Introduction
Knee Osteoarthritis (KOA) is one of the most common forms of Osteoarthritis, affecting millions of individuals and causing significant disability and suffering [10]. Pain is the most prevalent and troublesome symptom of KOA [6; 33], however, to date, traditional approaches to manage pain and other symptoms are still suboptimal [47]. Thus, treatment efforts often focus on the behavioral and psychological aspects of pain in order to improve patients’ quality of life. One such target is pain-catastrophizing, a cognitive and emotional coping style which is considered one of the most salient psychological factors contributing to heightened acute and chronic pain [36; 46]. In KOA, catastrophizing is associated with pain and disability [44] and is a risk factor for continuation of chronic pain following total knee arthroplasty [3; 18; 54].
Sleep disturbances are a significant comorbidity of KOA, with a prevalence rate of more than 70% [2; 32; 53], and an emerging target for intervention and improving quality of life [39; 51]. Poor sleep, especially short duration and/or fragmented sleep, are thought to enhance pain sensitivity in KOA [35; 38; 42] and may be heightened by pain-catastrophizing [4]. Pain-catastrophizing has been conceptualized as a form of repetitive negative thinking closely related to worry [12]. Pre-sleep worry, is common in both insomnia and chronic pain [15; 16; 41] and is associated with poor sleep [29]. Analyses of pre-sleep cognitions in those with chronic pain suggest that patients worry about both pain and sleep. Specifically, thoughts about pain were found to be more strongly associated with sleep-continuity disturbances than thoughts about sleep [40]. Importantly, sleep disturbance in chronic pain often includes periods of awakening during the night, which may also elicit worry that prolong awakenings.
For patients with comorbid chronic pain and insomnia, Cognitive Behavioral Therapy for Insomnia (CBT-I) is effective in improving insomnia symptoms, and is partially effective in reducing pain [11; 38; 39; 51]. Disrupted sleep may cause an increase in maladaptive coping responses to pain, which can be modified through CBT-I [51]. Recently, psychological sleep interventions have been shown to reduce pain-catastrophizing in pain populations with high pain-catastrophizing [22; 28; 48]. However, these studies have not investigated daily or nocturnal pain-catastrophizing. An additional gap in the literature remains in regards to the effect of behavioral sleep interventions on the pain-catastrophizing of patients with KOA, who tend to have lower levels of pain-catastrophizing [17; 18].
In the present study, secondary analyses from a randomized, double-blind controlled trial of CBT-I and an active control condition [39] evaluated changes in pain-catastrophizing in a sample of KOA with comorbid insomnia. Pain-catastrophizing was measured in three ways: (1) Pain Catastrophizing Scale (PCS), the most commonly used measure of general trait-like catastrophizing, (2) Evening daily-diary measuring daytime pain-catastrophizing, (3) Morning daily-diary measuring pain-catastrophizing during the previous night (nocturnal catastrophizing). Given the focus of CBT-I on sleep-related worry, we hypothesized a treatment effect on all three measures of pain-catastrophizing, and that the CBT-I group will show a greater reduction in pain-catastrophizing than the active control group.
Methods
Procedure
As part of a randomized controlled clinical trial assessing the effectiveness of CBT-I in KOA patients (ClinicalTrials.gov identifier: NCT00592449), questionnaires, and daily diaries (catastrophizing, pain and sleep) were collected at five different time points: baseline, mid-treatment, post-treatment and at 3 and 6 month follow-up. One hundred patients diagnosed with KOA and insomnia were randomized to receive either eight sessions of CBT-I or an active placebo intervention of behavioral desensitization. A detailed description of the study, including detailed information on recruitment and the interventions can be found in the main trial paper [39].
Outcome measures
Questionnaires
Catastrophizing
Pain catastrophizing was assessed by the Pain Catastrophizing Scale (PCS)[45], a 13-item self-report questionnaire in which participants rate the frequency with which they experience thoughts and feelings regarding their pain on a 5-point Likert scale (0=“not at all” to 4=“all the time”). Scores can range from 0–52 with higher scores representing higher catastrophizing. This is a well validated scale that assesses the trait-like aspects of pain catastrophizing [46]. Internal consistency values for baseline, mid-treatment, post-treatment, 3-month and 6-month follow-up were: Cronbach α = .92, .95, .94, .95 and .95 respectively.
Depressive symptoms
Depressive symptoms were assessed through the Center for Epidemiological Studies Depression Scale (CES-D) [37], a 20 item self-report questionnaire which has been validated in chronic pain populations [13]. Participants report the frequency they have experienced each symptom during the past week on a 4-point Likert scale (0-”none of the time” to 3-”all of the time”. Internal consistency for the baseline administration was Cronbach α = .87.
Diary data
Electronic personal digital assistants (PDAs) were used to capture daily sleep and pain-related information from each participant at each study time point. During each monitoring period, participants entered sleep-specific information as well as nocturnal pain ratings and other pain-related measures into the PDA diary each morning after waking. Pain ratings and other pain-related measures were completed each evening prior to bedtime. All entries were stamped with the time and date. At baseline, entries were made daily for approximately 2 weeks. Following randomization, each PDA sleep diary collection period spanned approximately 1 week before each visit.
Diary catastrophizing
Daytime Catastrophizing – each night before participants went to bed they were asked the following statement on their PDA system: “Indicate HOW MUCH you had each thought or feeling when you were in pain today, including now”.
Nocturnal Catastrophizing - In the morning upon awakening participants were asked the following statement on their PDA system: “The next few questions ask about your thoughts and feelings WHEN YOU HAD PAIN during the night. Rate how much you had each thought or feeling”.
Ratings for both daytime and nocturnal catastrophizing were made on an 11-point Likert scale ranging from “0” - not at all to “10” – very much. Scores were converted to a 100-point scale, as the items were presented on a digital display. Items for both daytime and nocturnal catastrophizing included three selected questions from the PCS: “I felt like I couldn’t stand the pain”; “I couldn’t stop thinking about how much it hurt” (see [50]). The third question was adapted from a PCS helplessness subscale item to be more appropriate for use in the daily and nocturnal ratings: “I felt like the pain was never going to get any better”. Internal consistency for both daytime and nocturnal catastrophizing at all time points was excellent and ranged between Cronbach α = .97–.99.
Diary pain
Diary Clinical Daytime Pain: Participants completed pain ratings of the “usual pain” they experienced during the entire day on a PDA through a sliding Visual Analogue Scale [(VAS) “0” equal to “no pain” and “100” equal to “the worst pain imaginable”]. Patients made ratings each night before they went to bed.
Diary Clinical Nocturnal Pain: Participants completed ratings of the pain they experienced during the night on a PDA through a sliding Visual Analogue Scale [(VAS) “0” equal to “no pain” and “100” equal to “the worst pain imaginable”]. Patients made ratings each morning upon awakening.
Diary sleep
Diary Wake After Sleep Onset: Although a variety of sleep continuity measures can be calculated from a sleep diary, the analyses in the present paper focus on Wake After Sleep Onset (WASO), which indexes the time spent awake in the middle of the night after initially falling asleep. We elected to limit our sleep-related analyses to this measure in order to reduce the number of statistical models tested and control alpha inflation. Furthermore, in our published primary outcomes analyses, WASO was the only sleep continuity measure to demonstrate significantly greater improvement in CBT-I compared to the active placebo control [39].
Analytic Strategy
Statistical analysis
In order to evaluate treatment related changes in the three measures of pain catastrophizing and their relation to pain and WASO, two sets of temporal parameters were used:
Temporal lags between data gathered at the primary assessment points: baseline, mid-treatment, post-treatment, 3-month and 6-month follow-up (Stable/trait catastrophizing analyses).
Day to day temporal lags were assessed through variables gathered by daily diaries.
1) Stable/trait catastrophizing analyses
Stable daytime and nocturnal catastrophizing was assessed by averaging daily reports within participant, over the days of each primary study assessment point. The PCS administered at each primary assessment point was also used as a stable measure of catastrophizing.
Multilevel modeling was used to evaluate longitudinal treatment associated changes in pain-catastrophizing across the primary assessment points. This was done by running three models (one for each measure of pain catastrophizing). The first set of models tested the rate of change in pain-catastrophizing between baseline and post-treatment and whether this change differed between the intervention groups. These same models were also run between the post-treatment and 6-month follow-up to test if changes were maintained after the end of treatment. The next set of models evaluated whether early changes in average daily pain and WASO moderated later changes in catastrophizing from mid-treatment to post-treatment. Residual change scores were created to represent early changes (baseline to mid treatment) in WASO and clinical pain (ΔWASO and ΔPain, respectively). These models, thus, included conditional interactions of ΔWASO X Time and ΔPain X Time in addition to the main effect of time. We tested if this early change in pain or WASO predicted the later change from mid-treatment to post-treatment. We choose to look at the contribution of pain and WASO to the change in pain-catastrophizing since results from the primary study indicated that both pain and WASO change early in treatment. We also tested the reverse relationship, namely, if early change in catastrophizing predicted later change in pain and WASO.
Mixed-effects models were used to account for random variation in intercepts, control for autoregression, and include all available data points. An autoregressive 1(AR1) variance-covariance matrix was chosen due to high autocorrelation between catastrophizing at the different assessment points. The following clinical and demographic covariates were entered into the preliminary models as conditional effects with Time: Age, sex, BMI, education level, race, and baseline depression. These covariates have been found in previous studies to be associated with sleep and catastrophizing. Non-significant conditional effects of these covariates with time (p > .20) were removed from the final models.
The Reliable Change Index (RCI) was used to estimate if the changes in pain catastrophizing scores from baseline to post-treatment were reliable and not due to the measure’s unreliability. The RCI was calculated by using the Cronbach’s alpha and standard deviation of each measure. The RCI provides a magnitude of change that is reliable at a significance level of .05 [20; 21].
2) Daily diary analyses
In order to test the relationships between catastrophizing, sleep and pain on a day-to-day basis, daily diary data was analyzed using multilevel modeling. Diary daytime and nocturnal catastrophizing, WASO and pain were computed by centering the daily scores around each individual’s mean scores across all the days in each main study assessment point resulting in daily within-person deviations.
Missing Data
The overall attrition rate was 27% and did not differ between the intervention groups (a full attrition analysis and information on treatment integrity have been previously reported [39]). Since no group differences were observed in attrition, we assume the data are missing at random.
In the daily diary analyses, at each assessment period the number of days participant completed their PDA varied based on their scheduled visits. At baseline, 66% of participants met the goal of 14 days of complete PDA data, 73% has at least 10 days of compete data and 95% had at least one week of complete data. At mid-treatment assessment 70% of participants had at least one week of complete data. At post-treatment assessment 76% of participants had at least one week of complete data. At the 3-month follow-up 92% of participants had at least one week of complete data, and at the 6-month follow-up 91% of the participants had at least one week of complete data. Instances in which data from the next day was missing were not included in the analyses.
All analyses were conducted using SPSS 24.
Results
1. Patient characteristics and preliminary analyses
Baseline demographic and clinical characteristics by intervention group can be found in Table 1. Out of the 100 randomized participants 79% were female, mean age was 59.4±9.5. Forty three percent were African American, 55% Caucasian and the rest were of other ethnicities.
Table 1.
Baseline demographic and clinical characteristics of the sample by treatment group
| CBT-I | BD | |
|---|---|---|
| Characteristic | ||
| Age, mean±SD years | 59.2±69.9 | 59.6±9.1 |
| Female sex | 38 (76%) | 41 (82%) |
| Ethnicity | ||
| African American | 21 (42%) | 22 (44%) |
| Caucasian | 28 (56%) | 27 (54%) |
| Asian | 0 (0%) | 1 (2%) |
| Multicultural | 1 (2%) | 0 (0%) |
| Education level | ||
| High school/some college | 31 (62%) | 17 (34%) |
| College graduate | 8 (16%) | 17 (34%) |
| Graduate studies | 11 (22%) | 16 (32%) |
| Clinical variables | ||
| Body mass index kg/m2 | 31.6±6.2 | 31.5±7.0 |
| CES-D score | 14.6±7.7 | 14.0±9.4 |
| WASO, mean±SD minutes | 65.9±37.8 | 68.6±43.1 |
| Average daytime diary pain | 47.4±17.2 | 46.8±18.9 |
| Average nocturnal diary pain | 35.6±19.9 | 37.8±20.1 |
CBT-I = Cognitive Behavioral Therapy for Insomnia; BD = Behavioral Desensitization; CES-D = Center for Epidemiological Studies Depression Scale; WASO = Wake After Sleep Onset;
PCS scores, daytime diary catastrophizing and nocturnal diary catastrophizing were autocorrelated between time points and ranged from r=.53–.82 for PCS, r=.72–.78 for daytime catastrophizing and r=.75–.80 for nocturnal catastrophizing. Baseline depression was moderately correlated with catastrophizing measures at all time points ranging from r=.23–.53, as was BMI ranging from r=.11–.37.
2. Treatment related change in the three measures of pain catastrophizing
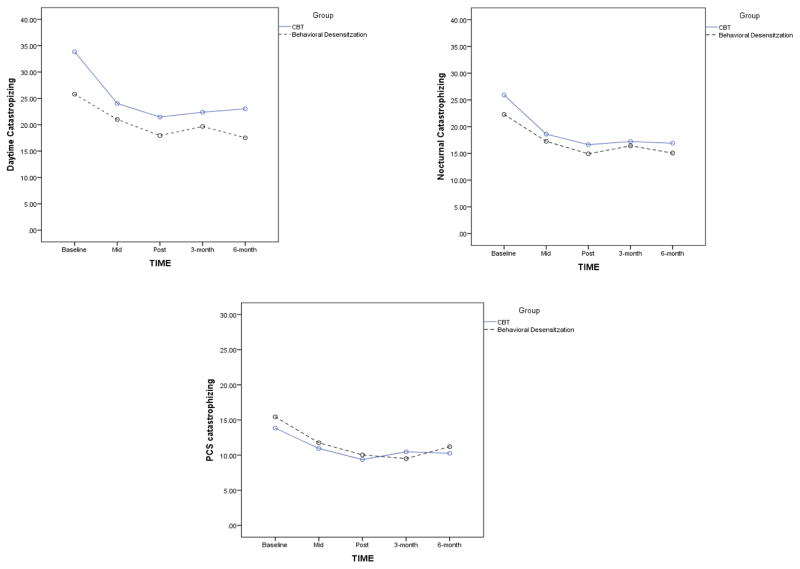
As shown in Figure 1, PCS scores, average daytime diary catastrophizing and average nocturnal diary catastrophizing decreased over time between baseline and post treatment assessment in both intervention groups and remained stable at the 3 and 6 month follow-up. Values of the three catastrophizing measures across the study time points are presented in Table 2. We used the initial mixed-effects model to test if the rate of change in pain-catastrophizing (represented by the Time variable) was significant and if it differed between the two intervention groups (represented by the Group X Time interaction) in the baseline to post-treatment assessment time period. Results indicate that Time was a significant predictor of changes in PCS (B =−2.34, SE = .62, t(185) = −3.77, p < .01, d=.52), daytime catastrophizing (B =−4.86, SE = .97, t(178) = −5.02, p < .01, d=.51) and nocturnal catastrophizing (B =−4.35, SE = .88, t(182) = −4.93, p < .01, d=.42), however, there was no Group X Time interaction in any of the models. Thus, it can be concluded that all measures of pain-catastrophizing decreased in response to treatment but there was no difference between the treatment groups in the rate of this change. None of the covariates were significant in this model and thus were removed and not used in further analyses.
Figure 1.
Treatment related changes in the three measures of pain catastrophizing by intervention group from baseline through the six-month follow-up.
CBT = Cognitive Behavioral Therapy for Insomnia; PCS = Pain Catastrophizing Scale
Table 2.
Values of catastrophizing across study time points by intervention group
| CBT-I group | N | BD group | N | |
|---|---|---|---|---|
| PCS | ||||
| Baseline | 14.82±14.83 | 50 | 15.18±10.16 | 49 |
| Mid-treatment | 11.86±9.31 | 45 | 13.17±12.99 | 46 |
| Posttreatment | 9.09±7.59 | 45 | 10.49±11.07 | 49 |
| 3-month follow-up | 10.87±9.47 | 35 | 9.95±11.06 | 41 |
| 6-month follow-up | 10.41±9.77 | 36 | 11.31±11.33 | 41 |
| Daytime catastrophizing | ||||
| Baseline | 30.30±19.70 | 48 | 28.97±22.49 | 49 |
| Mid-treatment | 21.19±21.03 | 43 | 24.46±23.51 | 47 |
| Posttreatment | 19.51±20.37 | 42 | 20.27±21.32 | 48 |
| 3-month follow-up | 21.70±22.26 | 36 | 23.12±24.06 | 42 |
| 6-month follow-up | 20.89±23.77 | 32 | 19.93±22.89 | 39 |
| Nocturnal catastrophizing | ||||
| Baseline | 23.09±19.23 | 50 | 24.50±20.81 | 48 |
| Mid-treatment | 17.21±18.73 | 44 | 20.43±21.79 | 46 |
| Posttreatment | 15.32±18.61 | 43 | 16.41±19.25 | 48 |
| 3-month follow-up | 16.83±17.45 | 32 | 18.89±21.46 | 42 |
| 6-month follow-up | 17.12±21.41 | 32 | 15.80±20.36 | 38 |
CBT-I = Cognitive Behavioral Therapy for Insomnia; BD = Behavioral Desensitization; PCS = Pain Catastrophizing Scale
To verify that there were no significant changes from post treatment to the follow-up assessments, the same models were run again only for the post-treatment to 6-month follow-up time points. Time was not a significant predictor in these models. This suggests that the treatment gains were maintained over time. Since the changes in pain-catastrophizing occurred between baseline and post treatment, we limited any further analyses to this time frame which also allowed us to maximize our data points due to attrition in the follow-up assessments.
The low pain-catastrophizing scores in our sample could result in a floor effect, making it harder to assess treatment benefits reliably through percent change scores. For this reason, the Reliable Change Index (RCI) was used to assess a statistically significant reliable change. According to the RCI, 39% of the sample obtained a reliable and significant change in PCS scores from baseline to post-treatment (reduction greater than 6.25 points), 33% obtained a significant change in daytime catastrophizing scores from baseline to post-treatment (reduction greater than 10.11 points) and 47% obtained a significant change in nocturnal catastrophizing scores from baseline to post-treatment (reduction greater than 9.57 points).
3. Contribution of early changes in WASO
In the second set of models we tested if early ΔWASO contributed to later changes in catastrophizing mid-treatment to post-treatment. The interaction of ΔWASO X Time was a significant predictor of change in PCS scores (B =−.05, SE = .02, t(82) = −2.81, p < .01) and also of nocturnal catastrophizing (B =−.08, SE = .04, t(81) = −2.17, p < .05), however the interaction of ΔWASO X Time did not significantly predict daytime catastrophizing (B =−.02, SE = .04, t(78) = −.60, p = .55).
In testing the reverse relationship, we did not find that early change in any of the catastrophizing measures (baseline to mid-treatment) predicted later change (mid to post treatment) in WASO.
4. Contribution of early change in pain
The third set of models was designed to test if early ΔPain contributed to later changes in catastrophizing from mid-treatment to post-treatment. The interaction of ΔPain X Time did not significantly predict any of the measures of catastrophizing: PCS (B =.09, SE = .06, t(85) = 1.41, p = .16), daytime catastrophizing (B =−.14, SE = .10, t(83) = −1.32, p = .19), nocturnal catastrophizing (B =−.03, SE = .10, t(84) = −.31, p = .76).
In testing the reverse relationship, we did not find that early change in any of the catastrophizing measures (baseline to mid-treatment) predicted later change (mid to post treatment) in average daytime pain (measured by PDA).
5. Analyses of daily diary data
In order to test if there were day to day associations between diary pain, WASO and daytime catastrophizing, a mixed-effects model was tested in which daytime catastrophizing was regressed upon prior night nocturnal pain and WASO during the baseline assessment period. Only nocturnal pain was a significant predictor of next-day daytime catastrophizing (b =.07, SE = .03, t(1020) = 2.47, p = .01). In a second model, same day pain and nocturnal catastrophizing were added as covariates into the model. Daytime catastrophizing from the previous day was also controlled for in order to account for autocorrelations between the different days. In this model, nocturnal pain was not a significant predictor (B =−.03, SE = .03, t(922) = −1.06, p = .29), however, nocturnal catastrophizing (B =.09, SE = .04, t(934) = 2.37, p < .05) and same day pain (B =.68, SE = .03, t(924) = 19.92, p < .001) predicted daytime catastrophizing. The second step added Time and the conditional effects of Time and nocturnal pain and WASO into the model and tested if the relationships found in the previous model changed as a response to treatment. Daytime catastrophizing did decrease over time (B =−4.98, SE = .37, t(2334) = −13.28, p < .001) however there was no significant interaction between Time and nocturnal pain (B =0.01., SE = .03, t(2312) = .45, p = .65), WASO (B =0.01., SE = .01, t(2311) = .04, p = .96) or any of the other covariates from the second model. Thus, it can be concluded that the prospective relationship between nocturnal pain and WASO and next-day catastrophizing did not change as a result of the intervention.
Discussion
Our results show that interventions focusing on sleep, including both CBT-I and an active control using desensitization, resulted in a significant reduction in all three measures of pain-catastrophizing in a sample of KOA patients with comorbid insomnia. Reductions occurred after eight weeks of treatment and were maintained at the six-month follow-up. Analyses of the timing of these changes indicate that larger reductions in WASO by mid-treatment were associated with later reductions in PCS and nocturnal catastrophizing, but not daytime catastrophizing. While reducing pain-catastrophizing, both as a general coping style (PCS) and as a daytime and nighttime pain coping strategy (daily diaries), the sleep interventions did not change the day-to-day relationships between nocturnal catastrophizing, WASO, and next-day catastrophizing.
Our most important finding is the reduction of pain-catastrophizing by both sleep interventions. We did not find that early changes in pain were associated with the magnitude of later change in pain-catastrophizing, nor did we find the reverse relationship. This implies that the changes in pain-catastrophizing are not simply a result of reduced pain. Because pain-catastrophizing plays a critical role in the experience of pain and is a risk factor for poor outcome [3; 46], the ability to reduce it through psychosocial intervention is an important clinical goal. Traditionally, pain-catastrophizing is one of the main targets of CBT for Pain (CBT-P), in which patients are taught to identify and change maladaptive catastrophic thoughts [8]. Despite being an effective treatment for KOA [43], CBT-P requires maintaining thought logs that rely heavily on reading and writing skills, making it harder for low-literacy patients to adhere to and benefit from treatment [49]. Sleep interventions rely less on cognitive monitoring and use diaries that monitor timing of sleep schedules. Thus, sleep interventions may be more engaging and achieve greater adherence than CBT-P for some groups. Moreover, stigma and embarrassment can prevent patients from seeking psychological help for their pain [19] or mental health [14]. Thus, targeting sleep may be an acceptable alternative for patients who perceive stigma regarding their chronic pain condition. This could be determined in future studies comparing the effectiveness of CBT-I and CBT-P. Our findings, combined with the primary results from the original trial [39], support the notion that by focusing on improving sleep and not explicitly focusing on cognitions about pain, we can also improve a host of pain related outcomes.
The magnitude of change in WASO early in treatment (baseline to mid-treatment) predicted the rate of reduction in pain-catastrophizing later in treatment for both trait and nocturnal catastrophizing. This is similar to the finding that improvements in sleep were associated with reduction of negative sleep cognitions [30]. Poor sleep and sleep fragmentation may cause individuals to be more prone to maladaptive coping [31]. In the present study, the consolidation of sleep appeared to reduce negative nighttime cognitions, as demonstrated by our finding of reduced nocturnal catastrophizing but also a more general reduction of negative pain cognitions as seen by the reduction in trait catastrophizing. The importance of these sequential effects – the change in WASO preceding the change in trait and nocturnal catastrophizing, is further bolstered by the absence of an effect of early changes in catastrophizing predicting later changes in WASO.
Our measure of nocturnal catastrophizing is novel and derives from our interest in sleep-pain interactions, and the rich insomnia literature demonstrating the major role of negative cognitions in initiating and perpetuating insomnia [9]. Nocturnal pain catastrophizing was monitored in a daily (morning) diary assessing catastrophizing about pain when awake during the night. The high correlation between nocturnal catastrophizing and daytime and trait catastrophizing suggests that patients who catastrophize during the day also tend to catastrophize when they are awake at night. Day-to-day analyses of the diary data show that nocturnal catastrophizing predicts next-day pain catastrophizing. Not surprisingly, when patients’ sleep improves and they are awake for less time during the night, they concurrently report less nocturnal catastrophizing. Yet, in addition to reduced opportunity, the findings on the other measures of pain-catastrophizing suggest this reduction is also due to a more general shift in the patient’s tendency to catastrophize. Negative cognitions about pain before sleep are associated with sleep discontinuity in chronic pain [40], and our findings support continued investigation of pain-related cognitions during the night. Future work should investigate the possibility that nocturnal and daytime catastrophizing could have differential effects on other sleep continuity measures as well as sleep quality and cortical arousal during sleep.
Both intervention groups demonstrated a similar reduction in all measures of catastrophizing explained in part by the change in WASO. Another mechanism shared by both groups and may account for these changes, is the use of daily symptom monitoring, which on its own can result in a significant reduction in pain-catastrophizing [48]. This may occur through a general process of increased awareness to negative thoughts that promotes various changes, including self-initiated strategies to decrease worry. Thus, the effect of sleep interventions on pain-catastrophizing might be part of a more general reduction in worry. Future work is needed to disentangle treatment effects on different types of worry (about sleep or pain) in patients with chronic pain and comorbid insomnia.
There may be other underlying mechanisms contributing to the reductions in pain-catastrophizing that differ between the interventions. Since similar cognitive processes are shared by pain and sleep [26; 40], the cognitive strategies designed to change negative sleep cognitions used in CBT-I may generalize to negative cognitions about pain. A different therapeutic mechanism likely underlies the effect in the desensitization intervention which addressed sleep-related conditional arousal by pairing neutral images with arousing thoughts and behaviors related to sleep. Examples of arousing thoughts and behaviors included worrying, clock watching and experiencing pain; examples of neutral images include preparing a meal or watching TV. During this exercise participants are asked to “close their eyes and clear their mind from any distraction”. Thus, this imaginary exercise may have acted as a relaxation technique which is known to be effective in reducing pain and related outcomes [1]. Although at least one major study has conceptualized the desensitization condition as a control intervention for CBT-I in primary insomnia [7], this desensitization intervention appears to have a more powerful effect on sleep in KOA patients with insomnia, which is consistent with a study of insomnia in older adults [23].
Despite our finding of the temporal relationship between change in WASO and treatment related changes in catastrophizing, we did not find a day-to-day relationship between WASO and next day catastrophizing. Nocturnal catastrophizing did predict next day catastrophizing, yet there was no change in this day-to-day relationship over the course of treatment. It appears that more stable measures of catastrophizing are malleable to and predicted by treatment-related changes in WASO, whereas temporally sensitive measures are not. More research is needed in order to understand the source of these differences and their contribution to clinical outcomes.
A number of limitations should be noted. Our sample exhibited low levels of pain-catastrophizing at baseline (Mean PCS=15.01) similarly to those reported in other studies of KOA patients [17; 18], but lower than baseline PCS scores reported in intervention studies in other chronic pain conditions [5; 22; 28]. This could have caused a floor effect, and possibly larger effects might be observed in patients with greater pain-catastrophizing. Alternatively, recent findings suggest that patients who are low in pain-catastrophizing may respond better to treatment [24; 52]. Thus, generalization of the findings to other populations with higher rates of pain-catastrophizing should be done with caution. Although pain-catastrophizing was not explicitly targeted in the interventions, its repeated assessment may have suggested its importance to the participants and contributed to the changes found. As there are known discrepancies between objective and subjective sleep measures [25] the use of another measure of WASO (e.g., polysomnography) could have led to different results; however, we believe the use of self-report electronic diaries is both theoretically and clinically appropriate for several reasons. First, we sought to control alpha inflation by avoiding the duplication of analyses with actigraphically-measured WASO. Second, our previous data suggest that actigraphy may not be a reliable estimate of WASO in distinguishing between KOA patients with and without insomnia [4]. Third, self-report daily diaries are most commonly used to monitor treatment gains in CBT-I and for modifying sleep schedules [34].
Conclusions
We find reductions in pain-catastrophizing in patients with KOA following two different interventions for sleep disturbance. As a whole, our findings support the relationship between catastrophizing and WASO, and specifically, we demonstrate that through a short intervention that focuses only on sleep, three measures of pain-catastrophizing, including a novel measure of nocturnal catastrophizing, can be significantly reduced. Reduction in pain-catastrophizing is particularly important in this population since high levels can increase the risk for poor recovery from knee surgery [3] which is a common procedure for KOA [27]. Future research is needed in order to test if these sleep related changes in pain-catastrophizing are also longitudinally associated with improved pain and quality of life.
Acknowledgments
This work was supported by the NIH (grants R01- AR-5487 and AR-059410 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases) awarded to M.T. Smith. We have no conflict of interest to report.
References
- 1.Integration of behavioral and relaxation approaches into the treatment of chronic pain and insomnia: Nih technology assessment panel on integration of behavioral and relaxation approaches into the treatment of chronic pain and insomnia. JAMA. 1996;276(4):313–318. doi: 10.1001/jama.1996.03540040057033. [DOI] [PubMed] [Google Scholar]
- 2.Allen KD, Renner JB, Devellis B, Helmick CG, Jordan JM. Osteoarthritis and sleep: the Johnston County Osteoarthritis Project. J Rheumatol. 2008;35(6):1102–1107. [PMC free article] [PubMed] [Google Scholar]
- 3.Burns LC, Ritvo SE, Ferguson MK, Clarke H, Seltzer Z, Katz J. Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: a systematic review. J Pain Res. 2015;8:21–32. doi: 10.2147/JPR.S64730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell CM, Buenaver LF, Finan P, Bounds SC, Redding M, McCauley L, Robinson M, Edwards RR, Smith MT. Sleep, Pain Catastrophizing, and Central Sensitization in Knee Osteoarthritis Patients With and Without Insomnia. Arthritis Care Res (Hoboken) 2015;67(10):1387–1396. doi: 10.1002/acr.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnall BD, Sturgeon JA, Kao MC, Hah JM, Mackey SC. From Catastrophizing to Recovery: a pilot study of a single-session treatment for pain catastrophizing. J Pain Res. 2014;7:219–226. doi: 10.2147/JPR.S62329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominick KL, Ahern FM, Gold CH, Heller DA. Health-related quality of life and health service use among older adults with osteoarthritis. Arthritis Rheum. 2004;51(3):326–331. doi: 10.1002/art.20390. [DOI] [PubMed] [Google Scholar]
- 7.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285(14):1856–1864. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 8.Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol. 2014;69(2):153–166. doi: 10.1037/a0035747. [DOI] [PubMed] [Google Scholar]
- 9.Espie CA. Understanding insomnia through cognitive modelling. Sleep Med. 2007;8(Suppl 4):S3–8. doi: 10.1016/S1389-9457(08)70002-9. [DOI] [PubMed] [Google Scholar]
- 10.Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42(1):1–9. v. doi: 10.1016/S0033-8389(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 11.Finan PH, Buenaver LF, Coryell VT, Smith MT. Cognitive-Behavioral Therapy for Comorbid Insomnia and Chronic Pain. Sleep Med Clin. 2014;9(2):261–274. doi: 10.1016/j.jsmc.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flink IL, Boersma K, Linton SJ. Pain catastrophizing as repetitive negative thinking: a development of the conceptualization. Cogn Behav Ther. 2013;42(3):215–223. doi: 10.1080/16506073.2013.769621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: a comparative analysis. Clin J Pain. 1997;13(2):163–170. doi: 10.1097/00002508-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Gulliver A, Griffiths KM, Christensen H. Perceived barriers and facilitators to mental health help-seeking in young people: a systematic review. BMC Psychiatry. 2010;10:113. doi: 10.1186/1471-244X-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40(8):869–893. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 16.Harvey AG, Greenall E. Catastrophic worry in primary insomnia. J Behav Ther Exp Psychiatry. 2003;34(1):11–23. doi: 10.1016/s0005-7916(03)00003-x. [DOI] [PubMed] [Google Scholar]
- 17.Helminen EE, Sinikallio SH, Valjakka AL, Vaisanen-Rouvali RH, Arokoski JP. Determinants of pain and functioning in knee osteoarthritis: a one-year prospective study. Clin Rehabil. 2016;30(9):890–900. doi: 10.1177/0269215515619660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hovik LH, Winther SB, Foss OA, Gjeilo KH. Preoperative pain catastrophizing and postoperative pain after total knee arthroplasty: a prospective cohort study with one year follow-up. BMC Musculoskelet Disord. 2016;17:214. doi: 10.1186/s12891-016-1073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson JE. Stigma, Liminality, and Chronic Pain: Mind-Body Borderlands. American Ethnologist. 2005;32(3):332–353. [Google Scholar]
- 20.Jacobson NS, Follette WC, Revenstorf D. Psychotherapy outcome research: Methods for reporting variability and evaluating clinical significance. Behav Ther. 1984;15(4):336–352. [Google Scholar]
- 21.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 22.Lami MJ, Martinez MP, Sanchez AI, Miro E, Diener FN, Prados G, Guzman MA. Gender Differences in Patients with Fibromyalgia Undergoing Cognitive-Behavioral Therapy for Insomnia: Preliminary Data. Pain Pract. 2016;16(2):E23–34. doi: 10.1111/papr.12411. [DOI] [PubMed] [Google Scholar]
- 23.Lichstein KL, Riedel BW, Wilson NM, Lester KW, Aguillard RN. Relaxation and sleep compression for late-life insomnia: a placebo-controlled trial. J Consult Clin Psychol. 2001;69(2):227–239. doi: 10.1037//0022-006x.69.2.227. [DOI] [PubMed] [Google Scholar]
- 24.Litt MD, Porto FB. Determinants of pain treatment response and nonresponse: identification of TMD patient subgroups. J Pain. 2013;14(11):1502–1513. doi: 10.1016/j.jpain.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8(3):175–183. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald S, Linton SJ, Jansson-Frojmark M. Avoidant safety behaviors and catastrophizing: shared cognitive-behavioral processes and consequences in co-morbid pain and sleep disorders. Int J Behav Med. 2008;15(3):201–210. doi: 10.1080/10705500802222675. [DOI] [PubMed] [Google Scholar]
- 27.Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, Jiranek WA, Berry DJ. Prevalence of Total Hip and Knee Replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386–1397. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez MP, Miro E, Sanchez AI, Diaz-Piedra C, Caliz R, Vlaeyen JW, Buela-Casal G. Cognitive-behavioral therapy for insomnia and sleep hygiene in fibromyalgia: a randomized controlled trial. J Behav Med. 2014;37(4):683–697. doi: 10.1007/s10865-013-9520-y. [DOI] [PubMed] [Google Scholar]
- 29.McGowan SK, Behar E, Luhmann M. Examining the Relationship Between Worry and Sleep: A Daily Process Approach. Behav Ther. 2016;47(4):460–473. doi: 10.1016/j.beth.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Morin CM, Blais F, Savard J. Are changes in beliefs and attitudes about sleep related to sleep improvements in the treatment of insomnia? Behav Res Ther. 2002;40(7):741–752. doi: 10.1016/s0005-7967(01)00055-9. [DOI] [PubMed] [Google Scholar]
- 31.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65(2):259–267. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 32.Parmelee PA, Tighe CA, Dautovich ND. Sleep disturbance in osteoarthritis: linkages with pain, disability, and depressive symptoms. Arthritis Care Res (Hoboken) 2015;67(3):358–365. doi: 10.1002/acr.22459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60(2):91–97. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive Behavioral Therapy for Insomnia: A session by Session Guide. New York: Springer; 2005. [Google Scholar]
- 35.Petrov ME, Goodin BR, Cruz-Almeida Y, King C, Glover TL, Bulls HW, Herbert M, Sibille KT, Bartley EJ, Fessler BJ, Sotolongo A, Staud R, Redden D, Fillingim RB, Bradley LA. Disrupted sleep is associated with altered pain processing by sex and ethnicity in knee osteoarthritis. J Pain. 2015;16(5):478–490. doi: 10.1016/j.jpain.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9(5):745–758. doi: 10.1586/ERN.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radloff LS. A self-reported depression scale for research in the general population. Applied Psychological Measures. 1977;1:385–401. [Google Scholar]
- 38.Salwen JK, Smith MT, Finan PH. Mid-Treatment Sleep Duration Predicts Clinically Significant Knee Osteoarthritis Pain reduction at 6 months: Effects From a Behavioral Sleep Medicine Clinical Trial. Sleep. 2017;40(2):zsw064–zsw064. doi: 10.1093/sleep/zsw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith MT, Finan PH, Buenaver LF, Robinson M, Haque U, Quain A, McInrue E, Han D, Leoutsakis J, Haythornthwaite JA. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: a randomized, double-blind, active placebo-controlled clinical trial. Arthritis & rheumatology (Hoboken, NJ) 2015;67(5):1221–1233. [Google Scholar]
- 40.Smith MT, Perlis ML, Carmody TP, Smith MS, Giles DE. Presleep cognitions in patients with insomnia secondary to chronic pain. J Behav Med. 2001;24(1):93–114. doi: 10.1023/a:1005690505632. [DOI] [PubMed] [Google Scholar]
- 41.Smith MT, Perlis ML, Smith MS, Giles DE, Carmody TP. Sleep Quality and Presleep Arousal in Chronic Pain. J Behav Med. 2000;23(1):1–13. doi: 10.1023/a:1005444719169. [DOI] [PubMed] [Google Scholar]
- 42.Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: a conceptual model. Curr Pain Headache Rep. 2009;13(6):447–454. doi: 10.1007/s11916-009-0073-2. [DOI] [PubMed] [Google Scholar]
- 43.Somers TJ, Blumenthal JA, Guilak F, Kraus VB, Schmitt DO, Babyak MA, Craighead LW, Caldwell DS, Rice JR, McKee DC, Shelby RA, Campbell LC, Pells JJ, Sims EL, Queen R, Carson JW, Connelly M, Dixon KE, Lacaille LJ, Huebner JL, Rejeski WJ, Keefe FJ. Pain coping skills training and lifestyle behavioral weight management in patients with knee osteoarthritis: a randomized controlled study. Pain. 2012;153(6):1199–1209. doi: 10.1016/j.pain.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Somers TJ, Keefe FJ, Pells JJ, Dixon KE, Waters SJ, Riordan PA, Blumenthal JA, McKee DC, LaCaille L, Tucker JM, Schmitt D, Caldwell DS, Kraus VB, Sims EL, Shelby RA, Rice JR. Pain catastrophizing and pain-related fear in osteoarthritis patients: relationships to pain and disability. J Pain Symptom Manage. 2009;37(5):863–872. doi: 10.1016/j.jpainsymman.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;(7):524–532. [Google Scholar]
- 46.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Sun MM, Beier F, Pest MA. Recent developments in emerging therapeutic targets of osteoarthritis. Curr Opin Rheumatol. 2017;29(1):96–102. doi: 10.1097/BOR.0000000000000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang NK, Goodchild CE, Salkovskis PM. Hybrid cognitive-behaviour therapy for individuals with insomnia and chronic pain: a pilot randomised controlled trial. Behav Res Ther. 2012;50(12):814–821. doi: 10.1016/j.brat.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Thorn BE, Day MA, Burns J, Kuhajda MC, Gaskins SW, Sweeney K, McConley R, Ward LC, Cabbil C. Randomized trial of group cognitive behavioral therapy compared with a pain education control for low-literacy rural people with chronic pain. Pain. 2011;152(12):2710–2720. doi: 10.1016/j.pain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turner JA, Mancl L, Aaron LA. Pain-related catastrophizing: a daily process study. Pain. 2004;110(1–2):103–111. doi: 10.1016/j.pain.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5(4):355–362. [PMC free article] [PubMed] [Google Scholar]
- 52.Wertli MM, Eugster R, Held U, Steurer J, Kofmehl R, Weiser S. Catastrophizing-a prognostic factor for outcome in patients with low back pain: a systematic review. The spine journal : official journal of the North American Spine Society. 2014;14(11):2639–2657. doi: 10.1016/j.spinee.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Wilcox S, Brenes GA, Levine D, Sevick MA, Shumaker SA, Craven T. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J Am Geriatr Soc. 2000;48(10):1241–1251. doi: 10.1111/j.1532-5415.2000.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 54.Witvrouw E, Pattyn E, Almqvist KF, Crombez G, Accoe C, Cambier D, Verdonk R. Catastrophic thinking about pain as a predictor of length of hospital stay after total knee arthroplasty: a prospective study. Knee Surg Sports Traumatol Arthrosc. 2009;17(10):1189–1194. doi: 10.1007/s00167-009-0817-x. [DOI] [PubMed] [Google Scholar]