Summary
Background
The economic burden on households affected by tuberculosis through costs to patients can be catastrophic. WHO's End TB Strategy recognises and aims to eliminate these potentially devastating economic effects. We assessed whether aggressive expansion of tuberculosis services might reduce catastrophic costs.
Methods
We estimated the reduction in tuberculosis-related catastrophic costs with an aggressive expansion of tuberculosis services in India and South Africa from 2016 to 2035, in line with the End TB Strategy. Using modelled incidence and mortality for tuberculosis and patient-incurred cost estimates, we investigated three intervention scenarios: improved treatment of drug-sensitive tuberculosis; improved treatment of multidrug-resistant tuberculosis; and expansion of access to tuberculosis care through intensified case finding (South Africa only). We defined tuberculosis-related catastrophic costs as the sum of direct medical, direct non-medical, and indirect costs to patients exceeding 20% of total annual household income. Intervention effects were quantified as changes in the number of households incurring catastrophic costs and were assessed by quintiles of household income.
Findings
In India and South Africa, improvements in treatment for drug-sensitive and multidrug-resistant tuberculosis could reduce the number of households incurring tuberculosis-related catastrophic costs by 6–19%. The benefits would be greatest for the poorest households. In South Africa, expanded access to care could decrease household tuberculosis-related catastrophic costs by 5–20%, but gains would be seen largely after 5–10 years.
Interpretation
Aggressive expansion of tuberculosis services in India and South Africa could lessen, although not eliminate, the catastrophic financial burden on affected households.
Funding
Bill & Melinda Gates Foundation.
Introduction
In May, 2014, the World Health Assembly ratified the End TB Strategy, which set aspirational worldwide objectives for tuberculosis control: reductions of 50% in global tuberculosis incidence and 75% in tuberculosis mortality by 2025, and of 90% and 95%, respectively, by 2035.1 National policy makers are now deciding which interventions to implement to achieve these targets. The TB Modelling and Analysis Consortium assessed in its TB Targets exercise2 how expanding control by an ambitious but feasible degree might affect the targets being achieved in China, India, and South Africa.
In China, sustained improvements in tuberculosis interventions led to a 70% decline in prevalence from 1990 to 2010,3, 4 and by 2015, tuberculosis incidence had reached 70 cases per 100 000 population.3 By contrast, in South Africa, where the tuberculosis epidemic has been greatly affected by HIV dynamics,5, 6 declines in tuberculosis incidence have been seen since only around 2010, and in 2015 incidence was 830 cases per 100 000.3 India has had relatively stable incidence of 200–300 cases per 100 000 population for the past 15 years, but faces challenges associated with a large and heterogeneous private sector that is used by many patients for diagnosis and care. By expanding government subsidies, India has attempted to improve the quality of tuberculosis care provided in the private sector.7
The TB Targets exercise was a modelling study done to assess the hypothetical effects of specific TB interventions and showed that the 2025 targets of the End TB Strategy would not be achieved by implementing any one intervention.2 In India and China, targets might only be met with aggressive implementation of a comprehensive set of currently available interventions and if new and improved technologies become available.2 Improvements need to be made in access to high-quality tuberculosis care, diagnosis, multidrug resistance (eg, by replacing smear microscopy with molecular diagnostic techniques), adherence and treatment success rates, active case finding in the general population, and use of isoniazid preventive therapy for people taking antiretroviral therapy.2 A concurrent economic evaluation found that such expansion was likely to be cost-effective and would avert large numbers of deaths per incremental tuberculosis budget dollar, but might need substantial increases in funding at the time of implementation,8 which in India and South Africa, would lead to annual tuberculosis service costs more than doubling and rising to 3–4% of current public health financing.
Research in context.
Evidence before this study
WHO's End TB Strategy proposes aggressive action to reduce tuberculosis incidence and mortality. Associated with those goals will be reductions in related out-of-pocket costs and impoverishment, which can be catastrophic when they place excessive economic burden on households. Although the economic burden of tuberculosis on households is known to be high, there is little evidence reported on the effects of financial-risk protection strategies in high-burden settings, such as India and South Africa. The TB Modelling and Analysis Consortium highlighted two reports on the End TB Strategy which suggested through estimation of costs incurred by patients in high-burden settings that substantial improvements in tuberculosis control could be cost-effective.
Added value of this study
We used models to estimate reductions in tuberculosis-related catastrophic costs with aggressive expansion of tuberculosis services in India and South Africa from 2016 to 2035. We quantified intervention effects as changes in the number of households incurring catastrophic costs, which were defined as the sum of direct medical and non-medical costs and indirect costs being more than 20% of the total household income. We estimated that improved care for drug-sensitive and multidrug-resistant tuberculosis would lead to reductions of up to 19% in India and South Africa, and found that benefits would be greatest in the poorest households. In South Africa, the expansion of access to care through intensified case finding could decrease tuberculosis-related household catastrophic costs by up to 20%.
Implications of all the available evidence
Aggressive expansion of tuberculosis services in India and South Africa could alleviate the financial burden on many patients. Nevertheless, improved social protection for people with tuberculosis will still be needed to meet the End TB Strategy target of eliminating catastrophic costs. Our results support efforts to scale up tuberculosis services and moving towards universal health coverage.
The TB Targets exercise suggested that expanding tuberculosis control would substantially reduce costs incurred by patients8 and thereby also diminish tuberculosis-related household impoverishment. This outcome would be important given the WHO's aim of universal health coverage and the fact that out-of-pocket medical costs are a leading cause of impoverishment in many low-income and middle-income countries.9 Survey evidence also suggests that in such settings tuberculosis leads to substantial losses in wages and work productivity for the affected individuals and their households.10
One of the three goals of the End TB Strategy is that no patient or their household should face catastrophic costs because of tuberculosis.11, 12 This goal is consistent with WHO having made protection from financial risks and prevention of medical impoverishment key elements of health-system performance13 and included financial protection in universal health coverage.14 Given the association between tuberculosis and poverty, and the recommendation to monitor related catastrophic costs,11, 12 it is essential to understand how different interventions might prevent impoverishment. Traditional cost-effectiveness analysis may be complemented by extended cost-effectiveness analysis (ECEA), which estimates the distributional and financial protection benefits to be gained by resource allocation15, 16 and, hence, when combined with modeling, can explore the financial protection impact of different tuberculosis interventions.
We developed a mathematical model to assess whether tuberculosis-related household catastrophic costs could be reduced by expansion of tuberculosis interventions in India and South Africa, building on the TB Targets exercise2, 8 and consistent with ECEA. We compared a subset of interventions highlighted by the TB Targets exercise with a base case derived from the standards of tuberculosis care in these countries at the start of the study.
Methods
Study design
We did a modelling study to assess effects of different interventions on catastrophic costs related to tuberculosis in the period 2016–35. We selected India and South Africa for this study because they are both major contributors to the global tuberculosis burden but differ in HIV and tuberculosis epidemiology, approaches to tuberculosis control, mix of public and private health-care provisions, and the availability of data on costs incurred by patients; although the main TB Targets analysis also included China, we only selected India and South Africa because of the criteria specified. India has the largest national tuberculosis burden worldwide and a large private sector providing care to about half of affected individuals.17 South Africa has the largest tuberculosis burden per person worldwide, and HIV and tuberculosis are closely linked in this country.6
Intervention scenarios
In each country, we consulted extensively with representatives from national tuberculosis programmes to decide on the activities that would be most relevant to assess with existing tools. We selected interventions with clear information on implementation and coverage, and with detailed future scenarios based on a framework of tuberculosis programme activities (table 1, appendix p 2). We assessed the effects of improvement in treatment quality for drug-sensitive and multidrug-resistant tuberculosis in South Africa and India. In India, there are many treatment providers of widely varying qualities,17, 18, 19 and, therefore, the treatment interventions allowed exploration of achievements if increased proportions of individuals with tuberculosis could access higher-quality care in this country (similar to optimum public sector care). We also assessed expansion of access to care through intensified tuberculosis screening at primary-care clinics in South Africa. In the intervention scenarios, high coverage was reached by 2025 and remained constant for 10 years over 2025–35. The models also considered local policy constraints and system capacities.2, 8
Table 1.
Intervention scenarios for India and South Africa8
| Activities | Programme targets | Mechanism of action for health effects | |
|---|---|---|---|
| India | |||
| Improving treatment quality | Improved private-sector quality through provider training, supervision, regulation, and subsidies; retention of patients in care by incentives, nutritional support, and link to social welfare programmes | Initial decrease in patients stopping treatment from 10% to 5% by 2015 for DS-TB and from 11% to 5% by 2020 for MDR-TB; treatment success measured as increases in adherence from 75% to 85% and from 48% to 67%, respectively | Improved retention of patients receiving care leading to increased cure rates |
| South Africa | |||
| Expanding access to care | Outreach clinics to underserved areas and symptom screening in primary care | Decrease population without access to care from 5% to zero by 2022 | Reduced duration of infectiousness and mortality risks by improved case detection |
| Improving treatment quality | Mobile health care, follow-up of patients in the community, counselling on adherence to treatment, and improved MDR-TB staffing | Initial decreases in patients stopping treatment from 17% to 5% by 2021 for DS-TB and from 30% to 15% by 2021 for MDR-TB; treatment success measured as increases in adherence from 76% to 85% and from 52% to 67%, respectively | Improved retention of patients receiving care leading to increased cure rates |
DS-TB=drug-sensitive tuberculosis. MDR-TB=multidrug-resistant tuberculosis.
In India, we took improvement in quality of treatment to mean that treatment adherence would increase from 75% to 85% for drug-sensitive tuberculosis and from 48% to 67% for multidrug-resistant tuberculosis. These changes would be achieved in both intervention scenarios through improved quality of private-sector treatment by provider training, supervision, regulation, and subsidies, and provision of patient-retention incentives, nutritional support, and linkage to social welfare programmes. In South Africa, we took improvement in quality of treatment to mean that treatment adherence would increase from 76% to 85% for drug-sensitive tuberculosis and from 52% to 67% for multidrug-resistant tuberculosis. These changes would be achieved in both intervention scenarios through provision of mobile health care and follow-up in the community, offering patients adherence counselling and psychosocial support, and tracing of patients to avert care dropout. Additional features to achieve improved treatment of multidrug-resistant tuberculosis would be increased staffing and decentralisation of the electronic register. We took expansion of access to care in South Africa to mean that all individuals seeking care would be screened for symptoms of tuberculosis.
We compared each intervention scenario with a base case, which assumed that coverage and treatment success rates at the start of the study would be maintained at a constant for the period 2016–35. The baseline trends in tuberculosis incidence and mortality projected in the base case were inferred from model calibration with 2012 survey data and past trends from 2000–15.2 The base case included country standards of care derived from the FIND study in India20 and the XTEND study in South Africa.21, 22
Calculation of catastrophic costs
We defined catastrophic costs as the sum of direct medical costs (eg, treatment costs), direct non-medical costs (eg, transport and food during visits), and indirect costs (eg, loss of earnings) to the patient exceeding 20% of total annual household income.23, 24 This definition followed that in WHO's protocol to estimate the proportion of households experiencing tuberculosis-related catastrophic costs.24 A simplifying assumption was made that each household could have a maximum of one case of tuberculosis.
We calculated household catastrophic costs for each intervention scenario and the base case. Indirect costs were estimated for the time before diagnosis of tuberculosis when patients were not taking treatment and, therefore, reductions due to interventions were quantified as estimated reductions in the time from onset of tuberculosis symptoms to diagnosis (table 2).
Table 2.
Parameters used to estimate catastrophic costs averted
| India | South Africa | References | ||
|---|---|---|---|---|
| Epidemiology | ||||
| Cumulative numbers (2016–35) in base case in Harvard model and TIME model | ||||
| Treated DS-TB cases | 49 785 000, 32 877 000 | 6 211 000, 5 342 000 | 2 | |
| Treated MDR-TB cases | 851 000, 1 258 000 | 160 000, 318 000 | 2 | |
| Tuberculosis-related deaths | 6 547 000, 8 210 000 | 1 316 000, 1 345 000 | 2 | |
| Estimated relative risk of tuberculosis, from poorest to richest quintile | 1·00, 0·66, 0·50, 0·28, 0·18 | 1·00, 0·66, 0·57,0·47, 0·17 | 25, 26 | |
| Estimated relative ratio of health-care use, from poorest to richest quintile | 0·18, 0·39,0·59, 0·80, 1·00 | 0·67, 0·75,0·83, 0·92, 1·00 | 27, 28 | |
| Direct medical costs to patients | ||||
| Monthly costs (US$) | ||||
| DS-TB care (base case) | 61 | 38 | 8, 10, 29, 30 | |
| MDR-TB care (base case) | 61 | 123 | 8, 29, 30, 31 | |
| Improved DS-TB care | 48 | 38 | 8, 10, 29, 30 | |
| Improved MDR-TB care | 48 | 123 | 8, 29, 30, 31 | |
| Fixed costs (per visit)* | 42 | N/A | 8, 10, 29, 30 | |
| Direct non-medical and indirect costs to patients | ||||
| Average time from onset to diagnosis in base case in Harvard model and TIME model (months) | 11·9, 20·4 | 9·7, 9·9 | 2 | |
| Average time from onset to diagnosis with expansion of access to care in Harvard model and TIME model (months) | N/A | 6·6, 6·9 | 2 | |
| Diagnosis cost per month ($)† | 60 | 66 | 8, 10, 29, 30 | |
| Funeral costs ($) | 300 | 1850 | 32, 33 | |
| Annual income per capita ($) | 1600 | 6890 | 34 | |
| Gini index‡ | 0·33 | 0·65 | 34 | |
| Distribution of annual income per capita by income quintile ($) | ||||
| 1 | <750 | <2760 | ·· | |
| 2 | 750–1170 | 2760–4530 | ·· | |
| 3 | 1170–1640 | 4530–6580 | ·· | |
| 4 | 1640–2320 | 6580–9630 | ·· | |
| 5 | >2320 | >9630 | ·· | |
DS-TB care implies 6 months of treatment and MDR-TB care implies 24 months of treatment. In the base case, all coverage levels and treatment success rates at the start of the study were assumed to be maintained at a constant for the period 2016–35. Costs are expressed in 2014 US$. TIME=TB Impact Model and Estimates. DS-TB=drug-sensitive tuberculosis. MDR-TB=multidrug-resistant tuberculosis. N/A=not applicable.
Include per-visit costs to physicians for diagnosis of tuberculosis.
Includes indirect social care and transport costs per month until tuberculosis is diagnosed. Reduction in indirect cost is then valued through the reduction of time to tuberculosis diagnosis after onset of interventions.
Measure of inequality of income distribution.
Modelling
Modelling involved four steps for each country. Step 1 involved collecting the number of tuberculosis cases and deaths for each intervention and year. In line with the TB Targets exercise, we used two dynamic deterministic compartmental models of tuberculosis transmission, the Harvard model developed by Menzies and colleagues35 and the model of the TB Impact Model and Estimates group (appendix p 1).36 Both models use the following factors to stratify populations: drug-sensitive or multidrug-resistant tuberculosis, treatment history for tuberculosis, HIV status, receiving antiretroviral therapy, and CD4 cell count. The Harvard model followed one age group (>15 years) and used Bayesian calibration, whereas the TB Impact Model and Estimates model tracked the whole population and used manual calibration.2 We used the following model outputs to estimate patient-incurred costs throughout the period 2016–35: the number of tuberculosis cases screened and diagnosed; the numbers of patients with tuberculosis who received treatment for drug-sensitive tuberculosis or for multidrug-resistant tuberculosis; time from onset of tuberculosis symptoms to starting treatment; and the number of tuberculosis-related deaths (table 2). The estimated numbers of tuberculosis cases screened, diagnosed, and treated for drug-sensitive or multidrug-resistant tuberculosis, as estimated by the models for the base case and the intervention scenarios, were distributed across income quintiles by an income distribution analysis. Data for tuberculosis prevalence25, 26 and health-care use27, 28 were obtained from the literature. We used case-fatality ratios to allocate tuberculosis-related deaths between people who were treated and those who remained untreated.37
In step 2, we used the estimates of direct and indirect costs incurred by patients (table 2), which were based on an analysis of surveys by Menzies and colleagues (appendix pp 3–7),8 to assess the effects of each intervention. These estimates provided the unit cost per model output from the patient's perspective and estimated a mean cost per tuberculosis-affected household. Indirect costs compared with average income were varied by income quintile (–20%, −10%, 0, 10%, 20% in quintiles 1 to 5, respectively), and we included costs for tuberculosis-related deaths (eg, funeral costs32, 33).
In step 3, we assigned an annual income for each tuberculosis-affected household, based on the income distribution defined for each tuberculosis case in step 1, drawn from a simulated γ distribution with parameters based on the country's gross domestic product per capita and Gini index (table 2).34, 38, 39 We then determined the size of each household with a Poisson model, with the average size taken from each country's national census (four per household in South Africa and five in India).40, 41 This approach allowed us to define household annual income in each quintile (table 2) and thus to which income quintile each case of tuberculosis should be assigned.
In step 4, we calculated the number of households incurring catastrophic costs per year and cumulatively in 2016–35, by income quintile. We combined the estimated household income for each quintile with the patient-incurred costs estimated in step 2 (table 2). To obtain the total number of cases of catastrophic costs averted by each intervention scenario, we subtracted the number of households incurring catastrophic costs in the base case from the number in the intervention scenario (appendix pp 8–9).
Further sensitivity and scenario analyses
We did three univariate sensitivity analyses to assess the effects of key factors, varying the following factors by 50% above and below baseline values: direct costs, funeral costs, and health-care use (appendix pp 24–35). Furthermore, we ran a probabilistic sensitivity analysis with Monte Carlo simulations (n=100 000 trials), where time to diagnosis, health-care use, income, and costs to patients were varied simultaneously with truncated normal distributions, with input means extracted from the model outputs and 20% of those means used to calculate SDs. From these data we extrapolated 2·5 and 97·5 percentiles to determine 95% uncertainty ranges.
We did four scenario analyses. First, we varied the burden distribution and assumed that tuberculosis cases were equally distributed across income quintiles (ie, removing the assumption that tuberculosis would occur more in the poor). Second, we varied the distribution of health-care use and assumed equal access to care across each quintile (ie, removing the assumption that the richer patients would seek care more than the poorer patients). Third, we modified the patient-incurred costs by removing costs arising from tuberculosis-related deaths and assuming equal indirect costs across quintiles. Fourth, we changed the catastrophic costs metric by changing the threshold from 20% to 10% or 40% of total household income42, 43, 44 or by using a different threshold for each quintile (eg, 20%, 25%, 30%, 35%, and 40%). All costs were expressed in 2014 US$. All analyses were done with RStudio (version 1.0.136).
Role of the funding source
The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The estimated numbers of households that incurred tuberculosis-related catastrophic costs in 2016–35, were around 20 million to 22 million in India and 1·1 million to 1·2 million in South Africa (Table 3, Table 4). We found a substantially higher risk of catastrophic costs being incurred in the bottom 40% of households in each country, among which the prevalence of tuberculosis was highest. In India, 30–40% of all catastrophic costs were estimated to be in the bottom income quintile, and in South Africa the proportion was about 80%.
Table 3.
Estimated numbers of households (in thousands) incurring tuberculosis-related catastrophic costs in India
| Total number of households (95% UR) |
Number of households (95% UR) per income quintile* |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Harvard model | ||||||
| Base case | 20 596 (16 848–24 278) | 8785 (8276–9203) | 5955 (4334–7394) | 4199 (3024–5449) | 1332 (920–1874) | 326 (197–512) |
| Improvement in DS-TB care | 19 544 (15 976–23 049) | 8357 (7864–8766) | 5638 (4104–7006) | 3979 (2864–5168) | 1261 (871–1775) | 309 (187–486) |
| Improvement in MDR-TB care | 20 475 (16 753–24 130) | 8739 (8232–9155) | 5917 (4309–7349) | 4171 (3005–5413) | 1324 (915–1860) | 324 (196–509) |
| TIME model | ||||||
| Base case | 21 926 (18 864–24 629) | 6757 (6442–7106) | 7101 (6370–7571) | 5383 (3854–6685) | 2036 (1425–2740) | 649 (493–826) |
| Improvement in DS-TB care | 20 547 (17 690–23 079) | 6381 (6065–6732) | 6635 (5950–7074) | 5027 (3602–6243) | 1898 (1329–2556) | 605 (459–771) |
| Improvement in MDR-TB care | 21 790 (18 742–24 485) | 6696 (6378–7049) | 7059 (6327–7526) | 5360 (3839–6659) | 2028 (1419–2731) | 647 (491–824) |
Catastrophic costs are defined as the sum of costs exceeding 20% of total household income. In the base case, all coverage levels and treatment success rates at the start of the study were assumed to be maintained at a constant for the period 2016–35. UR=uncertainty range. DS-TB=drug-sensitive tuberculosis. MDR-TB=multidrug-resistant tuberculosis. TIME=TB Impact Model and Estimates.
From poorest (quintile 1) to richest (quintile 5).
Table 4.
Estimated number of households (in thousands) incurring tuberculosis-related catastrophic costs in South Africa
| Total number of households (95% UR) |
Number of households (95% UR) per income quintile* |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Harvard model | ||||||
| Base case | 1184 (1031–1348) | 925 (805–1049) | 195 (163–231) | 59 (42–78) | 5 (0–11) | 0 (0–2) |
| Expansion of access to care | 1123 (972–1279) | 882 (769–999) | 199 (165–235) | 38 (22–57) | 3 (0–7) | 0 (0–2) |
| Improvement in DS-TB care | 964 (836–1103) | 757 (657–861) | 158 (129–190) | 45 (30–61) | 4 (0–9) | 0 (0–2) |
| Improvement in MDR-TB care | 965 (837–1105) | 759 (658–865) | 157 (129–190) | 44 (30–60) | 4 (0–9) | 0 (0–2) |
| TIME model | ||||||
| Base case | 1243 (1085–1396) | 984 (864–1096) | 172 (141–205) | 81 (58–103) | 7 (1–14) | 0 (0–1) |
| Expansion of access to care | 999 (874–1130) | 802 (709–898) | 143 (113–173) | 49 (33–68) | 5 (0–12) | 0 (0–1) |
| Improvement in DS-TB care | 1152 (1005–1297) | 915 (803–1021) | 159 (129–190) | 72 (52–93) | 6 (0–14) | 0 (0–1) |
| Improvement in MDR-TB care | 1171 (1016–1320) | 934 (816–1044) | 157 (127–189) | 73 (52–94) | 6 (0–14) | 0 (0–1) |
Catastrophic costs are defined as the sum of costs exceeding 20% of total household income. In the base case, all coverage levels and treatment success rates at the start of the study were assumed to be maintained at a constant for the period 2016–35. UR=uncertainty range. DS-TB=drug-sensitive tuberculosis. MDR-TB=multidrug-resistant tuberculosis. TIME=TB Impact Model and Estimates.
From poorest (quintile 1) to richest (quintile 5).
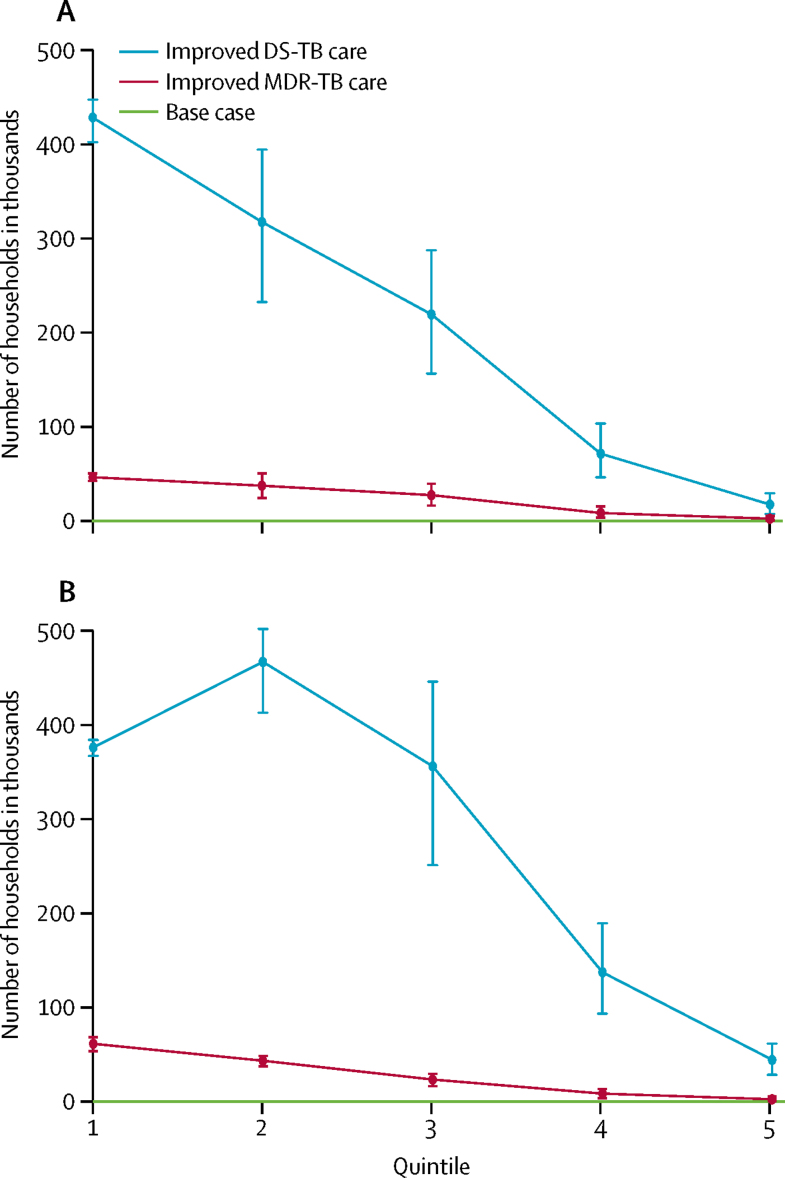
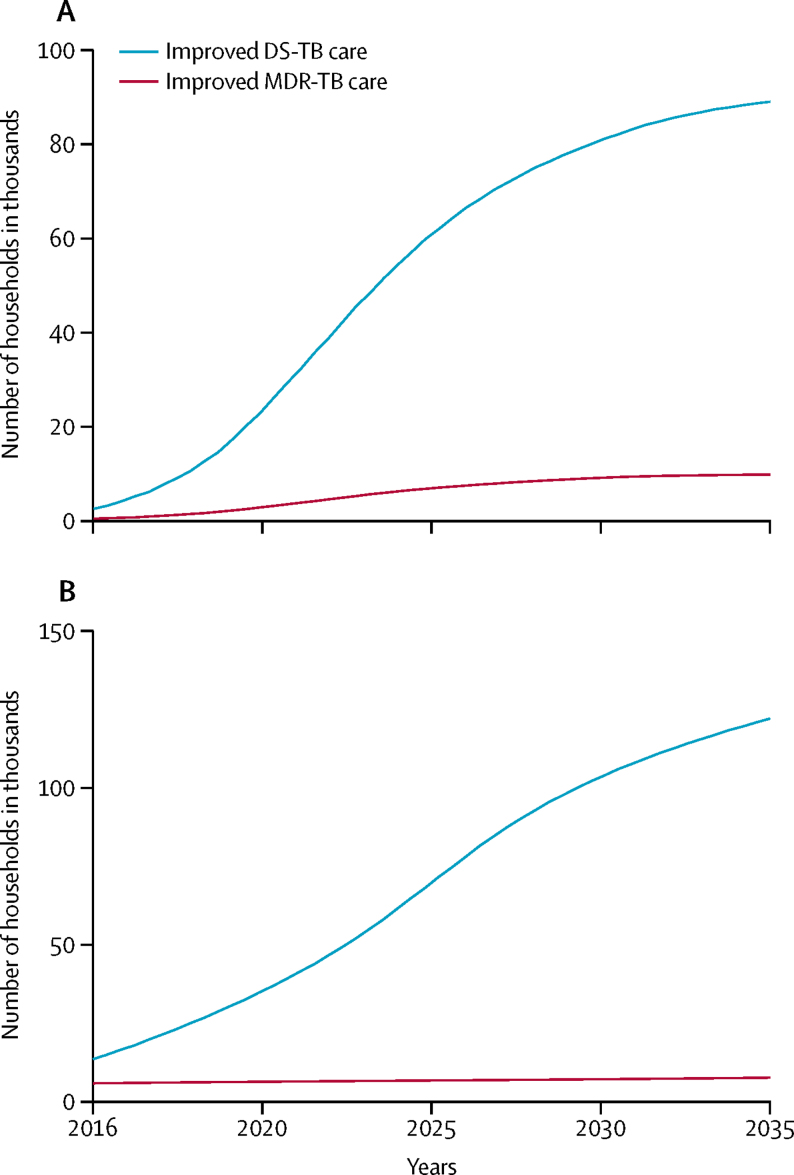
In India, more catastrophic costs would be averted by improvements in drug-sensitive tuberculosis care than by improvements in multidrug-resistant tuberculosis care (figure 1), and the difference between these two interventions would increase over time (figure 2). Over 2016–35, improvements in care for drug-sensitive tuberculosis would avert about 1·1 million to 1·4 million cases of household catastrophic costs (table 3), which was about 5–6% of all cases in the base case. Improvements in care of multidrug-resistant tuberculosis would avert about 120 000–140 000 cases or up to 1% of all cases incurred in the base case (table 3). These cost reductions would occur because of the provision of financial incentives to patients in the intervention and the prevention of additional tuberculosis transmission and infections.2, 8 The benefits of these two interventions would be greatest in the lowest two income quintiles (poorest 40% of households), where 60–70% of cases of catastrophic costs would be averted by improvements in care of drug-sensitive tuberculosis and 70–80% would be averted by improvements in care for multidrug-resistant tuberculosis. These reductions would be due to decreasing tuberculosis incidence, increasing health-care use, and decreasing likelihood of catastrophic costs with increasing income.
Figure 1.
Number of households in India with catastrophic costs averted by improved tuberculosis care, compared with the base case, by income quintile
(A) Harvard model. (B) TB Impact Model and Estimates model. The base case covers the period 2016–35, during which all coverage levels and treatment success rates were assumed to be maintained at a constant. Numbers of households are shown with 95% uncertainty ranges. Quintiles range from poorest (quintile 1) to richest households (quintile 5). DS-TB=drug-sensitive tuberculosis. MDR-TB=multidrug-resistant tuberculosis.
Figure 2.
Number of households in India per year with catastrophic costs averted by improved tuberculosis care over the period 2016–35
(A) Harvard model. (B) TB Impact Model and Estimates model. DS-TB=drug-sensitive tuberculosis. MDR-TB=multidrug-resistant tuberculosis.
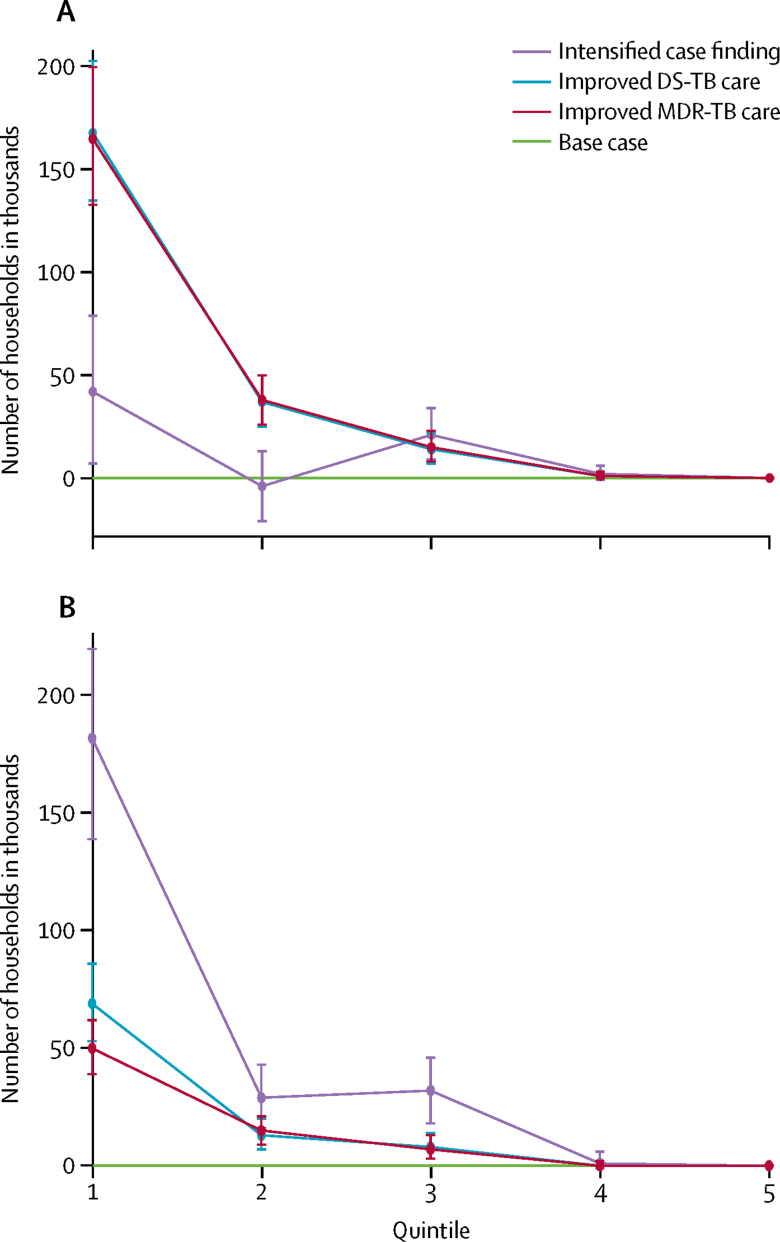
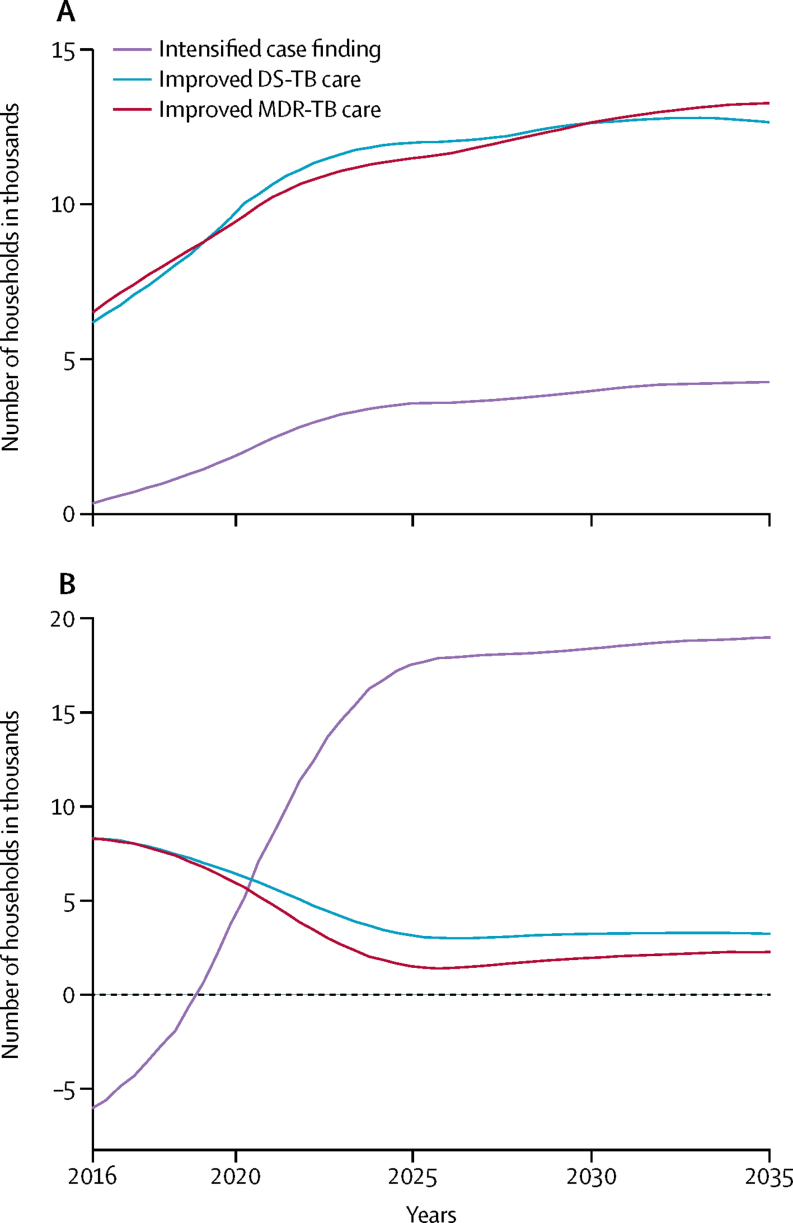
In South Africa, the numbers of cases of tuberculosis-related household catastrophic costs averted by improvements in care of drug-sensitive and multidrug resistant tuberculosis would be lower than in India (Figure 3, Figure 4). Expanding access to care through intensified case finding would avert about 60 000–240 000 cases of catastrophic costs in 2016–35 (table 4), which was about 5–20% of all cases incurred in the base case, although effects would not be seen for around 5–10 years (figure 4). With improvements in care of drug-sensitive tuberculosis, about 90 000–220 000 cases or 7–19% of all cases of catastrophic costs would be averted, as would 70 000–220 000 cases or 6–18% with improvements in care of multidrug-resistant tuberculosis (table 4). Households in the lowest two income quintiles would benefit the most from all three interventions. Expanded access to care would avert 65–90% of tuberculosis-related catastrophic costs in these quintiles, and improvements in care of drug-sensitive tuberculosis and multidrug-resistant tuberculosis, would each avert about 90% of cases.
Figure 3.
Number of households in South Africa with catastrophic costs averted by intensified case finding and improved tuberculosis care, compared with the base case, by income quintile
(A) Harvard model. (B) TB Impact Model and Estimates model. The base case covers the period 2016–35, during which all coverage levels and treatment success rates were assumed to remain at a constant. Numbers of households are shown with 95% uncertainty ranges. Quintiles range from poorest (quintile 1) to richest households (quintile 5). DS-TB=drug-sensitive tuberculosis. MDR-TB=multidrug-resistant tuberculosis.
Figure 4.
Number of households in South Africa per year with catastrophic costs averted by improved tuberculosis care and expanded access to care in 2016–35
(A) Harvard model. (B) TB Impact Model and Estimates model. DS-TB=drug-sensitive tuberculosis. MDR-TB=multidrug-resistant tuberculosis.
In the sensitivity analysis, assigning uniform distribution of tuberculosis lessened the difference in effects between quintiles (appendix pp 10–11). With equal use of health care across quintiles, in India and South Africa, reductions remained highest in the bottom income quintile (appendix pp 12–13). Removing funeral costs due to tuberculosis-related deaths had little effect on the number of cases of catastrophic costs averted in any quintile with improvements in care of drug-sensitive and multidrug-resistant tuberculosis in both countries (appendix pp 14–15). Uniform distribution of indirect costs across quintiles led to reductions in catastrophic costs in the higher quintiles and increases in the lower quintiles (appendix pp 16–17). When we varied the threshold for estimating catastrophic costs to 10% or 40%, or used varying thresholds by quintile, the numbers of cases of catastrophic costs increased with the lower thresholds and decreased with the upper thresholds (appendix pp 18–23).
Discussion
We developed an approach to assess the financial protection benefits of tuberculosis control in terms of cases of tuberculosis-related household catastrophic costs averted if selected interventions were implemented in India and South Africa from 2016–35. Without intervention, our base case indicated that tuberculosis-related household catastrophic costs would remain substantial in India and South Africa. Improvements in the quality of care for drug-sensitive and multidrug-resistant tuberculosis could reduce this financial burden, but even with aggressive efforts, the End TB Strategy target of no households facing tuberculosis-related catastrophic costs by 2030 would be far from being achieved in either country.
In the base case, the two lowest income quintiles bore most of the catastrophic costs, which is consistent with findings from other studies, for example in Nigeria45, 46 and Peru.23 We note, though, that although the gains in financial protection would be greatest in these two quintiles, the reductions would be less than 20%. Other policy actions, therefore, will be needed to reduce tuberculosis incidence and mortality. In India, the interventions included some payments to patients with tuberculosis to lessen the direct costs of accessing care, but these were not sufficiently large to combat the high indirect costs incurred. Tuberculosis is a debilitating illness that lasts long enough to adversely affect earning potential and, therefore, additional support is needed to alleviate these costs if households are to be protected. Based on a framework of tuberculosis programme activities and extensive discussions with national tuberculosis programme representatives, we studied the interventions of improved care for drug-sensitive and multidrug-resistant tuberculosis and expanded access to care through intensified case finding. Assessment of other interventions, such as expanding existing national social protection schemes for the poorest households and vulnerable populations, however, would be worthwhile, as has been proposed by WHO and partners of the newly established Health and Social Protection Action Research and Knowledge Sharing Network.47 The interventions we studied were focused on the delivery of core tuberculosis services, but in the future we might consider assessing systemic interventions that could reduce financial costs, such as decentralised care, fees, and transport support. Intervention coverage is only one element among many, including quality and financial architecture of health systems, that can improve care-seeking behaviour and health. The multifaceted benefits of tuberculosis interventions within the context of broader social development are intrinsically important to reductions in illness-related impoverishment.10, 48, 49
Our study builds on the substantial work of the TB Targets exercise,2, 8 including the use of two dynamic models of tuberculosis transmission.35, 36 Yet, our analysis had several limitations. First, we relied on modelled estimates of tuberculosis incidence and cost inputs2, 8, 35, 36 and on distributional assumptions. The lack of empirical evidence in these inputs points to the need for expanded data collection. Gathering information on tuberculosis-related costs and time losses for patients and caregivers will be very important. Tracking out-of-pocket expenditure and related catastrophic costs over time could enable testing of the effects of policies in terms of poverty reduction with the existing research platforms of randomised controlled trials or household surveys.
Second, our estimation of tuberculosis-related household catastrophic costs was rudimentary. For example, we did not consider which member of the household was affected (eg, if the main earner had tuberculosis rather than other members of the household, catastrophic costs might have been more likely) because age-stratified tuberculosis incidence was not available from the model outputs. However, how tuberculosis-related income losses by age could be differentiated without adding unnecessary complications and assumptions in the absence of empirical information is unclear. We applied the simplifying assumption of one tuberculosis case per household, whereas the number of cases might be concentrated in specific households, such as those of the poorest and most marginalised populations. Additionally, variation in indirect costs with income has a consequence on public policy valuation (ie, by placing a higher value on time losses among the higher income quintiles than those in the lower quintiles). These effects might make interventions that benefit people in the top quintiles seem to be most cost-effective, which would have implications for equitable access to care. Other elements the models might have included were financial implications related to death (eg, long-term income losses), multiple medical cost inputs (eg, because richer individuals often seek private care), discounting and income growth over time, and the presence of social-protection programmes (eg, paid sick leaves). These adjustments, though, might have led to unnecessary complexity in the findings without providing any additional insight.
Third, we represented financial protection in terms of cases of catastrophic costs averted. Other possible measures could have been cases of poverty or forced borrowing avoided. We chose household catastrophic costs averted because of its simplicity and widespread use,44, 50 but this approach has some drawbacks, such as exclusion of some individuals through the choice of threshold (eg 20% of total income).44 We used the sensitivity analyses to try to lessen this effect.
Fourth, while the use of two models was a strength of this study because it allowed us to consider some uncertainty in the model structure, full characterisation of uncertainty in estimates was not possible and, therefore, our findings should be interpreted with caution. Owing to the many unknowns in the intervention scenarios we assessed, we used univariate sensitivity analyses and scenario analyses to assess the effects of key factors, and did a probabilistic sensitivity analysis to determine the 95% uncertainty ranges.
Irrespective of potential limitations, we believe this study has some robust and important findings. First, substantial financial household burden is incurred because of tuberculosis in India and South Africa, with millions of households being affected. Second, improved tuberculosis control through implementation or expansion of selected services, such as those included in our models, could alleviate a notable proportion of that burden and disproportionately benefit the poorest households. Tuberculosis control alone, though, while necessary, will not be sufficient to achieve the End TB Strategy targets because the indirect costs are high. Other social-protection strategies and safety nets actions will be needed to substantially reduce catastrophic costs. In this respect, future work should estimate the additional financial resources needed to eliminate catastrophic costs by 2030, and assess whether governments should cover the full social cost of tuberculosis treatment for at-risk households in India and South Africa. This study has illustrated what financial protection might be achieved with various scenarios, but, importantly, our analysis remains only a stepping stone towards the essential process of involving policy makers and academics, who must make their own assessments of model assumptions so that our findings can best inform the policy dialogues in India and South Africa, particularly regarding feasibility, affordability, and value for money, and reflect the true scenarios of tuberculosis control in the two countries.
Acknowledgments
Acknowledgments
This work was funded by the TB Modeling and Analysis Consortium (TB-MAC, Bill & Melinda Gates Foundation, OPP1084276). We thank the economists and tuberculosis control experts who were part of the TB-MAC TB Targets exercise for their comments on our initial analyses, including Nimalan Arinaminpathy, Fiammetta Bozzani, Vineet Chadha, Salome Charalambous, Alison Grant, Yoko Laurence. RGW has received funding from the UK Medical Research Council and the UK Department for International Development under their concordat agreement that is also part of the EDCTP2 programme supported by the European Union (MR/P002404/1), the Bill & Melinda Gates Foundation (TB-MAC: OPP1084276/OPP1135288; SA Modelling for Policy: OPP1110334; CORTIS: OPP1137034; and Vaccines: OPP1160830), and UNITAID (4214-LSHTM-Sept15; PO #8477-0-600). NF has received funding from the Medical Research Council of South Africa as part of the National Health Scholars Programme, supported by the Public Health Enhancement Fund. We thank six reviewers for helpful and constructive comments and suggestions.
Contributors
SV and AV conceived and initiated the study. GBG, NAM, RMGJH, TS, ML, RGW, NF, and SC provided data and advice. SV and AV coordinated the research. CR-H and SV did the research and CR-H ran the analyses. SV wrote the first draft of the paper, which was reviewed by all authors.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Uplekar M, Weil D, Lönnroth K, on behalf of WHO's Global TB Program WHO's new End TB Strategy. Lancet. 2015;385:1799–1801. doi: 10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- 2.Houben RMGJ, Menzies NA, Sumner T. Feasibility of achieving the 2025 WHO global tuberculosis targets in South Africa, China, and India: a combined analysis of 11 mathematical models. Lancet Glob Health. 2016;4:e806–e815. doi: 10.1016/S2214-109X(16)30199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . Global tuberculosis report 2016. World Health Organization; Geneva: 2016. [Google Scholar]
- 4.Wang L, Zhang H, Ruan Y. Tuberculosis prevalence in China, 1990–2010: a longitudinal analysis of national survey data. Lancet. 2014;383:2057–2064. doi: 10.1016/S0140-6736(13)62639-2. [DOI] [PubMed] [Google Scholar]
- 5.Corbett EL, Watt CJ, Walker N. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 6.Churchyard GJ, Mametja LD, Mvusi L. Tuberculosis control in South Africa: successes, challenges, and recommendations. S Afr Med J. 2014;104:244–248. doi: 10.7196/samj.7689. [DOI] [PubMed] [Google Scholar]
- 7.Central TB Division. Directorate General of Health Services. Ministry of Health and Family Welfare . Revised National Tuberculosis Control Program. National strategic plan for tuberculosis control 2012–2017. Ministry of Health and Family Welfare; New Delhi: 2012. [Google Scholar]
- 8.Menzies NA, Gomez GB, Bozzani F. Cost-effectiveness and resource implications of aggressive TB control in China, India, and South Africa: a combined analysis of nine models. Lancet Glob Health. 2016;4:e816–e826. doi: 10.1016/S2214-109X(16)30265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu K, Evans DB, Carrin G. Protecting households from catastrophic health spending. Health Aff (Millwood) 2007;26:972–983. doi: 10.1377/hlthaff.26.4.972. [DOI] [PubMed] [Google Scholar]
- 10.Tanimura T, Jaramillo E, Weil D. Financial burden for tuberculosis patients in low-and middle-income countries: a systematic review. Eur Respir J. 2014;43:1763–1775. doi: 10.1183/09031936.00193413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO . Global Tuberculosis Report 2014. World Health Organization; Geneva: 2014. [Google Scholar]
- 12.Lönnroth K, Glaziou P, Weil D. Beyond UHC: monitoring health and social protection coverage in the context of tuberculosis care and prevention. PLoS Med. 2014;11:e1001693. doi: 10.1371/journal.pmed.1001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . The World Health Report 2000: health systems: improving performance. World Health Organization; Geneva: 2000. [Google Scholar]
- 14.WHO . The World Health Report 2010: health systems financing: the path to universal coverage. World Health Organization; Geneva: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verguet S, Laxminarayan R, Jamison DT. Universal public finance of tuberculosis treatment in India: an extended cost-effectiveness analysis. Health Econ. 2015;24:318–332. doi: 10.1002/hec.3019. [DOI] [PubMed] [Google Scholar]
- 16.Verguet S, Kim JJ, Jamison DT. Extended cost-effectiveness analysis for health policy assessment: a tutorial. Pharmacoeconomics. 2016;34:913–923. doi: 10.1007/s40273-016-0414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satyanarayana S, Nair SA, Chadha SS. From where are tuberculosis patients accessing treatment in India? Results from a cross-sectional community based survey of 30 districts. PLoS One. 2011;6:e24160. doi: 10.1371/journal.pone.0024160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achanta S, Jaju J, Kumar AM. Tuberculosis management practices by private practitioners in Andhra Pradesh, India. PLoS One. 2013;8:e71119. doi: 10.1371/journal.pone.0071119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bharaswadkar S, Kanchar A, Thakur N. Tuberculosis management practices of private practitioners in Pune municipal corporation, India. PLoS One. 2014;9:e97993. doi: 10.1371/journal.pone.0097993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sachdeva KS, Raizada N, Sreenivas A. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PLoS One. 2015;10:e0126065. doi: 10.1371/journal.pone.0126065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Churchyard G, Stevens WS, Mametja LD. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health. 2015;3:e450–e457. doi: 10.1016/S2214-109X(15)00100-X. [DOI] [PubMed] [Google Scholar]
- 22.Foster N, Vassall A, Cleary S. The economic burden of TB diagnosis and treatment in South Africa. Soc Sci Med. 2016;130:42–50. doi: 10.1016/j.socscimed.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 23.Wingfield T, Boccia D, Tovar M. Defining catastrophic costs and comparing their importance for adverse tuberculosis outcome with multi-drug resistance: a prospective cohort study, Peru. PLoS Med. 2014;11:e1001675. doi: 10.1371/journal.pmed.1001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO . Protocol for survey to determine direct and indirect costs due to TB and to estimate proportion of TB-affected households experiencing catastrophic costs: field testing version. World Health Organization; Geneva: 2015. [Google Scholar]
- 25.Harling G, Ehrlich R, Myer L. The social epidemiology of tuberculosis in South Africa: a multilevel analysis. Soc Sci Med. 2008;66:492–505. doi: 10.1016/j.socscimed.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Oxlade O, Murray M. Tuberculosis and poverty: why are the poor at greater risk in India? PLoS One. 2012;7:e47533. doi: 10.1371/journal.pone.0047533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balarajan Y, Selvaraj S, Subramanian S. Health care and equity in India. Lancet. 2011;377:505–515. doi: 10.1016/S0140-6736(10)61894-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maughan-Brown B, Lloyd ND, Bor J, Venkataramani SA. Increasing access to HIV testing: impacts on equity of coverage and uptake from a national campaign in South Africa. Working paper series number 145. 2015. http://opensaldru.uct.ac.za/bitstream/handle/11090/778/2015_145_Saldruwp.pdf?sequence=1 (accessed July 31, 2017).
- 29.Pantoja A, Floyd K, Unnikrishnan KP. Economic evaluation of public private mix for tuberculosis care and control, India. Part I. Socio-economic profile and costs among tuberculosis patients. Int J Tuberc Lung Dis. 2009;13:698–704. [PubMed] [Google Scholar]
- 30.John KR, Daley P, Kincler N, Oxlade O, Menzies D. Costs incurred by patients with pulmonary tuberculosis in rural India. Int J Tuberc Lung Dis. 2009;13:1281–1287. [PubMed] [Google Scholar]
- 31.Ramma L, Cox H, Wilkinson L. Patients' costs associated with seeking and accessing treatment for drug-resistant tuberculosis in South Africa. Int J Tuberc Lung Dis. 2015;19:1513–1519. doi: 10.5588/ijtld.15.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arokiasamy P, Bloom D, Lee J. Longitudinal aging study in India: vision, design, implementation, and some early results. In: Smith JP, Majumdar M, editors. Aging in Asia. Findings from new and emerging data initiatives. National Academics Press; Washington, DC: 2012. pp. 36–74. [Google Scholar]
- 33.Collins DL, Leibbrandt M. The financial impact of HIV/AIDS on poor households in South Africa. AIDS. 2007;21:S75–S81. doi: 10.1097/01.aids.0000300538.28096.1c. [DOI] [PubMed] [Google Scholar]
- 34.World Bank World development indicators. July 1, 2017. http://data.worldbank.org/data-catalog/world-development-indicators (accessed July 31, 2017).
- 35.Menzies NA, Cohen T, Lin H-H. Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: a dynamic simulation and economic evaluation. PLoS Med. 2012;9:e1001347. doi: 10.1371/journal.pmed.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houben RMGJ, Lalli M, Sumner T. TIME Impact—a new user-friendly TB model to inform TB policy decisions. BMC Med. 2016;14:1–10. doi: 10.1186/s12916-016-0608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borgdorff MW, Floyd K, Broekmans JF. Interventions to reduce tuberculosis mortality and transmission in low-and middle-income countries. Bull World Health Organ. 2002;80:217–227. [PMC free article] [PubMed] [Google Scholar]
- 38.Kemp-Benedict E. Income distribution and poverty: methods for using available data in global analysis. PoleStar technical note no. 4. May 17, 2001. http://gdrs.sourceforge.net/docs/PoleStar_TechNote_4.pdf (accessed July 31, 2017).
- 39.Salem A, Mount T. A convenient descriptive model of income distribution: the gamma density. Econometrica. 1974;42:1115–1127. [Google Scholar]
- 40.Statistics South Africa . Income and expenditure of households 2010/2011. Statistics South Africa; Pretoria: 2012. [Google Scholar]
- 41.Shah C. Average household size smallest in the southern states in 2011. Centre for Monitoring Indian Economy; 2014. [Google Scholar]
- 42.Pradhan M, Prescott N. Social risk management options for medical care in Indonesia. Health Econ. 2002;11:431–446. doi: 10.1002/hec.689. [DOI] [PubMed] [Google Scholar]
- 43.Ranson MK. Reduction of catastrophic health care expenditures by a community-based health insurance scheme in Gujarat, India: current experiences and challenges. Bull World Health Organ. 2002;80:613–621. [PMC free article] [PubMed] [Google Scholar]
- 44.Wagstaff A. Measuring financial protection in health. In: Smith PC, Mossialos E, Papanicolas I, Leatherman S, editors. Performance measurement for health system improvement. Cambridge University Press; Cambridge, UK: 2010. pp. 114–137. [Google Scholar]
- 45.Ukwaja KN, Alobu I, Abimbola S, Christy Hopewell P. Household catastrophic payments for tuberculosis care in Nigeria: incidence, determinants, and policy implications for universal health coverage. Infect Dis Poverty. 2013;2:21. doi: 10.1186/2049-9957-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ukwaja KN, Alobu I, Igwenyi C, Hopewell PC. The high cost of free tuberculosis servoces: patient and household costs associated with tuberculosis care in Ebonyi State, Nigeria. PLoS One. 2013;8:e73134. doi: 10.1371/journal.pone.0073134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.WHO . Health and Social Protection Action Research & Knowledge Sharing (SPARKS) Network: rationale, objectives and work plan. WHO/HTM/TB/2017.10. World Health Organization; Geneva: 2017. [Google Scholar]
- 48.Lönnroth K, Castro KG, Chakaya JM. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375:1814–1829. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 49.Lönnroth K, Weil DE. Mass prophylaxis of tuberculosis through social protection. Lancet Infect Dis. 2014;14:1032–1034. doi: 10.1016/S1473-3099(14)70964-8. [DOI] [PubMed] [Google Scholar]
- 50.Saksena P, Hsu J, Evans DB. Financial risk protection and universal health coverage: evidence and measurement challenges. PLoS Med. 2014;11:e1001701. doi: 10.1371/journal.pmed.1001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.