Abstract
Objective
To review temporary percutaneous mechanical circulatory support devices (pMCS) for the treatment of cardiogenic shock, including current evidence, contraindications, complications, and future directions.
Data Sources
A MEDLINE search was conducted with MeSH terms: cardiogenic shock, percutaneous mechanical circulatory support, extracorporeal membrane oxygenation (ECMO), Impella, and TandemHeart.
Study Selection
Selected publications included randomized controlled trial data and observational studies describing experience with pMCS in cardiogenic shock.
Data Extraction
Studies were chosen based on strength of association with and relevance to cardiogenic shock.
Data synthesis
Until recently, there were few options if cardiogenic shock was refractory to vasopressors or intra-aortic balloon pump (IABP) counterpulsation. Now, several pMCS devices, including Impella®, TandemHeart™, and ECMO, are more accessible. Compared with IABP, Impella provides greater hemodynamic support, but no reduction in mortality. Similarly, TandemHeart improves hemodynamic variables, but not survival. Comparative studies have been underpowered for mortality because of small sample size. Veno-arterial ECMO offers the advantage of biventricular circulatory support and oxygenation but there are significant vascular complications. Comparative studies with ECMO have not been completed. Despite lack of randomized controlled data, there has been a substantial increase in use of pMCS. Several ongoing prospective studies with larger sample sizes may provide answers, and newer devices may become smaller, easier to insert, and more effective.
Conclusions
Mortality from cardiogenic shock remains unacceptably high despite early coronary revascularization or other therapies. While evidence is lacking and complications rates are high, improvements and experience with pMCS may offer the prospect of better outcomes.
Keywords: Cardiogenic shock, mechanical circulatory support, percutaneous ventricular assist device
Introduction
Despite modern advances, cardiogenic shock still occurs in over 8% of ST-segment elevation myocardial infarction (MI) with mortality nearing 50%.1–3 Until recently, an intensivist’s armamentarium consisted of vasoactive agents and intra-aortic balloon pump (IABP) counterpulsation, neither of which have demonstrated mortality benefit in cardiogenic shock.4–7 Although introduction of IABP counterpulsation was hailed as a major advance, there was no mortality benefit at 30-day or 12 month follow-up in a major randomized controlled trial of IABP vs. medical therapy in 600 subjects eligible for revascularization (IABP-SHOCK II).6,8 The IABP-SHOCK II trial has been criticized because of a high cross-over rate, relatively smaller sample size, timing of IABP insertion, and lower mortality (40%) than reported earlier. Notably, there were positive trends in certain subsets, that some hypothesize could benefit from IABP support.9 Nevertheless, the recommendation for IABP use has been downgraded from Class I to IIa in the United States (US) and European guidelines. Percutaneous mechanical circulatory support (pMCS) carries a Class IIb recommendation.10,11
Similar to the IABP, short-term pMCS has not shown improved outcomes in cardiogenic shock.12 Despite this, use of percutaneous ventricular assist devices (pVADs) has increased 30-fold between 2007 and 2012, including in patients undergoing percutaneous coronary intervention (PCI) for acute MI (AMI) without cardiogenic shock.13,14 With so many options and little data, proper guidance has been lacking for clinicians, but is being developed.15 This review focuses on short-term advanced pMCS for cardiogenic shock, including current evidence, contraindications, complications, trends, and future directions.
Study Design
This review describes the current use of Impella® and TandemHeart™ devices, termed pVADs, as well as extracorporeal membrane oxygenation (ECMO), collectively termed pMCS. A MEDLINE search was performed with MeSH terms cardiogenic shock, percutaneous mechanical circulatory support, extracorporeal membrane oxygenation (ECMO), Impella, and TandemHeart. Randomized controlled trial data and observational studies were selected based on use of pMCS in cardiogenic shock. The IABP has been extensively reviewed elsewhere and was excluded.
Circulatory Support Devices
The common goal of pMCS devices is to improve cardiac function while awaiting reversal of the cause of cardiogenic shock.
Impella®
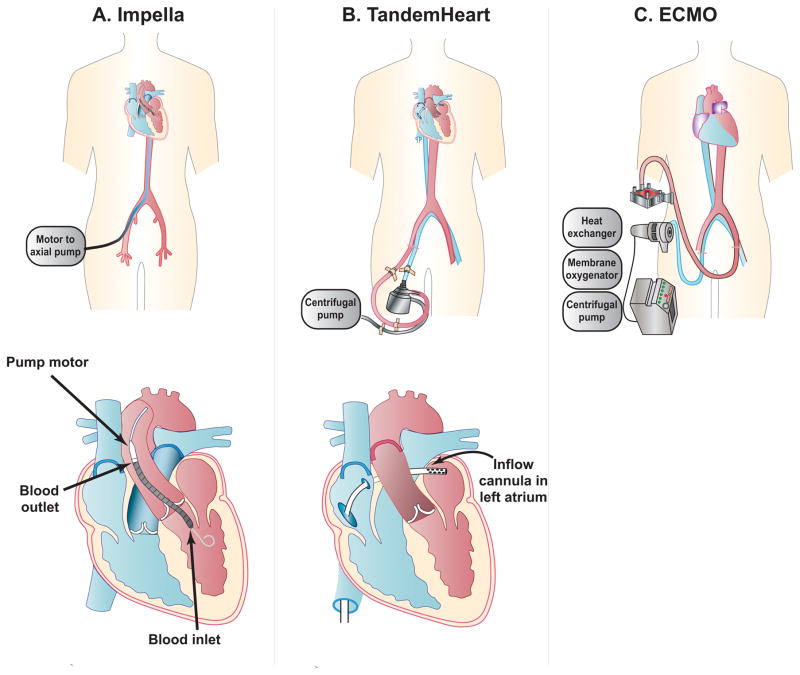
The Impella (Abiomed, Inc., Danvers, Massachusetts) devices are intracardiac pumps (Figure 1A). They produce nonpulsatile, axial-flow designed to pump blood from the left ventricle (LV) into the ascending aorta; Impella 2.5 L/min, Impella CP (Cardiac Power), and Impella 5.0 L/min (Table 1).15 In addition, the Impella RP (Right Percutaneous) is available for treatment of right heart failure.16 The Impella CP provides an intermediate level of support of 3.0–4.0 L/min of blood flow and is now available in the US.17 The smaller devices are inserted percutaneously via the femoral artery, or infrequently the axillary artery, and are advanced retrograde across the aortic valve. The larger Impella 5.0 has required a surgical cut down,18 but transcaval access is a novel approach designed to facilitate placement of large devices in patients ineligible for femoral artery access.19
Figure 1. Percutaneous Mechanical Circulatory Support Devices.
Schematic diagrams of percutaneous ventricular assist devices for cardiogenic shock including (A) Impella catheter; (B) TandemHeart; and (C) ECMO (extracorporeal membrane oxygenation).
Table 1.
Characteristics of peripheral mechanical circulatory support devices.
| Parameter | IABP | Impella 2.5 | Impella CP | Impella 5.0 | TandemHeart | ECMO |
|---|---|---|---|---|---|---|
| Blood flow (L/min) | 0.5–1.0 | 2.5 | 4.0 | 5.0 | 3.5–5.0 | ≥ 6.0 |
| Cannula size (Fr) | 7.0–8.0 | 12 | 14 | 21 | 21 venous, 15–19 arterial | 17–24 venous, 16–21 arterial |
| Insertion | Percutaneous | Percutaneous | Percutaneous | Surgical cutdown† | Percutaneous | Percutaneous and surgical |
| Insertion Time | + | 2+ | 2+ | 4+ | 4+ | 2+ |
| Biventricular Support | − | − | − | − | −* | + |
| Anticoagulation | +/− | + | + | + | + | + |
| Hemolysis | 1+ | 2+ | 2+ | 2+ | 2+ | 2+ |
| Limb Ischemia | 1+ | 2+ | 2+ | 2+ | 3+ | 3+ |
| Risk of Bleeding | 1+ | 1+ | 2+ | 2+ | 3+ | 3+ |
| Vascular Injury or stroke | 1+ | 1+ | 2+ | 2+ | 2+ | 2+ |
| Management complexity | + | 2+ | 2+ | 2+ | 4+ | 3+ |
Key: CP, Cardiac Power; ECMO, extracorporeal membrane oxygenation; Fr, French gauge; IABP, Intra-aortic balloon pump.
Placement in the right atrium and pulmonary artery can provide right ventricular support.
or transcaval access if ineligible for femoral artery access.
Unlike the IABP which decreases LV pressures and increases stroke volume (Figure 2A), Impella devices entrain blood from the LV to pump into the aorta in series, thus unloading the LV and reducing myocardial oxygen consumption and demand. The resulting decrease in LV pressures and volumes decreases cardiac workload (Figure 2B).15,20 Since Impella is positioned in the LV, there are obvious contraindications including significant aortic valve disease, mechanical aortic valve and LV thrombus.15,21
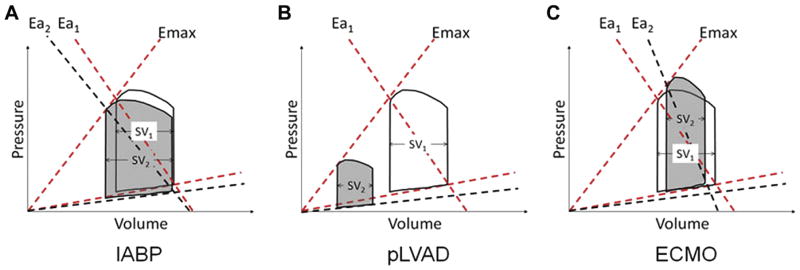
Figure 2. Pressure Volume Loops of Percutaneous Mechanical Circulatory Support Devices.
Cardiac effects of mechanical support. Illustrations of pressure volume (PV) loops before (non-shaded loops) and after activation of device therapy (shaded loops). Emax is load-independent contractility, defined as the maximal slope of the end-systolic PV point under various loading conditions. (A) Intra-aortic balloon pump (IABP) counterpulsation reduces both peak LV systolic and diastolic pressures and increases LV stroke volume. The net effect is a reduced slope of arterial elastance (from Ea1 to Ea2), (B) Percutaneous LV assist devices (pLVAD: Impella and TandemHeart) significantly reduce LV pressures, LV volumes, and LV stroke volume. The net effect is a significant reduction in cardiac workload. (C) Venoarterial Extra-corporeal Membrane Oxygenation (VA-ECMO) without a LV venting strategy increases LV systolic and diastolic pressure, while reducing LV stroke volume. The net effect is an increase in arterial elastance (from Ea1 to Ea2).(Reprinted from J Card Fail; Rihal CS, Naidu SS, Givertz MM, et al: SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care. 21; 499–518, 2015 with permission from Elsevier) 15
Key: Ea, arterial elastance; IABP = Intra-aortic balloon pump; ECMO = extracorporeal membrane oxygenation; pLVAD = percutaneous left ventricular assist device; SV, stroke volume.
There are two small randomized controlled trials comparing Impella and IABP for patients with cardiogenic shock (Table 2).16,17,22 One study randomized patients with AMI and cardiogenic shock to Impella 2.5 (n=12) vs. IABP counterpulsation (n=13). Compared with IABP, the Impella group had higher cardiac index (0.49L/min/m2 vs.0.11 L/min/m2; p=0.02) at 30 minutes after implantation, but 30-day mortality was roughly 50% in each group (p=0.97).22 More recently, Impella CP was compared with IABP in 48 patients with shock after AMI. Thirty-day mortality was about 50% in each group (p=0.92).17 Neither study was powered to detect differences in mortality. These trial data were combined with data from a study of Impella 2.5 vs. IABP in 21 subjects with cardiogenic pre-shock.23 The resultant meta-analysis reported no difference in mortality at 30 days (RR 0.99, [CI 0.62–1.58]; p=0.95) or 6 months (RR 1.15, [0.74–1.48]; p=0.53).24
Table 2.
Randomized controlled trials of percutaneous ventricular assist devices (pVAD) compared to intra-aortic balloon (IABP) counterpulsation for cardiogenic shock.
| Study | Date | Condition | Device | Control | Total Sample Size | Primary Outcome | Mortality at 30 days IABP vs. pVAD |
|---|---|---|---|---|---|---|---|
| Ouweneel et al17 | 2017 | Cardiogenic Shock | Impella CP n=24 |
IABP n=24 |
48 | 30-day mortality | 50% vs. 46%** |
| Ouweneel et al23 | 2016 | Cardiogenic Pre-Shock | Impella 2.5 n=12 |
IABP n=9 |
21 | LV ejection fraction at 4 months | 11% vs. 25% ** |
| Seyfarth et al22 | 2008 | Cardiogenic Shock | Impella 2.5 n=12* |
IABP n=13 |
26 | Cardiac index | 46% vs. 46%** |
| Thiele et al32 | 2005 | Cardiogenic Shock | TandemHeart n=21 |
IABP n=20 |
41 | Cardiac power index | 45% vs. 43%** |
| Burkhoff et al33 | 2006 | Cardiogenic Shock | TandemHeart n=19 |
IABP n=14 |
33 | Hemodynamic improvement | 64% vs. 53%** |
Key: CP, Cardiac Power; IABP, intra-aortic balloon pump; LV, left ventricular;
one patient died prior to implant;
Not significant
In 154 consecutive “real-world” patients from the USpella Registry with cardiogenic shock undergoing PCI, those receiving Impella 2.5 pre-PCI had reduced mortality compared to those receiving the device post-PCI (40.7% vs. 65.1%; p=0.003). Almost 90% of these patients had failed inotropes and/or IABP, and about 38% of them would have been considered too ill to be included in the IABP-SHOCK II trial.6,25
There are few studies, mostly non-randomized trials, assessing the utility of Impella 5.0. In a retrospective single center review comparing mortality in 34 patients with cardiogenic shock who received the Impella 2.5 or 5.0, the Impella 5.0 group had higher 30-day survival (33% (3/9) vs. 24% (6/25)). However, the study was not randomized or blinded and there were crossovers to Impella 5.0.26 Several other single center, retrospective studies have shown more favorable outcomes for cardiogenic shock when treated with Impella 5.0.27 In a multicenter, prospective, feasibility study without a control group, Impella 5.0 was used in 16 patients with postcardiotomy cardiogenic shock with 94% survival at 30 days.28
TandemHeart™
The TandemHeart (CardiacAssist, Inc., Pittsburgh, PA) is a continuous flow centrifugal extracorporeal assist device, withdrawing oxygenated blood from the left atrium and returning it to the femoral artery (Figure 1B). The inflow cannula is inserted percutaneously, via the femoral vein and advanced into the left atrium. This procedure requires a catheterization laboratory and experience in trans-septal puncture.21 Oxygenated blood is pumped through a femoral artery cannula at blood flow rates of 3.5–5.0 L/min depending on cannula size.29 Although TandemHeart can also support the right ventricle with placement of the inflow cannula in the right atrium and outflow cannula in the main pulmonary artery, this indication is not approved by the US Food and Drug Administration.15
Hemodynamic benefits include near-systemic blood flow rates, improved mean arterial pressure, and reduction in the pulmonary artery occlusion pressure.30 In simulated models, TandemHeart provides support intermediate between Impella 2.5 and Impella 5.0.21 Similar to Impella, TandemHeart reduces cardiac workload by decreasing LV pressures and volumes (Figure 2B),15 although, placement high in the aorta could increase afterload and offset LV unloading,31 especially in low cardiac output states.21 Contraindications include intracardiac thrombus and ventricular septal defect.15
In a review of 117 patients with refractory cardiogenic shock despite IABP counterpulsation or high-dose vasopressor support, insertion of TandemHeart improved hemodynamics significantly, and was associated with 40.2% 30-day mortality. This was lower than anticipated given that nearly half underwent cardiopulmonary resuscitation (CPR) preceding TandemHeart insertion.29 Two randomized studies have compared TandemHeart to IABP in patients with AMI complicated by cardiogenic shock.32,33 Each reported improved hemodynamic parameters but more complications in TandemHeart compared with IABP. Neither study reported a statistically significant difference in 30-day mortality. A subsequent meta-analysis that included the TandemHeart studies and the first Impella 2.5 randomized trial reported no mortality difference at 30-days (RR 1.06, 95% CI 0.68–1.66).12 The total population was small (n=100) and limited by heterogeneity in outcomes, likely due to combination of two different types of pVADs in the analyses.
Extracorporeal Membrane Oxygenation
Initially developed in the 1970s, ECMO has experienced recent improvements in technology, as well as a rise in cardiopulmonary usage.34 Venoarterial (VA)-ECMO (Figure 1C) includes a centrifugal (nonpulsatile) pump, heat exchanger, and membrane oxygenator allowing for full biventricular support (≥ 6 L/min) and gas exchange.35 VA-ECMO involves peripheral cannulation via the femoral vein and artery or centrally with cannulation of the right atrium and ascending aorta. Given the large diameter of the cannulas, reperfusion lines are often placed to allow blood flow distal to the insertion sites.36 Previously, ECMO was initiated in the operating room, but more recently, percutaneous cannulation has been performed at the bedside.35 While standard care includes a large, multidisciplinary team, initiation has occurred safely at remote institutions before transport to ECMO centers.37
Removal of venous blood reduces cardiac preload. Simultaneously, reinfusion of blood through the arterial cannula increases mean arterial pressure by increasing both systolic and diastolic pressures (Figure 2C).15 Depending on native LV function, the increase in afterload with ECMO can elevate left heart filling pressures.35 Several strategies to assist LV decompression while on ECMO include inotropic support or implantation of an Impella device or IABP or other LV venting plan.36,38
The evidence for the utility of ECMO in patients with cardiogenic shock is scant and there are no randomized controlled studies comparing ECMO with pVADs. In a retrospective review comparing patients with cardiogenic shock who received a pVAD (TandemHeart or Impella 5.0, n=18) vs. ECMO (n=61), there was no significant difference in rates of long-term support, complications, or in-hospital mortality.39 A recent meta-analysis of cohort studies found that patients treated with ECMO had a higher 30-day survival compared with IABP (p<0.001, NNT 13), but no difference when compared with pVADs (p=0.70).40 Another retrospective case series reported lower 30-day mortality in patients with cardiogenic shock undergoing ECMO-assisted PCI vs. ECMO-unassisted PCI (39.1% vs. 72%, respectively). However, the ECMO-unassisted group was a historic control group (1993–2002) and likely received a different standard of care.41
With the ability to deploy at the bedside and transport, another growing use of ECMO is in refractory cardiac arrest, termed extracorporeal CPR (E-CPR). The prospective, single-center CPR, Hypothermia, ECMO, and Early Reperfusion (CHEER) trial included 26 patients with refractory in-hospital and out-of-hospital cardiac arrest. Intensivists performed ECMO cannulation. Return of spontaneous circulation and full neurologic recovery occurred in 96% and 54% of patients, respectively.42
Complications
Given the large bore cannulas and need for systemic anticoagulation, there are many complications common to pMCS devices including limb ischemia, bleeding, vascular injury, infection, thromboembolic events, and hemolysis.15 Common contraindications include severe peripheral vascular disease, significant aortic valve disease, and the inability to tolerate systemic anticoagulation.
In general, Impella devices are associated with the most hemolysis amongst pMCS. If hemolysis persists and results in acute kidney injury, device removal should be considered. Alternatively, if hemodynamically tolerable, decreasing device motor speed may reduce the degree of hemolysis.23,43,44 Data from the USpella Registry and Impella-EUROSHOCK-registry estimate the frequency of hemolysis at 5.0–10%.25,44 Rarely, the Impella devices have been associated with LV perforation. When proper techniques are used, arterial complications of Impella 2.5 are similar to IABP.45 With the exception of vascular injury, complications rates for Impella CP and 5.0 are reportedly comparable with Impella 2.5.16,17,27
Proper placement of Impella is critically important, and requires close monitoring as migration can occur. Suction alarms suggesting inadequate blood volume for pump may indicate migration to the LV apex, but can also occur for other reasons, including acute hypovolemia from bleeding, dehydration, or RV failure. If a suction event occurs, the patient should be assessed for hypovolemia as well as device location by echocardiogram or fluoroscopy.45 Unique to Impella is a purge cassette with heparin solution, which is designed to prevent blood from entering the motor. Purge alarms can signal various complications along the system, including leakage, air, blood in the motor, or tube kinking.45
Due to cannula size, TandemHeart and ECMO are most often associated with limb ischemia, bleeding, and vascular injury.43 In a small trial comparing TandemHeart to IABP in cardiogenic shock, the TandemHeart group had more bleeding (42.1%vs.14.3%) and limb ischemia (21.1%vs.14.3%).32 Another study described development of disseminated intravascular coagulation in nearly all patients with TandemHeart after two or more days.33 Unique to TandemHeart is the possibility of residual atrial septal defect due to the trans septal approach.43 Dislodgement of the device into the right atrium can cause massive shunting, with deoxygenated blood being delivered to the arterial system.15 In a meta-analysis of the three randomized trials (n=100) comparing pVADs (Impella 2.5 and TandemHeart) to IABP for cardiogenic shock, the pVAD group had more bleeding (RR 2.35 [1.40–3.93], p<0.01) and a trend towards more limb ischemia (RR 2.59 [0.75–8.97]).12 Also, unlike ECMO, pVADs do not provide biventricular support, so in biventricular failure, LV pVAD insertion could lead to RV volume overload and worsening RV failure with a subsequent reduction in LV cardiac output.
Hemorrhage at multiple locations, including cannulation sites as well as the neurologic and pulmonary systems, remains one of the most devastating complications of ECMO.35,46,47 In an analysis of almost 1900 patients with cardiogenic shock or cardiac arrest requiring ECMO, complication rates were 40.8% for major bleeding; 16.9% for lower extremity ischemia; 5.9% for stroke; 30.4% for significant infection; and varying rates for both venous and arterial thrombus.47 Renal complications are also exceedingly common in patients on ECMO. Estimates of the incidence of acute kidney injury are as high as 80% with a subsequent increased mortality. Many patients progress to renal failure requiring continuous renal replacement therapy.48 However, the relationship is complex and multiple factors may contribute to worsening renal function including concomitant therapies, overall severity of illness or ECMO itself.
Femoral artery placement of the arterial cannula directs blood flow in a retrograde direction towards the heart. If native cardiac function is poor, the ECMO output perfuses both the cerebral and coronary circulation, regardless of pulmonary function. If cardiac function is robust or recovers, a watershed mixing point can develop opposite the ECMO flow. Supplying the heart with poorly oxygenated blood from the lungs could lead to hypoxemia to the upper half of the body, also known as North-South or Harlequin Syndrome. Oxygenation of the cerebral and coronary beds can be checked by sampling the right radial artery.35 Also, given the potential for LV over distension and worsening pulmonary edema with retrograde ECMO flow, there is increased risk of acute lung injury.49
Current Trends
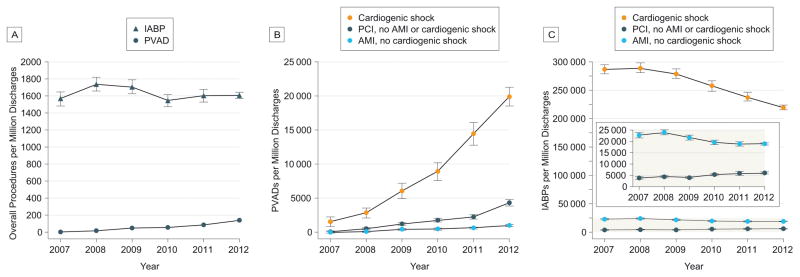
While studies have shown an improvement in hemodynamics with pMCS, there is no clear survival benefit.12,16,22,32,33 There is also lack of uniformity amongst professional societal guidelines.10,15 Nevertheless, data from the National Inpatient Sample reports that pVAD use has increased 30-fold from 2007 to 2011 (Figure 3).13,50 Further, the number of hospitals with an annual volume of 10 or more pVADs increased from 0 in 2007 to 102 in 2011 (p for trend <0.001). When compared with IABP, this increase in pVAD use was associated with a substantially increased cost.13 Yet, by comparison with surgically implanted hemodynamic support devices such as ECMO, analysis of a national administrative database (MedPAR) suggests that use of percutaneous VADs in cardiogenic shock are associated with lower cost (p<0.001).51 They also reported better survival and shorter length of stay with pVADS, but interpretation of these results are limited by the study’s observational design.
Figure 3. Calendar Year Trends in the Use of Percutaneous Ventricular Assist Devices and Intra-aortic Balloon Pumps in the United States, 2007 – 2012.
Estimated use of PVADs and IABPs per million discharges (± standard errors). A. Use of PVADs increased from 4.6 per million (2007) to 138 per million (2012; P for trend < .001). Use of IABPs decreased from 1738 per million (2008) to 1608 per million (2012; P for trend = .02). B. Use of PVADs increased in patients with cardiogenic shock, AMI without cardiogenic shock, and PCI without AMI or cardiogenic shock (P for trend < .001 for all). C. Use of IABPs decreased in patients with cardiogenic shock and AMI without cardiogenic shock but increased in patients who underwent PCI without cardiogenic shock or AMI (P for trend < .001 for all).
Reproduced with permission from JAMA Intern Med 2015; 175:941–950.13 Copyright© 2015 American Medical Association. All rights reserved.
Key: AMI, acute myocardial infarction; IABP, intra-aortic balloon pump; PCI, percutaneous coronary intervention; PVAD, percutaneous left ventricular assist device.
Gaps in Knowledge
Given the absence of a properly powered study to detect discrete outcomes like mortality, the true benefit or harm of temporary pMCS is unknown. In addition to small patient sample sizes, current studies have failed to detect differences in outcomes due to variance in timing of implantation, device management, and patient selection, as well as inherent difficulties in studying this population.23,52
As centers increase their yearly volume of pMCS implantation and multidisciplinary teams gain experience, complications rates have decreased.53 Analogous to door-to-balloon time, proper timing of intervention or “door-to-unloading” time has been suggested to prevent the progressive spiral of myocardial dysfunction seen in cardiogenic shock.3,43 Finally, the utility of employing temporary pMCS to improve candidacy of more durable VADs is unclear.
Device Selection
Once a decision to deploy mechanical support has been made for cardiogenic shock, the device should be inserted without delay to prevent progressive myocardial dysfunction. As discussed, definitive evidence for choice of device is lacking. Device selection should include assessment of familiarity, cost, consideration for right heart failure, degree of support needed, and institutional capabilities. In the absence of severe biventricular failure, the IABP remains a reasonable first option, because of lower cost, clinician familiarity, and option for bedside insertion. Consideration for pVAD becomes more practical in the setting of the cardiac catheterization laboratory. Although more expensive, with experience an Impella insertion may be as rapid as the IABP and is becoming first line for some centers.15 For patients with biventricular failure, concomitant respiratory failure, or cardiac arrest, ECMO is the best choice in experienced centers.
Future Directions
There are ongoing trials with higher patient enrollment, which could provide more answers. One notable trial for cardiogenic shock is the Danish Cardiogenic Shock Trial (DanShock; NCT01633502), which plans to randomize 360 patients to either the Impella CP or conventional circulatory support. Another is the ExtraCorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock (ECMO-CS; NCT02301819) trial with an estimated enrollment of 120 patients randomized to VA-ECMO or early conservative therapy.
Although great technological strides have occurred, future devices aim to be smaller and more powerful with faster insertion at the bedside and fewer complications. Currently in development, the i-cor system (Xenios AG, Heilbronn, Germany) is similar to an ECMO circuit and provides up to 8 L/min of blood flow. Novel to the i-cor device, continuous flow or diastolic augmentation with ECG-triggered pulsatile flow can be provided. The HeartMate PHP (Percutaneous Heart Pump, St. Jude, St. Paul, MN) is an axial flow circulatory device, which expands when across the aortic valve and provides up to 5 L/min of blood flow. It is currently being compared to the Impella 2.5 in high risk PCI patients. The Reitan Catheter Pump (CardioBridge GmbH, Hechingen, Germany), placed in the descending thoracic aorta distal to the subclavian artery, creates a pressure gradient similar to the IABP counterpulsation resulting in decreased afterload and increased perfusion distally. Also positioned in the descending aorta, the Aortix device (Procyrion Inc., Houston, TX) has expanding anchors and a transcutaneous charger allowing for sheath removal and potentially provides durable support.36
Conclusion
The prognosis for patients with cardiogenic shock remains poor despite current therapy. Temporary pMCS offers the opportunity to improve these outcomes, but still requires large studies powered to evaluate mortality as well as continued improvements in technology to decrease complications.
Acknowledgments
Funding: This work was supported by the National Institutes of Health Clinical Center.
We thank Judith Welsh, BSN, MLS, Clinical Informationist, NIH Library, National Institutes of Health for her assistance with literature searches. We also thank Ms. Kelly Byrne, NIH Clinical Center, for editorial assistance.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
Copyright form disclosure: All authors disclosed receiving support for article research from the National Institutes of Health.
References
- 1.Babaev A, Frederick PD, Pasta DJ, et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294(4):448–454. doi: 10.1001/jama.294.4.448. [DOI] [PubMed] [Google Scholar]
- 2.Jeger RV, Radovanovic D, Hunziker PR, et al. Ten-year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med. 2008;149(9):618–626. doi: 10.7326/0003-4819-149-9-200811040-00005. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117(5):686–697. doi: 10.1161/CIRCULATIONAHA.106.613596. [DOI] [PubMed] [Google Scholar]
- 4.Sjauw KD, Engstrom AE, Vis MM, et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J. 2009;30(4):459–468. doi: 10.1093/eurheartj/ehn602. [DOI] [PubMed] [Google Scholar]
- 5.Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015;36(20):1223–1230. doi: 10.1093/eurheartj/ehv051. [DOI] [PubMed] [Google Scholar]
- 6.Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 7.Unverzagt S, Buerke M, de Waha A, et al. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev. 2015;(3):CD007398. doi: 10.1002/14651858.CD007398.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382(9905):1638–1645. doi: 10.1016/S0140-6736(13)61783-3. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor CM, Rogers JG. Evidence for overturning the guidelines in cardiogenic shock. N Engl J Med. 2012;367(14):1349–1350. doi: 10.1056/NEJMe1209601. [DOI] [PubMed] [Google Scholar]
- 10.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):529–555. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 11.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(3):267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 12.Cheng JM, den Uil CA, Hoeks SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009;30(17):2102–2108. doi: 10.1093/eurheartj/ehp292. [DOI] [PubMed] [Google Scholar]
- 13.Khera R, Cram P, Lu X, et al. Trends in the use of percutaneous ventricular assist devices: analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med. 2015;175(6):941–950. doi: 10.1001/jamainternmed.2014.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandhu A, McCoy LA, Negi SI, et al. Use of mechanical circulatory support in patients undergoing percutaneous coronary intervention: insights from the National Cardiovascular Data Registry. Circulation. 2015;132(13):1243–1251. doi: 10.1161/CIRCULATIONAHA.114.014451. [DOI] [PubMed] [Google Scholar]
- 15.Rihal CS, Naidu SS, Givertz MM, et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care (Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention) J Card Fail. 2015;21(6):499–518. doi: 10.1016/j.cardfail.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Anderson MB, Goldstein J, Milano C, et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant. 2015;34(12):1549–1560. doi: 10.1016/j.healun.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2017;69(3):278–287. doi: 10.1016/j.jacc.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Gaudard P, Mourad M, Eliet J, et al. Management and outcome of patients supported with Impella 5.0 for refractory cardiogenic shock. Crit Care. 2015;19:363. doi: 10.1186/s13054-015-1073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frisoli TM, Guerrero M, O’Neill WW. Mechanical Circulatory Support With Impella to Facilitate Percutaneous Coronary Intervention for Post-TAVI Bilateral Coronary Obstruction. Catheter Cardio Inte. 2016;88(1):E34–E37. doi: 10.1002/ccd.26075. [DOI] [PubMed] [Google Scholar]
- 20.Kapur NK, Qiao X, Paruchuri V, et al. Mechanical Pre-Conditioning With Acute Circulatory Support Before Reperfusion Limits Infarct Size in Acute Myocardial Infarction. JACC Heart Fail. 2015;3(11):873–882. doi: 10.1016/j.jchf.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Naidu SS. Novel percutaneous cardiac assist devices: the science of and indications for hemodynamic support. Circulation. 2011;123(5):533–543. doi: 10.1161/CIRCULATIONAHA.110.945055. [DOI] [PubMed] [Google Scholar]
- 22.Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52(19):1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 23.Ouweneel DM, Engstrom AE, Sjauw KD, et al. Experience from a randomized controlled trial with Impella 2.5 versus IABP in STEMI patients with cardiogenic pre-shock. Lessons learned from the IMPRESS in STEMI trial. Int J Cardiol. 2016;202:894–896. doi: 10.1016/j.ijcard.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 24.Ouweneel DM, Eriksen E, Seyfarth M, Henriques JP. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump for Treating Cardiogenic Shock: Meta-Analysis. J Am Coll Cardiol. 2017;69(3):358–360. doi: 10.1016/j.jacc.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 25.O’Neill WW, Schreiber T, Wohns DH, et al. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol. 2014;27(1):1–11. doi: 10.1111/joic.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engstrom AE, Cocchieri R, Driessen AH, et al. The Impella 2.5 and 5.0 devices for ST-elevation myocardial infarction patients presenting with severe and profound cardiogenic shock: the Academic Medical Center intensive care unit experience. Crit Care Med. 2011;39(9):2072–2079. doi: 10.1097/CCM.0b013e31821e89b5. [DOI] [PubMed] [Google Scholar]
- 27.Lemaire A, Anderson MB, Lee LY, et al. The Impella device for acute mechanical circulatory support in patients in cardiogenic shock. Ann Thorac Surg. 2014;97(1):133–138. doi: 10.1016/j.athoracsur.2013.07.053. [DOI] [PubMed] [Google Scholar]
- 28.Griffith BP, Anderson MB, Samuels LE, Pae WE, Jr, Naka Y, Frazier OH. The RECOVER I: a multicenter prospective study of Impella 5.0/LD for postcardiotomy circulatory support. J Thorac Cardiovasc Surg. 2013;145(2):548–554. doi: 10.1016/j.jtcvs.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 29.Kar B, Gregoric ID, Basra SS, Idelchik GM, Loyalka P. The percutaneous ventricular assist device in severe refractory cardiogenic shock. J Am Coll Cardiol. 2011;57(6):688–696. doi: 10.1016/j.jacc.2010.08.613. [DOI] [PubMed] [Google Scholar]
- 30.Thiele H, Lauer B, Hambrecht R, Boudriot E, Cohen HA, Schuler G. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation. 2001;104(24):2917–2922. doi: 10.1161/hc4901.100361. [DOI] [PubMed] [Google Scholar]
- 31.Kono S, Nishimura K, Nishina T, et al. Autosynchronized systolic unloading during left ventricular assist with a centrifugal pump. J Thorac Cardiovasc Surg. 2003;125(2):353–360. doi: 10.1067/mtc.2003.100. [DOI] [PubMed] [Google Scholar]
- 32.Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26(13):1276–1283. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]
- 33.Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152(3):469 e461–468. doi: 10.1016/j.ahj.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 34.Paden ML, Conrad SA, Rycus PT, Thiagarajan RR, Registry E. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J. 2013;59(3):202–210. doi: 10.1097/MAT.0b013e3182904a52. [DOI] [PubMed] [Google Scholar]
- 35.Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol. 2014;63(25 Pt A):2769–2778. doi: 10.1016/j.jacc.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 36.Lawson WE, Koo M. Percutaneous Ventricular Assist Devices and ECMO in the Management of Acute Decompensated Heart Failure. Clinical Medicine Insights. Cardiology. 2015;9(Suppl 1):41–48. doi: 10.4137/CMC.S19701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beurtheret S, Mordant P, Paoletti X, et al. Emergency circulatory support in refractory cardiogenic shock patients in remote institutions: a pilot study (the cardiac-RESCUE program) Eur Heart J. 2013;34(2):112–120. doi: 10.1093/eurheartj/ehs081. [DOI] [PubMed] [Google Scholar]
- 38.Pappalardo F, Schulte C, Pieri M, et al. Concomitant implantation of Impella(R) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017;19(3):404–412. doi: 10.1002/ejhf.668. [DOI] [PubMed] [Google Scholar]
- 39.Chamogeorgakis T, Rafael A, Shafii AE, Nagpal D, Pokersnik JA, Gonzalez-Stawinski GV. Which is better: a miniaturized percutaneous ventricular assist device or extracorporeal membrane oxygenation for patients with cardiogenic shock? ASAIO J. 2013;59(6):607–611. doi: 10.1097/MAT.0b013e3182a8baf7. [DOI] [PubMed] [Google Scholar]
- 40.Ouweneel DM, Schotborgh JV, Limpens J, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. 2016;42(12):1922–1934. doi: 10.1007/s00134-016-4536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheu JJ, Tsai TH, Lee FY, et al. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med. 2010;38(9):1810–1817. doi: 10.1097/CCM.0b013e3181e8acf7. [DOI] [PubMed] [Google Scholar]
- 42.Stub D, Bernard S, Pellegrino V, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial) Resuscitation. 2015;86:88–94. doi: 10.1016/j.resuscitation.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Kar B, Basra SS, Shah NR, Loyalka P. Percutaneous circulatory support in cardiogenic shock: interventional bridge to recovery. Circulation. 2012;125(14):1809–1817. doi: 10.1161/CIRCULATIONAHA.111.040220. [DOI] [PubMed] [Google Scholar]
- 44.Lauten A, Engstrom AE, Jung C, et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail. 2013;6(1):23–30. doi: 10.1161/CIRCHEARTFAILURE.112.967224. [DOI] [PubMed] [Google Scholar]
- 45.Burzotta F, Trani C, Doshi SN, et al. Impella ventricular support in clinical practice: Collaborative viewpoint from a European expert user group. Int J Cardiol. 2015;201:684–691. doi: 10.1016/j.ijcard.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 46.Lorusso R, Barili F, Mauro MD, et al. In-Hospital Neurologic Complications in Adult Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation: Results From the Extracorporeal Life Support Organization Registry. Crit Care Med. 2016;44(10):e964–972. doi: 10.1097/CCM.0000000000001865. [DOI] [PubMed] [Google Scholar]
- 47.Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97(2):610–616. doi: 10.1016/j.athoracsur.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Askenazi DJ, Selewski DT, Paden ML, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol. 2012;7(8):1328–1336. doi: 10.2215/CJN.12731211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boulate D, Luyt CE, Pozzi M, et al. Acute lung injury after mechanical circulatory support implantation in patients on extracorporeal life support: an unrecognized problem. Eur J Cardiothorac Surg. 2013;44(3):544–549. doi: 10.1093/ejcts/ezt125. discussion 549–550. [DOI] [PubMed] [Google Scholar]
- 50.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64(14):1407–1415. doi: 10.1016/j.jacc.2014.07.958. [DOI] [PubMed] [Google Scholar]
- 51.Maini B, Gregory D, Scotti DJ, Buyantseva L. Percutaneous cardiac assist devices compared with surgical hemodynamic support alternatives: cost-effectiveness in the emergent setting. Catheter Cardiovasc Interv. 2014;83(6):E183–192. doi: 10.1002/ccd.25247. [DOI] [PubMed] [Google Scholar]
- 52.Briceno N, Kapur NK, Perera D. Percutaneous mechanical circulatory support: current concepts and future directions. Heart. 2016;102(18):1494–1507. doi: 10.1136/heartjnl-2015-308562. [DOI] [PubMed] [Google Scholar]
- 53.Henriques JP, Ouweneel DM, Naidu SS, et al. Evaluating the learning curve in the prospective Randomized Clinical Trial of hemodynamic support with Impella 2.5 versus Intra-Aortic Balloon Pump in patients undergoing high-risk percutaneous coronary intervention: a prespecified subanalysis of the PROTECT II study. Am Heart J. 2014;167(4):472–479. e475. doi: 10.1016/j.ahj.2013.12.018. [DOI] [PubMed] [Google Scholar]