Abstract
Purpose:
To characterize the effect of the relative motion of esophagus and tumor on radiation doses to the esophagus in patients treated with stereotactic body radiation therapy for central lung tumors.
Methods and Materials:
Fifty fractions of stereotactic body radiation therapy in 10 patients with lung tumors within 2.5 cm of the esophagus were reviewed. The esophagus was delineated on each treatment’s cone-beam computed tomography scan and compared to its position on the planning scan. Dose–volume histograms were calculated using the original treatment beams to determine the actual dose delivered to the esophagus for each fraction of stereotactic body radiation therapy.
Results:
Median interfraction right–left shift of the esophagus was 0.9 mm (range, −5.4 to 3.3 mm) toward the left. Median interfraction anteroposterior shift was 0.7 mm (range, −3.7 to 11.5 mm) posteriorly. The median percentage increase in dose to 1 cm3, dose to 3.5 cm3, and dose to 5 cm3 was 1.7%, 5.6%, and 6.6%, respectively. Two cases of significant late esophageal toxicity were observed, with change in esophageal position relative to the planning target volume resulting in significantly higher D5cc values than anticipated.
Conclusion:
Interfraction shifts between the internal target volume and esophagus can lead to unanticipated increases in the volume of esophagus receiving high doses when treating central lung tumors with stereotactic body radiation therapy. Certain practical steps, such as considering deep breath hold for internal target volume reduction, using a planning risk volume for esophagus, and carefully visualizing and considering esophageal position at the time of stereotactic body radiation therapy, can be taken to minimize unanticipated dose increases that could cause unexpected esophageal toxicity.
Keywords: SBRT, treatment planning, esophagus toxicity, lung metastasis, organ motion
Background
Stereotactic body radiation therapy (SBRT) is an effective and well-tolerated treatment modality for primary nonsmall cell lung cancer (NSCLC) and lung metastases.1,2 For central lung tumors treated with SBRT, the esophagus is often in close proximity, and complications can include esophagitis, tracheoesophageal (TE) fistula, and esophageal perforation.3 The Radiation Therapy Oncology Group (RTOG) 0813 protocol has specified normal tissue constraints to consider when treating tumors near the esophagus; however, the appropriate dose–volume constraints to prevent esophageal complications in SBRT remain uncertain. Our group has previously reported that doses to small volumes of the esophagus, particularly D5cc, may be predictive of esophageal toxicity.4
Stereotactic body radiation therapy treatment planning is based on a single simulation computed tomography (CT) scan, and the position of the esophagus relative to the tumor is assumed to be constant. However, the position of the esophagus relative to the target volume during treatment may be affected by a number of variables. Variations in esophageal position are not typically accounted for in SBRT treatment planning; therefore, the actual radiation dose received may differ from the planned dose if this variation is significant. Several studies have reported interfraction and intrafraction esophageal motion and proposed use of a planning risk volume (PRV) which adds a margin around the esophagus to account for this motion during radiation therapy (RT).5–8
Tumor position within the thorax may also vary significantly based on patient respiration and positioning during treatment. Image guidance with cone beam CT (CBCT) imaging is often used to assist in targeting accuracy, allowing for accurate delivery of dose to the tumor. For central lung tumors, there are numerous organs at risk including the trachea, proximal bronchial tree, heart, and esophagus, and patient shifts based on image guidance targeting the tumor may alter the relationship of the organs at risk to the high-dose region. A recently published study by Dahele et al estimated the delivered dose to the proximal bronchial tree during each fraction for tumors near the central lung zone treated with SBRT using pretreatment CBCT scans matched to planning CT scans and found that there was concordance between the planned and the estimated delivered dose.9 However, the effects of these shifts on the estimated delivered dose to the esophagus have not previously been studied. We therefore analyzed planning CT scans and CBCT scans performed throughout the RT treatment course to characterize the effect of interfraction esophageal and tumor motion on radiation doses to the esophagus, particularly with respect to dose–volume metrics such as dose to 1 cm3 (D1cc) and dose to 5 cm3 (D5cc).4,10
Methods and Materials
Patient Selection
The institutional review and privacy boards approved this study, and patient confidentiality was maintained as required by the Health Insurance Portability and Accountability Act. We retrospectively reviewed the records of patients treated with SBRT at our institution from 2008 to 2013 for lung tumors in the central lung zone, whose planning target volume (PTV) was within 2.5 cm of the esophagus as measured in the axial dimension and whose CBCT scans were analyzable in our current treatment planning software (Eclipse version 11; Varian Medical Systems, Palo Alto, California). Ten patients met our inclusion criteria. Eight patients were treated for localized NSCLC, whereas 2 patients were treated for a lung metastasis from another primary tumor. All patients with primary NSCLC had early stage (I-II) disease. No patients received concurrent chemotherapy. Nine patients received 45 Gy in 5 fractions, whereas 1 patient received 50 Gy in 5 fractions; therefore, the study sample consisted of 50 SBRT fractions in total. Patient characteristics are summarized in Table 1.
Table 1.
Patient Demographics.
| No. of patients | 10 |
| Male/female | 3/7 |
| Age, years, median (range) | 68 (61-85) |
| Race | |
| White, non-Hispanic | 8 |
| Hispanic | 2 |
| Primary site | |
| Lung | 8 |
| Biliary tree | 1 |
| Colon | 1 |
| Location | |
| Right upper | 3 |
| Right middle | 2 |
| Right lower | 2 |
| Left upper | 2 |
| Left hilum | 1 |
| Tumor size | |
| <3 cm | 8 |
| >3 cm | 2 |
| Tumor location | |
| Upper esophagus (superior to tracheal bifurcation) | 5 |
| Mid esophagus (tracheal bifurcation to 8 cm inferior) | 3 |
| Lower esophagus (>8 cm inferior to tracheal bifurcation) | 2 |
All patients were assessed by a multidisciplinary team and were considered to be medically inoperable or opted for SBRT over surgery after consideration of the risk and benefits. Patients with prior thoracic radiotherapy or treated synchronously for multiple tumors were excluded.
Stereotactic Body Radiation Therapy Procedure
Our SBRT technique has been described previously.11 Each patient underwent simulation including 4-dimensional CT (4DCT) scans. A moldable immobilization device (Alpha Cradle; Smithers Medical Products, Northern Canton, Ohio) was custom fitted for each patient with the patient’s arms abducted over their heads.
The planning scan was acquired with quiet free breathing. Gross tumor volume (GTV) was contoured on axial slices of the planning CT. The GTV was expanded to define the internal target volume (ITV) based on respiratory excursion to include the visualized tumor on a slice-by-slice basis as seen during each phase of the 4DCT. The ITV was expanded 2 to 3 mm to generate the clinical target volume (CTV), and the CTV was expanded 5 mm in all directions to generate the PTV.
All patients were treated with intensity modulated radiation therapy (IMRT) using treatment plans calculated with an in-house treatment planning system. The treatment dose was prescribed so that 100% of the PTV was covered by at least 95% of the prescription dose. Planning target volume coverage was kept as homogeneous as possible, with tolerance of a hotspot up to 110% of the prescription dose. Maximum point dose to the esophagus was limited to 30 Gy, but when this was not realistic due to proximity of the PTV to the esophagus, a maximum point dose of 45 Gy in 5 fractions to the esophagus was allowed.
Patients were treated with 4 to 7 coplanar 6megavolt photon beams. After initial positioning and alignment, a CBCT scan was acquired and the visualized tumor was manually registered by rigid translations (Aria; Varian Medical Systems) with the ITV of the planning CT scan. If necessary, the patient was shifted so that the boundary of the visualized tumor was no more than 2 mm from the ITV contour. The CBCT was repeated to confirm the accuracy of any shift; treatment commenced after the final registration was approved by the physician. Gross intrafraction motion during treatment was monitored with an in-house system of 3 infrared reflectors placed at stable points (hips, costal cartilage, and sternum) on the patient surface. A Polaris camera (Northern Digital, Inc, Ontario, Canada) was used to track the individual reflectors to an accuracy of 0.5 mm. Motion traces are displayed by in-house written software, and if any motion trace moved away from the baseline by more the 3 mm, treatment was manually stopped and the patient was reimaged and repositioned as needed.
Patient Follow-Up
Patients were assessed for toxicity during treatment and 1 month after treatment completion. Starting 3 months after treatment, a CT scan and follow-up visit occurred every 3 months for the first 2 years and every 6 to 12 months thereafter. Esophageal toxicity was scored according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The highest score was recorded for each patient.
Contouring the Esophagus
The esophageal contours on the planning scans were retrospectively reviewed for each patient. The planning scan and contours and all treatment beams’ directions and control points were transferred to the Eclipse treatment planning system, and the Analytic Anisotropic Algorithm (AAA, V11) was used to recalculate the dose to more accurately reflect the effects of tissue inhomogeneity on the doses delivered to the anatomy seen in the planning scan.12 The AAA is acceptable for dose calculation in RT protocols nationwide and is used at our institution for a broad range of disease sites including for lung SBRT.
The registered CBCTs and their registration matrices were already available to Eclipse because treatments had been performed using the Aria Treatment Management system. A single clinician (A.P.) delineated the outer contour of the esophagus as visualized on each day’s final CBCT scan (ie, the CBCT whose registration to the tumor had been used to establish that day’s treatment position) from 2 cm superior to 2 cm inferior to the PTV on the CBCT. Mediastinal windowing was used for contouring the esophagus on the CBCT. A second clinician (A.W.) reviewed the contours for consistency and accuracy. The esophageal contours were then copied from the CBCT to the planning CT scan using the registration matrix of the day. To assess for intraobserver variation, the clinician recontoured the esophagus on 1 CBCT scan for each of the 10 patients without reference to the first CBCT contours.
Dose–volume histograms (DVHs) were calculated in Eclipse using the original treatment beams to determine the dose delivered to the esophagus for each fraction of SBRT, assuming no additional motion during treatment. DVH end points evaluated included D5cc (minimum dose in Gy to the 5 cm3 of the esophagus receiving the highest dose), mean dose (Dmean), dose to 3.5 cm3 (D3.5cc), D1cc, and maximum point dose (Dmax), which have been previously identified in prior studies to impact the risk of esophageal toxicity.
Data Analysis
For the esophagus from each CBCT, the percentage of difference between D1cc, D3.5cc, and D5cc determined from the CBCT and the corresponding planned value was compared for each fraction: that is, 100 × (DCBCT − Dplanned) / Dplanned. In addition, doses were converted into biological equivalent doses, using α/β = 10 Gy (BED10) since the analyzed esophageal events were acute, to determine the absolute difference in dose.
The Dice similarity coefficients (DSC) were calculated to determine the similarity between the esophagus contours for each CBCT scan and the planning CT scan.13 The lower the DSC, the less 2 structures overlap; a DSC greater than 0.7 represents a good overlap. The smallest orthogonal distance between the edges of the PTV and esophagus in the axial plane was determined by measuring this distance in each slice containing PTV and finding either the shortest distance (if esophagus and PTV remain separate) or the maximum overlap if these 2 structures overlap. The orthogonal distance between the esophagus and PTV on the planning scan was subtracted from the distance found on the CBCT so that a positive number corresponds to an increase in orthogonal distance or a decrease in overlap. The displacement of the esophagus centroid relative to the PTV centroid in the anterior–posterior (AP) and right–left (RL) direction was also determined. A positive AP displacement indicates posterior motion, while a positive RL displacement indicates leftward motion. These changes in AP and RL direction, as well orthogonal distance, were calculated for each esophagus structure from the CBCT when compared to the planning scan.
Results
Interfraction Movement
In the interest of brevity, we only report the median and range for the RL and AP shifts. For the orthogonal distance, which we feel has greater dosimetric impact, we also report the frequency distribution of changes (Figure 1). Median interfraction RL shift of the esophagus was 0.9 mm (range: −5.4 to 3.3 mm) toward the left. Median interfraction AP shift was 0.7 mm (range: −3.7 to 11.5 mm) posteriorly. Median change in the orthogonal distance between the PTV and esophagus contour was 0.4 mm (range: −8.9 to 14.7 mm) with positive changes being away from the PTV as shown in Figure 1.
Figure 1.
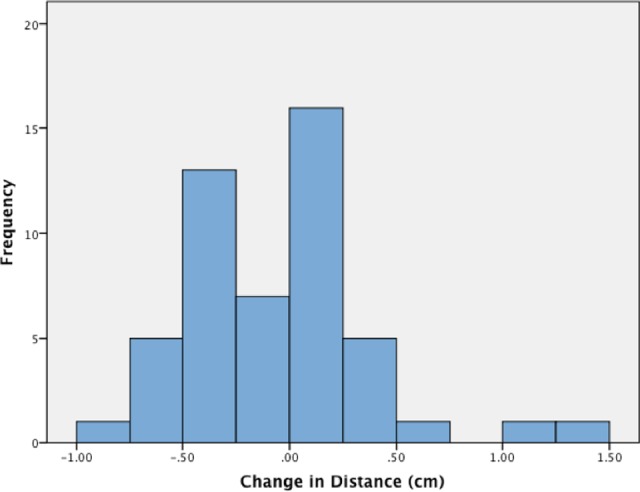
The orthogonal distance between planning target volume (PTV) and esophagus measured by examining each slice containing PTV and finding the closest orthogonal with either the shortest orthogonal distance between the edge of the PTV and the edge of the esophagus if esophagus and PTV remain separate or the maximum distance between the PTV and esophagus edges if these 2 structures overlap. The change in distance was found by subtracting the orthogonal distance on the planning scan from the distance on the cone beam computed tomography (CBCT) plan. Therefore, a negative change in distance corresponds to a change where the distance between esophagus and PTV decrease or the amount of overlap increases in the case of overlap.
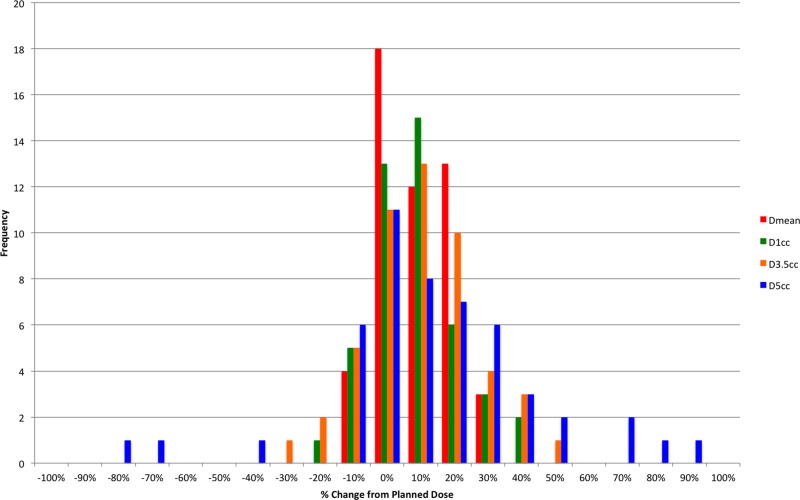
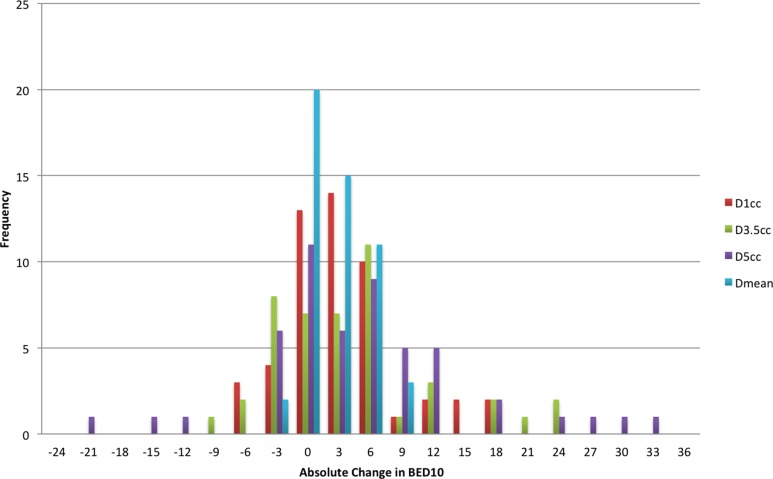
Over all patients, the median planned values for the full treatment course of the esophagus Dmax, D1cc, D3.5cc, D5cc, and Dmean were 29.2, 23.3, 20.1, 18.4, and 13.3 Gy, respectively. Distribution of the relative change in dose to D1cc, D3.5cc, D5cc, and Dmean between the planned dose and that predicted from the CBCT for each fraction are shown in Figure 2, whereas the absolute change in BED10 is shown in Figure 3. The median percentage change in D5cc was 6.6% (range: −80.4% to 86.6%), whereas the absolute change was 3.0 BED10 (range: −22.1 to 31.7). Median percentage change in D3.5cc was 5.6% (range: −36.9% to 43.9%), whereas the absolute change was 2.7 BED10 (range: −11.6 to 23.7). Median percentage change in D1cc was 1.7% (range: −20.2% to 5.7%), whereas the absolute change was 0.76 BED10 (range: −8.1 to 17.4). Eight patients received at least 1 fraction, where the D5cc, D3.5cc, and D1cc were greater than calculated from the plan, while 7 patients received at least 1 fraction, where Dmax and Dmean were greater than planned. Median DSC between the esophagus structures on the treatment plan and on the CBCTs was 0.63 (range: 0.04-0.83). In comparison, the median DSC between the original and the repeat esophagus structures from the intraobserver variation test was 0.82 (range: 0.77-0.88).
Figure 2.
Relative change in dose to 1 cm3 (D1cc), dose to 3.5 cm3 (D3.5cc), dose to 5 cm3 (D5cc), and mean distance (Dmean) between planned and predicted treatment dose for each fraction. For each fraction, the median percentage change in D5cc was 6.6% (range: −80.4% to 86.6%). Median percentage change in D3.5cc was 5.6% (range: −36.9% to 43.9%). Median percentage change in D1cc was 1.7% (range: −20.2% to 5.7%).
Figure 3.
Absolute change in dose to 1 cm3 (D1cc), dose to 3.5 cm3 (D3.5cc), dose to 5 cm3 (D5cc), and mean distance (Dmean) between planned and predicted treatment dose for each fraction in α/β = 10 Gy (BED 10). The absolute change in D5cc was 3.0 BED10 (range: −22.1 to 31.7). Median absolute change in D3.5cc was 2.7 BED10 (range: −11.6 to 23.7). Median absolute change in D1cc was 0.76 BED10 (range: −8.1 to 17.4).
Toxicity
Two patients experienced high-grade late esophageal toxicity. One patient received 45 Gy over 5 fractions for metastatic cholangiocarcinoma to the lung. At 23 months after completion of radiation, the patient developed a TE fistula. The other patient received 45 Gy over 5 fractions for NSCLC. This patient experienced grade 3 acute esophagitis after treatment and eventually developed a TE fistula at 14 months. In both cases of high-grade late toxicity, patients presented with dysphagia initially and a TE fistula was discovered on CT in both cases. The TE fistulas corresponded anatomically with the location of the SBRT, which was in the upper esophagus in one patient and in the lower esophagus in the other patient. Both patients were stented endoscopically with resolution of their symptoms.
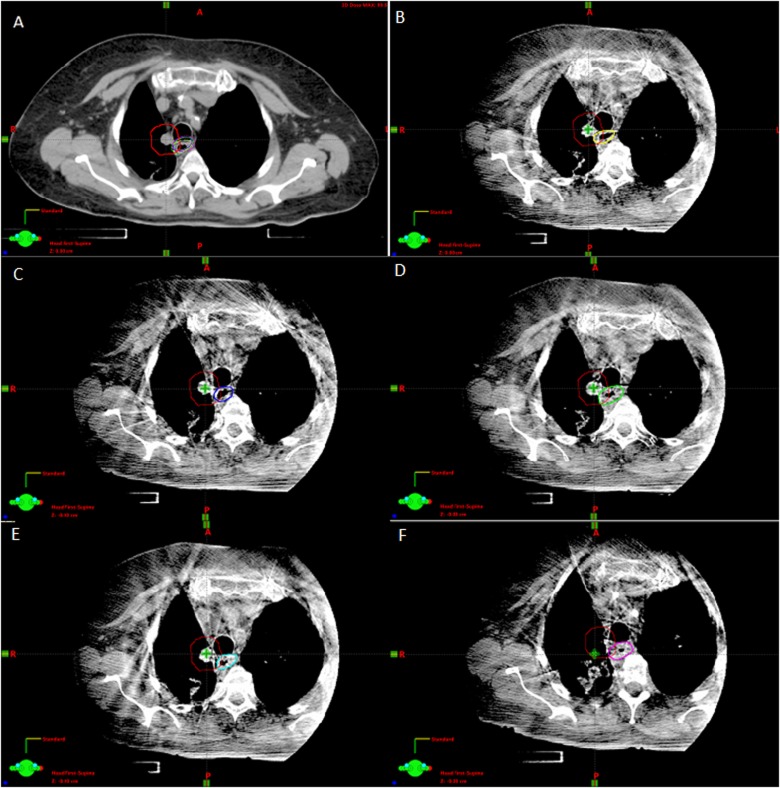
The patients who experienced high-grade toxicity had a calculated Dmax of 42.5 and 45 Gy, D1cc of 40.6 and 44.2 Gy, and D5cc of 18.4 and 26.4 Gy, respectively, based on the simulation CT and treatment plan that was recalculated in Eclipse. Review of the treatment plans for these patients demonstrated overlap between PTV and the esophagus on the original treatment plan of 0.34 cm3 and 0.44 cm3, respectively. In the first patient, overlap was greater on all CBCTs than at simulation with a median overlap volume of 1.24 cm3 (range: 0.85-1.55 cm3) as shown in Figure 4. The second patient had a median overlap of 0.38 cm3 (range: 0.25-0.57 cm3), with 2 fractions in which the overlap volume was 0.49 and 0.57 cm3. In both patients, the D5cc to the esophagus was greater than planned in all 5 fractions, with up to a 6.9 Gy increase with a median relative increase over all treatments for both patients of 36.51% (range: 5.1%-86.61%). Similarly, the D1cc to the esophagus was greater than planned in all 5 fractions for the first patient while it was greater in 3 of 5 fractions for the second patient. Table 2 summarizes these dose–volume metrics for the patients who experienced esophageal toxicity. No other symptomatic esophageal toxicities were observed.
Figure 4.
A, Treatment planning computed tomography (CT) for patient 1 with the esophagus contours from daily cone beam CT (CBCTs) seen in B-F superimposed. Planning target volume (PTV) is outlined in red. The planned overlap between PTV and esophagus volumes was 0.34 cm3. Shifts in relationship between the esophagus and tumor were measured. B, CBCT #1 with esophagus contoured in yellow. Overlap between PTV and esophagus volume was 1.00 cm3. C, CBCT #2 with esophagus contoured in blue. Overlap between PTV and esophagus volume was 1.28 cm3. D, CBCT #3 with esophagus contoured in green. Overlap between PTV and esophagus volume was 1.55 cm3. E, CBCT #4 with esophagus contoured in cyan. Overlap between PTV and esophagus volume was 0.85 cm3. F, CBCT #5 with esophagus contoured in magenta. Overlap between PTV and esophagus volume was 1.24 cm3.
Table 2.
Dosimetry for Patients who Experienced ≥3 Esophageal Toxicity After Treatment.a
| Patient | Site | Event Grade | D5cc, Gy | D3.5cc, Gy | D1cc, Gy | DMax, Gy | RL, mm | AP, mm | PTV, mm | DSC | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plan | Actual | Plan | Actual | Plan | Actual | Plan | Actual | |||||||
| 1 | RML | 3 | 18.4 | 30.5 | 27. 0 | 36.8 | 40.6 | 41.8 | 42.5 | 43.3 | −1.7 | −0.8 | −3.9 | 0.61 |
| 2 | RML | 3 | 26.4 | 30.5 | 36.4 | 38.2 | 44.1 | 44.2 | 45.0 | 45.0 | −0.4 | 1.1 | 1.0 | 0.80 |
Abbreviations: AP, anterior–posterior; CBCT, cone beam computed tomography; CT, computed tomography; DCS, Dice similarity coefficients; PTV, planning target volume; RL, right–left.
aThe actual dose is the median predicted treatment dose per fraction. AP: median posterior motion. PTV: median movement away (+) or towards (−) the PTV. DSC: median DSC between the esophagus contours for each CBCT scan versus the planning CT scan with a lower DSC representing overlap. RL: median leftward motion.
Discussion
Dose–volume histograms derived from the planning CT scan may incorrectly predict the delivered dose to the esophagus, which is mobile and also may be closer to the high-dose region after image-guided shifts based on tumor are applied. This may result in unexpected occurrence of high-grade esophageal adverse events in some patients treated with SBRT. We observed such events in patients who had an overlap of esophagus and PTV on the planning scan. Both patients met our planning constraints on the planning scan but analysis of their CBCTs revealed interfraction esophageal movement that had significant impact on volume of esophagus receiving high dose, such as measured by the D1cc and D5cc.
Stereotactic body radiation therapy has shown excellent local control of malignant disease.1,2 However, because of the high doses per fraction used in SBRT, less is known about the risk factors for normal tissue toxicity compared to conventionally fractionated RT. Several studies have analyzed rates of acute esophageal toxicity in conventionally fractionated treatment for a variety of dose–volume factors in an attempt to make definitive dosimetric recommendations which have been summarized by the QUANTEC review.3,14–16 There is a dose–response relationship, with mean esophagus dose higher than 34 Gy, V35 greater than 50%, V50 greater 40%, and V70 greater than 20% predicting for increased risk for esophageal toxicity. Similar studies have attempted to determine a dose–response model of esophageal toxicity in SBRT. A recent study by Stephans et al found that in SBRT for central NSCLC tumors, no significant late esophageal toxicity occurred when the Dmax was less than 50 Gy and the D1cc was less than 45 Gy.17 Higher than expected rates of esophageal toxicity due to SBRT were partially attributed to the use of chemotherapy and anti-vascular endothelial growth factor (VEGF) therapy. None of the patients in our study had prior chemotherapy or anti-VEGF modulating agents. Therefore, we would attribute esophageal toxicity directly to the esophageal radiation dose received. In a previously published study by our group of 125 patients with central lung tumors treated with SBRT which included the cohort in this analysis, we found that D5cc and Dmax best predicted for increased esophageal toxicity.4 The probability of complications at 2 years for those with D5cc >14.4 BED10 was 24% and for those with Dmax >29.6 BED10 was 21% compared to 2% and 7%, respectively, if the dose was less than or equal to those values. A literature review by Nuyttens et al compared a similar group of 58 patients with the 2 previous studies and found that the dose–response curves for grade 2 esophageal toxicity were similar to the findings of Wu et al.10 For 5 fraction treatments, they found that D1cc at a dose of 32.9 Gy and Dmax dose of 43.4 Gy corresponded to a complication probability of 50% for grade 2 toxicity. Despite these studies, there are no consensus dose limits for the esophagus for SBRT treatments.
The RTOG 0813 protocol limited esophagus dose to D5cc to less than 27.5 Gy and maximum point dose of 105% of prescription. Although our patients were treated prior to the inception of RTOG 0813, all of our study patients’ original treatment plans fulfilled the criteria for esophageal dose constraints laid out in this trial. Dosimetric constraints such as D1cc, D3.5cc, and D5cc were not implemented at our institution at that time. Evaluating both cases of high-grade toxicity, movement of the esophagus relative to the PTV resulted in cumulative doses that theoretically would have exceeded the constraints for D5cc (30.5 and 30.5 Gy, respectively). In addition, our findings that the actual esophageal dose may be significantly different than the planned dose (particularly for high-dose metrics such as Dmax or D5cc) complicate the efforts to ascertain predictive dose–volume thresholds, since these thresholds are invariably derived from planned rather than delivered esophageal dose.
Esophageal motion has been described in prior studies although many of these studies involved esophageal tumors, which may disrupt esophageal motion.6,7 A study of esophageal motion in patients with NSCLC by Dieleman et al found that a radial margin that incorporated 95% of lateral motion was 5 mm proximally, 7 mm in the mid-esophagus, and 9 mm in the distal esophagus.5 The majority of patients in our study had tumors near the upper esophagus. Combined with these prior studies, our findings further support consideration of planning organ-at-risk margins for the esophagus when planning high-dose SBRT for central lung tumors within 2 to 3 cm of the esophagus. Systematic analysis of a larger number of patients is needed to determine the appropriate margins, but based on our results and prior analyses, it appears that margins on the order of 5 to 10 mm should cover most cases. However, the trade-off with target coverage must also be considered when the esophagus overlaps the PTV.
Interfraction variations in the tumor position may also affect the relationship between the tumor and esophagus, independent of esophageal motion. Both intrafraction and interfraction mobility of lung tumors have been shown.18,19 A study by Chang et al used CBCT registered to the original treatment planning CT using the thoracic spine as a bony landmark to measure interfraction motion of lung tumor position.20 They found that the average centroid displacement between simulation CT and CBCT scans were 2.5 ± 2.7 mm, −2.0 ± 2.7 mm, and −1.5 ± 2.6 mm in the RL, AP, and superior–inferior directions. By verifying that the tumor visualized on the image acquired immediately before each treatment is within the ITV, IGRT reduces the effect of setup error or interfraction tumor motion on the dose delivered to the target volumes. However, realignment using IGRT may not account for the change in relationship between the tumor and the esophagus. The mobility of the lung tumor described by Chang et al could move the tumor so that the esophagus will receive a greater than expected dose, if image-guided shifts are based on tumor position at the time of treatment. Attention should be paid to ensure that such shifts do not inadvertently place the esophagus nearer to the high-dose region. In cases where esophagus was already the dose-limiting structure in planning SBRT, it may even be reasonable not to match directly on tumor in order to avoid including additional esophagus in the high-dose volume as long as the visualized tumor remains within the PTV.
More careful evaluation of the esophageal position should be considered both at simulation and at treatment, especially for patients with PTV abutting or overlapping the esophagus. Small shifts in relative position can result in a significant change in the volume of esophagus receiving high doses of radiation, which appears to be the most important risk factor for severe esophageal toxicity in SBRT. Although many patients with lung SBRT cannot sustain multiple deep breath holds due to comorbidities, we may consider using deep inspiration breath hold to reduce the ITV in capable patients whose esophagus is close to the tumor on the planning scan. Other ITV reduction methods such as abdominal compression or respiratory gating might also be considered. Esophageal contrast may help clinicians to visualize the location of the esophagus at the time of treatment as well as during simulation, particularly given that CBCT image quality is often suboptimal. If the visualized relationship between esophagus and PTV is significantly different on CBCTs compared to the simulation, the physician may even consider resimulating and replanning treatment. Finally, even if patient positioning or planning is not altered, the physician can consider the potential increased risk of esophageal complication when monitoring and counseling the patient after treatment.
This is the first study to attempt to investigate the effect of esophageal and tumor mobility on dosimetric variations in SBRT. Our study had several limitations including its retrospective nature and the fact that the results are based on a small study population and therefore a causal relationship between esophageal movement and toxicity cannot be determined. In addition, our contours are based on the clinical rigid registration between CBCT and planning scan, based on shifts made by therapists to place the tumor within the ITV as it appears on the CBCT. Since the registration is made to the ITV, the location of the esophagus at treatment relative to the planned high dose determines the dose to esophagus for each fraction of SBRT. Esophagus contours may have been transposed into anatomic areas on planning CT, which may not be an ideal surrogate for surroundings of the esophagus and could cause errors in the dose distribution. After reviewing the transposed CBCT contours, we found that in no case was any part of the registered esophagus transposed onto the lung. In some cases, slivers of the transposed esophagus encroached on the trachea instead of merely abutting it, but setting these structures to water density had minimal effect on the DVHs.
As this is a descriptive dosimetric study in a select group of patients rather than a systematic attempt to describe the risk of esophageal toxicity or find dosimetric predictors, a definite correlation between esophageal movement and toxicity cannot be proven. Although prior studies have identified small dose–volume constraints such at D5cc that are correlated with increased esophageal toxicity, this is not the purpose of this study. However, it is highly suggestive that in our 2 cases of significant late esophageal toxicity observed, variance in esophageal position relative to the PTV resulted in significantly higher small volume doses than anticipated, which we hypothesize may have enhanced their risk of toxicity. Certain practical steps, such as using a PRV for esophagus, and carefully visualizing and considering esophageal position at the time of SBRT, may also minimize unanticipated esophageal toxicity. In addition, our findings have implications for the discovery and interpretation of dose–volume constraints for the esophagus.
Conclusion
Interfraction shifts between the PTV and esophagus can lead to unanticipated increases in the volume of esophagus receiving high doses when treating central lung tumors with SBRT. These increases may increase the risk of esophageal toxicity for patients. Therefore, increased care should be taken to minimize unanticipated dose increases that could cause unexpected esophageal toxicity.
Abbreviations
- AAA
Analytic Anisotropic Algorithm
- AP
anterior–posterior
- BED10
α/β = 10 Gy
- CBCT
cone beam computed tomography
- DSC
Dice similarity coefficients
- DVH
dose–volume histogram
- Dmax
maximum point dose
- Dmean
mean dose
- D1cc
dose to 1 cm3
- D3.5cc
dose to 3.5 cm3
- D5cc
dose to 5 cm3
- GTV
gross target volume
- ITV
internal target volume
- NSCLC
nonsmall cell lung cancer
- PTV
planning target volume
- RL
right–left
- RT
radiation therapy
- RTOG
Radiation Therapy Oncology Group
- SBRT
stereotactic body radiation therapy
- TE
tracheoesophageal
- 4DCT
4-dimensional computed tomography.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grant P30 CA 008748.
ORCID iD: Abraham Jing-Ching Wu, MD http://orcid.org/0000-0002-2597-7091
References
- 1. Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer[Internet]. JAMA. 2010;303(11):1070–1076. http://jama.jamanetwork.com/article.aspx?articleid=185547. Accessed September 5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study[Internet]. Int J Radiat Oncol Biol Phys. 2009;75(3):677–682. http://www.sciencedirect.com/science/article/pii/S0360301608038509. Accessed September 5, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Werner-Wasik M, Yorke E, Deasy J, Nam J, Marks LB. Radiation dose-volume effects in the esophagus[Internet]. Int J Radiat Oncol Biol Phys. 2010;76(3 suppl):S86–S93. http://www.sciencedirect.com/science/article/pii/S0360301609032830. Accessed September 5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu AJ, Williams E, Modh A, et al. Dosimetric predictors of esophageal toxicity after stereotactic body radiotherapy for central lung tumors[Internet]. Radiother Oncol. 2014;112(2):267–271. http://www.thegreenjournal.com/article/S0167814014002874/fulltext. Accessed September 5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dieleman EMT, Senan S, Vincent A, Lagerwaard FJ, Slotman BJ, van Sörnsen de Koste JR. Four-dimensional computed tomographic analysis of esophageal mobility during normal respiration[Internet]. Int J Radiat Oncol Biol Phys. 2007;67(3):775–780. http://www.sciencedirect.com/science/article/pii/S0360301606033657. Accessed September 5, 2016. [DOI] [PubMed] [Google Scholar]
- 6. Cohen RJ, Paskalev K, Litwin S, Price RA, Feigenberg SJ, Konski AA. Esophageal motion during radiotherapy: quantification and margin implications[Internet]. Dis Esophagus. 2010;23(6):473–479. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2933373&tool=pmcentrez&rendertype=abstract. Accessed September 5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao K, Liao Z, Bucci MK, et al. Evaluation of respiratory-induced target motion for esophageal tumors at the gastroesophageal junction[Internet]. Radiother Oncol. 2007;84(3):283–289. http://www.ncbi.nlm.nih.gov/pubmed/17716759. Accessed September 5, 2016. [DOI] [PubMed] [Google Scholar]
- 8. Yaremko BP, Guerrero TM, McAleer MF, et al. Determination of respiratory motion for distal esophagus cancer using four-dimensional computed tomography[Internet]. Int J Radiat Oncol Biol Phys. 2008;70(1):145–153. http://www.ncbi.nlm.nih.gov/pubmed/17855008. Accessed September 5, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Dahele M, van Sörnsen de Koste JR, Verbakel WF, Slotman BJ, Senan S. An analysis of planned versus delivered airway doses during stereotactic lung radiotherapy for central tumors[Internet]. Acta Oncol. 2016;1–4. http://www.ncbi.nlm.nih.gov/pubmed/26878324. Accessed April 23, 2016. [DOI] [PubMed]
- 10. Nuyttens JJ, Moiseenko V, Mclaughlin M, Jain S, Herbert S, Grimm J. Esophageal dose tolerance in patients treated with stereotactic body radiation therapy. Semin Radiat Oncol. 2016;26(2):120–128. http://ac.els-cdn.com.libproxy2.usc.edu/S1053429615001162/1-s2.0-S1053429615001162-main.pdf?_tid=ddf4472a-194b-11e7-abc7-00000aacb35e&acdnat=1491320109_994f83b7f6eacc8c6bb32597e7cd57d4. Accessed April 4, 2017. [DOI] [PubMed] [Google Scholar]
- 11. Cuaron JJ, Yorke ED, Foster A, et al. Stereotactic body radiation therapy for primary lung cancers >3 centimeters[Internet]. J Thorac Oncol. 2013;8(11):1396–1401. http://www.ncbi.nlm.nih.gov/pubmed/24077457. Accessed September 5, 2016. [DOI] [PubMed] [Google Scholar]
- 12. Van Esch A, Tillikainen L, Pyykkonen J, et al. Testing of the analytical anisotropic algorithm for photon dose calculation[Internet]. Med Phys. 2006;33(11):4130 http://scitation.aip.org/content/aapm/journal/medphys/33/11/10.1118/1.2358333. Accessed January 10, 2015. [DOI] [PubMed] [Google Scholar]
- 13. Zou KH, Warfield SK, Bharatha A, et al. Statistical validation of image segmentation quality based on a spatial overlap index[Internet]. Acad Radiol. 2004;11(2):178–189. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1415224&tool=pmcentrez&rendertype=abstract. Accessed September 5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chapet O, Kong FM, Lee JS, Hayman JA, Ten Haken RK. Normal tissue complication probability modeling for acute esophagitis in patients treated with conformal radiation therapy for non-small cell lung cancer[Internet]. Radiother Oncol. 2005;77(2):176–181. http://www.sciencedirect.com/science/article/pii/S0167814005004263. Accessed September 5, 2016. [DOI] [PubMed] [Google Scholar]
- 15. Takeda K, Nemoto K, Saito H, Ogawa Y, Takai Y, Yamada S. Dosimetric correlations of acute esophagitis in lung cancer patients treated with radiotherapy[Internet]. Int J Radiat Oncol Biol Phys. 2005;62(3):626–629. http://www.sciencedirect.com/science/article/pii/S036030160500605X. Accessed September 5, 2016. [DOI] [PubMed] [Google Scholar]
- 16. Kahn D, Zhou S, Ahn SJ, et al. “Anatomically-correct” dosimetric parameters may be better predictors for esophageal toxicity than are traditional CT-based metrics[Internet]. Int J Radiat Oncol Biol Phys. 2005;62(3):645–651. http://www.sciencedirect.com/science/article/pii/S0360301604028500. Accessed September 5, 2016. [DOI] [PubMed] [Google Scholar]
- 17. Stephans KL, Djemil T, Diaconu C, et al. Esophageal dose tolerance to hypofractionated stereotactic body radiation therapy: risk factors for late toxicity[Internet]. Int J Radiat Oncol Biol Phys. 2014;90(1):197–202. http://www.ncbi.nlm.nih.gov/pubmed/25015204. Accessed September 5, 2016. [DOI] [PubMed] [Google Scholar]
- 18. Bissonnette JP, Franks KN, Purdie TG, et al. Quantifying interfraction and intrafraction tumor motion in lung stereotactic body radiotherapy using respiration-correlated cone beam computed tomography[Internet]. Int J Radiat Oncol Biol Phys. 2009;75(3):688–695. http://www.sciencedirect.com/science/article/pii/S0360301609001941. Accessed September 5, 2016. [DOI] [PubMed] [Google Scholar]
- 19. Weiss E, Wijesooriya K, Dill SV, Keall PJ. Tumor and normal tissue motion in the thorax during respiration: analysis of volumetric and positional variations using 4D CT[Internet]. Int J Radiat Oncol Biol Phys. 2007;67(1):296–307. http://www.sciencedirect.com/science/article/pii/S0360301606029671. Accessed September 5, 2016. [DOI] [PubMed] [Google Scholar]
- 20. Chang J, Mageras GS, Yorke E, et al. Observation of interfractional variations in lung tumor position using respiratory gated and ungated megavoltage cone-beam computed tomography[Internet]. Int J Radiat Oncol Biol Phys. 2007;67(5):1548–1558. http://www.sciencedirect.com/science/article/pii/S0360301606036613. Accessed September 5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]