Abstract
We investigated the impact of six protein diets on oxidation and anti-oxidation status in the muscle of young rats. Rats were fed six protein diets for 14 days, including casein (control), and proteins isolated from soy, fish, chicken, pork and beef. Grx1, Trx1 and other oxidative metabolic indices in muscle were quantified. Compared with the casein diet, the soy protein diet had a similar oxidation level, but higher GSH and lower SOD activities. The chicken and fish protein groups had lower GSH and higher SOD activities, the pork protein group showed lower Grx1 levels than the casein group and the beef protein group showed the highest GSH, Grx1 and Trx1 levels as reflected by RT-PCR, Western blotting and immunohistochemistry analyses. Intake of meat proteins showed higher ROS and T-AOC but lower MDA levels than non-meat proteins, which may be due to the increase in Grx1 and Trx1 expression and other antioxidants. Meat proteins are more conducive to muscle of growing rats.
Introduction
Contracted skeletal muscle produces reactive oxygen species (ROS), which brings about muscle performance interference, including muscle lassitude and oxidative stress1. ROS is a class of highly reactive free radicals or molecules containing oxygen, including superoxides, peroxides, hydroxyl radicals and singlet oxygen2. High ROS level may have a substantial influence on cell structures which further leads to a significant damage. ROS plays an important role in cell signaling3, homeostasis4 and Parkinson’s disease5. ROS production is inevitable, and thus the cells have anti-oxidation defense system to control the ROS production6. The total antioxidant capacity (T-AOC) includes enzymatic and non-enzymatic antioxidants in cellular and extracellular environments7. One of the vital enzymatic antioxidants in skeletal muscle is superoxide dismutase (SOD). It can convert ROS to hydrogen peroxides, and then other enzymes convert hydrogen peroxides to water8. The reduced SOD activity would promote lipid peroxidation to produce malondialdehyde (MDA) in the presence of metal ions. MDA is toxic to the cells, which could be coupled with protein molecules and induce apoptosis9. In addition, glutaredoxin (Grx) and thioredoxin (Trx) also belong to the antioxidant systems that are different from the above enzymes. Grx and Trx are small peptides encoded by genes Grx1 and Trx1 respectively10. Cancer cells can survive and proliferate under high concentrations of ROS because they can produce elevated levels of Grx and Trx that have the ability to remove ROS11–13. Grx catalyzes the reduction of disulfides via reduced glutathione (GSH)10. There is an active site, disulfide bond in Grx1. It can be reduced or oxidized depending on the cellular level of ROS11. Trx scavenges ROS and reduces disulfide bonds to maintain redox homeostasis with thioredoxin reductase and NADPH12.
Diet intake promotes a major oxidative response at the cellular level, and further alters the metabolic status of an organ including muscles14. Many previous studies have focused the induction of high-fat diets to metabolic dysfunction by oxidative stress in skeletal muscles15–17. Recent studies focused more on dietary supplements for antioxidant use in skeletal muscles18–22. Much concern has been taken about the effect of high-protein diets on obesity23–25 and muscle strength enhancement26–28. However, little is known about the impact of dietary proteins from different sources on the oxidative status of muscles at a recommended intake. Intake of different dietary proteins may result in the difference in anti-oxidative status in vivo.
To explore how dietary proteins of different sources affect the oxidative status and its regulation in muscle, we fed young male rats for 7 and 14 days with six protein diets. The six proteins were casein and proteins from soy, fish, chicken, pork and beef. We, for the first time, to evaluate the effect of different diet proteins at a standard intake of 20% on oxidative status and antioxidant activities in rat muscle. To get robust comparisons, we set the casein diet as control, and the diet impact was compared on the basis of the ratios of the soy and meat proteins to the control.
Results
Diets had different impacts on oxidative status in rats muscle
On day 7 of feeding, the soy protein group as same as casein had significantly lower ROS level but higher MDA level than fish, chicken and pork protein groups (P < 0.05, Fig. 1A and C). Among four meat protein groups, the fish protein group showed the highest ROS value and the beef protein group was the lowest (P < 0.05, Fig. 1A). However, the opposite results were observed for the MDA values (P < 0.05, Fig. 1C). On day 14, all meat protein diets showed higher ROS level than the casein and soy protein groups (P < 0.05, Fig. 1B). However, all meat protein groups, in particular to pork and beef protein groups, had lower MDA levels (P < 0.05, Fig. 1D). The highest MDA value was observed for the soy protein group but the lowest was the pork protein group (P < 0.05, Table 1).
Figure 1.
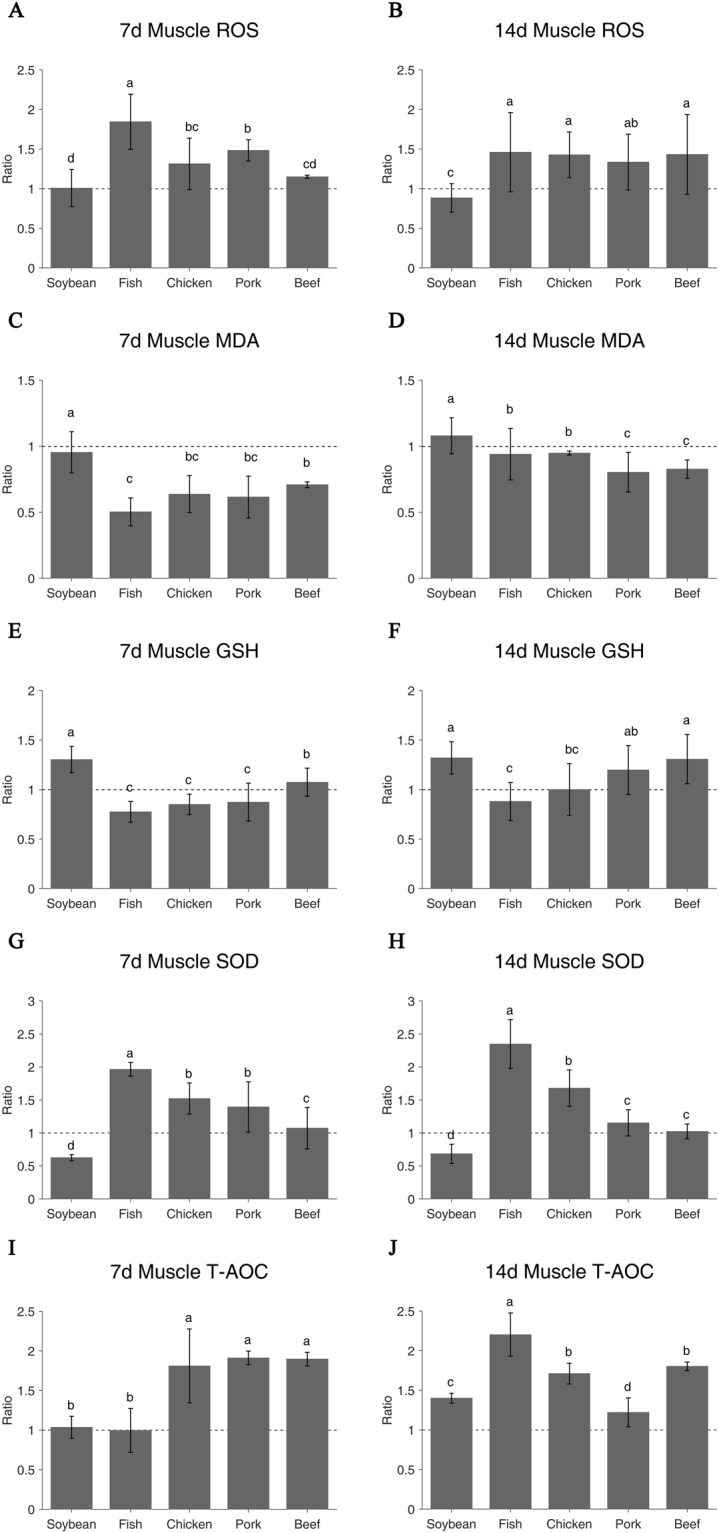
Oxidative status of different protein diets in muscle on day 7 and day 14. ROS (A,B), MDA (C,D), GSH (E,F), SOD (G,H) and T-AOC (I,J). Ratio of relative expression = Treatment group/Control group. Casein group is the control and the value is 1. The a/b/c/d indicates significant difference (P < 0.05).
Table 1.
Oxidation metabolic indices of six protein diets in muscle on day 7 and day 14.
| Protein diets | Casein | Soybean | Fish | Chicken | Pork | Beef | period |
|---|---|---|---|---|---|---|---|
| ROS | 48754 ± 18376 | 49151 ± 11391 | 89927 ± 16965 | 64066 ± 15843 | 72347 ± 6514 | 56104 ± 851 | 7d |
| F/mg pro | 46758 ± 14353 | 41330 ± 8391 | 68258 ± 23394 | 66701 ± 13512 | 62459 ± 16459 | 67025 ± 23591 | 14d |
| MDA | 4.743 ± 0.597 | 4.524 ± 0.742 | 2.387 ± 0.501 | 3.021 ± 0.663 | 2.915 ± 0.750 | 3.360 ± 0.105 | 7d |
| nmol/mg pro | 3.414 ± 0.632 | 3.685 ± 0.463 | 3.209 ± 0.664 | 3.236 ± 0.055 | 2.743 ± 0.512 | 2.822 ± 0.236 | 14d |
| GSH | 3.943 ± 0.082 | 5.140 ± 0.525 | 3.057 ± 0.413 | 3.356 ± 0.407 | 3.443 ± 0.755 | 4.240 ± 0.556 | 7d |
| μmol/g pro | 4.964 ± 0.392 | 6.555 ± 0.811 | 4.368 ± 0.951 | 4.971 ± 1.303 | 5.947 ± 1.225 | 6.493 ± 1.236 | 14d |
| SOD | 2.600 ± 0.259 | 1.623 ± 0.121 | 5.111 ± 0.268 | 3.957 ± 0.611 | 3.624 ± 0.993 | 2.788 ± 0.817 | 7d |
| U/mg pro | 2.245 ± 0.187 | 1.535 ± 0.325 | 5.271 ± 0.831 | 3.768 ± 0.619 | 2.587 ± 0.444 | 2.296 ± 0.253 | 14d |
| T-AOC | 0.141 ± 0.032 | 0.146 ± 0.020 | 0.141 ± 0.039 | 0.256 ± 0.066 | 0.270 ± 0.012 | 0.268 ± 0.012 | 7d |
| /mg pro | 0.184 ± 0.027 | 0.258 ± 0.012 | 0.406 ± 0.050 | 0.315 ± 0.024 | 0.225 ± 0.034 | 0.332 ± 0.010 | 14d |
Values are means ± SE; n = 8 per group.
Diets altered the antioxidant activities of GSH, SOD and T-AOC
After seven-day feeding, the soy protein group had higher GSH level than the casein and four meat protein groups (P < 0.05, Fig. 1E). The beef protein group showed the higher GSH activity than the four meat protein groups (P < 0.05). The 14-day GSH activities were in accordance with the 7-days results (Fig. 1F). On the contrary, the soy protein group showed the lowest SOD activity (P < 0.05, Fig. 1G) and among four meat protein groups, the SOD values were ranked as follows: fish > chicken and pork > beef (P < 0.05). The 14-day SOD results were consistent with the 7-day results (Fig. 1H). In terms of T-AOC, the soy and fish protein groups had the same T-AOC as the casein group (P > 0.05), while the other groups had much stronger T-AOC (P < 0.05, Fig. 1I). On day 14, T-AOC increased greatly in the soy and fish protein groups, but decreased in the pork protein group (Fig. 1J).
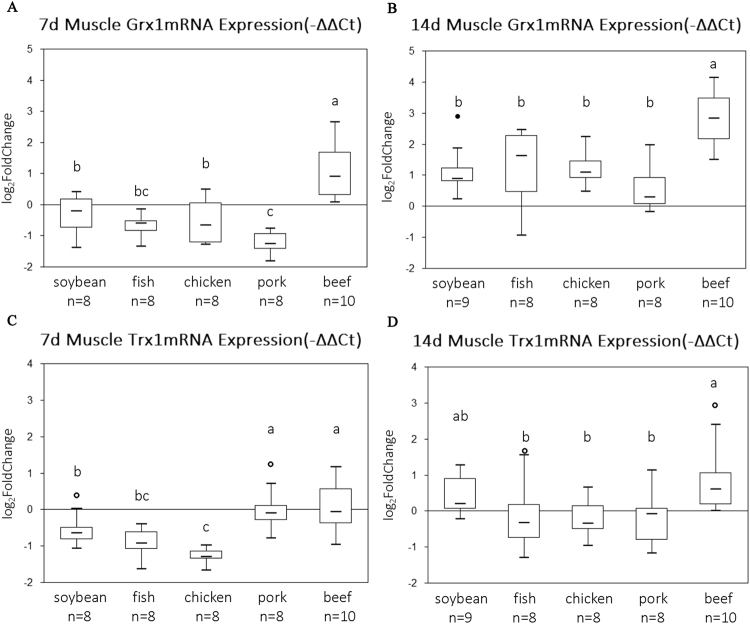
Grx1 and Trx1 mRNA showed significant responses to diets
On the mRNA level, the beef protein diet had the highest Grx1 value on day 7, while the lowest value was for the pork protein group (P < 0.05, Fig. 2A). On the day 14 of feeding, the highest Grx1 mRNA level was still for the beef protein group (P < 0.05, Fig. 2B), and there was no significant difference between any other diet groups (P > 0.05). The Trx1 mRNA levels in the pork and beef protein groups were the highest on day 7, but the lowest was for the chicken protein group (P < 0.05, Fig. 2C). On day 14, the highest value was still for the beef protein group (P < 0.05, Fig. 2D).
Figure 2.
The qRT-PCR data of Grx1 (A,B) and Trx1 (C,D) mRNA expression in rats muscle. The boxes represent the 25th through 75th percentiles, the horizontal lines represent the medians. The distance from the 25th to the 75th percentiles is the interquartile range (IQR). The hollow dot represents the outlier between 1.5 times and 3 times of IQR far away from 25th or 75th percentiles. The whiskers represent the minimum and maximum values except outliers. Casein group is the control and the value is 0. The a/b/c indicates significant difference (P < 0.05).
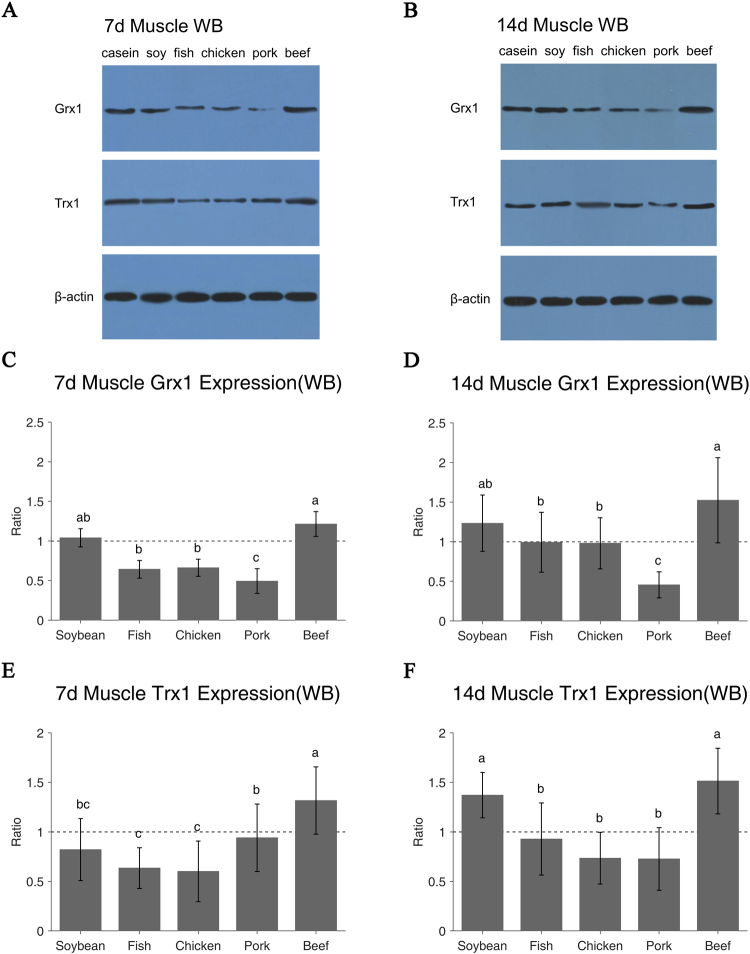
Grx1 and Trx1 proteins also showed significant responses
Western-blotting (WB) results (Fig. 3A and B) confirmed the RT-PCR results. The beef protein diet had higher Grx1 protein level than the casein and soy protein diets on day 7, while the intake of fish, chicken and pork proteins decreased the Grx1 levels (P < 0.05, Fig. 3C). On day 14, the Grx1 protein levels of all diets increased except that of the pork protein group. The beef protein group still showed the highest value but the pork protein group’s value was the lowest (P < 0.05, Fig. 3D). Intake of beef protein diet for 7 days resulted in the highest Trx1 protein level, but the soy, fish and chicken groups showed lower values as compared with the casein group (P < 0.05, Fig. 3E). After 14-day feeding, all diets increased the Trx1 protein levels except the pork protein group. The soy and beef protein groups showed the highest Trx1 protein expression (P < 0.05, Fig. 3F). There was no significant difference among fish, chicken and pork groups (P > 0.05, Fig. 3F).
Figure 3.
Grx1 and Trx1 protein expression of different protein diets in rats muscle on day 7 and day 14 were examined using Western blotting (A,B). β-Actin was used as reference. Relative expression of target protein = [Target protein (OD)/Reference protein(OD)] * 10, Ratio of relative expression = Treatment/Control. Casein group is control as 1 (C–F). Values are means ± SE; n = 8–10 per group. The a/b/c indicates significant difference (P < 0.05).
Immunohistochemical staining results of Grx1 and Trx1
Immunohistochemical (IHC) staining (Table 2) results further confirmed the results of RT-PCR and western blotting. The paraffin sections of each group indicated good quality of muscle tissues for IHC (Fig. 4A). Grx1 was observed in the muscle fibers (brownish yellow granules, Fig. 4C–F), but did not exist in negative control (Fig. 4B). Compared to the casein and soy protein groups, intake of beef protein diet induced stronger Grx1 staining, while weaker signals were observed in the fish, chicken and pork protein groups (P < 0.05, Fig. 4G). The pork and beef protein diets caused similar optical density (OD) values of Trx1 to the casein group (P > 0.05, Fig. 4I), while the soy, fish and chicken protein groups had lower OD values (P < 0.05, Fig. 4I). After 14-day feeding, the pork protein group had the lowest Grx1 IHC value (P < 0.05, Fig. 4H), while the beef protein group still had the highest OD values of Grx1 and Trx1 (P < 0.05, Fig. 4H and J). The white protein diets, fish and chicken, showed similar Grx1 and Trx1 IHC values (P > 0.05). Trx1 IHC value of the pork protein diet did not differ from the chicken and fish meat protein groups on day 14 (P > 0.05, Fig. 4J).
Table 2.
Grx1 and Trx1 IHC relative expression of six protein diets in rats muscle.
| Protein diets | Casein | Soybean | Fish | Chicken | Pork | Beef | period |
|---|---|---|---|---|---|---|---|
| Grx1 | 3.046 ± 0.261 | 3.046 ± 0.295 | 2.386 ± 0.253 | 2.364 ± 0.208 | 2.391 ± 0.224 | 3.757 ± 0.382 | 7d |
| OD | 3.086 ± 0.202 | 3.047 ± 0.167 | 3.111 ± 0.171 | 3.130 ± 0.159 | 2.376 ± 0.191 | 3.779 ± 0.243 | 14d |
| Trx1 | 3.718 ± 0.329 | 3.225 ± 0.345 | 2.628 ± 0.236 | 2.464 ± 0.297 | 3.765 ± 0.446 | 3.870 ± 0.434 | 7d |
| OD | 3.215 ± 0.170 | 3.581 ± 0.348 | 2.929 ± 0.338 | 2.944 ± 0.300 | 2.895 ± 0.210 | 4.088 ± 0.546 | 14d |
IHC relative expression of target protein = [Target protein(OD)] * 10, Values are means ± SE; n = 6 * 3 per group.
Figure 4.
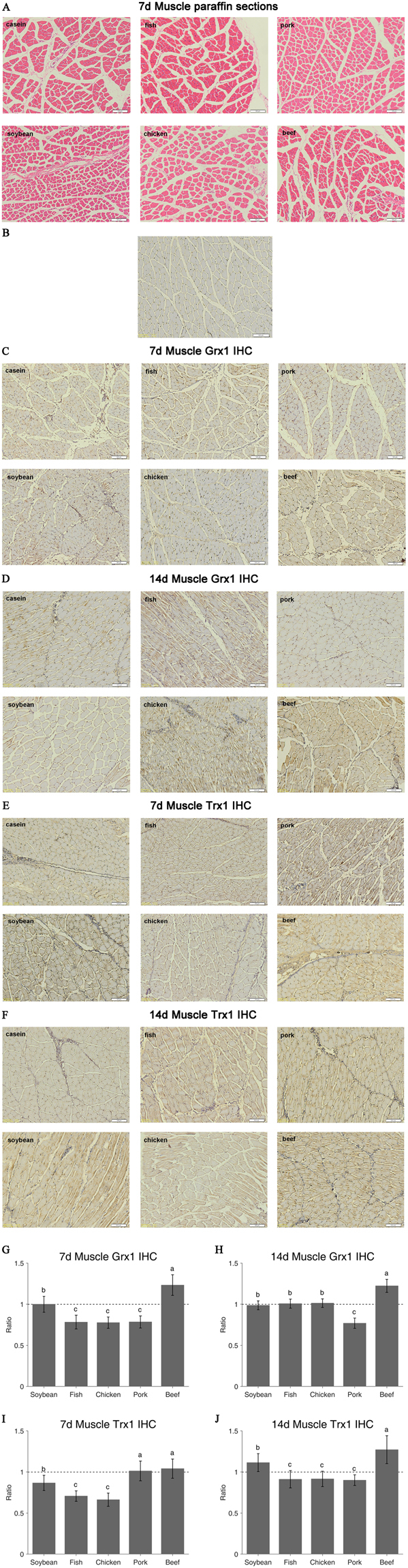
Paraffin sections of muscle from six diets for IHC (A, original magnification, *100); IHC staining of Grx1 and Trx1 in muscle on day 7 and day 14 are shown, including the negative slide without positive signal (B). The positive stains were yellow or brown signals and nuclei were stained blue with hematoxylin (C–F) and the OD of the yellow area was measured as semi-quantitative results (G–J). Ratio of relative expression = Treatment group/Control group. Casein group is the control and the value is 1. Values are means ± SE, n = 6 * 3 per group. The a/b/c indicates significant difference (P < 0.05).
Discussion
Intake of protein plays a certain role in the maintenance of metabolism balance in muscle29,30. During this process, ROS is produced. ROS accumulation would activate the enzymatic and non-enzymatic antioxidant responses31, including SOD8, GSH32 and antioxidant protein, Grx1 and Trx110. The total antioxidant system reduces ROS level and MDA generation. The T-AOC reflects a co-ordinate effect of the above antioxidant systems33.
Previous study showed that intake of meat proteins induced higher ROS level in rat muscle than the intake of casein and soy protein did. Meanwhile, SOD and T-AOC values were also higher in meat protein groups. One possible explanation for this is meat protein intake enhances muscle metabolism compared to intake of non-meat proteins. Previous research indicated that ROS may induce beneficial metabolic adaptations34. Recent studies indicated that ROS had a complex relationship with aging. Increasing ROS level in mitochondria can promote longevity, but high levels of ROS are toxic35. Contrary to ROS, as a marker of oxidative stress36 and oxidation end-product37, MDA was reduced by intake of meat proteins. It may be because meat proteins showed higher SOD levels than the casein and soy protein. Cells possess many pathways for neutralizing ROS, including a variety of SOD. Mice lacking SOD have high levels of oxidative damage in many tissues including skeletal muscle38. The SOD mRNA levels remained relatively stable in heat-stressed cells39, which may manifest that SOD results on day 14 of feeding were consistent with those of day 7 feeding. The fish protein group had the highest SOD level that may make a great contribution to T-AOC left in muscle. The beef and pork protein groups had higher GSH levels to protect against oxidative stress. GSH is a co-factor of several detoxifying enzymes and scavenges hydroxyl radical and singlet oxygen directly to reduce MDA40.
Meanwhile, Grxs play key roles in cellular redox regulation. Grx1 is diminished in the intermembrane space of mitochondria from aged heart41. Grx1 is also related to GSH, which glutaredoxins utilize the reducing power of GSH to maintain and regulate the cellular redox state and redox-dependent signaling pathways42. The fish and chicken protein groups had similar Grx1 levels based on western blotting and IHC results on day 14 of feeding since they have the similar GSH levels. On the other hand, Trxs have a remarkable number of functions in mammalian cells43 and are maintained independently from the GSH system. Moreover, Trx1 is required for the growth of the normal cells, while the Grx1 is not required44. The pork protein group had lower Grx1 and Trx1 levels than the casein group on the western blotting and IHC levels, which may cause lower T-AOC left in skeletal muscle. T-AOC could be useful to evaluate nutritional interventions for disease risk and prevention, including anti-aging strategies45. The beef protein group had much higher Grx1 and Trx1 expression and stronger T-AOC than the casein group. There may be a relevance between antioxidant indices and T-AOC values, but the measurement of T-AOC is considered as the cumulative effect of all the antioxidants in body, thus providing an integrated parameter rather than the simple sum of measurable antioxidants33. The 14-day intake had stronger gene expression and higher oxidation level than the 7-day intake, which indicates that a variety of dietary proteins are needed to avoid the damage caused by the long-term intake of a single dietary protein.
High quality diet protein is important for muscle growth46,47. Considering protein resources and costs, dietary protein supplements are always milk or plant proteins48,49, while animal protein would bring greater quantities of high-quality protein to people in developing countries50. Animal proteins, especially those from diets, seem to support better muscle protein synthesis than plant proteins51. The contents of leucine, isoleucine, phenylalanine + tyrosine, valine and lysine in soybean protein were significantly lower than those in these meat proteins52. The soy protein and casein diets for vegetarians showed no significant advantages for muscle health. As white meat, the fish and chicken protein groups showed similar results of oxidation and anti-oxitation in muscle because they had similar amino acid composition. And the chicken protein diet showed stable oxidative and anti-oxidative status from day 7 to day 14, which may be related the comprehensive and rich composition of amino acids according to amino acid score in previous publication52. However, beef protein was quite different from pork protein since they had different amino acid composition. The contents of leucine, lysine, isoleucine, phenylalanine + tyrosine, valine and serine in pork protein were significantly lower than those in beef protein. Previous studies showed that certain amino acids, including leucine, arginine and lysine, may play a critical role in optimizing efficiency of metabolic transformation to enhance muscle growth53. High dose of leucine may stimulate muscle protein synthesis and inhibit protein degradation in skeletal muscle54. Dietary l-arginine supplementation can increase muscle gain in growing-finishing pigs55. Lysine deficiency has been reported to reduce muscle mass56 and the activities of SOD in mice57. L-lysine treatment can enhance antioxidant activity by inhibiting the release of the inflammatory cytokine IL-6, which may involve upregulation of anti-inflammatory factors and subsequent downregulation of IL6. The fish protein group had the highest SOD activity, which could be due to the highest lysine concentration in the diet52.
In summary, we, for the first time, found that dietary meat proteins caused higher ROS production in young rat muscle than non-meat proteins and also higher antioxidant levels by increasing SOD activities, especially fish protein diet. As a result, the meat proteins had higher T-AOC but lower MDA levels. Meat proteins are more conducive to muscle of growing rats. The rats of beef protein diet group had the highest levels of Trx1 and Grx1 according to the RT-PCR, western blotting and IHC results, which is related to the highest GSH value of beef protein diet group. Nutritional benefits of mixtures of complementary protein sources are needed to avoid the damage caused by the long-term intake of a single dietary protein. The differences in oxidative status and antioxidant capacity may be related to the composition of amino acids in dietary proteins. Further work will be done to explore the underlying mechanism on how certain amino acids in diets regulate the oxidative status in muscle.
Materials and Methods
Diets
Diet were prepared by Jiangsu Xietong, Inc. (Nanjing, China) according to the formulation of AIN-93G diet58. The control diet was prepared by casein and the other five diets were made by replacing casein with purified proteins from soy, fish, chicken, pork and beef. The protein isolation was performed as previously described52.
Animals and sample collection
One hundred and twenty three-week-old male Sprague Dawley rats were bought from Shanghai Laboratory Animal Research Center (Shanghai, China) and housed in pairs at the Animal Center of Nanjing Agricultural University. All rats were kept in a pathogen-free environment. The procedures for care and use of animals were approved by the Ethics Committee of Nanjing Agricultural University [License No. SYXK 2011–0037] and all applicable institutional and governmental regulations concerning the ethical use of animals were followed. All rats were fed one-week control diet, and then randomly assigned to six protein diet groups (20 rats per group), i.e., casein diet (control), soy protein diet, fish protein diet, chicken protein diet, pork protein diet and beef protein diet. On day 7 and day 14, 10 rats from each diet group were decapitated and thigh muscles of posterior limb were collected, quick-frozen in liquid nitrogen and then stored at −80 °C.
Oxidation metabolic indices
Protein was quantified by the BCA Kit (A045-3). The oxidative status and antioxidant activities were quantified by ROS Kit (DCFH-DA, E004), MDA Kit (A003-1), T-AOC Kit (A015), GSH Kit (A006-2) and SOD Kit (A001-3), which were all from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). In brief, muscle sample (0.1 g) was homogenized in 0.9 ml saline at 4 °C, centrifuged at 2500 rpm for 10 min, kept the total protein supernatant at 4 °C. The absorbance at 562 nm was recorded by an Mze Microplate Spectrophotometer (MD, USA) for the total protein concentration (BCA Kit). For ROS, muscle sample (0.1 g) was homogenized in 1.9 ml, centrifuged at 1000 g for 10 min and the supernatant was kept. Protein concentration of the supernatant was quantified by the BCA Kit. A 190 μl of supernatant was mixed with 10 μl 1.0 mmol/L DCFH-DA working solution, and then incubated at 37 °C for 30 min. The fluorescence intensity was detected under excitation wavelength at 485 nm and emission wavelength at 525 nm. A blank control was used as PBS with working solution. The results were recorded as fluorescence intensity per milligram protein. For MDA, 50 μl 1% total protein supernatant was mixed with 1 ml working buffer, and incubated at 95 °C for 50 min. The reaction mixture was cooled down by running water and centrifuged at 4000 rpm for 10 min. The absorbance of the supernatant (200 μl) was measured at 532 nm. The blank control was 100% ethanol and the standard control was 10 nmol/ml reference solution. GSH was recorded as the absorbance at 405 nm. A blank control was used that did not contain sample, and a standard control was used that contained 20 μmol/L GSH. For SOD, 20 μl 10% total protein was mixed with 220 μl working solution, and incubated at 37 °C for 20 min, and then the absorbance was recorded at 450 nm. The value of T-AOC was measured as the absorbance at 520 nm. Double distilled water was used for a blank control.
RNA isolation and quantitative RT-PCR
Total RNA was isolated from muscles using the TAKARA MiniBEST Universal RNA Extraction Kit (No. 9767). The RNA concentration and quality were determined at 230 nm, 260 nm and 280 nm with a Nanodrop ND-2000 spectrophotometer (Thermo, Scientific, Waltham, MA). The samples were considered acceptable if A260/A280 and A260/A230 ratios were not smaller than 2.0. Then RNAs were reversely transcribed into cDNA using TAKARA PrimeScript Master Mix Kit (No. RR036A). All cDNAs were diluted to a minimum concentration of 111 ng/μl to serve as templates for subsequent PCR using the primers (Table 3). The quantitative melting curve of the target gene had one single peak, indicating that there was no nonspecific amplification and primer dimer formation in the amplification process59. RT-PCR was performed in QuantStudio™ 6 Flex Real-Time PCR System (Applied Biosystems, Foster, CA) with a total volume of 20 μl per well including 2 μl of the cDNA template and 18 μl of the TAKARA Master Mix Kit (No. RR420A). The results were normalized to the −ΔΔCT values of the casein group and a positive value indicated up-regulation on the mRNA level. The reference gene was β-actin. Four replicates were performed for each sample.
Table 3.
Primers for qRT-PCR.
| Gene | Primer sequence | Length |
|---|---|---|
| Trx-1 | Sense: 5′AGCCCTTCTTTCATTCCCTCTG3′ | 150 bp |
| (target) | Anti-Sense: 5′ACTCCCCAACCTTTTGACCCTT3′ | |
| Grx-1 | Sense: 5′TCAGTCTGGAAAGGTGGTCG3′ | 147 bp |
| (target) | Anti-Sense: 5′TCTTGAATCGCATTGGTGTTG3′ | |
| β-actin | Sense: 5′CCCATCTATGAGGGTTACGC3′ | 150 bp ordered |
| (reference) | Anti-Sense: 5′TTTAATGTCACGCACGATTTC3′ |
Primers were synthesized by the Shanghai Sangon Biotech Company (Shanghai, China). Primer-specific detection tools:http://www.ncbi.nlm.nih.gov/tools/primer-blast/.
Western Blotting
Muscle protein was extracted in chilled tissue protein extraction reagent (No. 78510, Thermo Pierce) with protease and phosphatase inhibitor cocktail (No. 78440, Thermo Pierce). Protein concentration was quantified by a BCA assay kit (No. P0010, Beyotime Biotech) and diluted to 10 μg/μl. Diluted protein mixture was mixed with an equal volume of loading buffer (Solarbio, Beijing, China) and heated at 95 °C for 5 min. Sixty micrograms of proteins were loaded on each well of 10% SDS polyacrylamide gel, and then separated at 80 V for 2 h. The gels were equilibrated for 30 min in ice cold transfer buffer, and then the proteins were transferred to PVDF membranes (IPVH00010, Millipore) at 100 V for 2 h at 4 °C. The PVDF membranes were soaked in methanol for 20 s and incubated in ice cold transfer buffer for 5 min. Membranes were blocked with 5% skim milk in TBS-T buffer (20 mM Tris, 137 mM NaCl, 5 mM KCl and 0.05% Tween 20) for 1 h and incubated in the primary antibodies (Table 4) overnight at 4 °C. The PVDF membranes were washed with TBS-T for four times (5 min/time), then incubated with the secondary antibodies (Table 4) for 1 h, which was followed by rinsing in TBS-T buffer for five times. The bands were detected by SuperSignal® West Dura Extended Duration Substrate (34075, Thermo Pierce) and ECL DualVue WB Marker (RPN810, GE). Finally, blots were detected and scanned with an ImageQuant LAS4000 (GE, Stockholm, Sweden). The band intensities were analyzed by the BandScan 5.0 software.
Table 4.
Antibodies for western blotting.
| 1st Antibody | Type | Dilution | kDa | |
|---|---|---|---|---|
| Grx1 | Abcam ab45953 | 1:500 | 12 | |
| Trx1 | Abcam ab26320 | 1:1500 | 12 | |
| β-actin | Santa Cruz SC-47778 | 1:1500 | 43 | |
| 2 nd Antibody | Type | Dilution | ||
| Goat anti-Mouse IgG (H + L) Secondary antibody | Thermo Pierce No: 31160 | 1:5000 | ||
| Goat anti-Rabbit IgG (H + L) Secondary antibody | Thermo Pierce No: 31210 | 1:5000 | ||
H & E staining
Muscle samples were fixed in 4% paraformaldehyde at 4 °C for 1 h, and then transferred to room temperature for 12 h. The fixed tissues were dehydrated by ethanol and xylene, and then embedded in paraffin. Cross-sections (7 μm) of fiber were cut and deparaffinized in xylene and then dehydrated in a graded series of ethanol. All sections were stained by hematoxylin-eosin (H & E) then examined by a microscope (BH-2, Olympus, Tokyo, Japan). Each muscle sample had three replicates and six visual fields.
Immunohistochemistry
After deparaffinization in xylene and dehydration in ethanol, the transverse muscle sections (7 μm) were washed with PBS (8 g NaCl, 0.2 g KCl, 1.44 g K2HPO4 and 0.24 g KH2PO4, pH = 7.4) for twice (5 min/time). After incubation with 10 mM citrate buffer (Beyotime, Nantong, China) for antigen unmasking, the sections were incubated in 3% H2O2 for 10 min to minimize endogenous peroxidase activity60, washed in PBS and then blocked in 10% goat serum (Solarbio, Beijing, China) for 10 min at room temperature. The sections were incubated with the primary antibodies of Grx1 (1 μg/ml, ab45953, Abcam, UK) and Trx1 (1:200, ab86255, Abcam, UK) in PBS overnight at 4 °C, and stored at 37 °C for 45 min. The PBS replaced primary antibody as the negative control. After twice rinse in PBS, the sections were incubated by biotinylated goat anti-rabbit antibody (1:1000, ab6721, Abcam, UK) at 37 °C for 30 min. After twice washes in PBS, the sections were treated with DAB Horseradish Peroxidase Color Development Kit (Jiancheng, Nanjing, China) and then treated by hematoxylin. Each slide had 6 visual fields for statistical analysis. The mean OD was used as a parameter for quantification by using ImageJ61. Images were transferred gray scale values to OD for each pixel and then a color threshold segmentation approach was implemented for selecting an area of interest. Finally, the mean OD was calculated through integrated OD within the region divided by a measure of the selected area.
Statistical analyses
All statistical analyses were performed by SAS (version 9.2, Statistics Analysis System). The effects of diet on measured variables were analyzed by ANOVA and means were compared by Duncan’s multiple range test method for multiple comparisons. Statistical significance was set at P < 0.05. Values were shown as means and standard error (SE).
Acknowledgements
This work was funded by grants 31471600 and 31530054 (National Natural Science Foundation of China). We would like to thank Dr. Yingqiu Li, Dr. Fan Zhao and Mengjie Li from Nanjing Agricultural University for their helpful suggestions in implementing the experiments.
Author Contributions
G.Z., C.L. and J.Z.: designed research; J.Z., H.Q., S.S. and X.L.: conducted research; J.Z., Z.G. and C.L.: analyzed data; J.Z., C.L., X.Y. and Z.G.: wrote the paper; G.Z. and C.L.: primary responsibility for final content. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Guanghong Zhou, Email: guanghong.zhou@hotmail.com.
Chunbao Li, Email: chunbao.li@njau.edu.cn.
References
- 1.Reid MB, et al. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J. Appl. Physiol. 1992;73:1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- 2.Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr. Pharm. Design. 2004;10:1611–1626. doi: 10.2174/1381612043384664. [DOI] [PubMed] [Google Scholar]
- 3.Martin KR, Barrett JC. Reactive oxygen species as double-edged swords in cellular processes: low-dose cell signaling versus high-dose toxicity. Hum. Exp. Toxicol. 2002;21:71–75. doi: 10.1191/0960327102ht213oa. [DOI] [PubMed] [Google Scholar]
- 4.Shi SY, et al. DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice. Nat. Commun. 2015;6:1–10. doi: 10.1038/ncomms8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang AH, Chung KK. Oxidative and nitrosative stress in Parkinson’s disease. BBA-Mol. Basis. Dis. 2009;1792:643–650. doi: 10.1016/j.bbadis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Quindry, J. C., Kavazis, A. N. & Powers, S. K. Exercise-induced oxidative stress: Are supplemental antioxidants warranted. Sports Nutr. 263-276 (2014).
- 7.Halliwell, B. & Gutteridge, J. M. Free radicals in biology and medicine. (Oxford University Press, USA 2015).
- 8.Lawler JM, Song W. Specificity of antioxidant enzyme inhibition in skeletal muscle to reactive nitrogen species donors. Biochem. Biophys. Res. Commun. 2002;294:1093–1100. doi: 10.1016/S0006-291X(02)00602-2. [DOI] [PubMed] [Google Scholar]
- 9.Franco R, Sánchez-Olea R, Reyes-Reyes EM, Panayiotidis MI. Environmental toxicity, oxidative stress and apoptosis: menage a trois. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009;674:3–22. doi: 10.1016/j.mrgentox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid. Redox sign. 2008;2:811–820. doi: 10.1089/ars.2000.2.4-811. [DOI] [PubMed] [Google Scholar]
- 11.Peltoniemi MJ, et al. Modulation of glutaredoxin in the lung and sputum of cigarette smokers and chronic obstructive pulmonary disease. Resp. Res. 2006;7:133–143. doi: 10.1186/1465-9921-7-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powis G, Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Curr. Opin. Pharmacol. 2007;7:392–397. doi: 10.1016/j.coph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Qu Y, et al. Thioredoxin-like 2 regulates human cancer cell growth and metastasis via redox homeostasis and NF-κB signaling. J. Clin. Invest. 2011;121:212–225. doi: 10.1172/JCI43144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamanti-Kandarakis E, Papalou O, Kandaraki EA, Kassi G. MECHANISMS IN ENDOCRINOLOGY: Nutrition as a mediator of oxidative stress in metabolic and reproductive disorders in women. Eur. J. Endocrinol. 2017;176:R79–R99. doi: 10.1530/EJE-16-0616. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, et al. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Natl. Acad. Sci. USA. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sparks LM, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 17.Bonnard C, et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Invest. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulford J, et al. Influence of dietary nitrate supplementation on human skeletal muscle metabolism and force production during maximum voluntary contractions. Pflugers Arch. 2013;465:517–528. doi: 10.1007/s00424-013-1220-5. [DOI] [PubMed] [Google Scholar]
- 19.Jia H, et al. Coffee intake mitigated inflammation and obesity-induced insulin resistance in skeletal muscle of high-fat diet-induced obese mice. Genes Nutr. 2014;9:1–10. doi: 10.1007/s12263-014-0389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musumeci G, Trovato FM, Imbesi R, Castrogiovanni P. Effects of dietary extra-virgin olive oil on oxidative stress resulting from exhaustive exercise in rat skeletal muscle: A morphological study. Acta histochem. 2014;116:61–69. doi: 10.1016/j.acthis.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Philp LK, Heilbronn LK, Janovska A, Wittert GA. Dietary enrichment with fish oil prevents high fat-induced metabolic dysfunction in skeletal muscle in mice. PLoS One. 2015;10:e0117494. doi: 10.1371/journal.pone.0117494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julien SG. Narciclasine attenuates diet-induced obesity by promoting oxidative metabolism in skeletal muscle. PLoS Biol. 2017;15:e1002597. doi: 10.1371/journal.pbio.1002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pesta DH, Samuel VT. A high-protein diet for reducing body fat: mechanisms and possible caveats. Nutr. Metab. (Lond). 2014;11:53. doi: 10.1186/1743-7075-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright C, Zhou J, Sayer R, Kim JE, Campbell W. Effect of a high protein, high egg diet on muscle composition, metabolic health and systemic inflammation in overweight and obese, older adults. FASEB J. 2015;29:270.6. [Google Scholar]
- 25.Verreijen AM, et al. Effect of a high protein diet and/or resistance exercise on the preservation of fat free mass during weight loss in overweight and obese older adults: a randomized controlled trial. Nutr. J. 2017;16:10. doi: 10.1186/s12937-017-0229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasiakos SM, et al. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: a randomized controlled trial. FASEB J. 2013;27:3837–3847. doi: 10.1096/fj.13-230227. [DOI] [PubMed] [Google Scholar]
- 27.Daly RM, et al. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: a cluster randomized controlled trial. Am. J. Clin. Nutr. 2014;99:899–910. doi: 10.3945/ajcn.113.064154. [DOI] [PubMed] [Google Scholar]
- 28.Antonio J, Ellerbroek A, Silver T, Vargas L, Peacock C. The effects of a high protein diet on indices of health and body composition-a crossover trial in resistance-trained men. J. Int. Soc. Sports Nutr. 2016;13:3. doi: 10.1186/s12970-016-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshizawa F, Kimball SR, Vary TC, Jefferson LS. Effect of dietary protein on translation initiation in rat skeletal muscle and liver. Am. J. Physiol. Endocrinol. Metab. 1998;275:E814–E820. doi: 10.1152/ajpendo.1998.275.5.E814. [DOI] [PubMed] [Google Scholar]
- 30.Paddon-Jones D, et al. Protein, weight management, and satiety. Am. J. Clin. Nutr. 2008;87:1558S–1561S. doi: 10.1093/ajcn/87.5.1558S. [DOI] [PubMed] [Google Scholar]
- 31.Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/S0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 32.Generally GSH. Multiple roles of glutathione in the central nervous system. Biol. Chem. 1997;378:793–802. [PubMed] [Google Scholar]
- 33.Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic. Biol. Med. 2000;29:1106–1114. doi: 10.1016/S0891-5849(00)00394-4. [DOI] [PubMed] [Google Scholar]
- 34.Mason S, Wadley GD. Skeletal muscle reactive oxygen species: a target of good cop/bad cop for exercise and disease. Redox Rep. 2014;19:97–106. doi: 10.1179/1351000213Y.0000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Raamsdonk JM. Levels and location are crucial in determining the effect of ROS on lifespan. Worm. 2015;4:e1094607. doi: 10.1080/21624054.2015.1094607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Moore K, Roberts LJ. Measurement of lipid peroxidation. Free Radic. Res. 1998;28:659–671. doi: 10.3109/10715769809065821. [DOI] [PubMed] [Google Scholar]
- 38.Wanagat J, Ahmadieh N, Bielas JH, Ericson NG, Van Remmen H. Skeletal muscle mitochondrial DNA deletions are not increased in CuZn-superoxide dismutase deficient mice. Exp. Gerontol. 2015;61:15–19. doi: 10.1016/j.exger.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kikusato M, Yoshida H, Furukawa K, Toyomizu M. Effect of heat stress-induced production of mitochondrial reactive oxygen species on NADPH oxidase and heme oxygenase-1 mRNA levels in avian muscle cells. J. Therm. Biol. 2015;52:8–13. doi: 10.1016/j.jtherbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Valko M, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Gao XH, et al. Aging-dependent changes in rat heart mitochondrial glutaredoxins implications for redox regulation. Redox Biol. 2013;1:586–598. doi: 10.1016/j.redox.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim. Biophys. Acta. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Holmgren A, et al. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem. Soc. Trans. 2005;33:1375–1377. doi: 10.1042/BST0331375. [DOI] [PubMed] [Google Scholar]
- 44.Trotter EW, Grant CM. Non-reciprocal regulation of the redox state of the glutathione-glutaredoxin and thioredoxin systems. EMBO Rep. 2003;4:184–188. doi: 10.1038/sj.embor.embor729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kusano C, Ferrari B. Total antioxidant capacity: a biomarker in biomedical and nutritional studies. J. Cell Mol. Biol. 2008;7:1–15. [Google Scholar]
- 46.Moore DR, Camera DM, Areta JL, Hawley JA. Beyond muscle hypertrophy: why dietary protein is important for endurance athletes1. Appl. Physiol. Nutr. Metab. 2014;39:987–997. doi: 10.1139/apnm-2013-0591. [DOI] [PubMed] [Google Scholar]
- 47.Reidy PT, et al. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J. Nutr. 2013;143:410–416. doi: 10.3945/jn.112.168021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friedman M. Nutritional value of proteins from different food sources. A review. J. Agric. Food Chem. 1996;44:6–29. doi: 10.1021/jf9400167. [DOI] [Google Scholar]
- 49.Davis J, Sonesson U, Baumgartner DU, Nemecek T. Environmental impact of four meals with different protein sources: case studies in Spain and Sweden. Food Res. Int. 2010;43:1874–1884. doi: 10.1016/j.foodres.2009.08.017. [DOI] [Google Scholar]
- 50.Wedin WF, Hodgson HJ, Jacobson NL. Utilizing plant and animal resources in producing human food. J. Anim. Sci. 1975;41:667–686. doi: 10.2527/jas1975.412667x. [DOI] [Google Scholar]
- 51.Gilbert JA, Bendsen NT, Tremblay A, Astrup A. Effect of proteins from different sources on body composition. Nutr. Metab. Cardiovasc. Dis. 2011;21:B16–B31. doi: 10.1016/j.numecd.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Song S, et al. Distinct physiological, plasma amino acid, and liver transcriptome responses to purified dietary beef, chicken, fish, and pork proteins in young rats. Mol. Nutr. Food Res. 2016;60:1199–1205. doi: 10.1002/mnfr.201500789. [DOI] [PubMed] [Google Scholar]
- 53.Wu G. Amino acids: metabolism, functions, and nutrition. Amino acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 54.Garlick PJ. The role of leucine in the regulation of protein metabolism. J. Nutr. 2005;135:1553S–1556S. doi: 10.1093/jn/135.6.1553S. [DOI] [PubMed] [Google Scholar]
- 55.Tan B, et al. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino acids. 2009;37:169–175. doi: 10.1007/s00726-008-0148-0. [DOI] [PubMed] [Google Scholar]
- 56.Rivera-Ferre MG, Aguilera JF, Nieto R. Muscle fractional protein synthesis is higher in Iberian than in Landrace growing pigs fed adequate or lysine-deficient diets. J. Nutr. 2005;135:469–478. doi: 10.1093/jn/135.3.469. [DOI] [PubMed] [Google Scholar]
- 57.Al-Malki AL. Suppression of acute pancreatitis by L-lysine in mice. BMC Complement. Altern. Med. 2015;15:193. doi: 10.1186/s12906-015-0729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reeves PG, Nielsen FH, Fahey GC., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 59.Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006;1:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- 60.Li M, et al. Meat proteins had different effects on oligopeptide transporter PEPT1 in the small intestine of young rats. Int. J. Food Sci. Nutr. 2016;67:995–1004. doi: 10.1080/09637486.2016.1210574. [DOI] [PubMed] [Google Scholar]
- 61.Rasband, W. S. & ImageJ. US National Institutes of Health, Bethesda, MD (1997).