Abstract
Purpose
To describe in detail cases with an initial diagnosis of Leber congenital amaurosis that were later found to have a hemizygous mutation in the CACNA1F gene.
Methods
The patients underwent a detailed ophthalmological evaluation and full-field electroretinography (ERG). Selective targeted capture and whole-exome next-generation sequencing (NGS) were used to find the disease-causing mutations.
Results
Patient 1 presented at age 3 months with nystagmus, normal visual attention, and a normal fundus exam. ERG responses were severely decreased. Patient 2 presented with nystagmus, severe hyperopia, esotropia, and visual acuity of 20/360 oculus dexter (OD) and 20/270 oculus sinister (OS) at age 5 months. His fundus exam showed slightly increased pigmentation around the foveae. The scotopic ERG responses were severely decreased and photopic responses mildly decreased. Based on the initial presentation, both patients received the clinical diagnosis of Leber congenital amaurosis (LCA). However, genetic testing showed no mutations in known LCA genes. Instead, broader genetic testing using NGS showed point mutations in the CACNA1F gene, which is reported to be associated with type 2 congenital stationary night blindness (CSNB2).
Conclusions
These two cases demonstrate the clinical overlap between LCA and CSNB in infants and young children. Genetic testing is an essential tool in these cases and provides a more accurate diagnosis and prognosis for patients with inherited retinal degenerative disorders.
Introduction
Several inherited retinal diseases present at birth, manifesting as nystagmus and decreased vision in infants [1]. These retinal conditions, such as Leber congenital amaurosis (LCA) and congenital stationary night blindness (CSNB), represent genetically and clinically heterogeneous groups of retinal disorders. Accurate diagnosis of an infant with decreased vision is often difficult, as presentations within the groups can overlap. Furthermore, LCA, achromatopsia, and CSNB can all present initially with a normal fundus appearance. Thus, precise diagnosis relies on electroretinography (ERG) and genetic diagnostic testing. In general, infants with severely attenuated or nonrecordable photopic and scotopic responses on ERG are diagnosed as having LCA, whereas those with absent cone-specific ERG responses and normal rod function are thought to have achromatopsia [1]. A ratio of the scotopic b-wave to a-wave amplitude of less than one (i.e., an electronegative waveform) indicates a possible diagnosis of CSNB [2]. However, performing ERGs in infants can be challenging, and ERG responses continue to mature throughout the first year of life, making it difficult to interpret responses measured in infancy and to attain a definitive diagnosis early in disease [3].
CSNB can be further classified as type 1 (CSNB1) or type 2 (CSNB2), depending on symptoms and abnormalities on the ERG [4]. CSNB1, or complete CSNB, is characterized by predominant night blindness, mildly decreased visual acuity, moderate to high myopia, and nystagmus that tends to lessen with time [4]. On ERG testing, the scotopic b-wave is unrecordable, but the photopic system is less abnormal, with typical broadening of the a-wave, due to absent photopic ON responses visible on photopic cone-specific ERG responses. The phenotype of CSNB2, or incomplete CSNB, is less distinct, and patients can present with impaired night vision, decreased visual acuity (VA), a variable degree of refractive error, nystagmus, light sensitivity, and the characteristic electronegative waveform on ERG [2,4]. ERG also shows a reduced but recordable scotopic b-wave, reduced photopic b-wave, and reduced 30-Hz flicker, due to abnormalities at the level of the scotopic and photopic ON and the photopic OFF responses [4,5]. CSNB2 can be difficult to diagnose due to its relatively low incidence and lack of specific symptoms. Furthermore, studies have shown that at least one of the major phenotypic features (night blindness, decreased visual acuity, myopia, and nystagmus) may be absent in up to three-quarters of cases [6-8].
The lack of specific symptoms and ocular findings in CSNB2 makes genetic testing a necessary component of diagnosis [8]. CSNB2 is most often caused by mutations in the CACNA1F gene (MIM#300110) [6,9,10], which encodes the α1F subunit of Cav1.4, a retina-specific voltage-gated L-type calcium-channel located in the membrane of photoreceptor and bipolar cell ribbon synapses [11,12]. CaV1.4 (α1F) knockout mouse models and mice with null mutations exhibit abnormally formed synaptic ribbons and cone photoreceptors, and outgrowth of rod bipolar and horizontal cell processes into the outer retina [13-15]. Furthermore, knock-in mice with the equivalent amino acid change of a known mutation in human CACNA1F, p.(Ile756Thr), also show immature ribbon synapses and diminished cone and rod photoreceptor terminals [16,17]. These findings support the long-postulated reduced signal transfer to ON and OFF bipolar cells as the reason for the decreased b-wave amplitudes and night blindness, reduced best-corrected visual acuity (BCVA), as well as photophobia in CSNB2 [10,11].
Interestingly, mutations in CACNA1F have also been linked to cone-rod dystrophy 3 (CORDX3), Åland island eye disease (AIED), and X-linked retinitis pigmentosa (XLRP), supporting the idea that one gene can be involved in multiple clinical entities [18-20]. Retinal dystrophies often present as a spectrum of clinical findings that do not always concur with a defined set of diagnostic characteristics, especially in infancy. Here, we describe two cases that further illustrate this theme. These patients were seen at the clinic with an initial presentation suggestive of LCA, including severely reduced photopic and scotopic functions on ERG and nystagmus, but upon genetic testing, they were found to have novel mutations in CACNA1F. This report demonstrates the significant overlap in phenotypes between LCA and CSNB2 and emphasizes that genotype often cannot be predicted by phenotype. We further propose that the classification of diseases by genotype (CACNA1F-associated retinal dystrophy) provides a more precise diagnosis for patients rather than the traditional phenotypic names (CSNB, LCA, etc.).
Methods
The study protocol was approved by the Institutional Review Boards of Massachusetts Eye and Ear Infirmary (MEEI), Boston Children’s Hospital (BCH), and the Children’s Hospital of Philadelphia (CHOP). Patients were recruited and clinically evaluated at three centers (Electroretinography Service at MEEI and BCH and the Ophthalmic Genetics & Visual Electrophysiology Clinic at CHOP), and the parents of the patients provided informed written consent for the study. This research adhered to the tenets of the Declaration of Helsinki and was conducted in accordance with the Health Insurance Portability and Accountability Act.
Clinical evaluation
The patients underwent a full ophthalmic examination which included best-corrected preferential looking and Kay picture tests, followed by Snellen visual acuity depending on age, visual field testing on the Goldmann perimeter with the V-4e white test light, the Lang Stereotest, the Ishihara 38 plate color test, the Farnsworth Dichotomous-15 color vision test, dark adaptation testing performed with the Goldmann-Weekers dark adaptometer, and full-field ERG testing. ERG responses were obtained following protocols as described elsewhere [21-23]. To summarize, a calibrated photodiode (IL1700; International Light, Newburyport, MA) with a scotopic or photopic filter was used at BCH to measure stimulus strength. For the dark-adapted eye, responses to full field, blue stimuli were recorded over a 5 log unit range (from -2 to 3 log scot td s) at increments of 0.3 log unit steps. Cone and cone-driven responses were recorded to a range of red flashes (0.3 – 35 cd·s/m2) presented on a steady, white rod-saturating background (25.5 cd/m2). Photopic function was also tested with a 30-Hz flickering stimulus of 2.25 cd·s/m2 [21]. At MEEI, full-field ERGs were elicited in response to single flashes of 0.5 Hz dim blue light, 0.5 Hz white light, and 30 Hz white light at durations of 10 µs [22]. At CHOP, full-field ERGs were obtained according to ISCEV standards as outlined previously [23]. 0.01 cd·s/m2 was used for rod stimulation, 3.0 cd·s/m2 for all other standard responses, and 30 cd/m2 for light adaptation and background luminance. After parents of the patients provided informed consent, blood samples were collected from the patients and available family members for DNA extraction. Blood samples were collected from the patients and available family members. Leukocyte DNA was purified using standard procedures.
Genetic analysis
Before the current study, both patients had undergone limited genetic diagnostic testing at several testing laboratories. The testing included testing for identified mutations in known LCA disease genes (the John and Marcia Carver Nonprofit Genetic Testing Laboratory, Iowa City, IA) and direct sequencing of the LCA and/or RP genes in-house and through the ARUP Laboratories (Associated Regional and University Pathologists, Inc., Salt Lake City, UT).
The patients then underwent more thorough genetic testing for the studies reported here. Patient 1 was genetically diagnosed with whole-exome sequencing of the patient and available family members. The whole-exome libraries (SureSelect Human All Exon V4, Agilent Technologies, - Santa Clara, CA) were sequenced on a HiSeq platform (Illumina, Inc., San Diego, CA) as previously reported [24]. Patient 2 was genetically screened with targeted exon sequencing with the Genetic Eye Disease (GEDi) test, as previously described [25]. Briefly, the custom SureSelect targeted enrichment GEDi capture kit (Agilent Technologies, Inc, Santa Clara, CA) was designed to capture and enrich coding exons and select deep intronic regions associated with 257 genes, including known IRD genes, early-onset glaucoma and optic atrophy genes, and candidate IRD disease genes. The targeted enrichment GEDi capture set included all currently known monogenic inherited retinal degeneration genes [25]. GEDi targeted enrichment sample sequencing was performed on a MiSeq NGS platform (Illumina, Inc.).
NGS sequencing data were analyzed using custom and publicly available tools. The Burrows-Wheeler Aligner (BWA) was used to align the sequence reads to the human reference genome (GRCh37) and SAMtools to remove potential duplicates and identify initial single nucleotide polymorphisms (SNPs) and insertions and deletions [26,27]. Custom and publicly available variant filtering programs were applied to remove likely false positive calls [27,28]. The resulting variant calls were annotated using a custom human base-pair codon resource and public resources [UCSC Genome Browser, ENSEMBL, 1000 Genomes Project, Exome Variant Server (EVS), SIFT, and PolyPhen-2] [29,30]. Variants were filtered by frequency (<0.15%) based on the EVS database and the Single Nucleotide Polymorphism Database (dbSNP), as before [31]. Non-synonymous variants, nonsense mutations, potential splice-site changes, and rare synonymous variants were considered. Rare synonymous changes were evaluated in terms of the possibility of affecting splicing.
Mutations in CACNA1F were confirmed through Sanger sequencing and cosegregation analysis in available family members. Regions of interest were PCR amplified (Taq DNA Polymerase, Life Technologies, Carlsbad, CA), purified (ExoSap-IT, Affymetrix, Santa Clara, CA), and sequenced (BigDye Terminator v3.1, ABI 3730xl, Life Technologies, Grand Island, NY). Regions of interest were PCR amplified for 35 cycles using the following conditions: denaturation at 95°C, annealing at 60°C, and extension at 72°C. Variant annotations were performed according to the transcript ENST00000376265.
Results
Case 1
Patient 1 (OGI 117_301) presented at age 3.5 months with nystagmus, which had been noted by his parents since 1 month after birth. He showed fixation on faces and seemed to have normal visual attention. The fundus exam was normal. The ERGs were notable for decreased scotopic and photopic responses with amplitudes of rod-specific, maximal combined rod-cone, and cone-specific responses at 10% of normal (Figure 1A). No obvious electronegative aspect of the maximal combined rod-cone response was noted at that time. A preliminary clinical diagnosis of LCA was made. However, genetic testing for mutations in previously identified LCA genes was negative.
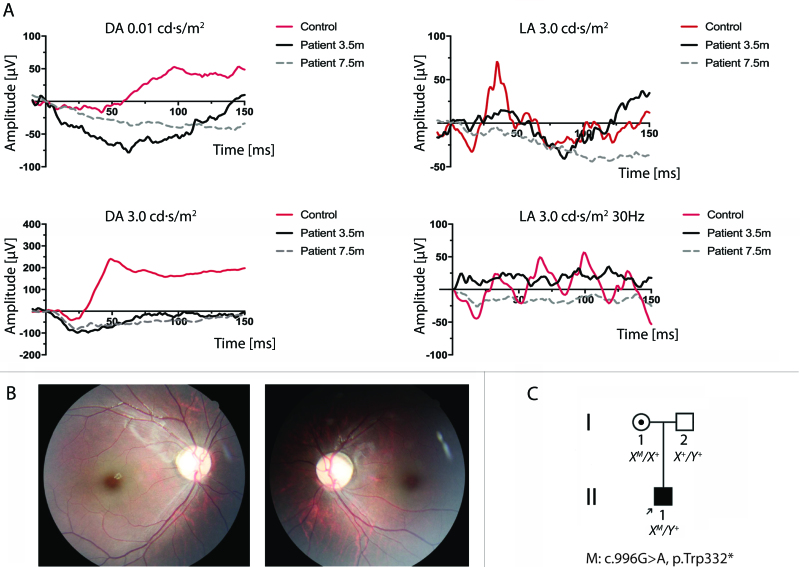
Figure 1.
Exam findings for Patient 1. A: Full-field flash electroretinography according to International Society for Clinical Electrophysiology of Vision (ISCEV) standards is shown at ages 3.5 months (black traces) and 7.5 months (gray traces). At 3.5 months, the electroretinogram (ERG) traces showed notably decreased amplitudes in the rod and cone responses, with the amplitudes in the rod-specific, maximal combined rod-cone, and cone-specific responses at 10% of normal. No obvious electronegative aspect was evident at that time. At 7.5 months, the ERG traces showed significantly reduced amplitudes in the rod and cone responses and an electronegative waveform on maximal responses. Control ERGs performed at the same institution for a healthy 8-month-old are included for comparison (red traces). DA = dark adapted; LA = light adapted. B: Right and left fundus photographs showing essentially normal fundi at age 4 years. C: Family pedigree of Patient 1.
At 7.5 months, the maximal combined ERG response began to show an electronegative waveform (Figure 1A), which was confirmed more clearly at age 4 years. The fundus exam was normal at that time (Figure 1B). His nystagmus had resolved by age 10 months, and he had no refractive error. His parents also noted that his fixation on objects and faces improved, and his BCVA was 20/60 oculus dexter (OD) and 20/70 oculus sinister (OS) at age 4 (Appendix 1). There was no history of vision loss or consanguinity in the family (Figure 1C). Using whole-exome sequencing, a new nonsense mutation in CACNA1F was discovered at c.966 G>A, resulting in truncation of the protein at codon 332 [p.(Trp332*)].
Case 2
The parents of Patient 2 (D226–1) brought him to the clinic after noticing “flickering of eyes” since age 3 weeks. On his first ophthalmologic exam at 5 months, the patient was interactive and visually responsive. He had vertical nystagmus, visual acuity of 20/270 with binocular viewing (20/360 OD and 20/270 OS) as measured with preferential looking, cycloplegic refraction of +9.50 S OD and +9.00 S OS, and esotropia of 30 prism diopters (Appendix 1). On the fundus exam, slightly increased pigmentation was noted in the parafoveal area. Scotopic ERG testing performed at BCH showed response amplitudes below the 99% prediction interval for normal. Photopic function was also considerably attenuated, with b-wave responses below the normal mean for his age by two standard deviations (Figure 2A). The responses did not demonstrate an electronegative waveform. The amplitudes of the 30-Hz flicker response were 10% of normal. His dark-adapted visual threshold was found to be elevated by 1.46 log units. These findings resulted in an initial diagnosis of LCA.
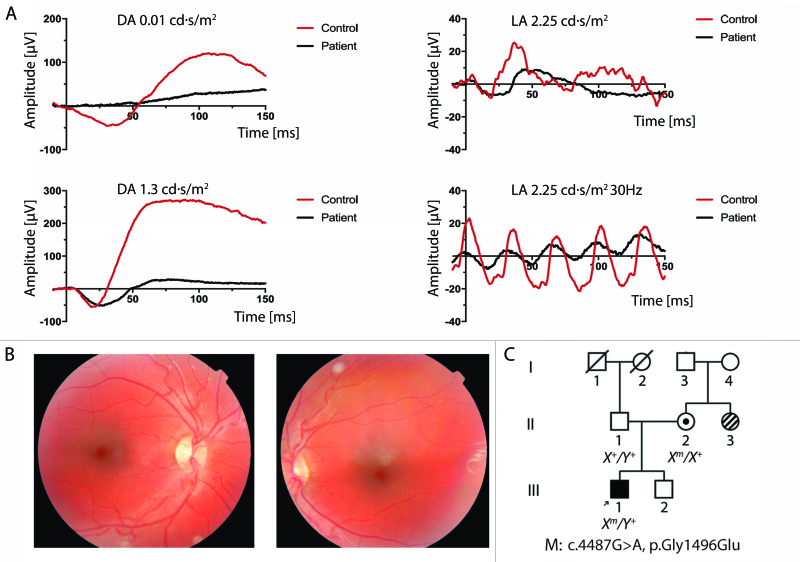
Figure 2.
Exam findings for Patient 2. A: Electroretinograms (ERGs) recorded from Patient 2’s right eye at 5 months old (black traces). Scotopic and photopic responses closest in flash strength to International Society for Clinical Electrophysiology of Vision (ISCEV) standards are shown. Mixed-response ERGs at 1.3 cd·s/m2 are shown instead of at the traditional 3.0 cd·s/m2 flash strength. Light-adapted ERGs at 2.25 cd·s/m2 are shown instead of at the traditional 3.0 cd·s/m2 flash strength. Scotopic ERG testing showed response amplitudes below the 99% prediction interval for normal, with prolonged b-wave implicit times [69]. Photopic function was also significantly attenuated, with b-wave responses below the normal mean for his age by two standard deviations and implicit times prolonged [70]. The amplitude of the 30-Hz flicker response was 10 µV, about 10% of normal. Control ERGs performed at the same institution for a healthy 10-month-old are included for comparison (red traces). DA = dark adapted; LA = light adapted. B: Right and left fundus photographs at age 3 years, showing red foveal areas and increased pigmentation in the parafoveal area. C: Family pedigree of Patient 2. Stripes indicate a maternal aunt with Stargardt disease.
Patient 2 was followed regularly at BCH, and his best-corrected visual acuity improved to 20/150 OD and 20/200 OS by age 3. His hyperopia increased slightly to +11.00 OU. He continued to have alternating esotropia of 30 prism diopters, and no stereopsis was demonstrated with the Lang test. At age 3, on a clinical exam, blunted foveal reflexes and a faint bull’s eye pattern in the macula were noted but were not evident on fundus photography (Figure 2B). At age 6, ERG testing showed improved scotopic responses compared to previous recordings, with b-wave amplitudes of approximately half the lower normal limit. Cone response amplitudes were 20% of normal. The amplitudes of the 30-Hz flicker were 20–25% of normal. At that visit, color vision testing was essentially normal, with only a few minor errors on the Ishihara plate test. Cycloplegic refraction was +11.50 sphere in both eyes. Best-corrected letter acuity was 20/100 OD and 20/70 −2 OS. Nystagmus and esotropia were present (Appendix 1).
At that time, further comprehensive genetic testing, which included sequencing all of the known inherited retinal disease (IRD) genes [25], showed a c.4487G>A mutation in CACNA1F, leading to a p.(Gly1496Glu) change. This position was highly conserved (Figure 3A), and this mutation was predicted to be deleterious (PolyPhen-2, SIFT, Protein Variation Effect Analyzer (PROVEAN), and MutationTaster; Table 1) [29,32-34]. Family history was significant for a maternal aunt with Stargardt disease (Figure 2C). There was no history of consanguinity in the family.
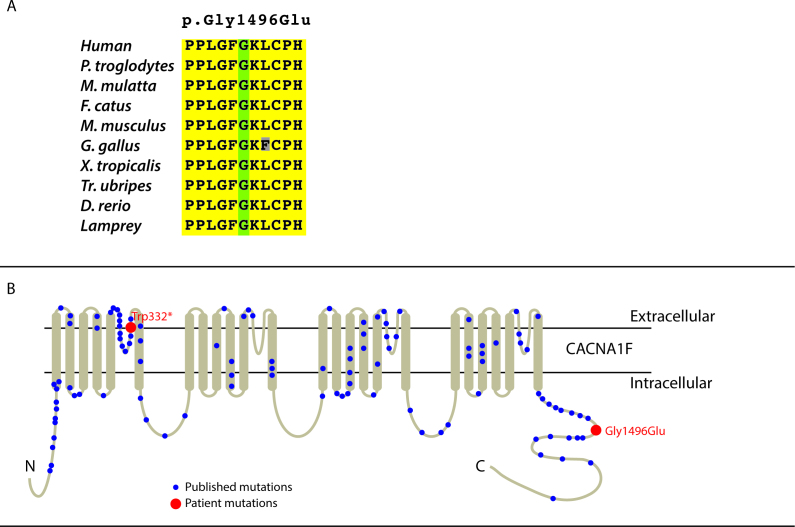
Figure 3.
Schematics. A: Schematic showing the conservation of the amino acid p.(Gly1496Glu) across the genomes of ten species. B: Schematic of the CACNA1F transmembrane protein and the positions of mutations p.(Trp332*) and p.(Gly1496Glu). (Adapted from [4]).
Table 1. Prediction of pathogenicity of CACNA1F c.4487G>A mutation leading to p.(Gly1496Glu) change.
| Variables | Prediction software | Prediction | Score |
|---|---|---|---|
| Amino-Acid change |
Polyphen-2 |
Probably Damaging |
1 |
| SIFT |
Damaging |
0 |
|
| Provean |
Deleterious |
−7.49 |
|
| MutationTaster |
Disease causing |
0.9999 |
|
| Nucleotide conservation | PhyloP |
Conserved |
5.512 |
| PhastCons |
Conserved |
1 |
|
| GERP | Conserved | 5.37 |
Discussion
The two patients described had early onset retinal degeneration and based on their clinical findings were initially diagnosed with LCA. Genetic diagnostic testing, however, did not identify mutations in known LCA genes. Instead, both patients were found to have mutations in the CACNA1F gene as the likely cause of their retinal disease, which is usually associated with CSNB2. These findings are important for several reasons. First, these findings demonstrate that phenotypes caused by mutations in different IRD genes overlap, and thus, it is difficult to predict genotype based on phenotypic characteristics. In particular, the phenotype of CSNB2 is intermediate between the archetypes of LCA (poor acuity, nystagmus, and hyperopia) and the more familiar form of CSNB1 (good acuity, less nystagmus in adulthood, and myopia). Further, clinical exams and electrophysiologic testing can be difficult in young children, compounding the clinical diagnostic challenge. The apparently atypical genetic diagnosis made for these patients emphasizes the importance of broad genetic diagnostic testing for patients with IRD, such as panel-based NGS tests [25,35-37]. Finally, the findings reported highlight the importance of continued studies of the genetic causality of IRD, including investigation of potential modifier alleles that may contribute to determining the severity of disease [38-43].
The mutations in CACNA1F in the two patients reported here are novel, and we strongly believe these mutations are the disease-causing variants. The positions of both mutations are near other known pathogenic mutations in CACNA1F (Figure 3B). The missense mutation [p.(Gly1496Glu)] in Patient 2 is predicted to be a deleterious change at a conserved position (Table 1), and Patient 1 has a nonsense mutation [p.(Trp332*)] that causes a truncation at exon 7 (out of 48 in total) of CACNA1F, which is presumed to result in a null allele. Further, neither patient had potential disease-causing mutations in other known IRD genes.
Given the genetic diagnoses of CACNA1F-associated retinal degeneration, the clinical findings in the patients described demonstrate the overlap in symptoms and clinical findings between LCA and CSNB. The clinical presentations of both patients were consistent with LCA: decreased vision and nystagmus in infancy and significantly reduced photopic and scotopic ERG responses. Patient 2 also had high hyperopia and an abnormal fundus exam, usually associated with LCA, whereas patients with CSNB tend to have myopia. Although hyperopia in CSNB is less common, it has been reported in other studies [44-46]. Neither patient underwent an electronegative ERG when they presented. Patient 1 did not show this finding until months to years after the initial presentation, and Patient 2 did not develop an electronegative ERG, even at 6 years of age. A possible contributing factor is Patient 2’s refractive error, as high hyperopia has been reported to correlate with increased b-wave amplitudes [47]. It has also been reported that the development of the characteristic electronegative waveform in CSNB can take time to manifest [48].
Both patients reported here were diagnosed with LCA but were found to have mutations in a “CSNB gene,” demonstrating that different types of IRD cannot be separated based on phenotype alone. In addition, distinguishing between different types of IRD, such as LCA and CSNB, based on ERG findings can be problematic, especially in young children. ERGs are difficult to interpret in young infants as their amplitudes are smaller and increase with age [3,49]. Thus, what may initially seem like severely reduced ERG amplitudes may improve on subsequent testing. This was observed in Patient 1, as his serial ERGs performed at one institution showed progressive development of retinal function over time. Furthermore, visual function during infancy can be initially profoundly subnormal and then improve months later, making early diagnosis challenging [48].
Adding to the already challenging task of differentiating LCA and CSNB is that some patients with LCA can exhibit improvements in visual function temporarily [50]. Several studies have reported that certain patients, such as those with mutations in RPE65 (Gene ID 6121, OMIM 180069), may show improved visual function over the first few years of life and deteriorate only as they reach their third to fifth decades [51,52].
One question raised by the findings presented here is why the retinal phenotypes produced by different mutations in genes associated with different types of retinal degeneration overlap. It does not appear that the primary mutations in the disease gene account completely for severity, as Patient 1 had clinically milder disease than Patient 2 but had a null allele of CACNA1F. This observation is common for patients with IRD, and it has been suggested that variants in additional genes may modify the severity of the phenotype from the primary mutation in CACNA1F, as has been suggested for other types of IRD [53-57]. Both patients had variants in other IRD genes (Table 2) that were ruled out as pathogenic but may function as modifier alleles.
Table 2. Additional IRD gene variants in CACNA1F patients.
| Patient ID | Gene | Transcript | Zygosity | cDNA position | Protein change | Polyphen-2 | SIFT | Provean | MutationTaster | dbSNP | ExAC Frequency |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
SEMA4A |
NM_022367.3 |
heterozygous |
c.2138G>A |
p.(Arg713Gln) |
Possibly damaging |
Tolerated |
Neutral |
Polymorphism |
rs41265017 |
A=4,297/ G=121,356 |
|
PDE6A |
NM_000440.2 |
heterozygous |
c.251A>T |
p.(Lys84Met) |
Benign |
Damaging |
Deleterious |
Polymorphism |
NA |
T=11/ A=121,396 |
|
|
PDE6B |
NM_000283.3 |
heterozygous |
c.1412C>T |
p.(Ala471Val) |
Benign |
Damaging |
Neutral |
Polymorphism |
rs182071364 |
T=17/ C=119,914 |
|
|
RPGR a |
NM_001034853.1 |
hemizygous |
c.1163C>T |
p.(Ala388Val) |
Benign |
Tolerated |
Neutral |
Polymorphism |
rs199661899 |
T=16/ C=85,833 |
|
| 2 |
USH2A |
NM_206933.2 |
heterozygous |
c.9286G>A |
p.(Val3096Met) |
Probably damaging |
Damaging |
Neutral |
Polymorphism |
rs147267500 |
A=6/ G=121,394 |
| PDE6A | NM_000440.2 | heterozygous | c.1214A>G | p.(Asn405Ser) | Probably damaging | Damaging | Deleterious | Disease causing | rs145107955 | G=20/ A=121,278 |
a Known polymorphism reported in [71].
The cases reported here exemplify the wide range of phenotypic variability encompassed by mutations in CACNA1F, which can present as CSNB2 or with LCA-like symptoms, or as AIED, CORDX3, or XLRP, as previously reported [18-20]. As indicated above, similar phenotypic variations are observed with many other forms of IRD. We believe these findings support broader use of genetic diagnostic testing for patients with IRD. This is especially true of making a correct diagnosis of the condition underlying nystagmus in the newborn. Multiple groups have reported successful application of panel-based tests and whole-exome sequencing to patients with IRD [25,58-62]. The present data suggest that panel-based testing is more accurate than exome sequencing at present, and as the costs of whole-genome sequencing continue to fall, this modality may be applied more broadly for clinical diagnostic testing [25,63]. In concert with increased use of genetic diagnostic testing, we encourage clinicians to adopt a genotype-based system of disease classification, as this approach is more precise and will facilitate identification of patients who may benefit from gene-based therapies for these disorders, which show great promise in ongoing clinical trials [64-68].
Acknowledgments
This research was accomplished through the support of the National Institutes of Health [RO1EY012910 (E.A.P.) and P30EY014104 (MEEI core support)], the Foundation Fighting Blindness USA (E.A.P., Q.L.), the Research to Prevent Blindness Medical Student Fellowship (C.M.), and the Fleming Family Foundation (K.M.B). B.P.L. is a Senior Clinical Investigator of the Research Foundation Flanders, Belgium (FWO). The authors have no financial disclosures.
Appendix 1. Clinical findings for patients 1 and 2.
To access the data, click or select the words “Appendix 1.”
References
- 1.Lambert SR, Taylor D, Kriss A. The infant with nystagmus, normal appearing fundi, but an abnormal ERG. Surv Ophthalmol. 1989;34:173–86. doi: 10.1016/0039-6257(89)90101-x. [DOI] [PubMed] [Google Scholar]
- 2.Miyake Y, Yagasaki K, Horiguchi M, Kawase Y, Kanda T. Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol. 1986;104:1013–20. doi: 10.1001/archopht.1986.01050190071042. [DOI] [PubMed] [Google Scholar]
- 3.Fulton AB, Hansen RM, Westall CA. Development of ERG responses: the ISCEV rod, maximal and cone responses in normal subjects. Doc Ophthalmol. 2003;107:235–41. doi: 10.1023/b:doop.0000005332.88367.b8. [DOI] [PubMed] [Google Scholar]
- 4.Zeitz C, Robson AG, Audo I. Congenital stationary night blindness: an analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Prog Retin Eye Res. 2015;45:58–110. doi: 10.1016/j.preteyeres.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Tremblay F, Laroche RG, De Becker I. The electroretinographic diagnosis of the incomplete form of congenital stationary night blindness. Vision Res. 1995;35:2383–93. doi: 10.1016/0042-6989(95)00006-l. [DOI] [PubMed] [Google Scholar]
- 6.Boycott KM, Maybaum TA, Naylor MJ, Weleber RG, Robitaille J, Miyake Y, Bergen AA, Pierpont ME, Pearce WG, Bech-Hansen NT. A summary of 20 CACNA1F mutations identified in 36 families with incomplete X-linked congenital stationary night blindness, and characterization of splice variants. Hum Genet. 2001;108:91–7. doi: 10.1007/s004390100461. [DOI] [PubMed] [Google Scholar]
- 7.Bijveld MM, Florijn RJ, Bergen AA, van den Born LI, Kamermans M, Prick L, Riemslag FC, van Schooneveld MJ, Kappers AM, van Genderen MM. Genotype and phenotype of 101 dutch patients with congenital stationary night blindness. Ophthalmology. 2013;120:2072–81. doi: 10.1016/j.ophtha.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Boycott KM, Pearce WG, Bech-Hansen NT. Clinical variability among patients with incomplete X-linked congenital stationary night blindness and a founder mutation in CACNA1F. Can J Ophthalmol. 2000;35:204–13. doi: 10.1016/s0008-4182(00)80031-9. [DOI] [PubMed] [Google Scholar]
- 9.Koenekoop RK, Lopez I, den Hollander AI, Allikmets R, Cremers FP. Genetic testing for retinal dystrophies and dysfunctions: benefits, dilemmas and solutions. Clin Experiment Ophthalmol. 2007;35:473–85. doi: 10.1111/j.1442-9071.2007.01534.x. [DOI] [PubMed] [Google Scholar]
- 10.Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:264–7. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- 11.Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Rüther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl A. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:260–3. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- 12.Morgans CW. Localization of the alpha(1F) calcium channel subunit in the rat retina. Invest Ophthalmol Vis Sci. 2001;42:2414–8. [PubMed] [Google Scholar]
- 13.Bayley PR, Morgans CW. Rod bipolar cells and horizontal cells form displaced synaptic contacts with rods in the outer nuclear layer of the nob2 retina. J Comp Neurol. 2007;500:286–98. doi: 10.1002/cne.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Specht D, Wu SB, Turner P, Dearden P, Koentgen F, Wolfrum U, Maw M, Brandstätter JH, tom Dieck S. Effects of presynaptic mutations on a postsynaptic Cacna1s calcium channel colocalized with mGluR6 at mouse photoreceptor ribbon synapses. Invest Ophthalmol Vis Sci. 2009;50:505–15. doi: 10.1167/iovs.08-2758. [DOI] [PubMed] [Google Scholar]
- 15.Raven MA, Orton NC, Nassar H, Williams GA, Stell WK, Jacobs GH, Bech-Hansen NT, Reese BE. Early afferent signaling in the outer plexiform layer regulates development of horizontal cell morphology. J Comp Neurol. 2008;506:745–58. doi: 10.1002/cne.21526. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Kerov V, Haeseleer F, Majumder A, Artemyev N, Baker SA, Lee A. Dysregulation of Ca(v)1.4 channels disrupts the maturation of photoreceptor synaptic ribbons in congenital stationary night blindness type 2. Channels (Austin) 2013;7:514–23. doi: 10.4161/chan.26376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regus-Leidig H, Atorf J, Feigenspan A, Kremers J, Maw MA, Brandstatter JH. Photoreceptor degeneration in two mouse models for congenital stationary night blindness type 2. PLoS One. 2014;9:e86769. doi: 10.1371/journal.pone.0086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauke J, Schild A, Neugebauer A, Lappa A, Fricke J, Fauser S, Rösler S, Pannes A, Zarrinnam D, Altmüller J, Motameny S, Nürnberg G, Nürnberg P, Hahnen E, Beck BB. A novel large in-frame deletion within the CACNA1F gene associates with a cone-rod dystrophy 3-like phenotype. PLoS One. 2013;8:e76414. doi: 10.1371/journal.pone.0076414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weleber RG, Pillers DA, Powell BR, Hanna CE, Magenis RE, Buist NR. Aland Island eye disease (Forsius-Eriksson syndrome) associated with contiguous deletion syndrome at Xp21. Similarity to incomplete congenital stationary night blindness. Arch Ophthalmol. 1989;107:1170–9. doi: 10.1001/archopht.1989.01070020236032. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Q, Cheng J, Yang W, Tania M, Wang H, Khan MA, Duan C, Zhu L, Chen R, Lv H, Fu J. Identification of a novel heterozygous missense mutation in the CACNA1F gene in a chinese family with retinitis pigmentosa by next generation sequencing. BioMed Res Int. 2015;2015:907827. doi: 10.1155/2015/907827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raghuram A, Hansen RM, Moskowitz A, Fulton AB. Photoreceptor and postreceptor responses in congenital stationary night blindness. Invest Ophthalmol Vis Sci. 2013;54:4648–58. doi: 10.1167/iovs.13-12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reichel E, Bruce AM, Sandberg MA, Berson EL. An electroretinographic and molecular genetic study of X-linked cone degeneration. Am J Ophthalmol. 1989;108:540–7. doi: 10.1016/0002-9394(89)90431-5. [DOI] [PubMed] [Google Scholar]
- 23.Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- 24.Falk MJ, Zhang Q, Nakamaru-Ogiso E, Kannabiran C, Fonseca-Kelly Z, Chakarova C, Audo I, Mackay DS, Zeitz C, Borman AD, Staniszewska M, Shukla R, Palavalli L, Mohand-Said S, Waseem NH, Jalali S, Perin JC, Place E, Ostrovsky J, Xiao R, Bhattacharya SS, Consugar M, Webster AR, Sahel JA, Moore AT, Berson EL, Liu Q, Gai X, Pierce EA. NMNAT1 mutations cause Leber congenital amaurosis. Nat Genet. 2012;44:1040–5. doi: 10.1038/ng.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Consugar M, Navarro-Gomez D, Place EM, Bujakowska KM, Sousa ME, Fonseca-Kelly ZD, Taub DG, Janessian M, Wang DY, Au ED, Sims KB, Sweetser DA, Fulton AB, Liu Q, Wiggs JL, Gai X, Pierce EA. Panel-based genetic diagnostic testing for inherited eye diseases is highly accurate and reproducible, and more sensitive for variant detection, than exome sequencing. Genet Med. 2015;17:253–61. doi: 10.1038/gim.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 28.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–4. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desmet FO, Hamroun D, Lalande M, Collod-Beroud G, Claustres M, Beroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bujakowska KM, Consugar M, Place E, Harper S, Lena J, Taub DG, White J, Navarro-Gomez D, Weigel DiFranco C, Farkas MH, Gai X, Berson EL, Pierce EA. Targeted exon sequencing in Usher syndrome type I. Invest Ophthalmol Vis Sci. 2014;55:8488–96. doi: 10.1167/iovs.14-15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi Y, Sims GE, Murphy S, Miller JR, Chan AP. Predicting the functional effect of amino acid substitutions and indels. PLoS One. 2012;7:e46688. doi: 10.1371/journal.pone.0046688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575–6. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 35.Shanks ME, Downes SM, Copley RR, Lise S, Broxholme J, Hudspith KA, Kwasniewska A, Davies WI, Hankins MW, Packham ER, Clouston P, Seller A, Wilkie AO, Taylor JC, Ragoussis J, Németh AH. Next-generation sequencing (NGS) as a diagnostic tool for retinal degeneration reveals a much higher detection rate in early-onset disease. Eur J Hum Genet. 2013;21:274–80. doi: 10.1038/ejhg.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glöckle N, Kohl S, Mohr J, Scheurenbrand T, Sprecher A, Weisschuh N, Bernd A, Rudolph G, Schubach M, Poloschek C, Zrenner E, Biskup S, Berger W, Wissinger B, Neidhardt J. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies. Eur J Hum Genet. 2014;22:99–104. doi: 10.1038/ejhg.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge Z, Bowles K, Goetz K, Scholl HP, Wang F, Wang X, Xu S, Wang K, Wang H, Chen R. NGS-based Molecular diagnosis of 105 eyeGENE((R)) probands with Retinitis Pigmentosa. Sci Rep. 2015;5:18287. doi: 10.1038/srep18287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coppieters F, Casteels I, Meire F, De Jaegere S, Hooghe S, van Regemorter N, Van Esch H, Matuleviciene A, Nunes L, Meersschaut V, Walraedt S, Standaert L, Coucke P, Hoeben H, Kroes HY, Vande Walle J, de Ravel T, Leroy BP, De Baere E. Genetic screening of LCA in Belgium: predominance of CEP290 and identification of potential modifier alleles in AHI1 of CEP290-related phenotypes. Hum Mutat. 2010;31:E1709–66. doi: 10.1002/humu.21336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, Zonneveld MN, Othman MI, Waseem N, Chakarova CF, Maubaret C, Diaz-Font A, MacDonald I, Muzny DM, Wheeler DA, Morgan M, Lewis LR, Logan CV, Tan PL, Beer MA, Inglehearn CF, Lewis RA, Jacobson SG, Bergmann C, Beales PL, Attié-Bitach T, Johnson CA, Otto EA, Bhattacharya SS, Hildebrandt F, Gibbs RA, Koenekoop RK, Swaroop A, Katsanis N. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet. 2009;41:739–45. doi: 10.1038/ng.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louie CM, Caridi G, Lopes VS, Brancati F, Kispert A, Lancaster MA, Schlossman AM, Otto EA, Leitges M, Gröne HJ, Lopez I, Gudiseva HV, O’Toole JF, Vallespin E, Ayyagari R, Ayuso C, Cremers FP, den Hollander AI, Koenekoop RK, Dallapiccola B, Ghiggeri GM, Hildebrandt F, Valente EM, Williams DS, Gleeson JG. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat Genet. 2010;42:175–80. doi: 10.1038/ng.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ebermann I, Phillips JB, Liebau MC, Koenekoop RK, Schermer B, Lopez I, Schäfer E, Roux AF, Dafinger C, Bernd A, Zrenner E, Claustres M, Blanco B, Nürnberg G, Nürnberg P, Ruland R, Westerfield M, Benzing T, Bolz HJ. PDZD7 is a modifier of retinal disease and a contributor to digenic Usher syndrome. J Clin Invest. 2010;120:1812–23. doi: 10.1172/JCI39715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fahim AT, Bowne SJ, Sullivan LS, Webb KD, Williams JT, Wheaton DK, Birch DG, Daiger SP. Allelic heterogeneity and genetic modifier loci contribute to clinical variation in males with X-linked retinitis pigmentosa due to RPGR mutations. PLoS One. 2011;6:e23021. doi: 10.1371/journal.pone.0023021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao KN, Zhang W, Li L, Ronquillo C, Baehr W, Khanna H. Ciliopathy-associated protein CEP290 modifies the severity of retinal degeneration due to loss of RPGR. Hum Mol Genet. 2016;25:2005–12. doi: 10.1093/hmg/ddw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pieh C, Simonsz-Toth B, Gottlob I. Nystagmus characteristics in congenital stationary night blindness (CSNB). Br J Ophthalmol. 2008;92:236–40. doi: 10.1136/bjo.2007.126342. [DOI] [PubMed] [Google Scholar]
- 45.Kurent A, Stirn-Kranjc B, Brecelj J. Electroretinographic characteristics in children with infantile nystagmus syndrome and early-onset retinal dystrophies. Eur J Ophthalmol. 2015;25:33–42. doi: 10.5301/ejo.5000493. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura M, Ito S, Terasaki H, Miyake Y. Incomplete congenital stationary night blindness associated with symmetrical retinal atrophy. Am J Ophthalmol. 2002;134:463–5. doi: 10.1016/s0002-9394(02)01541-6. [DOI] [PubMed] [Google Scholar]
- 47.Perlman I, Meyer E, Haim T, Zonis S. Retinal function in high refractive error assessed electroretinographically. Br J Ophthalmol. 1984;68:79–84. doi: 10.1136/bjo.68.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weleber RG, Tongue AC. Congenital stationary night blindness presenting as Leber’s congenital amaurosis. Arch Ophthalmol. 1987;105:360–5. doi: 10.1001/archopht.1987.01060030080031. [DOI] [PubMed] [Google Scholar]
- 49.Traboulsi EI. The Marshall M. Parks memorial lecture: making sense of early-onset childhood retinal dystrophies–the clinical phenotype of Leber congenital amaurosis. Br J Ophthalmol. 2010;94:1281–7. doi: 10.1136/bjo.2009.165654. [DOI] [PubMed] [Google Scholar]
- 50.Fulton AB, Hansen RM, Mayer DL. Vision in Leber congenital amaurosis. Arch Ophthalmol. 1996;114:698–703. doi: 10.1001/archopht.1996.01100130690009. [DOI] [PubMed] [Google Scholar]
- 51.Al-Khayer K, Hagstrom S, Pauer G, Zegarra H, Sears J, Traboulsi EI. Thirty-year follow-up of a patient with leber congenital amaurosis and novel RPE65 mutations. Am J Ophthalmol. 2004;137:375–7. doi: 10.1016/S0002-9394(03)00913-9. [DOI] [PubMed] [Google Scholar]
- 52.Paunescu K, Wabbels B, Preising MN, Lorenz B. Longitudinal and cross-sectional study of patients with early-onset severe retinal dystrophy associated with RPE65 mutations. Graefes Arch Clin Exp Ophthalmol. 2005;243:417–26. doi: 10.1007/s00417-004-1020-x. [DOI] [PubMed] [Google Scholar]
- 53.Bujakowska KM, Zhang Q, Siemiatkowska AM, Liu Q, Place E, Falk MJ, Consugar M, Lancelot ME, Antonio A, Lonjou C, Carpentier W, Mohand-Saïd S, den Hollander AI, Cremers FP, Leroy BP, Gai X, Sahel JA, van den Born LI, Collin RW, Zeitz C, Audo I, Pierce EA. Mutations in IFT172 cause isolated retinal degeneration and Bardet-Biedl syndrome. Hum Mol Genet. 2015;24:230–42. doi: 10.1093/hmg/ddu441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Badano JL, Leitch CC, Ansley SJ, May-Simera H, Lawson S, Lewis RA, Beales PL, Dietz HC, Fisher S, Katsanis N. Dissection of epistasis in oligogenic Bardet-Biedl syndrome. Nature. 2006;439:326–30. doi: 10.1038/nature04370. [DOI] [PubMed] [Google Scholar]
- 55.Badano JL, Kim JC, Hoskins BE, Lewis RA, Ansley SJ, Cutler DJ, Castellan C, Beales PL, Leroux MR, Katsanis N. Heterozygous mutations in BBS1, BBS2 and BBS6 have a potential epistatic effect on Bardet-Biedl patients with two mutations at a second BBS locus. Hum Mol Genet. 2003;12:1651–9. doi: 10.1093/hmg/ddg188. [DOI] [PubMed] [Google Scholar]
- 56.Baala L, Audollent S, Martinovic J, Ozilou C, Babron MC, Sivanandamoorthy S, Saunier S, Salomon R, Gonzales M, Rattenberry E, Esculpavit C, Toutain A, Moraine C, Parent P, Marcorelles P, Dauge MC, Roume J, Le Merrer M, Meiner V, Meir K, Menez F, Beaufrère AM, Francannet C, Tantau J, Sinico M, Dumez Y, MacDonald F, Munnich A, Lyonnet S, Gubler MC, Génin E, Johnson CA, Vekemans M, Encha-Razavi F, Attié-Bitach T. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet. 2007;81:170–9. doi: 10.1086/519494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tory K, Lacoste T, Burglen L, Morinière V, Boddaert N, Macher MA, Llanas B, Nivet H, Bensman A, Niaudet P, Antignac C, Salomon R, Saunier S. High NPHP1 and NPHP6 mutation rate in patients with Joubert syndrome and nephronophthisis: potential epistatic effect of NPHP6 and AHI1 mutations in patients with NPHP1 mutations. J Am Soc Nephrol. 2007;18:1566–75. doi: 10.1681/ASN.2006101164. [DOI] [PubMed] [Google Scholar]
- 58.Song J, Smaoui N, Ayyagari R, Stiles D, Benhamed S, MacDonald IM, Daiger SP, Tumminia SJ, Hejtmancik F, Wang X. High-throughput retina-array for screening 93 genes involved in inherited retinal dystrophy. Invest Ophthalmol Vis Sci. 2011;52:9053–60. doi: 10.1167/iovs.11-7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Audo I, Bujakowska KM, Léveillard T, Mohand-Saïd S, Lancelot ME, Germain A, Antonio A, Michiels C, Saraiva JP, Letexier M, Sahel JA, Bhattacharya SS, Zeitz C. Development and application of a next-generation-sequencing (NGS) approach to detect known and novel gene defects underlying retinal diseases. Orphanet J Rare Dis. 2012;7:8. doi: 10.1186/1750-1172-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neveling K, Collin RW, Gilissen C, van Huet RA, Visser L, Kwint MP, Gijsen SJ, Zonneveld MN, Wieskamp N, de Ligt J, Siemiatkowska AM, Hoefsloot LH, Buckley MF, Kellner U, Branham KE, den Hollander AI, Hoischen A, Hoyng C, Klevering BJ, van den Born LI, Veltman JA, Cremers FP, Scheffer H. Next-generation genetic testing for retinitis pigmentosa. Hum Mutat. 2012;33:963–72. doi: 10.1002/humu.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eisenberger T, Neuhaus C, Khan AO, Decker C, Preising MN, Friedburg C, Bieg A, Gliem M, Charbel Issa P, Holz FG, Baig SM, Hellenbroich Y, Galvez A, Platzer K, Wollnik B, Laddach N, Ghaffari SR, Rafati M, Botzenhart E, Tinschert S, Börger D, Bohring A, Schreml J, Körtge-Jung S, Schell-Apacik C, Bakur K, Al-Aama JY, Neuhann T, Herkenrath P, Nürnberg G, Nürnberg P, Davis JS, Gal A, Bergmann C, Lorenz B, Bolz HJ. Increasing the yield in targeted next-generation sequencing by implicating CNV analysis, non-coding exons and the overall variant load: the example of retinal dystrophies. PLoS One. 2013;8:e78496. doi: 10.1371/journal.pone.0078496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang F, Wang H, Tuan HF, Nguyen DH, Sun V, Keser V, Bowne SJ, Sullivan LS, Luo H, Zhao L, Wang X, Zaneveld JE, Salvo JS, Siddiqui S, Mao L, Wheaton DK, Birch DG, Branham KE, Heckenlively JR, Wen C, Flagg K, Ferreyra H, Pei J, Khan A, Ren H, Wang K, Lopez I, Qamar R, Zenteno JC, Ayala-Ramirez R, Buentello-Volante B, Fu Q, Simpson DA, Li Y, Sui R, Silvestri G, Daiger SP, Koenekoop RK, Zhang K, Chen R. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Hum Genet. 2014;133:331–45. doi: 10.1007/s00439-013-1381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christensen KD, Dukhovny D, Siebert U, Green RC. Assessing the costs and cost-effectiveness of genomic sequencing. J Pers Med. 2015;5:470–86. doi: 10.3390/jpm5040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell’Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 66.Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, Flotte TR, Fishman GA, Heon E, Stone EM, Byrne BJ, Jacobson SG, Hauswirth WW. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA. 2008;105:15112–7. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, Lotery AJ, Downes SM, Webster AR, Seabra MC. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–37. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiggs JL, Pierce EA. Genetic testing for inherited eye disease: who benefits? JAMA Ophthalmol. 2013;131:1265–6. doi: 10.1001/jamaophthalmol.2013.4509. [DOI] [PubMed] [Google Scholar]
- 69.Fulton AB, Hansen RM. The development of scotopic sensitivity. Invest Ophthalmol Vis Sci. 2000;41:1588–96. [PubMed] [Google Scholar]
- 70.Hansen RM, Fulton AB. Development of the cone ERG in infants. Invest Ophthalmol Vis Sci. 2005;46:3458–62. doi: 10.1167/iovs.05-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharon D, Bruns GA, McGee TL, Sandberg MA, Berson EL, Dryja TP. X-linked retinitis pigmentosa: mutation spectrum of the RPGR and RP2 genes and correlation with visual function. Invest Ophthalmol Vis Sci. 2000;41:2712–21. [PubMed] [Google Scholar]