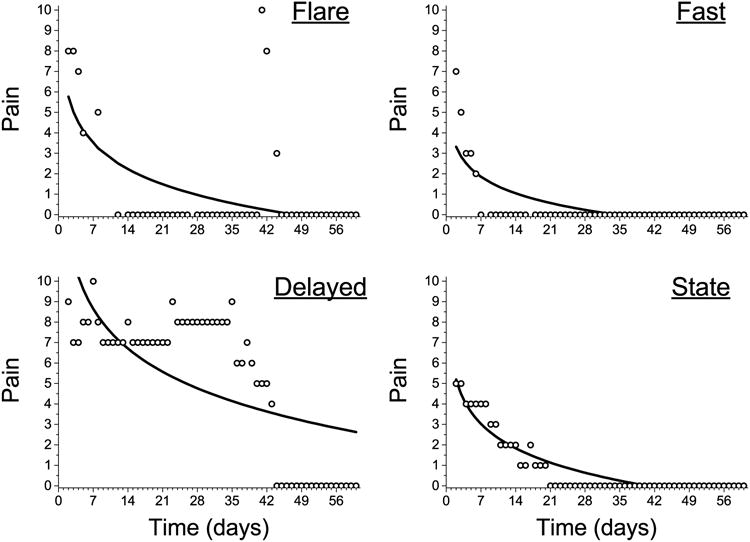
Examples of change processes that induce a pattern of errors into the residuals. In each example, the distance from the prediction line to the observed point contains a temporal pattern such that points nearby can be used to predict the error of subsequent points. This can be the case because of many “0” pain scores in a sequence (“fast”), because a small group of points are very aberrant (“flare”), because the change process is not typical of a log(time model) (delayed), or because the pain scores follow a state-like pattern where the only change levels occasionally.
3.4 Change-Point Models
A change-point model assumes that the nature of change (e.g., either the level or slope of change) itself changes during the observation period. Change-point models are often applied in time-series analyses when a model form abruptly changes due to any external reason. We applied these methods as they appear to be an intuitive fit for the nature of change after surgery/injury. In the recovery process, it is assumed that pain resolves fully for most individuals, and that at some point an individual experiences no pain due to the original injury. In such cases the point of complete recovery can be thought of as a change-point in that the pain reports afterward are far different than that before recovery.
We allowed each participant to have from zero to three change-points in their pain series, and fit a unique model optimizing the number and location of the change-points. Figure 5 displays the best fitting locations with the posterior probability of the change point occurring at a particular point in time (i.e., the probability that the change process has changed at that point). Nearly all individuals in both surgery types exhibited a change pattern where a change-point fit the data better than assuming a single model form. The location of the change point varied substantially across individuals, but generally followed a normal distribution with the majority of individuals exhibiting a change point between day 10 and 21 after surgery. Figure 3a (bottom panel) shows the predicted values from a log(time) model with a change point. Figure 3b displays the superior fit of the change-point models to the data (i.e., the residuals are more condensed), and Figure 3c demonstrates that by incorporating a change-point into the model, the degree of autocorrelation in the residuals is substantially reduced.
Figure 5.
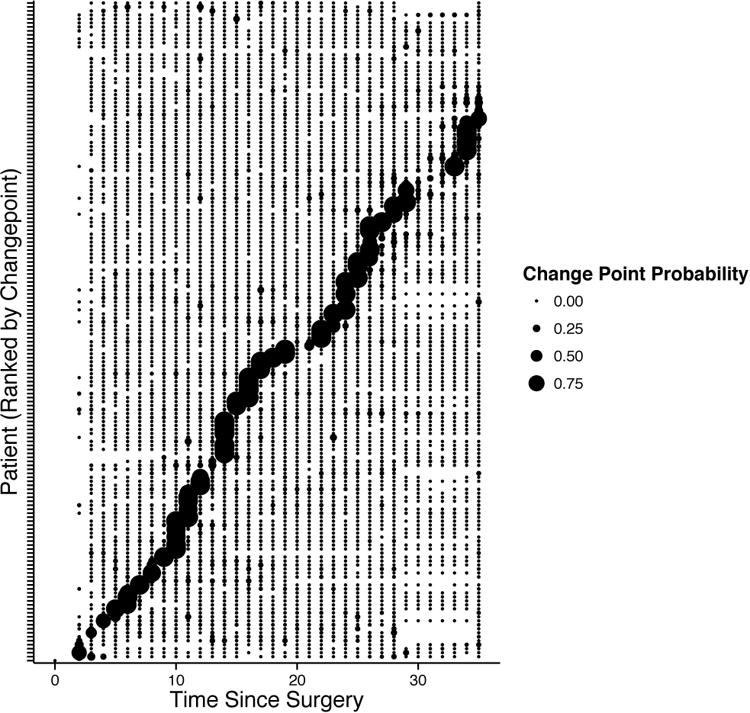
The location of the change-point (x-axis) for each individual participant (each row on the y-axis). The size of the circle reflects the probability that there is a change point at that time for that participant.
4. Discussion
In this study we pursued several distinct aims. We observed that daily diary data collection procedures are feasible in patients recovering from surgery. Additionally, we identified a simple model describing the resolution of pain after surgery (i.e., a log(time) model). When this model is employed as a pain resolution model (or recovery after surgery model), we can gain insight into a ‘typical’ pain experience for a typical person. Finally, we explored when this model did not fit the data well, and described several patterns of change that might lead to biased estimates of the resolution process. To accommodate the unique process of pain resolution, we devised a strategy using change-points that allow the model form to change over time in response to complete resolution or some new pain state.
4.1. Feasibility
That previous reports of pain after surgery have not used daily measures for several weeks suggests that investigators recognize the barriers to this approach. We examined feasibility in two populations: 1) elderly patients undergoing major surgery who exhibit a high incidence of postoperative cognitive dysfunction and who are often uncomfortable or unfamiliar interacting with novel electronic devices, and 2) young women undergoing surgery for childbirth who go home to a stressful although typically viewed as positive experience with a new baby, who are at low risk for postoperative cognitive dysfunction, and who are comfortable and familiar with digital technology. The TJA patients overwhelmingly preferred pencil and paper reporting, a method which suffers from the lack of a time stamp to confirm real time reporting rather than reliance on memory of previous pain. In a subsequent study in the TJA population (NCT02685735) we are utilizingtablet-based digital entry to a secure REDCap site with good success (data not shown).
In contrast to the TJA population, CD patients overwhelmingly preferred digital entry, usually through SMS or REDCap survey methods. These methods allowed study personnel daily to assess compliance on a daily basis and to provide reminders. It should be noted that these methods require dedicated research personnel to assess compliance and respond to hardware and software problems 7 days per week.
Regardless of the method used to acquire data, missing data are inevitable in this setting and missing observations complicate data analysis using traditional repeated measures ANOVA designs. If the data are missing at random (MAR), and factors that predict missingness can be included in the model, then the change estimates are unbiased [6]. If data are not MAR (e.g., patients with higher pain are more likely to drop out of the study early), then the estimates of change will be biased and further efforts must be made to reduce this bias.
4.2. Frequent assessments provide unique perspectives
These data and analyses add meaningfully to our understanding of recovery from pain after surgery in two important ways. First, they extend the period of frequent observations from 5-6 days while patients are in hospital [1; 4; 14] to a 2 month period. Interpretation of patterns of pain intensity scores in hospitalized patients is obscured by the analgesic interventions of varying efficacy applied, the titration of these interventions to pain intensity scores, and the ongoing trend to reduce the period of hospitalization after surgery. Extending these observations to 2 months is important, as the presence and intensity of persistent pain at this time period is a high risk factor (Odds Ratio 18.4) for pain lasting years [18]. Second, the current data include a considerably larger number of observations than has been previously acquired (Figure 6). As noted in Figure 6, the small number of time points has largely restricted analysis to simple models (linear, quadratic), and the only study using more complex modeling in a large number of subjects was restricted to only a few days in hospitalized patients [10].
Figure 6.
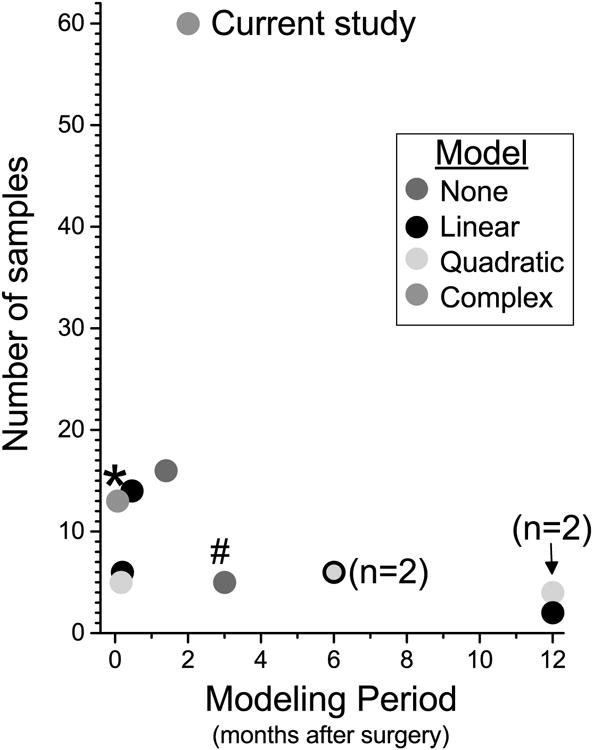
Summary of previous studies of recovery after surgery [1; 3; 4; 7; 11; 13-16] or with serial time sampling in medical patients [5; 10], showing number of samples obtained per patient and the modeling period. Each symbol represents a single study, unless otherwise noted, in when no formal model (red) or linear (black), quadratic (green), or complex (orange) models were fit to the data.
4.3. Application of growth curve modeling
In retrospect it is intuitively obvious that pain experience may not follow a simple form during recovery from injury. In most cases, pain completely resolves, in which case any model must be truncated, but in many cases pain may fluctuate around zero for a several days, complicating the decision of when to truncate the model. In other cases pain may initially change (either decrease or increase [4]) following a pattern that can be well fit, but then changes to a different pattern. Analysis of auto-correlations in the log(time) model in the current study suggested that this shift from one pattern to another occurs frequently in the postoperative population, leading to our application of a Bayesian change-point model. We note that the first (and often only) change point occurs approximately 10-21 days after surgery, a time of the most rapid return towards normal sensory processing leading. This may also be the time when processes which foster slow resolution of pain (e.g., the so-called transition to chronic pain) become manifest. Regardless of this interpretation, the enhanced granularity of understanding shifting time courses of recovery from pain provided by this time-change model approach may be useful in assessing efficacy of interventions aimed at speeding recovery in entire surgical populations or reducing the incidence of chronic postsurgical pain.
The current data suggest that there is a typical experience of pain resolution after surgery, but that meaningful subpopulations of experience may exist. Individuals reported a wide variety of day-to-day experiences after surgery ranging from very fast resolution of pain (i.e., 6 days) to much slower courses (> 40 days). Interestingly, the differences in pain resolution varied as much within surgery type as across surgeries, suggesting that individual differences play as large a role as surgical insult. Although the defining trend was pain resolution, there were patterns of experience that could be identified (e.g., “flare-ups”) that are difficult to model using only one model form.
In addition, identifying latent classes of subjects with initial recovery patterns or patterns after the first change point could substantially impact our appreciation for the experience of pain after surgery. Some individuals with fast resolution are often ignored by the chronic pain research community, whereas other individuals may initially appear to follow a trajectory towards chronic pain, only to have a rapid resolution later during the 6 months after surgery. An important question might be how these two types of individuals differ and how best to predict which individuals are in which category.
4.4 Limitations
This is an exploratory analysis that utilizes two disparate surgical populations, but it is difficult to generalize these results beyond these surgical procedures. The best fitting pain resolution models were remarkably similar between these two groups of patients, but the extent to which other surgeries might follow the same trajectories is unclear. Different procedures in different patient populations will result in different initial pain severity and time of resolution and that it is conceivable that in some cases the form could differ by procedure. However, variability in initial severity or time to resolution could also be considered a strength in this attempt to define the general form of recovery.
We considered a wide range of models in selecting the ‘best’ pain resolution model, but other candidates may fit better, or account for the variety of experiences in a more flexible way. We attempted to posit a simple model (e.g., log(time)), in the context of a change-point and perhaps this model could serve as a starting place for which to compare other more complex forms of change. For example, there may well be two forms of change in the recovery process- an initial recovery model and maintenance phases. We observed that many participants experienced pain even after ‘resolution’ (i.e., experienced occasional pain after pain reached ‘0’ for the first time), but the extent to which a model that assumed an inflection of change (e.g., a knot, or state-change model) fits better than our simple approach should be evaluated in a novel data set.
Our approach to characterizing the day-to-day experience of pain was ultimately qualitative, despite the fact that we based our characterizations on patterns in the autocorrelation function. Data driven procedures have been developed to optimize latent classes of models, but our modest sample size precluded the use of these procedures (e.g., latent class growth curve models). Future work should use such procedures as prudent.
4.5 Conclusions
These data demonstrate the feasibility of acquiring daily pain assessments for 2 months following surgery in young as well as older patients undergoing major surgery, although the burden on study personnel is large and missing data are frequent. Despite these problems, this approach yields an adequate number of observations for growth curve modeling, something that heretofore has not been possible at this level of detail in this 2 month time period. The Bayesian change-point model that best fit these data is biologically plausible, suggests a time period in which transitions to persistent pain or healing occurs, and applies in this modest sample size to disparate surgical procedures. Its application to a broader surgical population and replication will lay the foundation for its use as a novel outcome measure.
Acknowledgments
Supported in part by grant P01 GM113852 to JCE (PI) and to SM, JEL, TTH, RSC and CAA from the National Institutes of Health, Bethesda, MD, USA.
Footnotes
Conflict of Interest. JCE consults to Adynxx (San Francisco, CA, USA) and TEVA Pharmaceutical Industries (North Wales, PA, USA) regarding preclinical and clinical analgesic development of analgesics. Adynxx is developing agents to speed recovery from surgery, but does not use the methods described in this manuscript and did not participate in this work or the manuscript.
The remaining authors declare no conflicts of interest.
References
- 1.Althaus A, Arranz BO, Neugebauer E. Distinguishing between pain intensity and pain resolution: Using acute post-surgical pain trajectories to predict chronic post-surgical pain. EurJ Pain. 2014;18(4):513–521. doi: 10.1002/j.1532-2149.2013.00385.x. [DOI] [PubMed] [Google Scholar]
- 2.Brantley PJ, Waggoner CD, Jones GN, Rappaport NB. A daily stress inventory: Development, reliability, and validity. Journal of Behavioral Medicine. 1987;10(1):61–74. doi: 10.1007/BF00845128. [DOI] [PubMed] [Google Scholar]
- 3.Chapman CR, Davis J, Donaldson GW, Naylor J, Winchester D. Postoperative pain trajectories in chronic pain patients undergoing surgery: the effects of chronic opioid pharmacotherapy on acute pain. J Pain. 2011;12(12):1240–1246. doi: 10.1016/j.jpain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Chapman CR, Donaldson GW, Davis JJ, Bradshaw DH. Improving individual measurement of postoperative pain: the pain trajectory. J Pain. 2011;12(2):257–262. doi: 10.1016/j.jpain.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downie AS, Hancock MJ, Rzewuska M, Williams CM, Lin CWC, Maher CG. Trajectories of acute low back pain: a latent class growth analysis. Pain. 2016;157(1):225–234. doi: 10.1097/j.pain.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 6.Enders CK. Missing not at random models for latent growth curve analyses. Psychol Methods. 2011;16(1):1–16. doi: 10.1037/a0022640. [DOI] [PubMed] [Google Scholar]
- 7.Gaudilliere B, Fragiadakis GK, Bruggner RV, Nicolau M, Finck R, Tingle M, Silva J, Ganio EA, Yeh CG, Maloney WJ, Huddleston JI, Goodman SB, Davis MM, Bendall SC, Fantl WJ, Angst MS, Nolan GP. Clinical recovery from surgery correlates with single-cell immune signatures. Science translational medicine. 2014;6(255):255ra131. doi: 10.1126/scitranslmed.3009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansen A, Romundstad L, Nielsen CS, Schirmer H, Stubhaug A. Persistent postsurgical pain in a general population: prevalence and predictors in the Tromso study. Pain. 2012;153(7):1390–1396. doi: 10.1016/j.pain.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Kannampallil T, Galanter WL, Falck S, Gaunt MJ, Gibbons RD, McNutt R, Odwazny R, Schiff G, Vaida AJ, Wilkie DJ, Lambert BL. Characterizing the pain score trajectories of hospitalized adult medical and surgical patients: a retrospective cohort study. PAIN. 2016;157(12):2739–2746. doi: 10.1097/j.pain.0000000000000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenguerrand E, Whitehouse MR, Wylde V, Gooberman-Hill R, Blom AW. Pain and Function Recovery Trajectories following Revision Hip Arthroplasty: Short-Term Changes and Comparison with Primary Hip Arthroplasty in the ADAPT Cohort Study. PLoS One. 2016;11(10):e0164839. doi: 10.1371/journal.pone.0164839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 13.Miaskowski C, Cooper B, Paul SM, West C, Langford D, Levine JD, Abrams G, Hamolsky D, Dunn L, Dodd M, Neuhaus J, Baggott C, Dhruva A, Schmidt B, Cataldo J, Merriman J, Aouizerat BE. Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain. 2012;13(12):1172–1187. doi: 10.1016/j.jpain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MG, Katz J, Curtis K, Lutzky-Cohen N, Escobar EM, Clarke HA. Acute pain trajectories and the persistence of post-surgical pain: a longitudinal study after total hip arthroplasty. Journal of anesthesia. 2016;30(4):568–577. doi: 10.1007/s00540-016-2183-4. [DOI] [PubMed] [Google Scholar]
- 15.Page MG, Katz J, Romero Escobar EM, Lutzky-Cohen N, Curtis K, Fuss S, Clarke HA. Distinguishing problematic from nonproblematic postsurgical pain: a pain trajectory analysis after total knee arthroplasty. Pain. 2015;156(3):460–468. doi: 10.1097/01.j.pain.0000460327.10515.2d. [DOI] [PubMed] [Google Scholar]
- 16.Rabbitts JA, Zhou C, Groenewald CB, Durkin L, Palermo TM. Trajectories of postsurgical pain in children: risk factors and impact of late pain recovery on long-term health outcomes after major surgery. Pain. 2015;156(11):2383–2389. doi: 10.1097/j.pain.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rappaport BA, Cerny I, Sanhai WR. ACTION on the Prevention of Chronic Pain after Surgery: Public-Private Partnerships, the Future of Analgesic Drug Development. Anesthesiology. 2010;112(3):509–510. doi: 10.1097/ALN.0b013e3181cf4279. [DOI] [PubMed] [Google Scholar]
- 18.Romundstad L, Breivik H, Roald H, Skolleborg K, Romundstad PR, Stubhaug A. Chronic pain and sensory changes after augmentation mammoplasty: long term effects of preincisional administration of methylprednisolone. Pain. 2006;124(1-2):92–99. doi: 10.1016/j.pain.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Wallenstein S, Brem H. Statistical analysis of wound-healing rates for pressure ulcers. American journal of surgery. 2004;188(1A Suppl):73–78. doi: 10.1016/S0002-9610(03)00294-0. [DOI] [PubMed] [Google Scholar]