Abstract
Post-transplant lymphoproliferative disorders (PTLDs) are a heterogeneous group of lymphoid neoplasms associated with immunosuppression following transplantation. Among PTLDs, monomorphic PTLD (m-PTLD) is the largest category; however, its characteristics and survival outcome are not fully understood because of low incidence. This study enrolled 30 adult patients with m-PTLD after kidney-transplantation (KT, n = 17) and hematopoietic stem cell transplantation (HSCT, n = 13) from January 1998 to December 2014. The incidence rates of m-PTLD were 0.74 and 3.63% in the KT and HSCT groups, respectively. M-PTLD patients in the HSCT group were younger and showed earlier onset, with EBV-encoded small RNAs (EBER) more frequently identified. Diffuse large B cell lymphoma (DLBCL) was the main pathological type, and the digestive system was the most extranodal involvement site in m-PTLD after KT and HSCT. Among the 28 patients with DLBCL m-PTLD,the complete remission rate after rituximab treatment was higher than in patients not administered rituximab treatment (P = 0.038). With a median follow-up of 46 months after m-PTLD diagnosis, the estimated 5-year overall survival (OS) was 59.2 ± 9.1% in all patients, and 64.2 ± 11.8 and 52.7 ± 14.1% in the KT and HSCT groups, respectively (P = 0.741). ECOG PS, Ann Arbor stage, and CD68 IHC expression were independent prognostic factors for OS. M-PTLD is a rare but serious complication after transplantation. Ongoing efforts to standardize safe and effective treatment protocols would improve the poor overall survival. The independent prognostic factors contributed to risk-stratified treatment, and might be validated by larger studies.
Keywords: Post-transplant lymphoproliferative disorder, Monomorphic, Hematopoietic stem cell transplantation, Kidney transplantation, Tumor-associated macrophages, Rituximab
Introduction
Post-transplant lymphoproliferative disorders (PTLDs) are a heterogeneous group of lymphoid neoplasms associated with immunosuppression following solid-organ transplantation (SOT) and hematopoietic stem cell transplantation (HSCT). The World Health Organization classification introduced in 2008 distinguishes early lesions, polymorphic, monomorphic and classical Hodgkin lymphoma-type PTLD [1]. Among the PTLDs, monomorphic PTLD (m-PTLD) forms the largest category, and is indistinguishable from a subset of aggressive B-cell- and much less frequently T-cell/NK cell lymphomas observed in immunocompetent individuals [2]. The incidence of PTLD varies with the transplanted organ—from 1–2% following HSCT to as high as 10% after thoracic organ transplantation [3]. Meanwhile, the very low incidence of PTLD prevents well-validated prospective studies. Although several studies have attempted to retrospectively determine the clinical characteristics and long-term survival outcomes, the heterogeneity of PTLD and variable organ transplantation might disturb accurate analysis and validation of prognostic factors. This study analyzed m-PTLD after kidney transplantation (KT) and HSCT for baseline characteristics and survival outcomes, and identified m-PTLD specific parameters for more definite prognostic prediction.
Materials and Methods
Study Subjects
Between January 1998 and December 2014, a total of 2295 KT and 358 HSCT were performed in Beijing Friendship Hospital, Capital Medical University, Beijing, China. Of these, 30 adult patients were diagnosed with m-PTLD and clinically categorized into 2 groups according to graft types: (1) KT (n = 17) and (2) HSCT (n = 13) groups.
The two groups were respectively assessed for patient- and PTLD-related characteristics. Patient parameters included age, gender, graft type, time from transplantation to m-PTLD diagnosis, and age at m-PTLD diagnosis of; PTLD-related indexes were B symptoms, biochemical data (lactate dehydrogenase [LDH] and albumin), Eastern Cooperative Oncology Group performance state (ECOG PS) [4], Ann Arbor stage at diagnosis [5], and extranodal involvement.
Telephone survey and outpatient examination were carried out for follow-up every 3–6 months, recording medical history and laboratory data. Follow-up was completed in June 2016.
Diagnosis and Criteria
Diagnostic tissue samples were confirmed by pathologists and classified according to the World Health Organization (WHO) 2008 classification [1]. Immunohistochemical (IHC) staining was performed for CD68 (Dako Cytomation, Carpinteria, CA) to identify tumor-associated macrophages (TAMs), which were counted as proposed previously [6]. Briefly, macrophage counts were estimated in 5 high-power fields (HPFs) (40 × 10 magnification), and averaged. Only tumor-containing areas were analyzed by IHC, and areas containing only necrosis and fibrosis were excluded. Neutrophils were also excluded. The number of CD68-positive macrophages ranged from 5 to 200 cells per HPF. A cut-off of 100 cells per HPF was established to divide the patients into high and low CD68 expression groups, respectively. In situ hybridization for EBV-encoded small RNAs (EBER) was performed using a 30-mer digoxigenin-labeled oligonucleotide probe (Research Genetics, Huntsville, AL) as previously described [7]. Staining was semi-quantitatively evaluated, with 20% used as a cutoff for positivity.
Immunosuppressive Regimens
In the KT group, a calcineurin inhibitor and corticosteroids were provided after transplantation. Both cyclosporine A (CsA) and tacrolimus (FK506) were used, and mycophenolate mofetil (MMF) was added as a primary immunosuppressant from 2001. Use of FK506 was restricted to patients with more than three human leukocyte antigen (HLA) mismatches.
In the HSCT group, short-term methotrexate (15 mg/m2 at day +1 and 10 mg/m2 at days +3, +6, and +11) and CsA were provided to prevent acute graft-versus-host disease (aGVHD) in patients with HLA-matched related donors; intravenous anti-thymocyte globulin (ATG) (total dose 10 mg/kg, rabbit, Sang Stat, Lyon, France, distributed to administered at days −3 to −1), short-term methotrexate (15 mg/m2 at day +1 and 10 mg/m2 at day +3, +6, and +11), cyclosporine A, and mycophenolate mofetil were provided to prevent aGVHD in patients with HLA-haploidentical family donors and matched unrelated donors.
Treatment of m-PTLD
After m-PTLD diagnosis, active treatment was applied to KT group patients, except 1 that was managed with reduction in immunosuppressant (RIS) alone. A total of 12 patients were treated with rituximab (375 mg/m2) combined with cyclophosphamide + doxorubicin + vincristine + prednisone (R-CHOP) chemotherapy; 3 patients were treated by surgery alone and 1 was administered CHOP chemotherapy and surgery. During chemotherapy, immunosuppressant doses were reduced.
For the HSCT group, immunosuppressant doses were first reduced, unless the patients presented active signs or symptoms of GVHD. One patient was treated solely with RIS, and 3 additionally received rituximab (375 mg/m2); one patient was treated with rituximab (375 mg/m2) followed by donor lymphocyte infusion (DLI), while six patients were administered R-CHOP chemotherapy. The remaining two patients were treated, respectively, with CHOP and CHOP like regimen.
Standardize response criteria for m-PTLD patients receiving treatment were proposed previously [8].
Statistical Analysis
Statistical analysis was performed with SPSS for Windows Version 19.0. The χ2 test or Fisher exact test was used to compare categorical variables. The Kaplan–Meier method was used to assess overall survival (OS), with the Log-rank test used to compare subgroups. The Cox’s proportional hazard model was used to assess potential predictive factors. P < 0.05 was considered statistically significant for all analyses.
This study was approved by the ethics committee of Beijing Friendship Hospital, Capital Medical University, Beijing, China. Informed consent was obtained from all study participants included.
Results
Patient Characteristics
The incidence rates of m-PTLD were 0.74 and 3.63% in the KT and HSCT groups, respectively. Of the 17 patients in the KT group, the organs were received from 1 living relative and 16 deceased individuals. Of the 13 patients in the HSCT groups, donors were related haploidentical (n = 11), matched-unrelated (n = 1) and matched related (n = 1) individuals. After HSCT, the incidence of m-PTLD was 6.4% (11/172) in haploidentical, 1.1% (1/89) in matched-unrelated, and 1.0% (1/97) in matched related HSCT (P = 0.027). Overall, median age at transplantation, gender, time from transplantation to m-PTLD diagnosis, and age at m-PTLD diagnosis in the KT and HSCT groups are summarized in Table 1. Median age at transplantation was reduced in the HSCT group than the KT counterpart (29.2 vs. 52.8 years old, P = 0.014). Median times from transplantation to m-PTLD were 84.3 and 2.5 months in the KT in HSCT groups, indicating a significant difference between the two groups (P = 0.001). Median age at m-PTLD diagnosis was reduced in HSCT than in KT patients (29.4 vs. 56.5 years old, P = 0.010).
Table 1.
Compares of the characteristics of m-PTLD between KT and HSCT
| Characteristic | KT (n = 17) | HSCT (n = 13) | P value |
|---|---|---|---|
| Male/female | 8/9 | 9/4 | 0.391 |
| Age at Tx (year) | 52.8 (19–68) | 29.2 (18–45) | 0.014* |
| Time from Tx to m-PTLD (month) | 84.3 (8–253) | 2.5 (1–20) | 0.001* |
| Age at m-PTLD (year) | 56.5 (22–71) | 29.4 (18–46) | 0.010* |
| B symptoms | 0.011* | ||
| Yes | 4 | 12 | |
| No | 13 | 1 | |
| ECOG PS | 0.264 | ||
| <2 | 10 | 5 | |
| ≥2 | 7 | 8 | |
| Serum LDH | 0.205 | ||
| Normal | 11 | 4 | |
| Elevated | 6 | 9 | |
| Serum albumin | 0.861 | ||
| Normal | 9 | 7 | |
| Hypoalbuminemia | 8 | 6 | |
| Ann Arbor stage | 0.279 | ||
| I/II | 6 | 7 | |
| III/IV | 11 | 6 | |
| Extranodal involvement | 0.194 | ||
| ≤1 | 7 | 9 | |
| >1 | 10 | 4 | |
| Cell types | 0.729 | ||
| B-cell | 17 | 11 | |
| T-cell | 0 | 2 | |
| Pathological types | |||
| DLBCL | 17 | 11 | |
| NK/T | 0 | 1 | |
| PTCL-NOS | 0 | 1 | |
| EBER | 0.005* | ||
| Negative | 11 | 0 | |
| Positive | 6 | 13 | |
| CD68 expression | 0.384 | ||
| High | 6 | 7 | |
| Low | 11 | 6 |
KT kidney-transplantation, HSCT hematopoietic stem cell transplantation, Tx transplantation, m-PTLD monomorphic post-transplant lymphoproliferative disorder, LDH lactate dehydrogenase, DLBCL diffuse large B-cell lymphoma, NK/T NK/T cell lymphoma, PTCL-NOS peripheral T-cell lymphoma not otherwise specified, EBER EBV-encoded small RNAs
* meant significant difference
Disease-Related Characteristics
B symptoms, biochemical data (lactate dehydrogenase [LDH], albumin), ECOG PS, Ann Arbor stage, and extranodal involvement in the KT and HSCT groups are summarized Table 1. B symptoms occurred in 4 (23.5%) and 12 (92.3%) patients of the KT and HSCT groups respectively (P = 0.001).
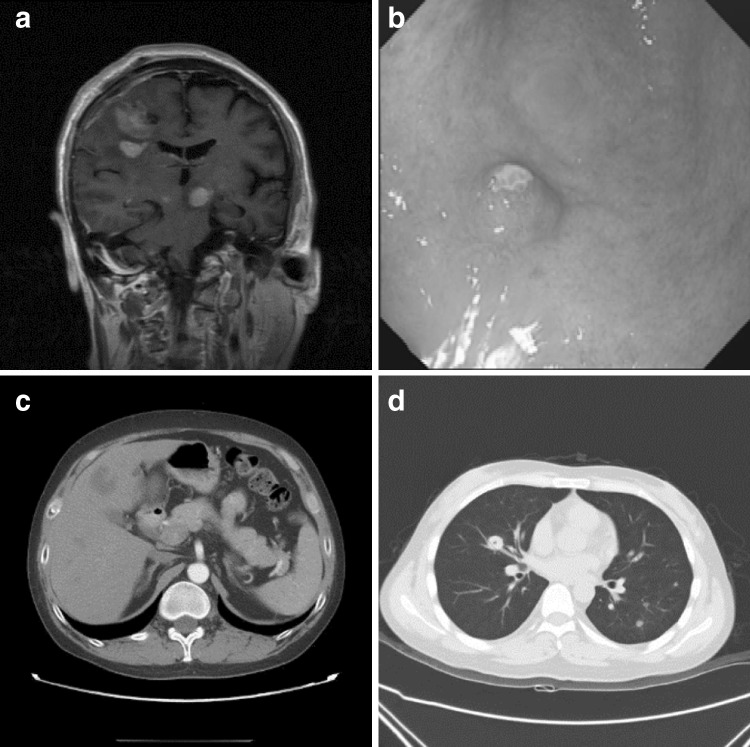
Lymph node involvement occurred in 4 (23.5%) and 10 (76.9%) patients in the KT and HSCT groups, respectively (P = 0.009); digestive system involvement was found in 7 (41.2%) and 6 (46.2%) in the two groups, respectively. Skin and soft tissue involvement occurred in 4 (23.5%) and 2 (15.4%) patients in the KT and HSCT groups, respectively; respiratory system involvement was found in 1 (5.9%) and 2 (15.4%), respectively. Urinary system involvement occurred in 1 (5.9%) and 1 (7.7%) patients in the KT and HSCT groups, respectively. Other systems involved were the central nervous (3 patients in the KT group) and reproductive (1 patient in the KT group) systems. Images of extranodal involvements are shown in Fig. 1.
Fig. 1.
Extranodal involvements. a Coronary scan in MRI showing extensive central nervous system disease in m-PTLD of DLBCL type after KT; b Gastroscopy showing solitary involvement of stomach in m-PTLD of DLBCL type after KT; c CT showing extensive liver involvement in m-PTLD of DLBCL type after KT; d CT showing multiple pulmonary parenchymal nodules in m-PTLD of DLBCL type after HSCT
Pathologic, Immunophenotypic, and in situ Hybridization Features
According to the 2008 World Health Organization (WHO) classification, all patients were diagnosed with diffuse large B-cell lymphoma (DLBCL) in the KT group; 11, 1, and 1 patients were diagnosed with DLBCL, NK/T cell lymphoma, and peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) in the HSCT group, respectively. EBER was positive in 6 patients (35.3%) and 13 patients (100%) of the KT and HSCT groups, respectively (P = 0.001). High CD68 expression in IHC was presented in five and eight patients of the KT and HSCT groups, respectively (P = 0.138). In situ hybridization data for EBER and CD68 IHC are shown in Fig. 2.
Fig. 2.
Immunohistochemistry (IHC) for CD68 detection and in situ hybridization for EBER. a Positive EBER in situ hybridization in m-PTLD of DLBCL type; b negative EBER in situ hybridization in m-PTLD of DLBCL type; c high CD68 expression by IHC in m-PTLD of PTCL-NOS type; d low CD68 expression by IHC in m-PTLD of DLBCL type
Therapeutic Outcome
After treatment, 12 patients had complete remission (CR), and 5 obtained progressive disease (PD) in the KT group. Among the 12 patients, 2 received second line chemotherapy and radiotherapy for recurrence after 10 and 15 months, respectively; 1 patient had stable disease and the other had complete remission again. Meanwhile, 8, 3 and 2 patients had CR, partial remission (PR), and PD in the HSCT group. Of the 28 patients with DLBCL m-PTLD,CR rates were 81.8% (18/22) and 33.3% (2/6), respectively, in patients administered rituximab and those without rituximab treatment, respectively, indicating a significant difference between the two groups (P = 0.038).
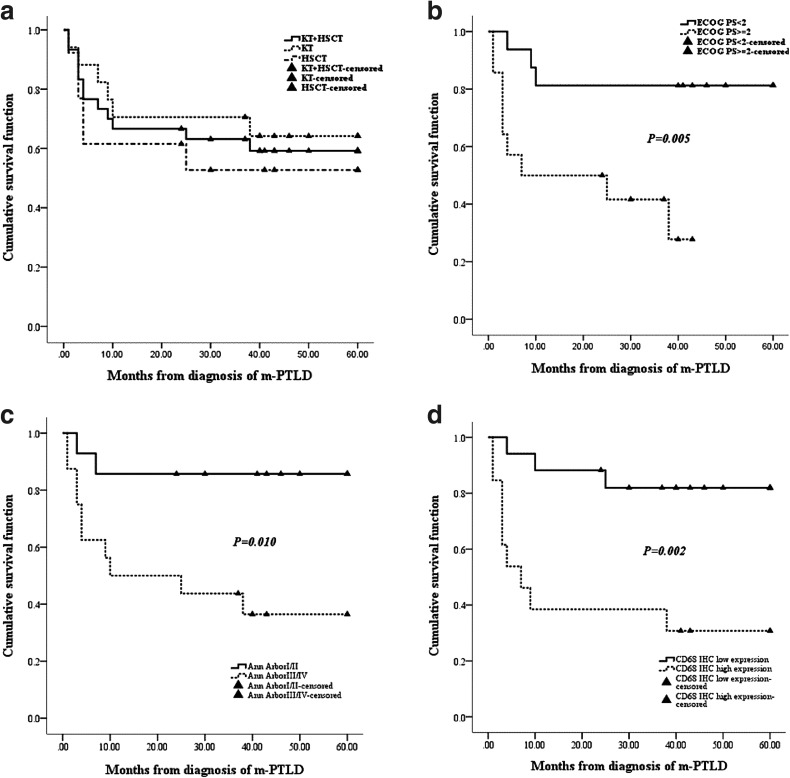
In the KT group, 17 patients were followed up for a median of 50 months (range: 1–189 months), with 11 survival and 6 survival and death cases, respectively, after m-PTLD diagnosis. The main causes of death were m-PTLD (n = 4) and infection (n = 2). In the HSCT group, 13 patients were followed up for a median of 39 months (range: 1–130 months), with 7 survival and 6 survival and death cases after m-PTLD diagnosis. The main causes of death were GVHD (n = 2), infection (n = 3), and leukemia recurrence (n = 1). With a median follow-up of 46 months (range: 1–189 months) after m-PTLD diagnosis, the estimated 5-year overall survival (OS) rate was 59.2 ± 9.1% in all patients, and values of 64.2 ± 11.8 and 52.7 ± 14.1% were obtained in the KT and HSCT groups, respectively, with no statistically significant difference between the two groups (P = 0.741) (Fig. 3).
Fig. 3.
Clinical outcomes of m-PTLD patients. a With a median follow-up of 46 months after m-PTLD diagnosis, the estimated 5-year overall survival (OS) rate was 59.2 ± 9.1% in all patients; values of 64.2 ± 11.8 and 52.7 ± 14.1% were obtained in the KT and HSCT groups (P = 0.741). Factors which showed significant values for prediction of worse survival outcomes: b ECOG PS ≥ 2 (P = 0.005), c Ann Arbor stage III/IV (P = 0.010), d CD68 IHC high expression (P = 0.002)
Determination of Risk Factors for OS
Among the 30 patients, the 5-year OS of patients with ECOG PS ≥ 2 at m-PTLD diagnosis was lower than that of individuals with ECOG PS < 2 (P = 0.005) by univariate analysis. Similar results were found for elevated LDH (P = 0.026), hypoalbuminemia (P = 0.037), Ann Arbor stage III/IV (P = 0.010), and high CD68 expression assessed by IHC (P = 0.002). Multivariate analysis showed that ECOG PS, Ann Arbor stage, and CD68 expression were associated with OS (P < 0.05). These findings suggested that ECOG PS, Ann Arbor stage, and CD68 expression were independent prognostic factors for OS in patients with m-PTLD (Fig. 3).
Discussion
In this study, the characteristics of m-PTLD after KT and HSCT, including clinical and histopathological features, therapeutic response, and disease prognosis, were analyzed. Although many PTLD types have been reported, only m-PTLD was selected in this study for two major reasons. First, the diagnosis of m-PTLD fulfills the histopathological criteria of lymphomas in immunocompetent hosts, so that m-PTLD characteristics after KT or HSCT could be compared with previously reported lymphoma states. Second, m-PTLD is analyzed with some variables used for lymphomas, which helped build a specific m-PTLD prognostic index. Despite the limitations of a retrospective study, with heterogeneous treatments and long-term follow-up period during which diagnostic techniques and treatment options may change, the clinicopathological characteristics and survival outcomes in m-PTLD after KT and HSCT were precisely described.
The current study aimed to comparatively analyze the characteristics of m-PTLD between KT and HSCT. The incidence rates of m-PTLD were 0.74 and 3.63% in the KT and HSCT groups, respectively. In HSCT patients, haploidentical HSCT had a higher incidence compared with matched related and unrelated HSCT (P = 0.027). Based on large transplant registries and small sample size single-center retrospective analyses, PTLD incidence seems to be highest in haploidentical HSCT (up to 20%), followed by kidney (1–1.5%), and matched related and unrelated HSCT (0.5–1%) [9, 10]. Median age of patients who underwent HSCT was reduced compared with the KT group (P = 0.014), as well as median time from HSCT to m-PTLD (P = 0.001). Therefore, median age at diagnosis of post-HSCT m-PTLD was significantly reduced compared with that of post-KT m-PTLD patients (P = 0.010). This might be caused by host immunity and the tendency of using immunosuppressive agents in all transplantation settings. In HSCT, the profound T cell-depleting conditioning regimen leads to lack of EBV-specific T cells, hence the often impressive and rapid growth of an EBV + clone, even within the first weeks [11]. Therefore, PTLD after HSCT is almost exclusively of donor origin and develops during the first 6 months after transplantation. Because immune reconstitution occurs in the first 6–12 months, and oral immune suppression can often be stopped, late PTLD is rare after HSCT. On the contrary, preserved early host immunity and inevitable long-term use of immunosuppressants with the aging process may cause late-onset PTLD in post-KT patients.
As the gold standard, EBER is recommended to determine EBV association in every biopsy sample [12]. EBER incidence in HSCT was higher than in KT (P = 0.001), and all patients diagnosed with m-PTLD in the HSCT group were EBER positive. In HSCT, conditioning regimens lead to compromised T cell function, and EBV + clones grow impressively and rapidly, which explains why PTLD after HSCT is always EBER positive [11]. In this study, survival rates showed no statistical significance between EBER positive and negative individuals. In a recently published analysis of 70 patients included in the international multicenter prospective phase II PTLD-1 trial, EBV association was also not shown to be a significant factor for overall survival or time to progression [13]. In classic Hodgkin lymphoma and follicular lymphoma, a high content of TAMs assessed by CD68 IHC was shown to correlate with inferior outcome [14, 15]. Meanwhile, their association with prognosis in diffuse large B-cell lymphoma (DLBCL) remains controversial [7, 16]. No report focused on the relationship between TAM levels and PTLD prognosis. Multivariate analysis in this study showed that CD68 IHC expression was a significant independent predictor of OS in m-PTLD. This finding firstly demonstrated the prognostic role of TAMs in m-PTLD.
This study indicated that the prognosis of transplant recipients with m-PTLD was still poor, with a 5-year survival rate of 59.2%; this was similar to those reported by Lawrence R et al. who found a 5-year survival rate in B-cell m-PTLD patients of approximately 53%, and Vahid Pourfarziani et al. who observed a 5-year survival rate of 59% in m-PTLD recipients after KT [16, 17]. Although most deaths were associated with progressive disease, up to 60% of patients would eventually die from unrelated cause (mainly infections), emphasizing the vulnerability of transplant patients, even in complete remission. In the last decade, several prognostic indices, including advanced age, advanced disease stage, poor performance status, elevated lactate dehydrogenase, CNS invasion, and hypoalbuminemia, were shown to negatively affect prognosis in PTLD by different trials [18–22]. In this study, advanced disease, poor performance status, and high CD68 by IHC were associated with poor prognosis in m-PTLD after KT and HSCT.
In summary, this study assessed clinical and histopathological features, as well as therapeutic response and prognosis of m-PTLD after KT and HSCT. Discrepant characteristics of m-PTLD according to the transplantation type were found, although OS was not significantly different. EBER was not a significant predictor of survival. Advanced disease, poor performance status, and high CD68 were associated with poor prognosis in m-PTLD after KT and HSCT.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no potential conflicts of interests.
References
- 1.Swerdlow SH, Campo E, Harris NL. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 2.Végso G, Hajdu M, Sebestyén A. Lymphoproliferative disorders after solid organ transplantation-classification, incidence, risk factors, early detection and treatment options. Pathol Oncol Res. 2011;17:443–454. doi: 10.1007/s12253-010-9329-8. [DOI] [PubMed] [Google Scholar]
- 3.Taylor AL, Marcus R, Bradley JA. Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Crit Rev Oncol Hematol. 2005;56:155–167. doi: 10.1016/j.critrevonc.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA, Tubiana M. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 6.Canioni D, Salles G, Mounier N, Brousse N, Keuppens M, Morchhauser F, Lamy T, Sonet A, Rousselet MC, Foussard C, Xerri L. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J Clin Oncol. 2008;26:440–446. doi: 10.1200/JCO.2007.12.8298. [DOI] [PubMed] [Google Scholar]
- 7.Chang KL, Chen YY, Shibata D, Weiss LM. Description of an in situ hybridization methodology for detection of Epstein-Barr virus RNA in paraffin-embedded tissues, with a survey of normal and neoplastic tissues. Diagn Mol Pathol. 1992;1:246–255. doi: 10.1097/00019606-199203000-00037. [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI sponsored international working group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 9.Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;42:222–230. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 10.Dierickx D, Tousseyn T, Sagaert X, Fieuws S, Wlodarska I, Morscio J, Brepoels L, Kuypers D, Vanhaecke J, Nevens F, Verleden G, Van Damme-Lombaerts R, Renard M, Pirenne J, De Wolf-Peeters C, Verhoef G. Single-center analysis of biopsy-confirmed posttransplant lymphoproliferative disorder: incidence, clinicopathological characteristics and prognostic factors. Leuk Lymphoma. 2013;54:2433–2440. doi: 10.3109/10428194.2013.780655. [DOI] [PubMed] [Google Scholar]
- 11.Heslop HE. How I treat EBV lymphoproliferation. Blood. 2009;114:4002–4008. doi: 10.1182/blood-2009-07-143545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuhlmann-Laeisz C, Oschlies I, Klapper W. Detection of EBV in reactive and neoplastic lymphoproliferations in adults-when and how? J Hematopathol. 2014;7:165–170. doi: 10.1007/s12308-014-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, Neuhaus R, Lehmkuhl H, Horst HA, Salles G, Morschhauser F, Jaccard A, Lamy T, Leithäuser M, Zimmermann H, Anagnostopoulos I, Raphael M, Riess H, Choquet S, PTLD German Study Group. European PTLD Network. German PTLD Study Group. European PTLD Network Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell posttransplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13:196–206. doi: 10.1016/S1470-2045(11)70300-X. [DOI] [PubMed] [Google Scholar]
- 14.Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, Klasa R, Voss N, Connors JM, Gascoyne RD. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL) Blood. 2005;106:2169–2174. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- 15.Tan KL, Scott DW, Hong F, Kahl BS, Fisher RI, Bartlett NL, Advani RH, Buckstein R, Rimsza LM, Connors JM, Steidl C, Gordon LI, Horning SJ, Gascoyne RD. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood. 2012;120:3280–3287. doi: 10.1182/blood-2012-04-421057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson LR, Nalesnik MA, Swerdlow SH. Impact of Epstein-Barr virus in monomorphic B-cell posttransplant lymphoproliferative disorders: a histogenetic study. Am J Surg Pathol. 2006;30:1604–1612. doi: 10.1097/01.pas.0000213317.59176.d2. [DOI] [PubMed] [Google Scholar]
- 17.Pourfarziani V, Taheri S, Lessan-Pezeshki M, Nourbala MH, Simforoosh N, Nemati E, Makhdoomi K, Ghafari A, Ahmadpour P, Nafar M, Einollahi B. Lymphoma after living donor kidney transplantation: an Iranian multicenter experience. Int Urol Nephrol. 2008;40:1089–1094. doi: 10.1007/s11255-008-9377-0. [DOI] [PubMed] [Google Scholar]
- 18.Evens AM, David KA, Helenowski I, Nelson B, Kaufman D, Kircher SM, Gimelfarb A, Hattersley E, Mauro LA, Jovanovic B, Chadburn A, Stiff P, Winter JN, Mehta J, Van Besien K, Gregory S, Gordon LI, Shammo JM, Smith SE, Smith SM. Multicenter analysis of 80 solid organ transplantation recipients with posttransplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol. 2010;28:1038–1046. doi: 10.1200/JCO.2009.25.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leblond V, Dhedin N, Mamzer Bruneel MF, Choquet S, Hermine O, Porcher R, Nguyen Quoc S, Davi F, Charlotte F, Dorent R, Barrou B, Vernant JP, Raphael M, Levy V. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2001;19:772–778. doi: 10.1200/JCO.2001.19.3.772. [DOI] [PubMed] [Google Scholar]
- 20.Ghobrial IM, Habermann TM, Maurer MJ, Geyer SM, Ristow KM, Larson TS, Walker RC, Ansell SM, Macon WR, Gores GG, Stegall MD, McGregor CG. Prognostic analysis for survival in adult solid organ transplant recipients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2005;23:7574–7582. doi: 10.1200/JCO.2005.01.0934. [DOI] [PubMed] [Google Scholar]
- 21.Hourigan MJ, Doecke J, Mollee PN, Gill DS, Norris D, Johnson DW, Gandhi MK. A new prognosticator for post-transplant lymphoproliferative disorders after renal transplantation. Br J Haematol. 2008;141:904–907. doi: 10.1111/j.1365-2141.2008.07149.x. [DOI] [PubMed] [Google Scholar]
- 22.Caillard S, Porcher R, Provot F, Dantal J, Choquet S, Durrbach A, Morelon E, Moal V, Janbon B, Alamartine E, Pouteil Noble C, Morel D, Kamar N, Buchler M, Mamzer MF, Peraldi MN, Hiesse C, Renoult E, Toupance O, Rerolle JP, Delmas S, Lang P, Lebranchu Y, Heng AE, Rebibou JM, Mousson C, Glotz D, Rivalan J, Thierry A, Etienne I, Moal MC, Albano L, Subra JF, Ouali N, Westeel PF, Delahousse M, Genin R, Hurault de Ligny B, Moulin B. Posttransplantation lymphoproliferative disorder after kidney transplantation: report of a nationwide French registry and the development of a new prognostic score. J Clin Oncol. 2013;31:1302–1309. doi: 10.1200/JCO.2012.43.2344. [DOI] [PubMed] [Google Scholar]