Abstract
Purpose
Light-induced photoreceptor cell degeneration and disease progression in age-related macular degeneration (AMD) involve oxidative stress and visual cell loss, which can be prevented, or slowed, by antioxidants. Our goal was to test the protective efficacy of a traditional Age-related Eye Disease Study antioxidant formulation (AREDS) and AREDS combined with non-traditional antioxidants in a preclinical animal model of photooxidative retinal damage.
Methods
Male Sprague-Dawley rats were reared in a low-intensity (20 lux) or high-intensity (200 lux) cyclic light environment for 6 weeks. Some animals received a daily dietary supplement consisting of a small cracker infused with an AREDS antioxidant mineral mixture, AREDS antioxidants minus zinc, or zinc oxide alone. Other rats received AREDS combined with a detergent extract of the common herb rosemary, AREDS plus carnosic acid, zinc oxide plus rosemary, or rosemary alone. Antioxidant efficacy was determined by measuring retinal DNA levels 2 weeks after 6 h of intense exposure to white light (9,000 lux). Western blotting was used to determine visual cell opsin and arrestin levels following intense light treatment. Rhodopsin regeneration was determined after 1 h of exposure to light. Gene array analysis was used to determine changes in the expression of retinal genes resulting from light rearing environment or from antioxidant supplementation.
Results
Chronic high-intensity cyclic light rearing resulted in lower levels of rod and cone opsins, retinal S-antigen (S-ag), and medium wavelength cone arrestin (mCAR) than found for rats maintained in low cyclic light. However, as determined by retinal DNA, and by residual opsin and arrestin levels, 2 weeks after acute photooxidative damage, visual cell loss was greater in rats reared in low cyclic light. Retinal damage decreased with AREDS plus rosemary, or with zinc oxide plus rosemary whereas AREDS alone and zinc oxide alone (at their daily recommended levels) were both ineffective. One week of supplemental AREDS plus carnosic acid resulted in higher levels of rod and cone cell proteins, and higher levels of retinal DNA than for AREDS alone. Rhodopsin regeneration was unaffected by the rosemary treatment. Retinal gene array analysis showed reduced expression of medium- wavelength opsin 1 and arrestin C in the high-light reared rats versus the low-light rats. The transition of rats from low cyclic light to a high cyclic light environment resulted in the differential expression of 280 gene markers, enriched for genes related to inflammation, apoptosis, cytokine, innate immune response, and receptors. Rosemary, zinc oxide plus rosemary, and AREDS plus rosemary suppressed 131, 241, and 266 of these genes (respectively) in high-light versus low-light animals and induced a small subset of changes in gene expression that were independent of light rearing conditions.
Conclusions
Long-term environmental light intensity is a major determinant of retinal gene and protein expression, and of visual cell survival following acute photooxidative insult. Rats preconditioned by high-light rearing exhibit lower levels of cone opsin mRNA and protein, and lower mCAR protein, than low-light reared animals, but greater retention of retinal DNA and proteins following photooxidative damage. Rosemary enhanced the protective efficacy of AREDS and led to the greatest effect on the retinal genome in animals reared in high environmental light. Chronic administration of rosemary antioxidants may be a useful adjunct to the therapeutic benefit of AREDS in slowing disease progression in AMD.
Introduction
Advancing age is the primary risk factor for vision loss in age-related macular degeneration (AMD), but oxidative stress induced by smoking increases that risk at least twofold [1-5]. A low serum antioxidant index is a related risk factor [1,6], and improving vascular health appears to correlate with improved outcomes in patients with AMD [7,8]. Drusen isolated from AMD eyes have higher levels of protein oxidation products, including carboxyethylpyrrole (CEP) adducts, than that found in unaffected age-matched controls [9]. Elevated levels of CEP adducts, formed from oxidized docosahexaenoic acid and free amino groups in proteins, and anti-CEP immunoreactivity, also were found in sera from patients with AMD [9,10]. Complement activation and chronic inflammation are additional interrelated factors affecting disease progression [11,12], with specific polymorphisms in complement factor H (CFH) associated with approximately 40% of AMD cases [11-14]. CFH normally binds factor C3b and accelerates the decay of C3 convertase, which inhibits the formation of the complement-mediated membrane attack complex [2,11,12]. CFH also binds proteins containing the fatty acid oxidation product malondialdehyde (MDA), thereby reducing the potential for further oxidative damage and inflammation [15]. Interestingly, micromolar zinc enhances CFH/C3b binding [16], whereas the CFH mutation Y402H reduces binding to factor C3b and MDA modified proteins [15]. These decreases in binding reduce the ability of CFH to regulate the membrane attack complex [17].
The protective efficacy of dietary treatment, using a combination of traditional antioxidants and minerals, has been demonstrated in clinical trials conducted by the Age-related Eye Disease Study group [6]. In the first trial (AREDS1), chronic administration of vitamins A (as beta carotene), C and E, along with zinc and cupric oxide (defined as AREDS hereafter), delayed disease progression in approximately 25% of patients who had intermediate AMD; however, zinc oxide accounted for approximately 70% of the benefit [6]. The more recent clinical trial (AREDS2) examined the addition of polyunsaturated fatty acids in conjunction with AREDS, and a modified formulation with a reduced level of zinc in one arm of the study and lutein and zeaxanthin substituted for beta carotene in another. Primary analysis revealed that no benefit versus placebo occurred [18], but subgroup analysis showed a benefit from lutein plus zeaxanthin in individuals with low carotenoid blood levels [19,20].
Animal models of acute or progressive retinal degeneration have provided useful insights into age-related ocular disease. For example, in a rat model of acute retinal phototoxicity, microglia migration and macrophage invasion occurred and were found to stimulate the alternative complement pathway and to mediate deposition of CFH and factors B and C3 in the area of photic lesions [21,22]. Marc et al. [23] compared end stage morphology in the light-damaged rat retina with late stage atrophic AMD and found remarkable anatomic similarities in retinal remodeling. In a murine model, Hollyfield et al. [24] reported that immunization with CEP adducted to mouse serum albumin led to inflammation of Bruch’s membrane and to the appearance of drusen like deposits. Acute treatment with zinc oxide or zinc combined with the common herb rosemary (Rosmarinus officinalis) led to a decrease in CEP-protein adduct levels in the rat retina and to an increase in visual cell survival following photooxidative damage [25,26]. In a long-term study, Kowluru and associates [27,28] fed an AREDS-based micronutrient mix to diabetic rats and found a reduction in the progression of retinopathy. Likewise, in the rapidly degenerating Rd1 mouse model of retinopathy, Komeima et al. [29] found that daily treatment with a combination of antioxidants, including vitamins C and E, decreased the levels of oxidative biomarkers and preserved cone cell function.
Although animal models can mimic some aspects of retinal disease, the mixed rod/cone photoreceptor mosaic in rodents differs from the high cone density found in the macula of primates [30]. In addition, AREDS antioxidants are normally given long term to patients with AMD, whereas laboratory antioxidant studies are often short term [30,31]. Despite these limitations, retinal gene expression studies in animal models have been extrapolated to humans, to help identify signaling mechanisms, or protein targets, for potential therapy. For example, Bedolla and Torre [32] reported that the genes Dio2 and Dio3 are strongly regulated by light and induce the formation of T3 thyroid hormone. Increased T3 levels upregulate phototransduction genes in human cells in vitro [33], and these same genes were found to be upregulated in a rat model. The connection between thyroid activity and retinal disease in the rat is also consistent with findings in patients with Graves’ disease [34]. Other studies have sought to extend genome-wide findings in rat models to human disease, including vasoregression [35], retinal injury [36-38], and oxygen-induced retinopathy [39,40].
Herein we describe studies to test the hypothesis that long-term dietary administration of AREDS supplemented with rosemary antioxidants will increase photoreceptor cell survival in a rat model of acute or chronic photooxidative retinal damage. Oral AREDS plus rosemary given daily enhanced protective efficacy against acute light-induced retinal degeneration, whereas AREDS by itself was ineffective. Compared with low-light rearing conditions, high-intensity cyclic light led to decreases in cone opsin, retinal DNA, and the mRNAs for opsin 1 and arrestin C. We also report the differential expression of 280 other retinal genes, including the prosenescence gene Gng11 [41]. Rosemary, rosemary plus zinc oxide, and AREDS plus rosemary suppressed changes in the retinal transcriptome induced by high cyclic light conditions. They also induced a subset of specific changes in gene expression, which were independent of light rearing conditions. AREDS plus carnosic acid [42] led to increased visual cell survival, following intense light treatment. These findings indicate that photoreceptor cell survival is enhanced by AREDS supplemented with rosemary antioxidants and suggest that complications [43,44] arising from photooxidative retinal damage can be diminished by their combination.
Methods
Animals, light rearing conditions, intense light treatment: Weanling male Sprague-Dawley rats were from Harlan Inc. (Indianapolis, IN) and maintained in one of two cyclic light rearing environments for 6 weeks. Environmental lighting was provided by a series of 7 W night lights spaced evenly above the animal cages, and the line voltage was regulated to provide either 20 lux (low) or 200 lux (high) light conditions. Lights were on for 12 h per day, beginning at 8 AM. At P63–65, some animals were dark adapted for 16 h and then euthanized in a saturated CO2 chamber under dim red illumination. Their retinas were isolated, without vitreous, by a modification of the Winkler technique [45,46]. In brief, the eye was proptosed, using a pair of full curved forceps, the cornea was sliced and the lens removed. Simultaneously, a slight change in the angle of the forceps causes the vitreous to protrude through the cut cornea. The vitreous was then removed with a pair of fine mouse-toothed forceps and the retina excised from the eyecup, by gently raising the full curved forceps. Other rats were dark adapted and then treated with intense white light for 6 h, beginning at 9 AM. The light exposure chambers consisted of 6-inch diameter clear Plexiglas cylinders surrounded by seven circular fluorescent lights, providing 9,000 lux of full spectrum (400–700 nm) light [26]. Following the intense light treatment, most rats were maintained in darkness for 2 weeks, allowing for removal of necrotic photoreceptor cell material and the recovery of still surviving visual cells. Other rats were kept in darkness for 2 days, to assess retinal protein expression and DNA damage. All animals received standard Rat Chow (Teklad, Madison, WI) ad libitum and had free access to water. The use of rats in this study conformed to the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and with Laboratory Animal Resource Committee guidelines at Wright State University.
Antioxidant supplements
Weanling rats were acclimated to their respective light environments for 1 day and then given daily antioxidant supplements for a period of 6 weeks. Every morning, each animal (n = 4–6 per treatment) was removed from its cage and placed in a clean cage without bedding but with a single piece of cracker infused with a small volume (about 50 μl) of antioxidant. The Group 1 antioxidant supplements consisted of AREDS complete (ICAPS soft-gels, Alcon Ltd, Fort Worth, TX), which contains the AREDS1 recommended doses of vitamins A (as beta carotene), C and E, zinc, and copper [6], AREDS antioxidants minus zinc, aqueous zinc oxide [25] (Alfa Aesar, Ward Hill, MA), or vehicle (1% Tween-80/16% ethanol/25% ROPUFA). The omega 3 polyunsaturated fatty acid ROPUFA mixture (75N-3 EE) was from DSM (Kaiseraugst, Switzerland). The Group 2 antioxidant supplements consisted of AREDS plus rosemary (17 mg/kg), zinc oxide plus rosemary, rosemary alone, or vehicle (1% Tween-80/16% ethanol/ 25% ROPUFA). Rosemary powder was obtained from LycoRed Ltd. (Beer Sheba, Israel) and emulsified as described [26]. A third group of animals (Group 3) was fed AREDS alone, AREDS plus rosemary (34 mg/kg), rosemary alone, or AREDS plus carnosic acid (Enzo Life Sciences, Farmingdale, NY), or AREDS plus ursolic acid (MP Biochemicals, Solon, OH) for a period of 1 week. Carnosic acid and ursolic acid were dissolved in 50% ethanol/ 50% ROPUFA and given at or above the concentrations found in 34 mg/kg of rosemary powder [26]. Other rats received antioxidant supplements as a single intraperitoneal (i.p.) injection 1 h before exposure to acute intense light. All rats were weighed every 2 days, and the volume of antioxidant was adjusted to provide the AREDS1 recommended daily dose. Daily feeding is less stressful than long-term oral gavage or multiple i.p. injections. The animals eagerly ingested the antioxidant-infused crackers, and their growth rates were identical to those fed only the house diet. Figure 1 describes the dietary paradigms used in this study, and Table 1 contains a summary of the antioxidant supplements, their daily doses, the total number of animals (n) for each antioxidant treatment, and the vehicles used.
Figure 1.
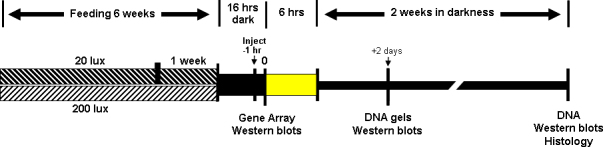
Antioxidant feeding and light rearing paradigms. Weanling rats were maintained in a 12 h cyclic light environment, consisting of either 20 lux or 200 lux white light, for 6 weeks and given supplemental antioxidants on a daily basis. Following dark adaptation, rats were exposed to 9 k lux white light for 6 h and then allowed to recover in darkness for 2 days or 2 weeks. Animals were euthanized in a saturated CO2 atmosphere, and their retinas excised under dim red light. The extent of visual cell survival was determined by retinal DNA measurements and histology 2 weeks after light exposure. Western blot analysis was at various times before or after photooxidative stress, whereas gene array profiles were determined using tissues excised after 6 weeks of dietary supplements, but without exposure to intense light.
Table 1. Antioxidant supplement compositions*.
| Antioxidant Supplement: 6 weeks mg/kg body weight | ||||||
|---|---|---|---|---|---|---|
| Group 1 |
Βeta-carotene |
Vitamin C |
Vitamin E |
Zn++ |
Cu++ |
Rosemary |
| AREDS (28) |
0.29 |
7.53 |
6.67 |
1.44 |
0.03 |
- |
| AREDS Antiox. (28) |
0.29 |
7.53 |
6.67 |
- |
- |
- |
| Zinc Oxide (28) |
- |
- |
- |
1.44 |
- |
- |
| Group 2 |
||||||
| Rosemary (18) |
- |
- |
- |
- |
- |
17 |
| AREDS + Rosemary (20) |
0.29 |
7.53 |
6.67 |
1.44 |
0.03 |
17 |
| Zinc + Rosemary (20) |
- |
- |
- |
1.44 |
- |
17 |
| Antioxidant Supplement: 1 week mg/kg body weight | ||||||
| Group 3 |
||||||
| AREDS (12) |
0.29 |
7.53 |
6.67 |
1.44 |
0.03 |
- |
| Rosemary (6) |
- |
- |
- |
- |
- |
34 |
| AREDS + Rosemary (10) |
0.29 |
7.53 |
6.67 |
1.44 |
0.03 |
34 |
| AREDS + Ursolic (6) |
0.29 |
7.53 |
6.67 |
1.44 |
0.03 |
5.3 (ursolic) |
| AREDS + Carnosic (12) | 0.29 | 7.53 | 6.67 | 1.44 | 0.03 | 7.9 / 15.8 (carnosic) |
*AREDS, AREDS antioxidants (Antiox.) and rosemary were solubilized with vehicle (1% Tween 80/16% ethanol/ 25% ROPUFA). Zinc oxide and copper oxide levels per AREDS1 formula [6]. Zinc oxide was dissolved in distilled water [25]. Carnosic acid and ursolic acid were solubilized with 50% pure ethanol/ 50% ROPUFA. Weanling male Sprague-Dawley rats were supplemented for 6 weeks before intense light exposure or sacrifice for gene array analysis. Short-term supplementation was for 1 week prior to intense light treatment and subsequent analysis. The numbers in parenthesis indicate the n for each sub-group of dietary treatment. Comparable numbers of vehicle and AREDS fed rats were used for these experiments.
Photoreceptor cell survival and antioxidant efficacy
The extent of visual cell loss was determined by measuring residual DNA in retinas from rats exposed to intense light, in comparison to retinal DNA measured in unexposed animals kept in darkness for the same 2-week post-light exposure period. DNA fluorescence was determined for individual retinas with the Hoechst (Calbiochem-Behring, La Jolla, CA) dye-binding assay, as described [26]. Photoreceptor cell DNA was calculated by subtracting the DNA content of the inner nuclear layers in 6-month old Royal College of Surgeons rat retinas from total retinal DNA. The relative level of protective efficacy was then determined by subtracting photoreceptor cell DNA in animals treated with vehicle and exposed to intense light from the DNA levels in rats treated with antioxidants and exposed to light, and from the DNA levels in unexposed-antioxidant-treated rats [25].
DNA damage
Rat retinas were excised 2 days after exposure to intense light, and DNA was isolated by using a Sigma Gene Elute kit. DNA was then pooled, and 1 μg aliquots were separated on 1.5% agarose neutral (pH 7.0) electrophoretic gels. Ethidium bromide and ultraviolet (UV) light were used to visualize apoptotic DNA ladders and higher molecular weight DNA fragments. These gels were run with DNA extracts from three to five retinas, isolated from different rats, whereas the fellow eyes were used for western blotting of retinal proteins.
Histology
Eyes were enucleated from rats treated with intense light, and unexposed rats, 2 weeks after light exposure and then fixed in 50% Karnovsky’s solution for 24 h. Before fixation, each eye was marked to identify the superior hemisphere and, after 10 min of fixation, the lens was removed. The next day, the eyecups were transferred to 0.1 M sodium cacodylate buffer (pH 7.4) and stored in that solution at 4 °C. For histology, the eyes were embedded in paraffin, sectioned vertically at 4 μm, and then stained with hematoxylin and eosin. Retinal tissue sections in the mid-superior hemisphere were examined.
Western blotting
Retinal homogenates were prepared in PBS (Cat #161-0780, Bio-Rad Inc. Hercules, CA) containing a protease inhibitor cocktail (P8340; Sigma, St. Louis, MO), 1 mM EDTA, 1 mM diethylenetriaminepentaacetic acid (DTPA), and 100 μM butylated hydroxytoluene (BHT; Sigma). The protein contained in three to five retinas from different rats was combined, measured with the Bradford technique (Bio-Rad Laboratories, Hercules, CA), and then extracted with 0.4% sodium dodecyl sulfate (SDS) in PBS (pH 6.8) at 4 °C for 30 min. Protein samples (20 μg/lane) were applied to 4% stacking/12.5% SDS-polyacrylamide running gels and separated in a Mini Protean 3 gel electrophoresis apparatus (Bio-Rad Laboratories). Following electrophoresis, the proteins were transferred to polyvinylidene fluoride (PVDF) membranes at 200 mA overnight, using 10 mM N-cyclohexyl-3-aminopropanesulfonic acid (CAPS) buffer (pH 11) containing 20% methanol. The PVDF membranes were blocked in PBS buffer, containing 2% non-fat dry milk and 0.2% Tween-20 for 1 h at 37 °C, and then incubated with antibodies. Subsequently, the PVDF membranes were stripped and reprobed with other antibodies. Western blot staining was visualized with chemiluminescence and exposure to X-ray film. Relative protein staining intensity was determined with Image J (NIH, Bethesda, MD). A summary of the antibodies used in this study appears as supplemental data in Appendix 1.
Rhodopsin regeneration
To assess the potential effects of antioxidants on the retinoid visual cycle [47], rats were given a single dose of rosemary extract (34 mg/kg, i.p.) 1 h before exposure for 1 h to bleaching light. After the light treatment, the animals were in darkness for various periods before being euthanized in a saturated CO2 atmosphere, and then both eyes were enucleated for rhodopsin measurement [26]. For each rat (n = 2–5), the rhodopsin values were averaged and the time course of regeneration was determined.
Retinal gene array analysis
Gene expression profiles were established for different groups of animals reared under high- or low-cyclic light conditions while being fed a dietary supplement consisting of rosemary, AREDS plus rosemary, zinc plus rosemary, or vehicle (R, A+R, Z+R, or V, respectively; see Figure 1). Retinas, with the vitreous removed, were excised from four to six rats after 6 weeks of daily dietary supplementation and stored in RNAlater (Qiagen, Inc., Valencia, CA) at −20 °C. Three independent groups of animals were used for each treatment condition to provide triplicate gene expression profiles. The fellow retinas were used for gel electrophoresis. RNA was extracted, using the E.Z.N.A. tissue RNA kit by Omega Bio-Tek (Norcross, GA), with some modifications to the manufacturer’s protocol. Briefly, the retinas were thawed on ice for approximately 5 min. RNAlater was then aspirated, and 500 μl TRK lysis buffer, supplemented with 50% more beta-mercaptoethanol than called for (30 μl/ml final), was added to each retina to prevent RNAse activity. The tissue was homogenized with a clean rotor-stator homogenizer and the lysate centrifuged at 13,000 ×g for 4 min. Supernatant was removed and then combined with 500 μl of 70% ethanol. Column purification was conducted according to the manufacturer’s instructions; the elution volume was 40 μl. RNA was quantified on a NanoDrop spectrophotometer (ThermoFisher Scientific, Waltham, MA) and an Agilent RNA Nano Bioanalyzer Chip (Agilent, Santa Clara, CA). The mean RNA integrity number (RIN) score was 7.01. One hundred ng total RNA was used as the input to prepare fragmented, single-stranded cDNA using the Ambion WT Expression Kit (ThermoFisher Scientific) according to the manufacturer’s directions. This cDNA was labeled using the GeneChip WT Terminal Labeling Kit from Affymetrix (Santa Clara, CA). The Affymetrix GeneChip Hybridization, Wash, and Stain Kit was used to hybridize against the Affymetrix Rat Gene 1.0 ST microarrays. Fluidics were performed on an Affymetrix Fluidics Station 450 using script FS450_0007. Arrays were scanned on an Affymetrix GeneChip Scanner 3000 7G. For a given treatment, each of the three biologic replicates was used to screen an independent Affymetrix GeneChip.
Gene array data analysis
CEL files generated from the scanned arrays were analyzed first in Affymetrix Expression Console build 1.3.1.187. CEL files were imported into Partek Genomics Suite version 6.6 (Partek Inc., St. Louis, MO) using the RMA import settings. Differentially expressed genes were detected using ANOVA, using the treatments as contrasts (light rearing condition (High and Low), and dietary supplement. A second analysis of the gene array data was performed using R version 3.0.1 using the BioConductor statistical analysis packages. The oligo package [48] was used for import, quality assessment, probe level modeling, and RMA normalization. Limma was used to produce linear models for identifying differential genes [49], and the annotate package was used to link gene names and symbols to microarray probe IDs. Results from both analyses were combined, and annotations were manually inspected to reconcile missing gene names where possible.
Statistical analysis
Differentially expressed genes for each condition were filtered to remove any genes that did not have a Benjamini-Hochberg false discovery rate (FDR)–adjusted p value of less than 0.05 (noted as p<0.05 hereafter) [50]. Even small differences in gene expression levels can be statistically significant if the difference is reproducible; however, the smaller the difference, the more difficult it is to differentiate it from background noise. Gene markers showing differential expression levels of greater than 1.3 fold-change or less than [–1.3] with p<0.05 were used for general bioinformatics and Gene Ontology analyses in Ingenuity Pathway Analysis (IPA, Qiagen, Redwood City, CA), or as otherwise noted in the text. For any pathway analysis or functional enrichment analysis, p values were established using a Fisher’s exact test. A standard goodness of fit test was used to determine whether a statistically significant variation existed between any two variables. Biochemical data are presented as the mean ± standard deviation (SD), or standard error of the mean (SEM), for four to eight retinas from different rats. Using a two-tailed t test, a p value of less than 0.05 was considered to represent a statistically significant difference.
Results
Antioxidant efficacy
To compare protective efficacy during photooxidative retinal damage, one sub-group of rats was fed an AREDS1 antioxidant and mineral mixture, with or without a rosemary supplement, and another sub-group was given zinc oxide plus rosemary. After 6 weeks of daily supplements, the rats were dark-adapted and then exposed to intense white light for 6 h. Other animals received the daily AREDS1 recommended dose, with or without rosemary, in a single 1.p. injection 1 h before light treatment. All rats were reared in the 20 lux cyclic light environment and allowed to recover for 2 weeks in darkness after exposure to intense light. Then, retinal DNA levels were determined to assess the extent of visual cell survival. Figure 2 shows that feeding rosemary alone, zinc oxide alone, or AREDS alone (fed or injected) did not prevent photooxidative damage. Protective efficacy for these treatments was less than 10%. Rosemary extract (17 mg/kg) added to the AREDS mixture or combined with zinc oxide (1.4 mg/kg) increased protective efficacy. For the fed and i.p. injected animals, efficacy was about 20%. AREDS supplemented with additional rosemary led to even greater visual cell survival. Rats fed or injected i.p. with AREDS plus 34 mg/kg rosemary exhibited 35–40% protective efficacy, indicating that bioavailability was nearly identical. Approximately 55% efficacy was found when the AREDS antioxidant and mineral mixture was given i.p. at five times the usual daily dose or when rosemary (34 mg/kg) was combined with zinc oxide at the dose normally present in a typical AREDS mixture (Table 1).
Figure 2.
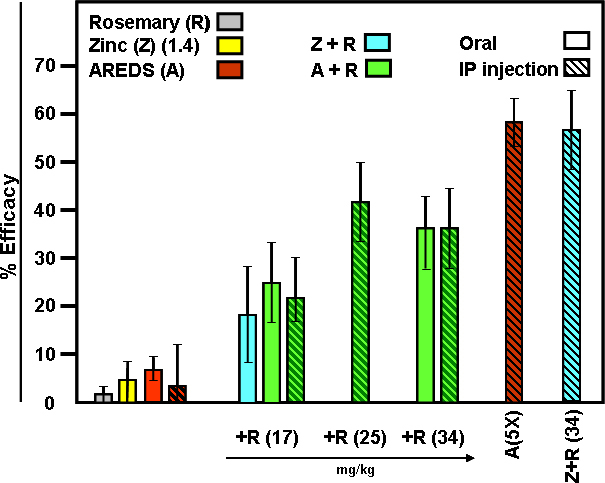
Protective efficacy of AREDS and rosemary on photooxidative retinal damage. Rats were reared in a 20 lux cyclic light environment and supplemented for 6 weeks with AREDS (A), AREDS + 17 mg/kg rosemary (A+R), zinc oxide (1.4 mg/kg) + 17 mg/kg rosemary (Z+R), rosemary alone (R), or zinc oxide alone (Z). Rats were exposed to intense light for 6 h (colored vertical bars), and allowed to recover in darkness for 2 weeks. One group of rats received A+R (34 mg/kg) daily for 1 week. Other animals were injected i.p. with A, or A+R (17, 25 or 34 mg/kg) 1 h before intense light treatment (diagonal hashed bars). One subgroup was given A at five times the daily recommended dose; a second subgroup received Z+R (1.4 and 34 mg/kg). Retinal DNA measurements were performed to determine protective efficacy, as described [25]. Results are presented as average percent (%) efficacy ± standard error of the mean (SEM) for four to eight animals per treatment. Chronic supplementation of rats with A, or acute i.p. administration, was ineffective in preventing photooxidative retinal damage. Oral R (17 mg/kg) or Z (1.4 mg/kg) alone were also ineffective. However, A+R (17 mg/kg), either given orally or by i.p. injection, resulted in 20–25% efficacy. A+R given i.p., with R at 25 or 34 mg/kg, or feeding A+R (34 mg/kg) for 1 week led to an average of 35% protective efficacy. AREDS (i.p.) at 5X the daily recommended dose or Z+R (34 mg/kg) provided 55–60% efficacy.
Western blotting of retinal proteins
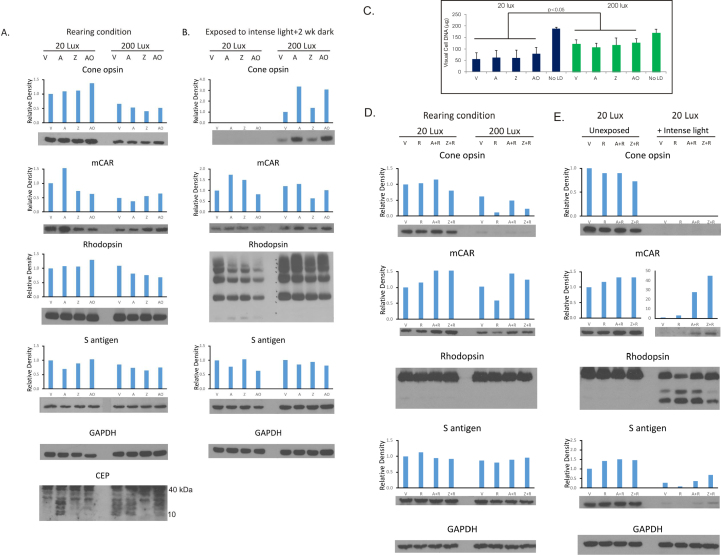
As shown in Figure 3, chronic high light rearing conditions had a dramatic effect on cone cell opsin. Rats kept for 6 weeks in the 200 lux cyclic light environment had approximately 50% of the cone opsin staining found in rats from the 20 lux environment (Figure 3A). The medium wavelength cone arrestin (mCAR) was also dramatically lower in the animals supplemented with AREDS and kept in 200 lux cyclic light. Antioxidant feeding did not appreciably alter the relative staining of the more abundant rhodopsin, or of rod cell S-antigen (S-ag). To assess the effects of chronic light rearing conditions on protein oxidation, CEP immunoreactivity was determined. A higher level of CEP-protein staining was seen in the retinal extracts from 200 lux–reared rats than for those from the 20 lux environment. Supplementation of rats with either AREDS complete or with AREDS antioxidants (see Group 1, in Table 1) did not lead to a decrease in the CEP-protein adduct levels. However, CEP adduct staining was decreased by supplemental zinc oxide in animals from the high light environment.
Figure 3.
Western blot analysis and retinal DNA levels in rats reared for 6 weeks in low- or high-cyclic light. Retinal proteins (20 μg/lane), pooled from four to five rats, were electrophoresed on 12.5% sodium dodecyl sulfate (SDS)-polyacrylamide gels, transferred to polyvinylidene fluoride (PVDF) membranes, and then probed with antibodies as indicated in Appendix 1. Rats exposed to intense light for 6 h and then kept in darkness for 2 weeks (B, E). The fellow eyes were used for DNA measurements (C), [n = 8 for No LD]. Density profiles determined by Image J analysis. A: Cone opsin levels were lower in all rats reared under high cyclic light than for those reared in low cyclic light, whereas medium wavelength cone arrestin (mCAR) staining was lower in only the rats fed AREDS. There were no major differences in rhodopsin and S-antigen levels for AREDS (A), zinc oxide (Z), or AREDS antioxidants minus zinc (AO). Multiple CEP protein adducts were present, with greater staining for the 200 lux cyclic light-reared rats than for those reared in 20 lux light. B: Two weeks after exposure to acute intense light, higher levels of cone- and rod-opsins were present in rats from the high light condition versus low cyclic light. S-antigen and mCAR levels were similar. C: Individual antioxidants had little effect on retinal DNA for rats reared in 20 lux cyclic light (55–79 μg/retina); DNA levels for 200 lux cyclic light animals (107–128 μg) were significantly higher (p<0.05). D: Rats fed vehicle (V), rosemary (R), AREDS + rosemary (A+R), or zinc oxide + rosemary (Z+R). Cone opsin levels were higher in rats reared in 20 lux cyclic light than for those reared in 200 lux light. Rhodopsin (monomer) and S-antigen levels were unchanged by higher light rearing conditions, or by antioxidant feeding E: Two weeks after photooxidative damage considerable loss of cone opsin and rhodopsin (monomer) occurred in rats originally from the 20 lux light environment, and rhodopsin was still undergoing degradation. mCAR levels were preserved in A+R and Z+R rats, whereas S-antigen levels were low in all retinas. Glyceraldehyde phosphate dehydrogenase (GAPDH) was the control for protein loading.
A second set of Group 1 animals was exposed to intense light for 6 h followed by recovery for 2 weeks in darkness. Figure 3B shows that cone opsin staining was undetectable in rats previously reared in the 20 lux light environment, whereas those from the 200 lux condition recovered substantial amounts. Compared with vehicle or zinc treatment, AREDS complete and the AREDS antioxidants fraction enhanced the level of cone opsin staining in rats from the 200 lux environment. mCAR levels were similar in rats reared in 20 lux or 200 lux light, while the 200 lux zinc-treated animals had slightly lower mCAR levels. Rhodopsin staining was greater for the rats reared in 200 lux light, including the monomer and higher molecular weight forms, whereas rhodopsin staining was appreciably lower in those reared in 20 lux light. Lower molecular weight degradation fragments of rhodopsin were also present. Likewise, retinal S-ag levels were slightly higher in rats reared in high light than in those reared in low light. Photoreceptor cell DNA was lower in rats originally from the 20 lux light environment (Figure 3C). The vehicle-treated rats recovered 55 μg DNA/retina compared with an average of 122 μg for those reared under the high light condition (p<0.05). Antioxidant-fed rats from the high light condition also had significantly higher retinal DNA than found in rats from the 20 lux environment, but there were no statistically significant differences among the various antioxidants. When compared to the DNA values in rats reared in low light, a modest decrease in photoreceptor cell DNA (10%) was found for the unexposed controls reared in higher intensity cyclic light.
The same high and low environmental light rearing effects were found for animals fed supplemental rosemary, AREDS plus rosemary, or zinc oxide plus rosemary (Group 2, Table 1; Figure 3D). Cone opsin staining was nearly undetectable in rats maintained in the high light condition. mCAR levels were lower in the rosemary-fed rats but appeared to be largely unaffected in rats fed rosemary combined with AREDS or with zinc oxide. Retinal rhodopsin and S-ag levels also appeared to be unaffected by the high light rearing environment. Major changes in retinal proteins occurred after intense photooxidative light treatment of rats reared in 20 lux cyclic light (Figure 3E). Cone opsin was undetectable in all animals, whereas mCAR staining was present in rats fed AREDS plus rosemary and in those fed zinc plus rosemary. The levels of rhodopsin (monomer) and retinal S-ag were also much lower. In addition, lower molecular weight rhodopsin fragments were present, indicating extensive rod cell damage, although these were somewhat reduced by the zinc plus rosemary treatment.
Short-term supplementation
To determine whether carnosic acid and ursolic acid, two major rosemary antioxidants [26], were effective, we fed AREDS alone or rosemary alone (34 mg/kg) to rats for 1 week. Other animals received AREDS plus rosemary, or AREDS plus carnosic acid at the concentration found in 34 mg of rosemary (7.9 mg/kg) or AREDS plus 5.3 mg/kg ursolic acid (Group 3, Table 1). The rats were dark adapted overnight and then treated with acute intense white light followed by 2 days in darkness. Previously, all rats had been in the 20 lux cyclic light rearing environment. Western blot analysis revealed that rosemary alone and AREDS alone were ineffective. Cone opsin staining was approximately 50% of that in unexposed control animals (Figure 4A). Treatment with AREDS plus rosemary or with AREDS plus carnosic acid resulted in cone opsin levels that were 75–80% of the level present in unexposed rats, while AREDS plus ursolic acid provided only modest protection. AREDS plus carnosic acid or ursolic acid also led to a slightly reduced level of Rac 1, a small GTPase induced by intense light [43,44] (Appendix 2).
Figure 4.
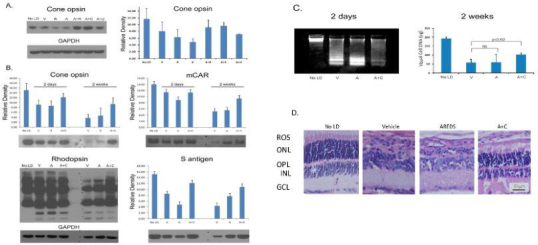
Rats reared in low cyclic light and fed antioxidants for 1 week prior to photooxidative stress. Western analysis of cone opsin 2 days after intense light treatment (A). Cone opsin, rhodopsin, mCAR and S-antigen levels 2 days or 2 weeks after intense light exposure (B). Vehicle (V), rosemary (R), AREDS (A), AREDS + rosemary (A+R), AREDS + carnosic acid (A+C), or AREDS + ursolic acid (A+U) treated rats, concentrations as shown in Table 1. Density determined by Image J analysis for 3 separate gels (20 µg protein/lane) from n=4-6 rats; error bars ± SD. Retinal DNA damage and DNA levels determined 2 days or 2 weeks (respectively) after photooxidative damage; No LD; n=8 retinas from 8 different rats (C). Retinal histology for V, A, or A+C fed rats 2 weeks after intense light treatment (D). A: Cone opsin staining was greater for rats fed A+R or A+C, than those fed R, A, or A+U (n=6). B: Two weeks after retinal light damage cone opsin and mCAR levels were lower than after 2 days, but the relative staining for A+C (15.8 mg /kg) fed rats remained higher than for those fed A alone. Two weeks after light damage overall rhodopsin staining was less than in rats after 2 days for those given V or A. Lower molecular weight degradation products were present after both 2 days and 2 weeks, but higher levels of rhodopsin (and its polymeric forms) were present in animals fed A+C. C: Two days after light exposure staining for low molecular weight apoptotic DNA fragments was greater for rats treated with V or A than seen for rats treated with A+C. As determined by ethidium staining of a gel run for only 5 min DNA loading was the same in all samples (Appendix 3). Two weeks after intense light a significantly higher level of visual cell DNA was found in rats fed A+C than seen for V treatment (p<0.02). D: Histology of fellow eyes revealed considerable damage in V and A treated rats (1 to 3 rows of nuclei in the ONL), and retention of 7-8 rows of nuclei in the ONL of rats fed A+C (bar =50 µm).
Another sub-set of Group 3 rats received AREDS alone or AREDS plus a higher level of carnosic acid (15.8 mg /kg) for 1 week. Following exposure to intense light, one-half of these animals recovered in darkness for 2 days, while the other half remained in darkness for 2 weeks. As shown in Figure 4B, AREDS plus the higher concentration of carnosic acid effectively reduced the loss of cone cell opsin. Two days after intense light treatment, cone opsin staining was 80% of the value in unexposed rats, whereas the rats treated with vehicle or with AREDS had approximately 60% of the opsin staining present in unexposed controls. Retinal mCAR staining was less impacted 2 days after light treatment. Figure 4B also shows that light-induced visual cell damage continued to progress during the dark recovery period. Compared with the level of cone opsin staining after 2 days, immunoreactivity was much lower 12 days later. Vehicle treatment or AREDS alone led to cone opsin levels that were approximately 25% of those in the unexposed control rats, and mCAR staining that was approximately 35% of control. However, rats treated with the AREDS and carnosic acid combination retained almost 65% of both proteins. Rod photoreceptors exhibited the same relative effects following photooxidative damage. Total rhodopsin staining was similar in all samples 2 days after light exposure, while S-ag staining was higher in the rats treated with AREDS plus carnosic acid than in rats treated with vehicle or with AREDS alone. The loss of rhodopsin was especially noticeable 2 weeks after intense light treatment. In the animals treated with vehicle or with AREDS, the higher molecular weight polymeric forms of rhodopsin were markedly decreased, whereas rats given AREDS plus carnosic acid exhibited higher levels of rhodopsin staining. Retinal S-antigen staining was also higher for rats treated with AREDS plus carnosic acid than for those given either vehicle or AREDS alone.
We determined retinal DNA damage by using neutral pH, agarose gel electrophoresis 2 days after intense light treatment, and then measured residual DNA 2 weeks later (Figure 4C). A typical low molecular weight apoptotic DNA ladder was present in rats treated with vehicle or with AREDS, while the DNA fragmentation pattern for the AREDS plus carnosic acid animals was less intense. As shown by the diminished staining for high molecular weight DNA, single strand breaks also occurred in all light-exposed rat retinas, but DNA loading was uniform in all samples (Appendix 3). Two weeks after exposure to intense light, visual cell DNA in rats fed vehicle or AREDS was approximately 50 μg per retina, while rats treated with AREDS plus carnosic acid retained an average of 100 μg DNA/retina, a value that is significantly higher (p<0.02) than for vehicle treatment. Staining for heme-oxygenase 1 (HO-1), a protein marker of oxidative stress, was absent in non-light-exposed rat retinas and in those from light-treated animals 2 weeks after exposure (Appendix 4). Two days after photooxidative damage, HO-1 staining was elevated in all light-exposed rat retinas, but the level was lower in AREDS plus carnosic acid tissues. To confirm the protective effect of AREDS plus carnosic acid, we performed retinal histology 2 weeks after intense light exposure. As shown in Figure 4D, only one to three rows of photoreceptor cell nuclei were present in the outer nuclei layer (ONL) of rats fed vehicle or AREDS. However, the AREDS plus carnosic acid (A+C) treated rat retina retained seven to eight rows of visual cell nuclei, resulting in an ONL nearly as thick as that in the non-light-treated control rat retina.
Rhodopsin regeneration
Because antioxidant treatment may interfere with the vitamin A visual cycle [47], we measured rhodopsin recovery at various times after a 1 h exposure to bleaching light. Regeneration of rhodopsin in the vehicle- and rosemary-treated rats was nearly identical and more than 75% of control following 90 min of darkness (Appendix 5).
Retinal gene array analysis
Pretreatment of animals with antioxidants, such as ascorbic acid, dimethylthiourea (DMTU), and others, ameliorates the effects of intense light treatment [31], presumably by reducing oxidative stress during the process of photooxidative visual cell death. In this study, we demonstrated that rosemary dietary supplementation also leads to amelioration of light-induced retinal degeneration. We examined the gene profiles of antioxidant- and vehicle-treated rats to determine whether rosemary-based antioxidant treatments also condition or alter the expression of retinal genes. By examining a high versus a low cyclic light rearing condition (which in itself does not compromise the retina significantly), we were able to examine early stages in retinal light damage and to determine whether environmental light preconditioning affects the retina’s susceptibility to subsequent intense damaging light. Gene expression profiles were established for different groups of animals reared in high (H) or low (L) cyclic light and fed one of the antioxidant dietary supplements (rosemary (R), zinc plus rosemary (Z+R), AREDS plus rosemary (A+R)), or the vehicle control (V) as described in the Methods section.
Differential analysis of the retinal gene profiles were performed in three sets of comparisons (Figure 5). MA (log ratio and mean average) plots/volcano plots for each set of gene array comparisons are shown in Appendix 6. Comparisons of antioxidant (AO) dietary treatment groups with the vehicle-treated (V) animals, all reared under low cyclic light (LAO/LV), identified one differentially expressed gene (Figure 5A). The A+R supplement comparison identified Oprk1, opioid receptor, Kappa 1, as a differentially expressed gene (FC = –1.48, p<0.05). Comparisons of the antioxidant treatment groups with the vehicle-treated animals, all high cyclic light-reared (HAO/HV), identified 143 differentially expressed gene markers (Figure 5B). R treatment lead to the identification of eight differentially expressed genes (three R specific), Z+R to 80 differentially expressed genes (20 Z+R specific), and A+R to 120 genes (60 A+R specific). For five of these gene markers (Egr1, Gng11, RGD1564999, RGD1564999, and Scn7a), all three antioxidant treatments resulted in higher relative expression levels than vehicle treatment alone (|FC|>1.3, p value<0.05). Differential comparisons of gene profiles derived from animals fed different rosemary-based antioxidants demonstrated that these treatments have an effect on retinal gene expression. Animals reared under high cyclic light conditions are more sensitive to antioxidant supplement associated changes in gene expression than animals raised in low cyclic light.
Figure 5.
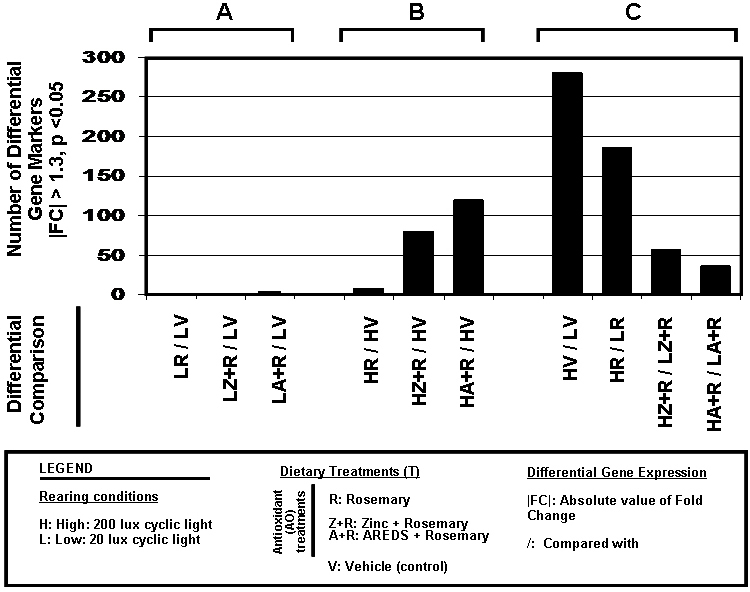
Tabulation of changes induced by light rearing and antioxidant diets in retinal gene expression. Gene expression profiles were established for different groups of animals raised with high (H) or low cyclic light rearing (L) conditions and treated with specific dietary supplements (rosemary [R], AREDS and rosemary [A+R], or zinc oxide and rosemary [Z+R], or vehicle [V]; see Figure 1). Differential analysis, of these profiles, was performed in three different sets of comparisons. A: The counts of differential gene markers from comparing different antioxidant-fed L-reared animals with vehicle-fed L-reared animals. B: Comparison of the antioxidant-fed H-reared animals with vehicle-fed H-reared controls. C: The findings when each dietary treatment is compared in animals reared in the high cyclic light environment with the same treatment in animals raised in low cyclic light conditions (genes listed in Appendix 7, Appendix 8, Appendix 9, Appendix 10 and Appendix 11). In all cases, the gene markers were categorized as differentially expressed between two different treatments if the absolute value of the fold change (FC) was ≥ 1.3, p<0.05 (|fold change (FC)| >1.3, p<0.05).
Comparisons between each treatment group (T) reared in high cyclic light with its respective dietary group reared in low cyclic light (HT/LT) provides a broader perspective to help define the effects of light rearing conditions and dietary supplementation (Appendix 7, Appendix 8, Appendix 9, Appendix 10 and Appendix 11). We established that the expression level of a given gene (with the exception of Oprk1) is such that gene levels at LV = LR = LZ + R = LA + R. Augmentation of differential status (H/L) for most genes by a given treatment is therefore co-mediated by a high light rearing environment. For any differential gene expression comparison, three trends are possible: The fold change goes up (U), the fold change goes down (D), or the fold change is not statistically significant (N). In total, we found 352 gene markers, defining 336 differentially expressed genes (U or D) across the four HT/LT analyses.
The count of differential gene markers decreased from (HV/LV > HR/LR > (HZ+R/LZ+R) > (HA+R/LA+R); Figure 5C). For a given gene, its expression trend status across all four H/L comparisons (V, R, Z+R, and A+R) can be collated to generate an expression phenotype. Twenty-two different-differential expression phenotypes were thus derived (Appendix 7, Appendix 8, Appendix 9, Appendix 10, and Appendix 11). The most abundant differential expression phenotype observed was UNNN (91 gene markers, Appendix 10), indicating that the primary response to rosemary-based dietary treatments is suppression of the inherent retinal response to shifting from a low cyclic light environment to a high cyclic light rearing environment. Next, we performed a trend analysis to determine the degree of similarity between each antioxidant treatment in high light versus vehicle (H/L) with HV/LV (Figure 6). The transition of rats from low cyclic light to a high cyclic environment light resulted in the differential expression of 280 gene markers; R, Z+R, and A+R treatment suppressed 131, 241, and 266 of these gene responses (respectively) in rats reared in high light versus low light. Although all three antioxidant (H/L) scenarios were significantly different from (HV/LV), the HV/LV differential gene profile had a higher degree match (similarity) with HR/LR than with (HZ+R/LZ+R) or (HA+R/ LA+R).
Figure 6.
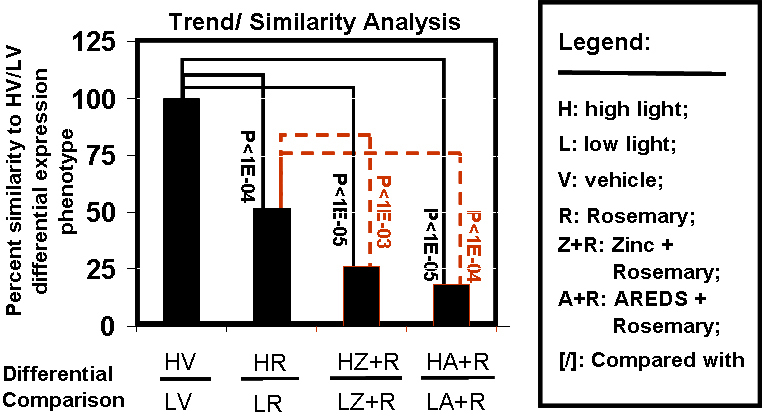
Expression trends. The differential status (up: U; down: D; not significant: N) for each gene marker in the list of 352 gene markers (Appendix 7, Appendix 8, Appendix 9, Appendix 10 and Appendix 11) were aligned and matched for (HV/LV) and each antioxidant [(HR/LR), (H Z+R/L Z+R), and (H A+R/L A+R)] treatment comparison. For each alignment, we scored the trend of the differential status whether they were the same or not. This information was used to derive a percent similarity measure for each antioxidant treatment under (H/L) conditions against the differential HV/LV gene profile. A goodness of fit test was performed between the percent similarities between the H/L antioxidant treatments, (HAO/LAO) comparisons with (HV/LV) to determine its statistical significance (p value). Although all three antioxidant (H/L) scenarios are all statistically significantly different than (HV/LV), the HV/LV differential gene profile is more similar to HR/LR than (H Z+R/L Z+R) or (H A+R/ L A+R).
The expression data were partitioned into vehicle effects (Figure 7; A ring, HV/LV) and antioxidant effects (Figure 7; B ring, HAO/LAO). From an overlapping comparison, three classes of differential genes become evident (Figure 7). 1) Nine gene markers with a similar fold change expression trend in all four analyses define class 1 genes (Appendix 7) corresponding to high light effects that could not be inhibited by antioxidant treatment. 2) 271 HV/LV differentially expressed gene markers, where the differential status is inhibited by at least one antioxidant treatment, corresponding to class 2 genes (light effects that can be modified by antioxidant treatment (Appendix 8, Appendix 9, and Appendix 10). 3) 72 class 3 genes, characterized by a HAO/LAO differential status in at least one of the antioxidant treatments, but not for HV/LV, thus defining purely antioxidant effects in the high light rearing environment (Appendix 11).
Figure 7.
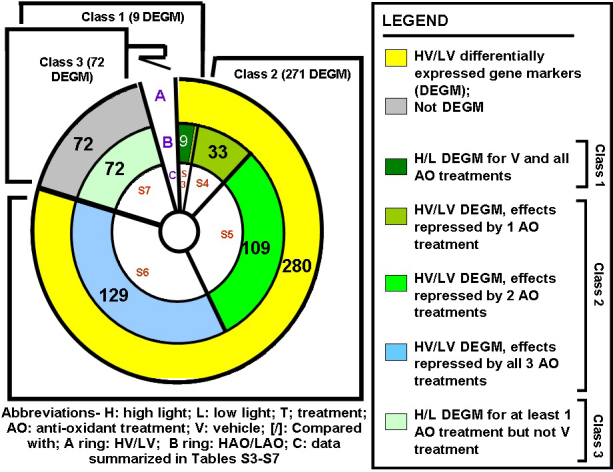
Three classes of induced gene markers. The (H/L) differential gene expression data across all four dietary treatments (352 gene markers) was partitioned into vehicle effects (A ring, HV/LV) and antioxidant (AO) effects (B ring, HAO/LAO); 280 of these gene markers define light (the transition from a low to high light rearing environment) responses. Nine gene markers with a similar fold change expression trend in all four analyses define class 1 genes (Appendix 7) that correspond to high light effects that cannot be inhibited with antioxidant treatment; 271 HV/LV differentially expressed gene markers where the differential phenotype is inhibited by at least one of the antioxidant treatments define class 2 genes (light effects that can be modified by an antioxidant treatment; Appendix 8, Appendix 9, Appendix 10 and Appendix 11); and 72 class 3 genes are characterized by a HAO/LAO differential status in at least one of the antioxidant treatments but not for HV/LV, thus defining purely antioxidant effects in a high light environment (Appendix 11).
Cell function trends
Two independent cell function analyses confirmed that the sets of differential genes from the four high light/low light (H/L) comparisons were distinct, implying that each treatment alters the retinal transcriptome toward a different tissue microenvironment (Figure 8). A focused analysis was then performed to determine whether specific categories of genes, defining specific cell responses, were enriched by the selection process and whether this selection was the same or different depending on the dietary supplement. The transition to a high- from a low-cyclic light rearing environment resulted in a marked enrichment in the expression of inflammation, apoptosis, cytokine, innate immune response, and receptor-related genes, a much more modest enrichment of growth factor and cell stress–related genes, and no enrichment of transcription-related genes (Figure 9A). Comparatively, rosemary treatment selectively decreased the enrichment for apoptosis, receptor, and growth factor–related genes while increasing the level of enrichment for stress response–related genes, relative to vehicle-treated animals (HV/LV). Z+R and A+R supplementation lead to decreased levels of all gene subgroups, except transcription factors, relative to vehicle treatment. Moreover, apoptosis- and receptor-related genes were highly enriched in HV/LV, but markedly depleted by all three antioxidant treatments.
Figure 8.
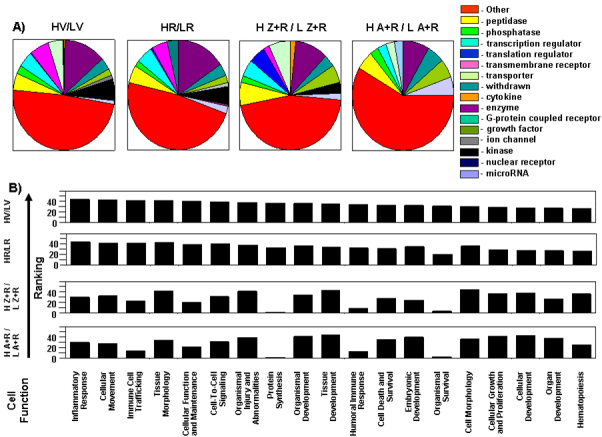
Molecular cell function analyses demonstrate differences in gene profiles. An Ingenuity Pathway Analysis (IPA) core analysis (Ingenuity® Systems) was performed using the differential gene marker data sets for (HV/LV), HR/LR, H Z+R/L Z+R, and H A+R/L A+R (Appendix 7, Appendix 8, Appendix 9, Appendix 10 and Appendix 11). A: Genes annotated by a single function (see the legend for designations) were extracted and used to generate a pie chart for each dietary treatment (H/L). Molecules with more complex multiple functions, or with unknown functions are categorized as “other.” Each dietary supplementation resulted in a different differential retinal gene profile with respect to a transition from a low to high light rearing environment. B: The information underlying the pathway analysis of each differential gene list and the IPA knowledge base was extracted. In total, 44 cell function or process categories were observed between the four differential comparisons. Not all categories are necessarily represented in each differential comparison. For each functional category in each comparison, the best matched pathway per category was ranked. The top 19 (HV/LV) ranked functional categories are graphed in reverse order. The corresponding ranking for the same functional categories for the different antioxidant supplemented comparisons are provided below the HV/LV graph. Comparison of individual rankings for each category from each differential comparison shows again that the functional nature of each set of genes is different. From a visual inspection HV/LV is more similar to the pattern seen in HR/LR and [H Z+R/LZ+R] is more similar with [H A+R/ L A+R].
Figure 9.
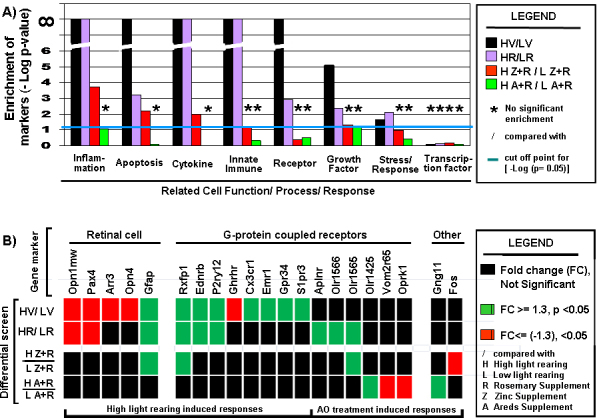
Cell function and cell response trends. A: Differential selection leads to enrichment and depletion of genes defining specific cell function and cell response. A focused cell function analysis was performed. A Boolean search for specific text strings was used to mine the NCBI gene database. We specifically mined for genes pertaining to the following categories (inflammation, apoptosis, cytokine, innate immune, receptor, growth factor, stress response, and transcription factor). Genes related to each text string were mined, and only rat genes were retrieved. Each gene list retrieved was aligned against a non-redundant gene list representing the microarray used, and this, in turn, was aligned to the differential comparison output data. From these alignments, we extracted the information needed to set up and perform a Fisher’s exact test for each functional category. P>0.05 indicates the absence of significant enrichment. –Log (p value) was plotted so changes in a single unit represent, a ten-fold difference. The blue line just above the value of 1 indicates the equivalent cut-off point for a p value of 0.05. B: Summary of the differential expression status for retinal cell associated gene markers and G-protein coupled protein associated gene markers.
Retina meta-analysis
The expression of several known retinal cell expressed genes provided insight into the state of the retina under the different rearing conditions and antioxidant dietary regimens (Appendix 7, Appendix 8, Appendix 9, Appendix 10 and Appendix 11, Figure 9B). A direct analysis of the differential data (HT/LT) for these associated gene markers demonstrated that rosemary supplementation results in a conservation of three of the five “retinal-specific” gene responses observed with vehicle treatment (Figure 9B). In contrast, the retinal expression response induced by comparing HV/LV was greatly inhibited by Z+R (four of five gene markers) or by A+R supplementation (five of five gene markers). Opn1mw levels for HV/LV and HR/LR were significantly decreased in that fold change (FC) was less than –1.3 and p<0.05. In the case of HZ+R/LZ+R and HA+R/LA+R, fold changes did not go below −1.3 (the cut-off threshold) although the p values were less than 0.05 (and therefore deemed not statistically significant). In addition, thyroid hormone receptor beta 2 (a nuclear hormone receptor, Thrb) is known to regulate the topography of green opsin (encoded by Opn1mw) and UV/blue opsin (Opn1sw) in the retina [51,52]. Thrb gene expression levels were decreased in the vehicle-treated rats (HV/LV) by 1.17 fold (p<0.05). However, the levels were consistent for all dietary treatments (with an average FC = –1.12 ± 0.04, for 12 independent retina samples on 12 independent arrays) and in each instance, p<0.05, making the Thrb gene a putative class 1 gene. Arrestin (Arr3) codes for a molecule functioning in the deactivation of G protein-coupled receptors involved in color vision (171107). Arr3 expression levels were decreased 1.45 fold for HV/LV but did not show statistically significant levels of variation for H/L for any of the antioxidant treatments. A similar trend was found for Opn4, the visual pigment of phototransducing retinal ganglion cells involved in setting the circadian clock (192223). Moreover, glial fibrillary acidic protein message (Gfap), a Müller cell stress marker and a retinal response to wounding, was elevated (2.27 ± 0.68) under the high light condition for animals treated with V, R, and Z+R, but showed no statistically significant difference in H/L levels for A+R.
Opn1mw and Opn4 are G-protein coupled receptors (GPCRs), a large and diverse family of proteins whose primary function is to transduce extracellular stimuli into intracellular signals [53]. High cyclic light rearing, versus low cyclic light, (H/L) caused significant changes in the expression levels of eight additional GPCR coding genes (Rxfp1, Ednrb, P2ry12, Ghrhr, Cx3cr1, Emr1, Gpr34, and S1pr3; highlighted in blue in Appendix 8 and Appendix 9 and in Figure 9B). All ten are class 2 genes; six did not show significant expression changes when treated with R, nine did not show statistically significant changes in levels when treated with Z+R, and all ten lacked differential status when treated with A+R. In addition, the expression levels of a different set of six GPCR genes (Aplnr, Olr1425, Olr1565, Olr1566, Oprk1, and Vom2r65; highlighted in yellow in Appendix 11, and Figure 9B) were unresponsive to the change from the low to high light rearing environment when the animals were fed vehicle but showed specific changes when the rats were fed at least one of the antioxidants. Thus, specific dietary treatments can, for some genes, suppress the inherent retinal transcriptome response to high light rearing (compared to low light rearing) and in other cases mediate antioxidant-driven changes in gene expression.
Dietary HA+R/LA+R (but not the other dietary treatments) resulted in the differential expression of Olr1425, Vom2r65, and Oprk1. Oprk1 is a GPCR that plays a role in the perception of pain and addiction. It binds endogenous endorphins and various synthetic opioids that result in signaling, via guanine nucleotide-binding proteins (G proteins), and inhibition of adenylate cyclase activity (P41145). G protein subunit gamma 11, Gng11, was observed to be elevated (2.43 fold, p<0.05; Appendix 7) in high light versus low light rearing conditions when animals were fed A+R, the dietary treatment that resulted in the highest degree of protection from photooxidative damage (Figure 2). Oprk1 and Gng11 are two components of several known canonical pathways, including Gas signaling, Gai signaling, and interleukin-8 (IL-8) signaling (IPA Database, Ingenuity® Systems). Gng11 promotes senescence in human fibroblasts [41]. It activates ERK1/2 but not Ras, and its expression can be induced by exposure to H2O2 [41]. An analysis of the proximal promoter for Gng11 revealed three separate binding sites for the transcription factor Pax4 and one for the related protein Pax6 (Table 2). We found that Pax4 was downregulated about 1.3 fold (p<0.05) by vehicle and rosemary treatment (H/L). No statistically significant variation occurred for the H/L reared animals treated with Z+R or A+R, implying that these two dietary supplements prevented the high light mediated downregulation of Pax4.
Table 2. Putative transcription factor binding sites within the proximal Gng11 promoter*.
| Factor Name | Position (strand) | Core Match | Matrix Match | Sequence (+ strand) |
|---|---|---|---|---|
| Pax-4 | -1763 (+) | 0.979 | 0.890 | attagTCAGGcgtggtagcgg |
| FOXD3 | -1086 (+) | 1.000 | 0.982 | caTTGTTttttt |
| Pax-4 | -993 (+) | 1.000 | 0.835 | aatggTCATGggtgtcttggg |
| HNF-1 | -952 (+) | 1.000 | 0.862 | aGTTAAtatttcacttg |
| Pax-6 | -863 (+) | 0.842 | 0.849 | tacatTCATGtttgatttttc |
| Pax-4 | -631 (+) | 0.986 | 0.856 | tggtgTCAAGtgtcacccaaa |
| Nkx2-5 | -626 (+) | 1.000 | 1.000 | tcAAGTG |
*The proximal 2000 bp upstream of the gene Gng11 was downloaded from the hg38 build of the human genome using the UCSC Genome Browser [51]. This sequence was used to query the Transfac 6.0 database using Match v.1.0 [52]. Only vertebrate matrices noted as “high quality” were used, and the cut-off selection for matrix groups was set to minimize false positives. * Position is given relative to the transcriptional start site. Matrix match is a score that describes the quality of a match between a positional weight matrix in TRANSFAC 6.0 and the input sequence. Core match denotes the quality of a match between the core sequence of a transcription factor binding site and the input sequence. For each, a score of 1 denotes an exact match. Capitalized letters in the sequence match the core sequence of the matrix, while the remaining positions of a matrix are lower case.
Discussion
This study showed that photooxidative retinal damage is reduced or prevented by an AREDS antioxidant mixture supplemented with non-traditional antioxidants present in the common herb rosemary. Chronic oral administration of AREDS alone, or acute i.p. injection, failed to prevent the light-induced loss of photoreceptor cell DNA or to increase the recovery of visual cell opsins (Figure 2, Figure 3, and Figure 4). Likewise, oral zinc oxide given daily for 6 weeks to rats (at the AREDS1-recommended dose) was ineffective unless supplemental rosemary was also given. Protective efficacy for the zinc and rosemary combination was dose dependent, as shown previously [26], with approximately 55% visual cell DNA retention using a 34 mg/kg dose of rosemary. Achieving a similar level of protection with AREDS required five times the normal recommended daily amount (Figure 2). At this higher concentration, the dose of zinc oxide would also be five times greater, which could account for the protective effect seen.
These findings also show that chronic high light rearing conditions result in lower levels of photoreceptor cell proteins (Figure 3A,D). Whereas these decreases occurred for cone opsin and rhodopsin, the higher environmental rearing light level had a remarkable effect on the less abundant cone cell opsin. Cone outer segment (COS) and rod outer segment (ROS) shortening is known to occur under intense light conditions in animal models [29,54] and in human retinal degenerations [55]. In this study, the accompanying decreases in visual cell arrestins suggests that some degree of photoreceptor cell loss also occurred because of the higher light rearing environment. To this end, we measured approximately 10% less visual cell DNA in retinas from rats reared in 200 lux cyclic light than found in rats from the 20 lux environment (Figure 3C). During light, mCAR appears to migrate from the cell body into COS [56] in a manner analogous to S-ag movement into ROS [57,58]. Because all animals were dark-adapted for 16 h before retinal excision, cone cell or rod cell arrestins should be localized within their respective photoreceptor cell bodies. Accordingly, this study confirms previous work [55] that showed mCAR and S-ag can serve as protein markers for cone or rod cell viability, and extends those findings by showing that coupled decreases in antibody staining for the membrane-bound opsins and the cytoplasmic arrestins are a reflection of acute or chronic photooxidative damage. This was most apparent following exposure to acute intense light for the animals originally reared in low cyclic light (Figure 3B,E and Figure 4B), where the loss of opsins and arrestins persisted 2 weeks after light damage. Conversely, rats reared in the 200 lux cyclic light environment appeared to have been preconditioned to limited levels of photooxidative stress and therefore were able to retain higher levels of both visual cell proteins.
The retinal gene expression data provide a potential mechanism for the preservation of cone opsin in animals reared in high light. The data are consistent with observations of the relationship between thyroid hormone and color vision [59]. Thyroid hormone receptor beta 2 (encoded by Thrb) increases the number of cone cells expressing green opsin (encoded by Opn1mw) and decreases the percentage of cells expressing UV/blue opsin (Opn1sw) [51]. We found that Thrb mRNA was downregulated 1.2-fold in the high light reared animals compared to the low-light animals (p<0.05). Similarly, high light animals also showed a 1.3-fold decrease in the expression of Opn1mw (p<0.05).
The protective efficacy of AREDS and rosemary combinations depends on the concentration of rosemary (Figure 2) and appears to result from carnosic acid. Ursolic acid, also present at a high level in rosemary [26], was less effective (Figure 4A). Because carnosic acid is unstable in aqueous solution [42], we used a hydrophobic ROPUFA/ethanol mixture to solubilize it, and as the vehicle for oral administration. We found higher levels of visual cell opsins and arrestins from feeding AREDS plus carnosic acid versus AREDS alone, along with a pattern of reduced DNA fragmentation 2 days after intense light. Although visual cell proteins continued to be lost during the 2-week dark recovery period, these were less pronounced in the rats treated with AREDS plus carnosic acid. The retention of photoreceptor cell DNA was also greater 2 weeks later, and the ONL was thicker in rats treated with AREDS plus carnosic acid (Figure 4B-D). For this study, we used carnosic acid at approximately 8 and 16 mg/kg, equal to, or double the amount in a 34 mg/kg dose of rosemary powder [26]. Additional work is required to assess relationships between dose and retinal up-take after oral administration, but carnosic acid (25 mg/kg), given i.p., has been shown to result in up-take by plasma and the retina [60]. One week of daily carnosic acid injections also led to a decrease in systemic oxidative stress and to an increase in visual cell survival following intense light [60].
The mechanism by which AREDS plus rosemary, or carnosic acid, imparts retinal protection may be related to their combined antioxidant capacity, their effects on retinal gene expression, and/or the ability of ionic zinc to reduce the formation of reactive oxygen species [61]. NADPH oxidase is a multisubunit membrane-bound enzyme that forms superoxide upon stimulation and can be inhibited by ionic zinc [62]. NADPH oxidase is present in the retina and requires the small GTPase Rac1 for its assembly and activity [43,44]. Exposure to intense light induces Rac1 activity [44], and conditional Rac1-knockout mice exhibit protection against photooxidative retinal damage [43]. We found a small increase in retinal Rac1 levels in rats treated with vehicle and AREDS 2 days after exposure to intense light, and a modest decrease in Rac1 levels in the rats treated with carnosic acid or ursolic acid (Appendix 2). The peak of Rac1 induction occurs approximately 6 h after exposure to intense light [43], and the level declines thereafter [44], which might explain the modest changes seen in the present study. A more comprehensive study of the time course of Rac1 induction and degradation should enhance our understanding of the mechanism of action of AREDS combined with supplemental antioxidants.
Gene profiling and differential comparisons of HV and LV gene profiles demonstrated 280 gene marker changes in the retinal genome that defines the transition between high and low light rearing conditions. This transition led to a modest enrichment of stress response genes (cell stress and oxidative stress). In contrast, a large enrichment in inflammation, apoptosis, cytokine, innate immune response, and receptor-related expressed genes was observed at the higher cyclic light intensity when compared with low light. This is not to say that oxidative stress does not exist because a higher level of CEP-protein staining was seen in retinal extracts from rats reared in the 200 lux environment than seen for those reared in the 20 lux environment. Moreover, differential expression (HV/LV, |FC|≥1.3, p<0.05)) for 40 known stress-related genes (including heat shock protein 1 (Hspb1), ceruloplasmin (Cp), glutathione peroxidase 8 (Gpx8), and glial fibrillary acidic protein (Gfap)) was observed (Appendix 7, Appendix 8, Appendix 9, Appendix 10 and Appendix 11).
The effect of dietary antioxidant treatment on retinal gene expression was twofold. First, the R, Z+R, and A+R treatments of high light reared rats compared with low light reared rats were found to suppress the majority of the high light preconditioning changes in the expression of genes (132, 242, and 267 gene markers, respectively). Second, each antioxidant treatment induced its own treatment-specific alterations in gene expression under the high light rearing condition that vehicle alone did not induce. Rosemary induced the differential expression of 38 gene markers in rats reared in high cyclic light, compared with low cyclic light, that were also independent of a light intensity effect. Z+R induced 18 gene marker changes that were independent of a light intensity effect, and A+R induced 22 markers. A+R resulted in the largest numbers of alterations to the normal high cyclic light preconditioned gene profile (suppression of 267 HV/LV differential gene markers and induction of 22 treatment-specific markers, totaling 289 gene expression modifications).
On a broader level of analysis, R treatment (H/L) was found to selectively decrease the level of enrichment for apoptosis-, receptor-, and growth factor–related genes while increasing the level of enrichment of stress response–related genes found for the vehicle-treated animals (HV/LV). Dietary Z+R and A+R, the two treatments resulting in the highest protective efficacy after intense acute light exposure (Figure 2), both led to a much more pronounced decrease in the enrichment of all gene subgroups considered (except transcription factors) relative to vehicle (HV/LV). Whereas the antioxidant effects of rosemary and AREDS are well-known, its actions as an anti-inflammation, anti-apoptotic, and anti-innate immune response agent are not well-known. Moreover, alterations in the expression of cytokine-, growth factor–, and receptor-related genes could greatly modify the way cells perceive their microenvironment and communicate with other cells. These data suggest that the increased protective efficacy observed for antioxidant treatments occurs, at least in part, by the activation or inhibition of transcriptional programs.
One of the most striking transcriptional changes revealed by microarray analysis was of the gene Gng11. This gene was upregulated in high light reared animals when supplemented with R alone and by co-treatment with A+R, or Z+R, all compared to vehicle treatment. Gng11 promotes senescence in fibroblasts [41] and is a lipid-anchored cell membrane protein with a role in cell signaling [63]. Analysis of the Gng11 proximal promoter identified three putative binding sites for the transcription factor Pax4 (Table 2). Pax4 is expressed in rat photoreceptors [64] and antagonizes the action of Pax6 [65-67], which is known to maintain photoreceptor progenitors in a multipotent state [65-67]. We found that Pax4 was downregulated by vehicle and R treatment (H/L), but no statistically significant variation was found for high versus low light reared animals that had been treated with Z+R or A+R. This suggests that these two antioxidant treatments prevented the high light-mediated downregulation of Pax4. It is possible that rosemary treatment acts through Pax4 to induce Gng11 as part of a program to promote the development of new photoreceptor cells. Gng11 and Oprk1 are two components of several known canonical pathways, including Gas signaling, Gai signaling, and IL-8 signaling. Oprk1 also mediates the positive regulation of dopamine secretion and the p38 mitogen-activated protein kinases (MAPK) cascade, as well as underlies roles in immune response and sensory perception. In previous work, we observed a peak dopamine receptor D4 (Drd4) expression level that coincided with retinal light damage susceptibility over a 24-h period [68], and disruption of the Drd4 gene (Drd4 knockout) leads to a light damage resistant state [69-71]. Finally, Oprk1 may affect the expression of Fos through its interaction in the p38 MAPK cascade, and deletion of Fos leads to a light damage resistant state [72].
The relevance of rodent animal models to AMD is still an open question [30]. Compelling evidence supports oxidative stress as an important factor in retinal light damage [31,43,44,60,61], and rodent models are known to recapitulate key aspects of human retinal pathology [21-24,27-29]. Oxidation coupled with inflammation also accelerates disease progression in AMD [1-6,11,12]. In this regard, photooxidative retinal damage provides an opportunity to study the mechanism and prevention, in a relatively inexpensive animal model, while gene array studies can identify proteins or pathways for potential future therapeutic intervention. Similarly, the impact of rod cell-derived factors on cone cell survival is amenable to study in rodent models [73]. This study shows a clear absence of protection by traditional AREDS antioxidants during photooxidative challenge, and improved rod cell and cone cell survival when supplemented by the non-traditional antioxidants in the common herb rosemary. It is entirely possible that an AREDS antioxidant/mineral mixture with enhanced antioxidant activity could effectively slow disease progression in a larger number of patients with AMD than currently benefit from traditional AREDS treatment.
Acknowledgments
This work was supported by funding from the International Retina Research Foundation, Birmingham, AL, the Ohio Lions Eye Research Foundation, Columbus, OH and the Petticrew Research Laboratory (DTO), NIH (NEI) P30EY006360, RPB, FFB (PW), and the Reunette Harris Professorship (PW). We acknowledge with thanks, Drs. John Crabb, Cheryl Craft, Larry Donoso and Krzysztof Palczewski for their generous gifts of antibodies used in this study. Special thanks to Dr. John Lang for providing the ROPUFA and rosemary powder used in this work, Christopher Waker and Richard Lee for computer graphics, and Linda Barsalou for DNA gel electrophoresis. Dr. D.T. Organisciak (dto@wright.edu) and Dr. P. Wong (pwong@emory.edu) are co-corresponding authors for this paper.
Appendix 1. Primary antibodies used for western analysis of retinal proteins.
To access the data, click or select the words “Appendix 1.”
Appendix 2. Retinal Rac1 levels determined by western analysis 48h after intense light exposure.
To access the data, click or select the words “Appendix 2.” Relative intensity is the average of 3 separate gels for n=6 animals per treatment; unexposed rat retinas (NoLD), vehicle (V), rosemary (R), AREDS (A), AREDS + rosemary (A+R), AREDS + carnosic acid (A+C), AREDS + ursolic acid (A+U).
Appendix 3. Neutral pH, agarose gel DNA levels as shown by ethidium staining following 5 min of running time.
To access the data, click or select the words “Appendix 3.”
Appendix 4. Heme-oxygenase (HO-1) immunoreactivity.
To access the data, click or select the words “Appendix 4.” Heme-oxygenase (HO-1) immunoreactivity determined 2 days and 2 weeks after intense light exposure of rats treated with vehicle (V), AREDS (A), or AREDS + carnosic acid (A+C). Two days after light exposure HO-1 staining was greater in V and AREDS treated rat retinas than in those treated with A+C. HO-1 activity was not present in any samples 2 weeks after photo-oxidative challenge.
Appendix 5. Rhodopsin regeneration following bleaching light exposure*
To access the data, click or select the words “Appendix 5.” *Male and female Sprague-Dawley rats were maintained from weaning in a cyclic light environment consisting of 140 lux for 12 h/day. Rats (P 50–60 days) were injected (i.p.) with rosemary extract (34 mg/kg), or vehicle (1% aqueous Tween-80/10% ethanol), 1 h before a 1 h light exposure and then placed in darkness. Eyes were enucleated under dim red light and rhodopsin extracted, as described [26]. Rhodopsin values were then calculated from the average of its levels measured in both eyes of n=2–5 animals.
Appendix 6. Distribution of fold-changes and p values.
To access the data, click or select the words “Appendix 6.” Differential expression between treatment groups was determined by 1-way ANOVA using Fisher's Least Significant Difference. Linear fold-change (x-axis) was plotted against p value (y-axis) to generate volcano plots. Each gene is colored by expression and treatments are abbreviated as in the embedded legend.
Appendix 7. High light induced gene expression (HV/LV), not modified by antioxidant treatments.
To access the data, click or select the words “Appendix 7.”
Appendix 8. High light induced gene expression (HV/LV), effects modified by one antioxidant treatment.
To access the data, click or select the words “Appendix 8.”
Appendix 9. High light induced gene expression (HV/LV), effect modified by two antioxidant treatments.
To access the data, click or select the words “Appendix 9.”
Appendix 10. High light induced gene expression (HV/LV), effect modified by all three antioxidant treatments.
To access the data, click or select the words “Appendix 10.”
Appendix 11. Antioxidant treatment induced gene expression (HAO/LAO), not affected by V treatment.
To access the data, click or select the words “Appendix 11.”
References
- 1.Eye Disease Case-Control Study Group Risk factors for neovascular age-related macular degeneration. Arch Ophthalmol. 1992;110:1701–8. doi: 10.1001/archopht.1992.01080240041025. [DOI] [PubMed] [Google Scholar]
- 2.Zarbin M, Sunness JS. Dry age-related macular degeneration and age-related macular degeneration pathogenesis. In: Levin LA, Albert DM, editors. Ocular Disease: Mechanisms and Management. London: Saunders Elsevier; 2010. p. 527–535. [Google Scholar]
- 3.Lederer DE, Cousins SW, Csaky KG. Neovascular age-related macular degeneration. In: Levin LA, Albert DM, editors. Ocular Disease: Mechanisms and Management. London: Saunders Elsevier; 2010. p. 536–543. [Google Scholar]
- 4.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, Yannuzzi LA, Willett W, Eye Disease Case-Control Study Group Dietary carotenoids, vitamin A, C, and E, and advanced age-related macular degeneration. JAMA. 1994;272:1413–20. [PubMed] [Google Scholar]
- 5.Berrow EJ, Bartlett HE, Eperjesi F, Gibson JM. Risk factors for age-related macular degeneration. European Ophthalmic Rev. 2011;5:143–53. [Google Scholar]
- 6.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene and zinc for age related macular degeneration. AREDS Report 8. Arch Ophthalmol. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGwin G, Xei A, Owsley C. The use of cholesterol-lowering medications and age-related macular degeneration. Ophthalmology. 2005;112:488–94. doi: 10.1016/j.ophtha.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Wilson HL, Schwartz DM, Bhatt HRF, McCulloch CE, Duncan JL. Statin and aspirin therapy are associated with decreased rates of choridal neovascularization among patients with age-related macular degeneration. Am J Ophthalmol. 2004;137:615–24. doi: 10.1016/j.ajo.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Solomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:14682–7. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278:42027–35. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 11.Klein RJ, Zeiss C, Chew EY, Tsai J-Y, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51:137–52. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haines JL, Hauser MA, Schmidt S, Scott WK, Olsen LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 14.Edwards AO, Ritter R, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 15.Weismann D, Hartvigen K, Lauer N, Bennet KL, Scholl HPN, Charbel Issa P, Cano M, Brandstatter H, Tsimikas S, Skerka C, Superti-Fuga G, Handa JT, Zipfe PF, Witztum JL, Binder CJ. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blom AM, Kask L, Ramesh B, Hillarp A. Effect of zinc on factor I cofactor activity of C4b-binding protein and factor H. Arch Biochem Biophys. 2003;418:108–18. doi: 10.1016/j.abb.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Donoso LA, Vrabec T, Kuivaniemi H. The role of complement factor H in age-related macular degeneration: a review. Surv Ophthalmol. 2010;55:227–46. doi: 10.1016/j.survophthal.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Age Related Eye Disease Study 2 Research Group Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration; the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–15. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 19.Chew EY. Nutrition effects on ocular diseases in the aging eye. Invest Ophthalmol Vis Sci. 2013;54:ORSF42–7. doi: 10.1167/iovs13-12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seddon JM. Genetic and environmental underpinnings to age-related ocular diseases. Invest Ophthalmol Vis Sci. 2013;54:ORSF28–30. doi: 10.1167/iovs.13-13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collier RJ, Wang Y, Smith S, Martin E, Ornberg R, Rhoades K, Romano C. Complement deposition and microglial activation in the outer retina in light-induced retinopathy: inhibition by a 5-HT 1A agonist. Invest Ophthalmol Vis Sci. 2011;52:8108–16. doi: 10.1167/iovs.10-6418. [DOI] [PubMed] [Google Scholar]
- 22.Rutar M, Natoli R, Kozulin P, Valter K, Gatenby P, Provis JM. Analysis of complement expression in light-induced retinal degeneration: Synthesis and deposition of C3 by microglia/macrophages is associated with focal photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2011;52:5347–58. doi: 10.1167/iovs.10-7119. [DOI] [PubMed] [Google Scholar]
- 23.Marc RE, Jones B, Watt C, Vazquez-Chona F, Vaughan DK, Organisciak DT. Extreme retinal remodeling triggered by light damage: implications for age related macular degeneration. Mol Vis. 2008;14:782–806. [PMC free article] [PubMed] [Google Scholar]
- 24.Hollyfield JG, Boniha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–8. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Organisciak D, Wong P, Rapp C, Darrow R, Ziesel A, Rangarajan R, Lang J. Light-induced retinal degeneration is prevented by zinc, a component in the age-related eye disease study formulation. Photochem Photobiol. 2012;88:1396–407. doi: 10.1111/j.1751-1097.2012.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Organisciak DT, Darrow RM, Rapp CM, Smuts JP, Armstrong DW, Lang JC. Prevention of retinal light damage by zinc oxide combined with rosemary extract. Mol Vis. 2013;19:1433–45. [PMC free article] [PubMed] [Google Scholar]
- 27.Kowluru RA, Kanwar M, Chan P-S, Zhang JP. Inhibition of retinopathy and retinal metabolic abnormalities in diabetic rats with AREDS-based micronutrients. Arch Ophthalmol. 2008;126:1266–72. doi: 10.1001/archopht.126.9.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowluru RA, Zhong Q. beyond AREDS: is there a place for antioxidant therapy in the prevention/treatment of eye disease? Invest Ophthalmol Vis Sci. 2011;52:8665–71. doi: 10.1167/iovs.10-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci USA. 2006;103:11300–5. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziess CJ. Animals as models of age-related macular degeneration: an imperfect measure of the truth. Vet Pathol. 2011;47:396–413. doi: 10.1177/0300985809359598. [DOI] [PubMed] [Google Scholar]
- 31.Organisciak D, Vaughan D. Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 2010;29:113–34. doi: 10.1016/j.preteyeres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bedolla DE, Torre V. A component of retinal light adaptation mediated by the thyroid hormone cascade. PLoS One. 2011;6:e26334. doi: 10.1371/journal.pone.0026334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Fu L, Chen D-G, Deeb SS. Identification of novel retinal target genes of thyroid hormone in the human WERI cells by expression microarray analysis. Vision Res. 2007;47:2314–26. doi: 10.1016/j.visres.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharanjeet-Kaur Dickinson CM, O’Donoghue E, Murray IJ. (1997) Spectral sensitivity in patients with dysthyroid eye disease. Ophthalmic Physiol Opt. 1997;17:232–8. [PubMed] [Google Scholar]
- 35.Feng Y, Wang Y, Li L, Wu L, Hoffmann S, Gretz N, Hammes HP. Gene expression profiling of vasoregression in the retina–involvement of microglial cells. PLoS One. 2011;6:e16865. doi: 10.1371/journal.pone.0016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agudo M, Pérez-Marín MC, Lönngren U, Sobrado P, Conesa A, Canovas I, Salinas-Navarro M, Miralles-Imperial J, Hallbook F, Vidal-Sanz M. Time course profiling of the retinal transcriptome after optic nerve transection and optic nerve crush. Mol Vis. 2008;14:1050–63. [PMC free article] [PubMed] [Google Scholar]
- 37.Vázquez-Chona F, Song BK, Geisert EE., Jr Temporal changes in gene expression after injury in the rat retina. Invest Ophthalmol Vis Sci. 2004;45:2737–46. doi: 10.1167/iovs.03-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vázquez-Chona FR, Lu L, Williams RW, Geisert EE. Genomic loci modulating the retinal transcriptome in wound healing. Gene Regul Syst Bio. 2008;1:327–48. [PMC free article] [PubMed] [Google Scholar]
- 39.Lee NE, Park YJ, Chung IY, Seo SW, Park JM, Yoo JM, Song JK. Gene expression changes in a rat model of oxygen-induced retinopathy. Korean J Ophthalmol. 2011;25:42–7. doi: 10.3341/kjo.2011.25.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishikawa K, Yoshida S, Kadota K, Nakamura T, Niiro H, Arakawa S, Yoshida A, Akashi K, Ishibashi T. Gene expression profile of hyperoxic and hypoxic retinas in a mouse model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2010;51:4307–19. doi: 10.1167/iovs.09-4605. [DOI] [PubMed] [Google Scholar]
- 41.Hossain MN, Sakemura R, Fujii M, Ayusawa D. G-protein gamma subunit GNG11 strongly regulates cellular senescence. Biochem Biophys Res Commun. 2006;351:645–50. doi: 10.1016/j.bbrc.2006.10.112. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y Smuts JP, Dodbiba E, Rangarajan R, Lang JC, Armstrong DW. Degradation study of carnosic acid, rosemarinic acid and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J Agric Food Chem. 2012;60:9305–14. doi: 10.1021/jf302179c. [DOI] [PubMed] [Google Scholar]
- 43.Haruta M, Bush RA, Kjellstrom S, Vijayasarathy C, Zeng Y, Le Y-Z, Sieving P. Depleting Rac1 in mouse photoreceptors protects them from photo-oxidative stress without affecting their structure or function. Proc Natl Acad Sci USA. 2009;106:9397–402. doi: 10.1073/pnas.0808940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belmonte MA, Santos MF, Kihara AH, Yan CYI, Hamassaki DA. Light-induced photoreceptor degeneration in the mouse involves activation of the small GTPase Rac1. Invest Ophthalmol Vis Sci. 2006;47:1193–200. doi: 10.1167/iovs.05-0446. [DOI] [PubMed] [Google Scholar]
- 45.Winkler BS. The electroretinogram of the isolated rat retina. Vision Res. 1972;12:1183–98. doi: 10.1016/0042-6989(72)90106-x. [DOI] [PubMed] [Google Scholar]
- 46.Organisciak D, Darrow R, Gu X, Barsalou L, Crabb JW. Genetic, age and light-mediated effects on crystallin protein expression in the retina. Photochem Photobiol. 2006;82:1088–96. doi: 10.1562/2005-06-30-RA-599. [DOI] [PubMed] [Google Scholar]
- 47.Mandal MNA, Moiseyev GP, Elliott MH, Kasus-Jacobi A, Li X, Chen H, Zheng L, Nikolaeva O, Floyd RA, Ma J-X, Anderson RE. α-phenyl-N-tert-butylnitrone (PBN) prevents light-induced degeneration of the retina by inhibiting RPE65 protein isomerohydrolase activity. J Biol Chem. 2011;286:32491–501. doi: 10.1074/jbc.M111.255877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carvalho BS, Irizarry RA. A Framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26:2363–7. doi: 10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smyth GK. Limma linear models for microarray data. In Bioinformatics and Computational Biology Solutions using R and Bioconductor. Gentleman R, Carey V, Dut S, Irizarry R, Huber W. editors, Springer, New York; 2005. p. 397–420. [Google Scholar]
- 50.Emig D, Salomonis N, Baumbach J, Lengauer T, Conklin BR, Albrecht M. AltAnalyze and DomainGraph: analyzing and visualizing exon expression data. Nucleic Acids Res. 2010;38:W755-62. doi: 10.1093/nar/gkq405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27:94–8. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- 52.Iwagawa T, Tanaka Y, Iida A, Itoh T, Watanabe S. Enhancer/promoter activities of the long/middle wavelength-sensitive opsins of vertebrates mediated by thyroid hormone receptor β2 and COUP-TFII. PLoS One. 2013;•••:8. doi: 10.1371/journal.pone.0072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kroeze WK, Sheffler DJ, Roth BL. G-protein-coupled receptors at a glance. J Cell Sci. 2003;116:4867–9. doi: 10.1242/jcs.00902. [DOI] [PubMed] [Google Scholar]
- 54.Sugawara T, Sieving PA, Bush RA. Quantitative relationship of the scotopic and photopic ERG to photoreceptor cell loss in light damaged rats. Exp Eye Res. 2000;70:693–705. doi: 10.1006/exer.2000.0842. [DOI] [PubMed] [Google Scholar]
- 55.John SK, Smith JE, Aguirre GD, Milam AH. Loss of cone molecular markers in rhodopsin-mutant human retinas with retinitis pigmentosa. Mol Vis. 2000;6:204–15. [PubMed] [Google Scholar]
- 56.Zhu X, Li A, Brown B, Weiss ER, Osawa S, Craft CM. Mouse cone arrestin expression pattern: light induced translocation in cone photoreceptors. Mol Vis. 2002;8:462–71. [PubMed] [Google Scholar]
- 57.Broekhuyse RM, Tolhuizen EFJ, Janssen APM, Winkes HJ. Light induced shift and binding of S-antigen in retinal rods. Curr Eye Res. 1985;4:613–8. doi: 10.3109/02713688508999993. [DOI] [PubMed] [Google Scholar]
- 58.Whelan JP, McGinnis JF. Light-dependent subcellular movement of photoreceptor proteins. J Neurosci Res. 1988;20:263–70. doi: 10.1002/jnr.490200216. [DOI] [PubMed] [Google Scholar]
- 59.Glaschke A, Weiland J, Del Turco D, Steiner M, Peichl L, Glosmann M. Thyroid hormone controls cone opsin expression in the retina of adult rodents. J Neurosci. 2011;31:4844–51. doi: 10.1523/JNEUROSCI.6181-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rezaie T, McKercher SR, Kosaka K, Seki M, Wheeler L, Viswanath V, Chun T, Joshi R, Valencia M, Sasaki S, Tozawa T, Satob T, Lipton SA. Protective effect of carnosic acid, a pro-electrophilic compound, in models of oxidative stress and light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2012;54:7847–54. doi: 10.1167/iovs.12-10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prunty MC, Aung MH, Hanif AM, Allen RS, Chrenek MA, Boatright JH, Thule PM, Kundu K, Murthy N, Pardue MT. In vivo imaging of retinal oxidative stress using a reactive oxygen species-activated fluorescent probe. Invest Ophthalmol Vis Sci. 2015;56:5862–70. doi: 10.1167/iovs.15-16810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cherny VV, DeCoursey TE. pH-dependent inhibition of voltage-gated H+ currents in rat alveolar epithelial cells by Zn2+ and other divalent cations. J Gen Physiol. 1999;114:819–38. doi: 10.1085/jgp.114.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Downes GB, Gautam N. The G protein subunit gene families. Genomics. 1999;15:544–52. doi: 10.1006/geno.1999.5992. [DOI] [PubMed] [Google Scholar]
- 64.Rath MF, Bailey MJ, Kim JS, Coon SL, Klein DC, Møller M. Developmental and daily expression of the Pax4 and Pax6 homeobox genes in the rat retina: localization of Pax4 in photoreceptor cells. J Neurochem. 2009;108:285–94. doi: 10.1111/j.1471-4159.2008.05765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith SB, Ee HC, Conners JR, German M. Paired-homeodomain transcription factor PAX4 acts as a transcriptional repressor in early pancreatic development. Mol Cell Biol. 1999;19:8272–80. doi: 10.1128/mcb.19.12.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petersen HV, Jørgensen MC, Andersen FG, Jensen J, Nielsen TF, Jørgensen R, Madsen OD, Serup P. Pax4 represses pancreatic glucagon gene expression. Mol Cell Biol Res Commun. 2000;3:249–54. doi: 10.1006/mcbr.2000.0220. [DOI] [PubMed] [Google Scholar]
- 67.Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 68.Wong P, Organisciak DT, Ziesel AC, Chrenek MA, Patterson ML. Circadian effects on Retinal Light Damage. In The Retina and Circadian Rhythms. Eds. G Tosini, PM Iuvone, DG McMahon, SP Collin. Springer Science+Business Media New York; 2014. p. 131–170. [Google Scholar]
- 69.Nir I, Harrison JM, Haque R, Low MJ, Grandy DK, Rubinstein M, Iuvone PM. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J Neurosci. 2002;22:2063–73. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson CR, Chaurasia SS, Zhou H, Haque R, Storm DR, Iuvone PM. Essential roles of dopamine D4 receptors and the type 1 adenylyl cyclase in photic control of cyclic AMP in photoreceptor cells. J Neurochem. 2009;109:148–57. doi: 10.1111/j.1471-4159.2009.05920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jackson CR, Chaurasia SS, Hwang CK, Iuvone PM. Dopamine D4 receptor activation controls circadian timing of the adenylyl cyclase 1/cyclic AMP signaling system in mouse retina. Eur J Neurosci. 2011;34:57–64. doi: 10.1111/j.1460-9568.2011.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wenzel A, Grimm C, Marti A, Kueng-Hitz N, Hafezi F, Niemeyer G, Remé CE. c-fos controls the “private pathway” of light-induced apoptosis of retinal photoreceptors. J Neurosci. 2000;20:81–8. doi: 10.1523/JNEUROSCI.20-01-00081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elachouri G, Lee-Rivera I, Clerin E, Argentini M, Fridich R, Blond F, Ferracane V, Yang Y, Raffelsberger W, Wan J, Bennett J, Sahel J-A, Zack DJ, Leveillard T. Thioredoxin rod-derived cone viability factor protects against photo-oxidative retinal damage. Free Radic Biol Med. 2015;81:22–9. doi: 10.1016/j.freeradbiomed.2015.01.003. [DOI] [PubMed] [Google Scholar]