Abstract
The rapid development of synthetic biology has conferred almost perfect modification on single cells, and provided methodological support for synthesizing microbial consortia, which have a much wider application potential than synthetic single cells. Co-cultivating multiple cell populations with rational strategies based on interacting relationships within natural microbial consortia provides theoretical as well as experimental support for the successful obtaining of synthetic microbial consortia, promoting it into extensive research on both industrial applications in plenty of areas and also better understanding of natural microbial consortia. According to their composition complexity, synthetic microbial consortia are summarized in three aspects in this review and are discussed in principles of design and construction, insights and methods for analysis, and applications in energy, healthcare, etc.
Keywords: Synthetic microbial consortium, Single/two/multiple species
1. Introduction
With the rapid development of synthetic biology, designing and constructing synthetic microbial consortia has raised extensive attention, becoming one of the important frontiers for the second wave of synthetic biology,1 but yet to be an important aspect of in-depth research.2 As summarized by Ron Weiss and Cynthia Collins, there are three advantages of taking microbial consortia as the research object to engineer specific routes: (1) different strains are functionally divided to fulfill many complex tasks at the same time; (2) relationships between cells are dynamically balanced, leading to stronger adaptability and stability to the fluctuant environment; (3) elements and modules from different sources and with different functions can be built in different strains, reducing the metabolic load on single chassis as well as avoiding the cross-influence of different functions.3, 4
There are mainly two ways for designing and constructing synthetic microbial consortia. The first one is to re-engineer naturally occurring microbial consortia, which is a top-down method.5 That is, based on multiple omics analysis,6, 7, 8, 9, 10, 11, 12 starting from the macroscopic microbial consortia, parsing the system principles, to explore the molecular mechanisms for the maintained systems. The other one is to design and construct artificial microbial consortia, which is a bottom-up method.5 That is, based on the genetic elements, modules, circuits and metabolic pathways or networks,13, 14, 15, 16 with the rational guidance of engineering principles, to obtain microbial consortia with higher efficiency, stability and controllability. Considering the complexity and practicability of synthetic biology, currently the bottom-up method is the mostly used for constructing microbial consortia from simple to complicated. Moreover, about the synthetic systems, there are different statements on the concept: co-cultures,17, 18 mixed cultures,19 microbial consortia,4, 20 and so on. Considering that the phrase “microbial consortia” indicates not only living together but also labor division, and covers all of conditions of their composition: by single, two, and multiple species,2, 6, 21 we use “microbial consortia” in this review.
This review summarized current synthetic microbial consortia reported in literature from three aspects according to their composition complexity (composed of single species, two species or multiple species) and then discussed their design and construction strategies based on the interactions within microbial communities, their mechanism analysis methods, as well as their applications in many fields such as medicine and energy, etc.
2. Synthetic microbial consortia composed of single species
Research on synthetic microbial consortia composed of single species mainly focuses on pattern microbes, such as Escherichia coli and Saccharomyces cerevisiae, which have clear genetic backgrounds and mature molecular technologies. It mainly focuses on cell–cell communications and interaction analysis within the ecosystems.
2.1. Design and construction of synthetic microbial consortia composed of single species
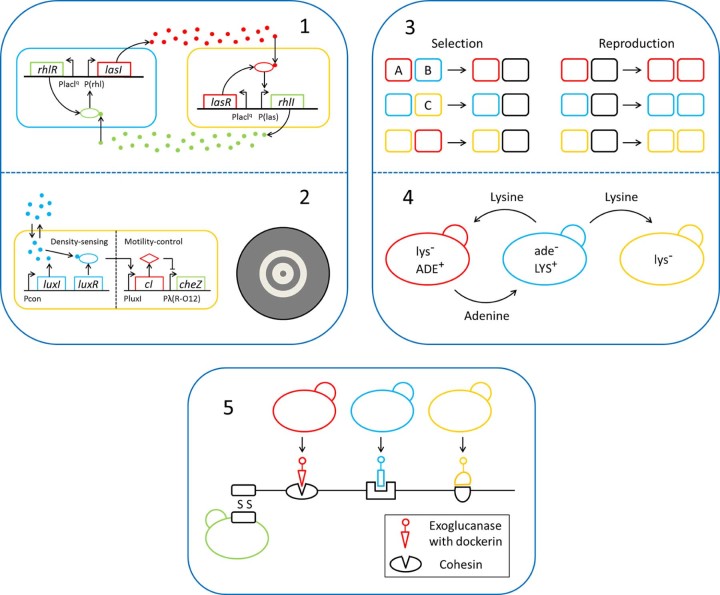
One of the basic cell–cell communications for constructing synthetic microbial consortia is quorum sensing (QS). The key of QS is some signaling molecules known as autoinducers, which diffuse from intracellular to extracellular. When reaching a certain threshold (usually in high cell concentration), they trigger or coordinate the expression of certain genes. Except communications within populations, one-way interactions between populations such as a pulse-generator system,22 a pattern formation programming system,23 and a sender–receiver communication network24 are equally employed. Besides, there are two-way interactions. Brenner et al.21 constructed a consortium with LasR/LasI and RhlR/RhlI QS systems in which gene-expression response if and only if both populations are present over a threshold cell densities (Fig. A1). These are all the basic interaction modes between microorganisms, revealing the molecular mechanisms, which provide the basis for the design of synthetic microbial consortia.
Fig. A.
Design, analysis and application of synthetic microbial consortia composed of single species. (1) A bidirectional QS system. (2) A spatio-temporal system coupled QS and density-sensing module. (3) Local reactions of designed rock–paper–scissors relationship. (4) Interactions of a cooperator–cheater system. (5) A minicellulosome yeast consortium.
Part 1 is adapted by permission from Proceedings of the National Academy of Sciences21 ©. Part 2 is adapted by permission from Science30 ©. Part 3 is adapted by permission from Macmillan Publishers Ltd: Nature43 ©. Part 4 is adapted by permission from Proceedings of the National Academy of Sciences45 ©. Part 5 is adapted by permission from Microbial Cell Factories49 ©.
The most well studied form of QS is spatio-temporal, by which population density is coupled with some special module, with the help of a fluorescent protein, the consortia is periodically distributed.25, 26, 27, 28, 29 For example,30 the coupling of LasR/LasI as a density-sensing module, and coupling it with motility-control modules in E. coli could command population behavior: high cell density stopped the motility while low cell density drove the movement, according to the density difference, light and dark circular pattern were gradually formed (Fig. A2).
Recently, QS has been developed with more complicated population behavior and more novel control. Using the LasR/LasI and two dispersal proteins, a colonizer–disperser consortium is designed to control the biofilm breakdown, movement and formation.31 Combining the LuxR/LuxI with a CcdA/CcdB toxin–antitoxin module, a typical Allee effect was constructed, which caused a tradeoff between population spread and survival.32 Payne et al.33 broke the traditional spatial cue for pattern formation which depended on morphogen gradient, using the morphogen served as a timing cue to trigger the formation and maintenance of the ring patterns.
Of course, QS is not the only principle for designing the communities. Based on nutrition complementarity, microbes can form symbiosis relationship, such as consortium composed of isoleucine auxotroph and leucine auxotroph, strains not only acquired what they lacked, but also over-supplied amino acids required for partnerstrains.34 On the basis of a stable system, further regulating the expression of genes encoded with amino acids could tune the metabolite exchanges, which changed the growth rate and ratio of the two strains in a wide range.35
Due to the relatively low complexity of single-species microbial consortia, it is more suitable for further mechanisms study, with the design and construction principle getting more and more systematic. However, single-species microbial consortia are limited to E. coli and S. cerevisiae. Besides, the universality of existing principles in other species still needs to be examined.
2.2. Analysis of synthetic microbial consortia composed of single species
Built ecosystems, as the mimic of natural ecosystems, demonstrated diverse relationships, such as mutualism, commensalism, parasitism or predation, competition, amensalism, and neutralism,5 promoting a better understanding of natural microbial consortia.
Balagadde et al.36 engineered a new predator–prey ecosystem, which is different from canonical predator–prey system in two aspects: first, the prey was an “antidote” rather than a food source; second, there was competition between predator and prey for nutrients, thus extinction, coexistence and oscillatory dynamics could be achieved under different conditions. Hu et al.37 constructed a symbiotic E. coli ecosystem, in which the two members had resistance to ampicillin and kanamycin (Kan), and they showed a variety of subtle relationships at the different concentration of antibiotics. For example, with ampicillin at 4 mg/mL and kanamycin at 1 mg/mL, they rely on each other's resistance, but KanR with high copy number have stronger degradation to antibiotics, thus dominating in the consortia.
However, in nature, even if a variety of interactions coexist, not every kind of interaction can be understood. To clarify the mechanism of cross-feeding, which is one of the common principles in ecosystems, many efforts have been made. Shou et al.38 constructed a synthetic obligatory cooperative system, finding that the consistently increased its ability to survive, but the mechanisms underlying the stable cooperation were unknown. To make it clear, Zhang et al.39 engineered a leucine (L) and lysine (K) auxotrophic community, and set the initial inoculation ratio to 1:1. Whenever OD reached 0.2 or so, the consortia would be inoculated into fresh medium for evolution, resulting a faster growth rate and lower mortality and improving the stability greatly. That was attributed to a virtuous circle: when L(K) had a higher productivity than K(L), K(L) dominated the population, correspondingly released more leucine, and therefore increased the proportion of L(K). Further, Pande et al.40 studied the long-lasting cross-feeding interactions of non-cooperating auxotrophic E. coli consortium, finding a speedup growth rate under competition condition. The reason was that the fitness cost of overproducing amino acids was less compared to being provided by their partner. Even though the ratio reached 1:100, the weak strain wouldn't die but gradually restored to 1:1. At the same time, a much more difficult work was done by Mee et al.,41 they devised a series of synthetic syntrophic communities (up to 14 members) to probe the complex interactions, finding that the amino acid biosynthesis has been broadly optimized to reduce individual metabolic burden, thus costly amino acid and common intermediates along branching pathways promoted stronger cooperative interactions.
On one hand, construction mechanism of ecosystem is gradually parsing and its capacity to endure fluctuant environment is stronger and stronger; on the other hand, why the biodiversity and stability is maintained is worth exploring, too. Kerr et al.42 synthesized a three-member E. coli ecosystem, in which there were resistant cells (R), colicinogenic cells (C) and sensitive cells (S). According to the design, S could displace R, R could displace C (in both cases the former has a growth-rate advantage) and C could displace S (C kills S). The rock–paper–scissors relationship maintained just under static and localized growth condition. Once mixed together, only R lived to the end. The phenomenon is interesting, but the reasons are not clear. Few years later, Reichenbach et al.43 did a deeper research on it in combination with spatial dispersal of static populations. Three members were arranged on a spatial lattice (Fig. A3), and the local reactions comprised selection and reproduction processes: selection was cyclic rock–paper–scissors, yielding an empty site (black); reproduction only occurred on empty neighboring sites. The mobility of individuals was realized by exchange of positions or occupation of an empty site. The competition between migration and local interactions could either promote or jeopardize biodiversity according to the value of mobility. At low values, the temporal development was dominated by interactions among neighbors, resulting in the long-term maintenance of species diversity; in contrast, when the values were high, spatial homogeneity caused biodiversity to disappear.
Another typical system is cooperator–cheater. In a two-member ecosystem established by Gore et al.,44 cooperator hydrolyzed sucrose into monosaccharides, while cheater took advantage of them and invaded cooperator. However, the cooperator could also invade cheater cells. No matter which had absolute advantage in the initial inoculum, they would finally converge toward a steady state. This is a relationship of both competition and cooperation: when competing, they consumed glucose rapidly, forcing cooperator to express sucrose invertase to produce glucose; but high concentration of glucose inhibited gene expression in turn, and cooperator fell into the competition with cheater again. Based on the study above, Waite et al.45 engineered a three-member ecosystem: two mutualist yeast strains and a cheater strain. Mutualists provided a limiting nutrient required by each other; however, the cheater used the nutrient secreted by mutualist but provided nothing in exchange. In a nutrient-limited environment, according to the different adaptive race between cheaters and cooperators, either could possibly destroy the other rapidly. But the final beneficiary were cooperators, allowing them to purge cheaters stochastically (Fig. A4). So, even though the selective pressure moldered the genetic variability impair to the evolution of cooperation, it did not molder the genetic variability necessary for the mutualism to persist. The capacity of mutualist partners to hitchhike with each other will help to maintain diversity in microbial communities.46
2.3. Applications of synthetic microbial consortia composed of single species
Although the construction and analysis of synthetic microbial consortia are booming, little achievement is gained in application of single species. That is probably because the main purpose for designing single-species consortia is exploring the natural relationships, and for improving the production efficiency which is difficult for single strain, so harsh tasks such as degrading toxic pollutant are out of the capacity of single-species consortia. Moreover, model strains have limited scopes, and are difficult to adapt to the complex and dynamic environments due to their low biodiversity. In addition, single species have similar background, which may impair the labor division and further confine them to few applications.
Using xylose-selective and glucose-selective E. coli strains, Eiteman et al.47 designed a simultaneous multi-sugar fermentation consortium that consumed substrate more quickly, saving about 15% time than the wild-type. Shin et al.48 constructed a binary microbial consortium, in which two E. coli strains hydrolyzed xylan and produced ethanol orderly, converted xylan hemicellulose to ethanol with a yield about 55% of the theoretical value. Chen et al.49, 50 constructed a minicellulosome yeast consortium that three members secreted different dockerin-tagged cellulases and the other member secreted a trifunctional scaffolding protein to match these cellulases, reaching a titer of 1.87 g/L (Fig. A5). Similarly, Tan et al.51 used nearly the same system obtaining ethanol at 1.138 g/L. The difference in productivity may be due to the different cellulases utilized and the un-optimized ratio. Two years later, they52 conducted tough work in enzyme–enzyme synergy, enzyme–proximity synergy, cellulose–enzyme–cell synergy, and the length of anchoring miniscaffolding, improving the titer to 1.412 g/L.
These studies devoted to the development of renewable lignocellulose with fast, effective and cost-effective methods. Though problems still exist in growth rate match, low production, long fermentation period, and so on, they are worth being popularized for a wider range of substrates utilization, such as arabinose, or even inhibitors frequently found in lignocellulosic hydrolysates, microbes combination, and more biorefinery products, such as cis,cis-muconic acid and 4-hydroxybenzoic acid.53
3. Synthetic microbial consortia composed of two species
Current synthetic microbial consortia composed of two species can be divided into four types: bacteria–bacteria, fungi–bacteria, archaea–bacteria and other microbes with bacteria. The design and construction principles are more diverse than single species, and the investigation focuses more on the material and cofactor communications between different strains. Although the mechanism has not been fully elucidated, the systems are extensively used.
3.1. Design and construction of synthetic microbial consortia composed of two species
When designing a two-species microbial consortium, the first to consider is how they coexist, and then the constructing principle to communicate across species, which probably results in more special mechanisms than single species, thus it is worth uncovering the signal molecules and their working mechanisms.
E. coli and Bacillus megaterium consortium was a QS system, but the difference was that the cell–cell communication relied on autoinducing peptides (AIP). To produce and secrete mature AIP, E. coli cells expressed the AgrD and AgrB proteins; to recognize AIP, B. megaterium cells expressed the two-component AgrCA. Then monomer AgrA activated gene expression and AgrC catalyzed AIP-dependent phosphorylation.54
For an auxotrophic consortium,55 engineered E. coli need glutamine, while what nutrients Dictyostelium discoideum need is unknown. To make it clear, 16 amino acids and 5 vitamins were tested in monoculture, the results were further selected in co-culture, and finally lipoic acid was found to be the best one.
In addition to relationships relying on nutrition, detoxifying can also be used as the basis for communication. When cultivated alone, E. coli absorbed glucose to produce acetate as end-metabolite, which inhibited the downstream pathway. But, rationally engineering Acinetobacter baylyi which was acetate dependent, resulted in an increase in biomass and proteins of E. coli.56
Another kind of communication mechanism is electron transfer. For example, under anoxic conditions, lactate permease of sulfate-reducing species dehydrogenized lactate and produced H+, which was further reduced into H2 with the help of electron carrier and hydrogenase, then hydrogenotrophic methanogens reacted with H2 and CO2 to produce CH4.57, 58 Rotaru et al.59 designed another methanogenesis consortium, in which Geobacter metallireducens and Methanosaeta harundinacea exchanged electrons not only by acetate molecule which is a typical way, but also directly via electrically conductive pili (Fig. B1).
Fig. B.
Design, analysis and application of synthetic microbial consortia composed of two species. (1) Electron transfer between G. metallireducens and M. harundinacea. (2) Metabolites exchange between B. megaterium and K. vulgare. (3) Distribute oxygenated taxanes metabolic pathway into E. coli and S. cerevisiae consortium. (4) Bacterial cell therapy systems in the future.
Part 1 is adapted by permission from Energy & Environmental Science59 ©. Part 2 is adapted by permission from Metabolomics12 ©. Part 3 is adapted by permission from Macmillan Publishers Ltd: Nature Biotechnology20 ©. Part 4 is adapted by permission from ACS Synthetic Biology81 ©.
Specially, physical connection nanotubes can also be the media for communication between species, but the two cells have to reside in proximity and they both need to have nanotubes.60, 61
Double-species microbial consortia have more choices to combine various species together. But this particularity makes it less regular. Development of these principles can just be horizontal to accumulate more fresh ideas, rather than be longitudinal to form scientific systems.
3.2. Analysis of synthetic microbial consortia composed of two species
Different from single species, analysis methods for interactions between two species are mainly metabolic profiling, metabolomics, genomics, proteomics, and dynamics analysis. The reasons for the difference may be because that though single species are reengineered to play many roles in the ecosystem, their similarities in genetic background and metabolic pathways limit the analysis at the macroscopic level. Zhou et al.62 investigated the metabolic cooperation mechanism of B. megaterium and Ketogulonicigenium vulgare, finding that K. vulgare converted peptides into amino acid for B. megaterium, and the latter provided erythrose, erythritol, and other metabolites for the former. At the same time, Ma et al.63 integrated proteomic and metabolomics analysis of the consortium, revealing the fact that the unselfish B. megaterium facilitated K. vulgare in purine biosynthesis and energy production. The community dynamics were further investigated by Du et al.12 illuminating that B. megaterium promoted K. vulgare's TCA cycle, nucleotide and amino acids metabolisms. Whether the latter promoted or inhibited the former depended on if it provided amino acids or biosynthesized 2-keto-L-gulonic acid (2-KLG) (Fig. B2). To improve the production of 2-KLG, a further study focused on cofactor balance. Du et al.64 found that overexpressing sorbose/sorbosone dehydrogenase together with the cofactor pyrroloquinoline quinone in the consortium could significantly enhance the production of 2-KLG.
3.3. Applications of synthetic microbial consortia composed of two species
Either construction and analysis or their application, E. coli and S. cerevisiae are far from the demand for two-species synthetic microbial consortia. More strains should be taken into account. However, the dependence of certain interactions on regulation of external environments just improved the specific indicators marginally. Reengineering these strains at the molecular level is also important.
In energy, biofuels are attracting more and more attentions. Usually, the substrates are lignocellulosics or its hydrolysate, and the products are ethanol, butanol and so on. At present, bioethanol research has obtained certain achievements in processing techniques, such as control of oxygen transfer,17 batch cells recycling fermentation,65 and development of new strain combination.66, 67 Among these approaches, one-pot method aided with cellulase-inducing substrate satisfied short time and high production at the same time. In studies on butanol, Minty et al.68 reached a titer of 1.88 g/L isobutanol through rigorous calculation and supplemented by experimental verification, but the mathematic process was cumbersome, and ultimately, yield was not high. For its isomer butanol,69 consolidated bioprocessing was used for simultaneous saccharification and fermentation of cellulose by complementary organisms under mesophilic conditions, resulting in a butanol concentration of 3.73 g/L. A higher titer 5.5 g/L was obtained from pretreated rice straw, and when exogenous cellulase was added into the consortium, up to 6.9 g/L butanol was produced.70
Another research direction toward energy is the microorganism electrogenesis, and the electron donors are various. Venkataraman et al.71 engineered a bioelectrochemical systems from Enterobacter aerogenes and Pseudomonas aeruginosa, and the former provided 2,3-butanediol by hydrolyzing glucose, which improved the electrogenesis capacity by 2-fold compared with taking glucose as substrate directly. Rosenbaum et al.18 compared the bioelectrochemical performance of Shewanella oneidensis in a pure-culture and in a co-culture with the homolactic acid fermenter Lactococcus lactis, enabling the system to convert glucose into current with a coulombic efficiency of ~17%, which was a very low efficiency. Similar microbial fuel cells consisted of E. coli and S. oneidensis were designed to figure out the process.72 The former fermented glucose to metabolites such as formate as electron donor for the latter, and the latter secreted flavin mediator to facilitate the former respiration on electrodes. Most importantly, flavin mediator promoted electron movement to the external charge collecting electrode to derive energy in MFCs. Qu et al.73 further improved the system. Nonexoelectrogen (E. coli) consumed oxygen to provide Geobacter sulfurreducens a hypoxic environment, and acetate was added for G. sulfurreducens, leading to a coulombic efficiency about 85%, but process for such a good result was not explored. To make up this shortage, analyses of metabolites were conducted to explore the process.74 Results showed that succinate played a key role in current variation. High level succinate decreased current density while removal of succinate was responsible for the increased current.
In natural products production, Zhou et al.20 distributed oxygenated taxanes and metabolic pathway into a E. coli and S. cerevisiae consortium. The fast-growing E. coli contained a taxadiene-producing module, and the S. cerevisiae expressed cytochrome P450s because of its excellent protein expression machinery. Through avoiding substrate competition and optimizing the module components, the consortium produced up to 33 mg/L oxygenated taxanes (Fig. B3). In addition to artificial pathways, natural pathways can also be regulated in synthetic consortia. For example,75 when co-cultivated, Aspergillus niger was rapidly colonized by Streptomyces coelicolor, which caused production of cyclic dipeptide cyclo(Phe-Phe) and 2-hydroxyphenylacetic acid in response to colonization. Further on, biotransformation studies with o-coumaric acid and caffeic acid resulted in the production of the novel compounds, which was analyzed by NMR-based metabolomics, proposing as an efficient approach to find new natural products.76
In healthcare, to kill the pathogenic P. aeruginosa, many consortia were synthesized with nearly the same mechanism:77, 78, 79 the engineered E. coli recognized and migrated toward P. aeruginosa, secreted toxin, and finally destroyed them. Further, Volzing et al.80 designed a consortium aiming at Gram-negative bacteria in which L. lactis produced and secreted heterologous antimicrobial peptides against pathogenic E. coli and Salmonella. In the future, drug delivery systems consisting of synthetic cells will be autonomous microbial “physicians” as envisioned by Fischbach et al.81 Bacterial cell therapy systems integrate the capacities to diagnose human disease, take appropriate treatment and bring it into effect, and self-eliminate when disease is alleviated (Fig. B4).
In the environment, most studies focused on harmful pollutants' degradation. During the process of wastewater management with consortia, purification is not the only purpose, and turning waste into wealth is much more meaningful. Products from wastewater are various, such as lipid,82, 83, 84 hydrogen,84 electricity,74, 85 etc. On the other hand, toxic compounds degradation is also an attractive field. Through proteomic analysis, Fazzini et al.19 compared pure cultures with a co-culture of Pseudomonas reinekei and Achromobacter xylosoxidans under simultaneous chemical and oxidative stress, and the latter showed enhanced biodegradation of 4-chlorosalicylate. Chen et al.86 mixed Pseudomonas sp. XM-01 and Acinetobacter sp. XM-02 to degrade alkane hydrocarbon and crude oil, showing a higher capability than the pure culture.
As for high-profile new energy development, researches on it emerge endlessly. However, the feasibility of industrialization amplification is still to be improved. Especially the strains have to satisfy with the time-, cost- and labor-intensive challenges.87 Drug production, human health and environmental management are confined to laboratory scale, while in practice, there are many problems to be considered: whether the complex metabolites of pharmaceutical producing microbes in the process of drug extraction have residues; whether the toxins are harmful to human body during pathogenic microorganism killing; whether new contaminants are introduced while treating the poisonous waste with microbial consortia in situ, etc. In these aspects, if we can draw lessons from the concept of green chemistry, new research fields may be developed in the future.
4. Synthetic microbial consortia composed of multiple species
Fewer studies are carried out on synthetic microbial consortia composed of multiple species due to their complicated interactions. Therefore, taking natural microbial ecosystems as blueprints,6, 10, 88 starting from their ecological structures, then studying the interactions among the members, enhancing stability and adaptability of synthetic systems, are keys to open the door of multiple-species synthetic microbial consortia.
4.1. Construction and analysis of synthetic microbial consortia composed of multiple species
Bacteria, fungi and mammalian cells can coexist in a programmed condition. S. cerevisiae (sender) metabolizes glucose to volatile acetaldehyde, which triggers the expression of sBLA in human embryonic kidney cells (receiver); the production of sBLA decreases ampicillin levels and promotes E. coli proliferation. Though E. coli competes nutrients and finally impairs mammalian cells, the consortium doesn't last long; the new communication mechanism across kingdoms provides a new insight into constructing synthetic ecosystems.88
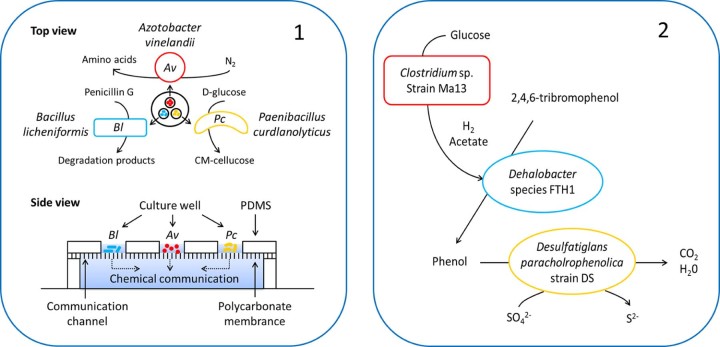
Kim et al.89 created a new cultivation method by using a microfluidic device. Three wild-type soil bacteria are separated in different culture wells, while their metabolites can communicate through the channel of polycarbonate membrane (Fig. C1). In a nutrient-poor medium, there are cellulose/N2/penicillin/as carbon source/nitrogen source/antibiotic pressures, which are utilized by Paenibacillus curdlanolyticus/Azotobacter vinelandii/B. licheniformis. The viability and biodiversity can maintain very well at intermediate separation distances compared with far away or well-mixed conditions. These findings provide new perspective for multi-species communities that require microscale spatial structure for stability, and the approach for controlling spatial structure may enable harnessing synthetic multispecies communities in the laboratory.
Fig. C.
Design, analysis and application of synthetic microbial consortia composed of multiple species. (1) Relationships of P. curdlanolyticus/A. vinelandii/B. licheniformi and microfluidic device for their cultivation. (2) Relationships of the 2,4,6-tribromophenol degradation system.
Part 1 is adapted by permission from Proceedings of the National Academy of Sciences89 ©. Part 2 is adapted by permission from Bioresource Technology91 ©.
Another method for studying microbial consortia is DNA microarray technology. Scholten et al.90 designed a four-member anaerobic community; lactate and propionate are the growth-limiting substrates, and can be oxidized to H2 by two syntrophic strains, and then H2 is converted into methane by two methanogens. Through 24-mer probes, the metabolic response of different strains to a 1 h exposure to air is detected. Syntrophobacter fumaroxidans up-regulates several genes involved in reactive oxygen species detoxification; Methanospirillum hungatei up-regulates heat shock protein to grasp ATP protecting cells against oxidative toxicity, while the other two seem to be less sensitive to the oxidative stress. With new analytical method, this work indicates that microarrays could provide novel viewpoint on the metabolism within microbial consortia.
4.2. Applications of synthetic microbial consortia composed of multiple species
Recently, Li et al.91 developed an anaerobic mineralized microbial consortia, in which Clostridium fermented glucose to supply hydrogen and acetate as reducing agent; then Dehalobacter reduced 2,4,6-tribromophenol to phenol; finally Desulfatiglans mineralized phenol into CO2 (Fig. C2). Another application study was property characterization of biosurfactants rhamnolipids.92 In fact, all of the three members had the capacity to produce rhamnolipids, and their mixture didn't show an advantage over single strain or combination of any two strains in production. That may be related to the quorum sensing control mechanism among them; however, the authors didn't concentrate on this. But the product of mixture had much more complex structure, which resulted in better emulsification ability, surface activity, phenanthrene solubilization in a comprehensive angle. Brethauer et al.93 engineered a consolidated bioprocessing from lignocellulose to ethanol in a batch membrane reactor, in which the process was similar to the one-pot method. Each member has a clear division of labor: Trichoderma reesei secreted cellulolytic enzyme; Scheffersomyces stipitis metabolized xylose; and S. cerevisiae produce ethanol with a 67% yield. The system makes a breakthrough of higher conversions and yields in shorter runtimes compared two-species consortia.17, 66 The most important is that the consortia are proven to be industrially robust, and thus have a good prospect of industrialization.
5. Conclusions and perspectives
Synthetic microbial consortia are getting more and more attentions with the rapid progress of synthetic biology. Academic authorities including Ron Weiss, Frances Arnold, Pam Silver are all working on the system design and construction, robust research, and dynamic stability analysis, etc. As one of the important aspects in synthetic microbial consortia, signals delivery and metabolites exchange are the basic principles and guidelines, which are reflected in QS21, 30 and cross-feeding39, 40, 41 systems. Ecosystems not only contain interaction among strains but also between microbial consortia and external environment, and are wildly used in natural relations simulation.36, 37 Evolution is important as well, and is mainly a supporting method in studies such as biodiversity and stability.42, 43, 44, 45 In this review, current synthetic microbial consortia are divided into three types according to their composition complexity, which are single species, two species or multiple species. Due to their relatively simple relationships, synthetic microbial consortia composed of single species have relatively more thorough understanding about the mechanisms. There are much more principles proposed to describe two species consortia, due to there much more complicated interactions. As for multiple species, breakthroughs have not been made yet; the supplementary metabolites or other signaling chemicals provided by other microbes in the communities are still mysterious.94
Hence there is a phenomenon that with the increase of species, principles for design and analysis of microbial consortia are getting more special and novel, but the mechanisms are getting less understood, and the strains are more inclined to wild type rather than genetically engineered in application. Several challenges need to be overcome for uncovering the veil of multispecies consortia, the foremost of these is to optimize media compositions that satisfy all the multiple species, which influence population dynamics and further metabolism greatly.5 Moreover, we need to take advantage of design and computational tools that take biological variability, uncertainty and evolution into account to provide rational guidance.1 In addition, more novel approaches should be introduced into this field such as micro bead encapsulation,95 13C-metabolic flux analysis,96 synthetic biofilm for sequential layering of microbes.97 Further, multispecies consortia finally put into application need to strengthen the robustness, especially in environment management or lignocellulose degradation in which survival conditions are tough for microbes, that is probably the reason why few genetically engineered strains are employed. One good solution to this may be to combine modified strains with natural microbial consortia derived strains together and evolve them to obtain a viability-improved synthetic microbial consortia.98
Therefore, a series of problems need to be addressed urgently, such as: how to expand research objects, jump out of the confine of model microbes; how to build functional pathways in the existing non-model systems with valuable applications; how to deeply illuminate ecological structures, interaction patterns, fluctuating environmental and evolutionary stresses94 of multiple species, in order to develop more powerful systems and broaden the application scope and depth? Correspondingly, the research methods and train of thoughts can't be limited into synthetic biology, which should also be combined with a systems biology approach. We envision that with the rational guidance of engineering principles, the advantages of synthetic microbial consortia over single strains enable multiple species make breakthrough in design and application of genetically engineered consortia in the future.
Acknowledgements
This work was funded by the National Basic Research Program of China (“973” Program: 2014CB745100), the National Natural Science Foundation of China (No. 21576197), and Tianjin Research Program of Application Foundation and Advanced Technology (No. 14JCQNJC06700).
We would like to say thanks to Dr. Kang Wu, assistant professor of the Department of Chemical Engineering, University of New Hampshire, for providing many valuable suggestions as well as particular attention to language use on this paper.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Purnick P.E., Weiss R. The second wave of synthetic biology: from modules to systems. Nat Rev Mol Cell Biol. 2009;10:410–422. doi: 10.1038/nrm2698. [DOI] [PubMed] [Google Scholar]
- 2.Brenner K., You L., Arnold F.H. Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 2008;26:483–489. doi: 10.1016/j.tibtech.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Gerchman Y., Weiss R. Teaching bacteria a new language. Proc Natl Acad Sci USA. 2004;101:2221–2222. doi: 10.1073/pnas.0400473101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shong J., Jimenez Diaz M.R., Collins C.H. Towards synthetic microbial consortia for bioprocessing. Curr Opin Biotechnol. 2012;23:798–802. doi: 10.1016/j.copbio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Großkopf T., Soyer O.S. Synthetic microbial communities. Curr Opin Microbiol. 2014;18:72–77. doi: 10.1016/j.mib.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woyke T., Teeling H., Ivanova N.N., Huntemann M., Richter M., Gloeckner F.O. Symbiosis insights through metagenomic analysis of a microbial consortium. Nature. 2006;443:950–955. doi: 10.1038/nature05192. [DOI] [PubMed] [Google Scholar]
- 7.Li B.Z., Yuan Y.J. Transcriptome shifts in response to furfural and acetic acid in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2010;86:1915–1924. doi: 10.1007/s00253-010-2518-2. [DOI] [PubMed] [Google Scholar]
- 8.Ma Q., Wang J., Lu S., Lv Y., Yuan Y. Quantitative proteomic profiling reveals photosynthesis responsible for inoculum size dependent variation in Chlorella sorokiniana. Biotechnol Bioeng. 2013;110:773–784. doi: 10.1002/bit.24762. [DOI] [PubMed] [Google Scholar]
- 9.Wang X., Jin M.J., Balan V., Jones A.D., Li X., Li B.Z. Comparative metabolic profiling revealed limitations in xylose-fermenting yeast during co-fermentation of glucose and xylose in the presence of inhibitors. Biotechnol Bioeng. 2014;111:152–164. doi: 10.1002/bit.24992. [DOI] [PubMed] [Google Scholar]
- 10.Greenblum S., Turnbaugh P.J., Borenstein E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proc Natl Acad Sci USA. 2012;109:594–599. doi: 10.1073/pnas.1116053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D.Z., Xie Z.X., Zhang S.F. Marine metaproteomics: current status and future directions. J Proteomics. 2014;97:27–35. doi: 10.1016/j.jprot.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Du J., Zhou J., Xue J., Song H., Yuan Y. Metabolomic profiling elucidates community dynamics of the Ketogulonicigenium vulgare-Bacillus megaterium consortium. Metabolomics. 2012;8:960–973. [Google Scholar]
- 13.Voigt C.A. Genetic parts to program bacteria. Curr Opin Biotechnol. 2006;17:548–557. doi: 10.1016/j.copbio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Canton B., Labno A., Endy D. Refinement and standardization of synthetic biological parts and devices. Nat Biotechnol. 2008;26:787–793. doi: 10.1038/nbt1413. [DOI] [PubMed] [Google Scholar]
- 15.Slusarczyk A.L., Lin A., Weiss R. Foundations for the design and implementation of synthetic genetic circuits. Nat Rev Genet. 2012;13:406–420. doi: 10.1038/nrg3227. [DOI] [PubMed] [Google Scholar]
- 16.Lim W.A. Designing customized cell signalling circuits. Nat Rev Mol Cell Biol. 2010;11:393–403. doi: 10.1038/nrm2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuroff T.R., Xiques S.B., Curtis W.R. Consortia-mediated bioprocessing of cellulose to ethanol with a symbiotic Clostridium phytofermentans/yeast co-culture. Biotechnol Biofuels. 2013;6:59. doi: 10.1186/1754-6834-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbaum M.A., Bar H.Y., Beg Q.K., Segrè D., Booth J., Cotta M.A. Shewanella oneidensis in a lactate-fed pure-culture and a glucose-fed co-culture with Lactococcus lactis with an electrode as electron acceptor. Bioresour Technol. 2011;102:2623–2628. doi: 10.1016/j.biortech.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 19.Bobadilla Fazzini R.A., Preto M.J., Poucas Quintas A.C., Bielecka A., Timmis K.N., Martins dos Santos V.A.P. Consortia modulation of the stress response: proteomic analysis of single strain versus mixed culture. Environ Microbiol. 2010;12:2436–2449. doi: 10.1111/j.1462-2920.2010.02217.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhou K., Qiao K., Edgar S., Stephanopoulos G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat Biotechnol. 2015;33:377. doi: 10.1038/nbt.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner K., Karig D.K., Weiss R., Arnold F.H. Engineered bidirectional communication mediates a consensus in a microbial biofilm consortium. Proc Natl Acad Sci USA. 2007;104:17300–17304. doi: 10.1073/pnas.0704256104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basu S., Mehreja R., Thiberge S., Chen M.T., Weiss R. Spatiotemporal control of gene expression with pulse-generating networks. Proc Natl Acad Sci USA. 2004;101:6355–6360. doi: 10.1073/pnas.0307571101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basu S., Gerchman Y., Collins C.H., Arnold F.H., Weiss R. A synthetic multicellular system for programmed pattern formation. Nature. 2005;434:1130–1134. doi: 10.1038/nature03461. [DOI] [PubMed] [Google Scholar]
- 24.Chen M.T., Weiss R. Artificial cell-cell communication in yeast Saccharomyces cerevisiae using signaling elements from Arabidopsis thaliana. Nat Biotechnol. 2005;23:1551–1555. doi: 10.1038/nbt1162. [DOI] [PubMed] [Google Scholar]
- 25.Chuang J.S., Rivoire O., Leibler S. Simpson's paradox in a synthetic microbial system. Science. 2009;323:272–275. doi: 10.1126/science.1166739. [DOI] [PubMed] [Google Scholar]
- 26.Prindle A., Samayoa P., Razinkov I., Danino T., Tsimring L.S., Hasty J. A sensing array of radically coupled genetic ‘biopixels‘. Nature. 2012;481:39–44. doi: 10.1038/nature10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabor J.J., Salis H.M., Booth Simpson Z., Chevalier A.A., Levskaya A., Marcotte E.M. A synthetic genetic edge detection program. Cell. 2009;137:1272–1281. doi: 10.1016/j.cell.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danino T., Mondragón-Palomino O., Tsimring L., Hasty J. A synchronized quorum of genetic clocks. Nature. 2010;463:326–330. doi: 10.1038/nature08753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song H., Payne S., Gray M., You L. Spatiotemporal modulation of biodiversity in a synthetic chemical-mediated ecosystem. Nat Chem Biol. 2009;5:929–935. doi: 10.1038/nchembio.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C.L., Fu X.F., Liu L.Z., Ren X.J., Chau C.K.L., Li S.H. Sequential establishment of stripe patterns in an expanding cell population. Science. 2011;334:238–241. doi: 10.1126/science.1209042. [DOI] [PubMed] [Google Scholar]
- 31.Hong S.H., Hegde M., Kim J., Wang X.X., Jayaraman A., Wood T.K. Synthetic quorum-sensing circuit to control consortial biofilm formation and dispersal in a microfluidic device. Nat Commun. 2012;3:613. doi: 10.1038/ncomms1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith R., Tan C., Srimani J.K., Pai A., Riccione K.A., Song H. Programmed Allee effect in bacteria causes a tradeoff between population spread and survival. Proc Natl Acad Sci USA. 2014;111:1969–1974. doi: 10.1073/pnas.1315954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Payne S., Li B.C., Cao Y.X.L., Schaeffer D., Ryser M.D., You L.C. Temporal control of self-organized pattern formation without morphogen gradients in bacteria. Mol Syst Biol. 2013;9:697. doi: 10.1038/msb.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosoda K., Suzuki S., Yamauchi Y., Shiroguchi Y., Kashiwagi A., Ono N. Cooperative adaptation to establishment of a synthetic bacterial mutualism. PLoS ONE. 2011;6:e17105. doi: 10.1371/journal.pone.0017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerner A., Park J., Williams A., Lin X.N. A programmable Escherichia coli consortium via tunable symbiosis. PLoS ONE. 2012;7:e34032. doi: 10.1371/journal.pone.0034032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balagaddé F.K., Song H., Ozaki J., Collins C.H., Barnet M., Arnold F.H. A synthetic Escherichia coli predator-prey ecosystem. Mol Syst Biol. 2008;4:187. doi: 10.1038/msb.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu B., Du J., Zou R.Y., Yuan Y.J. An environment-sensitive synthetic microbial ecosystem. PLoS ONE. 2010;5:10619. doi: 10.1371/journal.pone.0010619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shou W., Ram S., Vilar J.M. Synthetic cooperation in engineered yeast populations. Proc Natl Acad Sci USA. 2007;104:1877–1882. doi: 10.1073/pnas.0610575104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X., Reed J.L. Adaptive evolution of synthetic cooperating communities improves growth performance. PLoS ONE. 2014;9:e108297. doi: 10.1371/journal.pone.0108297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pande S., Merker H., Bohl K., Reichelt M., Schuster S., de Figueiredo L.F. Fitness and stability of obligate cross-feeding interactions that emerge upon gene loss in bacteria. ISME J. 2014;8:953–962. doi: 10.1038/ismej.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mee M.T., Collins J.J., Church G.M., Wang H.H. Syntrophic exchange in synthetic microbial communities. Proc Natl Acad Sci USA. 2014;111:E2149–56. doi: 10.1073/pnas.1405641111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerr B., Riley M.A., Feldman M.W., Bohannan B.J. Local dispersal promotes biodiversity in a real-life game of rock-paper-scissors. Nature. 2002;418:171–174. doi: 10.1038/nature00823. [DOI] [PubMed] [Google Scholar]
- 43.Reichenbach T., Mobilia M., Frey E. Mobility promotes and jeopardizes biodiversity in rock-paper-scissors games. Nature. 2007;448:1046–1049. doi: 10.1038/nature06095. [DOI] [PubMed] [Google Scholar]
- 44.Gore J., Youk H., van Oudenaarden A. Snowdrift game dynamics and facultative cheating in yeast. Nature. 2009;459:253–256. doi: 10.1038/nature07921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waite A.J., Shou W. Adaptation to a new environment allows cooperators to purge cheaters stochastically. Proc Natl Acad Sci USA. 2012;109:19079–19086. doi: 10.1073/pnas.1210190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nadell C.D., Foster K.R. Mutually helping microbes can evolve by hitchhiking. Proc Natl Acad Sci USA. 2012;109:19037–19038. doi: 10.1073/pnas.1217199109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eiteman M.A., Lee S.A., Altman E. A co-fermentation strategy to consume sugar mixtures effectively. J Biol Eng. 2008;2:3. doi: 10.1186/1754-1611-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin H.D., McClendon S., Vo T., Chen R.R. Escherichia coli binary culture engineered for direct fermentation of hemicellulose to a biofuel. Appl Environ Microbiol. 2010;76:8150–8159. doi: 10.1128/AEM.00908-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goyal G., Tsai S.L., Madan B., DaSilva N.A., Chen W. Simultaneous cell growth and ethanol production from cellulose by an engineered yeast consortium displaying a functional mini-cellulosome. Microb Cell Fact. 2011;10:89. doi: 10.1186/1475-2859-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai S.L., Goyal G., Chen W. Surface display of a functional minicellulosome by intracellular complementation using a synthetic yeast consortium and its application to cellulose hydrolysis and ethanol production. Appl Environ Microbiol. 2010;76:7514–7520. doi: 10.1128/AEM.01777-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan L.H., Zhang Z.J., Yu X.Y., Xue Y.X., Wang M.M., Tan T.W. In vitro assembly of minicellulosomes with two scaffoldins on the yeast cell surface for cellulose saccharification and bioethanol production. Process Biochem. 2013;48:430–437. [Google Scholar]
- 52.Fan L.H., Zhang Z.J., Yu X.Y., Xue Y.X., Tan T.W. Self-surface assembly of cellulosomes with two miniscaffoldins on Saccharomyces cerevisiae for cellulosic ethanol production. Proc Natl Acad Sci USA. 2012;109:13260–13265. doi: 10.1073/pnas.1209856109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H., Pereira B., Li Z., Stephanopoulos G. Engineering Escherichia coli coculture systems for the production of biochemical products. Proc Natl Acad Sci USA. 2015;112:8266–8271. doi: 10.1073/pnas.1506781112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marchand N., Collins C.H. Peptide-based communication system enables Escherichia coli to Bacillus megaterium interspecies signaling. Biotechnol Bioeng. 2013;110:3003–3012. doi: 10.1002/bit.24975. [DOI] [PubMed] [Google Scholar]
- 55.Kubo I., Hosoda K., Suzuki S., Yamamoto K., Kihara K., Mori K. Construction of bacteria-eukaryote synthetic mutualism. Biosystems. 2013;113:66–71. doi: 10.1016/j.biosystems.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Santala S., Karp M., Santala V. Rationally engineered synthetic coculture for improved biomass and product formation. PLoS ONE. 2014;9:e113786. doi: 10.1371/journal.pone.0113786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer B., Kuehl J., Deutschbauer A.M., Price M.N., Arkin A.P., Stahl D.A. Variation among Desulfovibrio species in electron transfer systems used for syntrophic growth. J Bacteriol. 2013;195:990–1004. doi: 10.1128/JB.01959-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker C.B., He Z.L., Yang Z.K., Ringbauer J.A., Jr, He Q., Zhou J. The electron transfer system of syntrophically grown Desulfovibrio vulgaris. J Bacteriol. 2009;191:5793–5801. doi: 10.1128/JB.00356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mallaá Shrestha P. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ Sci. 2014;7:408–415. [Google Scholar]
- 60.Dubey G.P., Ben-Yehuda S. Intercellular nanotubes mediate bacterial communication. Cell. 2011;144:590–600. doi: 10.1016/j.cell.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 61.Pande S., Shitut S., Freund L., Westermann M., Bertels F., Colesie C. Metabolic cross-feeding via intercellular nanotubes among bacteria. Nat Commun. 2015;6:1–13. doi: 10.1038/ncomms7238. [DOI] [PubMed] [Google Scholar]
- 62.Zhou J., Ma Q., Yi H., Wang L.L., Song H., Yuan Y.J. Metabolome profiling reveals metabolic cooperation between Bacillus megaterium and Ketogulonicigenium vulgare during induced swarm motility. Appl Environ Microbiol. 2011;77:7023–7030. doi: 10.1128/AEM.05123-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma Q., Zhou J., Zhang W.W., Meng X.X., Sun J.W., Yuan Y.J. Integrated proteomic and metabolomic analysis of an artificial microbial community for two-step production of vitamin C. PLoS ONE. 2011;6:e26108. doi: 10.1371/journal.pone.0026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du J., Bai W., Song H., Yuan Y.J. Combinational expression of sorbose/sorbosone dehydrogenases and cofactor pyrroloquinoline quinone increases 2-keto-l-gulonic acid production in Ketogulonigenium vulgare-Bacillus cereus consortium. Metab Eng. 2013;19:50–56. doi: 10.1016/j.ymben.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Ashoor S., Comitini F., Ciani M. Cell-recycle batch process of Scheffersomyces stipitis and Saccharomyces cerevisiae co-culture for second generation bioethanol production. Biotechnol Lett. 2015;37:2213–2218. doi: 10.1007/s10529-015-1919-9. [DOI] [PubMed] [Google Scholar]
- 66.Park E., Naruse K., Kato T. One-pot bioethanol production from cellulose by co-culture of Acremonium cellulolyticus and Saccharomyces cerevisiae. Biotechnol Biofuels. 2012;5:1–11. doi: 10.1186/1754-6834-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Costa R.L., Oliveira T.V., Souza Ferreira de J., Cardoso V.L., Batista F.R.X. Prospective technology on bioethanol production from photofermentation. Bioresour Technol. 2015;181:330–337. doi: 10.1016/j.biortech.2015.01.090. [DOI] [PubMed] [Google Scholar]
- 68.Minty J.J., Singer M.E., Scholz S.A., Bae C.H., Ahn J.H., Foster C.E. Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc Natl Acad Sci USA. 2013;110:14592–14597. doi: 10.1073/pnas.1218447110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z.Y., Cao G.L., Zheng J., Fu D.F., Song J.Z., Zhang J.Z. Developing a mesophilic co-culture for direct conversion of cellulose to butanol in consolidated bioprocess. Biotechnol Biofuels. 2015;8:1–9. doi: 10.1186/s13068-015-0266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kiyoshi K., Furukawa M., Seyama T., Kadokura T., Nakazato A., Nakayama S. Butanol production from alkali-pretreated rice straw by co-culture of Clostridium thermocellum and Clostridium saccharoperbutylacetonicum. Bioresour Technol. 2015;186:325–328. doi: 10.1016/j.biortech.2015.03.061. [DOI] [PubMed] [Google Scholar]
- 71.Venkataraman A., Rosenbaum M.A., Perkins S.D., Werner J.J., Angenent L.T. Metabolite-based mutualism between Pseudomonas aeruginosa PA14 and Enterobacter aerogenes enhances current generation in bioelectrochemical systems. Energy Environ Sci. 2011;4:4550–4559. [Google Scholar]
- 72.Wang V.B., Sivakumar K., Yang L., Zhang Q.C., Kjelleberg S., Loo S.C.J. Metabolite-enabled mutualistic interaction between Shewanella oneidensis and Escherichia coli in a co-culture using an electrode as electron acceptor. Sci Rep. 2015;5:860–863. doi: 10.1038/srep11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qu Y., Feng Y., Wang X., Logan B.E. Use of a coculture to enable current production by Geobacter sulfurreducens. Appl Environ Microbiol. 2012;78:3484–3487. doi: 10.1128/AEM.00073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bourdakos N., Marsili E., Mahadevan R. A defined co-culture of Geobacter sulfurreducens and Escherichia coli in a membrane-less microbial fuel cell. Biotechnol Bioeng. 2014;111:709–718. doi: 10.1002/bit.25137. [DOI] [PubMed] [Google Scholar]
- 75.Wu C.S., Zacchetti B., Ram A.F.J., van Wezel G.P., Claessen D., Choi Y.H. Expanding the chemical space for natural products by Aspergillus-Streptomyces co-cultivation and biotransformation. Sci Rep. 2015;5:10868. doi: 10.1038/srep10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu C., Kim H.K., van Wezel G.P., Choi Y.H. Metabolomics in the natural products field-a gateway to novel antibiotics. Drug Discov Today. 2015;13:11–17. doi: 10.1016/j.ddtec.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 77.Saeidi N., Wong C.K., Lo T.M., Nguyen H.X., Ling H., Leong S.S.J. Engineering microbes to sense and eradicate Pseudomonas aeruginosa, a human pathogen. Mol Syst Biol. 2011;7:521. doi: 10.1038/msb.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta S., Bram E.E., Weiss R. Genetically programmable pathogen sense and destroy. ACS Synth Biol. 2013;2:715–723. doi: 10.1021/sb4000417. [DOI] [PubMed] [Google Scholar]
- 79.Hwang I.Y., Tan M.H., Koh E., Ho C.L., Poh C.L., Chang M.W. Reprogramming microbes to be pathogen-seeking killers. ACS Synth Biol. 2013;3:228–237. doi: 10.1021/sb400077j. [DOI] [PubMed] [Google Scholar]
- 80.Volzing K., Borrero J., Sadowsky M.J., Kaznessis Y.N. Antimicrobial peptides targeting gram-negative pathogens, produced and delivered by lactic acid bacteria. ACS Synth Biol. 2013;2:643–650. doi: 10.1021/sb4000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Claesen J., Fischbach M.A. Synthetic microbes as drug delivery systems. ACS Synth Biol. 2014;4:358–364. doi: 10.1021/sb500258b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ling J., Nip S., Cheok W.L., Toledo de R.A., Shim H. Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresour Technol. 2014;173:132–139. doi: 10.1016/j.biortech.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 83.Wrede D., Taha M., Miranda A.F., Kadali K., Stevenson T., Ball A.S. Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PLoS ONE. 2014;9:e113497. doi: 10.1371/journal.pone.0113497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ren H.Y., Liu B.F., Kong F., Zhao L., Ren N. Hydrogen and lipid production from starch wastewater by co-culture of anaerobic sludge and oleaginous microalgae with simultaneous COD, nitrogen and phosphorus removal. Water Res. 2015;85:404–412. doi: 10.1016/j.watres.2015.08.057. [DOI] [PubMed] [Google Scholar]
- 85.Gieg L.M., Fowler S.J., Berdugo-Clavijo C. Syntrophic biodegradation of hydrocarbon contaminants. Curr Opin Biotechnol. 2014;27:21–29. doi: 10.1016/j.copbio.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 86.Chen Y., Li C., Zhou Z.X., Wen J.P., You X.Y., Mao Y.Z. Enhanced biodegradation of alkane hydrocarbons and crude oil by mixed strains and bacterial community analysis. Appl Biochem Biotechnol. 2014;172:3433–3447. doi: 10.1007/s12010-014-0777-6. [DOI] [PubMed] [Google Scholar]
- 87.Lee S.Y., Kim H.U. Systems strategies for developing industrial microbial strains. Nat Biotechnol. 2015;33:1061–1072. doi: 10.1038/nbt.3365. [DOI] [PubMed] [Google Scholar]
- 88.Weber W., Daoud-El Baba M., Fussenegger M. Synthetic ecosystems based on airborne inter-and intrakingdom communication. Proc Natl Acad Sci USA. 2007;104:10435–10440. doi: 10.1073/pnas.0701382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim H.J., Boedicker J.Q., Choi J.W., Ismagilov R.F. Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc Natl Acad Sci USA. 2008;105:18188–18193. doi: 10.1073/pnas.0807935105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scholten J.C., Culley D.E., Nie L., Munn K.J., Chow L., Brockman F.J. Development and assessment of whole-genome oligonucleotide microarrays to analyze an anaerobic microbial community and its responses to oxidative stress. Biochem Biophys Res Commun. 2007;358:571–577. doi: 10.1016/j.bbrc.2007.04.160. [DOI] [PubMed] [Google Scholar]
- 91.Li Z.L., Yoshida N., Wang A.J., Nan J., Liang B., Zhang C.F. Anaerobic mineralization of 2, 4, 6-tribromophenol to CO2 by a synthetic microbial community comprising Clostridium, Dehalobacter, and Desulfatiglans. Bioresour Technol. 2015;176:225–232. doi: 10.1016/j.biortech.2014.10.097. [DOI] [PubMed] [Google Scholar]
- 92.Hošková M., Ježdík R., Schreiberová O., Chudoba J., Sír M., Čejková A. Structural and physiochemical characterization of rhamnolipids produced by Acinetobacter calcoaceticus, Enterobacter asburiae and Pseudomonas aeruginosa in single strain and mixed cultures. J Biotechnol. 2015;193:45–51. doi: 10.1016/j.jbiotec.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 93.Brethauer S., Studer M.H. Consolidated bioprocessing of lignocellulose by a microbial consortium. Energy Environ Sci. 2014;7:1446–1453. [Google Scholar]
- 94.Song H., Ding M.Z., Jia X.Q., Ma Q., Yuan Y.J. Synthetic microbial consortia: from systematic analysis to construction and applications. Chem Soc Rev. 2014;43:6954–6981. doi: 10.1039/c4cs00114a. [DOI] [PubMed] [Google Scholar]
- 95.Jakiela S., Kaminski T.S., Cybulski O., Weibel D.B., Garstecki P. Bacterial growth and adaptation in microdroplet chemostats. Angew Chem Int Ed Engl. 2013;52:8908–8911. doi: 10.1002/anie.201301524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gebreselassie N.A., Antoniewicz M.R. 13C-Metabolic flux analysis of co-cultures: a novel approach. Metab Eng. 2015;31:132–139. doi: 10.1016/j.ymben.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burmølle M., Ren D., Bjarnsholt T., Sørensen S.J. Interactions in multispecies biofilms: do they actually matter? Trends Microbiol. 2014;22:84–91. doi: 10.1016/j.tim.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Ran D., Yan J.B., Li S.Z., Zhang L., Zhang S., Li J.H. Cellulosic ethanol production by natural bacterial consortia is enhanced by Pseudoxanthomonas taiwanensis. Biotechnol Biofuels. 2015;8:1–10. doi: 10.1186/s13068-014-0186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]