Abstract
Objective
The primary objective was to conduct a meta-analysis on published observational cohort data describing the association between acetyl-salicylic acid (aspirin) use prior to the onset of sepsis and mortality in hospitalized patients.
Study Selection
Studies which reported mortality in patients on aspirin with sepsis with a comparison group of patients with sepsis not on prior aspirin therapy were included.
Data sources
Fifteen studies described hospital-based cohorts (n=17,065), while one was a large insurance-based database (n=683,421). Individual-level patient data were incorporated from all selected studies.
Data Extraction
Propensity analyses with 1:1 propensity score matching at the study level were performed, using the most consistently available covariates judged to be associated with aspirin. Meta-analyses were performed to estimate the pooled average treatment effect of aspirin on sepsis-related mortality.
Data Synthesis
Use of aspirin was associated with a 7% (95%CI, 2% to 12%, p=0.005) reduction in the risk of death as shown by meta-analysis with considerable statistical heterogeneity (I2=61.6%).
Conclusions
These results are consistent with effects ranging from a 2% to 12% reduction in mortality risk in patients taking aspirin prior to sepsis onset. This association anticipates results of definitive studies of the use of low-dose aspirin as a strategy for reduction of deaths in patients with sepsis.
Keywords: Sepsis, septic shock, acetyl-salicylic acid, aspirin, mortality, death
Introduction
Sepsis therapy discovery has been bedeviled by failed attempts at proving efficacy of specific treatments (1). Numerous adjuvant treatments for sepsis that have targeted the inflammatory cascade have been ineffective when assessed in phase III or IV trials. Meanwhile, there has been a gradual reduction in mortality in patients admitted to Intensive Care Units (ICUs) owing to improvements in non-specific treatment of sepsis (2), although mortality due to septic shock remains high (20–40%) (2, 3).
Acetyl-salicylic acid (aspirin) may also have benefits in reducing sepsis severity. Observational studies of patients presenting to hospital with sepsis have calculated the impact of prior aspirin use on death in various cohorts (4–16). Other studies have considered aspirin’s influence on the various illnesses leading to acute lung injury (17–19).
In vitro data suggest that aspirin may have specific effects of potential benefit in sepsis that are not shared with other antiplatelets. Therefore, it is of value to consolidate the available information to assess whether aspirin has any benefit in improving sepsis outcomes. Quantifying the association of aspirin and other antiplatelet agents with reduced sepsis-associated mortality may support the specific benefit of aspirin therapy.
We present an individual patient data meta-analysis using study-level propensity score matched re-analysis to estimate the association between prior aspirin use and sepsis mortality.
Materials and Methods
Search strategy
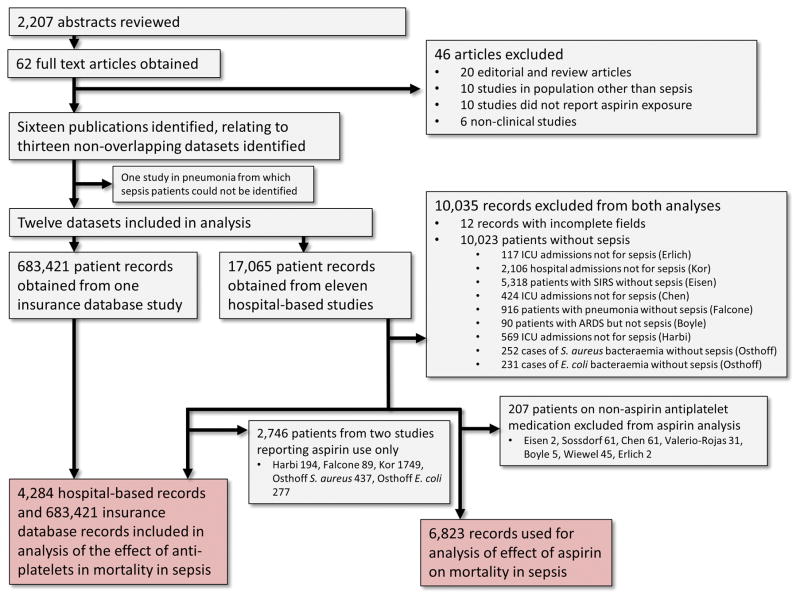
Our methods were prospectively registered with PROSPERO (CRD42015026690) and complied with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (20). The study profile is presented in Figure 1. We searched MEDLINE, Cochrane and PubMed databases for English language articles published from inception to 1st July 2016. A combination of MeSH (Medical Subject Headings) keywords was used. The search terms were “aspirin”, antiplatelet”, “acetyl-salicylic acid”, “nonsteroidal anti-inflammatory”, “NSAID”, “infection”, “sepsis”, “severe sepsis”, “septic shock”, “mortality” and “death”. This approach was supplemented with manual reviews of references from included studies and returned 2,207 abstracts, through which 62 potentially eligible full text articles were identified.
Figure 1.
Study flow diagram.
Study selection
Publications were selected for inclusion if they (i) included cohorts of patients with sepsis, (ii) included both patients treated and not treated with aspirin or anti-platelets and (iii) reported mortality as a consequence of the sepsis episode.
The search strategy identified 16 observational studies, which were subsequently found to describe thirteen non-overlapping datasets. All authorship groups agreed to participate and provided line-listed individual patient data. Of the thirteen datasets, four included sepsis patients only and eight were not limited to sepsis but patients with sepsis could be identified from provided data. One paper analyzed a dataset consisting of patients with pneumonia from which those with sepsis could not be distinguished and was excluded (4). The diagnosis of sepsis was made according to the prevailing consensus definitions in all but three of the included studies (11, 14, 15). One study (15) provided different covariates for two patient subgroups and therefore the approach to analysis differed for each group. Consequently, twelve datasets were included in the final analysis. One such dataset was an insurance database, which included 683,421 hospitalizations for sepsis and provided aggregate information on use of antiplatelet agents only (13). The remaining studies reported on hospital-based cohorts ranging in size from 161 to 6,131 hospital admissions.
Study-level propensity analyses for two-step meta-analyses
For the two-step meta-analyses, a standardized approach to study-level propensity analysis was first undertaken. Most studies provided individual-level data on aspirin use, allowing this exposure variable to be used for the primary analysis (7–9, 11, 12, 14, 15, 17, 19, 21). In one study, it was not possible to disaggregate aspirin use from that of other antiplatelets in the hospital-based cohort (13). However, as aspirin was the antiplatelet used by over 95% of patients in all other studies (6, 10, 16, 18), it is likely that it constituted the large majority of antiplatelet agents used in the analysis of antiplatelet exposure for this study.
For the analysis of the effect of aspirin, patients on other antiplatelet agents were excluded where information was specifically provided on these agents. For some studies, a single variable for antiplatelet agents was provided, while for others, variables were provided for individual medications (with clopidogrel, ticlopidine and prasugrel considered antiplatelets). All such patients treated with antiplatelets were excluded from the analysis of the effect of aspirin, whether treated with aspirin or not. For the analysis of the effect of antiplatelet agents, studies that only reported aspirin use and not use of any antiplatelet were excluded. Patients treated with non-steroidal anti-inflammatory drugs (NSAIDS) or with statins were included in all analyses.
Propensity scores were generated by 1:1 matching to nearest neighbours, using an approach to covariate selection that balanced (i) consistency with the approaches used by individual studies, (ii) consistency across studies given the differing covariates available (iii) breadth of inclusion variables (while also avoiding over-matching). Age and gender were used for all studies. Versions II or III of the Acute Physiology and Chronic Health Evaluation (APACHE) index were used where available (nine studies). Comorbidities of diabetes, stroke or cerebrovascular disease and coronary artery disease or cardiovascular disease were also included as covariates, as they were both consistently available from most studies and potentially associated with aspirin use. Other covariates that may have predicted aspirin use, such as hypertension or peptic ulcer disease, were not provided by enough studies. Covariates used for propensity matching by study are listed in Table 1.
Table 1.
Characteristics of Included Datasets
| Study (reference) | Patient cohort typea | Number of patients with sepsis / number of patients in received dataset | Male : Femaleb | Mean ageb | Exposure(s) reported | Time of death outcome assessment | Variables used in propensity scoring (in addition to age and gender) |
|---|---|---|---|---|---|---|---|
| Erlich et al. 2011 (26) | ICU admissions | 42 / 161 | 19 : 23 | 69.3 | Aspirin and anti-platelets | Hospital separation | APACHE III score, coronary artery disease, diabetesc |
| Kor et al. 2011 (25) | Hospital admissions at risk of ALI | 1,749 / 2,106 | 871 : 878 | 56.8 | Aspirin only | Hospital separation | APACHE II score, diabetes |
| O’Neal et al. 2011 (15) Chen et al. 2015 (27) | ICU admissions | 664 / 1,149 | 348 : 316 | 56.9 | Aspirin and anti-platelets | Hospital separation | APACHE II score, coronary artery disease, stroke, diabetes |
| Eisen et al. 2012 (16) | SIRS | 811 / 6,131 | 494 : 317 | 59.6 | Aspirin and anti-platelets | Hospital separation | APACHE III score, coronary artery disease, diabetes |
| Lösche et al. 2011 (14) | |||||||
| Otto et al. 2013 (17) | Sepsis | 918 / 979 | 603 : 315 | 63.9 | Aspirin and anti-platelets | Hospital separation | APACHE III score, coronary artery disease, diabetes |
| Sossdorf et al. 2013 (29) | |||||||
| Valerio-Rojas et al. 2013 (18) | Sepsis | 620 / 651 | 347 : 273 | 68.1 | Aspirin and anti-platelets | Hospital separationc | APACHE III score, cardiovascular disease, stroke, diabetes |
| Falcone et al. 2015 (20) | Community-acquired pneumonia | 89 / 1,005 | 51 : 38 | 73.0 | Aspirin only | 30 days | SOFA score, coronary artery disease, diabetes |
| Tsai et al. 2015 (21) | Sepsis | 683,421 / 683,421 | 376,612 : 306,809 | 67.5 | Anti-platelets only | Hospital separation | Coronary artery disease, stroke, diabetes |
| Boyle et al. 2015 (19) | ARDS | 106 / 202 | 58 : 48 | 57.9 | Aspirin and anti-platelets | Hospital separation | APACHE II score, coronary artery disease, diabetes |
| Al Harbi et al. 2016 (22) | ICU admissions enrolled in two RCTs | 194 / 763 | 110 : 84 | 62.4 | Aspirin only | Hospital separation | APACHE II score, cardiovascular disease, diabetes |
| Osthoff et al. 2016 (23) | S. aureus and E. coli bacteraemia | 714 / 1,440 | 385 : 329 | 62.2 | Aspirin only | Hospital separationc, d | Charlson score, diabetes (S. aureus cohort), Charlson score only (E. coli cohort) |
| Wiewel et al. 2016 (24) | Sepsis | 916 / 972 | 545 : 371 | 60.9 | Aspirin and anti-platelets | 30 days | APACHE IV score, diabetes |
For the seven studies that included patients with conditions other than sepsis, the analysis presented here is restricted to the subset of patients with sepsis.
Numbers included in the primary analysis.
Data for stroke available, but not used in propensity matching as it resulted in perfect prediction of the outcome for the primary analysis.
30 day mortality also available, but hospital separation used for analyses.
SIRS, systemic inflammatory response syndrome; APACHE, Acute Physiology and Chronic Health Evaluation; CAP, community-acquired pneumonia; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment; ARDS, acute respiratory distress syndrome; ALI, acute lung injury
Ten of twelve studies reported death at hospital separation and four reported death at thirty days from hospital admission, with two reporting both these outcomes. Therefore, death at hospital separation was used for the ten studies reporting this outcome and death at thirty days for the two for which this was not available. This approach was used to generate study-level point estimates and confidence intervals for the average treatment effect of aspirin and of antiplatelet agents on the risk of mortality after hospitalization, using the teffects psmatch command from Stata™ (College Station, USA).
Two-step meta-analyses
A meta-analysis was next undertaken at the study level, using the propensity matched results obtained as described above and standard meta-analytic methods (i.e. “two-step” meta-analysis). Due to statistical heterogeneity, random effects meta-analyses were performed. Pooled analyses of the effect of antiplatelet agents were performed with and without the large insurance database study (13) included, as its methodological approach was substantially different from that of the hospital-based studies and its size would have overwhelmed the contribution from the other studies.
One-step meta-analyses
Next, we undertook a single regression analysis across the entire pooled dataset by first performing 1:1 propensity matching using Stata’s psmatch2 command, which produces variables to indicate matched records. Logistic regression analyses were then performed with aspirin use and antiplatelet use as the only exposure variables to determine the odds ratio for death (at separation or 30 days) in the dataset restricted to matched patients only with incorporation of a clustering term for study of origin (i.e. one-step meta-analysis).
Results
Association between aspirin use and the risk of death
6,823 patients described in eleven studies from 2011 to 2016 were included in the analysis of the association between aspirin usage and mortality in patients hospitalised for sepsis. Point estimates for the average treatment effect from study level propensity analyses ranged from a 34% reduction to a 3% increase in the risk of death, although estimates from studies contributing more than one hundred patients ranged from a 15% reduction to a 3% increase in risk. Despite this, individual estimates from re-analysis of most (eight of twelve) included studies were non-significant. The two-step random effects meta-analysis suggested significant statistical heterogeneity (I2=61.6%, p=0.003), with a pooled estimate of a significant reduction in risk of death by 7% (95%CI, 2 to 12%, p=0.005, Figure 2). Multivariate meta-regression incorporating gender and the mean age of each included study population as exposure variables found non-significant trends towards male gender and increasing age being associated with a greater treatment effect (regression coefficient for proportion male: −0.42 (−0.89 to 0.55, p=0.08), regression coefficient per year of increasing age: −0.0098 (−0.20 to 0.00052, p=0.06)) (Data not shown). Funnel plots revealed no evidence of publication bias (Supplemental Figure 1).
Figure 2.
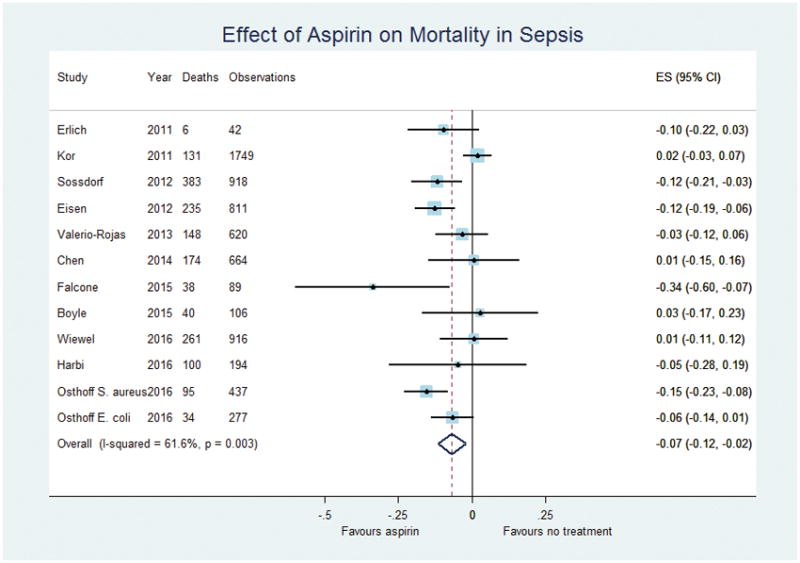
Forest plot of the effect of aspirin on mortality in patients with sepsis as determined by a two-step meta-analysis using a random effects model.
The odds ratio for death on the one-step propensity-matched regression analysis was 0.78 (0.57 to 1.06, p=0.11), non-significantly favoring aspirin (Table 2).
Table 2.
One-step propensity analyses of the effect of pre-sepsis onset aspirin usage.
| Endpoints studied | Aspirin or antiplatelet exposure | Age (per ten year increment) | Female gender | |||
|---|---|---|---|---|---|---|
| Effect of aspirin on death in sepsis, covariates excluded | 0.778 (0.569 – 1.06) | p = 0.114 | ||||
| Effect of aspirin on death in sepsis, covariates included | 0.714 (0.510 – 0.998) | p = 0.049 | 1.099 (1.026 – 1.178) | p = 0.007 | 0.910 (0.671 – 1.234) | p = 0.545 |
| Effect of anti-platelets on death in sepsis, covariates excluded | 0.390 (0.195 – 0.777) | p = 0.007 | ||||
Effect of antiplatelets on the risk of death
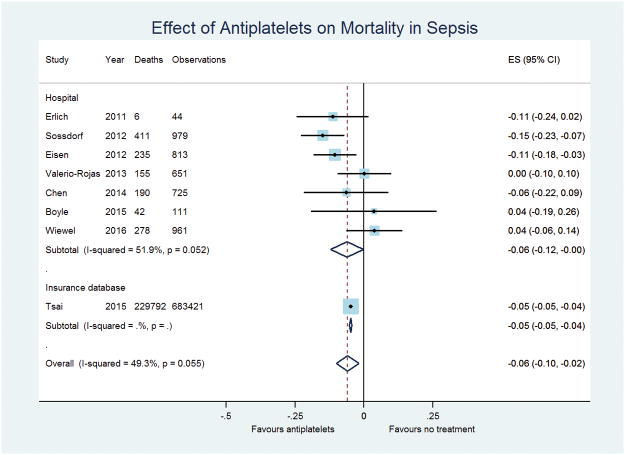
4,284 patients were included in the analysis of antiplatelet drug usage and mortality in patients hospitalized for sepsis from six hospital cohorts and a further 683,421 were included from the large insurance database study (13). Re-analyses of hospital-based studies using propensity scores revealed an overall effect size of a 6% reduction in mortality, with the 95% confidence interval ranging from 12% to virtually no effect. When the re-analyzed insurance database study was pooled with the hospital-based studies, statistical significance was clearer (average reduction in mortality with treatment 6%, 95%CI, 2 to 10%, p=0.004, Figure 3). It is important to note that the markedly larger sample size in the insurance database study has a considerable impact on this treatment effect. One-step regression analysis of these studies suggested a significant reduction in the odds of death (OR 0.39, 95%CI, 0.20 to 0.78, p=0.007) with all studies included.
Figure 3.
Forest plot of the effect of anti-platelet drugs on mortality in patients with sepsis.
Discussion
This combined analysis of all available observational data shows that antiplatelet drugs and aspirin usage in isolation prior to onset of sepsis are associated with a reduction in mortality. Propensity analyses are accepted as the optimal method for interrogating retrospective data, providing a method of pseudo-randomization. Several of the included datasets are from studies reporting markedly different effects from those we report for the same dataset using a standardised propensity analytic approach. Moreover, as most included studies showed non-significant effect sizes, only pooling of results demonstrated a measureable treatment effect, emphasizing the utility of our meta-analysis.
The included studies showed a considerable degree of heterogeneity, which may reflect differences in the cohorts considered. These were enrolled from different contexts and had considerably different baseline characteristics, as reported by the individual publications. However, despite these differences, the re-analyses of most included studies (nine of twelve) were consistent with the pooled effect size estimated from our two-step random effects meta-analyses. A large proportion of the heterogeneity was contributed by the study by Kor et al. (24), which was notable for having the lowest mortality of any included study. If this study is excluded from the two-step analysis, heterogeneity decreases markedly (I2=34.7%, p=0.121), with an average treatment effect estimate of 8% (95%CI 4% to 12%) and the confidence interval of all eleven remaining studies consistent with this point estimate.
Although the results for aspirin and antiplatelets are similar, these two analyses include many of the same individuals and so should not be seen as reinforcing the estimates provided by the other analysis. Rather, the meta-analysis of the effect of aspirin is intended to provide an effect size for the use of aspirin alone, while the meta-analysis of antiplatelets is intended to estimate the effect of these agents in general. Separate analyses were performed because we did not consider it reasonable to include all studies within the same meta-analysis given the differences in treatment exposure. The non-significant effect of age on meta-regression suggests that this variable is not an important modifier of the effect of aspirin at the study level, despite its apparent association with mortality.
The identified reduction in mortality in aspirin users has a biological basis. The following effects are either demonstrated or have a considerable likelihood of being produced by the low doses of aspirin used for cardioprotection.
Tumor necrosis factor (TNF) and interleukin-6 are pivotal pro-inflammatory cytokines. In sepsis, immune cell receptors recognize diverse pathogen-associated molecular patterns mediating intracellular signaling events that result in nuclear factor kappa-beta (NFκ-B) activation and transcription of TNF. NFκ-B activation is inhibited by aspirin and other NSAIDs, which is mediated by inhibition of IκB kinase. The concentration of NSAIDs required for this inhibition has been measured to be lower than aspirin (22), although the lower range of aspirin for this effect has not been defined.
Lipid mediators of sepsis have recently been described (23). Several of these molecules act not only as anti-inflammatories but also to restore homeostasis (24, 25). In sepsis, they reduce established inflammation by mechanisms including restoration of polymorphonuclear apoptosis, which limits continued production of pro-inflammatory cytokines in tissues (26). Low doses of aspirin have been shown to increase lipoxins (aspirin-triggered lipoxin, ATL) and resolvins in vitro (24, 25) and in human trials (27). Furthermore, aspirin reduces mortality in animal models when given either before or after onset of sepsis (28). Low-dose aspirin increases nitric oxide production as shown in experimental animals (29). Finally, in a human model, ATL accounts for prevention of skin blisters via both reduced inflammatory cell accumulation and endothelial adhesion (30).
Platelets become activated in sepsis due to interaction with invading bacteria via three broad mechanisms. These constitute; (i) binding of bacteria to plasma proteins which are ligands for platelet receptors, (ii) direct bacterial binding to platelet receptors and (iii) secretion of aspirin binding bacterial products such as toxins (31). Aspirin reduces activation of platelets by inhibition of cyclooxygenase I (32), thus reducing activated platelets contribution to microvascular occlusion and organ failure (33).
The minimum period of aspirin therapy required to improve patient outcomes prior to onset of infection remains unknown. However, aspirin’s inhibition of platelet activation lasts for their circulating lifetime (34). While aspirin-triggered lipoxin increase was measured after eight weeks of aspirin in a human randomized controlled trial, there are no data on the duration after aspirin cessation (27).
In this meta-analysis, we included all published data describing clinical outcomes in patients taking aspirin and other antiplatelets prior to sepsis onset. Because individual patient data were made available, we have been able to extract information on clinical outcomes and provide a comprehensive assessment of treatment effect despite the differences in study design. For example, we have been able to include the population-based study covering 98% of Taiwan’s population through analysis of the National Health Insurance program. This study includes 683,421 subjects. A nested case control study of 186,374 patients with recent antiplatelet use based on prescription up to 30 days before hospital admission showed an adjusted odds ratio for mortality of 0.90 (95%CI 0.87 to 0.94) (13). In another study with a primary endpoint of ICU mortality in patients with acute lung injury, univariate analysis of 111 subjects with sepsis showed no benefit from prior aspirin use (11).
Notably, a recent study of aspirin intervention for prevention of the adult respiratory distress syndrome mostly enrolled patients with sepsis due to pneumonia (35). This trial showed that aspirin did not prevent death which was a secondary outcome. While this trial will most likely discourage subsequent investigations of intervention in sepsis, the strategy we are describing here is a preventive one.
Limitations of this study include our reliance on observational data and the heterogeneity discussed above. The range of covariates reported in all the datasets and desire to maintain consistency of methods restricted our ability to propensity-match patients using a broad range of factors predicting aspirin use. The diagnosis of sepsis in all but three studies (11, 14, 15) used prevailing criteria (36, 37). Updated consensus definitions of sepsis (38) were published after conclusion of all the studies included herein. It was not possible to assess adherence to aspirin among study participants and individual data on aspirin dose were not universally available, prohibiting sensitivity analyses on these factors. There are no pediatric data due to concerns of the link between salicylate use and Reye Syndrome in febrile children. Finally, there is a paucity of critical care data in the developing world. However, the analysis’ strengths include incorporation of data from all published studies to date describing sepsis outcomes in a large group of patients using aspirin.
The demonstrated association between prior aspirin use and reduction in mortality in patients with sepsis supports a preventive approach for this prevalent syndrome. Primary prevention with aspirin appears to reduce all-cause mortality. A meta-analysis of 100,000 participants from aspirin primary prevention trials showed aspirin reduced all-cause mortality (OR 0.94, 95%CI, 0.88 to 1.00), due neither to reductions in cardiovascular nor cancer deaths (39). No analysis of non-cardiovascular or non-cancer mortality has been undertaken on data from primary or secondary aspirin prevention studies. It may be that one of the previously unmeasured means by which aspirin reduces all-cause mortality is via a reduction in sepsis deaths when aspirin is used for primary prevention.
A randomized placebo controlled trial of low-dose aspirin and prevention of sepsis deaths (The AspiriN To Inhibit SEPSIS trial (ANTISEPSIS)) in progress is a substudy of the ASPREE primary prevention study (40). The results of this propensity analysis based study are an important prelude to ANTISEPSIS, which will be powered to detect a reduction of sepsis mortality of equal magnitude to that shown here.
However, there are important factors that distinguish this meta-analysis and ANTISEPSIS. The mean age of patients we have studied was 61.6 at the onset of sepsis. By comparison, the ANTISEPSIS patients are considerably older, with 90% of patients in ANTISEPSIS over 70 at trial entry and an indeterminate time to sepsis onset. Additionally, the ANTISEPSIS primary endpoint of sepsis related death is measured in all hospitalized patients in contrast to most of the studies in this meta-analysis which only involve deaths in ICU. While results from ANTISEPSIS may show the benefit of a low cost, low-dose aspirin sepsis prevention strategy, this meta-analysis is of value due to the fundamental differences in the patient populations studied.
Conclusions
Our results are consistent with effects ranging from a negligible 2% reduction, through to a clinically important 12% decrease in the risk of death in patients hospitalized due to sepsis where they had prior aspirin treatment. While recent trial data show that aspirin treatment does not reduce mortality in patients with pneumonia, the available literature does show an association between prior aspirin use and reduction in sepsis deaths.
Supplementary Material
Supplemental Figure 1. Funnel plot.
Acknowledgments
Funding/support: This study did not receive any external funding.
Footnotes
Copyright form disclosure: Drs. Gong, Kor, O’Neal, and Ware received support for article research from the National Institutes of Health (NIH). Dr. Gong’s institution received funding from the National Heart, Lung, and Blood Institute (NHLBI). Dr. Kor’s institution received funding from the NIH, and he disclosed off-label product use of aspirin. Dr. McAuley received funding from Peptinnovate, Bayer, GlaxoSmithKline, Boehringer Ingelheim (consulting). Outside the submitted work, his institution has received funds from grants from the UK NIHR and others, and he is one of four named inventors on a patent US8962032 covering the use of sialic acid–bearing nanoparticles as anti-inflammatory agents issued to his institution, The Queen’s University of Belfast (http://www.google.com/patents/US8962032). Dr. Osthoff’s institution received funding from Pharming Technologies B.V. Dr. Ware’s institution received funding from the NIH, Boehringer Ingelheim, and Global Blood Therapeutics. Dr. Winning disclosed off-label product use of aspirin. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 2.Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 3.Kadri SS, Rhee C, Strich JR, et al. Estimating Ten-Year Trends in Septic Shock Incidence and Mortality in United States Academic Medical Centers Using Clinical Data. Chest. 2016;151:278–285. doi: 10.1016/j.chest.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winning J, Reichel J, Eisenhut Y, et al. Anti-platelet drugs and outcome in severe infection: clinical impact and underlying mechanisms. Platelets. 2009;20:50–57. doi: 10.1080/09537100802503368. [DOI] [PubMed] [Google Scholar]
- 5.Winning J, Neumann J, Kohl M, et al. Antiplatelet drugs and outcome in mixed admissions to an intensive care unit. Crit Care Med. 2010;38:32–37. doi: 10.1097/CCM.0b013e3181b4275c. [DOI] [PubMed] [Google Scholar]
- 6.Lösche WBJ, Kabisch B, Winning J, et al. Do aspirin and other antiplatelet drugs reduce the mortality in critically ill patients? Thrombosis. 2012;2012:720254. doi: 10.1155/2012/720254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Neal HR, Jr, Koyama T, Koehler EA, et al. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39:1343–1350. doi: 10.1097/CCM.0b013e3182120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisen DP, Reid D, McBryde ES. Acetyl salicylic acid usage and mortality in critically ill patients with the systemic inflammatory response syndrome and sepsis. Crit Care Med. 2012;40:1761–1767. doi: 10.1097/CCM.0b013e318246b9df. [DOI] [PubMed] [Google Scholar]
- 9.Otto GPSM, Boettel J, Kabisch B, et al. Effects of low-dose acetylsalicylic acid and atherosclerotic vascular diseases on the outcome in patients with severe sepsis or septic shock. Platelets. 2013;24:480–485. doi: 10.3109/09537104.2012.724482. [DOI] [PubMed] [Google Scholar]
- 10.Valerio-Rojas JC, Jaffer IJ, Kor DJ, et al. Outcomes of severe sepsis and septic shock patients on chronic antiplatelet treatment: a historical cohort study. Crit Care Res Pract. 2013;2013:782573. doi: 10.1155/2013/782573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle AJDGS, Hamid UI, Mottram LJ, et al. Aspirin therapy in patients with acute respiratory distress syndrome (ARDS) is associated with reduced intensive care unit mortality: a prospective analysis. Crit Care. 2015;19:1–8. doi: 10.1186/s13054-015-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falcone MRA, Cangemi R, Farcomeni A, et al. Lower mortality rate in elderly patients with community-onset pneumonia on treatment with aspirin. J Am Heart Assoc. 2015;4:e001595. doi: 10.1161/JAHA.114.001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai MJ, Ou SM, Shih CJ, et al. Association of prior antiplatelet agents with mortality in sepsis patients: a nationwide population-based cohort study. Intensive Care Med. 2015;41:806–813. doi: 10.1007/s00134-015-3760-y. [DOI] [PubMed] [Google Scholar]
- 14.Al Harbi SA, Tamim HM, Al-Dorzi HM, et al. Association between aspirin therapy and the outcome in critically ill patients: a nested cohort study. BMC Pharmacol Toxicol. 2016;17:5. doi: 10.1186/s40360-016-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osthoff M, Sidler JA, Lakatos B, et al. Low-Dose Acetylsalicylic Acid Treatment and Impact on Short-Term Mortality in Staphylococcus aureus Bloodstream Infection: A Propensity Score-Matched Cohort Study. Crit Care Med. 2016;44:773–781. doi: 10.1097/CCM.0000000000001554. [DOI] [PubMed] [Google Scholar]
- 16.Wiewel MA, de Stoppelaar SF, van Vught LA, et al. Chronic antiplatelet therapy is not associated with alterations in the presentation, outcome, or host response biomarkers during sepsis: a propensity-matched analysis. Intensive Care Med. 2016;42:352–360. doi: 10.1007/s00134-015-4171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kor DJ, Erlich J, Gong MN, et al. Association of prehospitalization aspirin therapy and acute lung injury: results of a multicenter international observational study of at-risk patients. Crit Care Med. 2011;39:2393–2400. doi: 10.1097/CCM.0b013e318225757f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erlich JM, Talmor DS, Cartin-Ceba R, et al. Prehospitalization antiplatelet therapy is associated with a reduced incidence of acute lung injury: a population-based cohort study. Chest. 2011;139:289–295. doi: 10.1378/chest.10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen WJD, Bastarache JA, May AK, et al. Prehospital aspirin use is associated with reduced risk of acute respiratory distress syndrome in critically ill patients: a propensity-adjusted analysis. Crit Care Med. 2015;43:801–807. doi: 10.1097/CCM.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Sossdorf MOG, Boettel J, Winning J, et al. Benefit of low-dose aspirin and non-steroidal anti-inflammatory drugs in septic patients. Crit Care. 2013;17:1–2. doi: 10.1186/cc11886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 23.Serhan CN, Haeggstrom JZ, Leslie CC. Lipid mediator networks in cell signaling: update and impact of cytokines. FASEB J. 1996;10:1147–1158. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- 24.Serhan CN, Chiang N. Lipid-derived mediators in endogenous anti-inflammation and resolution: lipoxins and aspirin-triggered 15-epi-lipoxins. Scientific World Journal. 2002;2:169–204. doi: 10.1100/tsw.2002.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arita M, Yoshida M, Hong S, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, et al. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang N, Bermudez EA, Ridker PM, et al. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci U S A. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Kebir D, Jozsef L, Pan W, et al. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180:311–319. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hennekens CH, Schneider WR, Pokov A, et al. A randomized trial of aspirin at clinically relevant doses and nitric oxide formation in humans. J Cardiovasc Pharmacol Ther. 2010;15:344–348. doi: 10.1177/1074248410375091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris T, Stables M, Hobbs A, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 31.Cox D, Kerrigan SW, Watson SP. Platelets and the innate immune system: mechanisms of bacterial-induced platelet activation. J Thromb Haemost. 2011;9:1097–1107. doi: 10.1111/j.1538-7836.2011.04264.x. [DOI] [PubMed] [Google Scholar]
- 32.Masotti G, Galanti G, Poggesi L, et al. Differential inhibition of prostacyclin production and platelet aggregation by aspirin in humans. Adv Prostaglandin Thromboxane Res. 1980;6:317–320. [PubMed] [Google Scholar]
- 33.Carvalho-Tavares J, Hickey MJ, Hutchison J, et al. A role for platelets and endothelial selectins in tumor necrosis factor-alpha-induced leukocyte recruitment in the brain microvasculature. Circ Res. 2000;87:1141–1148. doi: 10.1161/01.res.87.12.1141. [DOI] [PubMed] [Google Scholar]
- 34.Patrono C, Garcia Rodriguez LA, Landolfi R, et al. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 35.Kor DJ, Carter RE, Park PK, et al. Effect of Aspirin on Development of ARDS in At-Risk Patients Presenting to the Emergency Department: The LIPS-A Randomized Clinical Trial. JAMA. 2016;315:2406–2414. doi: 10.1001/jama.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 37.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 38.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seshasai SR, Wijesuriya S, Sivakumaran R, et al. Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:209–216. doi: 10.1001/archinternmed.2011.628. [DOI] [PubMed] [Google Scholar]
- 40.Nelson MR, Reid CM, Ames DA, et al. Feasibility of conducting a primary prevention trial of low-dose aspirin for major adverse cardiovascular events in older people in Australia: results from the ASPirin in Reducing Events in the Elderly (ASPREE) pilot study. Med J Aust. 2008;189:105–109. doi: 10.5694/j.1326-5377.2008.tb01932.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Funnel plot.