Abstract
The redox performance of CeO2 − x nanocrystals (nanoceria) is always accompanied by the switching of cerium oxidation state between Ce3+ and Ce4+. We monitored Ce3+ → Ce4+ oxidation of nanoceria stimulated by oxidant in aqueous colloidal solutions controlling the luminescence of Ce3+ ions located at different distances from nanoceria surface. The observed Ce3+ luminescence changes indicate that Ce3+ → Ce4+ reaction develops inside nanoceria being triggered by the diffusing oxygen originated from the water splitting on oxidized nanoceria surface. We present the first observation of the pronounced oscillations of Ce3+ luminescence intensity arising from Ce3+ ↔ Ce4+ reversible switching. This threshold effect is to be driven by uptaking and releasing oxygen by nanoceria, when the concentration of oxygen vacancies in nanoceria lattice, oxidant concentration in colloidal solution, and temperature reach certain critical values. So, the ability of nanoceria to uptake and release oxygen depending on the environmental redox conditions really makes it the self-sufficient eternal antioxidant.
Electronic supplementary material
The online version of this article (10.1186/s11671-017-2339-7) contains supplementary material, which is available to authorized users.
Keywords: Oxygen vacancies, Clusters, Luminescence, Antioxidants
Background
Today, nanocrystals with different structure and chemical composition are widely used in great diversity of modern applications [1–9]. Along with important engineering utilizations [3, 4], CeO2 nanocrystals (nanoceria) gave birth to promising biomedical developments [5–9] owing to its ability to work as a regenerative scavenger of reactive oxygen species (ROS). The main prerequisite that makes nanoceria so unique and useful is generally attributed to high content of oxygen vacancies (VO) and Ce3+ ions on its surface [10–14]. In nanoceria lattice, VO and Ce3+ ions are interrelated defects [10–14]; two Ce3+ ions are accounted for one VO [13]. The defect (Ce3+, VO) concentration in nanoceria can be controlled by particle size, special doping, and temperature treatment [11, 14, 15]. In general, the surface oxygen can assist the redox cycle through VO creation and healing or surface VO can act as binding sites for catalytically active species [3, 4, 14]. The surface Ce3+ ions of nanoceria are commonly supposed to provide ROS scavenging due to switching between 3+ and 4+ oxidation states [5–9]. ROS, namely superoxide ions , hydroxyl radicals OH˙, and hydrogen peroxide H2O2 at low concentrations, are critically important for the regulation of cell functions [5–9]. Unlike ordinary antioxidants, which disappear irretrievably after interaction with ROS [5–7], nanoceria, at particle sizes below 15 nm, can act as a self-regenerating antioxidant [5–9]. The critical dependence of nanoceria biological activity on its size, as well as the self-regeneration mechanism of nanoceria in biological environment, is still poorly understood [5–7], and discussions are continuing [8, 9]. It should be stressed that in in vitro and in vivo experiments [5–9], the nanoceria operate at a high defect concentration and water activity and its redox performance can be strongly masked by the cell antioxidant systems.
So, to understand the mechanism of nanoceria redox performance, we used more simple and controlled conditions: the nanoceria oxidation dynamics was studied in aqueous colloidal solutions for nanoceria specimens with variation of oxygen deficiency. As VO (Ce3+) concentration vary with the particle size [10–12], the nanoceria specimens of 3.0, 10.0, and 50.0 nm were used. According to the data [12], VO concentration in 3.0-nm nanoceria can reach up to ~ 20%. We determined that VO concentration in 10.0-nm nanoceria, as compared with 3.0-nm nanoceria, was twice less (see Additional file 1). In the case of 50.0-nm nanoceria, doping with Y3+ (or Eu3+) ions and vacuum annealing were used to generate VO and to create different conditions for oxygen diffusion in nanoceria lattice [14, 15]. In all 50.0 nm samples, VO concentration was made equivalent to 10.0-nm nanoceria. At Re3+ doping of 50.0-nm nanoceria, the concentrations of Y3+ and Eu3+ ions were at the level of 10 at.% (see Additional file 1). These concentrations were sufficiently lower than the values of corresponding solubility limits for these ions in ceria lattice, ~ 25 at.% [16] or even ~ 45 at.% [17] for Y3+ ions and ~ 30 at.% [18] for Eu3+ ions; so, formation of Y2O3 or Eu2O3 phases can be excluded. All colloidal solutions contained the same amount of the substance (1.0 g/l) and were characterized by initial pH ~ 7. For nanoceria oxidation, hydrogen peroxide (HP) and potassium periodate KIO4 (PP) were used. PP allowed us to exclude the chemical diversity of HP. The details of the nanoceria synthesis and characterization of the obtained specimens, as well as a description of the experiments, are presented in the Additional file 1.
Results and Discussion
Relying on our preliminary experiment [19], the Ce3+ luminescence (Fig. 1a) due to the dipole-allowed 5d → 4f optical transitions of Ce3+ ions [20] was used to monitor the oxidation dynamics of all tested nanoceria specimens. The increase of the Ce3+ band asymmetry with the decrease of particle size, that is, with the increase of the surface-to-bulk ratio (see insert in Fig. 1a) indicates clearly its inhomogeneity. The long-wave part of this band can be attributed to the Ce3+ luminescence from the nanoceria subsurface layer, and the remaining part of the Ce3+ band comes from deep-seated Ce3+ ions. The subsurface Ce3+ ions have the red-shifted luminescence spectra (see insert in Fig. 1a) because of the weakening of the crystal field acting on these ions as a result of the lattice parameter increase in the direction to the nanoceria surface [12]. Contrary to 3.0-nm nanoceria, for 50.0-nm nanoceria, the impact of these ions to the resulting luminescence spectrum is negligible (see insert in Fig. 1a). This attribution was confirmed by the stronger Forster quenching [21] of the long-wave part of the Ce3+ band (see Additional file 1 and Fig. 1b). The increase of the quencher concentration resulted in the luminescence quenching of deeper lying Ce3+ ions (Fig. 1b) because of the reduction of the donor-acceptor distance [21].
Fig. 1.
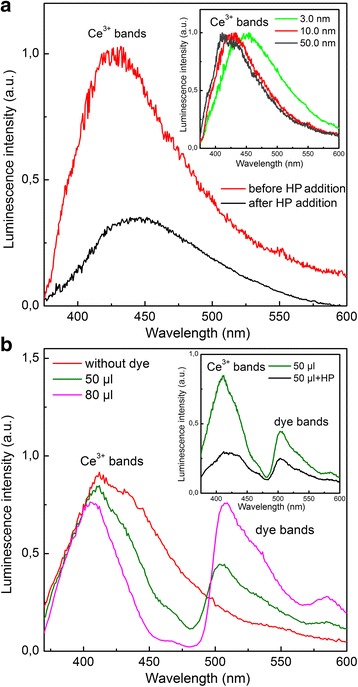
Luminescence spectra of nanoceria under different conditions. a 10.0-nm nanoceria before and 25 min after HP (C = 0.1 mM) addition. Insert: normalized spectra of different nanoceria samples. b Luminescence spectra of 10.0-nm nanoceria before and after addition of 50 and 80 μl of dye. Insert: spectra of 10.0-nm nanoceria after addition of 50 μl of dye and subsequent addition of HP (C = 0.1 mM)
Adding oxidant (HP or PP) to colloidal solutions resulted in a decrease of the Ce3+ band intensity for all tested nanoceria specimens (Fig. 1a). Moreover, one can see that under selective quenching of the Ce3+ band (Fig. 1b), the oxidant stimulated a drop in luminescence of the deep-seated Ce3+ ions (see insert in Fig. 1b). The Ce3+ → Ce4+ oxidation occurs for these ions as well in spite of the fact that the oxidant cannot penetrate into nanoceria. This effect is similar to annealing nanoceria in oxygen atmosphere (see Additional file 1). Hence, the oxidant stimulates the penetration of oxygen (its source will be determined below) inside nanoceria. It is really corroborated by the defect-controlled time evolution of Ce3+ luminescence under oxidant action (Fig. 2a). In this experiment, both HP and PP act in a similar way (see insert in Fig. 2a). As follows from Fig. 2a, the 3.0-nm nanoceria with the highest VO concentration demonstrated the fastest drop of the Ce3+ band intensity and the lowest residual Ce3+ luminescence. At the same VO concentration, the Y3+-doped nanoceria showed a stronger quenching rate of the Ce3+ band intensity and lower level of residual Ce3+ luminescence as compared to the Eu3+-doped nanoceria (Fig. 2a). It correlates with the fact that the activation energy of oxygen diffusion in cerium oxide increases in the presence of Eu3+ ions in a stronger way than of Y3+ ions [14, 15]. Hence, the Ce3+ band intensity decrease under oxidant action (Figs. 1 and 2a) is a result of Ce3+ → Ce4+ oxidation caused by oxygen penetration into nanoceria via the vacancy mechanism of diffusion [14, 15]. The addition of the reducing agent (for example, benzenetriol) to the colloidal solution upon nanoceria oxidation did not lead to the Ce3+ luminescence recovery, which is consistent with the proposed mechanism of Ce3+ → Ce4+ oxidation inside nanoceria.
Fig. 2.
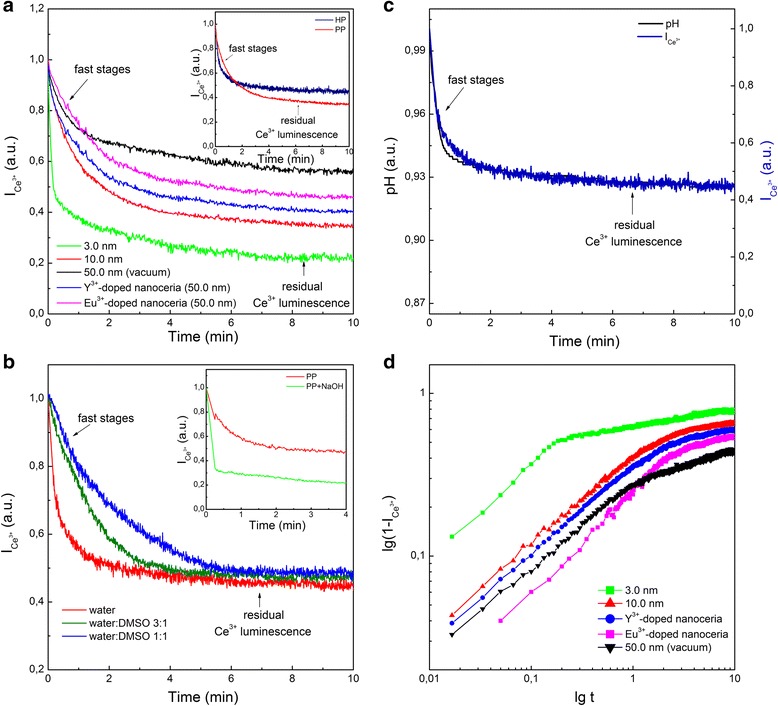
Time evolution of Ce3+ luminescence under the oxidant action (C = 0.1 mM) for different specimens of nanoceria. a After HP addition; insert—after HP and PP addition; b after PP addition to 10.0-nm nanoceria in water-DMSO solutions; insert—after PP addition and after NaOH and subsequent PP addition. c pH and Ce3+ band intensity () after PP addition for 10.0-nm nanoceria. d Curves of nanoceria filling by oxygen ρ(t)=1− shown on a log-log plot using data shown in a
The lower water concentration in the colloidal solution slowed down the dynamics of nanoceria oxidation (Fig. 2b), while the increase of initial pH accelerated this process (see insert in Fig. 2b). Excluding possible source of H+ in the case of HP application, we have also revealed the exact coincidence of the pH decrease with the Ce3+ band intensity drop under the nanoceria oxidation by PP (Fig. 2c). These facts indicate the water splitting, which can proceed with high efficiency with participation of the Ce4+ –Ce4+ (or Ce4+ –Ce3+) active sites on the nanoceria surface forming as a result of the oxidation of Ce3+-VO-Ce3+ sites (Fig. 3) [13]. There are two possible ways for that (Fig. 3): either the O2− ion occupies and two H+ ions are ejected to the solution or the O2− ion occupies resulting in the creation of hydroxyl, which makes one H+ ion to be ejected to solution (Fig. 3). The first jump of oxygen into nanoceria regenerates the Ce3+–VO–Ce3+ site for a new oxidation cycle (Fig. 3). This process can be repeated many times with different rates depending on oxidant concentration. The curve describes the filling of nanoceria with oxygen, and its initial stage fits well the ~t 1/2 function (see Fig. 2d). It means that oxygen penetrates into the nanoceria by the single-file diffusion through VO channels (Fig. 3), where oxygen atoms cannot bypass each other [22, 23]. The formation of large VO clusters opened onto the oxygen-terminated planes of nanoceria (Fig. 3) is unavoidable for two reasons: all tested nanoceria specimens contain high enough VO concentrations close to the percolation threshold [24] and VO concentration reaches its maximum value near the nanoceria surface [10–12]. The linear structures [25, 26] observed for the subsurface VO may be considered as VO channels or as components of large VO clusters.
Fig. 3.
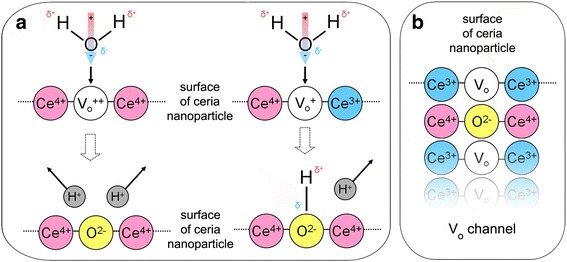
The stages of the nanoceria interaction with oxidant and water molecule. a Double-oxidized Ce4+––Ce4+ site and single-oxidized Ce4+––Ce3+ site on nanoceria surface and their interaction with H2O molecule. b Regeneration of Ce3+–VO–Ce3+ site for next oxidation cycle
The pronounced oscillations of the Ce3+ band intensity are observed when the oxidant (HP or PP) concentration in colloidal solutions exceeds ~ 0.5 mM, so that the dynamics of nanoceria Ce3+ → Ce4+ oxidation transform to Ce3+ ↔ Ce4+ redox scenario (Fig. 4). These oscillations did not appear immediately after the HP (C = 1.0 mM) or PP (C = 1.0 mM) addition but started to develop when the fast stage comes to completion (Fig. 4a, b). For all oxidants, after the oscillations disappeared, they could be repeated by adding new portions of the oxidant to the colloidal solutions (Fig. 4c). For comparison, the oscillations of the Ce3+ band intensity for all tested nanoceria specimens are shown in Fig. 4d. The base lines in Fig. 4d are actually the levels of the residual Ce3+ luminescence for each nanoceria specimen (Fig. 2a), and the Ce3+ band intensity oscillates above these levels. The variation of VO concentration in the Y3+ (or Eu3+)-doped 50.0-nm nanoceria showed clearly that the oscillations of the Ce3+ band intensity were observable only when the VO concentrations became equivalent to those in the 10.0-nm nanoceria (Fig. 4d). In the case of Eu3+-doped 50.0-nm nanoceria the oscillations were more irregular and less pronounced (Fig. 4d). As it was mentioned earlier, this fact is consistent with the suppression of oxygen diffusion in the presence of Eu3+ ions [14, 15]. In the annealed 50.0-nm nanoceria with thermodynamically nonequilibrium VO, the oscillations were not observed at all. The oscillations (see Fig. 4) were observable at the temperatures above 35 °C only.
Fig. 4.
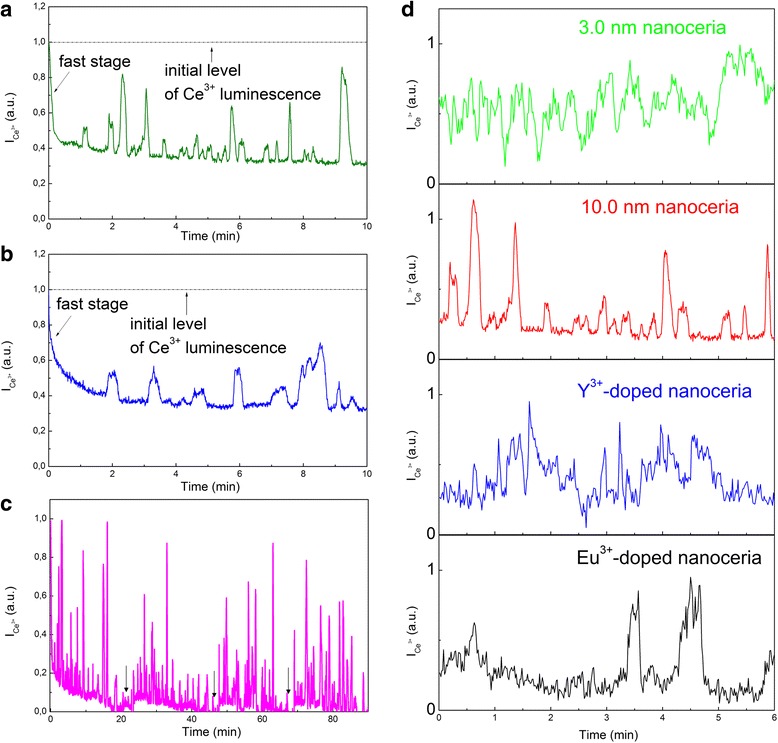
The oscillations of Ce3+ band intensity () stimulated by oxidant (C = 1.0 mM) in colloidal nanoceria: a after HP addition for 10.0-nm nanoceria; b after PP addition for 10.0-nm nanoceria; c after multiple HP addition for 10.0-nm nanoceria; d after HP addition for different nanoceria samples
The observed oscillations (Fig. 4) appeared when the population of VO by oxygen accumulated during the fast stage (Figs. 2a and 4a) became strongly nonequilibrium. The growth of the first peak of oscillations (Fig. 4a, b) is accompanied by the release of excess oxygen, and as long as the oscillations are observed, the nanoceria releases (Ce3+ luminescence increase) and uptakes (Ce3+ luminescence decrease) oxygen. For the annealed 50.0-nm nanoceria, the phase of oxygen release and, hence, the Ce4+ ↔ Ce3+ oscillations are impossible because the thermodynamically nonequilibrium VO are irreversibly healed by oxygen accumulated during oxidation (Figs. 2a and 4).
It should be noted that the time scale of the Ce3+ band intensity evolution (see Figs. 2 and 4) requires an anomalously high rate of oxygen diffusion in nanoceria at RT. Generally, in oxides, the ordinary oxygen diffusion is too slow owing to the large value of the activation energy [14, 15]. But in our case, the fast oxygen diffusion is provided by the loading-dependent reduction of the activation energy inherent for single-file diffusion [23]. The oxidant concentration in the colloidal solutions controls this effect via the rate and level of the filling of VO clusters with oxygen.
Conclusions
Our results suggest new vision of microscopic mechanisms behind the nanoceria redox performance. First of all, both the surface Ce3+ ions available for the oxidant and the deep-seated Ce3+ ions are involved in the oxidation dynamics of nanoceria due to oxygen diffusion supported by the open VO clusters. Such VO clusters are inevitably formed at a sufficiently small size (< 15 nm) of nanoceria that explains the strong size dependence of nanoceria antioxidant activity. The self-regeneration (reverse Ce4+ → Ce3+ reduction) of nanoceria in biological environment is a result of releasing the oxygen accumulated during its oxidation by ROS. Similar to the oscillations in heterogeneous catalysis [27], the oscillations of cerium oxidation state in nanoceria can be exploited for the development of high-performance antioxidants, which are extremely important for cell protection under high-intensity radiation (cancer radio-treatment, nuclear catastrophes, etc.). Overall, the ideas suggested in the paper allow to initiate a rational search for new nanomaterials that can be utilized not only as effective antioxidants, but also as unique catalytic materials in various technological areas.
Methods
Methods of Nanoceria Synthesis
Colloidal Synthesis of 3.0- and 10.0-nm Nanoceria
Aqueous solutions of ceria nanoparticles were obtained by the following method: CeCl3 solution (100 ml, 2 mM) was mixed with 100 ml of hexamethylenetetramine solution (4 mM) and stirred by means of magnetic stirrer for 3 h at room temperature. After that, 1.8 ml NH4OH and 0.6 ml of H2O2 were added into the solution. Then, the solution was put in round-bottom flask and refluxed for 5 h. As a result, transparent colorless solutions were obtained. The solution was evaporated in a rotary evaporator flask under vacuum at the bath temperature of 70 °C to 30 ml. A solution of 2 M NaCl was added to the obtained solution until the resulting solution became turbid. Then, the solid phase was precipitated by centrifugation. The precipitate was separated, and solution of sodium chloride was added again. The procedure of precipitate cleaning was repeated three times. After the last stage of centrifugation, solution of sodium citrate with molar ratio CeO2/NaCt of 1:1 was added to the precipitate. Size of nanoceria obtained from the mixture of cerium (III) chloride and hexamethylenetetramine (HMTA) taken in mole ratio 1:10 was ~ 10.0 nm. At further increase of HMTA, excess size of obtained nanoparticles decreases to ~ 3.0 nm. СеО2 − х nanoparticles were stabilized by sodium citrate with molar ratio 1:1. The solutions were additionally dialyzed for 24 h against deionized water to remove the excess of ions and organics species. Dialysis membrane tubing with a molecular weight cutoff of “Cellu Sep H1” 3.5 KDa was used. All sols were transparent in transmitted light and passed through membrane filters with pores of 100 nm without loss.
Sol–gel Synthesis of 50.0-nm Nanoceria
СеО2 − x, СеО2:Eu3+/Y3+(0.1–10 at.%) nanocrystals were obtained by Pechini method. Cerium oxide (СеO2) (99.995%, Sigma-Aldrich) was dissolved in the mixture of nitric acid (НNO3) and hydrogen peroxide (Н2О2) (in 1:1 volume ratio) at 60 °C. Europium oxide (Eu2O3) (99.999%, Sigma-Aldrich) and yttrium oxide (Y2O3) (99.999%, Sigma-Aldrich) were dissolved in the dilute НNO3 at 80 °C. The solution of 0.75 g of citric acid and 1 ml of ethylene glycol was added to 20 ml of cerium nitrate Се(NO3)3 (C = 1 M) solution or to 20 ml the stoichiometric mixture of cerium nitrate Се(NO3)3 (C = 1 M) and europium nitrate Eu(NO3)3/yttrium nitrate Y(NO3)3 (C = 1 M) solutions. All the resulting mixtures were treated at 80 °С during 10 h and then hydrolyzed by means of 10 mass.% NH3 aqueous solution. The precipitates were dried at 120 °С during 5 h and then dehydrated at 250 °С during 4 h. The nanocrystals were annealed during 2 h in vacuum at 1000 °C. After annealing, nanoparticles were dispersed in water at 1 g/l concentration.
Experimental Techniques
The photoluminescence of all types of nanoceria has been excited by a continuous-wave GKL-4UM He-Cd laser (λ~325 nm) and registered using the SDL-1 grating monochromator with the Hamamatsu R9110 PMT in the photon-counting mode.
Immediately after oxidant (hydrogen peroxide or potassium periodate) addition to aqueous colloidal solutions of nanoceria, the time evolution of Ce3+ luminescence intensity (taken at 390 nm) was determined by means of time-resolved measurements at CW excitation (He–Cd laser). Concentrations of nanoceria in aqueous solutions were similar in all experiments and equal to 1 g/l. Concentration of oxidant required to initiate non-reversible Ce3+ → Ce4+ redox reaction was equal to 0.1 mM; concentrations of oxidants for initiation of reversible Ce3+ ↔ Ce4+ redox reactions were equal to 1.0 mM for both H2O2 (HP) and KIO4 (PP).
The time dependence of pH value for the nanoceria aqueous colloidal solutions after oxidant addition was measured using pH meter. As the oxidants HP (C = 0.1 mM) and PP (C = 0.1 mM) were used, pH values were recorded with time intervals of 1 s after addition of the portions of oxidant to nanoceria aqueous colloidal solutions. The time dependence of pH value for distilled water after addition of the oxidant was taken as a control (initial pH value of distilled water was the same as the one for colloidal solutions of nanoceria (pH = 7)).
All experiments were realized at T = 37 °С.
Additional file
Supplementary materials. (DOCX 1515 kb)
Abbreviations
- HP
Hydrogen peroxide
- PP
Potassium periodate
- ROS
Reactive oxygen species
- VO
Oxygen vacancies
Authors’ Contributions
The idea of the mechanism of oscillations and antioxidant activity of nanoceria was developed by YM in assistance with NS. Nanocrystals were synthesized by VK. Spectroscopic investigations and interpretation of spectral bands were done by PM and VS. Manuscript was written by YM in assistance with VS. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s11671-017-2339-7) contains supplementary material, which is available to authorized users.
References
- 1.Gai S, Li C, Yang P, Lin J. Recent progress in rare earth micro/nanocrystals: soft chemical synthesis, luminescent properties, and biomedical applications. Chem Rev. 2013;114:2343–2389. doi: 10.1021/cr4001594. [DOI] [PubMed] [Google Scholar]
- 2.Tong X, Zhou Y, Jin L, Basu K, Adhikari R, Selopal GS, Zhao H, Sun S, Vomiero A, Wang ZM, Rosei F. Heavy metal-free, near-infrared colloidal quantum dots for efficient photoelectrochemical hydrogen generation. Nano Energy. 2017;31:441–449. doi: 10.1016/j.nanoen.2016.11.053. [DOI] [Google Scholar]
- 3.Reed K, Cormack A, Kulkarni A, Mayton M, Sayle D, Klaessig F, Stadler B. Exploring the properties and applications of nanoceria: is there still plenty of room at the bottom? Environ Sci: Nano. 2014;1:390–405. [Google Scholar]
- 4.Fu Q, Saltsburg H, Flutzani-Stephanopoulos M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science. 2003;301:935–938. doi: 10.1126/science.1085721. [DOI] [PubMed] [Google Scholar]
- 5.Rzigalinski BA, Meehan K, Davis RM, Xu Y, Miles WC, Cohen CA. Radical nanomedicine. Nanomedicine. 2006;1:399–412. doi: 10.2217/17435889.1.4.399. [DOI] [PubMed] [Google Scholar]
- 6.Celardo I, Pedersen JZ, Traversa EL, Ghibelli L. Pharmacological potential of cerium oxide nanoparticles. Nano. 2011;3:1411–1420. doi: 10.1039/c0nr00875c. [DOI] [PubMed] [Google Scholar]
- 7.Das S, Dowding JM, Klump KE, McGinnis JF, Self W, Seal S. Cerium oxide nanoparticles: applications and prospects in nanomedicine. Nanomedicine. 2013;8:1483–1508. doi: 10.2217/nnm.13.133. [DOI] [PubMed] [Google Scholar]
- 8.Pulido-Reyes G, Rodea-Palomares I, Das S, Sakthivel TS, Leganes F, Rosal R, Seal S, Fernández-Piñas F. Untangling the biological effects of cerium oxide nanoparticles: the role of surface valence states. Sci Rep. 2015;5(15613):1–14. doi: 10.1038/srep15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson BC, Johnson ME, Walker ML, Riley KR, Sims CM. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants. 2016;5(15):1–21. doi: 10.3390/antiox5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsunekawa S, Ishikawa K, Li ZQ, Kawazoe Y, Kasuya A. Origin of anomalous lattice expansion in oxide nanoparticles. PhysRevLett. 2000;85:3440–3443. doi: 10.1103/PhysRevLett.85.3440. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Wiesmann HJ, Moodenbaugh AR, Klie RF, Zhu Y, Welch DO, Suenaga M. Oxidation state and lattice expansion of CeO2-x nanoparticles as a function of particle size. Phys Rev B. 2004;69(125415):1–9. [Google Scholar]
- 12.Deshpande S, Patil S, Kuchibhatla S, Seal S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl Phys Lett. 2005;87:1–3. doi: 10.1063/1.2061873. [DOI] [Google Scholar]
- 13.Esch F, Fabris S, Zhou LT, Montini T, Africh C, Fornasiero P, Comelli G, Rosei R. Electron localization determines defect formation on ceria substrates. Science. 2005;309:752–755. doi: 10.1126/science.1111568. [DOI] [PubMed] [Google Scholar]
- 14.Trovarelli A. Fornasiero P catalysis by ceria and related materials. London: Imperial College Press; 2013. [Google Scholar]
- 15.Nakayama M, Martin M. First-principles study on defect chemistry and migration of oxide ions in ceria doped with rare-earth cations. PhysChemChemPhys. 2009;11:3241–3249. doi: 10.1039/b900162j. [DOI] [PubMed] [Google Scholar]
- 16.Coduri M, Scavini M, Allieta M, Brunelli M, Ferrero C. Defect structure of Y-doped ceria on different length scales. Chem Mater. 2013;25:4278–4289. doi: 10.1021/cm402359d. [DOI] [Google Scholar]
- 17.Chavan SV, Mathews MD, Tyagi AK. Phase relations and thermal expansion studies in the ceria–yttria system. J Am Ceram Soc. 2004;87:1977–1980. doi: 10.1111/j.1151-2916.2004.tb06349.x. [DOI] [Google Scholar]
- 18.Sharma A, Varshney M, Park J, Ha TK, Chae KH, Shin HJ. Bifunctional Ce1− xEuxO2 (0≤ x≤ 0.3) nanoparticles for photoluminescence and photocatalyst applications: an X-ray absorption spectroscopy study. Phys Chem Chem Phys. 2015;17:30065–30075. doi: 10.1039/C5CP05251C. [DOI] [PubMed] [Google Scholar]
- 19.Seminko V, Masalov A, Maksimchuk P, Klochkov V, Bespalova I, Viagin O, Malyukin Y (2016) Spectroscopic properties of nanoceria allowing visualization of its antioxidant action. Nanomaterials for Security 149–157
- 20.Masalov A, Viagin O, Maksimchuk P, Seminko V, Bespalova I, Aslanov A, Malyukin Y, Zorenko Y (2014) Formation of luminescent centers in CeO2 nanocrystals. J Lumin 145:61–64
- 21.Lakowicz JR. Principles of fluorescence spectroscopy. New York: Springer; 2006. [Google Scholar]
- 22.Lacasta AM, Sancho JM, Sagues F, Oshanin G. Propagation dynamics of a particle phase in a single-file pore. MRS Proc. 2000;651:T9–T1. doi: 10.1557/PROC-651-T9.1.1. [DOI] [Google Scholar]
- 23.Borman VD, Teplyakov VV, Tronin VN, Tronin IV, Troyan VI. Molecular transport in subnanometer channels. JETP. 2000;90:950–963. doi: 10.1134/1.559183. [DOI] [Google Scholar]
- 24.Stauffer D. Aharony a introduction to percolation theory. London: Taylor and Francis; 1994. [Google Scholar]
- 25.Murgida GE, Ganduglia-Pirovano MV. Evidence for subsurface ordering of oxygen vacancies on the reduced CeO2 (111) surface using density-functional and statistical calculations. PhysRevLett. 2013;110(246101):1–5. doi: 10.1103/PhysRevLett.110.246101. [DOI] [PubMed] [Google Scholar]
- 26.Namai Y, Fukui KI, Iwasawa Y. Atom-resolved noncontact atomic force microscopic observations of CeO2 (111) surfaces with different oxidation states: surface structure and behavior of surface. J Phys Chem B. 2003;107:11666–11673. doi: 10.1021/jp030142q. [DOI] [Google Scholar]
- 27.Schüth F, Henry BE, Schmidt LD. Oscillatory reactions in heterogeneous catalysis. Adv Catal. 1993;39:51–127. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials. (DOCX 1515 kb)


