Abstract
Rationale
TRPM2 (Transient Receptor Potential Melastatin-2) expressed in endothelial cells (ECs) is a cation channel mediating Ca2+ entry in response to intracellular generation of adenosine diphosphoribose (ADPR), the TRPM2 ligand.
Objective
Because polymorphonuclear leukocytes (PMN) interaction with endothelial cells (ECs) generates ROS, we addressed the possible role of TRPM2 expressed in ECs in the mechanism of transendothelial migration of PMNs.
Methods and Results
We observed defective PMN transmigration in response to LPS challenge in adult mice in which the EC expressed TRPM2 is conditionally deleted (Trpm2iΔEC). PMN interaction with ECs induced the entry of Ca2+ in ECs via the EC-expressed TRPM2. Prevention of generation of ADPR in ECs significantly reduced Ca2+ entry in response to PMN activation of TRPM2 in ECs. PMNs isolated from gp91phox−/− mice significantly reduced Ca2+ entry in ECs via TRPM2 as compared to WT PMNs and failed to induce PMN transmigration. Overexpression of the ADPR insensitive TRPM2 mutant channel (C1008→A) in ECs suppressed the Ca2+ entry response. Further the forced expression of TRPM2 C1008→A mutant channel or silencing of PARP-1 in ECs of mice prevented PMN transmigration.
Conclusion
Thus, endotoxin-induced transmigration of PMNs was secondary to TRPM2-activated Ca2+ signaling and VE-cadherin phosphorylation resulting in the disassembly of adherens junctions and opening of the paracellular pathways. These results suggest blocking TRPM2 activation in ECs is a potentially important means of therapeutically modifying PMN-mediated vascular inflammation.
Keywords: TRPM2, Inflammation, PMN Transmigration, PARP-1, ADPR
INTRODUCTION
The migration of polymorphonuclear neutrophils (PMNs) across the vascular endothelial barrier to site of infection constitutes the first line of defense against invading microorganisms (1). PMN transmigration requires an orchestrated interaction between the PMNs and endothelial cells (ECs) relying on the activation of adhesion molecules to initiate PMN stoppage followed by transmigration (1, 2). PMN transmigration is essential for sustaining the inflammatory response and killing bacteria until the infection subsides. Reactive oxygen species (ROS) produced by activated PMNs are important mediators of bacterial killing and disruption of the vascular endothelial barrier in inflammatory conditions such as acute respiratory distress syndrome and ischemia/reperfusion injury (1, 2). Oxidants also increase Ca2+ entry across the EC membrane (3), and as Ca2+ entry promotes the formation of inter-endothelial gaps, elevation in intracellular Ca2+ is a key factor contributing to endothelial barrier disruption (3). Studies have also described an important role of PMNs in breaching the endothelial barrier by the formation of inter-endothelial gaps secondary to disassembly of actin filaments in ECs (4). These results support the concept that ECs themselves actively participate in mediating PMN transmigration, although the underlying signaling mechanisms remain unclear.
Transient receptor potential melastatin (TRPM)2 is a ROS-activated channel belonging to the trp superfamily family of cation channels. TRPM2, a.k.a TRPC7 and LTRPC-2, is a non-selective cation channel expressed in mammalian tissue, including endothelial cells, blood cells such as PMNs, bone marrow cells, brain, spleen, heart, liver, and lungs (5). Expression of TRPM2 in macrophages and PMNs is important in regulating the bactericidal activity of phagocytic cells and PMN migration in tissue (6, 7). The binding of the intracellular second messenger adenosine diphosphoribose (ADPR) or related molecules to the Nudix box sequence motif (NUDT9-H) in its carboxyl-terminal domain regulates the gating of TRPM2 channel after exposure to oxidants (8–10). Because the Nudix box is homologous with the pyrophosphatase, NUDT9 ADP-ribose hydrolase, TRPM2 is dubbed a “chanzyme”(5). Hydrogen peroxide (H2O2) produced in the cytosol during oxidative stress is also known to activate ADPR formation in the nucleus and mitochondria (5), and thereby activate TRPM2 (8–10).
Here we studied the possible role of TRPM2 expressed in ECs in directing the trafficking of PMNs across the endothelial barrier. Using adult mice in which TRPM2 expression in ECs is conditionally deleted (Trpm2iΔEC), we observed markedly reduced PMN transmigration, vascular inflammation, and mortality in response to endotoxemia as compared to WT mice. ROS generation by PMNs played a central role in activating the TRPM2 channel in ECs, and did so in a PARP-1 dependent manner. PARP-1 in ECs was required for the generation of ADPR. We thus demonstrated the obligatory role of TRPM2 activated Ca2+ signaling in ECs and disruption of VE-cadherin junctions in mediating PMN transmigration and vascular inflammation induced by endotoxemia.
METHODS
Please see online supplement for complete Methods.
RESULTS
Deletion of TRPM2 in ECs (Trpm2iΔEC) prevents tissue PMN sequestration, inflammation, and mortality induced by endotoxemia
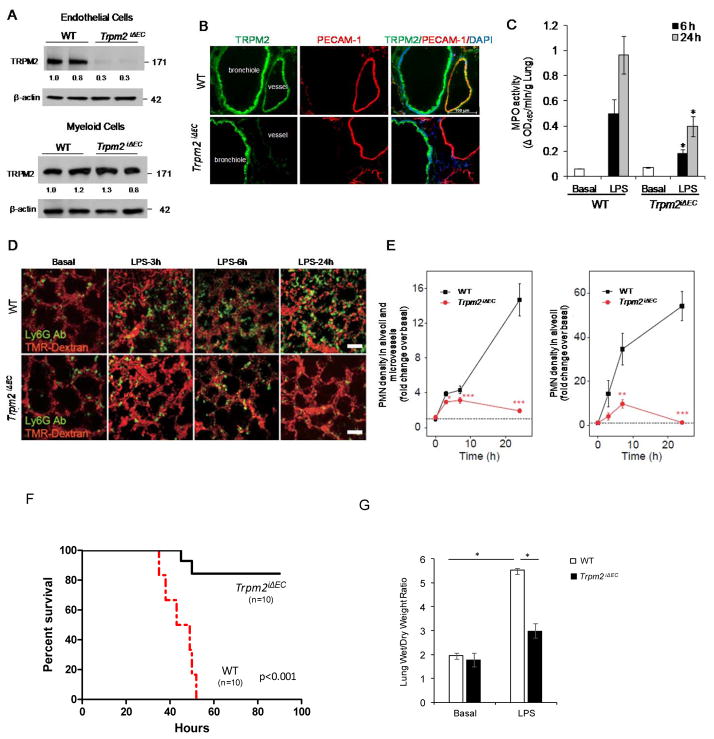
To investigate the role of TRPM2 expressed in ECs in recruiting PMNs and mediating vascular inflammation, we generated tamoxifen inducible EC-specific TRPM2 knockout mice (Trpm2iΔEC). Mice carrying floxxed Trpm2 gene were bred with End-SCL-Cre-ER(T) transgenic mice (11) (Supplemental Data). Tamoxifen treatment of these mice for 5 days (1mg/day i.p.) induced Trpm2 depletion in ECs but not in other cell types (Fig. 1A). Immunostaining of lung tissue from WT (Trpm2+/+) and Trpm2iΔEC mice with anti-TRPM2 and anti-CD31 antibodies showed the absence of TRPM2 in ECs (Fig. 1B). We challenged Trpm2iΔEC mice with 10 mg/kg LPS i.p. and measured PMN sequestration by assaying MPO activity. Trpm2iΔEC mice exhibited significantly reduced MPO activity at 6 h and 24h post-endotoxin challenge compared to WT mice (Fig. 1C). We used high resolution 2-photon excitation microscopy to study the infiltration of PMNs in lungs of WT and Trpm2iΔEC mice (Fig. 1D & E; Online Fig. II A). In WT mice, we observed a significant increase in tissue infiltration of PMNs at 6h and 24h post-LPS (Fig. 1D). These responses were significantly suppressed in Trpm2iΔEC mice (Fig. 1E and Online Fig. IIA). Hematoxylin and eosin staining also showed reduced vascular infiltration of leukocytes in Trpm2iΔEC mouse lungs at 6h post-endotoxin challenge compared to WT lungs; at 48h leukocyte infiltration returned to basal levels in both WT and Trpm2iΔEC mice (Online Fig. II C). In mortality studies, Trpm2iΔEC mice were challenged with lethal dose of LPS (20 mg/kg, i.p.). Trpm2 iΔEC mice showed 80% survival compared to WT mice over the 96h study period (Fig. 1E). Lung microvessels of Trpm2iΔEC mice were also resistant to endotoxin induced hyper-permeability measured by the EBA tracer (Online Fig. IIB), and there was significantly reduced edema formation in lungs in response to LPS challenge compared to WT mice (Fig. 1G). Notably, no differences were observed in the levels of MCP-1 and KC chemokines production in WT and Trpm2iΔEC after endotoxin challenge (Online Fig. III).
Figure 1. Conditional deletion of transient receptor potential melastatin-2 (TRPM2) in endothelial cells (Trpm2iΔEC) of mice prevents endotoxin induced PMN uptake, inflammatory lung injury, and mortality.
(A) Lung endothelial cells (ECs) isolated from Trpm2iΔEC mice and total cell lysates were used for immunoblot (IB) analysis of TRPM2 protein expression. (B) Lung tissue sections from WT (Trpm2+/+) and Trpm2iΔEC mice were immunostained with anti-TRPM2 and anti-CD31 antibodies and visualized by confocal microscopy. TRPM2 (Green), CD31 (Red), DAPI (Blue). Scale bar=100μm. TRPM2 is absent from the intima in Trpm2iΔEC mice. (C) WT and TrpmiΔEC mice were injected with i.p. endotoxin (LPS) or saline as above, lungs were collected at indicated time points, and used for measurement of MPO activity. n=5 mice per treatment. (D) in vivo imaging of PMNs and lung microvessels after LPS administration in wild-type and Trpm2iΔEC mice. Fluorescent-labeled LY6G antibody and TMR-Dextran were used to stain PMNs and lung microvascular structures, respectively. Colors are pseudocolors. Scale bar; 50 μm. (E) Quantitative analysis of PMN density in microvessels and alveoli (n=3–5 for each plot of wild-type, n=4–5 for each plot of Trpm2iΔEC). Fluorescent intensities of PMNs in field of view were quantified and value of no LPS condition in wild-type mice was normalized as 1 (Dotted line). * P < 0.05, *** P < 0.005 vs wild-type level. (E) Quantitative analysis of PMN density in alveoli (n=8–11 for each plot of wild-type, n=7–9 for each plot Trpm2iΔEC). Alveoli were outlined and fluorescent intensities of PMNs are quantified and the value of no LPS condition in wild-type mice was normalized as 1 (Dotted line). ** P < 0.01, *** P < 0.005 vs. wild-type level. (F) Age and weight-matched WT and Trpm2iΔEC mice were challenged with lethal dose of i.p. LPS (20 mg/kg, i.p.) and mortality was monitored for 96h. Trpm2iΔEC showed markedly increased survival compared with WT mice. n = 10 per group. *p<0.05. (G) WT and Trpm2iΔEC mice were challenged with PBS or 20 mg/kg LPS i.p. Wet and dry lung weights were recorded to determine edema. n = 6 /group. *p<0.05.
PMN interaction with ECs induces Ca2+ influx via TRPM2 expressed in ECs
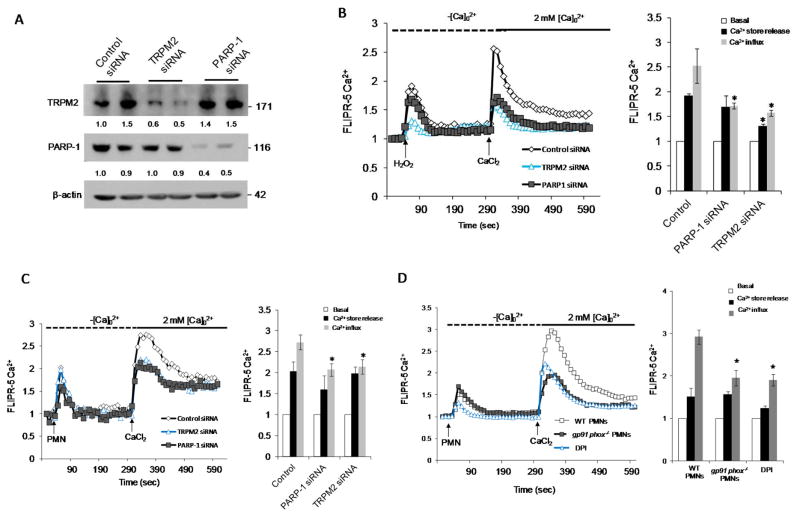
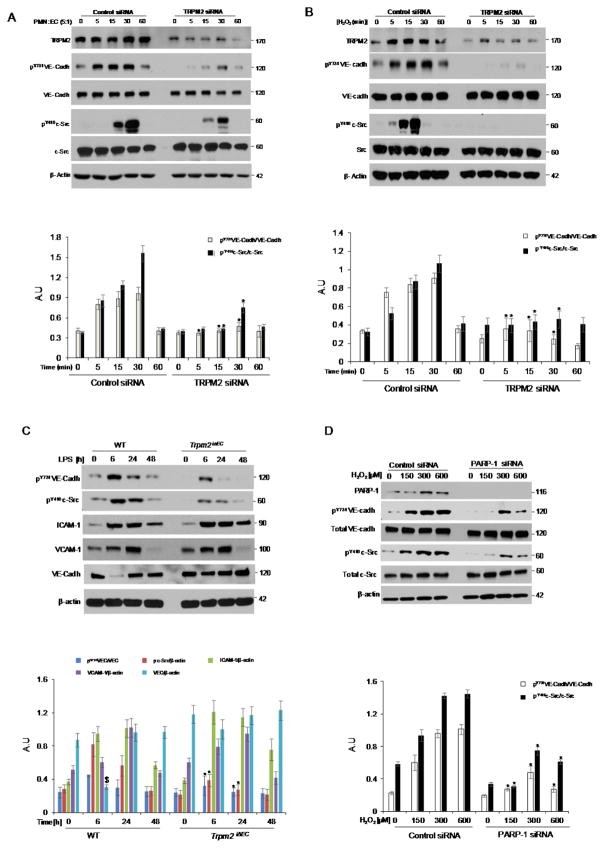
To determine the role of TRPM2 in mediating PMN-activated Ca2+ influx in ECs, HLMVECs were transfected with TRPM2 or PARP-1 siRNA. Depletion of TRPM2 or PARP-1 in HLMVECs (Fig. 2A) significantly reduced both H2O2- and PMN activated-induced Ca2+ influx. Depletion of TRPM2 in ECs also significantly reduced the H2O2 induced intracellular Ca2+ release (Fig. 2B and 2C). We observed that stimulation of mouse lung microvascular ECs with PMNs isolated from gp91phox−/− mice or PMNs from WT mice pretreated with DPI [NADPH oxidase inhibitor (Diphenyliodonium) showed significantly reduced Ca2+ entry (Fig. 2D). These results thus showed that PMN activation induced Ca2+ entry in ECs is dependent on activation of TRPM2 channel via ADPR generation in ECs.
Figure 2. Endothelial-expressed TRPM2 is essential for Ca2+ influx induced by activation of polymorphonuclear leukocytes (PMNs).
(A–C) HLMVECs were treated with either control-siRNA, TRPM2 siRNA, or poly ADP ribose polymerase-1 siRNA (PARP-1 siRNA) for 48h (A). HLMVECs were then loaded with FLIPR-5 (fluorescence imaging plate reader) Ca2+ sensitive dye for 30 min, followed by stimulation with either H2O2 (300 μM) (B) or fMLP (1μM)-stimulated PMNs (PMN-to-EC ratio of 5:1) (C). (D) PMNs isolated from gp91phox−/− mice or WT mice treated with indicated concentration of DPI (Diphenyliodonium) were used to measure Ca2+ entry in ECS as described above. Experiments were repeated at least twice with each experiment comprising of ≥6 replicates. Representative tracing of Ca2+ transients and mean values are shown as ± SEM. *p<0.05 compared to the respective control group.
PARP-1 mediates generation of ADPR in ECs secondary to PMN activation
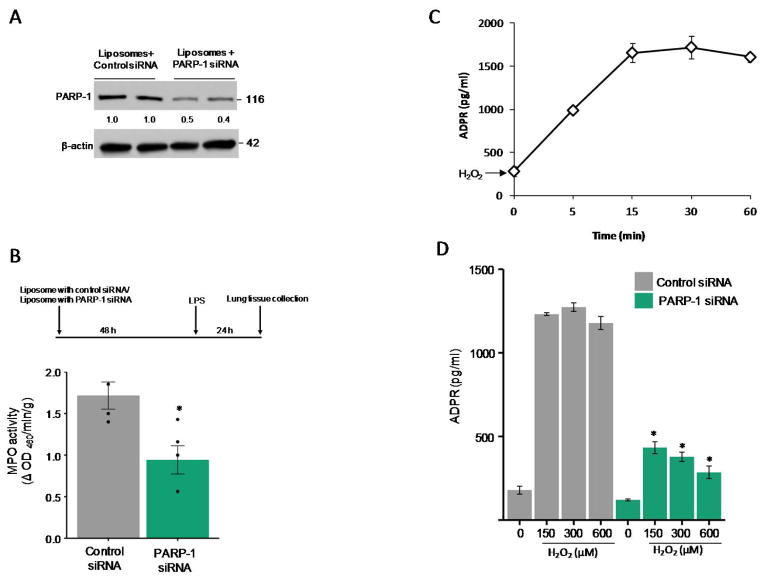
To address the mechanism by which TRPM2 expressed in ECs induces vascular inflammation, we first examined the role of PARP-1 in ECs in generating ADPR, the agonist responsible for TRPM2 activation (9). The role of PARP-1 was studied by in vivo silencing PARP-1 in ECs of mice. Here we observed that liposomal mediated delivery of PARP-1 siRNA in mice, which targets lung ECs (12, 13), suppressed lung MPO activity in response to LPS (Fig. 3A and 3B); thus indicating that PARP-1 dependent TRPM2 activation in ECs was responsible for the PMN uptake. We next assessed ADPR generation in ECs and effects of PARP-1 silencing on ADPR generation in ECs. Maximum ADPR generation in ECs was observed after 15 min of H2O2 stimulation, which plateaued at 30 min with a slight decline seen 1h (Fig. 3C). Silencing of PARP-1 in HLMVECs abrogated the ADPR generation (Fig. 3D), indicating requirement of PARP-1 in ECs in mediating the generation of ADPR.
Figure 3. Endothelial cell expressed PARP-1 is required for PMN uptake.
(A–B) C57BL/6 mice were injected i.v. with either liposomes alone or PARP-1 siRNA (30 μg) for 48h followed by i.p. LPS challenge (10 mg/kg) for 2h. PARP-1 protein expression reflecting protein present in ECs (A) and MPO activity (B) were then measured in lungs collected as described in Methods. n=6 mice per group. Data are shown as mean ± SEM. (C) Measurement of ADPR generation in HLMVECs treated with H2O2 for the indicated times. (D) Measurement of ADPR generation in HLMVECs treated with either control or PARP-1-siRNA, and stimulated with indicated concentrations of H2O2 for 30 min. Data are from at least three independent experiments and expressed as mean ± SEM. *p<0.05 compared to the respective control group.
ADPR-activation of TRPM2 in ECs signals Ca2+ influx and PMN transmigration
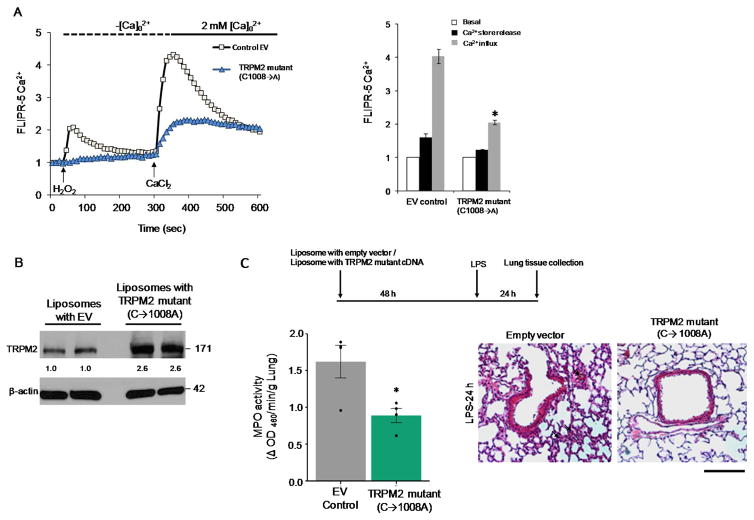
To address the role of Ca2+ entry via TRPM2 in ECs in mediating PMN transmigration, we measured Ca2+ influx in ECs after transfection of ADPR-insensitive mutant of TRPM2 (C1008→A). The TRPM2 mutant construct was generated by site-directed mutagenesis of cysteine residue at 1008 position to alanine in the putative pore region of TRPM2 as described (10, 14). Substitution of cysteine with alanine generated mutant channels that failed to be activated by ADPR. Transfection of this mutant in HLMVECs resulted in much reduced Ca2+ entry in response to PMN activation compared to control empty vector (Fig. 4A). We also performed liposome mediated delivery of TRPM2 (C1008→A) mutant (25 μg) in lung ECs in vivo (10), and assessed PMN infiltration by H&E staining and lung MPO activity after i.p. LPS challenge (10mg/Kg) (Fig. 4B). We observed that expression of TRPM2 mutant in lung ECs of mice prevented LPS induced increase in PMN transmigration and lung vascular permeability (Fig. 4C and Fig. IV).
Figure 4. Expression of ADPR-insensitive TRPM2 mutant (C1008→A) in endothelial cells impairs Ca2+ entry and endotoxin-induced PMN uptake.
(A–B) HLMVECs were transfected with empty vector or ADPR-insensitive TRPM2 mutant (C1008→A) plasmid for 24h (A). Cells were then loaded with FLIPR-5 Ca2+ sensitive dye for 30 min, followed by stimulation with H2O2 (300 μM). H2O2-induced Ca2+ influx was suppressed in cells expressing the TRPM2 mutant (B). Representative tracing obtained with each experiment consisting of ≥6 replicates. (B) C57BL/6 mice were injected i.v. with liposomes containing empty vector or TRPM2 mutant plasmid for 48h followed by LPS challenge (10 mg/kg, i.p.) for 24h. Lungs were collected and subjected to immunoblot analysis of TRPM2 protein expression (B) and MPO activity (C). Expression of TRPM2 mutant suppressed the LPS-induced PMN uptake in lungs as determined by MPO activity (left panel) and H&E staining. n= 6 mice per group. Data are shown as mean ± SEM. *p<0.05 compared to the respective control group. Scale bar- 100μm.
PMN activation TRPM2 in ECs phosphorylates VE-cadherin at Y731 to disassemble adherens junctions
Adhesion molecules expressed on the surface of ECs control transmigration of PMNs (15). VE-cadherin regulates the opening of endothelial adherens junctions (AJs), and facilitates PMN transmigration (15, 16). We observed that siRNA mediated depletion of TRPM2 in ECs suppressed the phosphorylation of VE-cadherin at Y731 (the domain critical for VE-cadherin internalization and disassembly of AJs (15, 16) in response to stimulation with activated PMNs or addition of H2O2 (Fig. 5A & B). We also observed that silencing of TRPM2 expression in ECs markedly reduced the phosphorylation of c-Src at Y416 in ECs (Fig. 5A & 5B). Further, in vivo challenge with LPS in mice suppressed VE-cadherin and c-Src phosphorylation in lungs of Trpm2iΔEC mice (Fig. 5C). We observed no degradation of VE-cadherin upon LPS stimulation at 6h in Trpm2iΔEC mice compared to WT mice (Fig. 5C). Moreover, levels of cell adhesion molecules ICAM-1 and VCAM-1 were not altered between WT and Trpm2iΔEC mice on LPS challenge (Fig. 5C).
Figure 5. Endothelial cell expressed TRPM2 is indispensible for PMN-induced VE-cadherin phosphorylation.
HLMVECs were transfected with either control-siRNA or TRPM2-siRNA for 48h, followed by stimulation with fMLP (1μM)-activated PMNs (A) or H2O2 (B) for the indicated times. Total cell lysates were used for immunoblot analysis. PMN stimulation or treatment with H2O2 induced time-dependent phosphorylation of VE-cadherin at Tyr-731 within 5 min, which returned to baseline at 60 min. TRPM2 silencing however suppressed PMN-induced VE-cadherin phosphorylation in HLMVECs. PMN (A) or H2O2 (B) stimulation also induced phosphorylation of active site in c-Src at Tyr-416, which as with VE-cadherin showed time dependent increase beginning at 5 min after addition of activated PMNs and returned to baseline by 60 min. This response was suppressed in endothelial cells transfected with TRPM2 siRNA. Densitometry analysis of n= 3 independent experiments is shown as a bar graph in the bottom panel. Similar studies as in (A) were also made using lung lysate from Trpm2iΔEC mice (C). We observed impaired phosphorylation of VE-cadherin at Tyr-731 and c-Src at Tyr-416. However, the levels of VE-cadherin, ICAM-1 and VCAM-1 remain unaffected in Trpm2iΔEC compared to WT mice. The densitometry analysis of n= 3 independent experiments is shown as a bar graph in the bottom panel. (D) Studies also determined the effects of PARP-1 depletion in HLMVECs to address the role of ROS (H2O2), known to activate TRPM2, in phosphorylating VE-cadherin and c-Src. Depletion of PARP-1 impaired phosphorylation of VE-cadherin at Tyr-731 as well as c-Src at Tyr-416. Representative image from three independent experiments are shown with β-actin used as loading control and densitometric analysis of n=3 experiments is shown in the bottom panel. *p<0.05 compared to the respective control group. $p<0.05 compared to WT group.
To investigate the role of PARP-1 in mediating disassembly of AJs, HLMVECs depleted of PARP-1 were challenged with H2O2 for 30 min. Here we observed that PARP-1 silencing abrogated H2O2-induced VE-cadherin and c-Src phosphorylation similarly to TRPM2 silencing (Fig. 5D). Moreover, depletion of TRPM2 or PARP-1 in HLMVECs prevented H2O2 induced disassembly of adherens junction as observed by immunofluorescent staining of VE-cadherin and recording of trans-endothelial electrical resistance (TEER) (Online Fig. V).
TRPM2 expression in Trpm2iΔEC ECs rescues defective PMN migration phenotype
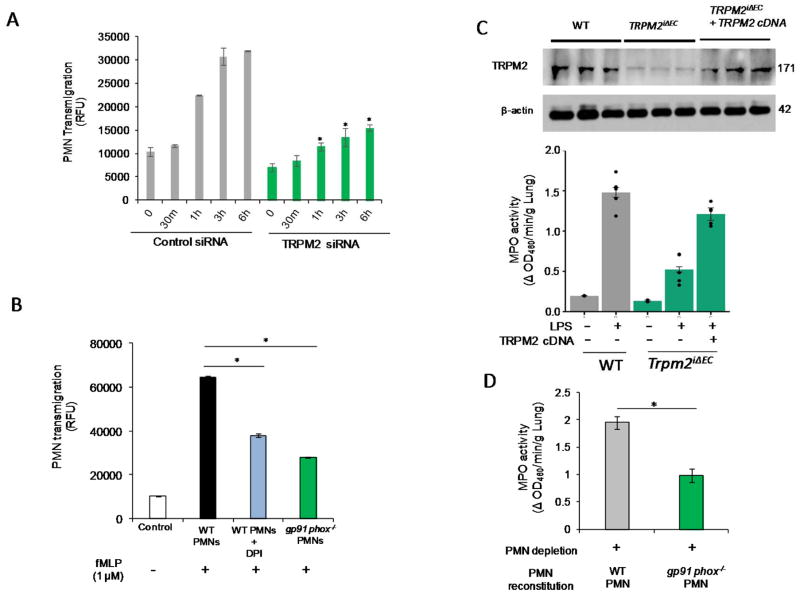
To test whether EC expressed TRPM2 is required for PMN transmigration, HLMVECs transfected with TRPM2 siRNA to deplete TRPM2 followed by addition of activated PMNs layered on ECs. TRPM2 silencing in ECs significantly reduced transmigration of PMNs (Fig. 6A). PMNs isolated from gp91phox−/− mice or WT PMNs treated with DPI also showed similarly impaired transmigration across WT mouse lung ECs (Fig. 6B). Next, we performed liposome-mediated delivery of WT-TRPM2 plasmid in lung ECs of Trpm2iΔEC mice followed by measurement of lung MPO activity. Expression of TRPM2 in Trpm2iΔEC mice rescued the defective PMN sequestration phenotype (Fig. 6C). Moreover, WT mice reconstituted with gp91phox−/− PMNs showed significantly reduced PMN uptake in lungs after LPS stimulation (10mg/Kg i.p.) (Fig. 6D), indicating that PMN-generated ROS activated TRPM2 in ECs to induce PMN transmigration and vascular inflammation.
Figure 6. Endothelial cell expressed TRPM2 is responsible for PMN transmigration whereas expression of TRPM2 in TRPM2iΔEC mice restores defective PMN uptake phenotype.
(A) Time dependent transmigration of fMLP (1μM)-activated PMNs in control and TRPM2-siRNA treated HLMVECs measured as described in Methods. TRPM2 silencing in endothelial cells suppressed PMN transmigration. (B) Transmigration of untreated WT PMNs, DPI treated PMNs or gp91phox−/− PMNs across mouse endothelial cells at 6h post-fMLP stimulation. PMNs treated with DPI or PMNs from gp91phox−/− mice showed defective transmigration across mouse endothelial cells. (C) TRPM2 overexpression in Trpm2iΔEC mice using liposomes restored the defective PMN uptake phenotype of Trpm2iΔEC mice. (D) PMNs in WT mice were depleted using Gr-1 antibody (i.p. 100 μg/mice) and after 24h of i.p. antibody injection, mice were reconstituted with gp91phox−/− PMNs and were simultaneously injected i.p. LPS for 24h and were then assayed for MPO activity in lungs. Mice reconstituted with gp91phox−/− PMNs showed defective PMN uptake in lungs compared to the WT PMNs reconstituted mice. *p<0.05 compared to the respective control group.
Activated PMNs mediate P-selectin surface localization through TRPM2 activation in ECs
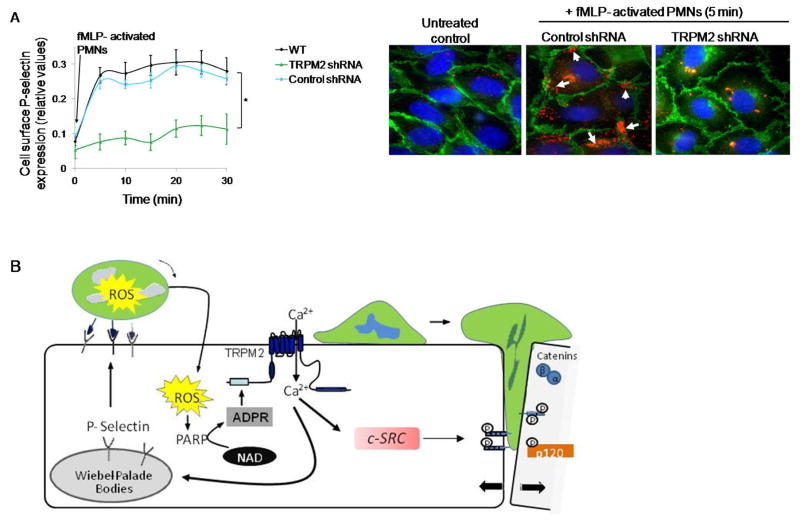
P-selectin translocates to the EC surface through Ca2+ signaling where it plays an essential role in mediating the initial phase of recruitment of PMNs (17). To investigate the involvement of TRPM2 in mediating P-selectin translocation in ECs secondary to activation of PMNs, HLMVECs transduced with shRNA to silence TRPM2 expression were stimulated with fMLP-activated PMNs. HLMVECs transduced with TRPM2 shRNA showed significantly reduced cell surface P-selectin expression (Fig. 7A). We also performed immunostaining in endothelial monolayers exposed to fMLP-activated PMNs and examined cell surface P-selectin expression. Depletion of TRPM2 prevented PMN activation-dependent EC surface redistribution of P-selectin (Fig. 7B).
Figure 7. Activated PMN-dependent P-selectin translocation to endothelial cell surface requires TRPM2.
(A) HLMVECs transduced with shRNA to silence TRPM2 expression were incubated with fMLP (1μM)-activated PMNs (WT, 1 EC:5 PMN ratio) for varying times. Cell surface expression of P-selectin was determined by enzyme linked immunosorbent assay (ELISA) using tetramethyl-benzidine substrate (results are shown as mean ± SEM; n= 3). (B) Endothelial monolayers layered with fMLP (1μM) -activated PMNs for 5 min, and then fixed and stained with Alexa 594 mouse IgG/Pselectin (red), Alexa-488 goat IgG/VE-cadherin (green), and DAPI (blue). Scale bar=20 μM. Each image is representative of three experiments. Control endothelial cells expressed little surface P-selectin whereas PMN activation increased P-selectin-expression along cell borders (arrows). Depletion of TRPM2 prevented PMN-elicited cell surface redistribution of P-selectin. (C) Model derived from our results. PMN interaction with ECs delivers a ROS signal which activates TRPM2-dependent gating of Ca2+ via the generation of ADPR through the catalytic activity of PARP-1. This in turn results in activation of c-Src and phosphorylation of VE-cadherin through intermediate steps, the disassembly of AJs and transmigration of PMNs. Another crucial TRPM2 Ca2+ gating step is the Ca2+ signaling dependent release of P-selectin from Weibel Palade bodies, which induces EC surface localization of P-selectin to promote PMN arrest on EC surface and thus may fine tune PMN interaction with ECs to amplify PMN dependent TRPM2 activation. *p<0.05 compared to the respective control group.
DISCUSSION
Studies have shown that adhesive interaction of PMNs with ECs elicits a rise in intracellular Ca2+ concentration and phosphorylation of myosin light chain in ECs, leading to a contractile dependent mechanism of inter-EC gap formation and PMN transmigration (2). The PMN-activated transcellular signaling is believed to increase endothelial permeability and facilitate transmigration of PMNs (2). A more recent study by Barzilae et al., (18) showed that PMNs use their nuclear lobes to generate gaps between and within ECs and thereby to cross the EC barrier through rapid turnover of actin filaments as opposed to EC contraction. Another recent study also showed the role of cation channel TRPC6 in ECs in mediating an increase in intracellular Ca2+ concentration that in turn triggered PMN transmigration downstream of PECAM interaction between PMNs and ECs (19). An important aspect of all these findings is that transcellular signaling events emanating from PMNs were essential for the PMNs to migrate across the endothelial barrier. We previously showed transcellular signaling in which NADPH oxidase activation in PMNs via the generation of ROS induced the activation of Toll-like receptor expression in ECs (20), indicating oxidant signaling is an important step initiating a dialog between PMNs and ECs. Studies showing that PMNs cross the continuous adherens junctions in ECs by disassembling actin filaments in ECs through Ca2+ signaling in ECs (4) raises key questions about the role of ROS in mediating PMN transmigration and PMN dependent vascular inflammation. In the present study, we demonstrated that the interaction of PMNs with ECs induces a rise in cytosolic Ca2+ through the activation of the ROS-sensitive TRPM2 channel in ECs. Downstream of ROS activation of TRPM2 in ECs, Src was activated in ECs resulting in the phosphorylation of Y731 residue on VE-cadherin and leading to VE-cadherin internalization and disassembly of adherens junctions. This thereby promoted PMN transmigration via the paracellular transendothelial pathway.
TRPM2 is highly expressed in ECs where it plays an important role in regulating key functions of the endothelium in innate immunity (21–23), endothelial barrier (3, 14, 24), and EC apoptosis (25). We observed that conditional deletion of TRPM2 in ECs (Trpm2iΔEC) of mice markedly reduced PMN transmigration and sequestration in tissue. Further, deletion of TRPM2 in ECs enhanced survival in mice in response to lethal dose of LPS as compared to WT controls. The primary mode of TRPM2 activation is through oxidant-induced generation of ADPR (9), which is a TRPM2 ligand that functions by binding to the Nudix box domain (NUDT9-H) in the TRPM2 C-terminal to activate the channel (9, 26),. Oxidant sensing by TRPM2 is dependent on the generation of the TRPM2 agonist ADPR (3) secondary to the activation of poly ADP-ribose polymerase enzyme (PARP-1). We observed that knockdown of PARP-1 in the endothelium replicated the defective PMN transmigration phenotype seen in Trpm2iΔEC mice. Further overexpression of an ADPR insensitive mutant of TRPM2 (C1008→A)(10), in which ADPR cannot activate the channel, resulted in defective transmigration of PMNs in vivo. Interestingly, there was no difference in the levels of ICAM-1, VCAM-1 and chemoattractants MCP-1 and KC upon endotoxin challenge in WT and Trpm2iΔEC mice, suggesting that the impaired PMN transmigration response in Trpm2iΔEC cannot be explained on this basis. Thus, multiple lines of genetic evidence provide prima facie evidence for the primary role of EC expressed TRPM2 in mediating PMN transmigration and thereby in contributing to vascular inflammation.
We observed that oxidants produced by PMNs were indispensable for TRPM2 activation in ECs. gp91phox−/− PMNs, which did not produce ROS, showed defective PMN transmigration and tissue sequestration. Thus it appears that oxidants generated by PMNs are the primary source of ROS required to activate TRPM2 in ECs and thereby transendothelial PMN migration. Weber et al., (19) described a role of TRPC6 as another trp channel mediating Ca2+ entry in ECs and PMN transmigration downstream of PECAM homophilic interactions between PMN and ECs. Because PMN transmigration is such a conserved host-defense response, it is likely that other means of Ca2+ entry and signaling are involved in mediating PMN transmigration to combat infection.
We showed that TRPM2 activation in ECs was dependent on oxidants generated by PMNs, which stimulated intracellular ADPR production via PARP-1 activation. We also showed that Ca2+ influx downstream of opening of TRPM2 channel induced the activation of c-Src and phosphorylation of VE-cadherin at Y731, the site reported to regulate VE-cadherin internalization and opening of AJs and leukocyte transmigration (27). Further we observed that P-selectin translocation to the EC plasma membrane was downstream of TRPM2 activation. This response may also be mediated by c-Src dependent tyrosine phosphorylation of P-selectin (17). These findings together are consistent with the model (Fig. 7B) that PMN interaction with ECs delivers the transcellular ROS signal that activates TRPM2 dependent gating of Ca2+ in ECs via PARP-1 mediating generation of ADPR. This in turn induces the activation of c-Src and through an intermediate step involving an unknown kinase phosphorylates VE-cadherin, resulting in the disassembly of AJs and transmigration of PMNs. Another crucial TRPM2 Ca2+ gating step is the Ca2+ signaling-dependent release of P-selectin from Weibel Palade bodies that induces EC surface localization of P-selectin and promotes PMN arrest on the EC surface. The significance of P-selectin translocation to the membrane is unclear. One possibility is that it facilitates PMN interaction with ECs and sets into motion the amplification of TRPM2 activation in ECs.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Reactive oxygen species (ROS)-mediated calcium (Ca2+) entry in endothelial cells triggers polymorphonuclear leukocytes (PMN) transmigration to promote vascular injury.
Redox-sensitive cation channel TRPM2 expressed in endothelial cells is activated by ROS.
What New Information Does This Article Contribute?
Inducible endothelial cell-restricted deletion of TRPM2 prevents endotoxin-induced neutrophil transmigration and lung vascular injury in mice.
PMN released ROS activates endothelial TRPM2 to facilitate PMN transendothelial migration secondary to calcium entry and subsequent disassembly of endothelial adherens junctions.
TRPM2 (Transient Receptor Potential Melastatin-2) channel expressed in endothelial cells (ECs) is activated by ROS; however, the role of endothelial TRPM2 in the mechanism of PMN transmigration is not known. Herein, we showed that PMN transmigration in response to endotoxin challenge was blocked in adult mice in which the EC expressed TRPM2 was conditionally deleted. Our results suggest that blocking TRPM2 activation in ECs is a potentially important therapeutic strategy for treating lung vascular inflammation.
Acknowledgments
We thank Dr. Peter Toth for technical support in 2-photon imaging.
SOURCES OF FUNDING
This work was supported by National Institute of Health Grants R01 HL122157, R01 HL128359, P01 HL077806, P01HL060678, 1S10RR025629-01A1 and Scientist Development Grant 16SDG30980061 from American Heart Association.
Nonstandard Abbreviations and Acronyms
- H2O2
Hydrogenperoxide
- ADPR
Adenosine diphosphoribose
- AJ
adherens junction
- Ca2+
Calcium
- Dil-Ac-LDL
Acetylated, DiI complex-Low Density Lipoprotein
- EBM2
endothelial basal medium 2
- fMLP
N-formylmethionyl-leucyl-phenylalanine
- EC
endothelial cell
- HLMVECs
Human lung microvascular endothelial cells
- HBSS
Hank’s Balanced Salt Solution
- NOX2
Nadph oxidase homolog 2
- NAD+
Nicotinamide adenine dinucleotide
- PARP
poly ADP ribose polymerase
- PBS
Phosphate buffer saline
- PECAM-1/CD31
Platelet endothelial cell adhesion molecule
- ROS
Reactive oxygen species
- SEM
Standard Error of Mean
- TEER
Trans endothelial electrical resistance
- TRPC
transient receptor potential canonical
- TRPM2
Transient receptor potential melastatin 2
- Tyr
Tyrosine residue
- VE-cadherin
vascular endothelial cadherin
Footnotes
DISCLOSURES
None.
AUTHORSHIP CONTRIBUTIONS
M.M., S.N., C.M.H, J.R., C.T., and A.B.M. designed research; M.M., S.N., and D.S. performed research; M.M., S.N., and C.T. analyzed data; and M.M., S.N., C.T., and A.B.M. wrote the paper.
References
- 1.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 2.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–67. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res. 2008;102:347–55. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 4.Mooren OL, Li J, Nawas J, Cooper JA. Endothelial cells use dynamic actin to facilitate lymphocyte transendothelial migration and maintain the monolayer barrier. Mol Biol Cell. 2014;25:4115–29. doi: 10.1091/mbc.E14-05-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hecquet CM, Ahmmed GU, Malik AB. TRPM2 channel regulates endothelial barrier function. Adv Exp Med Biol. 2010;661:155–67. doi: 10.1007/978-1-60761-500-2_10. [DOI] [PubMed] [Google Scholar]
- 6.Wang G, Cao L, Liu X, Sieracki NA, Di A, Wen X, et al. Oxidant Sensing by TRPM2 Inhibits Neutrophil Migration and Mitigates Inflammation. Dev Cell. 2016;38:453–62. doi: 10.1016/j.devcel.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di A, Kiya T, Gong H, Gao X, Malik AB. Role of the phagosomal redox-sensitive TRP channel TRPM2 in regulating bactericidal activity of macrophages. J Cell Sci. 2017;130:735–44. doi: 10.1242/jcs.196014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, Luckhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem. 2002;277:23150–6. doi: 10.1074/jbc.M112096200. [DOI] [PubMed] [Google Scholar]
- 9.Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–9. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 10.Mei ZZ, Mao HJ, Jiang LH. Conserved cysteine residues in the pore region are obligatory for human TRPM2 channel function. Am J Physiol Cell Physiol. 2006;291:C1022–C1028. doi: 10.1152/ajpcell.00606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gothert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, et al. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104:1769–77. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- 12.Zhou MY, Lo SK, Bergenfeldt M, Tiruppathi C, Jaffe A, Xu N, et al. In vivo expression of neutrophil inhibitory factor via gene transfer prevents lipopolysaccharide-induced lung neutrophil infiltration and injury by a beta2 integrin-dependent mechanism. J Clin Invest. 1998;101:2427–37. doi: 10.1172/JCI407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiruppathi C, Soni D, Wang DM, Xue J, Singh V, Thippegowda PB, et al. The transcription factor DREAM represses the deubiquitinase A20 and mediates inflammation. Nat Immunol. 2014;15:239–47. doi: 10.1038/ni.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittal M, Urao N, Hecquet CM, Zhang M, Sudhahar V, Gao XP, et al. Novel role of reactive oxygen species-activated Trp melastatin channel-2 in mediating angiogenesis and postischemic neovascularization. Arterioscler Thromb Vasc Biol. 2015;35:877–87. doi: 10.1161/ATVBAHA.114.304802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wessel F, Winderlich M, Holm M, Frye M, Rivera-Galdos R, Vockel M, et al. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat Immunol. 2014;15:223–30. doi: 10.1038/ni.2824. [DOI] [PubMed] [Google Scholar]
- 16.Alcaide P, Newton G, Auerbach S, Sehrawat S, Mayadas TN, Golan DE, et al. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood. 2008;112:2770–9. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maliba R, Brkovic A, Neagoe PE, Villeneuve LR, Sirois MG. Angiopoietin-mediated endothelial P-selectin translocation: cell signaling mechanisms. J Leukoc Biol. 2008;83:352–60. doi: 10.1189/jlb.0107056. [DOI] [PubMed] [Google Scholar]
- 18.Barzilai S, Yadav SK, Morrell S, Roncato F, Klein E, Stoler-Barak L, et al. Leukocytes Breach Endothelial Barriers by Insertion of Nuclear Lobes and Disassembly of Endothelial Actin Filaments. Cell Rep. 2017;18:685–99. doi: 10.1016/j.celrep.2016.12.076. [DOI] [PubMed] [Google Scholar]
- 19.Weber EW, Han F, Tauseef M, Birnbaumer L, Mehta D, Muller WA. TRPC6 is the endothelial calcium channel that regulates leukocyte transendothelial migration during the inflammatory response. J Exp Med. 2015;212:1883–99. doi: 10.1084/jem.20150353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan J, Frey RS, Malik AB. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J Clin Invest. 2003;112:1234–43. doi: 10.1172/JCI18696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, et al. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–47. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knowles H, Heizer JW, Li Y, Chapman K, Ogden CA, Andreasen K, et al. Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proc Natl Acad Sci U S A. 2011;108:11578–83. doi: 10.1073/pnas.1010678108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang G, Cao L, Liu X, Sieracki NA, Di A, Wen X, et al. Oxidant Sensing by TRPM2 Inhibits Neutrophil Migration and Mitigates Inflammation. Dev Cell. 2016;38:453–62. doi: 10.1016/j.devcel.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di A, Gao XP, Qian F, Kawamura T, Han J, Hecquet C, et al. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat Immunol. 2012;13:29–34. doi: 10.1038/ni.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecquet CM, Zhang M, Mittal M, Vogel SM, Di A, Gao X, et al. Cooperative Interaction of trp Melastatin Channel TRPM2 with its Splice Variant TRPM2-S is Essential for Endothelial Cell Apoptosis. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.114.302414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi N, Kozai D, Kobayashi R, Ebert M, Mori Y. Roles of TRPM2 in oxidative stress. Cell Calcium. 2011;50:279–87. doi: 10.1016/j.ceca.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, et al. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci. 2008;121:29–37. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.