Abstract
Deer are an iconic group of large mammals that originated in the Early Miocene of Eurasia (ca. 19 Ma). While there is some consensus on key relationships among their members, on the basis of molecular- or morphology-based analyses, or combined approaches, many questions remain, and the bony labyrinth has shown considerable potential for the phylogenetics of this and other groups. Here we examine its shape in 29 species of living and fossil deer using 3D geometric morphometrics and cladistics. We clarify several issues of the origin and evolution of cervids. Our results give new age estimates at different nodes of the tree and provide for the first time a clear distinction of stem and crown Cervidae. We unambiguously attribute the fossil Euprox furcatus (13.8 Ma) to crown Cervidae, pushing back the origin of crown deer to (at least) 4 Ma. Furthermore, we show that Capreolinae are more variable in bony labyrinth shape than Cervinae and confirm for the first time the monophyly of the Old World Capreolinae (including the Chinese water deer Hydropotes) based on morphological characters only. Finally, we provide evidence to support the sister group relationship of Megaloceros giganteus with the fallow deer Dama.
Introduction
Deer (Cervidae) are a family of antlered ruminants and, with 55 extant species, are one of the most diverse groups of artiodactyls. They are adapted to inhabit numerous climatic zones and ecotones on all continents, with the exception of Oceania and Antarctica1,2. In the last decade, molecular analyses have provided new input for a relative consensus of the phylogeny of extant taxa2–7. However, morphology-based analyses that allow the integration of extinct taxa and lead to a deeper understanding of the evolutionary history of the whole group do not always yield similar results for cervid phylogenetics8–11. While the distinction between the extant subfamilies Capreolinae and Cervinae is morphologically distinguishable by the condition of the lateral digits (distal part preserved only or “telemetacarpal” vs. proximal part preserved only or “plesiometacarpal”, respectively), the phylogenetic position and the genus-level relationships of many fossil species are still debated (see Grubb12 and Croitor13 for Plio-Pleistocene taxa). This has a strong effect on the ages used to calibrate deer phylogeny and, indeed, ruminant phylogeny as a whole. A classic uncertainty is the origin of the crown clade, as molecular-based approaches use the oldest occurrence of the extant Muntiacus (7 to 10 million years ago (Ma), Late Miocene3,7,14), whereas palaeontologists embrace Euprox furcatus (a Middle Miocene deer dated ca. 13.8 Ma, 4 to almost 7 million of years (My) older than the earliest Muntiacus) as the oldest crown deer and even as a member of the Muntiacinae15–18. Resolving these incongruences is therefore crucial for understanding the origin and evolutionary history of deer.
Antlers are classic morphological features of deer, and are paramount characters for the inclusion of fossil representatives in phylogenetic analyses19, having appeared in the fossil record ca. 19 Ma9,16,20–23. Antlers are deciduous cranial appendages that have developed a wide array of morphologies throughout the history of deer; from tiny multitomous antlers in Lagomeryx to small pointed spines in Pudu and huge spiked shovel-shaped structures in the extinct “Irish Elk”, Megaloceros, and the recent moose, Alces. Despite their good fossil record, studies based on antlers and other body parts have left many issues in deer phylogeny unresolved. The bony labyrinth (i.e., the organ of hearing and balance) has been recently shown to be an informative structure reflecting phylogenetic relationships in accordance with molecular-based hypotheses in various mammal groups e.g.24–30. Because molecular data for extinct species are unavailable, reconstructing the three-dimensional (3D) morphology of this structure throughout the history of a lineage thus has the potential to solve key questions regarding the origins and diversification of clades.
Here we reconstruct the bony labyrinth of 29 extant (N = 12) and extinct (N = 17) deer species spanning the 19 Ma of their evolutionary history (Supplementary data 1). We use a 3D geometric morphometric approach and the most comprehensive cladistic analysis (including fossil deer) to tackle phylogenetic issues at all levels of the tree. Stem taxa as well as key fossil species for the main tribes are included. Our analysis i) unquestionably separates stem from crown cervids; ii) sets the origin of crown deer earlier than previously proposed by molecular phylogenetic analyses and, in line with palaeontological data, confirms the position of Megaloceros in the Dama lineage; iii) adds data to the peculiar morphological disparity of New World cervids; iv) recalibrates the phylogenetic tree of cervids; and v) confirms the high potential of the bony labyrinth for resolving conflicting phylogenies in mammals, such as the phylogenetic position of the inermous Hydropotes.
Results
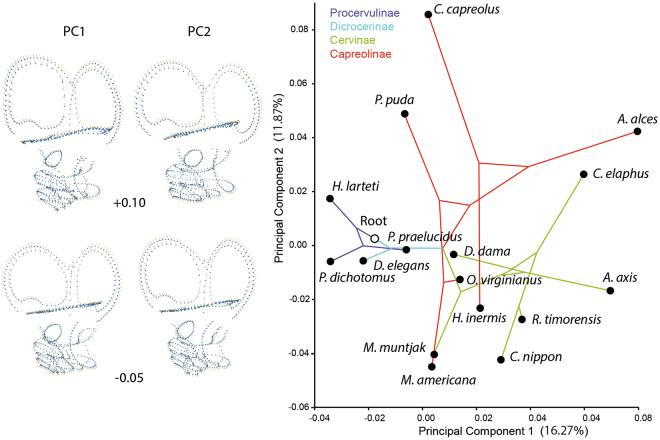
Principal Component Analysis of stem and crown Cervidae
Shape variation of the bony labyrinth among cervids is presented in Fig. 1. The p-value resulting from the permutation test, which tested the influence of the phylogenetic signal on the shape variation, is not significant (p-value = 0.3586). This indicates that we cannot reject the hypothesis that overall shape variation, across the tips of the tree is random. Nevertheless, the morphospace occupied by the bony labyrinth morphology of stem Cervidae differs from those of crown Cervidae along Principal Component 1 (PC1), with no overlap being observed between these groups (Fig. 1). The morphospace of extant Capreolinae is larger than that of the stem Cervidae and the extant Cervinae, indicating a higher variation in the shape of the bony labyrinth in the former clade (Fig. 1). In addition, there is no overlap between the Old World (Capreolus capreolus, Hydropotes inermis, and Alces alces) and the New World (Odocoileus virginianus, Mazama americana, and Pudu puda) Capreolinae, with the American ones being shifted toward stem Cervidae along the PC1 (Fig. 1).
Figure 1.
Principal component analysis (PCA) based on the 3D coordinates of the cervid bony labyrinth morphology with superimposed phylogenetic tree. PC shape variation is highighted by the hypothetical bony labyrinth shapes at the extreme scores −0.05 and +0.10 on PC1 and PC2.
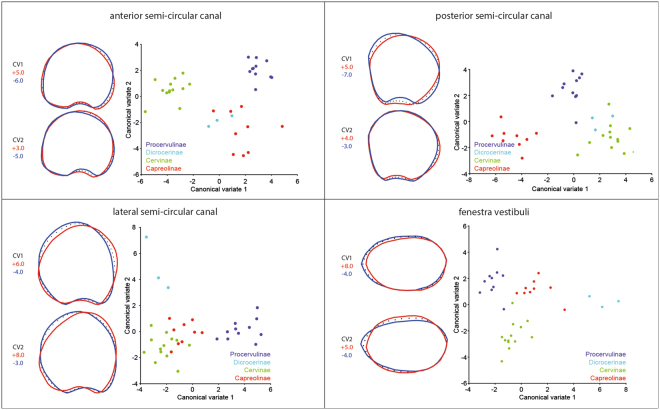
Canonical Variate Analysis of subfamilies
The Canonical Variate Analysis (CVA) statistically highlights discrimination between deer subfamilies based on bony labyrinth structures (Supplementary data 2). The discrimination of the Dicrocerinae is systematically less-pronounced than the other sub families due to the low number of specimens (Supplementary data 2). Nevertheless, the six CVA applied to specific areas of the cervid bony labyrinth show graphically the phylogenetically relevant structures allowing distinction between subfamilies when possible (Fig. 2). The following characters are continuous based on deduced measurements. Supplementary data 2 shows how the various measurements and ratios have been performed and Supplementary data 3 explains the terminology used in the following text and the cladistics analyses based on the bony labyrinth sole. Canonical Variate 1 (CV1) of the anterior semi-circular canal analysis allows a clear distinction of Procervulinae, Dicrocerinae, and Capreolinae from Cervinae, with the former having a rounded structure and the latter a squarer canal (Fig. 2). CV2 separates Cervinae and Procervulinae from Capreolinae and Dicrocerinae by a more anteriorly ovoid anterior canal, with the exception of Dama dama and Metacervocerus philisi (second morphotype) which show a more rounded canal. Moreover, Cervinae and Procervulinae have a more flattened anterior ampulla (Fig. 2). The posterior semi-circular canal is ovoid posteriorly in the Capreolinae, rounded in Procervulinae, and ovoid anteriorly in Dicrocerinae and Cervinae (except for Rusa timorensis). This canal is wider than high only in Procervulinae (Fig. 2). The posterior ampulla is strongly rounded in Capreolinae, rounded in Procervulinae, and flattened in Dicrocerinae and Cervinae (with the exception of R. timorensis). The lateral semi-circular canal is ovoid posteriorly in Capreolinae and Cervinae, while it is ovoid anteriorly in Procervulinae and Dicrocerinae (Fig. 2). Similarly, the lateral ampulla is flattened in Capreolinae and Cervinae and rounded in Procervulinae and Dicrocerinae. The fenestra vestibuli is elongated and narrow in Cervinae (stapedial ratio above 1.557), while it is more rounded in the remaining Cervidae (except in O. virginianus). The beginning of the first turn of the cochlea is narrow in Capreolinae and is rather enlarged in the other Cervidae. The thickness of the cochlear first turn is asymmetrical in Cervinae, while it remains symmetrical in the remaining Cervidae. The coil of the cochlear second turn is tightened in Cervinae and enlarged in the other Cervidae. Finally, the second turn of the cochlea is flattened in the Capreolinae and thick in the other Cervidae.
Figure 2.
Canonical variate analyses (CVA) of the different structures of the bony labyrinth (semi-circular canals, fenestra vestibuli) to maximise the similarities among subfamilies (Procervulinae, Dicrocerinae, Cervinae, and Capreolinae). The red outline corresponds to the CV shape variation at the positive extreme score and the blue outline to the negative extreme score.
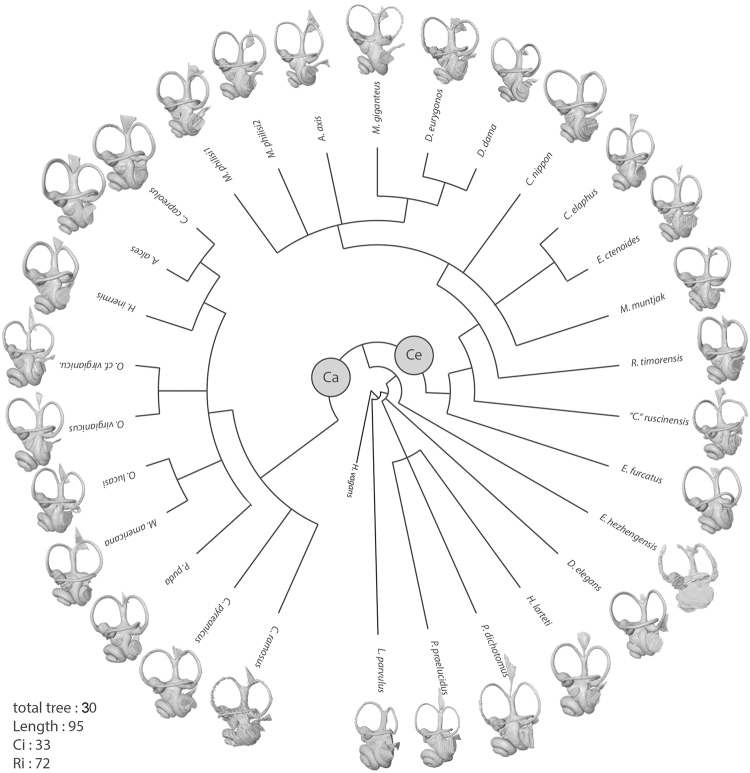
Cladistic analyses
Supplementary data 3 depicts the characters and character states on each node. The Nelson consensus compromise (collapse + consensus) tree of 30 most parsimonious trees shows a highly homoplasic and well-structured result (Consistency Index = 0.33, Retention Index = 0.72, see Fig. 3). Precise description of the topology of the tree and character distribution are found in Supplementary data 3.
Figure 3.
Phylogenetic tree of selected Cervidae based on 26 characters of the bony labyrinth (see Supplementary Information 3 for the description of the characters and character states and for the phylogenetic tree with the associated characters) using maximum an Euristic analysis and the Nelson consensus compromise (collaps + consensus) (Ci = 0.33, Ri = 0.72).
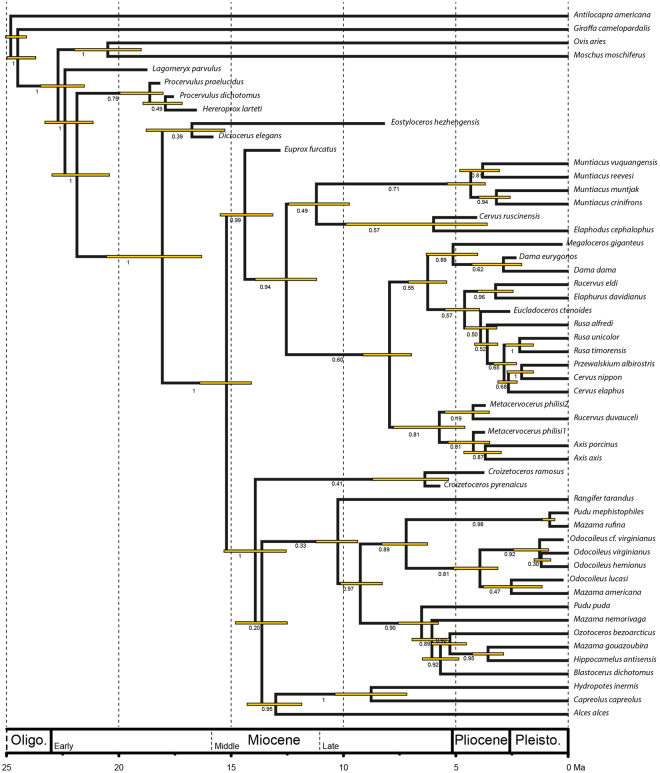
Calibrated tree
Despite the relatively small size of the dataset, visual inspection of the logs in Tracer v.1.6 showed that none of the analyses appeared to be reaching stationarity, with fluctuating parameters and extremely small effective sample sizes for some parameters. We believe this is because of the small size of the morphological dataset (26 characters, compared to a molecular dataset of >16,000 base pairs for most taxa), from which molecular rates had to be calculated for the whole tree. The uncertainty in the tree is also indicated by the very low posterior probability values on nodes that were not constrained. Nevertheless, repeated runs of 10 millions replications consistently yielded the same topology and similar divergence dates (even when certain parameters were changed, such as using a BDSKY tree model). This shows that the results presented here, while beset with uncertainty, are at least consistently reproducible with the current dataset. Figure 4 is one of the resulting calibrated trees. Precise description of the results are found in Supplementary data 4.
Figure 4.
Calibrated ruminant tree (see Supplementary Information 4 for the method and the results).
Discussion
The tree is structured by different groups of characters depending on the taxonomic level considered. All the characters of the cochlea (1, 6, 7, 24, 25, 26) contribute to the basic structure of the tree (main clades: Lagomeryx/more derived Cervidae; stem Cervidae/crown Cervidae, Capreolinae/Cervinae). Mennecart and Costeur29 and Costeur et al.31 revealed the ossification sequence of the bony labyrinth in different foetal stages of ruminants (Tragulidae and Bovidae). With the exception of structures that open in the brain cavity (vestibular aqueduct/endolymphatic sac and cochear aqueduct), the bony labyrinth mainly ossifies within three weeks, around mid gestation (Costeur et al.31). The cochlea and thus the pars cochlearis of the petrosal bone is the first part to ossify in the bony labyrinth31. The ossification timing differs from the developmental timing of the membranous inner ear (only known in humans) where the anterior, posterior, and lateral semicircular ducts are formed in that order, before the final coiling of the cochlea32. Nevertheles, the anterior canal (17 and 18) is the first fully ossified canal29. It contributes in structuring the tree at the subfamily level (i.e., Capreolinae and Cervinae). Terminal clades (O. lucasi + M. americana, O. virginianus + O. cf. virginianus, E. ctenoides + C. elaphus, M. giganteus + D. eurygonos + D. dama) are principally defined by characters of the endolymphatic sac (4, 9) and vestibular aqueduct (3, 8) both ossifying at last in the bony labyrinth29,31. The cochlear aqueduct still continues to grow after birth (Costeur et al.31). Characters of this structure (10, 11) are shown to distinguish closely related species (e.g. M. giganteus/D. dama lineage, A. axis/M. philisi, M. americana/O. lucasi). We show here the importance of reconstructing these structures precisely in a phylogenetic context.
Despite extensive data on well-studied fossils such as those of the putative Middle Miocene Muntiacini E. furcatus, calibrations of the origin of crown deer performed by molecular biologists often rely on fossil specimens attributed to extant genera (e.g. Muntiacus 3,7,14). Our identification of E. furcatus as a member of stem Cervinae allows us to recalibrate the cervid tree, as well as the timing of cladogenesis within the whole family (see Fig. 4). Our results indicate that crown Cervidae originated at least ca. 4 to 8 My earlier than previously estimated (from 8.5 Ma14 to 10.7 Ma7) at ca. 15.2 Ma. Similarly, the origin of the cervid subfamilies at crown level is suggested to be older than previously estimated. With an appearance at ca. 12.5 Ma for crown Cervinae and ca. 13.6 Ma for crown Capreolinae, respectively (Fig. 4), we set the origin of these clades ca. 4 to 5 My earlier (previous estimations lie between 7 and 9 Ma3,7,33) in the late Middle Miocene. Unfortunately, the cervid fossil record remains scarce for this period, especially in Asia where they originated. Cervavitus and Cervocerus from the Late Miocene (10 and 7 Ma, respectively) of Asia, the oldest known members of these clades, are either discussed as potential stem capreolines and cervines9 or even crown taxa34. Similarly, the estimation of the origin and diversification of the extant tribes is pushed back from 2 to 4 Ma in comparison to previous studies (see Fig. 4), occurring during the latest Miocene and Pliocene from ca. 6.5 to 3 Ma. This corresponds to a global climatic transition between the warm Late Miocene towards the onset of the Northern hemisphere glaciations35. In strong agreement with Duarte et al.36 and Hassanin et al.7, we find evidence for an early origin of the South American deer (early Late Miocene) long before the formation of the Panamian Isthmus. The oldest evidences of New World Cervidae are Eocoileus gentryorum, Odocoileus sp. and Bretzia pseudalces from the late Hemphillian, ca. 5 Ma37,38. These ruminants are rare and uncommon in Pliocene faunas39. However, their lineages were very successful, giving birth to several fossil genera (e.g. Navahoceros, Sangamona, Agalmoceros, Morenelaphus) and to the entire diversity of the extant South American Capreolinae1,37.
As a result of the cervid tree branch calibration, we propose new ages at different hierarchical levels of the ruminant tree, which are in some instances confirmed by earlier studies. The divergence of crown Pecora is estimated between 25 and 24 Ma, which corresponds to a peak of diversification of inermous pecoran ruminants of uncertain taxonomical affinities, first in Asia and then in Europe and North America40,41.
There is little consensus for the identification of the oldest stem deer8,9,16,20,21. It is mostly related to the presence of antlers in general and to their morphology. An array of Early and Middle Miocene Old World cervids bore branched cranial appendages similar to extant antlers but with a different morphology (among others, no beam and no burr)19. The identification of diagnostic characters for antlers has posed many problems. Evidence for deciduousness and regeneration of early “antlers”, fundamental features unique to deer today, add to the complexity16,19,21. In addition, some extant deer can i) lack antlers (Chinese water deer), ii) possess highly reduced antlers (tufted deer), or iii) show very tiny unbranched antlers (the Pudu). It stresses the high phenotypic plasticity and possible morphological convergences of this type of cranial appendage. “Protoantler” bearing taxa have often been hypothesised to be in their own family closely related to Cervidae, as for Lagomeryx or Ligeromeryx (Lagomerycidae e.g.,23), in different stem deer subfamilies, such as Procervulus and Heteroprox (Procervulinae16,20), and Dicrocerus, Acteocemas, or Stephanocemas (Dicrocerinae20), or even in crown deer, as again in Lagomeryx e.g.,8. Our phylogenetic analysis provides strong support that Lagomeryx, Procervulinae, and Dicrocerinae are not crown Cervidae, as preliminarily shown in Mennecart et al.30. However, the sister position of L. parvulus to all other cervids under study retrieved here prevents us from drawing any firm conclusion on its membership to the Cervidae lineage. Our shape analysis combined with the phylogenetic hypothesis unveil the modifications of the bony labyrinth that occur in deer evolution, such as thicker cochleas (character 6) and bulged lateral ampullas (character 21) in stem deer, becoming thinner and more flattened, respectively in crown species.
A surprising result is the stem position of the Late Miocene E. hezhengensis, which markedly differs from the general acceptance of a phylogenetic position deeply rooted within the Muntiacinae18,20,42–44—though all these analyses are mostly based on the shape of antlers. In agreement with our hypothesis, a recent phylogenetic analysis18 also places Eostyloceros blainvillei in an intermediate position between D. elegans and Muntiacus, the only crown cervid considered by the authors, thus preventing any firm conclusion on its crown or stem position. In addition, Deng et al.44 suggest a paraphyly of the genus Eostyloceros and exclude E. hezhengensis and all the species of Eostyloceros from the crown Cervidae. Despite the results of their phylogenetic analyses, the authors still consider Eostyloceros as a Muntiacinae due to its antler shape18,44. Our results confirm the published phylogenetic trees but firmly excludes Eostyloceros from crown Cervidae, and questions the widespread use of antler characteristics in phylogeny. Thus, stem deer may well have been still present during the Late Miocene in Asia.
Most molecular systematic analyses use the first appearance of the living muntjac’s genus Muntiacus (Muntiacus noringenensis at 9 Ma43) to calibrate the origin of crown deer e.g.,3,7,14. However, palaeontologists have proposed Euprox as the earliest crown deer— or even nested within Muntiacinae15–18,20,42,43,45. The first appearance of the genus (Euprox minimus) at ca. 14.5+/− 0.3 Ma is recorded in the Middle Miocene Austrian locality of Göriach46. Our phylogenetic analysis unambiguously proposes the hypothesis of E. furcatus as a stem Cervinae. Several characters (a rounded posterior ampulla, an elongated fenestra vestibuli, and a tightly coiled second cochlear turn) shared by E. furcatus and all the other analysed Cervinae support this hypothesis. Interestingly, its purported relationship with Muntiacus is not recovered. The single common character linking Euprox to other Muntiacinae is the strong backward inclination of the antlers’ pedicles also observed in Eostyloceros 16,20. This character is thus symplesiomorphic for crown deer. More recent phylogenetic analyses mentioned above18,44 do not seem to confirm the existence of a Muntiacinae clade that includes both Euprox and Eostyloceros, which is here (E. hezhengensis) excluded from Muntiacinae too and even from crown Cervidae. Several characters used as synapomorphies for Muntiacinae in these works have to be considered as symplesiomorphic for the crown Cervidae (e.g., true burr or centripetal mineralization of the antlers) and should not be used to define a subfamily or a tribe. E. furcatus is thus nested at the base of Cervinae pushing back the calibration of crown Cervidae by at least 4 My.
Our data provide new input for both the understanding and support of previous hypotheses on Plio-Pleistocene deer13. Dong47 considers the enigmatic Early Pliocene “C.” ruscinensis as a probable stem Cervidae, sister taxon to the poorly diagnosed Pliocervinae (see below and Croitor13). The result of our phylogenetic analysis places it within the stem Cervinae, in a more derived position than E. furcatus. The phylogenetic position of E. ctenoides within Cervini has often been discussed e.g.,48. Croitor13 indicates that species of this genus probably do not belong to the lineage of Przewalskium-Rusa-Cervus but does not conclude on any closer affinities with other deer taxa. In addition, even if Eucladoceros is not included into the Megacerines (sensu Vislobokova49), a close relationship with this group has been proposed10. Symplesiomorphic characters and a common evolutionary trend towards large size and heavy antlers could account for this49. Here we support close affinities of E. ctenoides from Senèze (in France) with C. elaphus. However, the well-defined Rusa-Cervus lineage is not clearly recovered in our analysis. Indeed, the insular species R. timorensis, which is considered to be more basal in our phylogenetic analysis, and C. nippon, included in a basal polytomy that includes all the Cervinae except R. timorensis, does not directly cluster with C. elaphus. Also, the position of R. timorensis and C. nippon in the bony labyrinth morphospace is very similar, distant from that of C. elaphus. C. nippon mainly differs from C. elaphus based on characters of the endolymphatic sac. Although nothing is yet known about how the bony labyrinth of ruminants evolves in an insular context (e.g. R. timorensis and possibly C. nippon), pressure release is known to induce significant morphological changes in the sense organs50. Accordingly, significant changes in the inner ear and bony labyrinth are also expected to occur. M. philisi is considered by Croitor51 as a sister taxon of the extant genus Axis based on cranial characteristics, while Valli48 and Pfeiffer10 found a closer relationship with Dama based on postcranial bones. Breda & Lister52 even attributed this species to the genus Pseudodama. The shape and position of the endolymphatic sac in M. philisi specimens is very similar to the conditions observed in Axis, being triangular in shape and starting at the level of the common crus. The relationships of Metacervocerus with Dama or Axis are not solved in our phylogenetic analysis and more than one species attributed to Metacervocerus could be present in Senèze. Indeed, the two Metacervocerus bony labyrinths under study display significant differences in orientation of the vestibular aqueduct recalling the Dama lineage. Croizetoceros pyreanicus and Croizetoceros ramosus have a very similar bony labyrinth, thereby confirming a close relationship13,20,47. They cluster together and are placed in a sister position to the crown Capreolinae. Their phylogenetic position has been discussed together with that of the extinct Pliocervinae because some raised arguments for the pliocervine Damacerus being their ancestor13. The Pliocervinae, observed to be holometacarpal deer (i.e., lateral digit full retention, a plesiomorphic cervid character), have a long history of debated affinities and could be a polyphyletic group containing early representatives of the Cervinae lineage, as well as Capreolinae and stem Cervidae (see e.g., Grubb12, Dong34, Croitor13 for competing opinions). In contrast, Croizetoceros is a plesiometacarpal deer48,53, but possible convergences in the reduction of the lateral digits of the foot and hand may have occurred13,15. C. pyreanicus emerges therefore as one of the oldest recognised Capreolinae, being already present in the Late Miocene of Spain13,20. In addition Croitor13 synonymises Pliocervinae with Capreolinae thus leaving a tribe Pliocervini within the Capreolinae subfamily, thus adding to the complex picture of the affinities of Plio-Pleistocene deer. A definitive conclusion about Croizetoceros cannot be proposed here because of the small number of characters involved in its position.
Excluding the enigmatic dromomerycine Surameryx acrensis (from the Late Miocene of Bolivia/Brazil, ca. 9 Ma54), Capreolinae are the only ruminants that have colonised South America. This could partly explain their prominent morphological and ecological diversity1,37. At least 12 species of South American Capreolinae are currently described, which constitutes over 20% of the whole diversity of Cervidae2,36. The basal polytomy of New World Capreolinae reflects the huge shape diversity of the bony labyrinth highlighted in Fig. 1. Bony labyrinth morphology of Old World Capreolinae differs from that of New World ones. This morphological shift of the bony labyrinth may be related to the release of ecological pressure or an ecological readjustement after the colonisation of America. The evolution of South American deer with the presence of ambush predator only “fosters low rates of reproduction, tiny young, low neonatal investment, and small adult body size” (sic. Geist1), that recalls insular evolutionary pressure and release of ecological constraints. Based on DNA data, Mazama seems to be a polyphyletic genus2,7,36,55. Interestingly, the species M. americana is part of the Odocoileus lineage, which coincides with earlier works7,14,36. Escobedo-Morales et al.55 and Heckeberg et al.2 even found evidence that some Mazama species are deeply nested within Odocoileini. In our tree M. americana clusters with the investigated specimen of O. lucasi from the Pleistocene (skull described by Rössner56) adding some more weight to the previous hypotheses. O. virginianus and O. cf. virginianus cluster together but some differences can be observed between the two specimens. Contrary to Heckeberg and Rössner57, we cannot support the view that they represent the same species and we suggest instead a sister relationship for them.
All the Old World Capreolinae (H. inermis, C. capreolus, and A. alces) cluster together, as already evidenced by numerous DNA analyses e.g.,2,3,6,7,58. The Chinese water deer H. inermis is the only antlerless deer, but with enormous upper canines instead. Its phylogenetic position has long been discussed since morphology, behaviour, and molecular markers led to contrasting hypotheses e.g.,2,7,8,11,58–60. Its primitive morphology and certain behavioural features (also found in tragulids, a sister group to pecoran ruminants) have led many of the aforementioned authors to propose Hydropotes as the sister taxon to antlered Cervidae. Only recent molecular data-based analyses unambiguously relate it to the Old World Capreolinae, close to the roe deer C. capreolus 2,3,6,7,58. Its telemetacarpal condition and its post-glenoid foramen support this hypothesis and strongly suggests that antlers were secondarily lost in the Chinese water deer. Our phylogenetic analysis places the bony labyrinth of H. inermis among those of the crown Capreolinae (which is supported by six characters) as a sister taxon of the Old World Capreolinae A. alces and C. capreolus. The latter monophyletic clade is supported by three characters (a symmetrical endolymphatic sac, a curved cochlear aqueduct, and an undulating posterior canal), with Hydropotes being excluded from the Alces-Capreolus group by a shorter cochlear aqueduct only. The bony labyrinth adds to the very few morphological features that relate Hydropotes to the Old World Capreolinae and confirms the results obtained from molecular data.
The phylogenetic position of the giant deer M. giganteus within the Cervinae is corroborated by both morphological and molecular analyses e.g.,1,5,6,61,62. The oldest Megacerine (and therefore crown Cervini) corresponds to the Late Miocene Praesinomegaceros (Tortonian, more than 7.25 Ma49,62). There is a growing consensus on the relationships of Megaloceros and the fallow deer Dama based on molecular and morphological data1,5,6,63. Some results had been obtained from these approaches that linked it to the Cervus lineage10,61, e.g., Kuehn et al.61 suggested that M. giganteus is conspecific to C. elaphus, but this result is probably due to modern contamination of the DNA samples. In our analysis, Dama species and Megaloceros share a similar cochlear aqueduct and endolymphatic sac and cluster together. Our results also support that D. eurygonos from Val d’Arno (ca. 1.5 Ma) belongs to an archaic lineage of fallow deer as suggested by Croitor51.
Material and Methods
Material
We used 49 specimens (see Supplemenatry data 1 for information about their origin, age, host institution, and scanning parameters) including Miocene (25 specimens from 7 species), Pliocene (2 specimens from 2 species), and Pleistocene (10 specimens from 8 species) deer from Europe, Northern America, and Asia, and extant (12 specimens from 10 genera and 12 species) deer. Our dataset therefore comprises more than half of the current cervid diversity (for a total of 1764, 1859, or 197 genera). The fossil species were selected in order to encompass two of the oldest known ruminants possessing deciduous cranial appendages (Lagomeryx parvulus and Procervulus praelucidus 22) and most of the Plio-Pleistocene genera, which are part of the Cervidae radiation during glacial episods. The nomenclature of Plio-Pleistocene fossils here used follows Croitor51. The terminology of the bony labyrinth follows Ekdale26, Macrini27, and Mennecart & Costeur28. The 3D data are available from the corresponding author on reasonable request.
Geometric morphometrics analyses
Left bony labyrinths were preferably selected when available. If not, the right one was included for analysis using the reflect application of Landmark Editor 3.6 software65. Digitizing of the specimens was also performed using this software. 77 curves of 10 semi landmarks and 1 landmark were digitised on the surface of the specimens following the protocol described in Mennecart and Costeur29. Shape variation in bony labyrinth morphology (disparity and similarity) was studied using the geometric morphometrics methods implemented in MorphoJ 1.06d software66. Principal component analysis is used to visualise the overall shape variation among specimens. It encompasses 3 species of Procervulinae (11 specimens), 1 species of Dicrocerinae (3 specimens), 6 living species of Capreolinae (7 specimens), and 6 living species of Cervinae (6 specimens) for which we have a phylogenetic control7,30. We included several specimens of fossil species as a control on the taxonomy and to increase the number of observations in the predefined groups of the following ordination method. A permutation test67 was performed to test the presence or absence of a phylogenetic signal in the overall shape of the bony labyrinth (randomised rounds: 1000), that could be assimilated to phenetics29. Mennecart & Costeur29 demonstrated, using this methodology, that intraspecific variability is lower than interspecific variation, and may be phylogenetically informative. This observation has been confirmed in many mammal groups (e.g. cetaceans68,69; primates70; primitive artiodactyls71) except for sloths, which exhibit extremely slow locomotion72.Thus, the hypothetical specimen mean (consensus of all specimens from a species) was used for the phylogenetic test to not artificially decrease the homoplasy. The phylogenetic tree was created using Mesquite 3.04 software73. Klingenberg & Gidaszewski67 remind us that geometric morphometrics results cannot be directly used as phylogenetic characters. An ordination method and splitting the shape into a set of multiple characters are methods already used (see Klingenberg & Gidaszewski67 for an exhaustive review). Mennecart & Costeur28 and Mennecart et al.30 have shown that morphological characters of the bony labyrinth may be phylogenetically significant when used separately in a cladistics analysis. Moreover, Costeur et al.31 have demonstrated the timing of the bony labyrinth ossification in an ontogenetic series of a pecoran ruminant. The different structures ossify diachronously and may be independent31. We should also highlight that the ecological impact on the morphology the bony labyrinth, if existing, is limited because all the studied ruminants possess a similar kind of locomotion and live in relatively similar environments. Mennecart & Costeur29 and Costeur et al.31 already pointed out that the open structures (i.e. the endolymphatic sac and the cochlear aqueduct) suffer from a strong intraspecific allometric effect due to continuous ossification of these structures long after birth (contrary to the rest of the bony labyrinth). Thus, these regions are not analyzed to avoid a Pinocchio effect. Similarly to Billet et al.25,74, we observed a weak allometric effect on bony labyrinth shape (see Supplementary data 5). The allometric effect is mainly based on the semi-circular canal proportions in comparison to the entire bony labyrinth. In our dataset, the centroid size may predict 8.5% of the total shape changes and there is a highly significant covariation between the bony labyrinth shape and the centroid size (p-value < 0.0001). However, the centroid size is not predictive for the bony labyrinth shape (R2 = 0.6672) and is not influenced by phylogeny (p-value = 0.2586). Since our data matrix for the phylogeny uses the semi-circular canal shape individually, and not the ratio between the canals and the bony labyrinth, the allometric effect does not affect our dataset. Thus, the semi-circular canals, the fenestra vestibuli, and the cochlea were treated here as separate and independent structures using an ordination method (CVA). This method was applied to maximise the between-group variation, relative to within-group groups according to the specified chosen grouping variable; here the well-defined cervid subfamilies Procervulinae, Dicrocerinae, Cervinae, and Capreolinae. Shape differences expressed along the axes are scaled morphological distances relative to within group variation. These shape differences are used as morphological characters in our cladistics matrix as additional characters to the matrix proposed by Mennecart and Costeur28 and coded for all the studied specimens (fossil and extant). We have included fossil specimens of well-known subfamilies (2 Capreolinae and 5 Cervinae), that were not in the PCA since their precise phylogenic position is still under discussion, to increase the number of observation per subfamilies (9 Procervulinae, 3 Dicrocerinae, 13 Cervinae, and 11 Capreolinae). Nevertheless, the low number of Dicrocerinae specimens may influence its morphospace relative to the other subfamilies. All statistical results are shown in the Supplementary data 5.
Cladistics analysis
A cladistic analysis based on a matrix of 26 characters (see Supplementary data 3) of the bony labyrinth was also performed to test the phylogenetic power of the bony labyrinth: 12 characters of the semi-circular canals, 7 on the vestibule and associated structures, and 7 characters on the cochlea. New characters expand the matrix of Mennecart and Costeur28 and are derived from the CVA (see Supplementary data 2, 3, 5, and 6). 29 species of antlered ruminants are included in these analyses (see Supplementary data 1) to test the phylogenetic relationships of the fossil L. parvulus, E. furcatus, Eostyloceros hezhengensis, “Cervus” ruscinensis, Croizetoceros pyreanicus, Croizetoceros ramosus, Metacervoceros philisi, and Megaloceros giganteus within the Cervidae lineage and find new characters to characterise Cervidae tribes. The earliest artiodactyl Homacodon vagans from the Eocene of North America was chosen as outgroup75,76. The small number of character states (60) in comparison to the number of taxa (30) cannot provide a robust relationship. We are testing here the relevance of the bony labyrinth solely as a tool for phylogeny and why this structure should be systematically used in character matrices. The analysis was performed using WinClada77. All characters were unordered and equally weighted. We ran a heuristic search (1000 maximum trees to keep, 5 replications), which resulted in 30 most parsimonious trees of 91 steps (retention index Ri: 0.35; consistency index Ci: 0.74) and used the Nelson consensus compromise (collaps + consensus) to optimise the graphic result (95 steps; Ri 33: Ci: 72). For each node, the list of non-ambiguous synapomorphies is provided in Supplementary data 3.
Calibrated tree
The complete mitochondrial genome matrix of Hassanin et al.7 was pruned down to include just cervids plus a few outgroup representatives of the remaining pecoran families. Cytochrome b gene data was added for Megaloceros giganteus (Genbank Accession number AM182644)6. After some tests of the combined morphological and molecular datasets, the early fossil artiodactyl Homacodon vagans (~47 Ma) was not included in the analysis because there is far too much of a temporal and phylogenetic gap between it and the next taxa for which morphological data was scored (Lagomeryx parvulus, Procervulus prealucidens, ~18 Ma). Its inclusion or exclusion did not significantly affect divergence estimates for the critical part of the tree (Pan-Cervidae), which, as detailed below, were largely controlled by the prior setting for the age of Pecora. The 34 partitions of the mtDNA data used in Hassanin et al.7 were analyzed in Partition Finder v.1.178 using the greedy search and GTR + G model. Best partitioning scheme indicated eight partitions. The morphological dataset comprised a ninth partition. Analysis xml files from the morphological and molecular data matrices were set up using Beastmaster79 and R80. The GTR + G model was used for all molecular partitions, the MK model for morphology. The SABDSKY treemodel was used, which allows for fossil tips to be ancestral nodes (though experimental runs with BDSKY produced similar results, not shown). Two main analyses were run. 1) A minimal constraint analysis in which the root age (Pecora) was set to a uniform distribution of 19-25 Ma (dubbed the ‘Min Constraint’ analysis), and only a single topological constraint forcing all extant fossil cervids and stem cervids to be monophyletic; and 2) an analysis with the same root prior plus many constraints (‘All Constraints’ analysis) following previous the topology of previous molecular studies as well as the parsimony analysis based only on the inner ear characters in this paper. This analysis constrained pecoran relationships with Antilocapra branching off first, followed by Giraffa, Bovidae + Moschidae, then Cervidae e.g.,7, Lagomeryx basal to all other pan-cervids, Euprox furcatus was united with the Cervinae, and Croizetocerus spp. with the Capreolinae. As in the ‘Min Constraint’ analysis, root age was set to a uniform 19–25 Ma distribution. A third experimental analyses was run same as the Min Constraint analysis, but with a wider prior on the root (19–35 Ma, ‘Old Pecora’ analysis). All analyses used the included fossil taxa as tip dates, with age assigned as a range based on the uncertainty of that taxon’s first appearance datum. This information is given for each taxon in a separate table (Supplementary data 4). The phylogenetic analysis was run in BEAST v.2.2.181 for 10 million generations. The morphological character matrix was separately analyzed using PAUP* 482 using a heuristic search of 1000 replicates with TBR and a random addition sequence. Bootstrap was run for 100 replicates, each holding a single TBR replicate.
Fossil host institutions
SNSB-BSPG – Staatliche Naturwissenschaftliche Sammlungen Bayerns - Bayerischen Staatssammlung für Paläontologie und Geologie, Munich (Germany); MNHN – Muséum National d’Histoire Naturelle, Paris (France); NMB – Naturhistorisches Museum Basel (Switzerland); ICP – Institut Català de Paleontologia Miquel Crusafont, Barcelona (Spain); IVPP – Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Science, Beijing (China); CCEC – Musée des Confluences, Lyon (France).
Electronic supplementary material
Acknowledgements
This research was supported by the Swiss National Foundation (SNF Project 200021_159854/1 and P2ZHP3_162102), the Stiftung zur Förderung des NMB, and the Kugler-Werdenberg Stiftung. We also thank support from the Spanish Ministerio de Economía y Competitividad (project CGL2016-76431-P) and the National Natural Science Foundation of China (Grant No. 41430102). We thank the MNHN UMS 2700, and the AST-RX, plate-forme for CT scanning, especially M. Garcia-Sanz (UMR7207). BMe warmly thanks Soledad De Esteban-Trivagno (Transmitting Science and ICP), Chris Klingenberg (University of Manchester) and Melissa Tallman (Grand Valley State University) for their precious help in geometric morphometrics through the Transmitting Science programm. Maeva Orliac (CNRS) is thanked for providing Homacodon’ data and Thomas Hiller for providing new comparative material. We are greatful to Christine Argot and Guillaume Billet for access to MNHN specimens, Didier Berthet and François Vigouroux for CCEC specimens. The authors are grateful for the constructive suggestions from the anonymous reviewers and the editor Adrian Lister.
Author Contributions
B.Me. and L.C. wrote the manuscript, designed, and organised the project. B.Me. designed the cladistics and the Geometric Morphometric analyses and analysed the data; F.B. did the DNA based analyses and the molecular dating; B.Me., G.R., G.M., G.S., D.D.M., S.W., J.N., B.Mü., and L.C. contributed to material collection and to the editing of the article. All authors approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-12848-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Geist, V. Deer of the world their evolution, behaviour, and ecology (Stackpole Books, 1998).
- 2.Heckeberg NS, Erpenbeck D, Wörheide G, Rössner GE. Systematic relationships of five newly sequenced cervid species. PeerJ. 2016;4:e2307. doi: 10.7717/peerj.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitra C, Fickel J, Meijaard E, Groves PC. Evolution and Phylogeny of Old World deer. Mol. Phylogenet. Evol. 2004;33:880–895. doi: 10.1016/j.ympev.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Kuznetsova MV, Kholodova MV, Danilkin AA. Molecular phylogeny of Deer (Cervidae: Artiodactyla) Russ. J. Genet. 2005;41(7):742–749. doi: 10.1007/s11177-005-0154-1. [DOI] [PubMed] [Google Scholar]
- 5.Lister AM, et al. The phylogenetic position of the “giant deer”. Megaloceros giganteus. Nature. 2005;438:850–853. doi: 10.1038/nature04134. [DOI] [PubMed] [Google Scholar]
- 6.Hugues S, et al. Molecular phylogeny of the extinct giant deer. Megaloceros giganteus. Mol. Phylogenet. Evol. 2006;40:285–291. doi: 10.1016/j.ympev.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Hassanin A, et al. Pattern and timing of diversification of Cetartiodactyla (Mammalia, Laurasiatheria), as revealed by a comprehensive analysis of mitochondrial genomes. C. R. Biol. 2012;335:32–50. doi: 10.1016/j.crvi.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Janis CM, Scott KM. The interrelationships of higher ruminant families with special emphasis on the members of the Cervoidea. Am. Mus. Novit. 1987;2893:1–85. [Google Scholar]
- 9.Gentry AW. The Miocene differentiation of old world Pecora (Mammalia) Hist. Biol. 1994;7(2):115–158. doi: 10.1080/10292389409380449. [DOI] [Google Scholar]
- 10.Pfeiffer T. The first complete skeleton of Megaloceros verticornis (Dawkins, 1868) Cervidae, Mammalia, from Bilshausen (Lower Saxony, Germany): description and phylogenetic implications. Mitt. Mus. Nat. Berlin. 2002;5:289–308. [Google Scholar]
- 11.Hernández Fernández M, Vrba ES. A complete estimate of the phylogenetic relationships in Ruminantia: a dated species-level supertree of the extant ruminants. Biol. Rev. 2005;80(2):269–302. doi: 10.1017/S1464793104006670. [DOI] [PubMed] [Google Scholar]
- 12.Grubb DCP. Valid and invalid nomenclature of living fossil deer, Cervidae. Acta Theriol. 2000;45(3):289–307. doi: 10.4098/AT.arch.00-30. [DOI] [Google Scholar]
- 13.Croitor R. Deer from the Late Miocene to Pleistocene of Western Palearctic: matching fossil record and molecular phylogeny data. Zitteliana B. 2014;32:115–153. [Google Scholar]
- 14.Gilbert C, Ropiquet A, Hassanin A. Mitochondrial and nuclear phylogenies of Cervidae (Mammalia, Ruminantia): Systematics, morphology, and biogeography. Mol. Phylogenet. Evol. 2006;40:101–117. doi: 10.1016/j.ympev.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Bubenik, G. A. & Bubenik, A. B. Horns, Pronghorns, and Antlers (Springer-Verlag 1990).
- 16.Azanza B. Sur la nature des appendices frontaux des cervidés (Artiodactyla, Mammalia) du Miocène inférieur et moyen. Remarques sur leur systématique et leur phylogénie. C. R. Acad. Sci. Paris. 1993;316:1163–1169. [Google Scholar]
- 17.Azanza B, Rössner GE, Ortiz-Jaureguizar E. The early Turolian (late Miocene) Cervidae (Artiodactyla, Mammalia) from the fossil site of Dorn-Dürkheim 1 (Germany) and implications on the origin of crown cervids. Palaeobiodivers. palaeoenviron. 2013;93:217–258. doi: 10.1007/s12549-013-0118-8. [DOI] [Google Scholar]
- 18.Hou S. A new species of Euprox (Cervidae, Artiodactyla) from the upper Miocene of the Linxia Basin, Gansu Province, China, with interpretation of its paleoenvironment. Zootaxa. 2015;3911(1):43–62. doi: 10.11646/zootaxa.3911.1.2. [DOI] [PubMed] [Google Scholar]
- 19.DeMiguel. D, Azanza B, Morales M. Key innovations in ruminant evolution: a paleontological perspective. Integr. Zool. 2014;9:412–433. doi: 10.1111/1749-4877.12080. [DOI] [PubMed] [Google Scholar]
- 20.Azanza BLosCervidae. (Artiodactyla, Mammalia) del Mioceno de Las Cuencas del Duero, Tajo, Calatayud-Teruel y Levante. Mem. Mus. Pal. Zarag. 2000;8:1–376. [Google Scholar]
- 21.Rössner GE. Odontologische und schädelanatomische Untersuchungen an Procervulus (Cervidae, Mammalia) Münchner Geowiss. Abh. A. 1995;29:1–127. [Google Scholar]
- 22.Heckeberg NS. Origination of antlerogenesis. J. Morphol. 2016;278(2):182–202. doi: 10.1002/jmor.20628. [DOI] [PubMed] [Google Scholar]
- 23.Azanza B, Ginsburg L. A revision of the large lagomerycid artiodactyls of Europe. Palaeontology. 1997;40(2):107–128. [Google Scholar]
- 24.Lebrun R, de Leon MP, Tafforeau P, Zollikofer C. Deep evolutionary roots of strepsirrhine primate labyrinthine morphology. J. Anat. 2013;216:368–380. doi: 10.1111/j.1469-7580.2009.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billet G, Hautier L, Lebrun R. Morphological diversity of the bony labyrinth (inner ear) in extant Xenarthrans and its relation to phylogeny. J. Mammal. 2015;96(4):658–672. doi: 10.1093/jmammal/gyv074. [DOI] [Google Scholar]
- 26.Ekdale EG. Comparative Anatomy of the Bony Labyrinth (Inner Ear) of Placental Mammals. PLoS ONE. 2013;10(8):e0137149. doi: 10.1371/journal.pone.0137149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macrini TE, Flynn JJ, Ni X, Croft DA, Wyss AR. Comparative study of notoungulate (Placentalia, Mammalia) bony labyrinths and new phylogenetically informative inner ear characters. J. Anat. 2013;223:442–461. doi: 10.1111/joa.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mennecart B, Costeur L. Dorcatherium (Mammalia, Ruminantia, Middle Miocene) petrosal bone and the tragulid ear region. J. Vert. Paleontol. 2016;36(6):e1211665. doi: 10.1080/02724634.2016.1211665. [DOI] [Google Scholar]
- 29.Mennecart B, Costeur L. Shape variation and ontogeny of the ruminant bony labyrinth, an example in Tragulidae. J. Anat. 2016;229(3):422–435. doi: 10.1111/joa.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mennecart B, et al. The petrosal bone and bony labyrinth of early to middle Miocene European deer (Mammalia, Cervidae) reveal their phylogeny. J. Morphol. 2016;277:1329–1338. doi: 10.1002/jmor.20579. [DOI] [PubMed] [Google Scholar]
- 31.Costeur L, Mennecart B, Müller B, Schultz G. Prenatal growth stages show the development of the ruminant bony labyrinth and petrosal bone. J. Anat. 2017;230(2):347–353. doi: 10.1111/joa.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toyoda S, et al. Morphogenesis of the inner ear at different stages of normal human development. Anat. Rec. 2015;298(12):2081–2090. doi: 10.1002/ar.23268. [DOI] [PubMed] [Google Scholar]
- 33.Bibi F. A. multi-calibrated mitochondrial phylogeny of extant Bovidae (Artiodactyla, Ruminantia) and the importance of the fossil record to systematics. BMC Evol. Biol. 2013;13(1):166. doi: 10.1186/1471-2148-13-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong W. Reconsideration of the systematics of the early Pleistocene Cervivatus (Cervidae, Artiodactyla, Mammalia) Estud. Geol. 2011;67(2):603–611. doi: 10.3989/egeol.40534.208. [DOI] [Google Scholar]
- 35.Hilgen, F. J., Lourens, L. J., & Van Dam, J. A. in A geological time scale (Gradstein, F. M., Ogg, J. G., Shmitz, M., & Ogg, G. ed.) 923–978 (Elsevier, 2013).
- 36.Duarte JMB, Gonzalez S, Maldonado JE. The surprising evolutionary history of South American deer. Mol. Phylogenet. Evol. 2008;49:17–22. doi: 10.1016/j.ympev.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Webb, S. D. In Antelopes, Deer, and Relatives (Vrba, E. S. & Schaller, G. B. ed.) 39–64 (Yale University Press, 2000).
- 38.Gustasfon EP. An Early Pliocene North American deer: Bretzia pseudalces, its osteology, biology, and place in cervid history. Bull. Mus. Nat. Hist. Oregon. 2015;25:1–75. [Google Scholar]
- 39.Webb, S. D. In Evolution of Tertiary mammals of North America (Janis, C. M., Scott, K. M., & Jacobs, L. L. ed.) 508–510 (Cambridge University Press, 1998).
- 40.Mennecart, B. The European Ruminants during the “Microbunodon Event” (MP28, Latest Oligocene): Impact of Climate Changes and Faunal Event on the Ruminant Evolution. PLoS ONE10, e0116830 10.1371/journal.pone.0116830 (2015). [DOI] [PMC free article] [PubMed]
- 41.Métais, G., Mennecart, B. & Ghazala, R. New assemblage of stem pecoran ruminants from the Oligocene Chitarwata Formation, Bugti Hills, Baluchistan, Pakistan. J. Asian Earth Sci. 136:40–49, 10.1016/j.jseaes.2016.09.009 (2017).
- 42.Dong W, Pan Y, Liu J. The earliest Muntiacus (Artiodactyla, Mammalia) from the Late Miocene of Yuanmou, southwestern China. C. R. Palevol. 2004;3:379–386. doi: 10.1016/j.crpv.2004.06.002. [DOI] [Google Scholar]
- 43.Dong W. New material of Muntiacinae (Artiodactyla, Mammalia) from the Late Miocene of the northeastern Qinghai-Tibetan Plateau, China. C. R. Palevol. 2007;6:335–343. doi: 10.1016/j.crpv.2007.05.002. [DOI] [Google Scholar]
- 44.Deng T, Wang S-Q, Shi Q-Q, Li Y-K, Li Y. A new species of Eostyloceros (Cervidae, Artiodactyla) from the Late Miocene of the Linxia Basin in Gansu, China. Zootaxa. 2014;3893(3):363–381. doi: 10.11646/zootaxa.3893.3.3. [DOI] [PubMed] [Google Scholar]
- 45.Wang L-H, Zhang Z-Q. A new species of Euprox (Cervidae, Mammalia) from the Middle Miocene of Daniao, Nei Mongol, China. Vertebrat. Palasiatic. 2011;49(4):365–376. [Google Scholar]
- 46.Aiglstorfer M, Rössner GE, Böhme M. Dorcatherium naui and pecoran ruminants from the late Middle Miocene Gratkorn locality (Austria) Palaeobiodivers. palaeoenviron. 2014;94(1):83–123. doi: 10.1007/s12549-013-0141-9. [DOI] [Google Scholar]
- 47.Dong W. Les Cervidae (Artiodactyla) Ruscinien (Pliocène) du Languedoc et du Roussillon (France) Bull. Mus. Nat. His. Nat. Paris. 1996;18:133–163. [Google Scholar]
- 48.Valli AMF. Les Cervidae du gisement Pliocène supérieur (Villafranchien moyen) de Saint-Vallier (Drôme, France) Géobios. 2004;37:S191–S232. doi: 10.1016/S0016-6995(04)80016-0. [DOI] [Google Scholar]
- 49.Vislobokova IA. Morphology, taxonomy, and phylogeny of Megacerines (Megacerini, Cervidae, Artiodactyla) Paleontol. J. 2013;47(8):833–950. doi: 10.1134/S0031030113080017. [DOI] [Google Scholar]
- 50.Köhler M, Moyà-Solà S. Reduction of brain and sense organs in the fossil insular bovid. Myotragus. Brain Behav. Evol. 2004;63(3):125–140. doi: 10.1159/000076239. [DOI] [PubMed] [Google Scholar]
- 51.Croitor R. Early Pleistocene small-sized deer ofEurope. Hell. J. Geosci. 2006;41:89–117. [Google Scholar]
- 52.Breda M, Lister A. Dama roberti, a new species of deer from the early Middle Pleistocene of Europe, and the origins of modern fallow deer. Quat. Sci. Rev. 2013;69:155–167. doi: 10.1016/j.quascirev.2013.01.029. [DOI] [Google Scholar]
- 53.Garrido G. Reflections on the artiodactyls of the upper Villafranchian represented in the fossil record of the Fonelas P-1 site. Cuad. Mus. Geominero. 2008;11:279–335. [Google Scholar]
- 54.Prothero DR, Campbell KE, Jr, Beatty BL, Frailey CD. New Late Miocene dromomerycine artiodactyl from the Amazon Basin: implications for interchange dynamics. J. Vert. Paleontol. 2014;88(3):434–443. doi: 10.1666/13-022. [DOI] [Google Scholar]
- 55.Escobedo-Morales LA, et al. First phylogenetic analysis of Mesoamerican brocket deer Mazama pandora and Mazama temama (Cetartiodactyla: Cervidae) based on mitochondrial sequences: implications on neotropical deer evolution. Mamm. Biol. 2016;81(3):303–313. doi: 10.1016/j.mambio.2016.02.003. [DOI] [Google Scholar]
- 56.Rössner GE. Teilschädel eines nordamerikanischen Berghirshes mit einem selten überlieferten Stadium des Gewihzyklus (Odocoileus lucasi Hay, 1927) SNSB-BSPG 2015 I 37. Jahresb. 2015 Mitt. Freunde Bayer. Staatssamml.Paläontol. Hist. Geol. München. 2015;44:59–60. [Google Scholar]
- 57.Heckeberg, N. S. & Rössner, G. E. Schädel eines pleistozänen Weißwedelhirsches (Odocoileus virginianus) aus den USA. Jahresb. 2012 Mitt. Freunde Bayer. Staatssamml.Paläontol. Hist. Geol. München41, 39–41 (2013).
- 58.Randi E, Mucci N, Pierpaoli M, Douzery EJP. New phylogenetic perspectives on the Cervidae (Artiodactyla) are provided by the mitochondrial cytochrome b gene. P. Roy. Soc. B. 1998;265:793–801. doi: 10.1098/rspb.1998.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Groves, C. P. & Grubb, P. In Biology and management of the Cervidae (Wemmer, C. M. ed.) 21–59 (Smithsonian Institution Press, 1987).
- 60.Dubost G, Charron F, Courcoul A, Rodier A. Social organization in the Chinese water deer. Hydropotes inermis. Acta Theriol. 2011;56(2):198–198. [Google Scholar]
- 61.Kuehn R, Ludt CJ, Schroeder W, Rottman O. Molecular phylogeny of Megaloceros giganteus – the giant deer or just a fiant red deer? Zool. Sci. 2005;22:1031–1044. doi: 10.2108/zsj.22.1031. [DOI] [PubMed] [Google Scholar]
- 62.Vislobokova IA. The most ancient megacerine deer from the late Miocene of Siberia and its implications to the evolution of the group. Palaeoworld. 2009;18:278–281. doi: 10.1016/j.palwor.2009.09.002. [DOI] [Google Scholar]
- 63.Immel A, et al. Mitochondrial genomes of giant deers suggest their late survival in Central Europe. Sci. Rep. 2015;5:10853. doi: 10.1038/srep10853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nowak, R. M. Walker’s mammals of the world (6th edition) (The John Hopkins University Press, 1999).
- 65.Wiley D. Landmark Editor 3.6. (Institute for Data Analysis and Visualization. Davis: University of California. 2006).
- 66.Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011;11:353–357. doi: 10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- 67.Klingenberg CP, Gidaszewski NA. Testing and quantifying phylogenetic signals and homoplasy in morphometric data. Syst. Biol. 2010;59(3):245–261. doi: 10.1093/sysbio/syp106. [DOI] [PubMed] [Google Scholar]
- 68.Aguirre-Fernández G, Mennecart B, Sánchez-Villagra MR, Sánchez R, Costeur L. A dolphin fossil ear bone from the northern Neotropics–insights into habitat transitions in iniid evolution. J. Vert. Paleontol. 2017 [Google Scholar]
- 69.Schnitzler JG, Frédérich B, Früchtnicht S, Schaffeld T, Baltzer J, Ruser A, Siebert U. Size and shape variations of the bony components of sperm whale cochleae. Sci. Rep. 2017;7:46734. doi: 10.1038/srep46734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gunz P, Ramsier M, Kuhrig M, Kuhrig M, Hublin JJ, Spoor F. The mammalian bony labyrinth reconsidered, introducing a comprehensive geometric morphometric approach. J. Anat. 2012;220:529–543. doi: 10.1111/j.1469-7580.2012.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orliac, M. J., Araújo, R., & Lihoreau, F. The petrosal and bony labyrinth of Diplobune minor, an enigmatic Artiodactyla from the Oligocene of Western Europe. J. Morphol. 10.1002/jmor.20702 (2017) [DOI] [PubMed]
- 72.Billet G, Hautier L, Asher RJ, Schwarz C, Crumpton N, Martin T, Ruf I. High morphological variation of vestibular system accompanies slow and infrequent locomotion in three-toed sloths. Proc. R. Soc. B. 2012;279:3932–3939. doi: 10.1098/rspb.2012.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maddison, W. P. & Maddison, D. R. Mesquite: a modular system for evolutionary analysis. Version 3.04. (2010).
- 74.Billet G, Germain D, Ruf I, Muizon Cde, Hautier L. The inner ear of Megatherium and the evolution of the vestibular system in sloths. J. Anat. 2013;223:557–567. doi: 10.1111/joa.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Orliac M, Benoit J, O’Leary MA. The inner ear of Diacodexis, the oldest artiodactyl mammal. J. Anat. 2012;221:417–426. doi: 10.1111/j.1469-7580.2012.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orliac M, O’Leary MA. Comparative anatomy of the petrosal bone of Dichobunoids, early members of Artiodactylamorpha (Mammalia) J. Mamm. Evol. 2014;21:299–320. doi: 10.1007/s10914-014-9254-9. [DOI] [Google Scholar]
- 77.Nixon, K. C. WinClada (BETA). Version 1.00.08. (Nixon, 2002).
- 78.Lanfear R, Calcott B, Ho SY, Guindon S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012;29:1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- 79.Matzke N. J. BEASTmasteR: R tools for automated conversion of NEXUS data to BEAST2 XML format, for fossil tip-dating and other uses, http://phylo.wikidot.com/beastmaster (2015).
- 80.R_Core_Team. R: A language and environment for statistical computing., R Foundation for Statistical Computing (2014).
- 81.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swofford, D. L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) Version 4. Sinauer Associates (2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.