Abstract
Plant cell walls, which are mainly composed of pectin, play important roles in plant defence responses to pathogens. Pectin is synthesised in a highly esterified form and then de-esterified by pectin methylesterases (PMEs). Because of this, PMEs are directly involved in plant defence. However, the molecular mechanisms of their interactions with pectins remain unclear. In this study, we compared the expression level and enzyme activities of PMEs in a banana Cavendish cultivar (Musa AAA ‘Brazilian’) inoculated with Fusarium oxysporum f. sp. cubense pathogenic races 1 (Foc1) and 4 (Foc4). We further examined the spatial distribution of PMEs and five individual homogalacturonans (HGs) with different degree of pectin methylesterification (DM). Results suggested that the banana roots infected with Foc1 showed lower PME activity than those infected with Foc4, which was consisted with observed higher level of pectin DM. The level of HGs crosslinked with Ca2+ was significantly higher in roots infected with Foc1 compared with those infected with Foc4. Therefore, banana exhibited significantly different responses to Foc1 and Foc4 infection, and these results suggest differences in PME activities, DM of pectin and Ca2+-bridged HG production. These differences could have resulted in observed differences in virulence between Foc1 and Foc4.
Introduction
Fusarium oxysporum f. sp. cubense (Foc), the causal agent of banana Fusarium wilt, can cause severe losses in yield and quality. The pathogen penetrates banana roots, colonises and blocks vascular tissues, causes a reddish-brown discolouration of the rhizome and pseudostem, and eventually leads to leaf collapse and plant death1. Susceptible banana plants can only be grown in pathogen-free soil because Foc can survive in the soil for decades2. According to the ability to cause disease to certain cultivars in the field, pathogenic variability within Foc causes its subdivision into races. Three races (1, 2 and 4) of the pathogen affect banana. Foc race 1 (Foc1) attacks dessert bananas, such as ‘Gros Michel’ (Musa spp., AAA-group), and caused the 20th century epidemic resulting in the inability to grow that cultivar for the mass market. Foc race 2 (Foc2) affects ‘Bluggoe’ (Musa spp., ABB-group) and its closely related cultivars. Foc race 4 (Foc4) causes disease to most commercially grown ‘Cavendish’ banana cultivars (Musa spp., AAA-group), as well as the hosts of Foc1 and Foc2.
Cell walls serve as external barriers to prevent pathogens from infecting or spreading within a plant. Homogalacturonans (HGs) are the main constituents of cell wall pectin, and therefore play a crucial role in the first defence against pathogen attack3. Necrotrophic fungi usually degrade pectin to achieve a successful infection4. F. oxysporum, a hemibiotrophic and/or necrotrophic pathogen, releases a series of cell wall-degrading enzymes (CWDEs) that digest host plant cell walls3,5. The pectin methylesterases (PMEs), one of the CWDEs, remove methyl esters from pectin and improve the cell wall accessibility to other CWDEs6,7.
A series of strategies, such as hydroxylation of plant cell wall components and production of inhibitor proteins, is used by plants against pathogenic CWDEs to prevent degradation of pectin8,9. Plants finely tune pectin methylesterification and regulate PME activities during normal growth and development. In plant–pathogen interactions, the activities of PMEs10, level of PMEs inhibitor (PMEI)9, degree of methylesterfication (DM) of HGs11 and molecular properties of HGs8 are associated with plant resistance to pathogens. The extensive degradation of HGs by pathogens causes the production of oligogalacturonides (OGs) that act as elicitors of defence responses in the host3,12 including changes in the expression of defence-associated genes12. However, during the interaction between plant and pathogen, the specific roles of PMEs and molecular mechanisms of PMEs action on pectin remain unclear, and conflicting results also were occasionally reported about the level of PME expression5,13, and changes in cell wall composition14,15 that occurs when different plants species are infected by different pathogens.
During the interaction between banana and Foc, both of Foc1 and Foc4 can infect ‘Brazilian’ banana, but only Foc4 can cause ‘Brazilian’ banana disease16. To date, most of the research work about Foc has focused on molecular detection17,18, pathogenesis-related genes19, the infection process of Foc420, resistance-related genes of host21, and disease control22,23. There was a report that the spatial distributions of PMEs and different pectin DM affect susceptibility of different banana cultivars to Foc424. Nevertheless, no study addressed the differences in pathogenicity between Foc1 and Foc4 using cell histological observations. In addition, whether and how PMEs affect differences in observed virulence between pathogen races remains unclear.
In the present study, we mainly focused on examining whether there are differences in the enzyme activities of PMEs and DMs of HGs in ‘Brazilian’ banana challenged with Foc1 and Foc4. Furthermore, whether or not PMEs affect the pathogenicity of Foc1 and Foc4 on ‘Brazilian’ banana also was addressed. To this end, the enzyme activities and expression of PME1 of Musa accuminata (MaPME1) gene were compared in ‘Brazilian’ banana challenged with Foc1 and Foc4. Likewise, observations of spatial distributions of PMEs and pectin with different DMs were made, and the relationship with of these obseversations with observed pathogenicity of Foc1 and Foc4 was determined by measuring the abundance of Foc in plants inoculated by Foc, as well as the expression level of six defence genes in roots treated with OGs, Foc1 and Foc4. Based on our results, we could make conclusions whether or not plant PME activities, methyl-esterified levels of pectin, relatively DM of pectin, and production of HG-bridged by Ca2+ could impact pathogenicity of different Foc races on ‘Brazilian’ banana.
Results
Differences in the activities of PMEs and the DMs of pectin
The PME activity of the roots infected with Foc1 was remarkably lower than that of the roots inoculated with Foc4 (Fig. 1a). However, the general change of PME activities in roots inoculated respectively with both Foc1 and Foc4 was similar. In brief, the PME activities increased from 0 to 6 h, and kept in a lower and stable state from 12 h to 48 h. However, the PME activities were still higher at 48 h after infection than those of controls.
Figure 1.
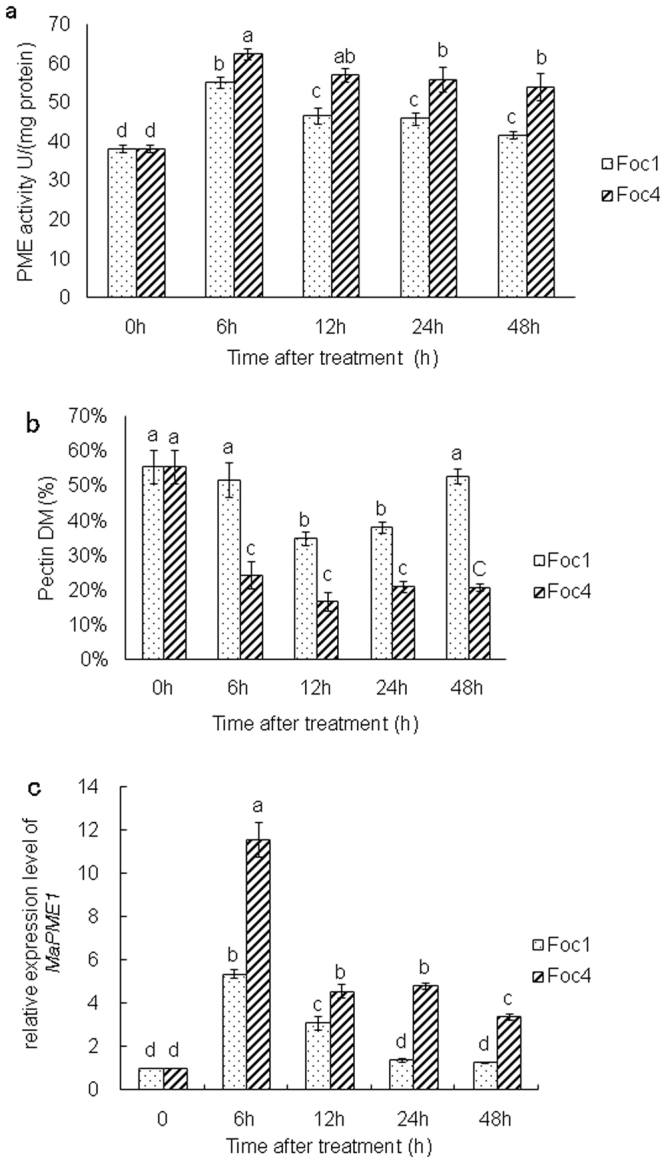
The changes in PME activities (a), pectin DMs (b) and in transcript levels of MaPME1 (c)in banana (Musa spp. AAA) roots different hours after infection with Foc1 and Foc4 of Fusarium oxysporum f. sp. cubense, respectively. Data represent an average of three replicates ± SD. Values followed by the same letter are not significantly different using Duncan’s multiple range test at P < 0.05 after angular transformation of the data. Data of the DMs were expressed as the ratio of mean methanol content (in moles; n = 3) to 1 mol GalA (n = 3).
The DM of pectin in the roots inoculated with Foc1 was significantly higher compared with those of roots inoculated with Foc4. The DM of the roots inoculated with Foc1 showed no considerable change at 6 h, decreased markedly from 12 h to 24 h after infection and finally almost restored the level of the controls. By contrast, the DM of the roots inoculated with Foc4 considerably decreased at 6 h, and maintained a lower level in any other time point (Fig. 1b). The higher DM in the roots infected with Foc1 coincided with low PME activity. In addition, the DM of the roots infected with Foc1 represented an almost 3-fold difference in the DM of roots inoculated with Foc4.
Changes in relative expression levels of MaPME1
We investigated the transcript levels of MaPME1 to confirm the results of PME activity measurements by qRT-PCR. The amplification of the standard dilution series yielded linear and reliable results (MaPME1: R2 = 0.993; RPS2: R2 = 0.979), as well as the high efficiency of the amplification (MaPME1: Eff = 100.2%; RPS2: Eff = 100.1%). The MaPME1 showed significantly different expression at any time point in the roots infected with Foc1 compared with the roots inoculated with Foc4 (Fig. 1c). The qRT-PCR validation of this gene was in agreement with the above study. The transcript levels of MaPME1 were highest at 6 h in the roots infected with the two pathogenic races, but the transcript level of MaPME1 in roots inoculated with Foc4 was twice as high as that in roots infected with Foc1. The transcript level of MaPME1 in the roots infected with Foc1 decreased from 12 h to 24 h and then returned to level similar to controls. Nevertheless, the transcript level of MaPME1 in the roots inoculated with Foc4 showed a decrease after 12 h, no changes from 12 h to 24 h, and decreased expression after 48 h. In general, the transcript level of MaPME1 in the roots inoculated with Foc4 was greater than that in the roots infected with Foc1 at all time-points.
Spatial and temporal distribution of PMEs
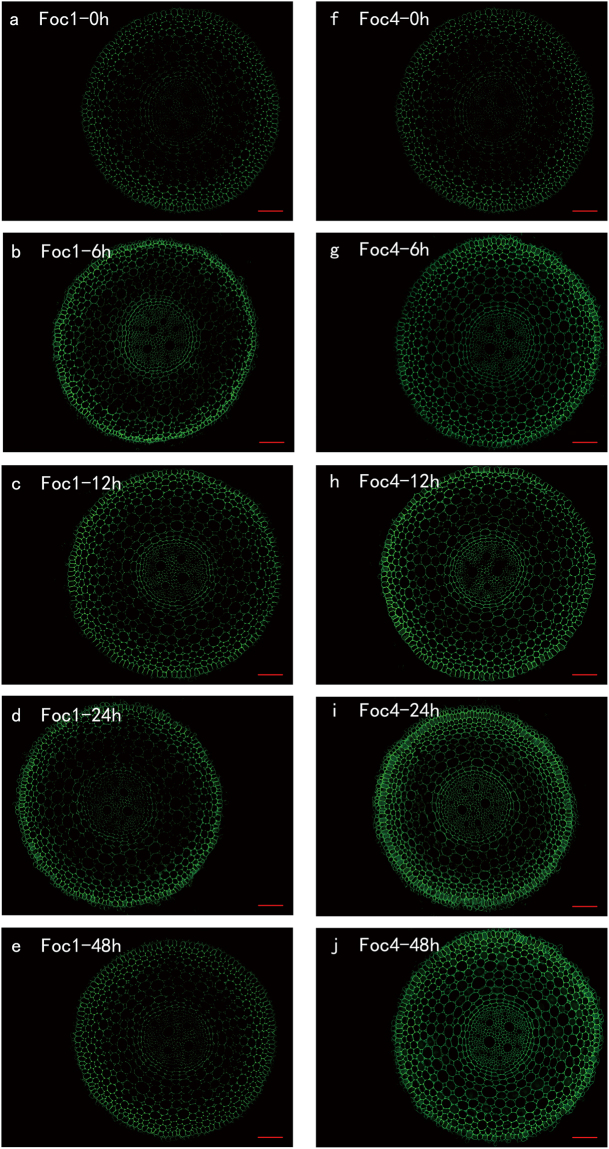
Before the treatment with pathogens, strong signals were observed in the epidermis, endodermis and cortical cells close to the epidermis; by contrast, weak signals emerged in the most cortical cells and vascular cylinder (Fig. 2a,f). PME immunolabelling of roots infected with Foc1 was lower than that of roots infected with Foc4 during the entire experiment. First, the labelling in the roots inoculated with Foc1 and Foc4 increased mainly in the epidermis, endodermis and steles at 6 h after infection (Fig. 2b, g). Afterwards, the level of epitope decreased slowly from 12 h to 48 h in roots infected with Foc1, particularly in epidermis and stele (Fig. 2c–e), but that of roots inoculated with Foc4 showed sharp increase at all the times, thereby the strong signals observed in the whole root sections at 48 h (Fig. 2g–j). Moreover, the signals of roots inoculated with Foc4 were fourfold higher than those of roots infected with Foc1 (see Supplementary Fig. S1). The results indicated that the variation trends were similar to those in the above experiments. These results might mean that higher activities of PMEs resulted in the stronger pathogenicity of Foc4 compared with that of Foc1 in ‘Brazilian’ banana.
Figure 2.
Immunolocalization by a pan-specific PME antibody recognizing more PMEs isoforms (a–j) in banana (Musa spp. AAA) roots at different hours (0, 6, 12, 24, 48 h) after infection with Foc1 and Foc4. The banana roots infected by Foc1: (a–e); the banana roots infected by Foc4: (f–j) The immunolabelling observed before the treatment (a,f), 6 hours after infection (b,g), 12 hours after infection (c,h), 24 hours after infection (d,i) and 48 hours after infection (e,j) are presented. Bars represent 100 µm.
Changes in subcellular distribution of HGs with different DMs during host–pathogen interactions
We used several antibodies, which bound different degrees of methyl-esterified HGs, to monitor the dynamic changes in the subcellular distribution of HGs with different DMs during host–pathogen interactions and subsequently understand the mechanism of how PMEs modify pectin and how the subcellular distribution of HGs with different DMs changes during host–pathogen interactions.
LM20
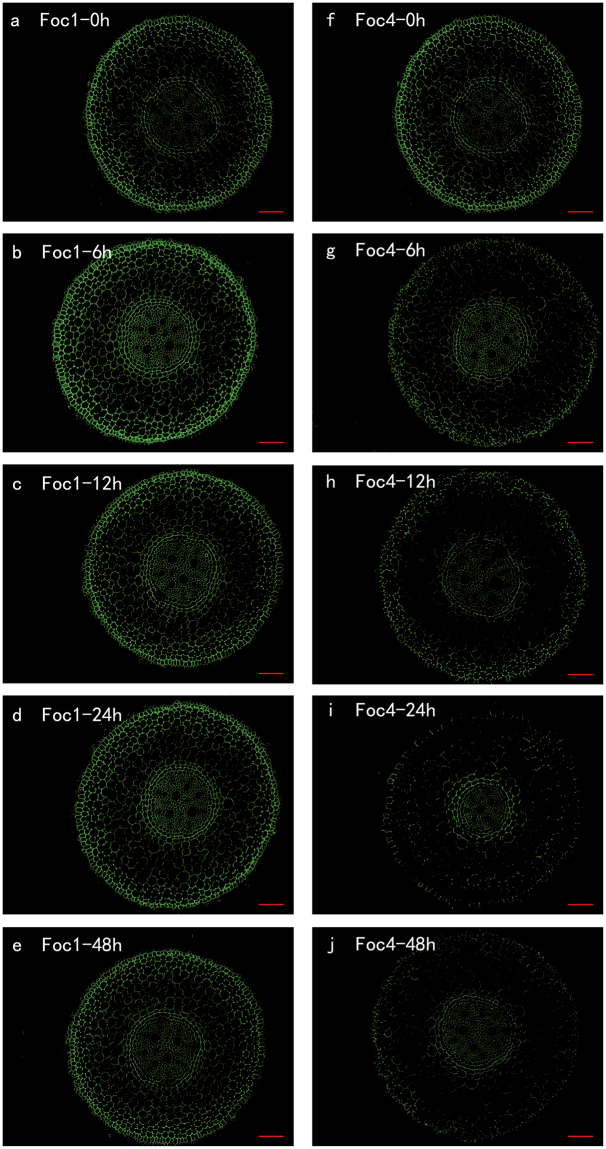
LM20 antibody recognises highly methyl-esterified HGs. After the roots were treated with Foc, the changes in labelling were mainly observed in the cortical cells and steles. The binding of LM20 was stronger in the roots infected with Foc1 than in the roots infected with Foc4 (Fig. 3a–j). These results were consistent with the results of PME activities. During the co-incubation of the roots with Foc1, the signal reduced in the steles and then in the cortical cells at 6 and 12 h after infection (Fig. 3a–c); starting from 24 h, the antigen levels showed an upward trend and almost recovered the level of the control. By contrast, the abundance of antigen decreased almost linearly in the roots inoculated with Foc4 (Fig. 3f–j). Additionally, the level of antigen in roots inoculated with Foc1 was fourfold of that in roots inoculated with Foc4 after 48 h (Fig. 3e,j; see Supplementary Fig. S2). These two treatments presented no substantial difference before 12 h, but a crosscurrent from 24 h to 48 h (Fig. 3d,e,i,j; see Supplementary Fig. S2).
Figure 3.
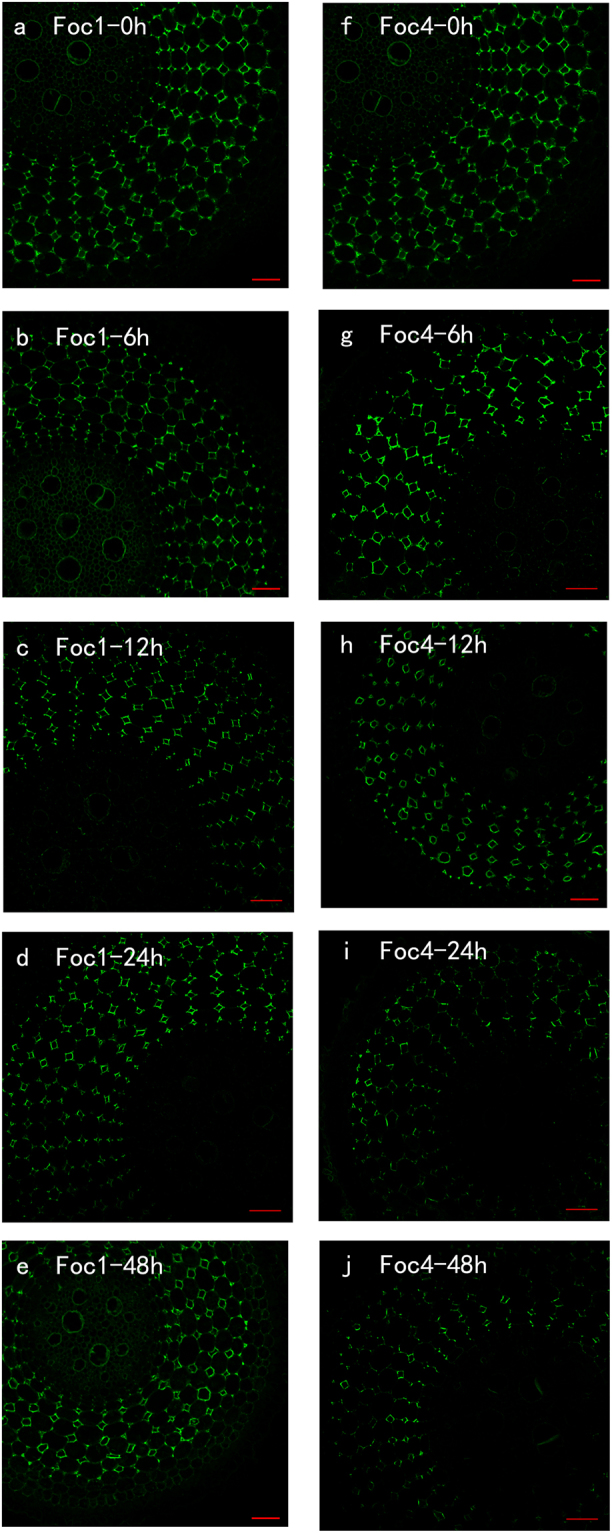
The changes in immunolocalization by LM20 antibody in banana (Musa spp. AAA) roots at different hours (0, 6, 12, 24, 48 h) after infection by Fusarium oxysporum f. sp. cubense. The banana roots infected by Foc1: (a–e); the banana roots infected by Foc4: (f–j). The immunolabelling observed before the treatment (a,f), 6 hours after infection (b,g), 12 hours after infection (c,h), 24 hours after infection (d,i) and 48 hours after infection (e,j) are presented. Bars represent 50 µm.
LM19
LM19 antibody binds low methyl-esterified or demethyl-esterified HGs. LM19 immunolabelling was evenly distributed over the whole root section when the roots were non-treated with Foc (Fig. 4a,f). The labelling, in the roots after 6 h of infection with Foc, was less intense compared with the controls, and no considerable difference was observed (Fig. 4b,g). However, the corresponding antigen of roots inoculated with Foc4 was higher than that of roots infected with Foc1 from 12 h to 48 h (Fig. 4c–e and h–j). From the perspective of fluorescence signal intensity, the binding of LM19 in roots inoculated with Foc4 was twice less after 48 h than that of mock inoculation. Furthermore, the binding of LM19 in roots infected with Foc1 was fourfold lower than that of mock inoculation (see Supplementary Fig. S3).
Figure 4.
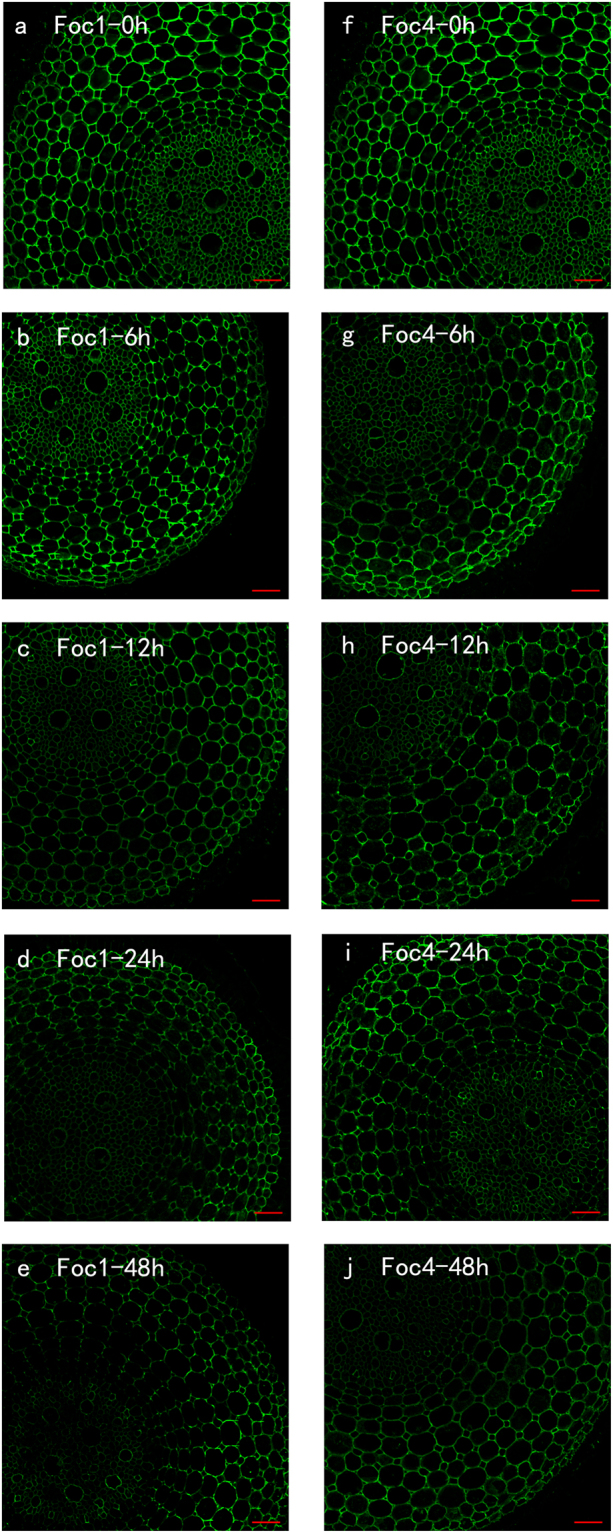
The changes in immunolocalization by LM19 antibody in banana (Musa spp. AAA) roots at different hours (0, 6, 12, 24, 48 h) infection by Fusarium oxysporum f. sp. cubense. The banana roots infected by Foc1: (a–e); the banana roots infected by Foc4: (f–j). The immunolabelling observed before the treatment (a,f), 6 hours after infection (b,g), 12 hours after infection (c,h), 24 hours after infection (d,i) and 48 hours after infection (e,j) are presented. Bars represent 50 µm.
JIM7
JIM7 binds to methyl-esterified oligouronide epitopes in HGs whose degree of methyl esterification ranges between 35% and 81% (high methyl-esterified HGs). Except for epidermis, the distribution of JIM7 pectin covered the whole section evenly before the roots were infected with Foc (Fig. 5a,f). The epitope was tapering off with time after the samples were treated with Foc. Nonetheless, the signals were higher in roots infected with Foc1 than those in roots inoculated with Foc4 (Fig. 5a–j; see Supplementary Fig. S4). In addition, the label of roots inoculated with Foc4 reduced significantly in the cells enclosed by the endodermis at 48 h after infection (Fig. 5j). More roots (16.5–19.94) infected with Foc1 were labelled compared with roots inoculated with Foc4 (3.7–14.71), and the roots infected with Foc1 showed about twice the intension at 24 h and fourfold at 48 h after infection compared with roots inoculated with Foc4 (see Supplementary Fig. S4).
Figure 5.
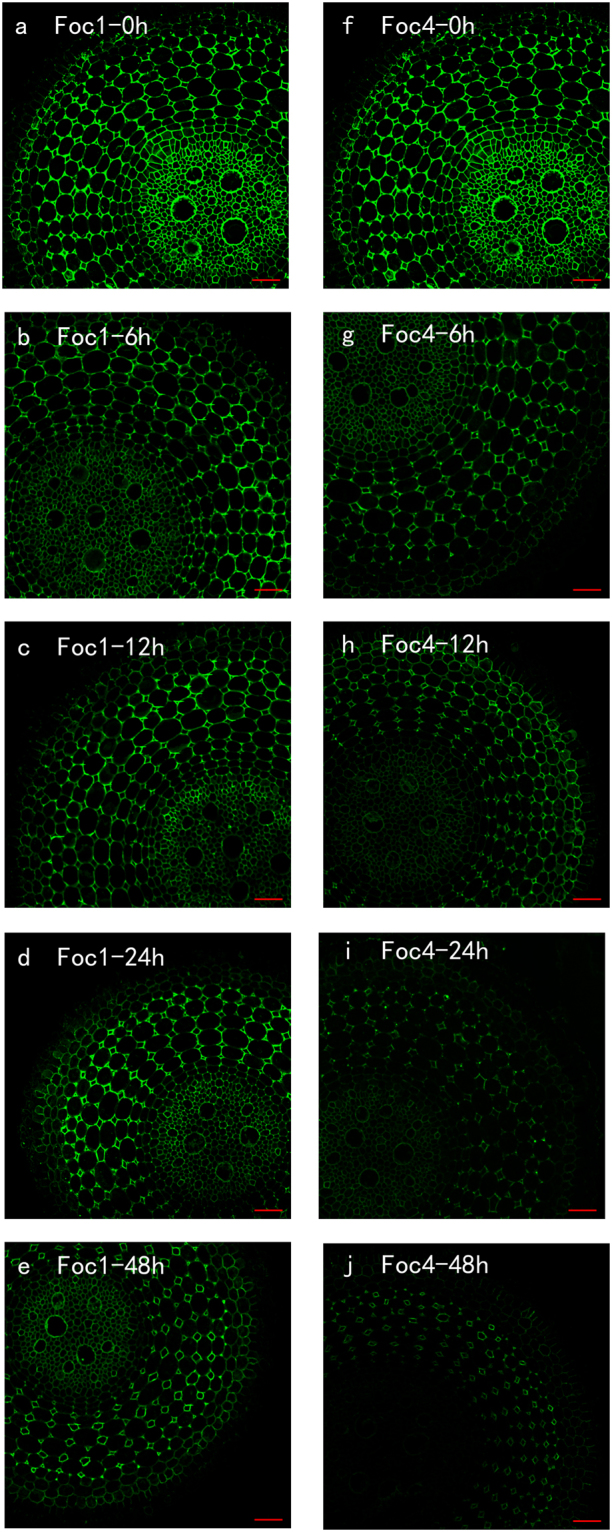
The changes in immunolocalization by JIM7 antibody in banana (Musa spp. AAA) roots at different hours (0, 6, 12, 24, 48 h) infection by Fusarium oxysporum f. sp. cubense. The banana roots infected by Foc1: (a–e); the banana roots infected by Foc4: (f–j). The immunolabelling observed before the treatment (a,f), 6 hours after infection (b,g), 12 hours after infection (c,h), 24 hours after infection (d,i) and 48 hours after infection (e,j) are presented. Bars represent 50 µm.
JIM5
JIM5 recognises several epitopes containing 3–9 fully non-esterified oligogalacturonates25 within HGs with approximately 40% degree of methyl esterification26. The decreasing trend in roots infected with both of Foc1 and Foc4 was observed particularly in the epidermis and cells close to the epidermis; this result was similar to that observed with the monoclonal antibody JIM7. Nevertheless, the roots inoculated with Foc4 showed a more remarkable change compared with Foc1 during the entire process (Fig. 6a–j; see Supplementary Fig. S5). Prior to treatment with pathogens, the corresponding epitopes were distributed evenly all over the root section (Fig. 6a,f). At 6 h, the JIM5 pectin notably reduced in roots inoculated with Foc, and the change of epitope in epidermis was the most obvious (Fig. 6b,g). No differential expression was observed when the roots were infected with Foc4 at 12, 24 and 48 h, but significantly different results were presented by roots infected with Foc1, which showed continued downward trend (Fig. 6a–j). Finally, the binding of JIM5 in roots inoculated with Foc4 was threefold as that of roots infected with Foc1 at 48 h (see Supplementary Fig. S5). The roots infected with Foc4 exhibited a stronger signal in each time point compared with the roots infected with Foc1.
Figure 6.
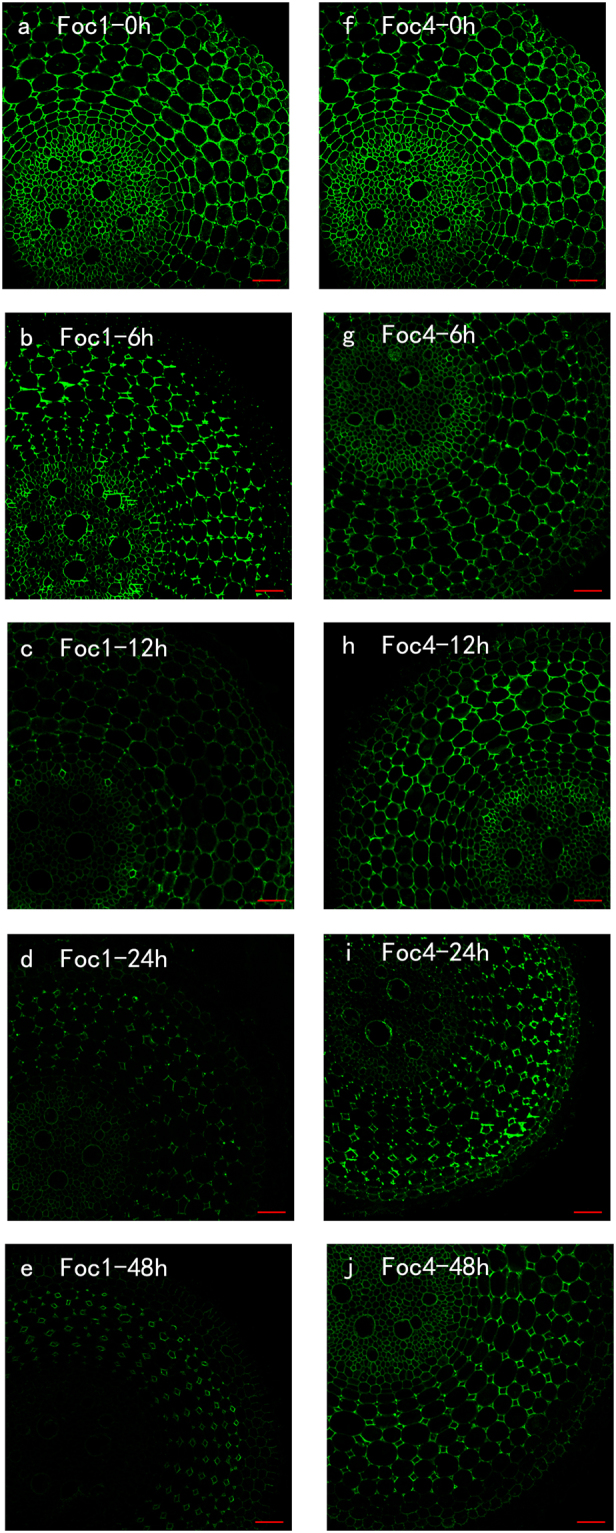
The changes in immunolocalization by JIM5 antibody in banana (Musa spp. AAA) roots at different hours (0, 6, 12, 24, 48 h) infection by Fusarium oxysporum f. sp. cubense. The banana roots infected by Foc1: (a–e); the banana roots infected by Foc4: (f–j). The immunolabelling observed before the treatment (a,f), 6 hours after infection (b,g), 12 hours after infection (c,h), 24 hours after infection (d,i) and 48 hours after infection (e,j) are presented. Bars represent 50 µm.
2F4
2F4 antibody recognises a conformational dimeric HGs epitope induced by a given ionic fraction between calcium and sodium. The dimer consists of two sequences of at least nine non-esterified GalA crosslinked with calcium ions, that is, the ‘egg-box’ structure27, which is assumed to induce gel formation and thus strengthen the cell wall27. The signals, recognised by 2F4, mainly concentrated on the cells of epidermis and endodermis (Fig. 7a–j). After 6 h infection with Foc1, the root sections contained a large amount of antigen epitopes (Fig. 7b). On the contrary, the corresponding epitopes of the roots inoculated with Foc4 decreased significantly at 6 h (Fig. 7g). The 2F4 labelling of the roots infected with Foc1 and those inoculated with Foc4 showed a similar tendency, where the intension decreased slowly from 12 h to 48 h (Fig. 7c–e and h–j). The main difference was that the signals of the roots infected with Foc1 were higher than those of the roots inoculated with Foc4 (Fig. 7e–j).
Figure 7.
The changes in immunolocalization by 2F4 antibody in banana (Musa spp. AAA) roots at different hours (0, 6, 12, 24, 48 h) infection by Fusarium oxysporum f. sp. cubense. The banana roots infected by Foc1: (a–e); the banana roots infected by Foc4: (f–j). The immunolabelling observed before the treatment (a,f), 6 hours after infection (b,g), 12 hours after infection (c,h), 24 hours after infection (d,i) and 48 hours after infection (e,j) are presented. Bars represent 100 µm.
Differences in Foc growth over time
We analyzed different rates of fungal growth in plants inoculated with Foc in different time-points by qPCR with special Foc primer sets (Fig. 8). Our results showed that the levels of Foc1 was not significant different than that of Foc4 in roots at 6 h and 12 h, but afterward Foc4 had greater increases in growth, while levels of Foc1 slowly decreased after 24 h infection.
Figure 8.
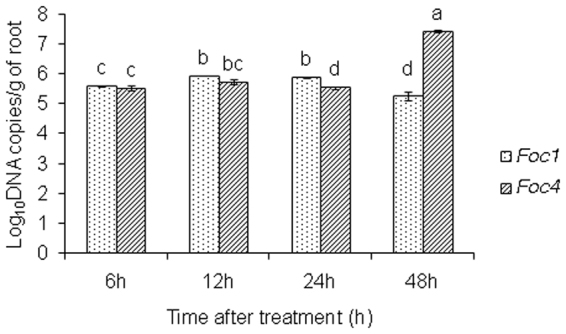
The dynamic changes of Foc1 and Foc4 of Fusarium oxysporum f. sp. cubense in banana (Musa spp. AAA) roots at different hours after infection. Data represent an average of three replicates ± SD. Values followed by the same letter are not significantly different using Duncan’s multiple range test at P < 0.05 after angular transformation of the data.
The induction of defence-related genes
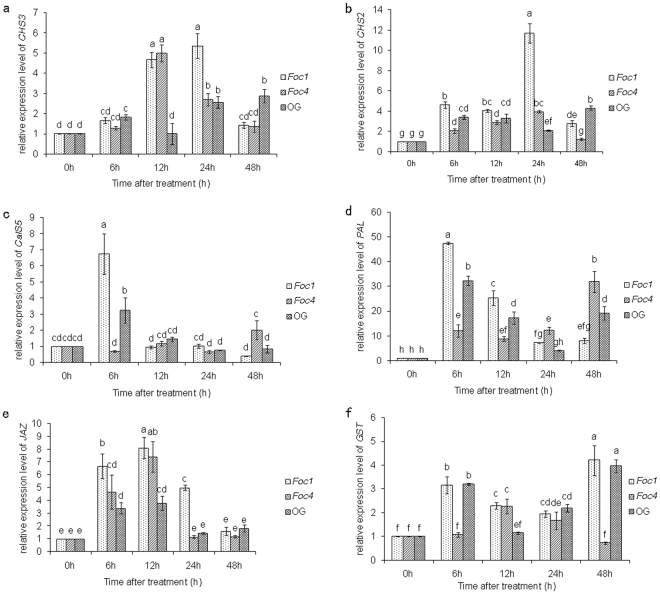
Because there were differences in the expression levels of PME, we hypothesized that mediate increase of PME may promote the production of OG, and excessive PME may result in the collapse of cell wall. To investigate this, by RT-qPCR, we measured the transcriptional levels of six defence-related genes respectively, which encode Chalcone synthase 2 and 3 (CHS2, CHS3), Callose synthase 5 (CalS5), phenylalanine ammonia-lyase (PAL), Jasmonate ZIM-domain protein (JAZ) and glutathione-S-transferase (GST) (Supplementary Table S1). Our results showed that all of six genes were induced significantly by OG, Foc1 and Foc4 respectively, but the pattern of induction was different (Fig. 9). Because the obvious difference of these gene expression levels in the plants treated with sterile ddH2O at every time point was not discovered, the samples treated with Foc at 0 h were utilized as the control to analyse the data. In all measured genes, the increases, induced by Foc1, were considerable higher than by Foc4 at most time points. The results suggested that OG could indeed induce the defence responses in banana, and the difference of OG accumulation might be one of influence factors on the pathogenicity difference between Foc1 and Foc4. In consideration of PME activities and PME expression levels, we concluded that appropriate increases of PME promoted the accumulation of OG, but overdoses on PME content resulted in the rapid necrocytosis.
Figure 9.
The transcriptional expression of six defence genes treated by OG, Foc1 and Foc4 of Fusarium oxysporum f. sp. cubense in banana (Musa spp. AAA) roots at different hours after infection. (a) The relative expression level of CHS3 treated by OG, Foc1 and Foc4. (b) The relative expression level of CHS2 treated by OG, Foc1 and Foc4. (c) The relative expression level of CalS5 treated by OG, Foc1 and Foc4. (d) The relative expression level of PAL treated by OG, Foc1 and Foc4. (e) The relative expression level of JAZ treated by OG, Foc1 and Foc4. (f) The relative expression level of GST treated by OG, Foc1 and Foc4. Data represent an average of three replicates ± SD. Values followed by the same letter are not significantly different using Duncan’s multiple range test at P < 0.05 after angular transformation of the data.
Discussion
A positive relationship exists between the production of enzymes by Fusarium spp. and its virulence, symptoms of disease or degrees of damage28,29. PMEs are related to the location of the pectin in the cell wall30, and thus they may play an important role in the development of disease.
In dicotyledonous plants and non-graminaceous monocots, pectin is a major plant cell wall polysaccharides31, and HGs are the main component of pectin. Pectin structure and DMs are involved in plant defence9,13,32. Lionetti et al. indicated that the pectin DMs differing between resistant and susceptible wheat genotypes possibly were related to the resistance against a fungal necrotroph33. Ma et al. reported that the spatial distributions of PMEs and different DMs of pectin were involved in the susceptibility of two different resistant banana cultivars to pathogens24. However, no reports addressed the different pathogenicity of Foc1 and Foc4 using histological observations. In the present study, we aimed to understand how PMEs play the role in the interaction of host and different pathogenic races of Foc.
In addition to roles in remodelling the plant cell wall during growth and development, PMEs participate in the pathogenic mechanism of pathogens to plant. In Arabidopsis, AtPME3 is induced upon infection with Botrytis cinerea and Pectobacterium carotovorum and its expression becomes a susceptibility factor towards both microbes13. However, a decrease in PME expression was measured in flax (Linum usitatissimum L.) upon infection with Fusarium 5. We showed that two pathogenic races of Foc with different types of pathogenicity, induced variation in the degree of PMEs activity in the host plant. Specifically, PME activities were lower in the banana infected with Foc1 compared with those infected with Foc4. Moreover, an increase in expression of MaPME1 was observed after infection by Foc. In this study, the PME monoclonal antibodies against purified total PME protein of banana were used. Due to the differences of structures and characters of PME between fungus and plant34,35, we speculated that the PME monoclonal antibodies are specified against plant PMEs and not those produced by Foc. Combining the observations of PMEs with observations of Foc1 and Foc4 growth in the roots at different time points (Fig. 8), we considered that fungal growth might not be responsible for alterations in PME activity in these samples. Based on these findings, we believed that the plant PME activities in roots infected by Foc4 were higher than that of the roots infected by Foc1, although it was possible that Foc1 and Foc4 had different ability to produce PMEs as controlled by differences in their genetic makeup, or that expression patterns or activities of specific fungal PMEs were different between the races. Ma et al. also reported high increase of PME activities in the susceptible banana cultivar against Foc424. Other pectin-degrading enzymes only degrade pectin possessing to a certain level of de-methyl esterification10. Thus, the increased PME activities improve the cell wall accessibility to other CWDEs, and consequently accelerate cell wall damage and indirectly promote the production of small pectic fragments, namely OGs, which can activate the defence response of host. Additionally, structural requirements for the highest elicitor activity of OGs include a low DM and a size between 10 and 15 GalA residues36,37. Our results showed that OGs induced the expression of defence-related genes, which were induced in plants infected with Foc, and the degrees of induction of the defence genes were higher in plants infected by Foc1 than Foc4 at most time-points (Fig. 9). De-methylesterified pectin is a preferred substrate of pectin degrading enzymes, and should result in increased production of OGs. Further, previous study suggested that de-methylesterified OGs were more likely to bind Ca2+ 38. However, in our results, the DM of pectin and the induced degree of these defence genes were lower in roots infected with Foc4 than that of Foc1. These results might indicate that the increase of PME activities promoted the degradation of pectin. However, it was not confirmed that pectin fragments which were excessively degraded by CWDEs were active OGs. In light of our other results, we speculated that most of these degraded smaller fragments in roots infected with Foc4 may have been inactive. In addition, we observed that the roots infected with Foc4 at 72 h post-inoculation had rot and were so soft that the paraffin section could not obtained (data not shown). Therefore, we speculate that the induced mild increase of PME by pathogens promotes the production of OGs, which induce defence responses, and as a result of decreased the pathogenicity of Foc1. However, the excessive level of PME activities may have resulted in the rapid collapse of cell walls in banana plants infected with Foc4.
Marty et al. found an additional PME isoform in potato cultivars susceptible to Erwinia carotovora 39. Lionetti et al.33 and Zega & D’Ovidio40 indicated that the identification of specific PME isoforms specifically were repressed or induced in resistant or susceptible wheat cultivars to Fusarium head blight, respectively. Multiple enzyme isoforms and PMEIs could finely regulate PME activity35. In addition, Bethke et al. suggested that not total activity, but some specific effort of PMEs determined the pathogenicity of Pseudomonas syringae in Arabidopsis10. However, only one isoform MaPME1 had been studied in banana. Therefore, it is not clear that which isoforms involve in the process of infection. Plant PMEs catalyse the specific demethylesterification of HGs within plant cell walls, thereby releasing methanol, protons and the remaining pectin with negatively charged carboxyl groups41.
The factors involved in the pathogenic differences of Fusarium on banana are uncertain. Demethylesterification of HGs by PMEs and subsequent split by other pectinases might result in the accumulation of OG to induce the defence response, or decrease in physical strength and rigidity of the cell wall barrier, which favored pathogen invasion.
In this study, we took advantage of several cell wall antibodies to analyse the methylesterification of HGs. LM20 and JIM7 recognise highly methyl-esterified HGs. LM19 and JIM5 bind preferentially to un-esterified HGs. 2F4 are specific for a dimeric association of pectin chains, and not for isolated chains or multimeric associations27. However, LM19 can recognise to all pectins (pectin treated with an Aspergillus niger PME or with orange peel PME), showing little discrimination in relation to the extent or pattern of methyl-esterification. It was in contrast to JIM5 which bound considerably weakly to P16 (pectin treated with orange peel PME until 16% esterification remained). In contrast to JIM7, LM20 showed reduced recognition of F19 (pectin treated with an Aspergillus niger PME until 19% methyl-esterification remained)42. While there is some overlap in the functionality of these antibodies, each one has unique features. Immunofluorescence mapping indicated that pectic epitopes recognised by LM20 and LM19 antibodies presented distinct localisations at the same time points. High methyl-esterified HG domains, recognised by LM20, were detected in roots of cortical cells, whereas low methyl-esterified HG, corresponding to LM19 antibody, distributed evenly in the roots. Unlike the pectic epitopes recognised by LM20 and LM19, pectic epitopes, recognised by JIM7 presented a similar localisation with pectic epitopes recognised by JIM5. Compared with controls, no obvious differences were observed in the HGs corresponding to LM20 antibody at 48 h after infection in roots infected with Foc1, and considerable reductions were respectively observed in the HGs recognised by LM19, JIM5 and JIM7 antibodies. At 6 h after infection, increased PME activity in the roots inoculated with Foc1 was in accordance with decreased DMs. The following decrease of PME activities might result in the restoration of HGs corresponding to LM20 antibody. These findings suggested that PME activities regulate the change of pectin components, specifically those recognised by LM20 antibody, which are likely to determine the non-pathogenicity of Foc1 on ‘Brazilian’ banana.
Additionally, we compared the mapping difference of the roots inoculated with Foc1 and Foc4. Higher PME activities were observed in the roots infected with Foc4 than those infected with Foc1. This result was consistent with the weak signals of LM20 and JIM7, which recognised highly methyl-esterified HGs. Furthermore, a continued decrease was observed in the labelling of LM19, JIM7 and JIM5 in roots inoculated with Foc. The binding of LM20 and JIM7 was lower but that of LM19 and JIM5 was higher in the roots infected with Foc4 than in those infected with Foc1. Besides that, measurement of pectin DMs with colorimetry indicated that the DM of the roots infected with Foc4 was lower than that with Foc1. These results might suggest that the roots infected with Foc1 contained a large amount of highly methyl-esterified HGs and relatively high pectin DMs, although all treatments were carried out at the same time points. On the basis of these results, we concluded that a high abundance of highly DMs of HGs and relatively high pectin DMs might result in the low pathogenicity of Foc1 on banana. In addition, the high DM of pectin, which cannot be degraded by other CWDEs, possibly influenced the nutrient supply for Foc1.
During the interaction between host and pathogen, many reports showed that decrease of HGs recognised by JIM5 and JIM7 is involved in the response of most plants to the pathogen15,43,44, but the increase of JIM7 labelling is also reported14. Increase of HGs recognised by LM19 is a common response of many hosts to the pathogens10. However, an opposite result was obtained in our study, and it might be related with the content of other cell wall degrading enzymes. Unlike the decrease of the other plants10,45, the variation tendency of LM20 in the roots inoculated with Foc1 was an inverted-bell type, that is, increasing levels after an initial decrease, but the research of Ma et al. suggested that the binding of LM20 was increased at 6 h during the interaction between resistant banana and Foc424. Although it is not clear why the binding of LM20 increased at a certain time point during the host and Foc1, the present results provided some information on the dynamic changes of PMEs, pectin DMs and individual HG levels, thereby suggesting that the difference of sampling timing, plant species and pathogens might result in the differences of results.
The activities of PMEs on pectin can result in two major mechanical outcomes: low DM pectin is a target for polygalacturonases, which cleave it further and likely intensify gel softness; low DM pectin is also ready to cross-like with Ca2+ to increase gel rigidity44. HGs with sufficiently low DMs (<40%) and at least nine consecutive un-esterified GalA residues can interact with Ca2+, which result in calcium bridges also known as ‘egg-box’ structure27.
The antibody 2F4 is specific to calcium crosslinked HGs, and these bridged HGs might be more resistant to fungal invasion than unbridged epitopes46. These 2F4 antibodies are specific for a dimeric association of pectin chains such as the one described as the ‘egg-box’ model, and not for isolated chains or multimeric associations27. However, we did not clear the ratio of a dimeric association of pectin chains. Therefore, we indicated that there may be no direct or inevitable correlation between the 2F4 binding and the binding of LM19 and JIM5.
The labelling of 2F4 mainly concentrated on the cells of epidermis and endodermis. In the banana-Foc4 interaction, the occurrence of the 2F4-epitope was gradually lower. Ma et al. also reported that the attack of Foc4 results in the decrease of 2F4 labelling24. Nevertheless, the labelling of 2F4 remains stable during the interaction between banana and Foc1, except for the increase at 6 h post infection. These results indicated that the increase of pectin recognised by 2F4 might be responsible for the non-pathogenicity of Foc1 on ‘Brazilian’ banana, and its decrease might be an element of the strong pathogenicity of Foc4.
In summary, we observed that the infection of Foc induced the varying degrees of PME activities, and the activity was lower level in banana inoculated with Foc1 than that with Foc4. The increase of enzyme activity might trigger the immune response induced by OG, and the excessive enzyme activity probably would result in the direct damage of cell wall. In addition, the banana infected with Foc1 showed higher abundance of highly DMs of HGs and relatively higher pectin DMs than that with Foc4. This result suggested that high abundance of highly DMs of HGs and relatively high pectin DMs might decrease the pathogenicity of Foc1. Therefore, the degrees of PME activities induced by pathogen and the level of highly DMs of pectin may contribute the observed difference in pathogenicity between Foc1 and Foc4. Thus, our observations provide new insights regarding how different pathogenic races in banana may differ in ability to cause disease.
Materials and Methods
Plant materials and pathogen inoculation
Musa spp. AAA cv. ‘Brazilian’ was used in our study. Banana plantlets were propagated under a sterile tissue culture condition. We previously performed inoculation experiments with Foc and confirmed that ‘Brazilian’ cultivar is susceptible to Foc4 and resistant to Foc147,48. Tissue-cultured plants, incubated at 28 ± 2 °C under cool-white light (20 μmol m−2 s−1) on a shaker at 90 rpm, were cultured in liquid rooting medium24. First, the roots were induced for two weeks. Second, one root of each seedling was cut off for pathogen penetration. Third, these treated plants were transferred to a new medium, which contained Foc1 or Foc4 at a final concentration of 5 × 102 ml−1 or contained OGs (elicityl-oligothch, France, DP: 10–15) at a final concentration of 20 μg ml−1. Seedlings immersed in the culture medium without the pathogen (mock inoculation) were used as a control. Afterwards, the infected roots were harvested at 6, 12, 24 and 48 h after the inoculation. All tissue samples were collected at the same time points to minimise differences. The control samples were also collected at the same time points after mock inoculation.
Enzyme activity assays
Samples (0.2 g), washed in distilled water, were homogenised in 1.5 ml of extraction solution (0.25 M NaCl, 0.1 M acetic acid–sodium acetate buffer, pH 5.0) at low temperature. The suspensions were centrifuged at 10000 rpm for 20 min at 4 °C. Titration of generated carboxyl groups, which is a modification of the method developed by Marcus and Schejter49, was used to determine the PME activity in protein extracts. Aliquots of the supernatant (0.3 ml) were added to 3 ml of PME substrate (1% citrus pectin). PME activity was measured at pH 6.5 via continuous automatic titration with 0.01 M NaOH of the carboxyl groups released during the enzyme reaction. The titration volume was used to determine generated carboxyl groups analytically. A PME activity unit was defined as the number of microequivalents of carboxyl groups cleaved by 1 mg of enzyme min−1 at 30 °C and pH 6.5.
Protein determination
Protein content was determined by the method of Bradford using the protein dye reagent (Sigma Chemical Co.). A calibration curve was made with bovine serum albumin (BSA)50.
Alcohol insoluble residue (AIR) preparation
Plant cell wall material was prepared according to the method described by Louvet et al.51 with some modification. Briefly, AIR preparation was carried out as follows: the roots were gently cut and washed thrice with 70% ethanol (v/v) at 70 °C for 30 min. After centrifugation, the supernatant was removed. The pellet was crushed in liquid nitrogen and freeze dried.
Measurement of pectin DMs with colorimetry
The DMs were determined via saponification of AIR and enzymatic oxidation of methanol released by alcohol oxidase. The DMs were calculated by the proportion of methanol content to galacturonic acid (GalA) molar content. The methanol assay was adopted from the methods described by Louvet et al.52 and Klavons and Bennett53. GalA content was determined by the metahydroxydiphenyl assay (MHDP) adapted from Blumenkrantz and Asboe-Hansen54. Three replicates were prepared for each treatment, and each experiment was repeated thrice. GalA was calculated as micrograms of GalA per gram of AIR.
Quantitative real-time PCR (RT-qPCR) assay for defence-related genes
Total RNA was extracted using the QIAGEN RNeasy plant mini kit (QIAGEN, CA) and treated with RNasefree DNAse I (Promega, USA). RNA was reverse transcribed in 20 μl of reaction system using the PrimeScriptTM RT Master Mix Kit (TaKaRa). The sequence of RPS2 gene was used as the reference gene51. Gene-specific primers designed based on the gene sequences using Primer 5.0 software. The primer sequences are listed in Table S1. The RT-qPCR was performed using the SYBR Premix Ex Taq Kit (TaKaRa) according to the manufacturer’s protocol. All RT-qPCR reactions were carried out using TP800 Thermal Cycler Dice (Takara, Japan). Standard curves based on threshold cycles for 10-fold dilution series of cDNA were obtained. A total of five 10-fold dilution steps of cDNA were run in triplicate on each well plate. PCR efficiency and correlation coefficient (R2) were calculated from the threshold cycles of these standard dilution steps. The reactions were run with primer pairs at annealing temperature of 57 °C. The conditions were as follows: initial holding at 95 °C for 30 s, followed by a two-step program of 95 °C for 15 s and annealing temperature for 30 s for 40 cycles. Each sample was analysed in three technical replicates. The relative changes in gene expression levels were calculated using the formula 2−ΔΔCT 55.
Immunofluorescence labeling
For immunolocalisation of PMEs and HGs with different DMs, the roots were treated with Foc1 and Foc4 for 6, 12, 24 and 48 h and harvested at the same time points to minimise the effect of other factors. The protocol for fixation, embedding of samples and immunolabelling was carried out as described by Xu et al.56. We labelled the sections with primary monoclonal antibodies PME, 2F4, LM19, LM20, JIM5 and JIM7. The anti-mouse lgG-FITC (F9006, Sigma) was used as secondary antibody for PME and 2F4 antibodies, and the anti-rat lgG-FITC (F6258, Sigma) was used for the other antibodies. Sections marked only with secondary antibodies were used as controls. At least three replicates were prepared for each antibody. Images were collected using a LSM 7 DUO laser scanning confocal microscope (ZEISS, Germany) and Olympus BH-2-FRCA microscope, and fluorescence signals were examined with LSM 7 DUO laser scanning confocal microscope (ZEISS, Germany) by the function “Histo” to determine an average fluorescence signal of a whole root section.
Real-time PCR amplification and quantification for abundance of Foc
Fungal mycelium and Foc-infected banana roots were immediately frozen in liquid nitrogen and ground to fine powder. Genomic DNA (gDNA) was extracted according to Lin et al.57, and the DNA samples were dissolved in 0.1× TE buffer (1 mM Tris-HCl, pH 8; 0.01 mM EDTA). In order to detect Foc1 and Foc4 isolates, a specific primer set Foc-1/Foc-2 (5′-CAGGGGATGTATGAGGAGGCT/5′-GTGACAGCGTCGTCTAGTTCC) was used for Foc457 and another specific primer set W1805F/W1805R (5′ GTTGAGTCTCGATAAACAGCAAT/5′GACGAGGGGAGATATGGTC) were used for Foc158. A 242-bp size fragment was produced by PCR from Foc4 gDNA and a 354-bp from Foc1 gDNA. The Foc fragments were gel-purified, cloned into pMDTM18-T vector (TAKARA), and sequenced. After plasmid purification, the plasmid DNA concentration was quantified using spectrophotometer (NANODROP ONE, USA). The copy number of standard plasmids was calculated according to plasmid (2692 bp) plus insert lengths (242 bp/354 bp) and assuming a molecular mass of 660 Da for a base pair. Standard DNA stock solutions of 1010 copies of plasmid µL− 1 were prepared. Ten-fold serial dilutions of the target DNA, ranging from 108 to 103 copies per reaction, were performed for standard curve plotting and melting-curve analysis of real-time PCR amplification and yielded linear and reliable results (correlation coefficient, R2 > 0.99).
Next, the real-time PCR assay was used to detect Foc in Foc-infected banana. The Foc-infected banana samples were surface-sterilized with 0.1% Clorox bleach for 1 min, rinsed with sterile water three times, and airdried. The surface-sterilized dried samples were picked for PCR amplification. The tested DNA samples were extracted from roots for RT-qPCR assays. When testing DNA samples, we used RPS2 gene as the reference gene to make samples to be normalized.
Statistical analysis
Statistical analyses were carried out using ANOVA by the statistical program SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA). At least three replicates were used in the experiments. Data are expressed as the mean ± SD. Multiple differences among means were evaluated using Duncan’s multiple range tests at a 5% probability level.
Electronic supplementary material
Acknowledgements
The study was supported by the earmarked fund for Modern Agro-industry Technology Research System (CAR-32-05) in China. PME monoclonal antibody was generated by Abmart (Shanghai, China) using purified total PME protein of banana. JIM antibodies and the other antibodies used in this work were from PlantProbe (Leeds University, UK). The authors sincerely thank Dr. Christopher M. Wallis of USDA-ARS SJVASC for his critical reading of the manuscript.
Author Contributions
H.F. carried out the enzyme activity assays, measurement of pectin DMs, RT-qPCR assay. H.F., H.D., C.X., J.L. and B.H., performed the Immunofluorescence labelling. H.D., J.Y. and G.M. prepared the all materials and carried out the inoculation of banana. C.X., H.F. and H.L. discussed the results and commented on the manuscript. H.L. and C.X. supervised all of the work.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-13625-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stover, R. H. Fusarial Wilt (Panama disease) of Bananas and Other Musa Species. (Commonwealth Mycological Institute UK, 1962).
- 2.Ploetz, R. C. & Pegg, K. G. Fungal diseases of root, corm and pseudostem. Diseases of Banana, Abaca, and Enset. (ed. Jones, D.R.) 143–172 (CABI Publishing, Wallingford, 2000).
- 3.Vorwerk S, Somerville S, Somerville C. The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci. 2004;9:203–209. doi: 10.1016/j.tplants.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Kars I, et al. Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris. The Plant Journal. 2005;43:213–225. doi: 10.1111/j.1365-313X.2005.02436.x. [DOI] [PubMed] [Google Scholar]
- 5.Wojtasik W, Kulma A, Kostyn K, Szopa J. The changes in pectin metabolism in flax infected with Fusarium. Plant Physiology and Biochemistry. 2011;49:862–872. doi: 10.1016/j.plaphy.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Pogorelko G, Lionetti V, Bellincampi D, Zabotina O. Cell wall integrity: targeted post-synthetic modifications to reveal its role in plant growth and defense against pathogens. Plant Signaling & Behavior. 2013;8:e25435. doi: 10.4161/psb.25435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovane A, et al. Pectin methylesterase inhibitor. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2004;1696:245–252. doi: 10.1016/j.bbapap.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Cantu D, Vicente AR, Labavitch JM, Bennett AB, Powell ALT. Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends Plant Sci. 2008;13:610–617. doi: 10.1016/j.tplants.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Lionetti V, et al. Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol. 2007;143:1871–1880. doi: 10.1104/pp.106.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bethke G, et al. Arabidopsis pectin methylesterases contribute to immunity against Pseudomonas syringae. Plant Physiol. 2014;164:1093–1107. doi: 10.1104/pp.113.227637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lionetti V, Raiola A, Cervone F, Bellincampi D. How do pectin methylesterases and their inhibitors affect the spreading of tobamovirus? Plant signaling & behavior. 2014;9:e972863. doi: 10.4161/15592316.2014.972863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aziz A, Heyraud A, Lambert B. Oligogalacturonide signal transduction, induction of defense-related responses and protection of grapevine against Botrytis cinerea. Planta. 2004;218:767–774. doi: 10.1007/s00425-003-1153-x. [DOI] [PubMed] [Google Scholar]
- 13.Raiola A, et al. Pectin methylesterase is induced in Arabidopsis upon Infection and is necessary for a successful colonization by necrotrophic pathogens. Mol. Plant Microbe. 2011;24:432–440. doi: 10.1094/MPMI-07-10-0157. [DOI] [PubMed] [Google Scholar]
- 14.Simon UK, Bauer R, Rioux D, Simard M, Oberwinkler F. The intercellular biotrophic leaf pathogen Cymadothea trifolii locally degrades pectins, but not cellulose or xyloglucan in cell walls of Trifolium repens. New Phytol. 2005;165:243–260. doi: 10.1111/j.1469-8137.2004.01233.x. [DOI] [PubMed] [Google Scholar]
- 15.Boudjeko T, et al. Loss of pectin is an early event during infection of cocoyam roots by Pythium myriotylum. Planta. 2006;223:271–282. doi: 10.1007/s00425-005-0090-2. [DOI] [PubMed] [Google Scholar]
- 16.Guo L, et al. Differential colonization patterns of bananas (Musa spp.) by physiological race 1 and race 4 isolates of Fusarium oxysporum f.sp cubense. J. Phytopathol. 2015;163:807–817. doi: 10.1111/jph.12378. [DOI] [Google Scholar]
- 17.Peng J, et al. Rapid and quantitative detection of Fusarium oxysporum f. sp cubense race 4 in soil by real-time fluorescence loop-mediated isothermal amplification. J. Appl. Microbiol. 2014;117:1740–1749. doi: 10.1111/jam.12645. [DOI] [PubMed] [Google Scholar]
- 18.Li B, et al. Development of a loop-mediated isothermal amplification assay for rapid and sensitive detection of Fusarium oxysporum f. sp. cubense race 4. Eur. J. Plant Pathol. 2013;135:903–911. doi: 10.1007/s10658-012-0136-9. [DOI] [Google Scholar]
- 19.Fraser-Smith S, et al. Sequence variation in the putative effector gene SIX8 facilitates molecular differentiation of Fusarium oxysporum f. sp cubense. Plant Pathol. 2014;63:1044–1052. doi: 10.1111/ppa.12184. [DOI] [Google Scholar]
- 20.Li C, et al. The use of GFP-transformed isolates to study infection of banana with Fusarium oxysporum f. sp cubense race 4. Eur J Plant Pathol. 2011;131:327–340. doi: 10.1007/s10658-011-9811-5. [DOI] [Google Scholar]
- 21.Peraza-Echeverria S, Dale JL, Harding RM, Smith MK, Collet C. Characterization of disease resistance gene candidates of the nucleotide binding site (NBS) type from banana and correlation of a transcriptional polymorphism with resistance to Fusarium oxysporum f.sp cubense race 4. Mol. Breeding. 2008;22:565–579. doi: 10.1007/s11032-008-9199-x. [DOI] [Google Scholar]
- 22.Akila R, et al. Combined application of botanical formulations and biocontrol agents for the management of Fusarium oxysporum f. sp. cubense (Foc) causing Fusarium wilt in banana. Biol. Control. 2011;57:175–183. doi: 10.1016/j.biocontrol.2011.02.010. [DOI] [Google Scholar]
- 23.Zacky FA, Ting ASY. Biocontrol of Fusarium oxysporum f. sp cubense tropical race 4 by formulated cells and cell-free extracts of Streptomyces griseus in sterile soil environment. Biocontrol Sci. Techn. 2015;25:685–696. doi: 10.1080/09583157.2015.1007921. [DOI] [Google Scholar]
- 24.Ma L, et al. Wound-induced pectin methylesterases enhance banana (Musa spp. AAA) susceptibility to Fusarium oxysporum f. sp. cubense. J. Exp. Bot. 2013;64:2219–2229. doi: 10.1093/jxb/ert088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clausen MH, Willats WGT, Knox JP. Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohyd Res. 2003;338:1797–1800. doi: 10.1016/S0008-6215(03)00272-6. [DOI] [PubMed] [Google Scholar]
- 26.Vandenbosch KA, et al. Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. The EMBO journal. 1989;8:335–341. doi: 10.1002/j.1460-2075.1989.tb03382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liners F, Letesson JJ, Didembourg C, Van Cutsem P. Monoclonal antibodies against pectin: recognition of a conformation induced by Calcium. Plant Physiol. 1989;91:1419–1424. doi: 10.1104/pp.91.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Have AT, Mulder W, Visser J, van Kan JAL. The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol. Plant Microbe Interact. 1998;11:1009–1016. doi: 10.1094/MPMI.1998.11.10.1009. [DOI] [PubMed] [Google Scholar]
- 29.Lang C, Dornenburg H. Perspectives in the biological function and the technological application of polygalacturonases. Appl. Microbiol. Biotechnol. 2000;53:366–375. doi: 10.1007/s002530051628. [DOI] [PubMed] [Google Scholar]
- 30.Wolf S, Mouille G, Pelloux J. Homogalacturonan methyl-esterification and plant development. Mol. Plant. 2009;2:851–860. doi: 10.1093/mp/ssp066. [DOI] [PubMed] [Google Scholar]
- 31.Lionetti V, Cervone F, Bellincampi D. Methyl esterification of pectin plays a role during plant–pathogen interactions and affects plant resistance to diseases. J. Plant Physiol. 2012;169:1623–1630. doi: 10.1016/j.jplph.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Bethke G, et al. Pectin biosynthesis is critical for cell wall integrity and immunity in Arabidopsis thaliana. The Plant Cell. 2016;28:537–556. doi: 10.1105/tpc.15.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lionetti V, et al. Cell wall traits as potential resources to improve resistance of durum wheat against Fusarium graminearum. BMC Plant Biol. 2015;15:6. doi: 10.1186/s12870-014-0369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.L’Enfant M, et al. Substrate specificity of plant and fungi pectin methylesterases: identification of novel inhibitors of PMEs. Int. J. Biol. Macromol. 2015;81:681–691. doi: 10.1016/j.ijbiomac.2015.08.066. [DOI] [PubMed] [Google Scholar]
- 35.Pelloux J, Rusterucci C, Mellerowicz E. New insights into pectin methylesterase structure and function. Trends Plant Sci. 2007;12:267–277. doi: 10.1016/j.tplants.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Ferrari, S. Oligogalacturonides: plant damage-associated molecular patterns and regulators of growth and development. Front Plant Sci. 4, 10.3389/fpls.2013.00049 (2013). [DOI] [PMC free article] [PubMed]
- 37.Cabrera JC, Boland A, Messiaen J, Cambier P, Van Cutsem P. Egg box conformation of oligogalacturonides: the time-dependent stabilization of the elicitor-active conformation increases its biological activity. Glycobiology. 2008;18:473–482. doi: 10.1093/glycob/cwn027. [DOI] [PubMed] [Google Scholar]
- 38.Osorio S, et al. Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca) The Plant Journal. 2008;54:43–55. doi: 10.1111/j.1365-313X.2007.03398.x. [DOI] [PubMed] [Google Scholar]
- 39.Marty P, Jouan B, Bertheau Y, Vian B, Goldberg R. Charge density in stem cell walls of Solanum tuberosum genotypes and susceptibility to blackleg. Phytochemistry (Oxford). 1997;44:1435–1441. doi: 10.1016/S0031-9422(96)00766-2. [DOI] [Google Scholar]
- 40.Zega A, Ovidio D. R. Genome-wide characterization of pectin methyl esterase genes reveals members differentially expressed in tolerant and susceptible wheats in response to Fusarium graminearum. Plant Physiol. Bioch. 2016;108:1–11. doi: 10.1016/j.plaphy.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 41.Moustacas AM, Nari J, Borel M, Noat G, Ricard J. Pectin methylesterase, metal ions and plant cell-wall extension. The role of metal ions in plant cell-wall extension. The Biochemical journal. 1991;279(Pt 2):351–354. doi: 10.1042/bj2790351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verhertbruggen Y, Marcus SE, Haeger A, Ordaz-Ortiz JJ, Knox JP. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohyd Res. 2009;344:1858–1862. doi: 10.1016/j.carres.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Digonnet C, et al. Deciphering the route of ralstonia solanacearum colonization in arabidopsis thaliana roots during a compatible interaction: focus at the plant cell wall. Planta. 2012;236:1419–1431. doi: 10.1007/s00425-012-1694-y. [DOI] [PubMed] [Google Scholar]
- 44.Vincent RR, Williams MAK. Microrheological investigations give insights into the microstructure and functionality of pectin gels. Carbohyd Res. 2009;344:1863–1871. doi: 10.1016/j.carres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Kim JS, Gao J, Daniel G. Ultrastructure and immunocytochemistry of degradation in spruce and ash sapwood by the brown rot fungus Postia placenta: characterization of incipient stages of decay and variation in decay process. Int. Biodeter Biodegr. 2015;103:161–178. doi: 10.1016/j.ibiod.2015.05.005. [DOI] [Google Scholar]
- 46.Bowling AJ, Vaughn KC, Hoagland RE, Stetina K, Boyette CD. Immunohistochemical investigation of the necrotrophic phase of the fungus Colletotrichum gloeosporioides in the biocontrol of hemp sesbania (Sesbania exaltata; Papilionaceae) AM. J. BOT. 2010;97:1915–1925. doi: 10.3732/ajb.1000099. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, et al. Development of a single-tube duplex real-time fluorescence method for the rapid quantitative detection of Fusarium oxysporum f. sp. cubense race 1 (FOC1) and race 4 (FOC4) using TaqMan probes. Crop Prot. 2015;68:27–35. doi: 10.1016/j.cropro.2014.11.004. [DOI] [Google Scholar]
- 48.Ting-Ting Bai, W. X. P. Z. & Jie Sun, X. R. H. L. Transcriptome and expression profile analysis of highly resistant and susceptible banana roots challenged with Fusarium oxysporum f. sp. cubense tropical race 4. Plos One. 8, 10.1371/journal.pone.0073945 (2013). [DOI] [PMC free article] [PubMed]
- 49.Marcus L, Schejter A. Single step chromatographic purification and characterization of the endopolygalacturonases. Physiological Plant Pathology. 1983;22:1–13. doi: 10.1016/0048-4059(83)90031-0. [DOI] [Google Scholar]
- 50.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 51.Chen L, et al. Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta. 2011;234:377–390. doi: 10.1007/s00425-011-1410-3. [DOI] [PubMed] [Google Scholar]
- 52.Louvet R, et al. Major changes in the cell wall during silique development in Arabidopsis thaliana. Phytochemistry. 2011;72:59–67. doi: 10.1016/j.phytochem.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Klavons JA, Bennett RD. Determination of methanol using alcohol oxidase and its application to methyl ester content of pectins. J. Agr. Food Chem. 1988;34:597–599. doi: 10.1021/jf00070a004. [DOI] [Google Scholar]
- 54.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 55.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 56.Xu, C., Takac, T., Burbach, C., Menzel, D. & Samaj, J. Developmental localization and the role of hydroxyproline rich glycoproteins during somatic embryogenesis of banana (Musa spp. AAA). BMC Plant Biol. 11, 38, 10.1186/1471-2229-11-38 (2011). [DOI] [PMC free article] [PubMed]
- 57.Lin Y, et al. Development of a molecular marker for specific detection of Fusarium oxysporum f. sp. cubense race 4. Eur J Plant Pathol. 2009;123:353–365. doi: 10.1007/s10658-008-9372-4. [DOI] [Google Scholar]
- 58.Li M, et al. Rapid detection and identification of Fusarium oxysporum f. sp. cubense race 1 and race 4. Scientia Agricultura Sinica. 2012;45:3971–3979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.