Abstract
Aim
The Neoadjuvant PI3K inhibition in HER2 OverExpressing Breast cancEr (NeoPHOEBE) trial evaluated the efficacy and safety of buparlisib, a pan-phosphatidylinositol 3-kinase (PI3K) inhibitor, plus trastuzumab and paclitaxel as neoadjuvant treatment for human epidermal growth factor receptor-2 positive (HER2+) breast cancer.
Methods
NeoPHOEBE was a neoadjuvant, phase II, randomised, double-blind study. Women with HER2+ breast cancer were randomised within two independent cohorts by PIK3CA mutation status and, in each cohort stratified by oestrogen receptor (ER) status to receive buparlisib or placebo plus trastuzumab (first 6 weeks) followed by buparlisib or placebo with trastuzumab and paclitaxel. Primary end-point was pathological complete response (pCR) rate; key secondary end-point was objective response rate (ORR) at 6 weeks. Exploratory end-points were evaluation of Ki67 levels and change in tumour infiltrating lymphocytes (TILs) in intermediate biopsies at day 15.
Results
Recruitment was suspended mainly due to liver toxicity after enrolment of 50 of the planned 256 patients. In each arm (buparlisib n = 25; placebo n = 25) 21 patients (84%) had wild type PIK3CA and 4 patients (16%) had mutant PIK3CA. Overall, pCR rate was similar between buparlisib and placebo arms (32.0% versus 40%; one-sided P = 0.811). A trend towards higher ORR (68.8% versus 33.3%; P = 0.053) and a significant decrease in Ki67 (75% versus 26.7%; P = 0.021) was observed in buparlisib versus placebo arm in the ER+ subgroup (Pinteraction = 0.03).
Conclusions
Addition of the pan-PI3K inhibitor buparlisib to taxane-trastuzumab-based therapy in HER2+ early breast cancer was not feasible. However, the higher ORR and Ki67 reduction in the ER+, HER2+ subgroup indicates a potential role for PI3K-targeted therapy in this setting and may warrant further investigation with better-tolerated second-generation PI3K inhibitors.
Trial registration identifier
Keywords: NeoPHOEBE, Buparlisib, pCR, HER2, Primary breast cancer, Neoadjuvant
1. Background
The human epidermal growth factor receptor-2 (HER2) is overexpressed in approximately 15–20% of breast cancers [1,2]. In the neoadjuvant setting, treatment with HER2-targted agents such as trastuzumab, lapatinib or pertuzumab, in combination with chemotherapy has improved pathological complete response (pCR) rates in patients with HER2+ early breast cancer (EBC) [3–10]. Emerging evidence also suggests that dual HER2-targeted strategy may be more efficacious than a single HER2-targeted agent [3–7,9].
Although the majority of patients with HER2+ breast cancer respond to HER2-targeted therapy, resistance to HER2-targeted therapy remains a clinical challenge. One of the mechanisms implicated in HER2 treatment resistance is the activation of phosphoinosi-tide 3 kinase (PI3K)/Akt/mammalian (or mechanistic) target of rapamycin (mTOR) [PI3K/AKT/mTOR] pathway [11]. Preclinical data suggest that alterations in the PI3K/AKT/mTOR pathway, such as PIK3CA mutations, render cell lines resistant to HER2-targeted agents which can be reversed using PI3K inhibitors [12–15]. In the recently reported combined analysis of the neoadjuvant GeparQuattro, GeparQuinto and GeparSixto studies, pCR rates were lower in patients with PIK3CA mutant tumours, even when treated with the dual HER2-targeting approach [16–19]. These data suggest that targeting the PI3K pathway, in addition to HER2 targeting, may improve outcomes in patients with HER2+ breast cancer.
Buparlisib (BKM120), an orally bioavailable pan-PI3K inhibitor targeting all the known isoform of PI3K (p110α, β, γ and δ), has demonstrated synergistic growth inhibitory activity when combined with HER2-targeted agents in preclinical studies [20]. In early phase clinical trials, buparlisib demonstrated promising efficacy and manageable safety profile providing the rationale for further clinical evaluation of buparlisib [21–24]. The Neoadjuvant PI3K inhibition in HER2 OverExpressing Breast cancEr (NeoPHOEBE) trial was designed to evaluate the efficacy and safety of buparlisib plus trastuzumab and paclitaxel as neoadjuvant treatment for patients with untreated HER2+ primary breast cancer and the potential predictive value of PIK3CA mutations for higher tumour responses.
2. Materials and methods
2.1. Study design and participants
NeoPHOEBE (NCT01816594) was a phase II, randomised, double-blind, placebo-controlled, parallel-cohort, two-stage trial. Women aged ≥18 years were eligible if they had not received prior treatment for unilateral, histologically confirmed, newly diagnosed HER2+ primary breast cancer. Primary breast cancer was defined as non-inflammatory breast cancer >2 cm by clinical examination and/or >1.5 cm by ultrasound or magnetic resonance imaging (MRI). Oestrogen receptor (ER) status, HER2 status and PIK3CA status (wild type/mutant) were centrally assessed prior to enrolment, and patients with unknown status were excluded. Eligible patients were required to have Eastern Cooperative Oncology Group (ECOG) performance status 0–1 and adequate bone marrow and organ function at baseline. Patients with bilateral breast cancer, inflammatory disease or metastatic breast cancer and those with active cardiac disease or with a history of cardiac dysfunction were ineligible. Patients with Left Ventricular Ejection Fraction (LVEF) below 50%, corrected QT interval, QTcF > 480 m sec, ventricular arrhythmias (except for benign premature ventricular contractions), supraventricular and nodal arrhythmias requiring a pacemaker or not controlled with medication, conduction abnormality requiring a pacemaker or other cardiac arrhythmia not controlled with medication were not allowed. Patients scoring ≥12 on the Patient Health Questionnaire-9 (PHQ-9), those responding 1, 2 or 3 to question number 9 on the PHQ-9 questionnaire regarding potential for suicidal thoughts or ideation (independent of the total score of the PHQ-9), those with Generalized Anxiety Disorder Assessment-7 (GAD-7) mood scale score ≥ 15 or those having ≥ US National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) grade 3 anxiety were excluded. Patient with a medically documented history of or active major depressive episode, bipolar disorder (type I or II), obsessive-compulsive disorder, schizophrenia, a history of suicidal attempt or ideation, or homicidal ideation (e.g. risk of doing harm to self or others), or with an active severe personality disorders (defined according to Diagnostic and Statistical Manual, Fourth edition, (DSM-IV)) were ineligible.
Written informed consent was obtained from all patients prior to enrolment. The study was performed in accordance with the Good Clinical Practice guidelines and the Declaration of Helsinki. The study protocol was approved by an independent ethics committee or institutional review board at each site.
2.2. Randomisation
Patients were stratified upfront into two cohorts based on the PIK3CA mutation status (wild type/mutant) and within each cohort were randomised 1:1 to receive buparlisib plus trastuzumab or placebo plus trastuzumab for 6 weeks. After 6 weeks, paclitaxel was added to the buparlisib/placebo plus trastuzumab combination regimen and treatment continued for an additional 12 weeks. Randomisation was stratified by ER status.
2.3. Procedures
The overall 18-week treatment was divided into two treatment periods – the ‘biologic treatment window’ consisting of buparlisib/placebo plus trastuzumab for 6 weeks followed by a chemotherapy treatment phase in which paclitaxel was added to the buparlisib/placebo plus trastuzumab combination for a duration of 12 weeks. Buparlisib or matching placebo was administered orally on a continuous schedule at a dose of 100 mg/day for the first 6 weeks and reduced to 80 mg/day when administered along with paclitaxel for 12 weeks. Buparlisib dosing was based on previously published data which suggested that buparlisib 100 mg daily in combination with either paclitaxel or trastuzumab was generally well tolerated, however the reduced dose of buparlisib (80 mg daily) in combination with weekly paclitaxel/trastuzumab was expected to offer a better safety profile while maintaining sufficient antitumour activity [23–26]. Intravenous trastuzumab was administered at a loading dose of 4 mg/kg followed by a weekly maintenance dose of 2 mg/kg for 18 weeks. Paclitaxel was administered intravenously at a weekly dose of 80 mg/m2 for 12 weeks, after completion of the 6-week buparlisib/placebo plus trastuzumab treatment window. Buparlisib/placebo modification and/or discontinuation were allowed in cases of unmanageable toxicity, withdrawal of consent and disease progression. Definitive surgery was planned 14–28 days after the last dose of paclitaxel or trastuzumab. Post-surgery chemotherapy was as per investigator’s discretion, however, an anthracycline-based regimen (e.g. 3–4 cycles of 5-fluorouracil-epirubicin-cyclophosphamide (FEC)) was recommended. After completion of adjuvant chemotherapy, it was suggested that trastuzumab alone should be continued for a total trastuzumab therapy of 1 year as per local institutional practice. Further adjuvant radiotherapy and endocrine therapy was at the discretion of the investigator.
Cardiac safety was monitored by LVEF measurement, electrocardiogram [ECG] (standard 12 lead) and evaluation of cardiac signs or symptoms as well as cardiac enzymes (high sensitivity troponin I or T and N-terminal pro-B type natriuretic peptide (NTproBNP)). These assessments were performed at baseline, 6 weeks (Day 1 of Week 7) after randomisation and at the end of study (within 30 days of the last dose of study treatment). LVEF assessments were repeated at the investigators’ discretion after starting adjuvant treatment. The frequency of cardiac monitoring, including assessments of LVEF were performed every 3 months and then repeated until function returned to normal if the investigator deemed that an adverse event (AE) was related to cardiac dysfunction.
Serial biopsies were collected at baseline (prior to therapy), on day 15 (+/− 4 days) and tumour tissue was obtained at surgery. The biopsy on day 15 and the tumour tissue samples obtained at surgery were for translational research objectives and were obtained as per the investigator’s discretion with patient’s consent. PIK3CA status (wild-type, exon 9 mutant, exon 20 mutant or both exon 9 and 20 mutant) was assessed from formalin-fixed paraffin-embedded (FFPE) tumour samples obtained at baseline using Sanger sequencing. Ki67 levels and percentage of tumour infiltrating lymphocytes (TILs) were assessed at baseline and on day 15 using standardised methodology [27,28].
2.4. Outcomes
The primary end-point was pCR assessed according to the National Surgical Adjuvant Breast and Bowel Protocol (NSABP) definition [29,30] and was defined as absence of invasive disease in the breast (ypT0/ypTis; any ypN) at the time of surgery. The key secondary endpoint was ORR at 6 weeks measured using ultrasound or MRI and assessed according to the World Health Organisation (WHO) criteria. Other secondary endpoints included pCR analysed by additional definitions (German Breast Group [GBG] [31] and MD Anderson [32] definitions), pCR and ORR by hormone receptor status (ER+ versus ER−) and safety. Exploratory endpoints were assessment of change in Ki67 levels and percentage of stromal tumour infiltrating lymphocytes (TILs) from baseline to day 15 and their impact (on pCR [28,33]). Ki67 levels were determined by standardised image analysis [27]. A low proliferation rate was defined as a Ki67 of ≤20%. TILs were assessed based on previously published guidelines [28].
2.5. Statistical analysis
The study was originally planned as a 2-stage study to be conducted independently in each cohort (PIK3CA mutated versus wild type) which would allow early stopping for lack of efficacy. The original sample size calculation of 128 patients per cohort was based on a minimax two-stage randomised, phase II approach to detect a treatment difference of 18% with 80% power at α = 0.15. However, due to the early termination of the study after recruitment of 50 patients, a one-sided Fisher exact test was used to compare pCR rates between arms (pooled population and for each cohort) with α = 0.15, instead of the originally planned two-stage analysis. The pooled population was used for reporting demographics, baseline characteristics and safety analyses. pCR, ORR, treatment discontinuation and treatment duration analyses were performed cohort-wise. Confidence intervals at 95% for pCR rates and ORR were calculated using the Clopper and Pearson method. The one-sided Fischer exact test was used for comparison of pCR rates and ORR in the pooled population and in the cohorts, the two-sided Fisher’s exact-test was used for biomarker analyses. The p-values were reported without adjustments for multiple comparisons. Correlation between biomarkers and pCR was assessed using univariate and multivariate logistic regression models adjusted for the treatment arm with hormone receptor status (ER+ versus ER−) and PIK3CA mutation status (wild type versus mutant) as the covariates.
3. Results
3.1. Patient and disease characteristics
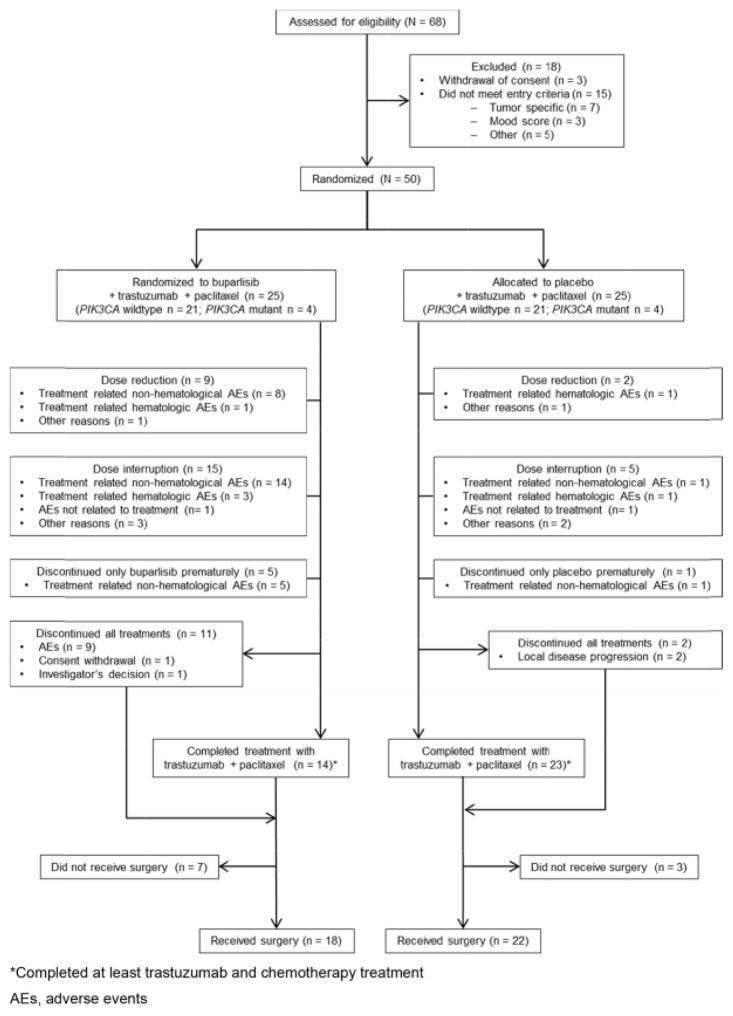
Between 3rd September 2013 and 6th October 2014, 50 of the planned 256 patients were enrolled across 17 sites in four countries and randomised equally to either the buparlisib arm (n = 25) or placebo arm (n = 25). In each arm, 21 patients (84%) belonged to the wild-type PIK3CA cohort and 4 patients (16%) belonged to the PIK3CA mutant cohort. Recruitment was suspended due to AEs leading to early treatment discontinuations in the buparlisib arm. Of the 25 patients in the buparlisib arm, 11 patients discontinued prematurely the planned 18 weeks treatment, the primary reason being AEs (n = 9). Of the 50 randomised patients, 40 patients (18 in buparlisib arm and 22 in placebo arm) underwent surgery. The data from all 50 randomised patients were included in the efficacy and safety analyses (Fig. 1).
Fig. 1.
Consort statement.
Baseline and treatment characteristics were well balanced between the two treatment arms. The majority of patients had stage 2 tumours (84% in buparlisib arm; 96% in placebo arm) and no lymph node metastases (68% in buparlisib arm; 64% in placebo arm). Tumours were ER+ in 64% of patients in the buparlisib arm and 60% of patients in the placebo arm (Table 1). PIK3CA mutations were detected in overall 16% (n = 8) of the patients (Table 1).
Table 1.
Baseline characteristics.
| Characteristic | Buparlisib + trastuzumab + paclitaxel n = 25 |
Placebo + trastuzumab + paclitaxel n = 25 |
|---|---|---|
| Age, median (range), years | 50.0 (35.0–72.0) | 50.0 (26.0–78.0) |
| BMI, median (range), kg/m2 | 25.1 (18.1–38.6) | 23.9 (19.7–35.9) |
| Race, n (%) | ||
| Caucasian/white | 21 (84.0) | 24 (96.0) |
| Black | 0 (0.0) | 0 (0.0) |
| Asian | 3 (12.0) | 1 (4.0) |
| Other | 1 (4.0) | 0 (0.0) |
| ECOG performance status, n (%) | ||
| ECOG 0 | 25 (100) | 25 (100) |
| Menopausal status, n (%) | ||
| Premenopausal | 15 (60.0) | 17 (68.0) |
| Postmenopausal | 10 (40.0) | 8 (32.0) |
| Tumour size, median (range), mma | 27.0 (17.0–57.0) | 23.0 (13.0–40.0) |
| Ki67, median (range), % | 30.0 (20.0–95.0) | 40.0 (14.0–60.0) |
| Ki67, n (%)b | ||
| ≤20% | 5 (22.7) | 5 (22.7) |
| >20% | 17 (77.3) | 17 (77.3) |
| Clinical tumour stage, n (%)a | ||
| cT1 | 3 (12.0) | 1 (4.0) |
| cT2 | 21 (84.0) | 24 (96.0) |
| cT3 | 1 (4.0) | 0 (0.0) |
| Clinical lymph node status, n (%)a | ||
| cN0 | 17 (68.0) | 16 (64.0) |
| cN1 | 7 (28.0) | 7 (28.0) |
| cN2 | 1 (4.0) | 2 (8.0) |
| ER status, n (%) | ||
| Positive | 16 (64.0) | 15 (60.0) |
| Negative | 9 (36.0) | 10 (40.0) |
| PgR status, n (%) | ||
| Positive | 10 (40.0) | 12 (48.0) |
| Negative | 15 (60.0) | 13 (52.0) |
| HER2 status, n (%) | ||
| Positive | 25 (100.0) | 25 (100.0) |
| Negative | 0 (0.0) | 0 (0.0) |
| PIK3CA status, n (%) | ||
| Mutant | 4 (16.0) | 4 (16.0) |
| Wild type | 21 (84.0) | 21 (84.0) |
| PIK3CA exon-specific mutations, n (%) | ||
| Wild type | 21 (84.0) | 21 (84.0) |
| Exon 9 | 2 (8.0) | 2 (8.0) |
| Exon 20 | 2 (8.0) | 2 (8.0) |
| Both exon 9 and exon 20 | 0 (0.0) | 0 (0.0) |
BMI, body mass index, ECOG, Eastern Cooperative Oncology Group; ER, oestrogen receptor; HER2, human epidermal growth factor receptor-2; PgR, progesterone receptor.
Assessed using ultrasound or magnetic resonance imaging.
Assessed before randomisation.
3.2. Treatment duration, dose reductions/interruptions and discontinuations
The overall median duration of treatment was 18.0 weeks in both arms. Median treatment duration of buparlisib was significantly shorter than placebo (9.0 weeks versus 18.0 weeks) (Table 2). Dose reduction was more frequent in the buparlisib arm (36%) compared to the placebo arm (8.0%) (P = 0.019). Similarly, more patients in the buparlisib arm had dose interruptions versus placebo (60.0% versus 20.0%). Occurrence of non-haematological AEs was the most common reason for dose reduction (32%) and dose interruption (56%) in the buparlisib arm. Buparlisib was discontinued prematurely in 14 patients and placebo was discontinued in one patient. Paclitaxel dose was reduced in three patients in the buparlisib arm and one patient in the placebo arm, the most common reason being treatment related non-haematological toxicity (two patients in buparlisib arm and one patient in placebo arm) Overall 13 patients across both treatment arms discontinued all treatments (Fig. 1). The most common reason for treatment discontinuation was increase in liver enzymes/hepatotoxicity. Discontinuations occurred early, within 6–8 weeks of treatment initiation.
Table 2.
Duration of treatment of buparlisib, trastuzumab and paclitaxel.
| Treatment duration, median (range), weeks | Buparlisib + trastuzumab + paclitaxel n = 25 |
Placebo + trastuzumab + paclitaxel n = 25 |
|---|---|---|
| Overall treatment duration | 18.0 (2.0–19.1) | 18.0 (4.1–18.9) |
| Buparlisib/placebo | 9.0 (2.0–19.1) | 18.0 (4.1–18.9) |
| Trastuzumab | 17.0 (2.0–18.0) | 18.0 (4.0–18.0) |
| Paclitaxel | 11.0 (0.0–12.0) | 12.0 (0.0–12.0) |
3.3. Efficacy
Based on the 50 patients, the addition of buparlisib to trastuzumab and paclitaxel did not improve pCR rates in either the overall population or in patients in the PIK3CA wild-type/mutant cohorts or those stratified by ER status. pCR rate (ypT0/is) in the overall population was similar in the buparlisib arm versus placebo arm (32.0% versus 40%; P = 0.811). Similarly, there was no significant increase in pCR rates between buparlisib and placebo arms in the PI3KCA wild-type cohort (33.3% versus 42.9%; P = 0.830) or PIK3CA mutant cohort (25% in both arms; P = 0.786). Buparlisib did not improve pCR rates in the ER+ (31.3% versus 26.7%; P = 0.546) and ER− subgroups (33.3% versus 60.0%; P = 0.949) (Table 3).
Table 3.
pCR rates in overall population and subgroups by definition of primary end-point and additional pCR definitions.
| Buparlisib+ trastuzumab + paclitaxel
|
Placebo +trastuzumab + paclitaxel
|
P-Valuea | |||
|---|---|---|---|---|---|
| n | pCR, n (%) [95% CI] | n | pCR, n (%) [95% CI] | ||
| ypT0/is | ypT0/is | ||||
| All patients | 25 | 8 (32.0) [14.9%–53.5%] | 25 | 10 (40.0) [21.1%–61.3%] | 0.811 |
| PIK3CA status | |||||
| PIK3CA WT | 21 | 7 (33.3) [14.6%–57.0%] | 21 | 9 (42.9) [21.8%–66.0%] | 0.830 |
| PIK3CA MT | 4 | 1 (25.0) [0.6%–80.6%] | 4 | 1 (25.0) [0.6%–80.6%] | 0.786 |
| ER status | |||||
| ER+ | 16 | 5 (31.3) [11.0%–58.7%] | 15 | 4 (26.7) [7.8%–55.1%] | 0.546 |
| ER− | 9 | 3 (33.3) [7.5%–70.1%] | 10 | 6 (60.0) [26.2%–87.8%] | 0.949 |
| ypT0/is ypN0 | ypT0/is ypN0 | ||||
| All patients | 25 | 8 (32.0) [14.9%–53.5%] | 25 | 9 (36.0) [18.0%, 57.5%] | 0.724 |
| ER status | |||||
| ER+ | 16 | 5 (31.3) [11.0%–58.7%] | 15 | 3 (20.0) [4.3%–48.1%] | 0.382 |
| ER− | 9 | 3 (33.3) [7.5%–70.1%] | 10 | 6 (60.0) [26.2%–87.8%] | 0.949 |
| ypT0 ypN0 | ypT0 ypN0 | ||||
| All patients | 25 | 5 (20.0) [6.8%–40.7%] | 25 | 7 (28.0) [12.1%–49.4%] | 0.840 |
| ER status | |||||
| ER+ | 16 | 2 (12.5) [1.6%–38.3%] | 15 | 2 (13.3) [1.7%–40.5%] | 0.725 |
| ER− | 9 | 3 (33.3) [7.5%–70.1%] | 10 | 5 (50.0) [18.7%, 81.3%] | 0.885 |
CI, confidence interval; ER, oestrogen receptor; MT, mutant; pCR, pathological complete response; WT, wild-type.
One-sided Fisher exact test.
ORR at the end of biologic window (6 weeks) was numerically higher in the buparlisib arm versus placebo arm in the overall population (56% versus 44%; P = 0.286) and PIK3CA wild-type cohort (61.9% versus 42.9%; P = 0.177). However, this difference was not statistically significant. A trend towards higher ORR with buparlisib was observed in the ER-positive subgroup (68.8% versus 33.3%; P = 0.053) but not in the ER-negative subgroup (33.3% versus 60%; P = 0.949) (Table 4). The interaction test for ER status and buparlisib treatment was significant for ORR (Pinteraction = 0.032).
Table 4.
Best overall response at week 6.
| Response at the end of biologic window | Buparlisib + trastuzumab + paclitaxel n (%) |
Placebo + trastuzumab + paclitaxel n (%) |
P-Valuea |
|---|---|---|---|
| All patients | n = 25 | n = 25 | |
| CR | 0 (0.0) | 1 (4.0) | |
| PR | 14 (56.0) | 10 (40.0) | |
| SD | 7 (28.0) | 9 (36.0) | |
| PD | 1 (4.0) | 3 (12.0) | |
| Missing | 3 (12.0) | 2 (8.0) | |
| ORR (CR or PR) | 14 (56.0) | 11 (44.0) | 0.286 |
| 95% CI for ORR | 34.9%–75.6% | 24.4%–65.1% | |
| PIK3CA status | |||
| PIK3CA WT | n = 21 | n = 21 | |
| CR | 0 (0.0) | 1 (4.8) | |
| PR | 13 (61.9) | 8 (38.1) | |
| SD | 5 (23.8) | 8 (38.1) | |
| PD | 1 (4.8) | 2 (9.5) | |
| Missing | 2 (9.5) | 2 (9.5) | |
| ORR (CR or PR) | 13 (61.9) | 9 (42.9) | 0.177 |
| 95% CI for ORR | 38.4%–81.9% | 21.8%–66.0% | |
| PIK3CA MT | n = 4 | n = 4 | |
| CR | 0 (0.0) | 0 (0.0) | |
| PR | 1 (25.0) | 2 (50.0) | |
| SD | 2 (50.0) | 1 (25.0) | |
| PD | 0 (0.0) | 1 (25.0) | |
| Missing | 1 (25.0) | 0 (0.0) | |
| ORR (CR or PR) | 1 (25.0) | 2 (50.0) | 0.929 |
| 95% CI for ORR | 0.6%–80.6% | 6.8%–93.2% | |
| ER status | |||
| ER+ | n = 16 | n = 15 | |
| ORR (CR or PR) | 11 (68.8) | 5 (33.3) | 0.053b |
| 95% CI for ORR | 41.3%–89.0% | 11.8%–61.6% | |
| ER− | n = 9 | n = 10 | |
| ORR (CR or PR) | 3 (33.3) | 6 (60.0) | 0.949 |
| 95% CI for ORR | 7.5%–70.1% | 26.2%–87.8% | |
CI, confidence interval; CR, complete response; ER, oestrogen receptor; MT, mutant; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease; WT, wild-type.
One-sided Fisher exact test.
Significant interaction.
3.4. Safety
The most common any-grade non-haematological AEs in the buparlisib versus placebo arms were increased alanine aminotransferase (ALT) (84% versus 72%), increased aspartate aminotransferase (AST) (76% versus 36%), mucositis (76% versus 48%) and maculopapular rash (60% versus 48%). Anaemia (68% versus 72%), leukopenia (44% versus 60%) and lymphopenia (44% versus 32%) were the most common any-grade haematological AEs in buparlisib arm versus placebo arm. No febrile neutropenia was reported. The most common grade 3/4 AEs in the buparlisib versus placebo arms were increased ALT (48% versus 8%), increased AST (28% versus 0%) and maculopapular rash (20% versus 0%) (Table 5). The incidence of serious AEs (SAEs) was higher in the buparlisib arm versus placebo arm (36% versus 8%). In the buparlisib arm, nine patients (36%) discontinued treatment due to AEs. Liver toxicity typically occurred within the first few weeks of treatment, consisted typically of isolated transaminitis and was rarely associated with symptoms or signs of impaired liver function. All patients recovered and no Hy’s law cases were observed [34,35]. There were no treatment discontinuations due to AEs in the placebo arm. No deaths occurred during the study.
Table 5.
Incidence of adverse events (≥10% any grade incidence in any arm).
| Buparlisib + trastuzumab + paclitaxel N = 25 |
Placebo + trastuzumab + paclitaxel N = 25 |
|||
|---|---|---|---|---|
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Non-haematological AEs, n (%) | ||||
| Increased ALT | 21 (84.0) | 12 (48.0) | 18 (72.0) | 2 (8.0) |
| Increased AST | 19 (76.0) | 7 (28.0) | 9 (36.0) | 0 (0.0) |
| Mucositis | 19 (76.0) | 2 (8.0) | 12 (48.0) | 0 (0.0) |
| Alopecia | 18 (72.0) | n.a. | 17 (68.0) | n.a. |
| Maculo-papular rash | 15 (60.0) | 5 (20.0) | 12 (48.0) | 0 (0.0) |
| Diarrhoea | 15 (60.0) | 1 (4.0) | 10 (40.0) | 1 (4.0) |
| Increased total cholesterol | 14 (58.3) (missing: n = 1) | 0 (0.0) | 14 (60.9) (missing: n = 2) | 0 (0.0) |
| Peripheral sensory neuropathy | 14 (56.0) | 0 (0.0) | 16 (64.0) | 1 (4.0) |
| Fatigue | 13 (52.0) | 0 (0.0) | 14 (56.0) | 1 (4.0) |
| Increased FPG | 13 (52.0) | 3 (12.0) | 8 (32.0) | 1 (4.0) |
| Infection | 12 (48.0) | 1 (4.0) | 19 (76.0) | 1 (4.0) |
| Increased aPTTa | 5 (45.5) (missing: n = 14) | 0 (0.0) | 6 (40.0) (missing: n = 10) | 0 (0.0) |
| Nausea | 11 (44.0) | 0 (0.0) | 8 (32.0) | 0 (0.0) |
| Pruritus | 10 (40.0) | 0 (0.0) | 5 (20.0) | 0 (0.0) |
| Decreased calcium | 9 (36.0) | 0 (0.0) | 11 (44.0) | 0 (0.0) |
| Decreased sodium | 9 (36.0) | 1 (4.0) | 7 (28.0) | 0 (0.0) |
| Increased GGT | 8 (32.0) | 2 (8.0) | 7 (28.0) | 0 (0.0) |
| Fever without neutropenia | 7 (28.0) | 0 (0.0) | 3 (12.0) | 0 (0.0) |
| Increased uric acid | 6 (24.0) | 0 (0.0) | 10 (40.0) | 0 (0.0) |
| Depression | 6 (24.0) | 1 (4.0) | 8 (32.0) | 0 (0.0) |
| Increased AP | 6 (24.0) | 0 (0.0) | 6 (24.0) | 0 (0.0) |
| Decreased potassium | 6 (24.0) | 0 (0.0) | 2 (8.0) | 0 (0.0) |
| Increased triglycerides | 5 (20.8) (missing: n = 1) | 0 (0.0) | 6 (26.1) (missing: n = 2) | 0 (0.0) |
| Decreased serum albumin | 5 (20.8) (missing: n = 1) | 0 (0.0) | 2 (8.3) (missing: n = 1) | 0 (0.0) |
| Anxiety | 5 (20.0) | 1 (4.0) | 3 (12.0) | 0 (0.0) |
| Anorexia | 5 (20.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) |
| Constipation | 4 (16.0) | 0 (0.0) | 7 (28.0) | 0 (0.0) |
| Increased sodium | 4 (16.0) | 0 (0.0) | 2 (8.0) | 0 (0.0) |
| Increased potassium | 3 (12.0) | 0 (0.0) | 11 (44.0) | 1 (4.0) |
| Insomnia | 3 (12.0) | 0 (0.0) | 4 (16.0) | 1 (4.0) |
| Vomiting | 3 (12.0) | 0 (0.0) | 2 (8.0) | 0 (0.0) |
| Haematological AEs, n (%) | ||||
| Anaemia | 17 (68.0) | 0 (0.0) | 18 (72.0) | 1 (4.0) |
| Leukopenia | 11 (44.0) | 0 (0.0) | 15 (60.0) | 0 (0.0) |
| Lymphopenia | 11 (44.0) | 0 (0.0) | 8 (32.0) | 2 (8.0) |
| Neutropenia | 6 (24.0) | 1 (4.0) | 9 (36.0) | 0 (0.0) |
| Thrombopenia | 4 (16.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
AEs, adverse events; ALT, alanine aminotransferase; AP, alkaline phosphatase aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; FPG, fasting plasma glucose; GGT, gamma-glutamyl transferase; na, not available.
Assessed if clinically indicated.
3.5. Biomarker analysis
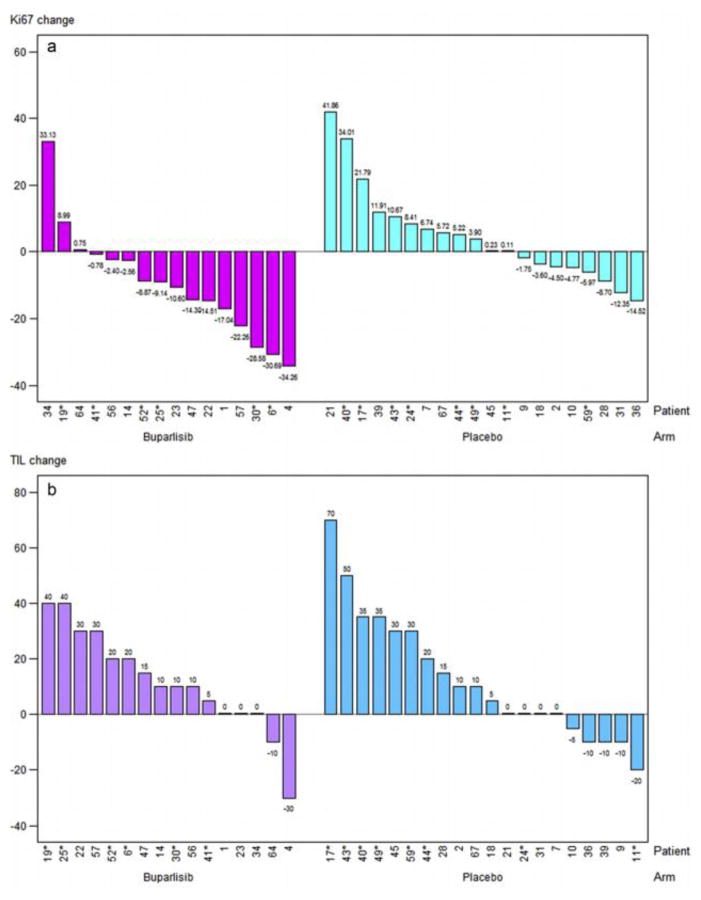
At baseline, there was no difference in percentage of low Ki67 (22.7%) between both arms. In day 15 samples, drop of Ki67 below 20% was observed in the complete study cohort, and the percentage of tumours with low proliferation increased to 43.9%. The rate of patients with Ki67 below 20% at day 15 was significantly higher in the buparlisib arm than in the placebo arm (66.7% versus 26.1%, p = 0.013) (Fig. 2a). The reduction in proliferation was stronger in the ER-positive subgroup (75% versus 26.7% with low Ki67; P = 0.021) than in the ER-negative subgroup (50% versus 25%; P =0.580).
Fig. 2.
Change in Ki67 levels and percentage of TILs. a: Change in Ki67 levels from baseline up to day 15 with buparlisib versus placebo. b: Change in percentage of TILs from baseline up to day 15 with buparlisib versus placebo. TIL, tumor infiltrating lymphocytes.
Baseline TIL values were not associated with pCR in the whole cohort. However, absolute changes (increase) from baseline to day 15 in TILs were significantly associated with pCR (odds ratio per 10% absolute change = 1.94 [95% CI 1.14–3.28]; P = 0.014), but not in Ki67 (odds ratio = 1.08 [95% CI 0.67–1.73]; P = 0.764) independently predicted pCR (Fig. 2b).
4. Discussion
The NeoPHOEBE trial evaluated a combination of a pan-PI3K inhibitor with HER2-targeted therapy and chemotherapy for HER2+ early breast cancer in cohorts defined by PIK3CA genotype, unlike contemporary trials which have evaluated dual HER2-targeted therapies plus chemotherapy in the neoadjuvant setting. The study was terminated after enrolment of 50 of the planned 256 (19%) patients mainly due to toxicity. As such, the study was underpowered and did not meet its primary objective of demonstrating improvement in pCR in the investigational arm. The dosing of buparlisib was limited by toxicity likely reducing its efficacy. Only eight patients had PIK3CA mutation which limited our ability to see differences in the PIK3CA wild-type/mutant cohort, a key objective of the study. Only 56.0% of the patients in the buparlisib arm completed the planned study treatment compared to 92.0% in the placebo arm. Discontinuations typically occurred during the first 6–8 weeks on study treatment, resulting in shorter buparlisib treatment exposure, and also to some extent shorted trastuzumab and paclitaxel exposure in the buparlisib arm. The overall safety profile (elevated liver enzymes, rash, hyperglycaemia, psychiatric disorders [i.e. depression and anxiety]) was consistent with that expected for buparlisib [22–24,26,36]. However, the frequency of these in this study was much higher than previously reported [26,37].
ORR at the end of biologic window (week 6), the key secondary objective of the study, trended in favour of buparlisib versus placebo (56% versus 44%) suggesting a higher clinical response. This effect was more pronounced in the ER-positive subgroup of patients where ORR in the buparlisib arm was 69% compared to 33% in the placebo arm and showed an opposite effect in the ER-negative subgroup. These findings were supported by exploratory biomarker data that showed a significant decrease in Ki67 levels after 2 weeks in the buparlisib arm versus placebo arm of the overall population (66.7% versus 26.1%; P = 0.013) and the ER-positive subgroup (75% versus 26.7%; P = 0.021). Patients with ER+/HER2+ breast cancer derive, in general, a lower benefit from neoadjuvant chemotherapy plus anti-HER2 treatment. Data from trials evaluating HER2-targeted therapies as well as from trials of the mTOR inhibitor everolimus suggest that patients with HER2+ hormone receptor-positive disease derived lower clinical benefit compared to patients with HER2+ hormone receptor-negative disease, highlighting an unmet need [38–42]. The promising ORR trend and Ki67 decrease with buparlisib suggest that the PI3K pathway is still a rational target and warrants further investigation in the future with more tolerable agents.
The absolute change in TILs from baseline to day 15 in both arms in the context of trastuzumab without chemotherapy was significantly associated with higher chance of achieving a pCR. This data further support the concept that trastuzumab monotherapy can enhance a functional antitumour immunity that results in augmented tumour responses [43]. This potentially underscores the clinical importance of the host immune response in this breast cancer subtype.
5. Conclusions
Results of the NeoPHOEBE study demonstrated that the addition of neoadjuvant buparlisib to trastuzumab and paclitaxel was not feasible and did not result in significantly improved pCR in patients with HER2+ primary breast cancer. In the buparlisib arm, a trend towards a higher ORR at the end of the biologic window was observed, predominantly in the ER-positive subgroup, supported by a significant Ki67 decrease after 2 weeks of treatment. Investigations using more specific second-generation PI3K inhibitors (such as alpelisib, taselisib) which are expected to have a better safety profile are ongoing [44,45]. Similarly, analysis of biomarker data from recent trials evaluating agents targeting the PI3K/AKT/mTOR pathway and those targeting HER2 may provide insights into the identifying of the subset of population which would benefit from a combination of a PI3K inhibitor and a HER2-targeted agent.
Acknowledgments
Role of the study sponsor
This study was funded by Novartis Pharmaceuticals Corporation and was performed in collaboration with the German Breast Group (GBG), the Spanish Oncology Group (SOLTI), and the Breast International Group (BIG). The sponsor provided study drugs and participated in regulatory and ethics approval along with the collaborators. The study protocol was designed by study investigators and data management, statistical evaluation and reporting was performed by GBG. Authors had full access to data for interpretation and analysis, were involved in development and approval of the manuscript and had the final responsibility for the decision to submit for publication.
The NEOPHOEBE study was funded by Novartis Pharmaceuticals Corporation and was performed in collaboration with the German Breast Group (GBG), the Spanish Oncology Group (SOLTI), and the Breast International Group (BIG). We thank the patients who took part in this trial, the investigators, study nurses and clinical research associates from the individual trial centres who supported this trial.
We thank the following groups and sites for their active contribution towards the conduct of this study: German Breast Group (GBG) (Germany), Luisenkrankenhaus Düsseldorf (Germany), Kliniken Essen-Mitte, Germany (Kümmel S, Reinisch M), University Hospital Erlangen, (Lux M), Helios Klinikum Berlin-Buch, Germany (Untch M), University Hospital Schleswig–Holstein Lübeck, Germany (Fischer D, Liedtke C), University Hospital Münster, Germany (Tio J), Sana-Klinikum Offenbach, Germany (Jackisch C, Loibl S, Seiler S), University Hospital Schleswig–Holstein, Kiel (Schem Ch), SRH Waldklinikum Gera, Germany (Zahm D), University Hospital Essen, Germany (Aktas B), St. Johannes Hospital, Dortmund, Germany (Kunz G), SOLTI Breast Cancer Research Group (Spain), Hospital Clinico Universitario de Valencia (Spain), Hospital San Joan de Reus, Spain (Mele M), Hospital Puerta de HierroMajadahonda, Spain (Cantos B), Hospital Universitario Virgen del Rocio, Spain (Gonzalez-Mancha MR), Cancer Therapeutics Research Group (CTRG), Singapore, National University Cancer Institute; Singapore (Lee SC), Australian New Zealand Breast Cancer Trials Group (ANZBCTG) (Australia), Concord Repatriation General Hospital, Australia (Beale P), Austrian Breast Cancer Study Group (ABCSG), International Breast Cancer Study Group (IBCSG) and Breast International Group (BIG) (Belgium). We thank Amol Hosing (Novartis Health-care Pvt Ltd) for providing medical editorial assistance with this manuscript.
Footnotes
Previous publications from the same study: Presented as poster at the 2015 San Antonio Breast Cancer Symposium (SABCS); Dec 08–Dec 12, San Antonio, Texas, USA.
Authors’ contributions
S Loibl and S Loi contributed to study concepts. The study was designed by S Loibl, S Loi, VN and SM. VN performed the quality control of data and algorithms, and statistical analysis. The manuscript was prepared and edited by S Loibl, S Loi, ST and PU. All the authors contributed to data acquisition, data analysis and interpretation, and manuscript review.
Conflict of interest statement
S Loibl has received funding from Novartis. LSC has received funding from Novartis, Roche, Pfizer, GlaxoSmithKline, Esai Co Ltd, ASLAN Pharmaceuticals, Roche and AstraZeneca. SK has a consulting/advisory role for Novartis. GS has received funding from Novartis, AstraZeneca, Pfizer, Celegne, Teva, Roche and Lilly. ML has received funding from Novartis. MJP has received funding from Roche. GVM has received funding from Novartis. JB has a leadership role at Infinity Pharmaceuticals and ownership interest in GRAIL. S Loi has received funding from Roche, Pfizer, Novartis and Merck. ST and PU are Novartis employees. All other authors declare no conflict of interest.
References
- 1.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200:290–7. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 3.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–40. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multi-centre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 5.Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol – Off J Am Soc Clin Oncol. 2012;30:1989–95. doi: 10.1200/JCO.2011.39.0823. [DOI] [PubMed] [Google Scholar]
- 6.Robidoux A, Tang G, Rastogi P, Geyer CE, Jr, Azar CA, Atkins JN, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1183–92. doi: 10.1016/S1470-2045(13)70411-X. [DOI] [PubMed] [Google Scholar]
- 7.Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol – Off J Eur Soc Med Oncol/ESMO. 2013;24:2278–84. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 8.Untch M, Fasching PA, Konecny GE, Hasmuller S, Lebeau A, Kreienberg R, et al. Pathologic complete response after neo-adjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol – Off J Am Soc Clin Oncol. 2011;29:3351–7. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 9.Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 2012;13:135–44. doi: 10.1016/S1470-2045(11)70397-7. [DOI] [PubMed] [Google Scholar]
- 10.Carey LA, Berry DA, Cirrincione CT, Barry WT, Pitcher BN, Harris LN, et al. Molecular heterogeneity and response to neo-adjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol – Off J Am Soc Clin Oncol. 2016;34:542–9. doi: 10.1200/JCO.2015.62.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukohara T. PI3K mutations in breast cancer: prognostic and therapeutic implications. Breast Cancer (Dove Med Press) 2015;7:111–23. doi: 10.2147/BCTT.S60696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/-phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68:9221–30. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kataoka Y, Mukohara T, Shimada H, Saijo N, Hirai M, Minami H. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol – Off J Eur Soc Med Oncol/ESMO. 2010;21:255–62. doi: 10.1093/annonc/mdp304. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9:1489–502. doi: 10.1158/1535-7163.MCT-09-1171. [DOI] [PubMed] [Google Scholar]
- 15.Rexer BN, Chanthaphaychith S, Dahlman K, Arteaga CL. Direct inhibition of PI3K in combination with dual HER2 inhibitors is required for optimal antitumor activity in HER2+ breast cancer cells. Breast Cancer Res BCR. 2014;16:R9. doi: 10.1186/bcr3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loibl S, von Minckwitz G, Schneeweiss A, Paepke S, Lehmann A, Rezai M, et al. PIK3CA mutations are associated with lower rates of pathologic complete response to anti-human epidermal growth factor receptor 2 (her2) therapy in primary HER2-overexpressing breast cancer. J Clin Oncol – Off J Am Soc Clin Oncol. 2014;32:3212–20. doi: 10.1200/JCO.2014.55.7876. [DOI] [PubMed] [Google Scholar]
- 17.Guarneri V, Dieci MV, Frassoldati A, Maiorana A, Ficarra G, Bettelli S, et al. Prospective biomarker analysis of the randomized CHER-LOB study evaluating the dual anti-HER2 treatment with trastuzumab and lapatinib plus chemotherapy as neoadjuvant therapy for HER2-positive breast cancer. Oncologist. 2015;20:1001–10. doi: 10.1634/theoncologist.2015-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loibl S, Darb-Esfahani S, Huober J, Klimowicz A, Furlanetto J, Lederer B, et al. Integrated analysis of PTEN and p4EBP1 protein expression as predictors for pCR in HER2-positive breast cancer. Clin Cancer Res – Off J Am Assoc Cancer Res. 2016;22:2675–83. doi: 10.1158/1078-0432.CCR-15-0965. [DOI] [PubMed] [Google Scholar]
- 19.Majewski IJ, Nuciforo P, Mittempergher L, Bosma AJ, Eidtmann H, Holmes E, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol – Off J Am Soc Clin Oncol. 2015;33:1334–9. doi: 10.1200/JCO.2014.55.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11:317–28. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 21.Ando Y, Inada-Inoue M, Mitsuma A, Yoshino T, Ohtsu A, Suenaga N, et al. Phase I dose-escalation study of buparlisib (BKM120), an oral pan-class I PI3K inhibitor, in Japanese patients with advanced solid tumors. Cancer Sci. 2014;105:347–53. doi: 10.1111/cas.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma CX, Luo J, Naughton M, Ademuyiwa F, Suresh R, Griffith M, et al. A phase I trial of BKM120 (buparlisib) in combination with fulvestrant in postmenopausal women with estrogen receptor-positive metastatic breast cancer. Clin Cancer Res – Off J Am Assoc Cancer Res. 2016;22:1583–91. doi: 10.1158/1078-0432.CCR-15-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martín M, Chan A, Dirix L, O’Shaughnessy J, Hegg R, Manikhas A, et al. Abstract A166: BELLE-4: a phase II study of buparlisib and paclitaxel in women with HER2− advanced breast cancer, with or without PI3K pathway activation. Mol Cancer Ther. 2015;14:A166. [Google Scholar]
- 24.Rodon J, Bendell J, Abdul RA, Homji N, Trandafir L, Quadt C, et al. P3-16-01: safety profile and clinical activity of single-agent BKM120, a pan-class I PI3K inhibitor, for the treatment of patients with metastatic breast carcinoma. Cancer Res. 2011;71 P3-16-01. [Google Scholar]
- 25.Martín M, Chan A, Dirix L, O’Shaughnessy J, Hegg R, Manikhas A, et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2− advanced breast cancer (BELLE-4) Ann Oncol. 2017;28:313–20. doi: 10.1093/annonc/mdw562. [DOI] [PubMed] [Google Scholar]
- 26.Saura C, Bendell J, Jerusalem G, Su S, Ru Q, De Buck S, et al. Phase Ib study of buparlisib plus trastuzumab in patients with HER2-positive advanced or metastatic breast cancer that has progressed on trastuzumab-based therapy. Clin Cancer Res – Off J Am Assoc Cancer Res. 2014;20:1935–45. doi: 10.1158/1078-0432.CCR-13-1070. [DOI] [PubMed] [Google Scholar]
- 27.Klauschen F, Wienert S, Schmitt WD, Loibl S, Gerber B, Blohmer JU, et al. Standardized Ki67 diagnostics using automated scoring–clinical validation in the GeparTrio breast cancer study. Clin Cancer Res – Off J Am Assoc Cancer Res. 2015;21:3651–7. doi: 10.1158/1078-0432.CCR-14-1283. [DOI] [PubMed] [Google Scholar]
- 28.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol – Off J Eur Soc Med Oncol/ESMO. 2015;26:259–71. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol – Off J Am Soc Clin Oncol. 1997;15:2483–93. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 30.Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681–95. doi: 10.1002/cncr.10741. [DOI] [PubMed] [Google Scholar]
- 31.Sinn HP, Schmid H, Junkermann H, Huober J, Leppien G, Kaufmann M, et al. Histologic regression of breast cancer after primary (neoadjuvant) chemotherapy. Geburtshilfe Frauenheilkd. 1994;54:552–8. doi: 10.1055/s-2007-1022338. [DOI] [PubMed] [Google Scholar]
- 32.Green MC, Buzdar AU, Smith T, Ibrahim NK, Valero V, Rosales MF, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol – Off J Am Soc Clin Oncol. 2005;23:5983–92. doi: 10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- 33.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol – Off J Am Soc Clin Oncol. 2015;33:983–91. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 34.Reuben A. Hy’s law. Hepatology. 2004;39:574–8. doi: 10.1002/hep.20081. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Drug-induced liver injury: a national and global problem. Available at: https://www.fda.gov/downloads/Drugs/.../Guidances/UCM174090.pdf.
- 36.Mayer IA, Abramson VG, Isakoff SJ, Forero A, Balko JM, Kuba MG, et al. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol – Off J Am Soc Clin Oncol. 2014;32:1202–9. doi: 10.1200/JCO.2013.54.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saura C, Sachdev J, Patel M, Cervantes A, Juric D, Infante J, et al. Ph1b study of the PI3K inhibitor taselisib (GDC-0032) in combination with letrozole in patients with hormone receptor-positive advanced breast cancer. Presented at the San Antonio Breast Cancer Conference; San Antonio, TX. December 09–13; 2014. [Google Scholar]
- 38.Andre F, O’Regan R, Ozguroglu M, Toi M, Xu B, Jerusalem G, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:580–91. doi: 10.1016/S1470-2045(14)70138-X. [DOI] [PubMed] [Google Scholar]
- 39.Baselga J, Cortes J, Im SA, Clark E, Ross G, Kiermaier A, et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol – Off J Am Soc Clin Oncol. 2014;32:3753–61. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 40.Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano MS, Neciosup SP, et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015;16:816–29. doi: 10.1016/S1470-2045(15)00051-0. [DOI] [PubMed] [Google Scholar]
- 41.Krop IE, Kim SB, Gonzalez-Martin A, LoRusso PM, Ferrero JM, Smitt M, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:689–99. doi: 10.1016/S1470-2045(14)70178-0. [DOI] [PubMed] [Google Scholar]
- 42.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knutson KL, Clynes R, Shreeder B, Yeramian P, Kemp KP, Ballman K, et al. Improved survival of HER2+ breast cancer patients treated with trastuzumab and chemotherapy is associated with host antibody immunity against the HER2 intracellular domain. Cancer Res. 2016;76:3702–10. doi: 10.1158/0008-5472.CAN-15-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickler M, Saura C, Oliveira M, Richards D, Krop I, Cervantes A, et al. Abstract P6-12-01: phase II study of taselisib (GDC-0032) plus fulvestrant in HER2-negative, hormone receptor-positive advanced breast cancer: analysis by <em>PIK3CA</em> and <em>ESR1</em> mutation status from circulating tumor DNA. Cancer Res. 2017:77. P6-12-01–P6-12-01. [Google Scholar]
- 45.Jain S, Santa-Maria CA, Rademaker A, Giles FJ, Cristofanilli M, Gradishar WJ. Phase I study of alpelisib (BYL-719) and T-DM1 in HER2-positive metastatic breast cancer after trastuzumab and taxane therapy. J Clin Oncol. 2017;35:1026. doi: 10.1007/s10549-018-4792-0. [DOI] [PubMed] [Google Scholar]