Summary
Background
Alpha-tocopherol (α-TP) supplementation is recommended for the prevention of various equine neuromuscular disorders. Formulations available include RRR-α-TP acetate powder and a more expensive but rapidly water-dispersible liquid RRR-α-TP (WD RRR-α-TP). No cost-effective means of rapidly increasing serum and cerebrospinal fluid (CSF) α-TP with WD RRR-α-TP and then sustaining concentrations with RRR-α-TP acetate has yet been reported.
Objectives
To evaluate serum, CSF and muscle α-TP concentrations in an 8-week dosing regimen in which horses were transitioned from WD RRR-α-TP to RRR-α-TP acetate.
Study design
Non-randomised controlled trial.
Methods
Healthy horses with serum α-TP of <2 μg/mL were divided into three groups and followed for 8 weeks. In the control group (n = 5), no α-TP was administered. In the second group (Group A; n = 7), 5000 IU/day RRR-α-TP acetate was administered. In the third group (Group WD–A; n = 7), doses of 5000 IU/day of WD RRR-α-TP were administered over 3 weeks, followed by a 4-week transition from WD RRR-α-TP to RRR-α-TP acetate, and a final 1 week of treatment with RRR-α-TP acetate. Serum samples were obtained weekly; muscle biopsies were obtained before, at 2.5 weeks and after supplementation. CSF samples were obtained before and after the 8-week period of supplementation.
Results
Serum α-TP increased significantly in Group WD–A at week 1 and remained significantly higher than in Group A and the control group throughout the transition, with inter-individual variation in response. Serum α-TP increased significantly by week 7 in Group A. CSF α-TP increased significantly in Group WD–A only. Muscle α-TP concentrations did not differ significantly across groups. Serum and CSF α-TP were closely correlated (r = 0.675), whereas serum and muscle-α-TP concentrations were not correlated.
Main limitations
The study duration was short and data on pre-transition CSF was lacking.
Conclusions
The administration of 5000 IU/day of water-dispersible RRR-α-TP rapidly increases serum α-TP. Serum and CSF α-TP concentrations are sustained with a gradual transition to 5000 IU/day of RRR-α-TP acetate. Periodic evaluation of serum α-TP concentrations is recommended because responses vary among individuals.
Keywords: horse, antioxidant, equine motor neuron disease, equine degenerative myeloencephalopathy, nutrition, supplements
Introduction
An adequate intake of vitamin E (α-tocopherol [α-TP]) and adequate concentrations of α-TP within the central nervous and muscular systems are important to the prevention of neuroaxonal dystrophy/equine degenerative myeloencephalopathy [1,2], equine motor neuron disease [3], vitamin E-deficient myopathy [4] and nutritional myodegeneration (along with adequate selenium) [5]. The recommended daily dietary intake of vitamin E in adult horses is 1–2 IU/kg bwt of α-TP per day [6]. The usual source of vitamin E for horses is green grass; however, in the USA grazing pasture has decreased significantly as a result of recent drought conditions [7]. Additionally, metabolic diseases often require horses to be maintained on dry forage such as hay, which contains significantly lower concentrations of α-TP than grass [8]. As a result, dietary supplementation with α-TP has become increasingly important. There are two commercial sources of α-TP. Synthetic α-TP (all-rac-α-TP) has equal amounts of the eight stereoisomers, whereas natural α-TP (RRR-α-TP) consists of only one isomer. Of the isomers in synthetic α-TP, its preferential uptake by the liver makes the RRR stereoisomer (RRR-α-TP) the most bioavailable isoform [9]. There are currently two formulations of commercially available RRR-α-TP: an acetate formulation is available as a powder or pellet and a micellised water-dispersible (WD) formulation is available as a liquid.
Synthetic all-rac-α-TP acetate at high doses (10,000 IU/day for 14 days) increases serum α-TP from deficient to normal ranges, but has no impact on cerebrospinal fluid (CSF) α-TP concentrations [10]. Thus, this formulation does not appear to be adequate for horses with neurological disease. Powdered formulations of RRR-α-TP acetate have levels of bioavailability roughly twice as high as those of synthetic all-rac-α-TP acetate and serum α-TP concentrations increase gradually with supplementation in a dose-dependent manner [11,12]. By contrast, WD RRR-α-TP is 5–6 times more bioavailable than synthetic all-rac-α-TP acetate and its administration results in a rapid increase in serum α-TP concentrations (within 12 h) [12]. Two-week dosage trials have shown that CSF α-TP increases within 2 weeks when horses are given 10,000 IU/day of WD RRR-α-TP [10]. The effects of lower dosages on CSF RRR-α-TP are unknown. However, at present, doses of up to 10,000 IU/day of WD α-TP are commonly used in horses with neurological disease.
The duration for which horses should be treated with WD RRR-α-TP and the best means of ceasing or transitioning the supplementation to another formulation are unclear from the current literature. The cessation of supplementation with WD RRR-α-TP results in a rapid decline of serum α-TP within 6 days of discontinuation [12]. A gradual transition to RRR-α-TP acetate appears to be a logical as well as an economical choice once clinical signs have resolved and serum and CSF concentrations have stabilised because acetate formulations are approximately 1.3 times less expensive than WD RRR-α-TP.
The purpose of the current study was to determine whether a transitioning regimen using WD and acetate α-TP formulations over an 8-week period could induce a rapid increase in and sustain high concentrations of α-TP in serum, muscle and CSF in vitamin E-deficient horses. We hypothesised that a gradual transition from WD RRR-α-TP to RRR-α-TP acetate over 8 weeks would result in a sustained increase in serum α-TP concentrations and the maintenance of higher serum, muscle and CSF α-TP levels than supplementation with an equivalent dose of RRR-α-TP acetate alone.
Materials and methods
Pilot trial
A preliminary study was performed in eight of the horses in the University of Minnesota teaching herd that were used in the supplementation trial described below. The purpose of the pilot trial was to determine the dose of α-TP and the method of transitioning from WD to acetate RRR-α-TP for the longer-term supplementation trial. In the pilot trial, horses received 3000 IU/day of WD RRR-α-TP for 14 days and were then transitioned to 3000 IU/day of RRR-α-TP acetate for 14 days. Blood samples were obtained prior to and at the end of each 14-day period and handled as described for the longer supplementation trial. Serum α-TP concentrations increased significantly (paired t test, P<0.01) after the administration of WD RRR-α-TP over 14 days from a mean ± s.d. of 1.60 ± 0.56 μg/mL to 3.24 ± 1.41 μg/mL and declined to 1.83 ± 0.65 μg/mL after 14 days of treatment with RRR-α-TP acetate. As a result, in the longer full supplementation trial, a protocol involving a higher dose of RRR-α-TP acetate and a combination of both WD and acetate RRR-α-TP was devised for the transition period prior to full RRR-α-TP acetate supplementation.
Supplementation trial
Horses
Nineteen healthy horses from the University of Minnesota teaching herd were included in this study. Horses included 17 mares and two geldings with a mean ± s.d. age of 13.4 ± 3.7 years (range: 8–22 years) and a mean ± s.d. bodyweight of 540 ± 42 kg (range: 460–635 kg). The sample population included Quarter Horses (n = 9), Thoroughbreds (n = 2), Paint crosses (n = 2), Warmbloods (n = 1), Appaloosas (n = 3), Quarter Horse–Irish Sports Horse cross (n = 1) and one horse of unknown crossbreeding.
For at least 6 months prior to the study, horses were fed free choice grass hay cubes (Happy Horse Maintenance Formula Biscuits™a) and some horses received a ration balancer (Empower Balance Grass Formula Supplementb) or other concentrate. At day −18, the diet of all horses was standardised to include only free choice grass hay cubes.a Any previously fed ration balancer, concentrates and hand-grazing were discontinued. All horses were housed on a dry lot before and throughout the trial at an accredited facility.
Study design
Eighteen days prior (day −18) to the 8-week supplementation trial, blood was collected to determine serum α-TP concentrations. Horses were classified based on sex and serum α-TP concentrations. As the number of geldings was small, geldings were allocated non-randomly to groups (one in each of the acetate and combination-treatment groups, and two in the control group). A random number generator was used to assign mares to one of three groups and a non-parametric Mann–Whitney test was used to determine a difference between groups at day −18. Assignments were assessed for mean serum α-TP concentrations; to ensure group means did not differ significantly among the three groups at baseline (P>0.9), four mares were re-allocated amongst groups to produce a mean serum α-TP of approximately 2 μg/mL in each group. Sample size calculations using mean serum concentrations of α-TP-deficient horses from the pilot trial described above (effect size: 1.76) determined that a minimum sample size of seven horses per group was required to achieve 80% power, with an α-value of 0.05.
The three groups consisted of: Group A, in which horses received 5000 IU/day RRR-α-TP acetate powder (Elevate® Maintenance Powderc) (n = 7); Group WD–A, in which horses received 5000 IU/day RRR-α-TP WD liquid (Elevate® W.S.c) and were gradually transitioned to 5000 IU/day RRR-α-TP acetate powderc (n = 7), and a control group, in which horses received no additional α-TP (n = 5). A dose of 5000 IU α-TP was equivalent to a dose of 9.3 IU/kg bwt (range: 7.8–10.8 IU/kg bwt). The transition protocol applied in Group WD–A involved the administration of 5000 IU/day WD RRR-α-TP during weeks 1–3, the reduction of WD RRR-α-TP by 1000 IU per week during weeks 4–8 (from 4000 IU in week 4 to 0 IU in week 8), and the increase of RRR-α-TP acetate by 1000 IU per week (from 1000 IU in week 4 to 5000 IU in week 8) to maintain a total supplement of 5000 IU RRR-α-TP per day. During week 8, horses received 5000 IU/day of RRR-α-TP acetate (Table 1).
TABLE 1.
Transition of RRR-α-tocopherol (RRR-α-TP) supplementation in Group WD–A (horses transitioning from treatment with water-dispersible [WD] to acetate RRR-α-TP)
| Week | WD RRR-α-TP, IU | RRR-α-TP acetate, IU | All-rac-α-TP acetate, IU |
|---|---|---|---|
| 1 | 5000 | 0 | 300 |
| 2 | 5000 | 0 | 300 |
| 3 | 5000 | 0 | 300 |
| 4 | 4000 | 1000 | 300 |
| 5 | 3000 | 2000 | 300 |
| 6 | 2000 | 3000 | 300 |
| 7 | 1000 | 4000 | 300 |
| 8 | 0 | 5000 | 300 |
To ensure that horses received their entire daily dose of RRR-α-TP, Group A and Group WD–A horses were housed in stalls at the same time each day. A feeder bag (Dura-Tech® Mesh Feed Bagd) containing the daily dose of α-TP mixed with 0.23 kg of a ration balancerb was placed over the nose of each horse. The ration balancer contained an additional 300 IU of all-rac-α-TP acetate. Feedbags were checked to ensure there was no residual feed or supplement before removal. Control horses received no α-TP supplementation or ration balancer.
Sampling of CSF
On day 0 of the trial, a jugular catheter was placed, horses were premedicated with xylazine (1.1 mg/kg bwt i.v.) and anaesthesia was induced with ketamine hydrochloride (2.2 mg/kg bwt i.v.) and diazepam (0.08 mg/kg bwt i.v.). Horses were placed in lateral recumbency and CSF was obtained at the atlanto-occipital site using an 8.3-cm, 18-gauge spinal needle. A minimum of 8–10 mL of a free flow of CSF was obtained. Samples were collected into plastic vials protected from light, stored on ice and centrifuged within 3 h. The supernatant was stored at −80°C prior to analysis. The procedure was repeated on day 55 at the end of the trial. Gross evidence of blood contamination was not observed in any sample.
Serum sampling
Baseline serum samples were obtained prior to beginning α-TP supplementation (day −18) and day 0 samples were collected prior to the CSF tap. At all timepoints, blood was collected via jugular venipuncture into serum separator vacuum tubes and immediately protected from light. The samples were stored on ice, refrigerated, protected from light and allowed to clot prior to serum separation, which occurred within 3 h after collection. Following serum separation, samples were transferred to polypropylene cryovials and stored at −80°C until analysis. Serum samples were taken weekly (days 0, 6, 13, 18, 26, 33, 40, 47) prior to the administration of that day’s supplement on the day of the next potential dosage alteration. Final serum samples were taken on completion of the trial (day 55), 24 h following the last administration of RRR-α-TP and prior to the CSF tap.
Muscle sampling
Gluteus medius muscle samples were obtained while horses were anaesthetised for CSF spinal taps at days 0 and 55. Additionally, muscle biopsies were obtained in standing horses at day 18 under sedation (0.4 mg/kg bwt xylazine i.v.). Samples were obtained at a standardised site previously described and repeat samples were taken from alternate sides [13]. Briefly, the site was aseptically prepared and a stab incision made at a point one-third of the distance on a line from the tuber sacrale to the tuber coxae and a Bergstrom biopsy needle used to obtain a muscle sample (minimum: 250 mg). Tissue was immediately freeze-dried and stored at −80°C until analysis.
Analysis of α-TP
Serum α-TP concentrations were assayed at Michigan State University by high-performance liquid chromatography with fluorescence detection as previously described [1].
Cerebrospinal fluid α-TP was analysed at the California Animal Health and Food Safety Laboratory System at the University of California Davis using inductively coupled argon plasma spectrometry as previously described [14].
Muscle α-TP was analysed at Michigan State University by chromatography as for serum. Approximately 250 mg of tissue was weighed and homogenised in distilled deionised water and methanol. Ascorbic acid and dibutylhydroxytoluene were added to the reagents prior to homogenisation, and lipids extracted from the homogenate with hexane. Duplicate analysis was performed on two separate aliquots of tissue from the same samples in eight horses.
Data analysis
Data for serum and CSF α-TP in the supplementation trial were normally distributed when assessed by a Kolmogorov–Smirnov test. A general linear model (GLM) ANOVA with post hoc Tukey–Kramer multiple comparisons was used to determine if there were differences among groups in serum α-TP at timepoints day −18 and day 0. To determine if serum α-TP changed over time, a repeated-measures ANOVA with post hoc Tukey–Kramer multiple comparison testing that included interactions for treatment and time was performed. A paired Student’s t test was used to determine if there were differences in CSF α-TP concentrations between pre- and post-trial CSF samples within each supplementation group. Muscle α-TP concentration was not normally distributed when assessed by a Kolmogorov–Smirnov test. Kruskal–Wallis and Dunn’s multiple comparison tests were performed on muscle α-TP concentrations. A Pearson product moment correlation was performed to determine correlations between serum α-TP concentrations and CSF or muscle α-TP concentrations. Coefficients of variation were calculated for duplicate muscle samples using the formula:coefficient of variation = s.d. of replicates/mean of replicates.
Values were expressed as the mean ± s.d. and significance indicated by a P<0.05. Analysis was performed using NCSS Statistical Software Version 10.0e and GraphPad Prism Version 5.0.f
Results
Baseline serum α-TP
Mean ± s.d. serum α-TP concentrations at day −18 were: control group, 2.04 ± 0.69 μg/mL (range: 1.62–3.11 μg/mL); Group A, 2.07 ± 0.52 μg/mL (range: 1.31–2.83 μg/mL), and Group WD–A, 2.06 ± 0.54 μg/mL (range: 1.37–2.87 μg/mL). Mean serum α-TP concentrations at day 0 were: control group, 1.38 ± 0.16 μg/mL (range: 1.57–1.54 μg/mL); Group A, 1.76 ± 0.46 μg/mL (range: 1.26–2.47 μg/mL), and Group WD–A, 1.33 ± 0.40 μg/mL (range: 0.59–1.70 μg/mL). There was a significant decline (P<0.001) in serum α-TP concentrations between day −18 and day 0 when all previous supplements and any hand-grazing of horses were terminated. There were, however, no statistically significant differences in mean α-TP concentrations among the control group, Group A and Group WD–A at either day −18 or day 0 (P = 0.45).
Serum α-TP in the control group
In the control group, mean serum α-TP concentrations did not change significantly (P = 0.98) between days 0 and 55, and mean values remained below 1.38 ± 0.32 μg/mL (Fig 1a).
Fig 1.
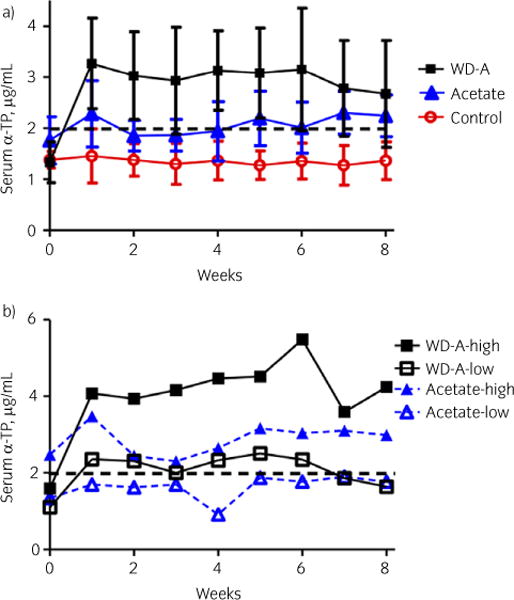
a) Mean ± s.d. serum α-tocopherol (α-TP) concentrations in control horses receiving no supplemental α-TP, horses receiving 5000 IU/day of RRR-α-TP acetate and horses receiving 5000 IU/day of water-dispersible (WD) RRR-α-TP for 3 weeks, followed by a tapering dose of WD RRR-α-TP and increasing dose of RRR-α-TP acetate, culminating in 5000 IU RRR-α-TP acetate for the final week. b) Serum concentrations of α-TP in the horse with the highest and horse with the lowest responses to acetate and combination α-TP supplementation. There was inter-individual variation in response to supplementation. The dotted line indicates the lower limit of the normal range [22].
Serum α-TP in Group A
Mean serum α-TP concentrations in Group A horses increased significantly during the supplementation trial by days 47 and 55 (P<0.01).
Serum α-TP in Group WD–A
Mean serum α-TP concentrations in Group WD–A horses increased significantly during the supplementation trial (P<0.01). Mean serum α-TP concentration increased significantly from day 0 to day 6 and remained elevated, with no significant difference in mean concentrations between days 6 and 55.
Differences in serum α-TP among groups
There was a significant treatment effect on mean serum α-TP concentrations (P<0.002) with significantly higher (P<0.0001) serum α-TP in Group WD–A horses than in Group A or control group horses. There was a significant interaction between treatment group and time (P = 0.000).
Concentrations of CSF α-TP with treatment
Mean CSF α-TP concentrations increased significantly during the supplementation trial in Group WD–A (P = 0.01), but did not change significantly with treatment in Group A (P = 0.51) or the control group (P = 0.79) (Fig 2a).
Fig 2.
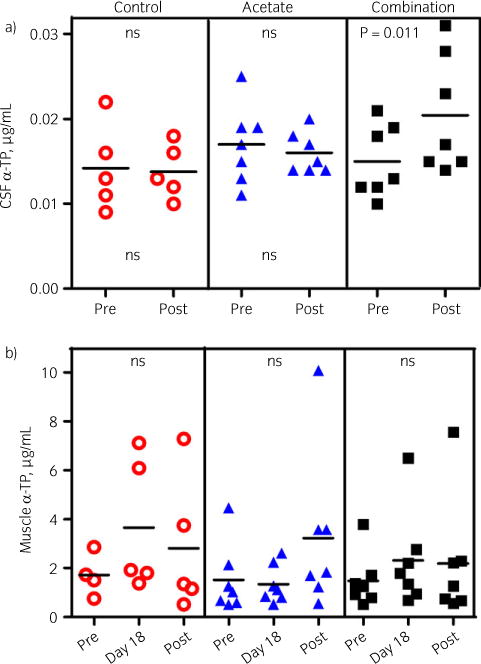
a) Individual values for cerebrospinal fluid (CSF) α-tocopherol (α-TP) in control horses, horses on 5000 IU/day of RRR-α-TP acetate and 5000 IU/day of a combination of water-dispersible (WD) and acetate RRR-α-TP before and at the end of the 8-week trial. Bars represent the mean values. CSF concentrations increased significantly only on the combination. b) Individual values for muscle α-TP in control horses, horses on 5000 IU/day of RRR-α-TP acetate and 5000 IU/day of a combination of WD and acetate RRR-α-TP before, at day 18 and at the end of the 8-week trial. Bars represent the mean. Muscle concentrations were highly variable and did not change significantly on any treatment. ns, not significant.
Differences in CSF α-TP among groups
There were no significant differences in mean CSF α-TP concentrations among Group A, Group WD–A and the control group (P = 0.2), although the power to detect differences was low (0.4). There was no significant interaction between group and time (P = 0.2).
Correlation between serum and CSF α-TP
A significant correlation (P<0.01, r = 0.68, r2 = 0.46) was found between serum and CSF α-TP concentrations (Fig 3a).
Fig 3.
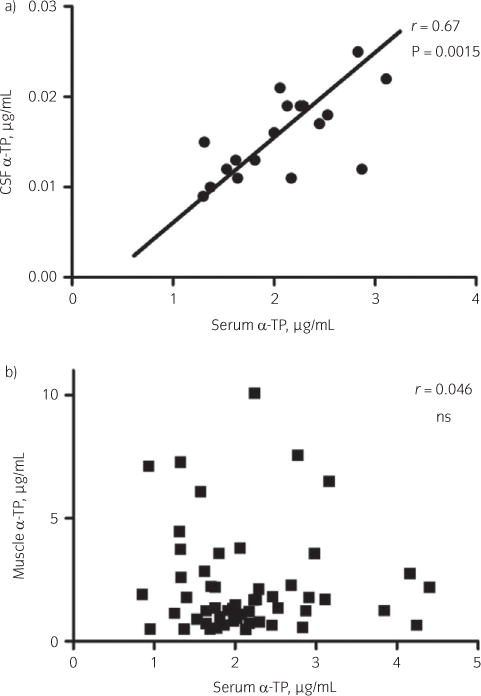
a) Significant positive correlation between cerebrospinal fluid (CSF) and serum α-tocopherol (α-TP) concentrations. b) A significant correlation was not found between muscle and serum α-TP concentrations. ns, not significant.
When horses with a serum α-TP concentration of <2 μg/mL were evaluated regardless of supplementation group (26 of 38 horses; serum α-TP 1.44 ± 0.3 μg/mL), mean CSF α-TP concentration was 15 ± 3 ng/mL (range: 9–22 ng/mL). In horses with a serum α-TP level of ≥2 μg/mL, regardless of supplementation group (12 of 38 horses; serum α-TP 2.69 ± 0.7 μg/mL), mean CSF α-TP was 20 ± 6 ng/mL (range: 14–31 ng/mL).
Muscle α-TP
There were no significant differences in time or treatment (P = 0.5) in muscle α-TP concentrations during the supplementation trial (Fig 2b). The coefficient of variation for duplicate analysis of muscle samples was low at 6.02%.
Correlation between serum and muscle α-TP
Serum and muscle α-TP were not significantly correlated (P = 0.8, r = 0.03, r2<0.01) (Fig 3b).
Discussion
Serum α-TP deficiency can be a common occurrence in horses without access to grass pasture as demonstrated in the herd of horses maintained on dry lots at the University of Minnesota. Based on price per IU, the most economical choice for an α-TP supplement would have been synthetic all-rac-α-TP acetate. However, previous studies have shown that doses as high as 8000 IU/day of synthetic all-rac-α-TP acetate are not effective in increasing serum α-TP even after 56 days of supplementation [12]. A dose of 10,000 IU/day of synthetic all-rac-α-TP acetate would appear to be required as this dose was shown to increase serum α-TP within 7 days in another study [10]. The 300 IU of all-rac-α-TP acetate in the ration balancer was considered unlikely to contribute to α-TP levels, and thus the balancer was used as a vehicle for the administration of supplementation when treated horses were brought in each day. In an ideal design, the control group would also have been fed the ration balancer. It is also relevant to note that this study used a non-random design to allocate horses to the treatment groups because we felt it was necessary to ensure that each group had a similar baseline serum α-TP concentration.
The use of RRR-α-TP acetate to increase serum α-TP concentrations in the university herd of horses was also based on economic considerations. The bioavailability of RRR-α-TP acetate is twice as high as that of synthetic all-rac-α-TP acetate [11]. The results of the current study showed that after 47 days, a dose of 5000 IU/day of RRR-α-TP acetate can effectively increase mean serum α-TP to within the normal range. Serum α-TP deficiency persisted in the control group in the current study, reinforcing the finding that changes in serum α-TP were the result of supplementation. Similar increases in serum α-TP were reported by Pagan et al. using a 5000-IU dose of RRR-α-TP acetate [11]. Thus, for horses showing no clinical signs of α-TP deficiency, supplementation with RRR-α-TP acetate at approximately 10 IU/kg bwt per day over the long term is a viable means of achieving normal serum α-TP. However, supplementation with 5000 IU of RRR-α-TP acetate does not appear to be a good choice for horses with neurological disease as no change in CSF α-TP concentrations occurred in the RRR-α-TP acetate group in the current trial.
Horses with clinical signs of equine motor neuron disease or vitamin E-deficient myopathy require an immediate increase in serum and CSF α-TP concentrations. Water-dispersible RRR-α-TP is five to six times more bioavailable than synthetic all-rac-α-TP acetate and has been shown to more than double serum concentrations within 12 h to 3 days of the administration of doses of 5000 IU [11] to 10,000 IU [10] once per day. In the current study, normal serum α-TP concentrations were also reached within the first sampling point 1 week after the initiation of 5000 IU/day of WD RRR-α-TP. Values in the current study were approximately half those achieved in a previous study on day 6 of supplementation with 10,000 IU/day of WD RRR-α-TP [10]. Ideally, a WD α-TP control group would have been included in the current study; however, there is previous evidence that 5000 IU/day results in a sustained increase in serum α-TP concentrations over 56 days [11]. In our pilot trial, a dose of 3000 IU/day for 14 days also achieved normal concentrations of α-TP (mean: 3.24 μg/mL). The administration of doses as low as 1000 IU/day of WD RRR-α-TP has previously been shown to produce values well within the normal range, but slightly lower (mean: 2.98 μg/mL at day 6) than those achieved in the current study (mean: 3.27 μg/mL at day 6) [12]. As serum concentrations were within the normal range in the first 2 weeks of 1000–5000 IU/day with supplementation, this range of dosage of WD RRR-α-TP seems to be a reasonable choice for the supplementing of horses deficient in vitamin E that require a rapid increase in serum concentrations. It is important to note that there was a great deal of individual variation in response to WD RRR-α-TP, where one horse demonstrated a 5.5-fold increase and one horse showed no change in serum α-TP concentrations (Fig 1b). Therefore, in a clinical setting, serum α-TP concentrations should be reassessed periodically to ensure horses are responding appropriately to the dose being utilised and higher doses initiated in horses with a poor response.
The primary goal of α-TP supplementation in horses with neurological disease, however, is to increase CSF rather than serum α-TP concentrations. Increases in CSF α-TP have been demonstrated previously after 10–14 days of supplementation with 10,000 IU/day of WD RRR-α-TP, achieving mean CSF α-TP concentrations of 23.2 ng/mL [12] and ~41 ng/mL [10]. Using a lower dose of 5000 IU/day of WD RRR-α-TP and a transition to 5000 IU/day of RRR-α-TP acetate, we were able to demonstrate a prolonged increase in CSF α-TP concentrations at 8 weeks after beginning supplementation, with mean CSF values of 16 ± 2 ng/mL in Group WD–A. Thus, the results of our supplementation trial would suggest that a dose of 5000 IU/day of WD RRR-α-TP is on average sufficient to increase CSF α-TP concentrations to within the normal range.
Based on our pilot trial and on a previous study, discontinuation of WD RRR-α-TP can result in a rapid decline in serum α-TP, even when horses are switched to an equivalent dose of RRR-α-TP acetate [12]. The tapering regime used in the current study avoided this rapid decline, producing a sustained increase in serum α-TP concentrations through the transition to 5000 IU/day RRR-α-TP acetate. Thus, this appears to be an ideal means of transitioning horses to a more cost-effective RRR-α-TP supplement. The decision to transition to RRR-α-TP acetate formulations in horses with neurological disease should be made on a case-by-case basis based on the severity of clinical signs, the response of clinical signs to treatment and serum α-TP concentrations. As serum α-TP concentrations showed a degree of decline during the final weeks of 5000 IU/day acetate supplementation, it would be prudent to re-evaluate serum α-TP levels after several weeks on the acetate regime to ensure concentrations remain within the normal range. It would have been ideal to continue the trial for several more weeks to determine if serum α-TP concentrations remained within the normal range in the transitioned group. Furthermore, the inclusion of a tapering WD formulation without α-TP acetate would have been ideal to better assess the effect of the addition of the acetate formulation to the tapering course. Unfortunately, this was not feasible in the current study.
In agreement with previous studies, we found a strong correlation between serum and CSF α-TP concentrations, indicating that low serum concentrations of α-TP provide good evidence of a deficiency in central nervous system concentrations of α-TP. Striatal brain tissue α-TP concentrations are significantly correlated with CSF concentrations in rats [15]. In addition, across species α-TP CSF concentrations are used to predict nervous tissue α-TP concentrations when brain biopsies are not feasible [16,17]. Previous studies have suggested normal ranges for adult equine CSF α-TP at 4.1–13.5 ng/mL [12,18] and 9.4–25.4 ng/mL calculated from a serum-CSF α-TP correlation and a desired serum α-TP level of 2.96 μg/mL [12]. The current study identified a range of 9–22 ng/mL (mean: 15 ng/mL) for CSF α-TP in horses with deficient serum α-TP, and a range of 14–31 ng/mL (mean: 20 ng/mL) for CSF α-TP in horses with adequate serum α-TP. Cytology was not performed on CSF to assess normalcy, but samples were collected after fluid had run for several seconds to avoid blood contamination, and no gross contamination was appreciated. Blood contamination of CSF by up to 9550 red blood cells (RBCs)/μL does not appear to alter CSF α-TP and selenium concentrations [18].
Muscle α-TP levels did not increase significantly with any form of α-TP supplementation in the current study. By contrast, a longer-term study found an increase in muscle α-TP concentrations after 112 days of supplementation with all-rac-α-TP acetate [19]. The peak in muscle α-TP concentration occurred at approximately week 12 in the previous study and concentrations of α-TP remained elevated for 7 weeks following the discontinuation of supplementation. The shorter duration of supplementation in the current study relative to the previous study may explain the lack of significant tissue response to α-TP supplementation. Uptake of α-TP into muscles requires lipoprotein lipase (LPL) [20]. Muscle α-TP concentrations have been shown to be inversely correlated with body mass index (BMI) in human subjects [21], with high adiposity and decreased muscle LPL activity leading to preferential deposition of α-TP in adipose tissue. Lipid has a higher vitamin E content than muscle tissue [19]. It is possible that variations in BMI among the horses in the trial or the inclusion of a variable amount of lipid within muscle fascicles caused the high degree of variability found in muscle α-TP concentrations in the present study. Methodological error in measuring muscle α-TP is unlikely as the coefficient of variation for muscle concentrations in duplicate samples was small. A study of vitamin E-deficient myopathy found instances of normal serum α-TP and low muscle α-TP concentrations, further indicating that there may not be a strong correlation between muscle and serum concentrations [4].
Conclusions
The results of the current study show that it is critical to select the most appropriate formulation and dose of α-TP in order to achieve normal and sustained serum and CSF α-TP concentrations. In horses with neurological disease, a rapid increase in α-TP concentrations to within the normal range is desired and was achieved with 5000 IU/day of WD RRR-α-TP, although individual variation occurred. A tapered regime that included a gradual transition from 5000 IU of WD RRR-α-TP to 5000 IU of RRR-α-TP acetate was effective in avoiding a precipitous drop in serum α-TP concentrations. In α-TP-deficient horses, normal serum but not normal CSF α-TP concentrations can be achieved within 47 days of treatment with 5000 IU/day RRR-α-TP acetate.
Acknowledgments
The authors would like to thank Sue Penny and Kelly Vallandingham, Veterinary Population Medicine, University of Minnesota, St Paul, MN, USA, for their help in executing the study.
Source of funding
This study was supported by funds provided by the University of Minnesota Equine Center and contributions from the Minnesota Racing Commission. The analyses of cerebrospinal fluid α-tocopherol and α-tocopherol products were supported by funds provided by Kentucky Performance Products LLC (Versailles, KY, USA).
Footnotes
Authors’ declaration of interests
None declared.
Ethical animal research
All procedures were approved by the University of Minnesota’s Institutional Animal Care and Use Committee.
Authorship
S.J. Valberg and C.J. Finno developed the study design. M. Hogg, J.C. Brown and S.J. Valberg executed the study, with C.J. Finno contributing to data collection. J.C. Brown, S.J. Valberg and C.J. Finno contributed to the interpretation of data and the preparation of the manuscript. All authors approved the final manuscript for submission.
Square Meal Feeds LLC, Cokato, Minnesota, USA.
Nutrena, Cargill, Inc., Minneapolis, Minnesota, USA.
Kentucky Performance Products LLC, Versailles, Kentucky, USA.
Dura-Tech™, Schneider Saddlery Co., Inc., Chagrin Falls, Ohio, USA.
NCSS, LLC, Kaysville, Utah, USA.
GraphPad Software, Inc., San Diego, California, USA.
References
- 1.Finno CJ, Higgins RJ, Aleman M, Ofri R, Hollingsworth SR, Bannasch DL, Reilly CM, Madigan JE. Equine degenerative myeloencephalopathy in Lusitano horses. J Vet Intern Med. 2011;25:1439–1446. doi: 10.1111/j.1939-1676.2011.00817.x. [DOI] [PubMed] [Google Scholar]
- 2.Mayhew IG, deLahunta A, Whitlock RH, Geary JC. Equine degenerative myeloencephalopathy. J Am Vet Med Assoc. 1977;170:195–201. [PubMed] [Google Scholar]
- 3.Divers TJ, Cummings JE, deLahunta A, Hintz HF, Mohammed HO. Evaluation of the risk of motor neuron disease in horses fed a diet low in vitamin E and high in copper and iron. Am J Vet Res. 2006;67:120–126. doi: 10.2460/ajvr.67.1.120. [DOI] [PubMed] [Google Scholar]
- 4.Bedford HE, Valberg SJ, Firshman AM, Lucio M, Boyce MK, Trumble TN. Histopathologic findings in the sacrocaudalis dorsalis medialis muscle of horses with vitamin E-responsive muscle atrophy and weakness. J Am Vet Med Assoc. 2013;242:1127–1137. doi: 10.2460/javma.242.8.1127. [DOI] [PubMed] [Google Scholar]
- 5.Dill SG, Rebhun WC. White muscle disease in foals. Compend Contin Educ Pract Vet. 1985;7:627–635. [Google Scholar]
- 6.National Research Council. Nutrient Requirements of Horses. 6th. National Academies Press; Washington, DC: 2007. [Google Scholar]
- 7.National Drought Mitigation Center. US Drought Monitor. 2016 [WWW document]. Available at: http://droughtmonitor.unl.edu/Home.aspx (Accessed 16 March 2016)
- 8.McDowell LR. Vitamins in Animal Nutrition. Academic Press; San Diego, CA: 1989. pp. 93–131. [Google Scholar]
- 9.Schneider C. Chemistry and biology of vitamin E. Mol Nutr Food Res. 2005;49:7–30. doi: 10.1002/mnfr.200400049. [DOI] [PubMed] [Google Scholar]
- 10.Pusterla N, Puschner B, Steidl S, Collier J, Kane E, Stuart RL. α-Tocopherol concentrations in equine serum and cerebrospinal fluid after vitamin E supplementation. Vet Rec. 2010;166:366–368. doi: 10.1136/vr.b4802. [DOI] [PubMed] [Google Scholar]
- 11.Pagan JD, Kane E, Nash D. Form and source of tocopherol affects Vitamin E status in Thoroughbred horses. Pferdeheilkunde. 2005;21:101–102. [Google Scholar]
- 12.Higgins JK, Puschner B, Kass PH, Pusterla N. Assessment of vitamin E concentrations in serum and cerebrospinal fluid of horses following oral administration of vitamin E. Am J Vet Res. 2008;69:785–790. doi: 10.2460/ajvr.69.6.785. [DOI] [PubMed] [Google Scholar]
- 13.Lindholm A, Piehl K. Fibre composition, enzyme activity and concentrations of metabolites and electrolytes in muscles of standardbred horses. Acta Vet Scand. 1974;15:287–309. doi: 10.1186/BF03547460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melton LA, Tracy ML, Moller G. Screening trace elements and electrolytes in serum by inductively coupled plasma emission spectrometry. Clin Chem. 1990;36:247–250. [PubMed] [Google Scholar]
- 15.Vatassery GT, Brin MF, Fahn S, Kayden HJ, Traber MG. Effect of high doses of dietary vitamin E on the concentrations of vitamin E in several brain regions, plasma, liver, and adipose tissue of rats. J Neurochem. 1988;51:621–623. doi: 10.1111/j.1471-4159.1988.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 16.Vatassery GT, Nelson MJ, Maletta GJ, Kuskowski MA. Vitamin E (tocopherols) in human cerebrospinal fluid. Am J Clin Nutr. 1991;53:95–99. doi: 10.1093/ajcn/53.1.95. [DOI] [PubMed] [Google Scholar]
- 17.Vatassery GT, Adityanjee, Quach HT, Smith WE, Kuskowski MA, Melnyk D. Alpha and gamma tocopherols in cerebrospinal fluid and serum from older, male, human subjects. J Am Coll Nutr. 2004;23:233–238. doi: 10.1080/07315724.2004.10719366. [DOI] [PubMed] [Google Scholar]
- 18.Finno CJ, Estell KE, Katzman S, Winfield L, Rendahl A, Textor J, Bannasch DL, Puschner B. Blood and cerebrospinal fluid α-tocopherol and selenium concentrations in neonatal foals with neuroaxonal dystrophy. J Vet Intern Med. 2015;29:1667–1675. doi: 10.1111/jvim.13618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roneus BO, Hakkarainen RV, Lindholm CA, Tyopponen JT. Vitamin E requirements of adult Standardbred horses evaluated by tissue depletion and repletion. Equine Vet J. 1986;18:50–58. doi: 10.1111/j.2042-3306.1986.tb03537.x. [DOI] [PubMed] [Google Scholar]
- 20.Sattler W, Levak-Frank S, Radner H, Kostner GM, Zechner R. Muscle-specific overexpression of lipoprotein lipase in transgenic mice results in increased alpha-tocopherol levels in skeletal muscle. Biochem J. 1996;318(Pt 1):15–19. doi: 10.1042/bj3180015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meydani M, Fielding RA, Cannon JG, Blumberg JB, Evans WJ. Muscle uptake of vitamin E and its association with muscle fiber type. Nutr Biochem. 1997;8:74–78. [Google Scholar]
- 22.Finno CJ, Valberg SJ. A comparative review of vitamin E and associated equine disorders. J Vet Intern Med. 2012;26:1251–1266. doi: 10.1111/j.1939-1676.2012.00994.x. [DOI] [PubMed] [Google Scholar]


