Summary
Regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking in response to neuronal activity is critical for synaptic function and plasticity. Here, we show that neuronal activity induces the binding of ephrinB2 and ApoER2 receptors at the postsynapse to regulate de novo insertion of AMPA receptors. Mechanistically, the multi-PDZ adaptor glutamate-receptor-interacting protein 1 (GRIP1) binds ApoER2 and bridges a complex including ApoER2, ephrinB2, and AMPA receptors. Phosphorylation of ephrinB2 in a serine residue (Ser-9) is essential for the stability of such a complex. In vivo, a mutation on ephrinB2 Ser-9 in mice results in a complete disruption of the complex, absence of ApoER2 downstream signaling, and impaired activity-induced and ApoER2-mediated AMPA receptor insertion. Using compound genetics, we show the requirement of this complex for long-term potentiation (LTP). Together, our findings uncover a cooperative ephrinB2 and ApoER2 signaling at the synapse, which serves to modulate activity-dependent AMPA receptor dynamic changes during synaptic plasticity.
Keywords: GRIP1, AMPA receptors, synaptic plasticity, LTP, ApoER2, ephrinB
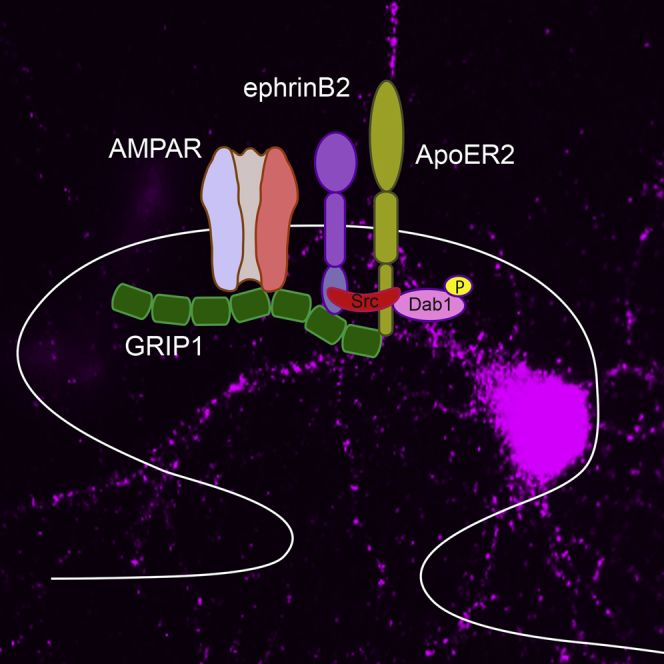
Graphical Abstract
Highlights
-
•
GRIP1, ephrinB2, ApoER2, and AMPA receptors form a complex at the synapse
-
•
The complex forms upon Reelin stimulation and induction of neuronal activity
-
•
Phosphorylation of a serine residue in ephrinB2 regulates the assembly of such complex
-
•
GRIP1 and ephrinB2 mediate ApoER2-induced AMPA receptor insertion at the synapse
Activity-dependent AMPA receptor dynamic changes modulate synaptic plasticity. Pfennig et al. show that insertion of new AMPA receptors at the synapse is mediated by the formation of a macromolecular complex at the membrane that includes ApoER2, ephrinB2, and AMPA receptors bridged by the multi-PDZ adaptor protein GRIP1.
Introduction
Neuronal activity at the synapse induces changes in synaptic strength by altering the abundance of receptors at the synaptic membrane. Thus, changes in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor abundance are thought to underlie the regulation of synaptic strength during synaptic plasticity and homeostatic synaptic scaling. During long-term potentiation (LTP), AMPA receptors are incorporated into the postsynaptic membrane, thereby increasing postsynaptic potentials at that synapse (Makino and Malinow, 2009). Elucidating the machinery regulating the new insertion of AMPA receptors at the synapse is therefore essential to understand the basic molecular events underlying synaptic transmission, learning, and memory. The apolipoprotein E receptor 2 (ApoER2) mediates the functions of Reelin in the developing and adult nervous system (Cooper, 2008, D’Arcangelo et al., 1995, Trommsdorff et al., 1999, Weeber et al., 2002, Rogers et al., 2011, Trotter et al., 2013). Upon Reelin binding to the receptors, ApoER2 and very-low-density lipoprotein receptor (VLDLR), Src family kinases (SFKs) become activated and phosphorylate the intracellular adaptor protein Dab1, thereby initiating a complex signaling cascade leading to correct neuronal positioning during the development of the neocortex, hippocampus, and cerebellum (Bock and Herz, 2003, D’Arcangelo et al., 1999, Howell et al., 1999). We have previously shown that ephrinBs, transmembrane ligands for Eph receptors, are required for Reelin signaling to regulate neuronal migration during brain development (Sentürk et al., 2011). EphrinBs regulate the clustering and activation of SFKs, Dab1, and ApoER2/VLDLR at the membrane of migrating neurons. Reelin expression remains in the adult brain in a subset of GABAergic interneurons that regulate excitatory neuronal networks and therefore are essential for synaptic transmission and plasticity. In this context, it has been shown that Reelin controls synaptic plasticity in an ApoER2- and Dab1-dependent manner (Weeber et al., 2002, Rogers et al., 2011, Trotter et al., 2013). Several mechanisms have been postulated for the enhancement of synaptic transmission by Reelin. ApoER2 associates with NMDA receptors at postsynaptic sites (Beffert et al., 2005), and Reelin-induced phosphorylation of NMDA receptors enhances NMDA receptor currents. Reelin has also been shown to facilitate the new insertion of AMPA receptors at the synapse (Qiu et al., 2006), although the molecular mechanisms that link AMPA receptors and ApoER2 at the synaptic membrane remain still poorly characterized.
Apart from its functions in regulating maturation of dendritic spines, ephrinB ligands possess an active signaling role in regulating hippocampal plasticity in CA3-CA1 synapses (Segura et al., 2007, Grunwald et al., 2004, Bouzioukh et al., 2007). The molecular mechanisms underlying the function of ephrinB2 at the CA1 postsynaptic site involve the phosphorylation of a serine residue on the cytoplasmic tail of ephrinB2 (serine-9 [Ser-9]) and the recruitment of GRIP1, a multiple-PDZ-domain-containing adaptor molecule that also binds to the GluR2 subunit of AMPA receptors (Essmann et al., 2008). Because ephrinB ligands play important roles regulating Reelin signaling during neuronal migration (Sentürk et al., 2011), we hypothesize that ephrinB/GRIP1 complexes might mediate the functions of ApoER2 at the synapse.
Here, we show that neuronal activity induces the clustering of ApoER2 and ephrinB2 at postsynaptic sites and downstream signaling, resulting in Dab1 phosphorylation. EphrinB2 is required for the activity-induced and ApoER2-mediated de novo insertion of AMPA receptors in dendrites. We identify the serine residue Ser-9 in the cytoplasmic tail to be essential for the regulatory function of ephrinB2 in ApoER2 signaling at the synapse. Mechanistically, we show that GRIP1 molecules bridge a complex consisting of ephrinB2/ApoER2/GluR2. Using compound genetics, we show the requirement for such a complex for the function of ApoER2 in regulating AMPA receptor insertion and LTP.
Results
Neuronal Activity Induces Co-clustering of EphrinBs with ApoER2
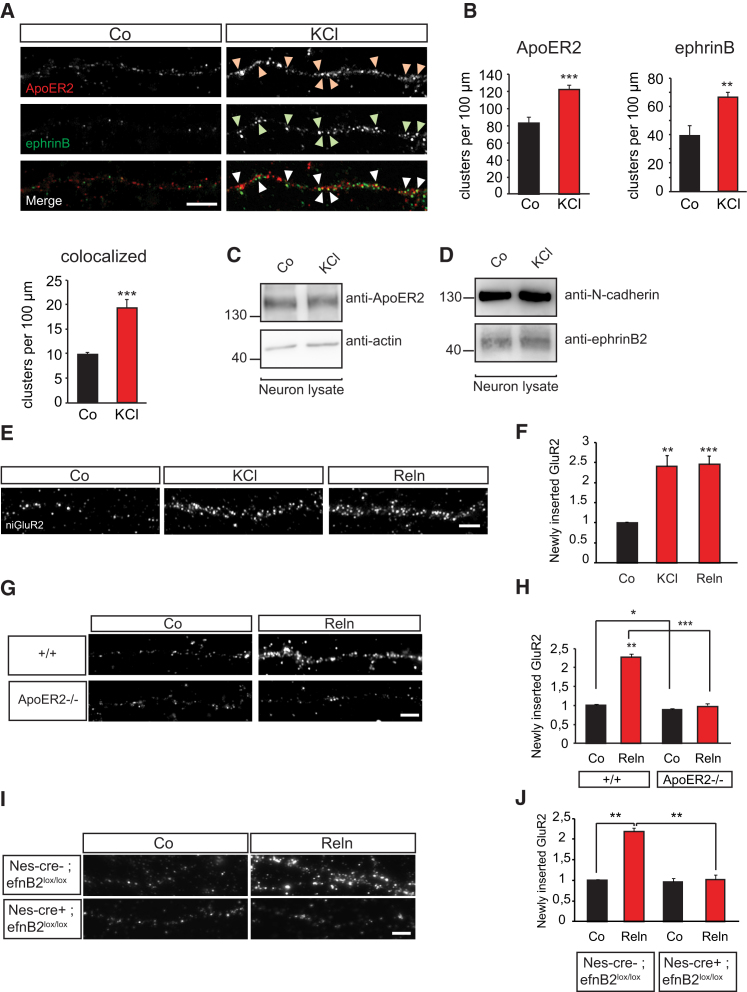
We have previously shown that in order to stabilize AMPA receptors at the membrane, ephrinB proteins cluster at postsynaptic sites, and such clustering occurs following induction of neuronal activity by membrane depolarization (Essmann et al., 2008). During development, ApoER2 signaling requires the activation of SFKs by ephrinB proteins (Sentürk et al., 2011). Therefore, we initially addressed whether this association is also important for functions of ApoER2 in adult stages during synaptic plasticity. We first investigated whether ApoER2 would cluster together with ephrinB ligands and whether this clustering could be regulated by neuronal activity. We changed the membrane potential of primary hippocampal neurons in culture by applying a depolarizing potassium chloride (KCl) solution for 10 min. Immunofluorescence analysis showed increased cluster formation and co-localization of ApoER2 and ephrinB following induction of neuronal activity (Figures 1A and 1B), suggesting a cooperative function of these two receptors in regulating activity-induced responses in neurons. Depolarizing the neurons with KCl for 10 min did not affect the expression levels of ApoER2 or ephrinB2 (Figures 1C and 1D).
Figure 1.
ApoER2 and EphrinB2 Are Required for Activity-Induced New AMPA Receptor Insertion
(A and B) Potassium chloride (KCl) stimulation co-clusters ephrinBs with ApoER2. Primary hippocampal neurons were isolated from wild-type mice at E17.5 and stimulated with 10 mM KCl for 10 min at 14 DIV to activate neuronal activity. Microscopic images show fluorescent staining of ApoER2 and ephrinB in dendrites. Arrowheads indicate co-clustering of ApoER2 (red) and ephrinB (green) upon KCl stimulation (A). Quantification of ApoER2, ephrinB, and ApoER2-ephrinB colocalized clusters is shown for n = 5 neurons (B).
(C and D) Neuronal membrane depolarization by KCl does not alter the expression levels of ApoER2 and ephrinB2. Hippocampal neuron cultures were treated with 10 mM KCl for 10 min at 14 DIV to induce neuronal activity and subjected to subsequent western blot analysis. Western blots showing ApoER2 expression levels and control actin levels (C) and ephrinB2 and control N-cadherin expression levels (D).
(E and F) Induction of neuronal activity and activation of ApoER2 induces AMPA receptor insertion in dendritic membranes. Wild-type hippocampal neurons (E17.5) were subjected to AMPA receptor membrane insertion assays. During the assay, neurons were stimulated with 10 mM KCl or concentrated Reelin (Reln) supernatants. Stimulations were conducted for 10 min (KCl) or 3 hr (Reln). Fluorescent images of GluR2 inserted into the dendritic membrane in response to KCl or Reelin (E). Relative fluorescence intensities of newly inserted GluR2 in dendrites of neurons (n = 6) (F).
(G and H) ApoER2 is essential for Reelin-induced membrane insertion of AMPA receptors. AMPA receptor membrane insertion was analyzed in wild-type and ApoER2 knockout hippocampal neurons upon stimulation with Reelin for 3 hr. Fluorescent images represent newly inserted GluR2 in dendritic branches of wild-type (+/+) and ApoER2 knockout (ApoER2−/−) neurons upon Reelin stimulation (G). Quantification shows relative intensities of GluR2 insertion in wild-type and ApoER2 knockout neurons (n = 3) (H).
(I and J) EphrinB2 mediates the membrane insertion of AMPA receptors upon Reelin stimulation. Control and ephrinB2 knockout neurons were subjected to AMPA receptor membrane insertion assays. Images of dendrites showing newly inserted GluR2 in response to Reelin stimulation in control (Nes-cre−; efnB2lox/lox) and ephrinB2 knockout (Nes-cre+; efnB2lox/lox) hippocampal neurons (I). Quantification of relative GluR2 intensities in neuronal dendrites (n = 3) (J).
Scale bars represent 5 μm (A, E, G, and I). Bar graphs show mean ± SEM (shown as error bars). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S1.
ApoER2 and EphrinB2 Are Required for Activity-Induced New AMPA Receptor Insertion
Neuronal activity induces synaptic plasticity via insertion of additional AMPA receptors at the dendritic membrane (Hayashi et al., 2000, Collingridge and Singer, 1990). In fact, ApoER2 has been shown to regulate the insertion of AMPA receptors into the dendritic membrane (Qiu et al., 2006). To analyze whether ephrinB2 and ApoER2 signaling co-operates to control AMPA receptor insertion into the membrane, we took advantage of an AMPA receptor membrane insertion assay (Lu et al., 2001, Man et al., 2003). A primary mouse anti-GluR2 antibody is applied on the neurons, followed by an unlabeled secondary anti-mouse antibody to block GluR2 subunits residing in the membrane prior to the assay (Figure S1A). The new insertion of AMPA receptors is then monitored by staining (in non-permeabilizing conditions) the receptors at the surface using the same anti-GluR2 antibody in combination with a fluorophore-labeled secondary antibody. After imaging, fluorescence intensities are measured and represent the population of AMPA receptors that has been inserted at the membrane following a given stimulus. We first treated primary hippocampal neurons at 14 days in vitro (DIV) with a depolarizing KCl solution or Reelin (to activate ApoER2 receptors) and examined newly inserted AMPA receptors. Induction of neuronal activity by KCl as well as activation of ApoER2 with Reelin led to an enhancement of AMPA receptor membrane insertion compared to the non-stimulated controls (Figures 1E and 1F). The effect of Reelin on new insertion of AMPA receptors required ApoER2, since hippocampal neurons isolated from ApoER2 knockouts failed to respond to Reelin and showed decreased AMPA receptor insertion at the dendritic membrane (Figures 1G and 1H). We next addressed the requirement of ephrinB2 and activation of Src kinases for the Reelin-induced and ApoER2-mediated insertion of new AMPA receptors. We generated neuron-specific knockout of ephrinB2 by using conditional homozygous ephrinB2lox/lox mice expressing one copy of cre under the Nestin promoter (Nes-cre+; efnB2lox/lox). We then isolated hippocampal neurons from Nes-cre-positive ephrinB2lox/lox mice at embryonic day 17.5 (E17.5) and Nes-cre-negative control neurons from littermates and cultured them for 14 DIV. The AMPA receptor insertion induced by stimulation with Reelin was blocked in neurons that did not express ephrinB2, suggesting that ephrinB2 proteins are required for this function of Reelin (Figures 1I and 1J). In agreement with a requirement for SFKs in this process, AMPA receptor insertion following ApoER2 activation by Reelin was impaired when neurons were pre-incubated with the SFK-specific inhibitor SU6656 (Figures S1B and S1C). These results suggest that in hippocampal neurons, activation of ApoER2 receptors results in the insertion of new AMPA receptors, and this function requires ephrinB2 and the activation of SFKs.
Neuronal Activity Induces Dab1 Phosphorylation at the Synapse through ApoER2/EphrinB2
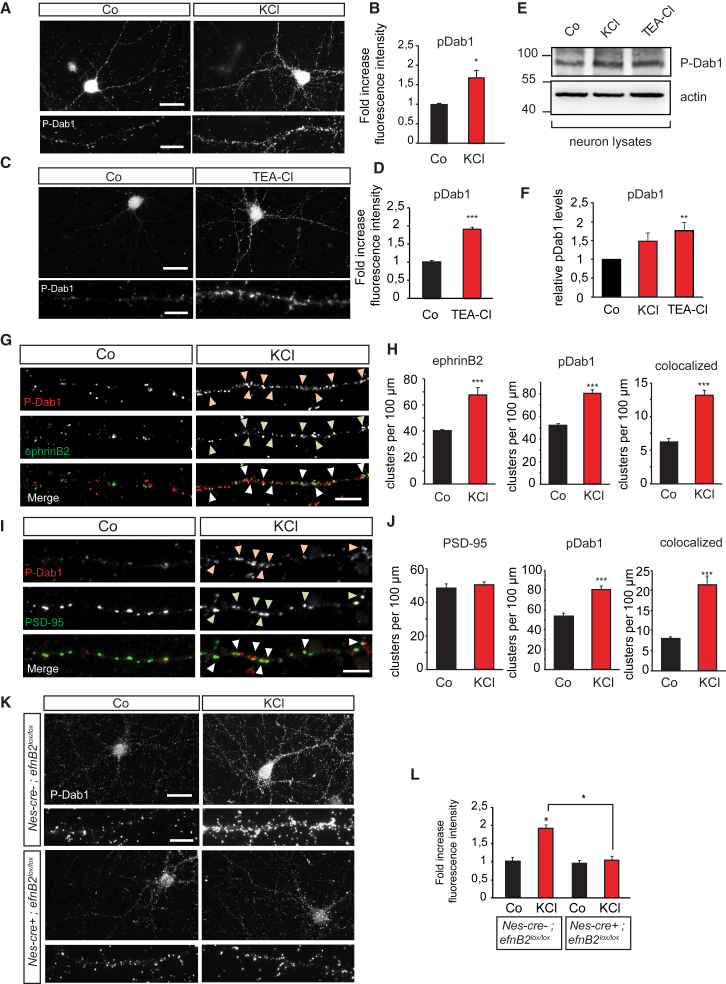
Next, we tested whether canonical signaling through the phosphorylation of Dab1 adaptor protein also occurs following induction of neuronal activity in hippocampal neurons. Membrane depolarization with KCl as well as induction of chemical LTP by tetraethylammonium chloride (TEA-Cl) application resulted in increased Dab1 phosphorylation in hippocampal neurons as assessed by immunofluorescence with an anti-P-Dab1 (Figures 2A–2D) and by western blot analysis (Figures 2E and 2F). The specificity of the anti-P-Dab1 antibody was confirmed by phospho-Tyrosine immunoprecipitation from Dab1 knockout brain lysates (Figure S2A). The phosphorylation of Dab1 upon induction of neuronal activity was comparable to the one obtained by directly stimulating ApoER2 with Reelin (Figures S2B and S2C). Neuronal activity also induced the accumulation of P-Dab1 at the postsynaptic sites in clusters with ephrinB2 (Figures 2G–2J and S3A–S3D). Moreover, stimulation of hippocampal neurons with Reelin and the ectodomain of EphB4 fused to Fc (EphB4-Fc) (to activate ephrinB2) resulted in increased P-Dab1 clusters and recruitment to PSD-95-positive sites (Figures S3E–S3H).
Figure 2.
Neuronal Activity Induces Dab1 Phosphorylation at the Synapse through EphrinB2
(A–D) Neuronal activity induces Dab1 phosphorylation in primary hippocampal neurons. Levels of phosphorylated Dab1 were assessed by immunocytochemistry. Fluorescent images showing dendritic pDab1 staining upon stimulation with KCl for 10 min (A) to depolarize the membrane or 25 mM TEA-Cl for 10 min to induce chemical LTP (C). Quantification of relative pDab1 fluorescence intensity is shown in (B; n = 4) and (D; n = 3).
(E and F) Hippocampal neurons were stimulated with 10 mM KCl or 25mM TEA-Cl for 10 min and subjected to western blot analysis. Western blot showing increased levels of pDab1 upon KCl stimulation (E). Quantification of relative pDab1 levels (n = 4) (F).
(G and H) Induction of neuronal activity causes co-clustering of ephrinB and pDab1 at postsynaptic sites. Hippocampal neurons were treated with KCl and immunocytochemical analysis was performed. Microscopic images show dendritic pDab1 and ephrinB staining. Arrowheads indicate co-clustering of pDab1 (red) and ephrinB (green) upon KCl stimulation (G). Quantification of ephrinB2, pDab1, and ephrinB2-pDab1 colocalized clusters upon KCl stimulation (n = 5 neurons) (H).
(I and J) Co-staining of pDab1 (red) and PSD-95 (green) in response to KCl is shown by arrowheads (I). Quantification of PSD-95, pDab1, and colocalized clusters of PSD-95-pDab1 is shown (n = 5 neurons) (J).
(K and L) Dab1 phosphorylation upon KCl stimulation depends on ephrinB2. Primary hippocampal neurons were isolated from conditional neuronal specific ephrinB2 knockout embryos at E17.5. At 14 DIV, neurons were stimulated with 10 mM KCl for 10 min and immunocytochemistry for pDab1 was performed. Fluorescent images showing pDab1 in control (Nes-cre−; efnB2lox/lox) and ephrinB2 knockout (Nes-cre+; efnB2lox/lox) neurons stimulated with KCl (K). Quantification of relative pDab1 fluorescence intensity in control and ephrinB2 knockout neurons (n = 3) (L).
Scale bars represent 20 μm in (A), (C), and (K) and 5 μm in the higher magnifications in (A), (C), and (K) as well as in (G) and (I). Bar graphs show mean ± SEM (shown as error bars).∗p < 0.05, ∗∗∗p < 0.001. See also Figures S2–S4.
To confirm the requirement for ephrinB2 and ApoER2 for the phosphorylation of Dab1 induced by activity, we isolated hippocampal neurons from Nes-cre-positive ephrinB2lox/lox mice at E17.5 and Nes-cre-negative control neurons from littermates and depolarized the membrane by KCl treatment. Depolarization of the membrane failed to induce Dab1 phosphorylation in neurons lacking ephrinB2 (Figures 2K and 2L). Likewise, neurons isolated from ApoER2 knockouts (ApoER2−/−) also failed to activate Dab1 in response to membrane depolarization (Figures S4A and S4B). These results suggest that both ApoER2 and its co-receptor, ephrinB2, are necessary for Dab1 phosphorylation at the synapse in response to neuronal activity.
GRIP1 Binds to ApoER2 and Scaffolds an EphrinB2/ApoER2/AMPA Receptors Complex
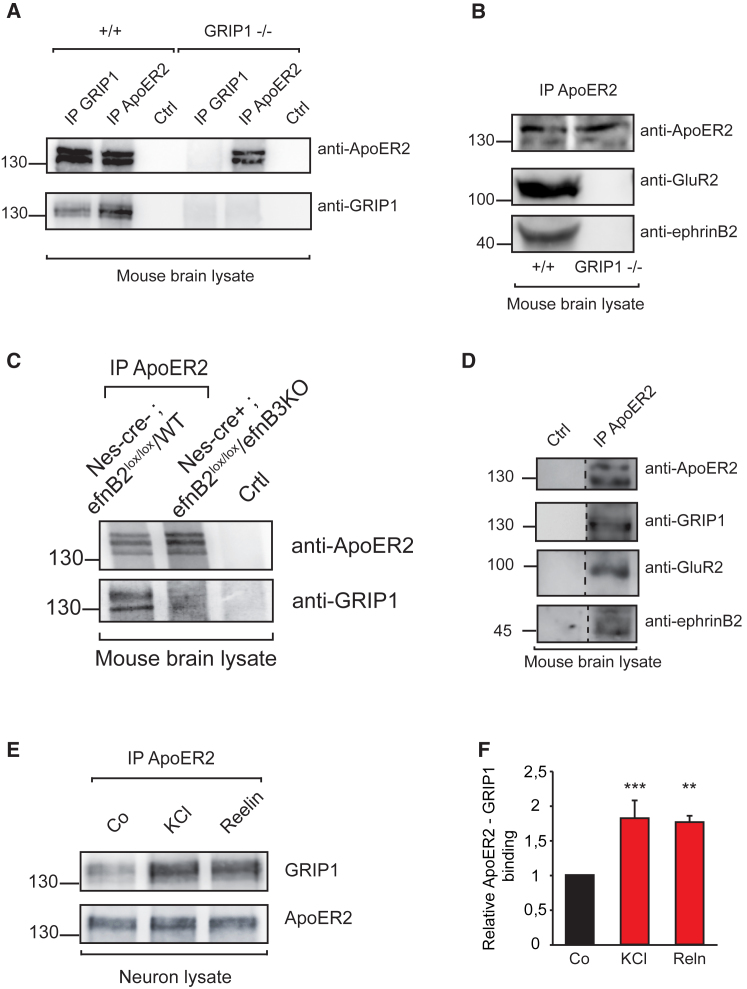
We have previously shown that the PDZ-containing scaffold protein GRIP1 binds to ephrinB2 and AMPA receptors at postsynaptic sites (Essmann et al., 2008). Thus, we postulated that GRIP1 might be a candidate for scaffolding a complex consisting of ApoER2, ephrinB2, and AMPA receptors at the membrane in response to neuronal activity. Therefore, we first analyzed whether ApoER2 binds to GRIP1. Immunoprecipitation of endogenous ApoER2 from brain lysates showed endogenous GRIP1 binding that was abrogated when ApoER2 was immunoprecipitated from GRIP1−/− lysates (Figure 3A). We next confirmed that ApoER2 and AMPA receptors interact biochemically. To test whether this interaction requires the linker protein GRIP1, we prepared total brain lysates of wild-type and GRIP1−/− mice and performed immunoprecipitation with anti-ApoER2 antibody. We observed binding of GluR2 to ApoER2 in wild-type lysates that was impaired in GRIP1−/− lysates (Figure 3B), suggesting that GRIP1 in fact bridges ApoER2 and AMPA receptors. Moreover, also binding between ApoER2 and ephrinB2 was impaired in GRIP1−/− lysates (Figure 3B).
Figure 3.
GRIP1 Binds to ApoER2 and Scaffolds an EphrinB2/ApoER2/AMPA Receptor Complex
(A) GRIP1 and ApoER2 co-immunoprecipitate in mouse brain lysates. Total brain lysates from adult wild-type (+/+) and GRIP1 knockout (GRIP−/−) mice were immunoprecipitated with anti-GRIP1, anti-ApoER2, or an unrelated antibody generated in rabbit (Ctrl) and analyzed by western blot for GRIP1 and ApoER2.
(B) GRIP1 bridges ApoER2, GluR2, and ephrinB2. Wild-type (+/+) and GRIP1 knockout (GRIP1−/−) brain lysates were immunoprecipitated using anti-ApoER2 antibody, and binding to GluR2 and ephrinB2 was analyzed by western blot.
(C) EphrinB ligands mediate the interaction between ApoER2 and GRIP1. Brain lysates from adult control (Nes-cre−; efnB2lox/lox/efnB3+/+) and double ephrinB2/ephrinB3 knockout (Nes-cre+; efnB2lox/lox/efnB3−/−) mice were immunoprecipitated with anti-ApoER2 antibody or normal rabbit immunoglobulin G (IgG; Ctrl) and analyzed by western blot using anti-GRIP1 antibody.
(D) Co-immunoprecipitation using anti-ApoER2 antibody or an unrelated rabbit antibody (Ctrl) shows the macromolecular complex formed by ApoER2, GRIP1, GluR2, and ephrinB2.
(E and F) Interaction between ApoER2 and GRIP1 is increased upon stimulation with KCl or Reelin in primary hippocampal neuron cultures. Western blots showing GRIP1 co-immunoprecipitation by using an anti-ApoER2 antibody (E). Quantification of relative binding of GRIP1 to ApoER2 upon KCl or Reelin stimulation is shown (n = 3) (F).
Bar graphs show mean ± SEM (shown as error bars).∗∗p < 0.01, ∗∗∗p < 0.001.
In order to investigate the role of ephrinB2 in the binding of ApoER2 to GRIP1 and take into account potential compensatory mechanisms by ephrinB3, we crossed our neuron-specific ephrinB2 knockout mice to a global ephrinB3 knockout mouse line. We prepared total brain lysates from double-knockout and control mice and performed immunoprecipitation with anti-ApoER2 antibody. We observed that the binding of GRIP1 to ApoER2 was impaired in the double-knockout mice, suggesting that ephrinB ligands are involved in the binding of ApoER2 to GRIP1 (Figure 3C). Importantly, immunoprecipitation of ApoER2 from wild-type brain lysates yields together the entire complex formed by ApoER2, ephrinB2, GRIP1, and AMPA receptors (Figure 3D).
We next investigated if the binding of GRIP1 to ApoER2 is regulated by neuronal activity. For this purpose, we induced membrane depolarization in hippocampal neurons isolated from wild-type mice and immunoprecipitated ApoER2. Western blot analysis showed that induction of neuronal activity in hippocampal neurons leads to an increase in the recruitment of GRIP1 to ApoER2 (Figures 3E and 3F). Moreover, activation of ApoER2 by stimulation of hippocampal neurons with Reelin also led to increased binding of scaffold protein GRIP1 (Figures 3E and 3F).
Taken together, these data show the formation of a complex in which GRIP1 acts as a scaffold for ApoER2, ephrinB2, and AMPA receptors and that the binding of the bridging molecule GRIP1 is increased upon induction of neuronal activity in hippocampal neurons.
GRIP1 Is Required for Activity-Induced ApoER2 Downstream Signaling
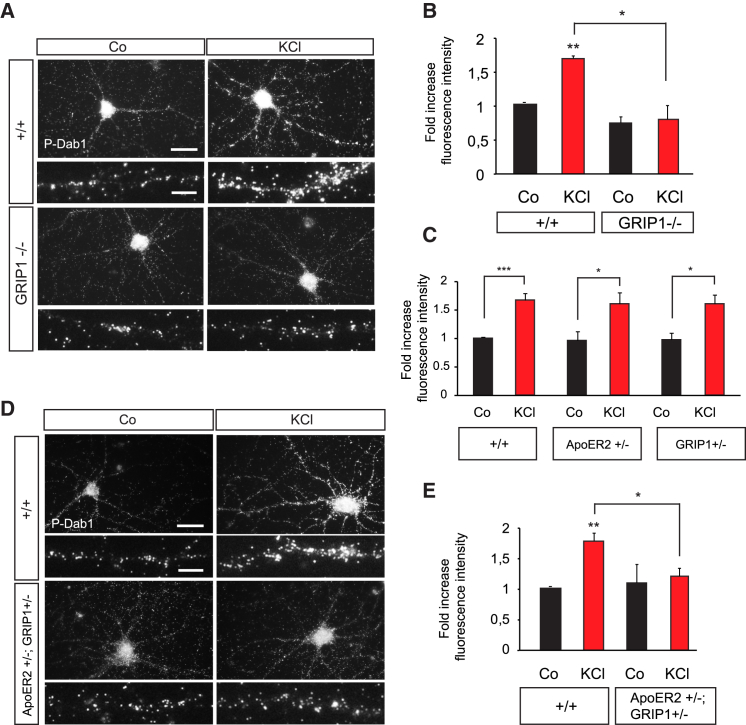
We next investigated the requirement of GRIP1 for the function of ApoER2 in hippocampal neurons. Our previous results suggested that Dab1 transduces the signals downstream of the complex ApoER2/ephrinB2 induced at the postsynaptic site by neuronal activity (Figure 2). In order to investigate whether GRIP1 is required for the functions of ephrinB2/ApoER2 in vivo we isolated hippocampal neurons from GRIP1−/− embryos and assessed Dab1 phosphorylation upon induction of neuronal activity. Phosphorylation of Dab1 following membrane depolarization with KCl was blocked in hippocampal neurons isolated from GRIP1−/− embryos in comparison to wild-type littermates (Figures 4A and 4B).
Figure 4.
GRIP1 Is Required for Activity-Induced Dab1 Phosphorylation Mediated by ApoER2
(A and B) Dab1 phosphorylation in response to neuronal activity was examined in primary hippocampal neurons prepared from wild-type (+/+) and GRIP1 knockout (GRIP1−/−) embryos at E15.5. Images of immunocytochemical pDab1 staining in dendrites stimulated with 10mM KCl for 10 min (A). Quantification of pDab1 fluorescence intensities is shown (n = 3) (B).
(C) Single-heterozygous neurons for ApoER2 and GRIP1 show normal levels of pDab1. Quantification of relative pDab1 fluorescence intensities in wild-type, ApoER2+/−, and GRIP1+/− neurons (n = 3–6).
(D and E) ApoER2 and GRIP1 genetically interact in the phosphorylation of Dab1 upon KCl stimulation. Primary hippocampal neurons from wild-type and ApoER2+/−; GRIP1+/− compound mice were stimulated with KCl, and pDab1 levels were assessed by immunocytochemistry. Fluorescent images represent pDab1 in wild-type (+/+) and ApoER2+/−; GRIP1+/− compound neurons (D). Quantification of relative pDab1 fluorescence intensity in wild-type and ApoER2+/−; GRIP1+/− compound neurons is shown (n = 3) (E).
Scale bars represent 20 μm in (A) and (D) and 5 μm in the higher magnifications in (A) and (D). Bar graphs show mean ± SEM (shown as error bars). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Moreover, in order to show that binding of GRIP1 to ApoER2 is required for the function of ApoER2 in vivo, we used genetic interaction strategies and generated compound mice with a reduction on the gene dosage for both proteins. We have used these strategies in the past to identify functional interactions of proteins in the same signaling pathway (Sentürk et al., 2011). We isolated hippocampal neurons from wild-type (+/+), ApoER2 heterozygous (ApoER2+/−), heterozygous GRIP1 (GRIP1+/−), and compound double-heterozygous (ApoER2+/−; GRIP1+/−) mice and assessed activity-induced Dab1 phosphorylation. The response to membrane depolarization of hippocampal neurons isolated from heterozygous mice for either ApoER2 or GRIP1 was indistinguishable from the one obtained in neurons isolated from wild-type mice (Figure 4C). However, when the dosage of both proteins was reduced to half in the compound mice (ApoER2+/−; GRIP1+/−), these neurons phenocopied the defects found in the ApoER2−/− and in the GRIP1−/− homozygous mutants individually and showed no induction of phosphorylation of Dab1 following membrane depolarization (Figures 4D and 4E). These results indicate that both ApoER2 and GRIP1 are acting in the same pathway to allow phosphorylation of Dab1 at the synapse.
GRIP1 Is Required for ApoER2-Mediated AMPA Receptor Insertion
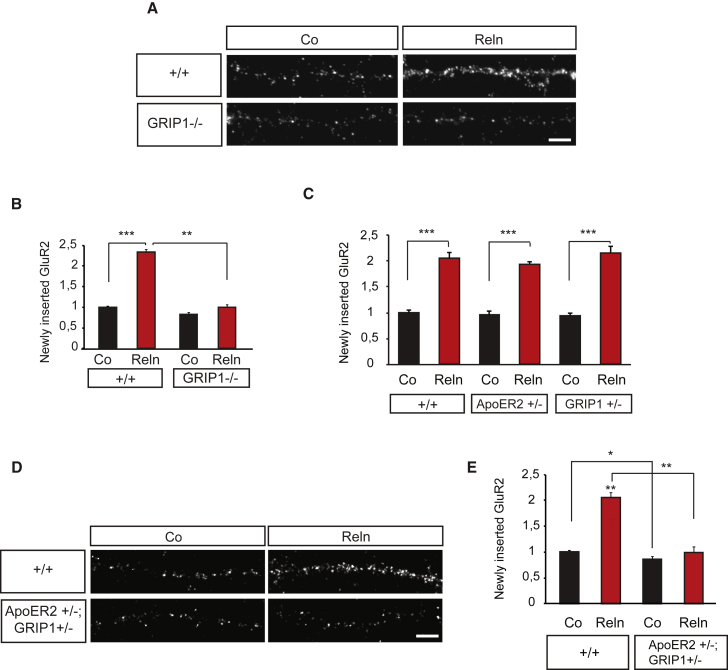
We next investigated the role of GRIP1 in the ApoER2-mediated new insertion of AMPA receptors at the dendritic membrane. For this, we stimulated hippocampal neurons with Reelin to activate ApoER2 receptors and performed the newly inserted AMPA receptor assay as described above. Hippocampal neurons from GRIP1−/− embryos failed to respond to stimulation of ApoER2 by Reelin and to insert new AMPA receptors (Figures 5A and 5B). Compound genetics also verified the interaction of ApoER2 and GRIP1 required to insert AMPA receptors upon Reelin stimulation. Heterozygous single-mutant ApoER2+/− or GRIP1+/− neurons inserted new subunits of AMPA receptors upon Reelin stimulation as efficiently as neurons obtained from the respective wild-type littermates (Figure 5C). However, compound mice (ApoER2+/−; GRIP1+/−), in which both protein dosages have been reduced by half, recapitulated the impairment in inserting AMPA receptors (Figures 5D and 5E) observed in GRIP1−/− (Figures 5A and 5B) and ApoER2−/− (Figures 1G and 1H) single mutants.
Figure 5.
GRIP1 Is Required for ApoER2-Mediated AMPA Receptor Insertion
(A and B) GRIP1 is required for AMPA receptor membrane insertion mediated by ApoER2. GluR2 insertion was examined in GRIP1 knockout neurons upon Reelin stimulation. Staining of newly inserted GluR2 in wild-type (+/+) and GRIP1 knockout (GRIP1−/−) dendrites (A). Statistical analysis of GluR2 fluorescence intensities (n = 3) (B).
(C) Hippocampal neurons generated from single-heterozygous ApoER2 (ApoER2+/−) and GRIP1 (GRIP+/−) embryos show normal levels of newly inserted GluR2 upon Reelin stimulation (n = 3–4).
(D and E) Functional interaction between ApoER2 and GRIP1 is necessary for new AMPA receptor insertion. ApoER2+/−; GRIP1+/− compound hippocampal neurons were subjected to AMPA receptor membrane insertion assays with Reelin stimulation. Microscopic images show newly inserted GluR2 after Reelin stimulation in wild-type (+/+) and ApoER2+/−; GRIP1+/− compound neurons (D). Quantification of relative fluorescence intensities of newly inserted GluR2 in wild-type and compound neurons (n = 3) (E).
Scale bars in (A) and (D) represent 5 μm. Bar graphs show mean ± SEM (shown as error bars). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
EphrinB2 Ser-9 Regulates ApoER2/EphrinB2/AMPAR/GRIP1 Interactions and Activity-Induced AMPA Receptor Insertion In Vivo
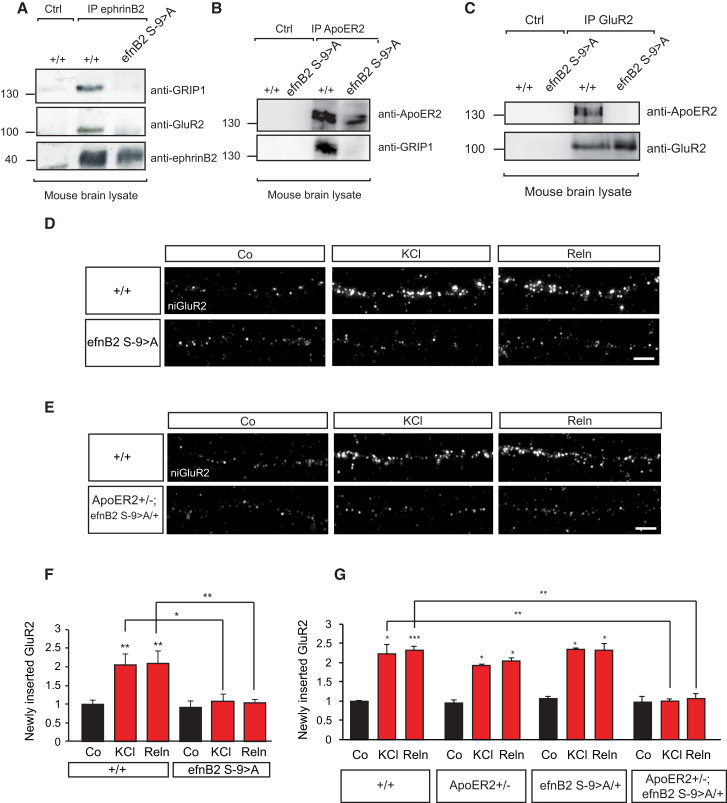
We next aimed to obtain further mechanistic insights on the regulation of the ApoER2/ephrinB2/GRIP1 complex at the synapse. Previously, we have identified a serine residue in the cytoplasmic tail of ephrinB2 at position −9 whose phosphorylation was required for the interaction with GRIP1 (Essmann et al., 2008). In order to investigate the function of this serine residue in the regulation of the ApoER2/ephrinB2/AMPAR/GRIP1 complex at the synapse in vivo, we generated a knockin mouse mutant where the endogenous ephrinB2 allele was replaced by the ephrinB2 cDNA in which the serine −9 in the cytoplasmic domain was mutated to alanine (efnB2 S-9 > A mouse), therefore rendering a non-phosphorylatable ephrinB2. The expression levels of the knockin ephrinB2 were comparable to wild-type levels as assessed by immunoblot of cultured hippocampal neurons (Figure S5A). We first characterized whether GRIP1 would still be able to stably form a complex with AMPA receptors and the mutant ephrinB2 in vivo. Brain lysates from wild-type and efnB2 S-9 > A mice were immunoprecipitated with antibodies against ephrinB2, GRIP1, and the subunit GluR2 of AMPA receptors. Immunoprecipitation of ephrinB2 or GRIP1 failed to bring together the triple complex (ephrinB2/GRIP1/AMPA receptor) in efnB2 S-9 > A mice (Figures 6A and S5B). These results suggest that the serine residue in ephrinB2 regulates the recruitment of GRIP1 and that mutated ephrinB2 fails to scaffold AMPA receptors in one complex.
Figure 6.
Ser-9 of EphrinB2 Is Essential for the ApoER2-Mediated Insertion of AMPA Receptors at the Post-synaptic Membrane
(A–C) The macromolecular complex consisting of ApoER2, GRIP1, GluR2, and ephrinB2 is disrupted in ephrinB2 S-9 > A mice. Ser-9 of ephrinB2 is necessary for the binding of GRIP1 and GluR2 to ephrinB2. Wild-type (+/+) and ephrinB2 S-9 > A (efnB2 S-9 > A) total brain lysates were immunoprecipitated by using an ephrinB2 antibody or an unrelated goat antibody (Ctrl), and binding of GRIP1 and GluR2 was investigated by western blot (A). The interaction between ApoER2 and GRIP1 is lost in ephrinB2 S-9 > A mutant mice. Wild-type (+/+) and ephrinB2 S-9 > A knockin (efnB2 S-9 > A) brain lysates were immunoprecipitated using anti-ApoER2 antibody or an unrelated rabbit antibody (Ctrl), and the interaction with GRIP1 was analyzed by western blot (B). ApoER2 and GluR2 co-immunoprecipitation in mouse brain lysates is dependent on Ser-9 of ephrinB2. Total brain lysates from adult wild-type (+/+) and ephrinB2 S-9 > A knockin (efnB2 S-9 > A) mice were immunoprecipitated with anti-GluR2 antibody or an unrelated mouse antibody (Ctrl) and analyzed by western blot for ApoER2 and GluR2 (C).
(D–G) Ser-9 of ephrinB2 is required for AMPA receptor membrane insertion and functionally interacts with ApoER2 upon stimulation with KCl and Reelin. Wild-type (+/+) and ephrinB2 S-9 > A knockin (efnB2 S-9 > A) neurons were subjected to AMPA receptor membrane insertion assays upon stimulation with 10 mM KCl or concentrated Reelin (Reln) supernatant. Fluorescent images of newly inserted GluR2 staining (D). Fluorescent images of newly inserted GluR2 in wild-type (+/+) and ApoER2+/−; ephrinB2S-9>A/+ compound neurons upon KCl or Reelin (Reln) stimulation (E). Shown are quantification of newly inserted GluR2 intensities in wild-type (+/+) and ephrinB2 S-9 > A (efnB2 S-9 > A) neurons (n = 10) (F) and quantification of fluorescence intensities in wild-type, ApoER2+/−, ephrinB2S-9>A/+, and compound neurons (n = 3–4) (G).
Scale bars in (D) and (E) represent 5 μm. Bar graphs show mean ± SEM (shown as error bars). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figures S5 and S6.
We next investigated whether the formation of the ApoER2/ephrinB2/AMPAR/GRIP1 complex was altered in the efnB2 S-9 > A mouse. Immunoprecipitation of endogenous ApoER2 from brain lysates isolated from wild-type showed endogenous GRIP1 binding that was impaired in lysates from efnB2 S-9 > A (Figure 6B). Likewise, immunoprecipitation of GluR2 showed binding of ApoER2 in brain lysates from wild-type mice, but not from efnB2 S-9 > A mice (Figure 6C). These results indicate that serine phosphorylation on serine −9 in ephrinB2 regulates the formation of the ApoER2/ephrinB2/AMPAR/GRIP1 in vivo.
In order to explore the functional consequences of a mutation on serine −9 in ephrinB2 on ApoER2-mediated functions, we first isolated hippocampal neurons from mice carrying the mutation on ephrinB2 serine −9 (efnB2 S-9 > A) and wild-type littermates at E17.5 and assessed AMPA receptor insertion at 14 DIV. The AMPA receptor insertion induced by stimulation with Reelin was abolished in neurons expressing a mutant ephrinB2, suggesting that the binding of GRIP1 to phosphorylated ephrinB2 is essential for the ApoER2-mediated AMPA receptor insertion (Figures 6D and 6F). Moreover, the new insertion of AMPA receptors following membrane depolarization in neurons was also prevented in neurons with an ephrinB2 serine mutant (Figures 6D and 6F). We next proceeded to demonstrate a genetic interaction between the ApoER2 receptor and ephrinB2 in the function of ApoER2 during synaptic plasticity and insertion of new AMPA receptors at the dendritic membrane. Thus, we generated compound mice heterozygous for ApoER2 and crossed in one allele of the ephrinB2 serine mutation (ApoER2+/−; efnB2S-9>A/+). Analysis of new insertion of AMPA receptors following stimulation with Reelin and induction of neuronal activity was performed in hippocampal neurons from E17.5 wild-type (+/+) mice, heterozygous ApoER2 (ApoER2+/−) mice, mice heterozygous for the ephrinB2 serine mutation (efnB2S-9>A/+), and compound mice carrying only one allele of ApoER2 and one of the ephrinB2 serine mutations (ApoER2+/−; efnB2S-9>A/+). Mice heterozygous for ApoER2 and mice heterozygous for the ephrinB2 serine mutation individually did not show any decrease in AMPA receptor insertion after Reelin stimulation and KCl treatment (Figure 6G). However, under the same stimuli, the mice compound (ApoER2+/−; efnB2S-9>A/+) heterozygous for the ApoER2 receptor failed to induce AMPA receptor insertion when one allele of the ephrinB2 was mutated in the serine residue (Figures 6E and 6G). Moreover, activity-induced and ApoER2-mediated downstream signaling at the synapse through Dab1 phosphorylation was also impaired in hippocampal neurons isolated from compound ApoER2+/−; efnB2S-9>A/+ mice (Figures S6A and S6B). Hippocampal neurons isolated from wild-type mice (+/+), heterozygous ApoER2+/− mice, and mice with one mutated allele of ephrinB2 responded to treatment with KCl by inducing comparable levels of phosphorylation of the adaptor protein Dab1 (Figure S6B).
ApoER2 Binding to GRIP1 and EphrinB2 Is Required for the Induction of Synaptic Plasticity
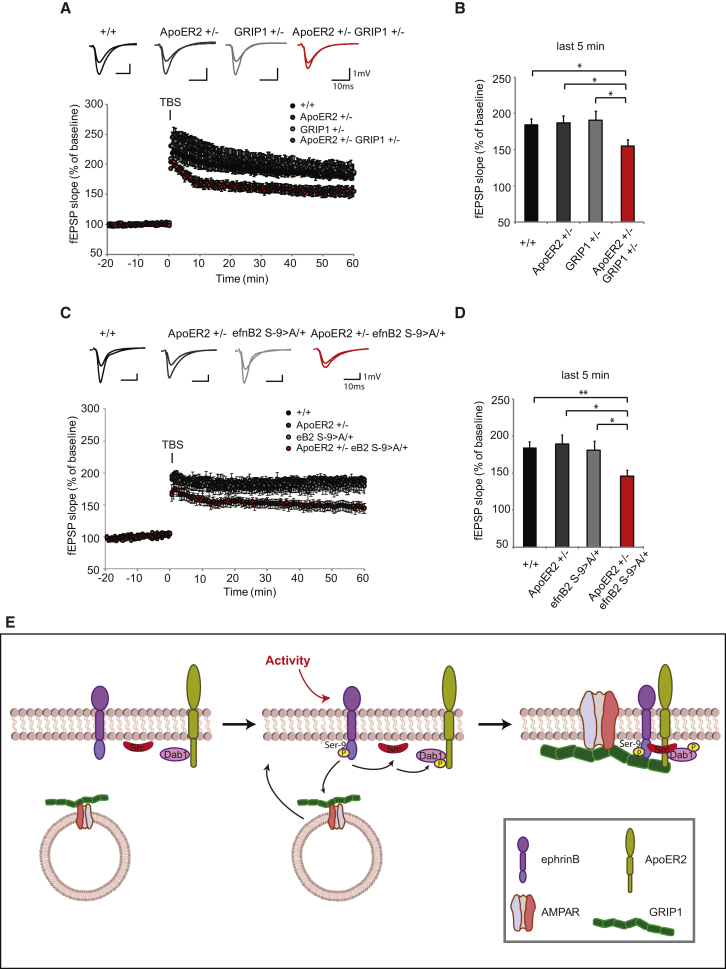
To examine the requirement of ApoER2-GRIP1 and ApoER2-ephrinB2 interactions for synaptic plasticity in a physiological context, we performed LTP measurements in hippocampal slices. During the expression phase of LTP, the insertion of new AMPA receptors into the postsynaptic membrane is a crucial step. We recorded LTP in acute hippocampal slices from wild-type, single-heterozygous ApoER2+/−, GRIP1+/− or efnB2S-9>A/+ mice and double-heterozygous compound mice (ApoER2+/−; GRIP1+/− or ApoER2+/−; efnB2S-9>A/+). For both combinations, LTP magnitude was comparable between single-heterozygous mice and wild-type littermates, whereas the double-heterozygous compound mice showed significantly reduced LTP (Figures 7A–7D).
Figure 7.
ApoER2, GRIP1, and EphrinB2 Genetically Interact at the Synapse
(A–D) ApoER2, GRIP1, and ephrinB2 genetically interact in LTP. ApoER2+/−; GRIP1+/− compound mice (A and B) and ApoER2+/−; efnB2S-9>A/+ compound mice (C and D) show reduced LTP. LTP was induced by theta burst stimulation (TBS) of Schaffer collaterals and field excitatory postsynaptic potentials (fEPSPs) were recorded in the stratum radiatum of the CA1 region. Representative traces of the compound mice and their respective single-heterozygous and wild-type littermates (+/+) are shown. (ApoER2+/−; GRIP+/− in A; ApoER2+/−; eB2S9>A/+ in C) LTP is significantly reduced in ApoER2+/−; GRIP1+/− compound mice (B) and in ApoER2+/−; efnB2S-9>A/+ compound mice (D) compared to their respective control genotypes at 55–60 min after TBS.
(E) GRIP1 regulates ApoER2/ephrinB2 functions at the synapse. Neuronal activity induces ephrinB clustering, leading to the recruitment of Src and its activation, which in turn phosphorylates the adaptor protein Dab1 recruited by ApoER2 receptors. Upon phosphorylation of ephrinB2 in a serine residue (Ser-9), glutamate-receptor-interacting protein 1 (GRIP1) binds to ApoER2 and bridges a complex including ApoER2, ephrinB2, and AMPA receptors at the synapse in an activity-dependent manner. The formation of such a complex is necessary for activity induced synaptic plasticity.
For (A)–(D), bar graphs show mean ± SEM as calculated across slices (shown as error bars). ApoER2-GRIP1: n = 7 wild-type mice (16 slices), 6 ApoER2+/− mice (13 slices), 9 GRIP1+/− mice (16 slices), and 8 ApoER2+/−; GRIP1+/− compound mice (21 slices). ApoER2-ephrinB2: n = 6 wild-type mice (13 slices), 4 ApoER2+/− mice (9 slices), 4 efnB2S-9>A/+ mice (8 slices), and 6 ApoER2+/−; efnB2S-9>A/+ compound mice (15 slices). ∗p < 0.05; ∗∗p < 0.01. See also Figure S7.
To test whether the attenuated LTP in ApoER2+/−; GRIP1+/− and ApoER2+/−, efnB2S-9>A/+ compound mice result from altered basal synaptic transmission, we measured input-output curves at various stimulus intensities. No significant effect was found among the genotypes (Figures S7A and S7C). Additionally, to investigate whether presynaptic properties are altered in the mutant mice, we analyzed paired-pulse ratio as a measure for presynaptic release probability. No differences were found among the genotypes, indicating that presynaptic function is normal in ApoER2+/−; GRIP1+/− mice and ApoER2+/−; efnB2 S-9 > A/+ compound mice (Figures S7B and S7D). These experiments suggest that basal synaptic properties are unchanged in ApoER2+/−; GRIP1+/− mice and ApoER2+/−; efnB2S-9>A/+ compound mice and that the reduced LTP most likely results from a defect in expression or maintenance of LTP due to an impairment of plasticity-induced insertion of additional AMPA receptors into the postsynaptic membrane.
Discussion
Reelin, its receptors ApoER2 and VLDLR, and the intracellular adaptor protein Dab1 all continue to be expressed in the adult brain and have been proposed to play roles during synaptic plasticity and memory formation (Weeber et al., 2002, Beffert et al., 2005, Trotter et al., 2013). We have previously shown that the Reelin receptor ApoER2 interacts with ephrinB proteins to regulate neuronal migration during the development of laminated structures in the mouse brain (Sentürk et al., 2011). In this study, we unravel the cooperation of ephrinB2 and ApoER2 to regulate new AMPA receptor membrane insertion in response to neuronal activity (Figure 7E), demonstrating that the previously identified function of ephrinBs in regulating Reelin signaling goes beyond the control of developmental functions of Reelin and extends to other roles in adulthood such as synaptic plasticity.
ApoER2-deficient mice were shown to exhibit impairments in LTP (Weeber et al., 2002). The mechanism underlying the plasticity functions of ApoER2 receptors has been in part attributed to the modulation of the activity of NMDA receptors. Reelin signaling induces NMDA receptor phosphorylation and synaptic currents in a Dab1- and Src-family-kinase-dependent manner (Chen et al., 2005). In addition to the modulation of NMDA receptor activity, ApoER2 and Dab1 have been shown to regulate new insertion of GluR1-containing AMPA receptors following long-lasting treatment of hippocampal neurons in culture with Reelin (Qiu et al., 2006). This mechanism, which seems to apply to insertion of newly synthesized AMPA receptors during the late phases of LTP, is dependent on the activation of phosphatidylinositol 3-kinase (PI3K) by Reelin and appears to be independent of SFK activation. Interestingly, PI3K signaling has been shown to be activated by phosphorylated Dab1 and to be involved in AMPA receptor insertion via the ApoER2-Dab1 pathway (Qiu et al., 2006). PI3K activation may lead to activation of Akt, which has been found to exhibit reduced phosphorylation and reduced activation of its downstream substrates in Dab1 knockout hippocampus, causing defects in synaptic plasticity (Trotter et al., 2013).
We now show a mechanism that seems to act in the early phases after the induction of neuronal activity and that results in a rapid clustering of ApoER2 and ephrinB2 and the new insertion of GluR2-containing AMPA receptors at the postsynaptic membrane. Loss-of-function studies with both receptors and the use of compound genetics show the requirement of ephrinB for this ApoER2-mediated AMPA receptor insertion induced both by neuronal activity as well as by short-term stimulation with Reelin. Interestingly, insertion of AMPA receptors in this early phase following Reelin stimulation appears to depend on the activation of SFKs. The kinetics of such events suggest that the clustering of ApoER2 and ephrinB2 regulates the new insertion of AMPA receptors from the intracellular compartment pool and is important for induction of LTP. In agreement with this, postnatal ablation of ephrinB2 in the nervous system leads to severe defects in hippocampal LTP (Grunwald et al., 2004).
In the current study, we show that Dab1 phosphorylation is increased by neuronal depolarization. We found that induction of neuronal activity by membrane depolarization clusters ephrinB, ApoER2, and the intracellular adaptor molecule Dab1 at postsynaptic sites. Direct activation of ephrinB2 or ApoER2 by EphB4 receptor or Reelin, respectively, caused Dab1 phosphorylation at postsynaptic sites. Loss of function of any of both receptors showed an impairment to phosphorylate Dab1 upon neuronal activity induction, suggesting that the cooperation of both receptors is required for Dab1-dependent signaling during synaptic plasticity. Consistently with our results, Reelin-induced phosphorylation of Dab1 via SFKs has been shown to be required for the formation and maintenance of dendritic spines (Niu et al., 2008), and Dab1 deletion in the adult brain results in impaired induction and maintenance of LTP (Trotter et al., 2013).
We had previously identified a new phosphorylation site in the cytoplasmic tail of ephrinB2, on the serine −9 from the C terminus (Essmann et al., 2008). Using constructs where this phosphorylation was either prevented (serine mutated to alanine [S9 > A]) or mimicked (serine mutated to glutamic acid [S9 > E]) to generate a loss or gain of function, respectively, we showed that binding of GRIP1 to ephrinB2 was impaired in cells expressing the S9 > A construct, but constitutive upon the expression of the S9 > E plasmid. Furthermore, by expressing similar constructs in neurons isolated from neuron-specific ephrinB2 knockout mice, we showed that AMPA receptor internalization was increased in the ephrinB2 knockout mice, a phenotype that was rescued in neurons expressing the S9 > E mutation, but not S9 > A. In other words, the stabilization of AMPA receptors at the membrane by ephrinB2 requires the phosphorylation of ephrinB2 at the residue Ser-9 (Essmann et al., 2008). To analyze whether serine phosphorylation of ephrinB2 regulates the co-operative functions of ephrinB2 and ApoER2, we generated a knockin mouse mutant where the Ser-9 of ephrinB2 was replaced by alanine in order to prevent phosphorylation at that residue (efnB2 S-9 > A mouse). Hippocampal neurons from efnB2 S-9 > A mice were unable to insert new AMPA receptors upon KCl or Reelin stimulation. The same impairment was observed in compound mice double heterozygous for ApoER2 and the mutated ephrinB2, while the single heterozygous mice were unaffected, showing the requirement of ephrinB2 for ApoER2 function and underlining the importance of ephrinB2 serine phosphorylation for this interaction. Importantly, also the phosphorylation of Dab1 was impaired in compound mice double heterozygous for ApoER2 and mutated ephrinB2. In agreement with these findings, we found that biochemical interaction of ApoER2 and the AMPA receptor subunit GluR2 was disrupted in efnB2 S-9 > A mouse brain lysates.
It seems likely that different cooperative signaling complexes assemble at the intracellular domain of ApoER2 to regulate baseline and Reelin-induced LTP. These complexes include the interaction of Dab1 to the NPXY domain of ApoER2 and of other adaptor proteins to exon 19 of ApoER2. The exon 19 was shown to be required for the Reelin-modulated functions of ApoER2 during synaptic plasticity (Beffert et al., 2005). Exon 19 of ApoER2 contains a PDZ-binding domain and binds PDZ-containing proteins, such as the postsynaptic protein PSD-95 and the multi-adaptor protein X11α, a regulator of processing and trafficking of the amyloid precursor protein APP; both interactions regulate the insertion of ApoER2 into the membrane (Tomita et al., 1999, Gotthardt et al., 2000, Hoe et al., 2006, Minami et al., 2010). Indeed, PSD95 has been postulated to mediate the interaction between ApoER2 and NMDA receptors (Beffert et al., 2005). We have shown previously that AMPA receptor membrane stabilization regulated by ephrinB2 phosphorylation at serine −9 depends on the recruitment of the scaffolding molecule GRIP1, which bridges ephrinB2 and AMPA receptors (Essmann et al., 2008). Here, we have unraveled an interaction between GRIP1 and ApoER2 receptors that is necessary for the regulation of activity- and ApoER2-mediated AMPA receptor insertion. We show biochemically that GRIP1 acts as a scaffolding molecule bridging ephrinB2, ApoER2, and AMPA receptors in a complex and that GRIP1 loss of function results in impaired formation of such a complex in vivo. As a consequence of the disruption of such complex, hippocampal neurons isolated from the single ApoER2−/−, Nes-cre ephrinB2−/−, ephrinB2 S-9 > A and GRIP1−/− as well as compound mutants ApoER2+/−; GRIP1+/− and ApoER2+/−; ephrinB2S-9>A/+ show impairments in new AMPA receptor insertion at the synaptic membrane. Interestingly, and in line with our results, it has also been previously shown that AMPA receptor recycling upon NMDA-induced activity is impaired in neurons lacking GRIP1 and GRIP2 (Mao et al., 2010), showing that GRIP1 is an essential key player in the regulation of AMPA receptor availability.
In order to further assess the physiological function of the ApoER2/GRIP1/ephrinB2-mediated insertion of AMPA receptors, we performed field recordings in compound ApoER2+/−; GRIP1+/− and in compound ApoER2+/−; efnB2S-9>A/+ double-heterozygous mice. Our results show a significant reduction in LTP in both compound mice, while the corresponding single-heterozygous mice appear unaffected. The input-output curves and paired-pulse ratios reveal no difference between the genotypes, excluding an issue with basal presynaptic transmission. Consistently, presynaptic properties in ApoER2−/− mice are unchanged (Weeber et al., 2002). The impaired LTP observed in compound GRIP1; ApoER2 double-heterozygous mice, as well as in compound efnB2 S-9 > A; ApoER2 mice, is therefore most likely due to postsynaptic defects, such as the AMPA receptor insertion into the dendritic membrane, thus corroborating our molecular findings.
We suggest a model in which neuronal activity causes clustering of ephrinB2, which in turn leads to the recruitment of GRIP1 molecules. GRIP1 provides a scaffold for a multiprotein signaling complex including ApoER2, AMPA receptors, and ephrinB2 to activate downstream signaling cascades necessary for the insertion of new AMPA receptors. Our findings open the possibility to explore potential new therapeutic targets for neurological disorders and cognitive dysfunction associated with Reelin signaling loss of function such as schizophrenia and Alzheimer disease.
Experimental Procedures
Animals
The generation of conditional ephrinB2lox/lox knockout mice has been described previously (Grunwald et al., 2004). ApoER2 knockout mice were kindly provided by Joachim Herz and genotyped as described previously (Trommsdorff et al., 1999). GRIP1 knockout mice were kindly provided by Richard Huganir (Takamiya et al., 2004). To generate ApoER2+/−; GRIP1+/− compound mice, heterozygous ApoER2 knockout mice were crossed with heterozygous GRIP1 knockout animals.
To generate the ephrinB2 S-9 > A mouse line, a knockin strategy previously used to generate ephrinB2 ΔC mice (Adams et al., 2001) and ephrinB2 ΔValin mice and ephrinB2 5Y mice (Mäkinen et al., 2005) was used. For more details, please refer to Supplemental Experimental Procedures. To generate ApoER2+/−; ephrinB2 S-9 > A/+ compound mice, heterozygous ApoER2-knockout mice were crossed with heterozygous ephrinB2 S-9 > A knockin animals.
All the experiments involving animal were conducted according to the institutional guidelines and approved by the German authorities (Hessian government).
Primary Hippocampal Neuron Cultures
Primary hippocampal neurons from wild-type, ephrinB2 conditional knockout, ApoER2 knockout, ApoER2+/−; GRIP1+/− compound, ephrinB2 S-9 > A knockin, and ApoER2+/−; ephrinB2S-9>A/+ compound mice and control littermates were prepared at E17.5. Neurons from GRIP1 knockout mice were isolated at E15.5. Isolation and cultivation of hippocampal neurons was carried out as described previously (Segura et al., 2007).
Immunoprecipitation and Western Blot Analysis
Co-immunoprecipitation assays and western blot analysis were performed as previously described (Essmann et al., 2008). As immunoprecipitation control, either unrelated primary antibodies or normal immunoglobulin Gs (IgGs) produced in the same species as the experimental antibody were used.
Statistical Analysis
The numbers (n) stated in the figure legends represent the number of means on which the presented results, expressed as the mean ± SEM, and the statistical significance, determined by the two-tailed Student’s t test, are based. These n values are independent experiments in all figures, except when otherwise mentioned. For electrophysiological recordings, an average of 14 slices in 4–9 animals per condition were recorded, from which the mean ± SEM were calculated. Statistical significance was assumed when p < 0.05. In figures, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗ p < 0.001.
Author Contributions
A.A.-P. conceived the project, supervised, analyzed experiments and, together with S.P., wrote the manuscript. S.P., F.F., D.B., E.H., and J.C.T. designed, performed, and analyzed experiments. M.S., together with all other authors, edited the manuscript and prepared the figures.
Acknowledgments
We would like to thank U. Bauer, D. Schmelzer, T. Belefkih, and K. Happich for technical support. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB834, SFB1080, FOR2325, SFB1193, EXC115, and EXC147), the ERC (grant 669742 Neurovessel), the Max Planck Fellow Program and Gutenberg Research College (GRC) at Johannes Gutenberg University Mainz (A.A.-P.), and the EU (grant EU-CIG 293902, M.S.).
Published: October 3, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2017.09.019
Supplemental Information
References
- Adams R.H., Diella F., Hennig S., Helmbacher F., Deutsch U., Klein R. The cytoplasmic domain of the ligand ephrinb2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Beffert U., Weeber E.J., Durudas A., Qiu S., Masiulis I., Sweatt J.D., Li W.-P., Adelmann G., Frotscher M., Hammer R.E., Herz J. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–579. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Bock H.H., Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr. Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- Bouzioukh F., Wilkinson G.A., Adelmann G., Frotscher M., Stein V., Klein R. Tyrosine phosphorylation sites in ephrinB2 are required for hippocampal long-term potentiation but not long-term depression. J. Neurosci. 2007;27:11279–11288. doi: 10.1523/JNEUROSCI.3393-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Beffert U., Ertunc M., Tang T.S., Kavalali E.T., Bezprozvanny I., Herz J. Reelin modulates NMDA receptor activity in cortical neurons. J. Neurosci. 2005;25:8209–8216. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G.L., Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol. Sci. 1990;11:290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Cooper J.A. A mechanism for inside-out lamination in the neocortex. Trends Neurosci. 2008;31:113–119. doi: 10.1016/j.tins.2007.12.003. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G., Miao G.G., Chen S.C., Soares H.D., Morgan J.I., Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G., Homayouni R., Keshvara L., Rice D.S., Sheldon M., Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Essmann C.L., Martinez E., Geiger J.C., Zimmer M., Traut M.H., Stein V., Klein R., Acker-Palmer A. Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat. Neurosci. 2008;11:1035–1043. doi: 10.1038/nn.2171. [DOI] [PubMed] [Google Scholar]
- Gotthardt M., Trommsdorff M., Nevitt M.F., Shelton J., Richardson J.A., Stockinger W., Nimpf J., Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J. Biol. Chem. 2000;275:25616–25624. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- Grunwald I.C., Korte M., Adelmann G., Plueck A., Kullander K., Adams R.H., Frotscher M., Bonhoeffer T., Klein R. Hippocampal plasticity requires postsynaptic ephrinBs. Nat. Neurosci. 2004;7:33–40. doi: 10.1038/nn1164. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Shi S.H., Esteban J.A., Piccini A., Poncer J.C., Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hoe H.-S., Pocivavsek A., Chakraborty G., Fu Z., Vicini S., Ehlers M.D., Rebeck G.W. Apolipoprotein E receptor 2 interactions with the N-methyl-D-aspartate receptor. J. Biol. Chem. 2006;281:3425–3431. doi: 10.1074/jbc.M509380200. [DOI] [PubMed] [Google Scholar]
- Howell B.W., Herrick T.M., Cooper J.A. Reelin-induced tyrosine [corrected] phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 1999;13:643–648. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W., Man H., Ju W., Trimble W.S., MacDonald J.F., Wang Y.T. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Makino H., Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen T., Adams R.H., Bailey J., Lu Q., Ziemiecki A., Alitalo K., Klein R., Wilkinson G.A. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man H.-Y., Wang Q., Lu W.-Y., Ju W., Ahmadian G., Liu L., D’Souza S., Wong T.P., Taghibiglou C., Lu J. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/s0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Mao L., Takamiya K., Thomas G., Lin D.-T., Huganir R.L. GRIP1 and 2 regulate activity-dependent AMPA receptor recycling via exocyst complex interactions. Proc. Natl. Acad. Sci. USA. 2010;107:19038–19043. doi: 10.1073/pnas.1013494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami S.S., Sung Y.M., Dumanis S.B., Chi S.H., Burns M.P., Ann E.-J., Suzuki T., Turner R.S., Park H.-S., Pak D.T.S. The cytoplasmic adaptor protein X11α and extracellular matrix protein Reelin regulate ApoE receptor 2 trafficking and cell movement. FASEB J. 2010;24:58–69. doi: 10.1096/fj.09-138123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S., Yabut O., D’Arcangelo G. The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J. Neurosci. 2008;28:10339–10348. doi: 10.1523/JNEUROSCI.1917-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S., Zhao L.F., Korwek K.M., Weeber E.J. Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J. Neurosci. 2006;26:12943–12955. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.T., Rusiana I., Trotter J., Zhao L., Donaldson E., Pak D.T.S., Babus L.W., Peters M., Banko J.L., Chavis P. Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn. Mem. 2011;18:558–564. doi: 10.1101/lm.2153511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura I., Essmann C.L., Weinges S., Acker-Palmer A. Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat. Neurosci. 2007;10:301–310. doi: 10.1038/nn1858. [DOI] [PubMed] [Google Scholar]
- Sentürk A., Pfennig S., Weiss A., Burk K., Acker-Palmer A. Ephrin Bs are essential components of the Reelin pathway to regulate neuronal migration. Nature. 2011;472:356–360. doi: 10.1038/nature09874. [DOI] [PubMed] [Google Scholar]
- Takamiya K., Kostourou V., Adams S., Jadeja S., Chalepakis G., Scambler P.J., Huganir R.L., Adams R.H. A direct functional link between the multi-PDZ domain protein GRIP1 and the Fraser syndrome protein Fras1. Nat. Genet. 2004;36:172–177. doi: 10.1038/ng1292. [DOI] [PubMed] [Google Scholar]
- Tomita S., Ozaki T., Taru H., Oguchi S., Takeda S., Yagi Y., Sakiyama S., Kirino Y., Suzuki T. Interaction of a neuron-specific protein containing PDZ domains with Alzheimer’s amyloid precursor protein. J. Biol. Chem. 1999;274:2243–2254. doi: 10.1074/jbc.274.4.2243. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M., Gotthardt M., Hiesberger T., Shelton J., Stockinger W., Nimpf J., Hammer R.E., Richardson J.A., Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Trotter J., Lee G.H., Kazdoba T.M., Crowell B., Domogauer J., Mahoney H.M., Franco S.J., Müller U., Weeber E.J., D’Arcangelo G. Dab1 is required for synaptic plasticity and associative learning. J. Neurosci. 2013;33:15652–15668. doi: 10.1523/JNEUROSCI.2010-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber E.J., Beffert U., Jones C., Christian J.M., Forster E., Sweatt J.D., Herz J. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem. 2002;277:39944–39952. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.