Abstract
We present a rare complication of deep venous thrombosis with pulmonary embolism that threatened the patient with systemic embolization. A 36-year-old female was referred to the hospital after five days of progressive shortness of breath and chest pain. Preceding onset of symptoms, she had undergone surgery leading to reduced physical activity and had just returned from vacation by a long flight. Investigations with transthoracic and transesophageal echocardiography revealed a thromboembolism-in-transit across a patent foramen ovale. Thoracic CT showed submassive bilateral pulmonary embolism. Hemodynamic parameters were stable. The patient was treated surgically with extraction of the thrombus, closure of the foramen ovale and removal of the bilateral pulmonary emboli. She was discharged after an uneventful hospital stay.
Learning points:
Thromboembolism-in-transit across a patent foramen ovale usually occurs in the presence of deep venous thrombosis with pulmonary embolism. The abrupt rise in pulmonary arterial pressure may contribute to the migration of the thrombus across the atrial septum to the systemic circulation.
If any abnormal structures are seen in the left atrium by TTE in a patient with pulmonary embolism, a TEE should be performed to rule out an embolus entrapped in a patent foramen ovale.
When acute pulmonary hypertension cannot be assessed by conventional methods, additional parameters such as shortened right ventricular outflow tract acceleration time and a mid-systolic notching of the pulse wave Doppler profile in the right ventricular outflow tract may be useful.
Mortality is highest during the initial 24 h after onset of chest symptoms; thus, optimal treatment must commence urgently.
The choice of treatment in each individual patient must be made after a thorough discussion in a multidisciplinary heart team.
Keywords: transthoracic echocardiography, transesophageal echocardiography, patent foramen ovale, pulmonary embolism, surgery
Background
Thromboembolism-in-transit across a patent foramen ovale (PFO), also referred to as impending paradoxical embolism, is a rare condition. It is most often seen in conjunction with deep venous thrombosis (DVT) and pulmonary embolism (PE). Due to the abrupt rise in pulmonary arterial pressure, the direction of the shunt through the PFO may change to right to left and allow migration of the thrombus across the atrial septum to the systemic circulation. This predisposes to potentially fatal complications, embolization to the brain being the number one threat. Treatment options are anti-thrombotic therapy, thrombolysis and surgery. Which of these is the best has not yet been settled and is prone to individual assessment.
Case presentation
A 36-year-old female was referred to the hospital after five days of progressive shortness of breath and chest pain. She had undergone a partial conchotomy of concha bullosa and endoscopic ethmoidectomy bilaterally six weeks earlier. This led to a period of physical immobilization. Her medical history further included a disc prolapse, obesity and hypothyroidism. Apart from thyroxine substitution, she was taking oral contraceptives.
Immediately preceding symptom onset, she had returned from vacation, traveling five and a half hours by plane. Three days after returning home, she visited the casualty clinic. Her symptoms of dyspnea and cough were perceived as pneumonia. Upon admission, she reported dyspnea class III–IV according to the New York Heart Association (NYHA) classification. The patient was then acutely admitted to the emergency room.
Investigation
Physical examination revealed an overweight female with malaise, pronounced respiratory distress (26/min), tachycardia (143 bpm) and oxygen saturation of 92% without additional oxygen supply. She was hypertensive with a blood pressure of 166/113 mmHg, but otherwise hemodynamically stable. Height: 174 cm, weight: 126 kg. The clinical examination was otherwise normal.
The electrocardiogram (ECG) confirmed sinus tachycardia, S wave in leads I and II and Q wave in lead III, concordant with right heart strain.
A blood gas was sampled revealing pH 7.43, pCO2 3.3 kPa, pO 9.3 kPa, base excess (BE) −7.4 mmol/L, bicarbonate (HCO3) 16 mmol/L, lactate 3.3 mmol/L. She had an elevated troponin-T (TnT) of 74 ng/L, a proBNP of 253 pmol/L and a CRP of 39 mg/L. D-dimer was >4.00 mg/L. Hypercoagulability tests were negative.
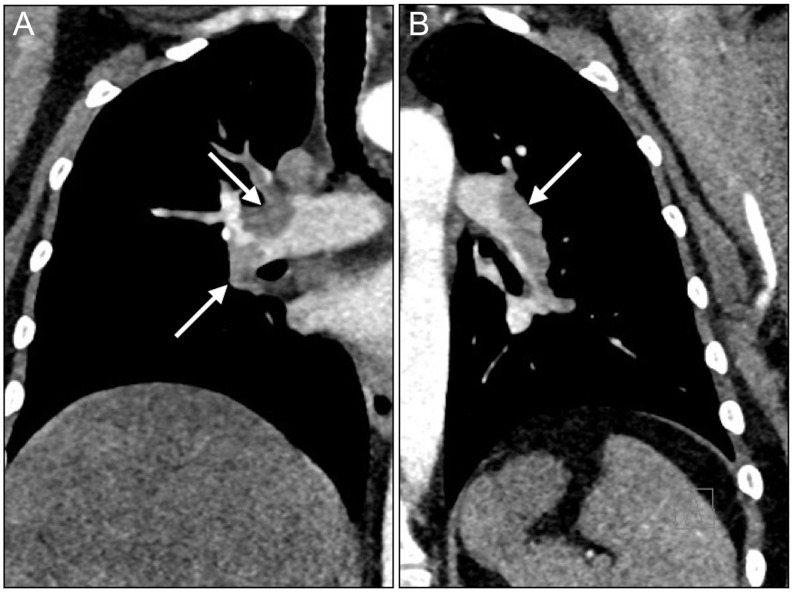
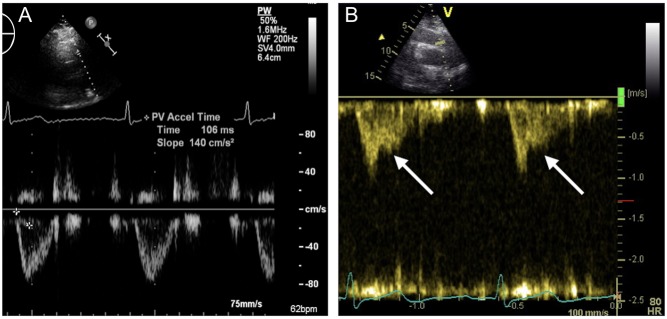
A CT scan revealed central bilateral PE branching out to the segmental arteries of all lobes (Fig. 1). No thrombosis was seen in the superior and inferior vena cava (SVC; IVC) or in the iliac vessels. Anti-thrombotic therapy was initiated, but due to persistent sinus tachycardia, hypoxemia and shortness of breath at rest, the patient was referred to transthoracic echocardiography (TTE). This showed a slightly dilated right ventricle (4.3 cm basal part and 3.3 cm mid-ventricular), but with a preserved systolic function. Left ventricular ejection fraction was 60%. No systolic pulmonary artery pressure could be measured due to lack of a tricuspid regurgitant jet and pressure gradient over the tricuspid valve. However, a shortened right ventricular outflow tract acceleration time (78 ms) and a mid-systolic notching of the pulse wave Doppler profile in the right ventricular outflow tract (Fig. 2) were both present, suggesting acute pulmonary hypertension.
Figure 1.
Submassive bilateral pulmonary embolism. The emboli in the right (A) and left (B) lungs are indicated by arrows.
Figure 2.
Echocardiographic image of the right ventricular outflow tract with pulsed wave Doppler profile. In a normal subject (A) the signal is smooth and parabolic with a normal right ventricular outflow tract acceleration time. In acute pulmonary hypertension (B) a mid-systolic notching (arrows) with shortened right ventricular outflow tract acceleration time is evident.
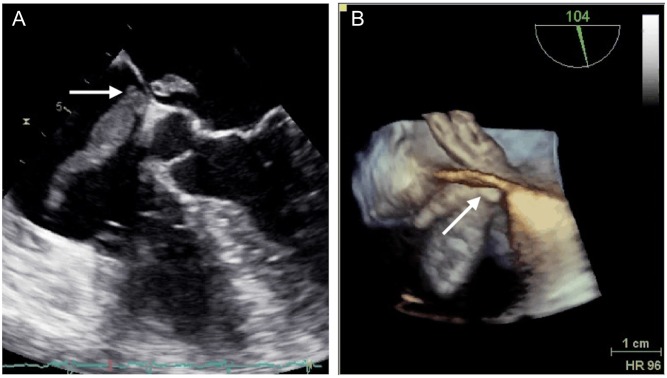
In the parasternal long-axis view and apical four chamber view, a long mobile structure in the left atrium was detected (Fig. 3), prompting a transesophageal echocardiography (TEE; Fig. 4 and Videos 1 and 2). This revealed a thromboembolism-in-transit across a PFO.
A long mobile structure in the left atrium was detected on transthoracic echocardiography (TTE), prompting a transesophageal echocardiography (TEE). The videos show TEE 2D recordings on the left and 3D on the right, visualizing the thromboembolism-in-transit across the atrial septum. View Video 1 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0043/video-1.
Download Video 1 (904.9KB, mp4)
A long mobile structure in the left atrium was detected on transthoracic echocardiography (TTE), prompting a transesophageal echocardiography (TEE). The videos show TEE 2D recordings on the left and 3D on the right, visualizing the thromboembolism-in-transit across the atrial septum. View Video 2 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0043/video-2.
Download Video 2 (869.4KB, mp4)
Figure 3.
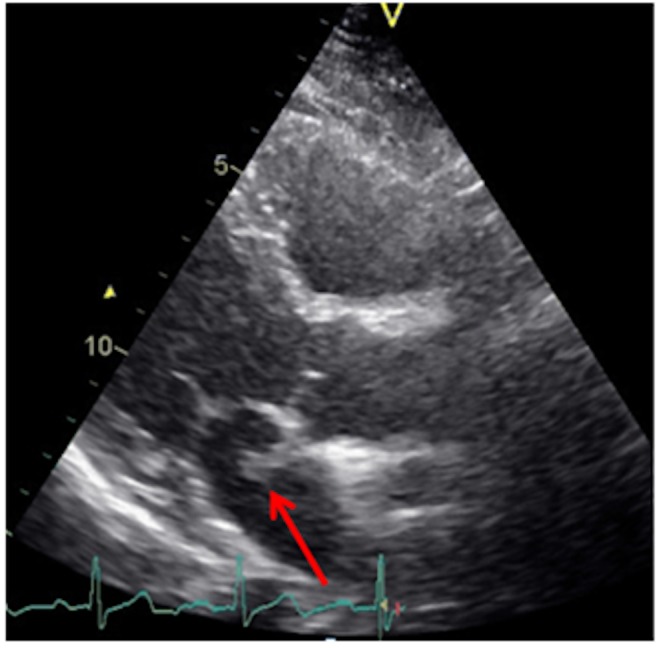
Parasternal long-axis view by transthoracic echocardiography showing a long mobile structure in the left atrium (arrow).
Figure 4.
The atrial thrombus (arrow) extending from the right to the left atrium through the PFO in mid-esophageal four chamber view by transesophageal echocardiography (A) and 3D echocardiography (B).
During work-up, a cerebral MRI was performed to detect eventual subclinical cerebral events. This showed three small foci consistent with microembolization. No clinical implications of these findings were recorded.
Treatment and outcome
The risks and benefits of embolectomy were extensively discussed with the patient, her family, hematologists and within the multidisciplinary heart team. Due to the imminent risk of further embolization with potentially fatal consequences in a young patient, surgery became the treatment of choice and was considered less risky and more beneficial than medical treatment. This would permit a concomitant pulmonary embolectomy as well.
The patient was operated within 24 h after the diagnosis of a thromboembolism-in-transit was settled. This diagnosis was, however, delayed by nearly one week due to the lack of systemic symptoms and thus a failure of recognition.
The operation was performed through a median sternotomy with extracorporeal circulation, exercising extreme caution to avoid unnecessary manipulation with the risk of embolization. Snared bicaval venous cannulation gave access to the right atrium. Intraoperative findings were consistent with the preoperative TEE.
The right atrial portion of the pendulating thrombus was approximately 6 cm in length (Fig. 5). To ensure removal in toto, the PFO was enlarged by incising the septum. This gave full visibility of and access to the 3 cm long left atrial portion of the thrombus.
Figure 5.
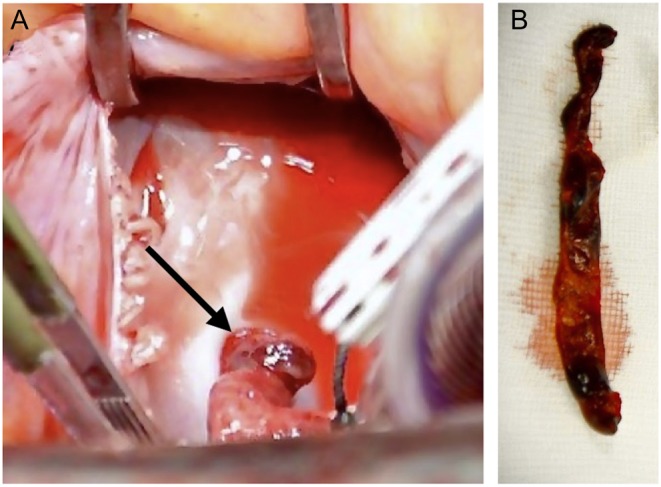
Thrombus in PFO. Viewed through the incision in the right atrium the thrombus can be seen entrapped in the PFO (arrow; A). Thrombus after extraction (B).
The septal incision and PFO were closed using a double-layered Prolene 4-0 (Ethicon, Somerville, NJ, USA) suture. The pulmonary artery was then incised, and the PE was removed mechanically under full visualization.
She was discharged on the 8th postoperative day after an uneventful postoperative hospital stay, except for two episodes of visual flare. Examination by an ophthalmologist revealed no pathology and the symptoms subsided spontaneously.
Discussion
The annual incidence of DVT including PE is approximately 1 per 1000 (1). Based upon autopsies, a PFO is present in 20–30% of the population (2). Despite this seemingly high likelihood of catching a thromboembolism-in-transit across the PFO, it is seldom encountered in clinical practice. This has raised the question of unrecorded events masking the real incidence and triggered the proposition of a screening echocardiogram in all patients with DVT (3). The delay of the thromboembolism-in-transit diagnosis in this case report emphasizes this statement.
Several known risk factors for DVT were present in this patient: obesity, inactivity and the use of oral contraceptives. All of these may have contributed to her condition, although hypercoagulability tests were negative.
Acute massive PE may lead to right ventricle pressure overload and systolic dysfunction, which can be easily detected by echocardiography (4). The detection of right ventricle dilatation either by echocardiography or CT scan of the thorax is also useful for the risk stratification and PE severity index. Generally, TTE is the method of choice for the assessment of pulmonary hypertension, right ventricle pressure overload and systolic dysfunction, as well as thrombus in the left ventricle. In particular, right ventricular function, a major determinant of morbidity and mortality in pulmonary hypertension, can be easily assessed by TTE (5). Indirect measures like tricuspid annular plane systolic excursion (TAPSE) and tissue Doppler at the RV free wall may be reduced in right ventricular dysfunction. Conventionally, systolic pulmonary artery pressure is derived from the recording of peak tricuspid regurgitant jet and the tricuspid systolic gradient, adding the right atrial pressure on the basis of inferior vena cava size and its inspiratory collapse (6). However, when the measurement of tricuspid regurgitation and pressure gradient are not available for the assessment of systolic pulmonary artery pressure, indirect assessment of pulmonary hypertension may be useful. Normally, one would expect a smooth parabolic signal of the right ventricular outflow tract pulsed-wave Doppler profile (Fig. 2A). In both acute and chronic pulmonary hypertension, a shortened right ventricular outflow tract acceleration time and a mid-systolic notching of the pulse wave Doppler profile in the right ventricular outflow tract may be observed (Fig. 2B).
If any abnormal structures are seen in the left atrium in a patient with PE on TTE, a prompt TEE should be performed to rule out an embolus entrapped in the PFO. Furthermore, TEE is also useful in diagnosing left-sided cardiac valve disease, intrapulmonary shunt, atrial septal abnormalities and in evaluating size, morphology and function of the left atrium and left atrial appendage (7).
Initial treatment of massive PE is still thrombolysis; however, if the effect is poor and the patient hemodynamically unstable, surgical embolectomy should be carried out and should perhaps play a stronger role than previously considered (8). How to treat a patient with a thromboembolism-in-transit with a submassive PE is up for discussion. One review article concludes with a trend toward improved survival and a significantly reduced risk of systemic embolism and mortality if the patient is operated rather than conservatively treated (9). This conclusion is not necessarily applicable in every patient, considering age and comorbidity. An additional effect of surgery is that the pulmonary emboli may also be removed, thus promoting immediate respiratory improvement and reduced pulmonary pressure. The choice of treatment in each individual patient must be made after a thorough discussion in a multidisciplinary heart team. Mortality of paradoxical embolism is high, approximately one-in-five, despite optimal treatment. Two-thirds of these die within the initial 24 h, so treatment ought to be initiated urgently (9).
Declaration of interest
The authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartiality of this case report.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent has been received.
References
- 1.Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. 2014. Secular trends in occurrence of acute venous thromboembolism: the Worcester VTE Study (1985–2009). American Journal of Medicine 127 829.e5–839.e5. ( 10.1016/j.amjmed.2014.03.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagen PT, Scholz DG, Edwards WD. 1984. Incidence and size of patent foramen ovale during the first 10 decades of life: an Autopsy Study of 965 Normal Hearts. Mayo Clinic Proceedings 59 17–20. ( 10.1016/S0025-6196(12)60336-X) [DOI] [PubMed] [Google Scholar]
- 3.Hui DS, Fleischman F, McFadden PM. 2016. Thromboembolism-in-transit and patent foramen ovale: should screening echocardiogram be routine for thromboembolic disease? Ochsner Journal 16 321–323. [PMC free article] [PubMed] [Google Scholar]
- 4.Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Çetin Erol CD, Fagard R, Ferrari R, Hasdai D, et al. 2014. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Endorsed by the European Respiratory Society (ERS). European Heart Journal 35 3033–3073. ( 10.1093/eurheartj/ehu283) [DOI] [PubMed] [Google Scholar]
- 5.Harjola V-P, Mebazaa A, Čelutkienė J, Bettex D, Bueno H, Chioncel O, Crespo-Leiro MG, Falk V, Filippatos G, Gibbs S, et al. 2016. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. European Journal of Heart Failure 18 226–241. ( 10.1002/ejhf.478) [DOI] [PubMed] [Google Scholar]
- 6.Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. European Heart Journal 37 67–119.26320113 [Google Scholar]
- 7.Saeed S, Gerdts E. 2017. Echocardiography in ischemic stroke in young adults In Ischemic stroke in the young, ch 10. Eds Tatlisumak T, Thomassen L. Oxford, UK: Oxford University Press. [Google Scholar]
- 8.Haaverstad R, Vitale N. 2016. Surgical pulmonary embolectomy: should we extend its role? Journal of Thoracic and Cardiovascular Surgery 152 879–880. ( 10.1016/j.jtcvs.2015.12.002) [DOI] [PubMed] [Google Scholar]
- 9.Myers PO, Bounameaux H, Panos A, Lerch R, Kalangos A. 2010. Impending paradoxical embolism: systematic review of prognostic factors and treatment. Chest 137 164–170. ( 10.1378/chest.09-0961) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A long mobile structure in the left atrium was detected on transthoracic echocardiography (TTE), prompting a transesophageal echocardiography (TEE). The videos show TEE 2D recordings on the left and 3D on the right, visualizing the thromboembolism-in-transit across the atrial septum. View Video 1 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0043/video-1.
Download Video 1 (904.9KB, mp4)
A long mobile structure in the left atrium was detected on transthoracic echocardiography (TTE), prompting a transesophageal echocardiography (TEE). The videos show TEE 2D recordings on the left and 3D on the right, visualizing the thromboembolism-in-transit across the atrial septum. View Video 2 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-17-0043/video-2.
Download Video 2 (869.4KB, mp4)



 This work is licensed under a
This work is licensed under a