ABSTRACT
Hepatitis E virus (HEV) causes an acute, self-limiting hepatitis in healthy individuals and leads to chronic disease in immunocompromised individuals. HEV infection in pregnant women results in a more severe outcome, with the mortality rate going up to 30%. Though the virus usually causes sporadic infection, epidemics have been reported in developing and resource-starved countries. No specific antiviral exists against HEV. A combination of interferon and ribavirin therapy has been used to control the disease with some success. Zinc is an essential micronutrient that plays crucial roles in multiple cellular processes. Zinc salts are known to be effective in reducing infections caused by few viruses. Here, we investigated the effect of zinc salts on HEV replication. In a human hepatoma cell (Huh7) culture model, zinc salts inhibited the replication of genotype 1 (g-1) and g-3 HEV replicons and g-1 HEV infectious genomic RNA in a dose-dependent manner. Analysis of a replication-defective mutant of g-1 HEV genomic RNA under similar conditions ruled out the possibility of zinc salts acting on replication-independent processes. An ORF4-Huh7 cell line-based infection model of g-1 HEV further confirmed the above observations. Zinc salts did not show any effect on the entry of g-1 HEV into the host cell. Furthermore, our data reveal that zinc salts directly inhibit the activity of viral RNA-dependent RNA polymerase (RdRp), leading to inhibition of viral replication. Taken together, these studies unravel the ability of zinc salts in inhibiting HEV replication, suggesting their possible therapeutic value in controlling HEV infection.
IMPORTANCE Hepatitis E virus (HEV) is a public health concern in resource-starved countries due to frequent outbreaks. It is also emerging as a health concern in developed countries owing to its ability to cause acute and chronic infection in organ transplant and immunocompromised individuals. Although antivirals such as ribavirin have been used to treat HEV cases, there are known side effects and limitations of such therapy. Our discovery of the ability of zinc salts to block HEV replication by virtue of their ability to inhibit the activity of viral RdRp is important because these findings pave the way to test the efficacy of zinc supplementation therapy in HEV-infected patients. Since zinc supplementation therapy is known to be safe in healthy individuals and since high-dose zinc is used in the treatment of Wilson's disease, it may be possible to control HEV-associated health problems following a similar treatment regimen.
KEYWORD: hepatitis E virus
INTRODUCTION
Hepatitis E virus (HEV) is a single-stranded, positive-sense RNA virus belonging to the family Hepeviridae (1). It is a major cause of acute, sporadic hepatitis in many developing countries. HEV is primarily transmitted through the fecal-oral route. Although HEV-induced hepatitis is self-limiting, the mortality rate ranges from 0.5 to 3% in young adults and increases up to 30% in pregnant women (2). The virus that infects mammals is classified into seven genotypes and one serotype. Genotype 1 (g-1) and genotype 2 (g-2) viruses exclusively infect humans, and no animal reservoir is known for them. Genotype 3 (g-3) and genotype 4 (g-4) are zoonotic, with an expanded host range, and are highly diverse. Genotype 5 and 6 viruses infect wild boar, and genotype 7 virus infects camel (1). Cases of chronic hepatitis E have been reported in immunocompromised persons, such as organ transplant recipients, patients receiving cancer chemotherapy, and HIV-infected persons (3–5). Emerging evidence also demonstrates the ability of HEV to infect extrahepatic tissues such as placenta, intestine, gallbladder, and neuronal cells (6–10). No specific antiviral therapeutic exists against HEV. Ribavirin monotherapy or combined therapy together with pegylated interferon has been reported to clear viruses in transplantation patients (11–13). Nevertheless, these broad-spectrum antivirals have to be used with care in transplant patients and are not ideal for use in pregnant women. Therefore, a specific antiviral against HEV is warranted.
Zinc is an essential trace element for humans and other animals. It is required for the catalytic activity of many cellular enzymes, and it is also an essential component of the zinc finger motif-containing proteins, many of which act as transcription factors. It plays a significant role in metabolic and immune homeostasis (14, 15). Zinc has been shown to possess broad-spectrum antimicrobial activity. Among the viruses, human immunodeficiency virus (HIV), transmissible gastroenteritis virus (TGEV), herpes simplex virus (HSV), vaccinia virus, severe acute respiratory syndrome coronavirus (SARS-CoV), equine arteritis virus (EAV), rhinovirus, and respiratory syncytial virus (RSV) are known to be inhibited by zinc salts (16–22). The antiviral effects of zinc on these viruses are mediated via different mechanisms, such as inhibition of virus entry, blocking of polyprotein processing, or inhibition of viral RNA-dependent RNA polymerase (RdRp) activity.
We investigated the antiviral activity of zinc salts against HEV. Here, we report that zinc salts inhibit the replication of both g-1 and g-3 HEVs. Our in vitro and in vivo studies demonstrate that zinc salts act on HEV by inhibiting the activity of viral RdRp. The significance of these findings in the context of HEV infection is discussed.
RESULTS
Zinc salts inhibit the replication of hepatitis E virus.
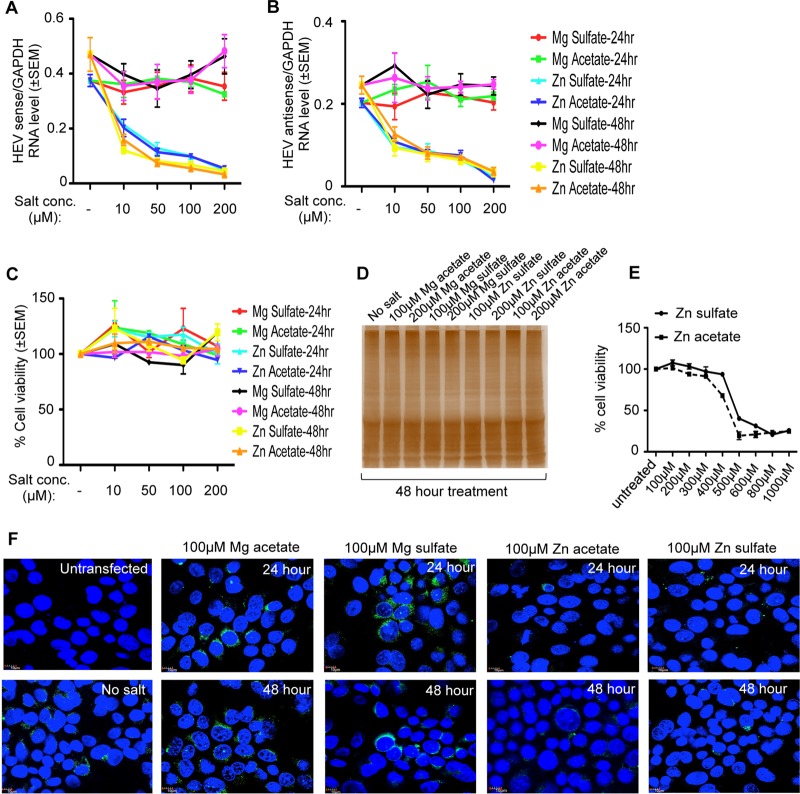
Huh7 cells were transfected with in vitro-synthesized capped genomic RNA of a g-1 HEV replicon expressing enhanced green fluorescent protein (HEV-EGFP) and treated with increasing concentrations (10, 50, 100, and 200 μM) of zinc sulfate (Zn sulfate) and zinc acetate (Zn acetate) for 24 and 48 h to evaluate their effects on viral replication. Similar concentrations of magnesium sulfate (Mg sulfate) and magnesium acetate (Mg acetate) were used in parallel to ensure specificity of the effect of zinc. Both zinc sulfate and zinc acetate inhibited viral sense and antisense RNA levels by approximately 50% at a working concentration of 10 μM whereas neither magnesium sulfate nor magnesium acetate had any effect (Fig. 1A and B). An increase in the zinc salt concentration further decreased the level of viral sense and antisense RNA in a dose-dependent manner, with approximately 95% inhibition at 200 μM (Fig. 1A and B). A possible cytotoxic effect of zinc sulfate and zinc acetate on Huh7 cells was ruled out by measuring the viability of aliquots of the cells (used above for RNA estimation) using an assay based on tetrazolium salt [MTT; 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] (Fig. 1C). Since zinc salts have been reported to inhibit cellular translation in a neuronal cell line (23), the global protein level of Huh7 cells treated for 48 h with high doses of zinc and magnesium salts (100 and 200 μM) was monitored by silver staining of the whole-cell extract, which revealed equal protein levels in all the samples (Fig. 1D). Further, a toxic dose of zinc sulfate and zinc acetate in Huh7 cells was measured by treating the cells for 24 h with increasing amounts of the salts, followed by measurement of cell viability by MTT assay. Fifty percent toxicity was observed at ∼450 μM and 500 μM Zn acetate and Zn sulfate treatment, respectively (Fig. 1E), which clearly ruled out the role of zinc-induced cytotoxicity in reducing the level of HEV RNA. RNA analysis data were further supplemented by monitoring the level of EGFP fluorescence in Huh7 cells expressing the g-1 HEV-EGFP replicon and treated with different zinc salts. In agreement with quantitative real-time PCR (qRT-PCR data), 100 μM zinc sulfate or zinc acetate treatment significantly decreased EGFP fluorescence at 24- and 48-h time points (Fig. 1F).
FIG 1.
Zinc salts inhibit the activity of g-1 HEV-EGFP replicon. (A) qRT-PCR detection of the HEV sense-strand RNA level in Huh7 cells expressing in vitro-synthesized capped RNA of a g-1 HEV-EGFP replicon treated with different salts, as indicated. Values were normalized to the level of GAPDH and are represented as means ± SEM of triplicate samples. (B) qRT-PCR detection of the HEV antisense-strand RNA level in the samples described in panel A. Values were normalized to the level of GAPDH and are represented as means ± SEM of triplicate samples. (C) MTT assay-mediated cell viability estimation of aliquots of the samples described in panel A. The value for an untreated (no salt) sample was considered to be 100%, and all other values were calculated with reference to that. Values are means ± SEM of triplicate samples. (D) Silver staining of total protein of whole-cell extract prepared from Huh7 cells treated with different salts for 48 h, as indicated. (E) MTT assay-mediated cell viability estimation of Huh7 cells treated for 24 h with increasing concentrations of Zn sulfate and Zn acetate, as indicated. The value for the untreated (no salt) sample was considered to be 100%, and all other values were calculated with reference to that. Values are means ± SEM of triplicate samples. (F) Detection of EGFP (green) and 4′,6′-diamidino-2-phenylindole (nucleus, blue) in Huh7 cells expressing in vitro-synthesized capped genomic RNA of g-1 HEV-EGFP replicon, treated with different salts, as indicated. Untransfected and no-salt cells refer to Huh7 cells lacking g-1 HEV-EGFP and g-1 HEV-EGFP-containing Huh7 cells without any additional salt treatment, respectively.
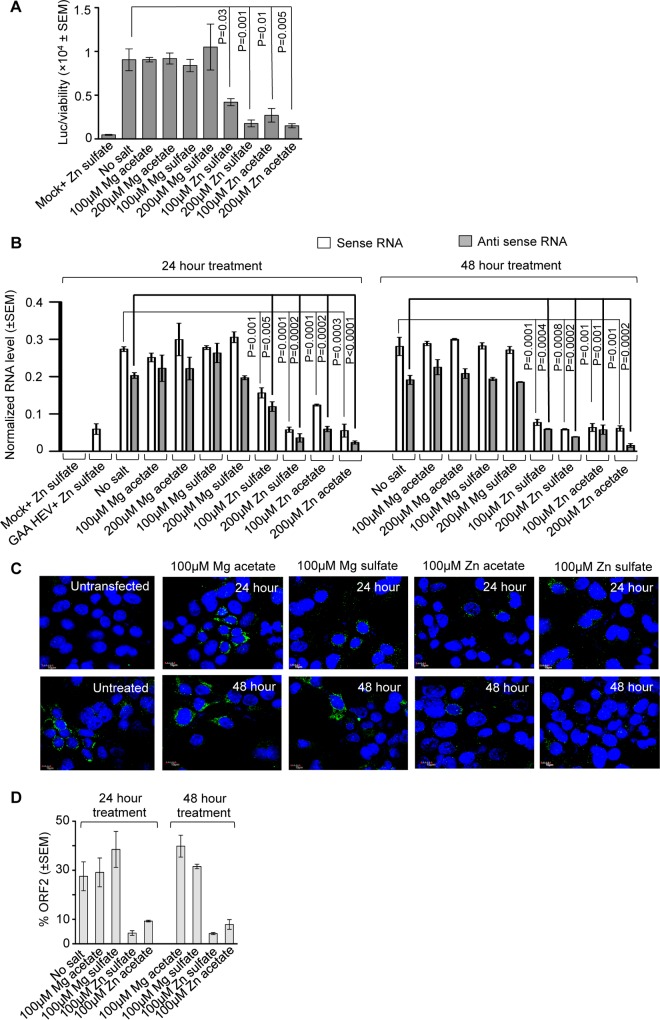
Next, a Huh7 cell-based model of g-3 HEV replicon expressing luciferase (clone P6 HEV-Luc) was used to estimate the effect of zinc salts on the replication of g-3 HEV. Huh7 cells expressing in vitro-synthesized capped genomic RNA of P6 HEV-Luc replicon were treated with 100 and 200 μM concentrations of different zinc and magnesium salts for 48 h, followed by measurement of Renilla luciferase activity and cell viability. Both zinc sulfate and zinc acetate significantly reduced luciferase activity, indicating an inhibition of viral replication (Fig. 2A). Data obtained from the replicon models were confirmed using a Huh7 cell-based infectious g-1 HEV model. Huh7 cells were transfected with in vitro-synthesized capped genomic RNA of g-1 HEV and treated with different concentrations of zinc and magnesium salts for 24 and 48 h. Viral sense and antisense RNA levels were reduced by zinc sulfate and zinc acetate at both 100 μM and 200 μM concentrations at both time points (Fig. 2B). A replication-defective mutant of the g-1 HEV genome, GAA HEV (24), was used in parallel to ensure the specificity of the RT-PCR data, which did not produce any antisense RNA, as expected (Fig. 2B). An immunofluorescence assay was performed using g-1 HEV-expressing Huh7 cells to assess the effect of zinc salts on the level of viral ORF2 protein. Treatment with 100 μM zinc sulfate and zinc acetate for 24 or 48 h, followed by immunofluorescence staining using anti-ORF2 antibody, demonstrated a clear reduction in ORF2 signal without any cytotoxic effect (evident from almost similar numbers of cells and normal nuclear morphology in all the samples) (Fig. 2C). Quantification of the number of ORF2-positive cells in 12 random fields revealed a significant reduction in ORF2 levels in cells treated with zinc salts (Fig. 2D).
FIG 2.
Inhibition of a g-3 HEV replicon and infectious g-1 HEV replication by zinc salts. (A) Measurement of Renilla luciferase activity in Huh7 cells expressing in vitro-synthesized capped RNA of a P6 HEV-Luc replicon, treated for 48 h with different salts, as indicated. Renilla luciferase values were normalized to the value of the cell viability assay and are represented as means ± SEM of triplicate samples. Mock treatment represents cells transfected with the transfection reagent only (without RNA). (B) qRT-PCR detection of HEV sense and antisense RNA levels in Huh7 cells expressing in vitro-synthesized capped genomic RNA of g-1 HEV or its replication-defective mutant (GAA HEV) and treated with different salts, as indicated. Values were normalized to the value of GAPDH and are represented as means ± SEM of triplicate samples. Mock treatment represents cells transfected with the transfection reagent only (without RNA). (C) Immunofluorescence detection of ORF2 (green) and nucleus (blue) in Huh7 cells expressing in vitro-synthesized capped genomic RNA of g-1 HEV treated with different salts, as indicated. Untransfected and no-salt cells represent Huh7 cells lacking the g-1 HEV genome and g-1 HEV genome-containing Huh7 cells without any additional salt treatment, respectively. (D) Quantitation of ORF2-positive cells in 12 random fields of the immunofluorescence slides represented in panel C. The percentage of green fluorescent cells with reference to blue fluorescent cells (total nuclear staining) is shown. Values are means ± SEM of two experiments.
In the case of viral RNA analysis in the g-1 HEV-EGFP replicon and infectious g-1 HEV and quantification of the ORF2 level in infectious g-1 HEV-expressing cells, it was observed that the 24- and 48-h treatments with zinc salts had almost similar effects. To evaluate whether replenishment of zinc salts at 24-h intervals would further enhance the inhibitory effect, we measured viral sense and antisense RNA levels in g-1 HEV-expressing cells treated once or twice (at a 24-h interval) with Zn acetate for 48 h. Repeating the treatment at a 24-h interval further reduced the level of viral sense and antisense RNA (Fig. 3A). An MTT assay of similarly treated cells revealed that two treatments with Zn acetate did not cause cytotoxicity (Fig. 3B).
FIG 3.
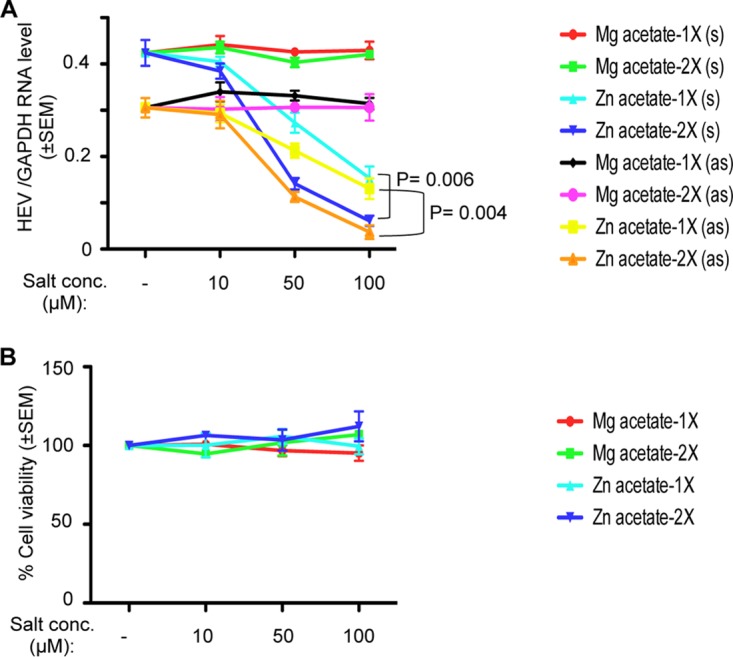
Repeat treatment with zinc salts at 24-h intervals more effectively inhibits HEV replication. (A) Huh7 cells were transfected with in vitro-synthesized capped genomic RNA of g-1 HEV. At day 6 posttransfection, cells were treated once (1×) or twice (2×; treatment repeated at a 24-h interval) with the indicated concentrations of different salts. At 24 and 48 h posttreatment, total RNA was isolated from the cells, followed by qRT-PCR measurement of viral sense, antisense, and host GAPDH RNA levels. GAPDH-normalized sense (s) and antisense (as) RNA levels are represented as means ± SEM of triplicate samples. (B) Simultaneously processed samples (described for panel A) were used to measure cell viability by MTT assay. The value for the untreated (no salt) sample was considered to be 100%, and all other values were calculated with reference to that. Values are means ± SEM of triplicate samples.
Zinc salts inhibit viral replication in HEV-infected cells.
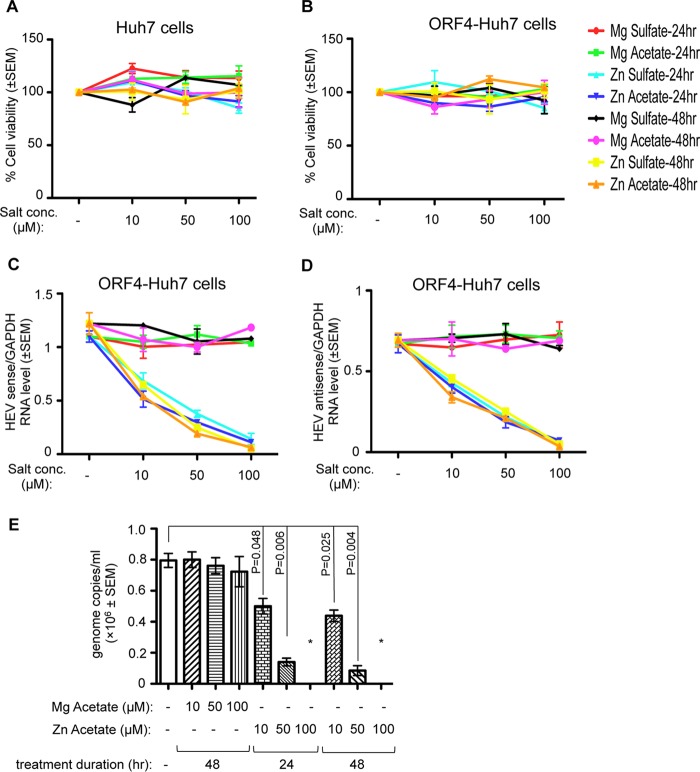
The effect of zinc salts on HEV replication was further evaluated in a cell line-based infection model using a naturally circulating g-1 HEV clinical isolate obtained from an acute HEV patient. Virus was isolated from the fecal sample of the patient and concentrated by polyethylene glycol (PEG) precipitation; the viral genotype was confirmed by sequencing, and genome copy number was estimated by quantitative real-time PCR (qRT-PCR) (see Materials and Methods). Initial infection studies were carried out in Huh7 and ORF4-Huh7 cells to identify the optimal duration and the cell line that supports efficient replication of the infected virus. Note that our earlier studies have demonstrated that Huh7 cells constitutively expressing the ORF4 protein of g-1 HEV (ORF4-Huh7 cell line) permit efficient replication of g-1 HEV (24). A total of 4 × 105 Huh7 or ORF4-Huh7 cells were infected with 8 × 106 genome copies of the virus; this was followed by qRT-PCR-mediated detection of viral sense and host glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA levels at different days, up to 2 weeks. Viral RNA values were normalized to the level of GAPDH to equalize the input RNA quantity. Maximum viral RNA was detected between 12 and 14 days postinfection (Fig. 4). Between 8 and 14 days postinfection, ORF4-Huh7 cells harbored approximately 10-fold more viral RNA than Huh7 cells, demonstrating that the former permit more efficient replication of the virus (Fig. 4). Thus, ORF4-Huh7 cells were used to evaluate the effect of zinc salts on the replication of the naturally circulating g-1 HEV clinical isolate. Next, the possible cytotoxicity levels of different zinc and magnesium salts on Huh7 and ORF4-Huh7 cells were compared. On the 8th day postinfection, g-1 HEV clinical isolate-infected ORF4-Huh7 cells were treated for 24 and 48 h with increasing concentrations of different salts. None of the salts showed any cytotoxicity at the concentrations tested, indicating that there was no gross difference between Huh7 and ORF4-Huh7 cells with respect to zinc metabolism (Fig. 5A and B). qRT-PCR analysis of viral sense and antisense RNA levels in ORF4-Huh7 cells revealed a significant reduction in their levels in the presence of zinc sulfate and zinc acetate, in agreement with the earlier data (Fig. 5C and D). Zinc salt-mediated inhibition of viral replication in HEV-infected ORF4-Huh7 cells was also demonstrated by measuring the number of viruses released to the culture medium of the infected cells. Fecal HEV-infected ORF4-Huh7 cells were treated for 24 or 48 h with different concentrations of zinc acetate on the 9th or 8th day postinfection, respectively, followed by collection of the culture medium, precipitation of the virus particles using PEG 6000, isolation of RNA, and quantification of viral genome copies. Zinc acetate significantly reduced the amount of virus particles released from the infected cells (based on genome copy number), further supporting its role in inhibiting HEV replication (Fig. 5E). Mg acetate treatment for 48 h did not alter virus release under similar conditions (Fig. 5E).
FIG 4.
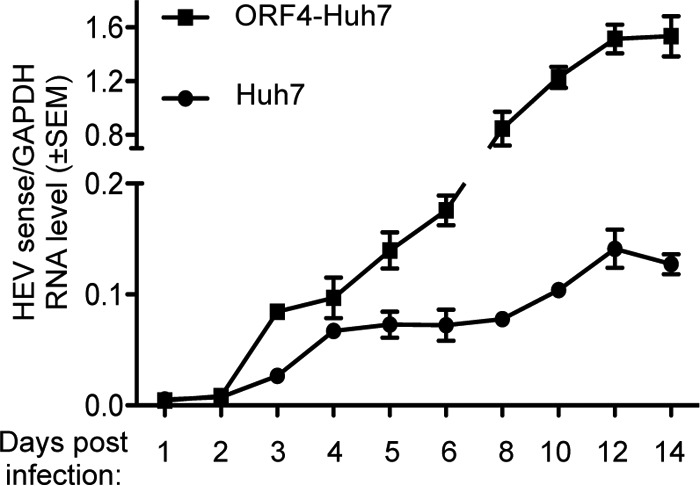
Comparison of the replication efficiency of a g-1 HEV clinical isolate in Huh7 and ORF4-Huh7 cells. qRT-PCR detection of the HEV sense-strand RNA level in Huh7 or ORF4-Huh7 cells infected with a g-1 HEV clinical isolate. Total RNA was isolated at different days postinfection, followed by estimation of HEV sense and GAPDH RNA levels. The ratios of HEV sense/GAPDH values are represented as means ± SEM.
FIG 5.
Zinc salts inhibit the replication of a g-1 HEV clinical isolate. (A) MTT assay-mediated estimation of cell viability in Huh7 cells infected with a g-1 HEV clinical isolate and treated with different salts, as indicated. The value for the untreated (no salt) sample was considered to be 100%, and all other values were calculated with reference to that. Values are means ± SEM of the triplicate samples. (B) MTT assay-mediated estimation of cell viability in ORF4-Huh7 cells infected with a g-1 HEV clinical isolate treated with different salts, as indicated. The value for the untreated (no salt) sample was considered to be 100%, and all other values were calculated with reference to that. Values are means ± SEM of the triplicate samples. (C) qRT-PCR detection of the HEV sense-strand RNA level in ORF4-Huh7 cells infected with a g-1 HEV clinical isolate treated with different salts, as indicated. On days 8 and 9 postinfection, 48- and 24-h treatments, respectively, were started, and all samples were processed for RNA isolation on the 10th day. HEV sense RNA values were normalized to the value for GAPDH and are represented as means ± SEM of triplicate samples. (D) qRT-PCR detection of the HEV antisense-strand RNA level in ORF4-Huh7 cells infected with a g-1 HEV clinical isolate treated with different salts, as indicated. Treatment was done as described for panel B, and values were normalized to the value of GAPDH and are represented as means ± SEM of triplicate samples. (E) qRT-PCR detection of the HEV sense-strand RNA level in HEV particles recovered from the medium of ORF4-Huh7 cells infected with a g-1 HEV clinical isolate, followed by treatment with different salts, as indicated. Treatment was done as described for panel B, and 10th-day medium from each plate was used for PEG precipitation. No amplification was observed in samples treated with 100 μM Zn acetate (denoted by *).
Zinc salts act by inhibiting the activity of HEV RNA-dependent RNA polymerase.
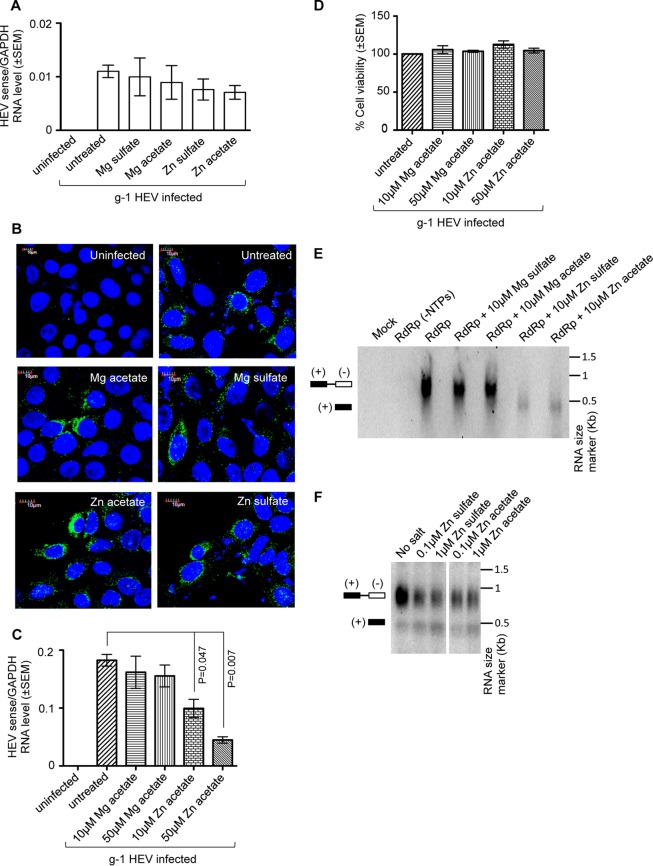
Zinc salts have been reported to inhibit viral entry as well as replication in a few viruses. We tested the effect of zinc salts on the entry of g-1 HEV into ORF4-Huh7 cells. A total of 4 × 105 ORF4-Huh7 cells were treated with different salts (100 μM final concentration); 15 min later, they were infected with 8 × 106 genome copies of the virus for 1 h, followed by washes to remove the unbound virus and measurement of the intracellular level of viral sense RNA. Zinc or magnesium salts had no effect on intracellular viral RNA levels, indicating that virus entry was not affected (Fig. 6A). In order to ensure whether a 1-h infection was sufficient for virus entry into the cell and if the viral sense RNA measured by qRT-PCR was actually recovered from internalized virus rather than virus attached to the extracellular surface, cells incubated in parallel were processed for immunofluorescence assay to detect the intracellular ORF2 protein. ORF2 was equally detected in all samples, irrespective of the presence or absence of different salts, further suggesting that zinc salts had no effect on HEV entry (Fig. 6B).
FIG 6.
Zinc salts act by inhibiting the activity of HEV RNA-dependent RNA polymerase. (A) qRT-PCR detection of the HEV sense RNA level in ORF4-Huh7 cells infected for 1 h with a g-1 HEV clinical isolate. Cells were treated with the indicated salts at 15 min prior to the infection and maintained throughout the HEV infection period. HEV sense RNA values were normalized to the value of GAPDH and are represented as means ± SEM of triplicate samples. (B) Immunofluorescence detection of ORF2 (green) and nucleus (blue) in ORF4-Huh7 cells infected and treated with different zinc salts as described for panel A. (C) ORF4-Huh7 cells were pretreated with the Mg acetate or Zn acetate for 10 h, followed by infection with a g-1 HEV clinical isolate for 1 h. Infected cells were maintained in the presence of Mg acetate or Zn acetate for 3 days. On day 4, total RNA was isolated from the cells, followed by qRT-PCR detection of HEV sense and host GAPDH RNA levels. HEV sense RNA values were normalized to the level of GAPDH and are represented as means ± SEM of triplicate samples. (D) MTT assay-mediated cell viability estimation of aliquots of the samples described in panel C. The value for the untreated (no salt) sample was considered to be 100%, and all other values were calculated with reference to that. Values are means ± SEM of triplicate samples. (E) HEV RdRp assay in the presence of different zinc and magnesium salts, as indicated. Different salts were added to the reaction mixture containing RdRp protein and template RNA and maintained throughout the incubation period. Mock and RdRp (−NTPs) denote reaction mixtures devoid of RdRp protein and nucleoside triphosphates (ATP, CTP, GTP, and UTP), respectively. (F) HEV RdRp assay in the presence of increasing amounts of zinc salts, as indicated. Salt treatment was done as described for panel E.
To evaluate whether the observed inhibitory effect of zinc salts on HEV replication might be an indirect outcome of the interference of the zinc salts with any cellular process, ORF4-Huh7 cells were pretreated with different concentrations of Zn or Mg acetate for 10 h, followed by a 1-h infection with fecal HEV and further incubation in the maintenance medium in the presence of Zn or Mg acetate for 3 days (salt treatment was repeated at 24-h intervals). On the fourth day postinfection, total RNA was isolated, and the HEV sense RNA level was measured by qRT-PCR. As expected, the HEV sense RNA level was significantly reduced in the zinc acetate-treated samples (Fig. 6C). Cell viability of the samples was monitored by MTT assay in parallel, which ruled out any cytotoxic effect of zinc acetate (Fig. 6D).
Next, an in vitro RNA-dependent RNA polymerase (RdRp) assay was performed to evaluate the effect of zinc salts on HEV RdRp activity (24, 25). Flag affinity-purified RdRp was incubated with the template RNA in the presence of different salts, followed by chemiluminescent detection of the double-stranded RNA (dsRNA) product. Magnesium sulfate or magnesium acetate at 10 μM had no effect on HEV RdRp activity whereas 10 μM zinc sulfate or zinc acetate completely inhibited its activity, indicating the specificity of the inhibition exhibited by the zinc salts (Fig. 6E). Moreover, 0.1 μM zinc sulfate or zinc acetate could significantly inhibit viral RdRp activity, further supporting the specific effect of zinc sulfate and zinc acetate on HEV RdRp activity (Fig. 6F).
Effect of cotreatment with zinc salt and ribavirin on HEV replication.
Ribavirin treatment is known to inhibit HEV replication (26). We wondered whether cotreatment of zinc and ribavirin would enhance or antagonize the inhibitory effect of either of them. To demonstrate that, Huh7 cells expressing in vitro-synthesized capped genomic RNA of a P6 HEV-Luc replicon were treated with 100 μM zinc sulfate along with 10 μM or 50 μM ribavirin for 3 days (treatment repeated at 24-h intervals). Only ribavirin-treated (10 and 50 μM) cells were maintained in parallel as controls. Note that in our cell viability assay, 100 μM ribavirin showed some cytotoxicity, so it was not possible to include it in the experiment. Analysis of normalized Renilla luciferase values revealed that 100 μM Zn sulfate treatment displayed inhibition almost similar to that of 50 μM ribavirin, and cotreatment of 100 μM Zn sulfate and 50 μM ribavirin marginally yet significantly increased inhibition efficiency compared to that observed with 100 μM Zn sulfate or 50 μM ribavirin treatment alone (Fig. 7).
FIG 7.
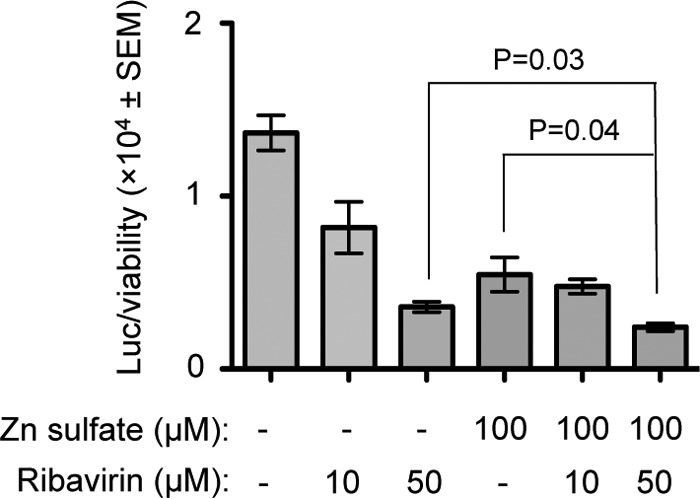
Cotreatment with Zn sulfate and ribavirin marginally increases the inhibitory effect of either of them on HEV replication. Renilla luciferase activity was measured in Huh7 cells expressing in vitro-synthesized capped RNA of the P6 HEV-Luc replicon, treated every day for 3 days with 100 μM Zn sulfate and/or 10, 50 μM ribavirin, as indicated. Luciferase values were normalized to the value of the cell viability assay (detected by MTT assay) and are represented as means ± SEM of triplicate samples.
DISCUSSION
The current study identifies the ability of zinc salts such as zinc sulfate and zinc acetate to inhibit the replication of HEV. Employing both an in vitro-synthesized capped genomic RNA-based replication model of g-1 and g-3 HEVs and an infection model using a clinical isolate of g-1 HEV, our data clearly demonstrate that the inhibitory effect of zinc salts on HEV replication is specific. These findings suggest the possibility of evaluating the potential benefit of zinc in controlling HEV infection-associated health problems in humans as well as in farm animals such as swine. Note that although cell lines may not be ideal for evaluation of the therapeutic value of zinc salts, based on our data from Huh7 and ORF4-Huh7 cells, the therapeutic index (ratio of 50% toxicity concentration to 50% inhibitory concentration) of Zn sulfate and Zn acetate was found to be approximately 45 and 50, respectively.
Zinc is an important micronutrient involved in many different cellular processes as it is essential for the function of many cellular enzymes and transcription factors. It is crucial for maintaining metabolic and immune homeostasis. Zinc deficiency affects both innate and adaptive immune systems and induces oxidative stress in humans and animals, which can be corrected by zinc supplementation (15, 27, 28). Zinc has been reported to potentiate the antiviral action of human alpha interferon (IFN-α), alleviate the symptoms of rhinovirus-induced colds, and increase the response rate of IFN-α therapy in chronic hepatitis C cases, and topical zinc treatment has been used to control HSV infection (18, 29–31). Although several reports have demonstrated the inhibitory effect of zinc salts on viral replication, very few studies have identified the underlying mechanism. In the case of rhinovirus, zinc is reported to block the binding of virus to the host cell surface and inhibit the activity of viral protease and RdRp (32, 33). In the case of EAV and SARS-CoV, zinc inhibits the activity of viral RdRp in vitro whereas in the case of RSV, zinc inhibits both viral penetration as well as replication (20, 22). Our results reveal that zinc inhibits HEV RdRp activity without affecting entry of the virus. There might be a common mechanism underlying zinc-mediated inhibition of the activity of HEV, EAV, and SARS-CoV RdRp. Analysis of the structure and activity of RdRp of these three viruses should provide a better insight into the mechanism by which zinc inhibits their function.
One of the limitations of zinc supplementation-mediated therapy is attributed to the tight regulation of bioavailability of zinc by the host metabolic processes. The liver is the primary organ involved in zinc metabolism, and zinc is present as both rapidly mobilized and slowly mobilized forms in the liver. Glucocorticoids, insulin, and glucagon hormones control zinc metabolism in the liver, which in turn influences the plasma zinc level (31). Normal plasma zinc concentration ranges between 10 and 20 μM, and approximately 10 to 20% of the ingested zinc is absorbed (34). Therefore, ingestion of a high dose of zinc supplement may not proportionately increase the bioavailability of zinc in all individuals. The net bioavailable quantity of zinc will be dependent on the level of zinc available before the onset of zinc supplementation. It is also important to note that a high level of zinc supplementation may lead to toxicity (35). In all our experiments involving measurement of viral replication in cells, 10 μM zinc sulfate or zinc acetate had a moderate effect. A minimum 100 μM concentration was required to achieve a ∼75% inhibitory effect. However, in vitro, 10 μM zinc salts was able to completely inhibit the activity of viral RdRp. Therefore, the requirement of a high concentration of zinc salts in cell-based assays might be linked to the status of zinc metabolism in Huh7 or ORF4-Huh7 cells, which limits the net level of bioavailable zinc for acting on viral RdRp. A similar situation might be prevailing in vivo in HEV-infected patients, which may counter the therapeutic benefit of zinc supplementation. However, since a high incidence of HEV infection is generally observed among populations of economically weak or developing countries, which remain zinc deficient due to malnutrition or other associated causes, zinc supplementation is likely to have a therapeutic benefit in controlling HEV infection not only by inhibiting viral RdRp activity but also by stimulating the overall immune response (36, 37).
While evaluating the effect of cotreatment of zinc salts and ribavirin on HEV replication, we observed a moderate enhancement in inhibition when both zinc acetate and ribavirin were added. This appears to be an interesting observation since such a therapy might prove to be a better strategy to manage severe cases. Future studies should provide conclusive evidence of the possible therapeutic value of zinc salts and their cooperativity with ribavirin in controlling HEV infection-induced hepatitis.
MATERIALS AND METHODS
Plasmids, viral RNA, cell culture, transfection and salt treatment.
The pSK-HEV2 (GenBank accession no. AF444002.1) (g-1 HEV) and pSK P6-Luc (GenBank accession no. JQ679013.1) (g-3 HEV) plasmids containing cDNA of g-1 HEV and the P6 HEV-Luc replicon were generously provided by S. Emerson (38, 39). The sequences between nucleotides 6220 and 6970 (from the 5′ end of genome), falling within the ORF2 region of pSK-HEV2, was replaced in frame by enhanced green fluorescent protein (EGFP) sequence to generate the HEV-EGFP replicon. Nucleotides 3970 to 6251 (from the 5′ end of genome) of g-1 HEV was PCR amplified using the primers 5′-CCACGCTCGTGGGCCGTTAT-3′ (forward primer [FP]) and 5′-TCTCAGTGCTAGCCGCTATCCCGCGGCC-3′ (reverse primer [RP]) and digested with SfiI and NheI. EGFP sequence was PCR amplified using the primers 5′-TCTCAGTGCTAGCGTGAGCAAGGGCGAGGA-3′ (FP) and 5′-TCTCAGTGCTAGCACCTTGTACAGCTCGTCCATGC-3′ (RP) and digested with NheI. pSK-HEV2 was digested with SfiI and NheI; a 7-kb band was gel extracted and ligated with the digested PCR products to generate pSK-HEV-EGFP. The clone was confirmed by sequencing. The replication-defective mutant of HEV (GAA HEV) has been described previously (24). g-1 HEV, g-1 HEV-EGFP, and P6 HEV-Luc genomic RNAs were in vitro synthesized, as described previously (38); size and integrity were monitored by formaldehyde agarose gel electrophoresis. Huh7 human hepatoma cells were originally obtained from the laboratory of C. M. Rice (40). The ORF4-Huh7 cell line has been reported previously (24). Cells were maintained in Dulbecco's modified eagle medium (DMEM) containing 10% fetal calf serum (FCS) and 50 IU/ml penicillin and streptomycin in 5% CO2. Hygromycin (200 μg/ml) was added to ORF4-Huh7 cells during routine maintenance (24). Cells were transfected using Lipofectamine 3000 (Life Technologies, USA) for g-1 HEV and g-1 HEV-EGFP RNA or electroporated (Bio-Rad Gene Pulser Xcell; Bio-Rad, USA) for P6 HEV-Luc RNA in a 4-mm cuvette at 200 V, 950 μF, and infinite resistance, according to the manufacturer's guidelines. Stock solutions of magnesium sulfate, magnesium acetate, zinc sulfate, and zinc acetate (230391, M5661, 96495, and 379786, respectively; Sigma, USA) were prepared in water and added to the cells at the final concentration mentioned in the text and shown in the figures on the 6th day of transfection; cells were maintained for 24 and 48 h (unless mentioned otherwise) before being used for cell viability assays, RNA isolation, silver staining, or immunofluorescence assays. Ribavirin (R9644; Sigma, USA) was prepared in dimethyl sulfoxide (DMSO) and added to the cells at the indicated final concentrations. Every day, culture medium was changed, and fresh ribavirin and Zn sulfate were added for 3 days.
Cell viability assay, luciferase assay, qRT-PCR, silver staining, immunofluorescence, and RdRp assay.
A cell viability assay was performed using a Cell Titer 96 Non-Radioactive Cell Proliferation Assay (MTT) kit (Promega, USA) according to the manufacturer's instructions. A luciferase assay was done as described previously (24). Total RNA from cells was isolated using TRI reagent (MRC, USA), and RNA from a PEG-precipitated virus suspension was isolated using a QIAamp viral RNA minikit (Qiagen, USA) according to the manufacturer's instructions. Reverse-transcription (RT) and quantitative real-time PCR (qRT-PCR) were done as described previously (24). Briefly, to measure the HEV sense and host GAPDH RNA levels, cDNA was synthesized using random hexamers, and to measure the HEV antisense RNA level, an HEV antisense RT primer (5′-CGTGTCATGGTGGCGAATAAGCAGACCACATATGTGGTCGAT) was used. Superscript III (Thermo Fisher Scientific, USA) was used for cDNA synthesis. The following primers were used for subsequent quantitative real-time PCR: HEV sense, 5′-GAACTACATATGTTGCGCGGACAGCAA (FP) and 5′-AGACTGAATTCGGAGCAGCAGCAAATGAGG (RP); human GAPDH (hGAPDH), 5′-GAGTCAACGGATTTGGTCGT (FP) and 5′-TTGATTTTGGAGGGATCTCG (RP); HEV antisense, 5′-GTGTCATGGTGGCGAATAAG (FP) and 5′-AACGGTGGACCACATTAGGA (RP). Silver staining was done using a Pierce silver stain kit (Thermo Fisher Scientific, USA). An immunofluorescence assay was done using rabbit polyclonal anti-ORF2 antibody, as described previously (24). An RdRp assay was done as described previously (24) with the modification that EDTA was removed from the assay buffer, and, in the case of salt-treated samples, the indicated concentrations of salts (Fig. 6E and F) were added to the reaction mixture before addition of RdRp protein.
Infection assays using a g-1 HEV clinical isolate.
With informed consent, a fecal sample was obtained from an acute HEV patient admitted to the Gastroenterology Department of the All India Institute of Medical Sciences, New Delhi, India, according to institutional guidelines. The fecal sample was suspended in 10% (wt/vol) phosphate-buffered saline (PBS) without calcium and magnesium and centrifuged at 7,000 × g for 1 h at 4°C, and the supernatant was collected. The collected supernatant was passed through a 0.2-μm-pore-size filter. The viral suspension was concentrated by PEG precipitation for 16 h at 0°C using PEG 6000 at a working concentration of 8% made in 0.4 M NaCl, according to a previously described protocol (41). The precipitated virus-containing sample was centrifuged at 18,000 × g for 30 min at 4°C, followed by resuspension of the pellet in PBS and buffer exchange to remove the PEG 6000. The final virus-containing suspension was filtered through a 0.2-μm-pore-size filter under sterile conditions and stored in aliquots at −80°C. Total RNA was isolated from an aliquot of the filtered viral suspension, followed by cDNA synthesis and RT-PCR using HEV-specific primers (FP, 5′-GATACTAAGCTTATCATGGTGCGCGGACAG-3′; RP, 5′-AGATCGAATTCGGAGCAGCAGCAAATGAGG-3′). The PCR product was purified and cloned into pJET 1.2 vector (CloneJET PCR cloning kit; Thermo Fisher Scientific, USA) according to the manufacturer's instructions, and two independent clones were sequenced to confirm their identity with the g-1 HEV sequence. For estimating genome copy number of the viral suspension, known quantities of the pSK HEV2 plasmid that contains the g-1 HEV genome were serially diluted and amplified by qRT-PCR to generate a standard plot. cDNA from the viral suspension was amplified in parallel and quantified from the standard curve. Genome copy number was estimated to be 4 × 107 copies/ml using the following formula: number of copies = [ng × (number/mole)]/[bases × (ng/g)(g/mole of bases)].
For comparing the replication efficiency of the infectious virus in cell lines, 4 × 105 Huh7 or ORF4-Huh7 cells were infected with 8 × 106 genome copies of the virus, according to a previously reported protocol (42). After a 1-h infection, cells were washed three times in PBS and maintained for the indicated periods (up to 2 weeks) (Fig. 4). For estimation of the viral sense RNA level in infected cells, total RNA was isolated at different time points, followed by qRT-PCR analysis of HEV sense and GAPDH RNA levels. HEV sense RNA values were normalized to the GAPDH level to equalize the input quantity. For studies involving salt treatment, at 8 days postinfection, fresh medium (DMEM, 10% FCS, penicillin, streptomycin) was added to the infected ORF4-Huh7 cells, followed by addition of different salts and further incubation for 24 or 48 h. Simultaneously, processed cells were used for cell viability assays and quantification of intracellular sense and antisense RNA levels.
For estimation of the amount of virus released into the culture medium, medium from the above-mentioned salt-treated cells was collected on the 10th day postinfection and clarified by centrifugation at 7,197 × g at 4°C for 15 min. Virus particles in the clarified medium were concentrated by precipitation in 8% PEG 6000, as described above for the fecal virus. The precipitated samples were resuspended in TRI reagent (MRC, USA), followed by isolation of RNA and measurement of the HEV sense RNA level by qRT-PCR. Simultaneously, processed cells were used to measure cell viability.
For studies involving evaluation of the effect of different salts on HEV entry, ORF4-Huh7 cells were infected with the virus suspension for 1 h as described above, followed by three washes of the cells with PBS to remove the unbound virus and processing of the cells for RNA analysis and immunofluorescence assay. Different salts were added to the culture medium at 15 min prior to addition of the virus and maintained throughout the infection period.
For studies involving pretreatment of cells with zinc salt, ORF4-Huh7 cells were treated with different concentrations of Zn acetate or Mg acetate for 10 h, followed by treatment with maintenance medium containing the above salts and infection with fecal HEV. At 1 h postinfection, cells were washed in PBS as described above, and fresh medium containing the above salts was added. Medium (with Zn and Mg acetate) was changed at 24-h intervals, and cells were maintained for 3 days. On the 4th day, total RNA was isolated from the cells, and the levels of HEV sense and GAPDH RNAs were estimated by qRT-PCR.
Statistics.
Data are presented as means ± standard errors of the means (SEM) of three independent experiments unless indicated otherwise and were analyzed using GraphPad Prism and a Student t test. A P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
We thank S. Emerson for the pSK HEV2 and pSK P6-Luc plasmids.
This work was supported by core funds from THSTI and a DBT-RGYI grant by Department of Biotechnology, Government of India, to M.S.; N.K., C.S., and S.A. are supported by senior research fellowships from the University Grants Commission, Council of Scientific and Industrial Research, and Department of Science and Technology, Government of India, respectively; C.T.R.K. is supported in part by a grant from the Department of Biotechnology, Government of India.
REFERENCES
- 1.Smith DB, Simmonds P, Jameel S, Emerson SU, Harrison TJ, Meng XJ, Okamoto H, Van der Poel WHM, Purdy MA. 2014. Consensus proposals for classification of the family Hepeviridae. J Gen Virol 95:2223–2232. doi: 10.1099/vir.0.068429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar A, Devi SG, Kar P, Agarwal S, Husain SA, Gupta RK, Sharma S. 2014. Association of cytokines in hepatitis E with pregnancy outcome. Cytokine 65:95–104. doi: 10.1016/j.cyto.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Kamar N, Selves J, Mansuy J-M, Ouezzani L, Péron J-M, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel J-P, Izopet J, Rostaing L. 2008. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med 358:811–817. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 4.Ollier L, Tieulie N, Sanderson F, Heudier P, Giordanengo V, Fuzibet JG, Nicand E. 2009. Chronic hepatitis after hepatitis E virus infection in a patient with non-Hodgkin lymphoma taking rituximab. Ann Intern Med 150:430–431. doi: 10.7326/0003-4819-150-6-200903170-00026. [DOI] [PubMed] [Google Scholar]
- 5.Dalton HR, Bendall R, Keane FE, Tedder R, Ijaz S. 2009. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med 361:1025–1027. doi: 10.1056/NEJMc0903778. [DOI] [PubMed] [Google Scholar]
- 6.Bose PD, Das BC, Hazam RK, Kumar A, Medhi S, Kar P. 2014. Evidence of extrahepatic replication of hepatitis E virus in human placenta. J Gen Virol 95:1266–1271. doi: 10.1099/vir.0.063602-0. [DOI] [PubMed] [Google Scholar]
- 7.Fujioka K, Nishimura T, Seki M, Kinoshita M, Mishima N, Irimajiri S, Yamato M. 2016. Genotype 1 hepatitis E virus infection with acute acalculous cholecystitis as an extrahepatic symptom: a case report. Trop Med Health 44:18. doi: 10.1186/s41182-016-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams TPE, Kasorndorkbua C, Halbur PG, Haqshenas G, Guenette DK, Toth TE, Meng XJ. 2001. Evidence of extrahepatic sites of replication of the hepatitis E virus in a swine model. J Clin Microbiol 39:3040–3046. doi: 10.1128/JCM.39.9.3040-3046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazerbachi F, Haffar S, Garg SK, Lake JR. 2015. Extra-hepatic manifestations associated with hepatitis E virus infection: a comprehensive review of the literature. Gastroenterol Rep (Oxf) 4:1–15. doi: 10.1093/gastro/gov042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haffar S, Bazerbachi F, Lake JR. 2015. HEV-associated cryoglobulinaemia and extrahepatic manifestations of hepatitis E. Lancet Infect Dis 15:268. doi: 10.1016/S1473-3099(15)70034-4. [DOI] [PubMed] [Google Scholar]
- 11.Kamar N, Rostaing L, Abravanel F, Garrouste C, Lhomme S, Esposito L, Basse G, Cointault O, Ribes D, Nogier MB, Alric L, Peron JM, Izopet J. 2010. Ribavirin therapy inhibits viral replication on patients with chronic hepatitis E virus infection. Gastroenterology 139:1612–1618. doi: 10.1053/j.gastro.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Radenne S, Coilly A, Garrigue V, D'Alteroche L, Buchler M, Couzi L, Lebray P, Dharancy S, Minello A, Hourmant M, Roque-Afonso A-M, Abravanel F, Pol S, Rostaing L, Mallet V. 2014. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med 370:1111–1120. doi: 10.1056/NEJMoa1215246. [DOI] [PubMed] [Google Scholar]
- 13.Kamar N, Rostaing L, Abravanel F, Garrouste C, Esposito L, Cardeau-Desangles I, Mansuy JM, Selves J, Peron JM, Otal P, Muscari F, Izopet J. 2010. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis 50:e30–e33. doi: 10.1086/650488. [DOI] [PubMed] [Google Scholar]
- 14.Prasad AS. 1995. Zinc: an overview. Nutrition 11(1 Suppl):93–99. [PubMed] [Google Scholar]
- 15.John E, Laskow TC, Buchser WJ, Pitt BR, Basse PH, Butterfield LH, Kalinski P, Lotze MT. 2010. Zinc in innate and adaptive tumor immunity. J Transl Med 8:118. doi: 10.1186/1479-5876-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haraguchi Y, Sakurai H, Hussain S, Anner BM, Hoshino H. 1999. Inhibition of HIV-1 infection by zinc group metal compounds. Antiviral Res 43:123–133. doi: 10.1016/S0166-3542(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 17.Wei Z, Burwinkel M, Palissa C, Ephraim E, Schmidt MFG. 2012. Antiviral activity of zinc salts against transmissible gastroenteritis virus in vitro. Vet Microbiol 160:468–472. doi: 10.1016/j.vetmic.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahba A. 1980. Topical application of zinc-solutions: a new treatment for herpes simplex infections of the skin? Acta Derm Venereol 60:175–177. [PubMed] [Google Scholar]
- 19.Katz E, Margalith E. 1981. Inhibition of vaccinia virus maturation by zinc chloride. Antimicrob Agents Chemother 19:213–217. doi: 10.1128/AAC.19.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.te Velthuis AJW, van den Worm SHE, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. 2010. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog 6:e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korant BD, Kauer JC, Butterworth BE. 1974. Zinc ions inhibit replication of rhinoviruses. Nature 248:588–590. doi: 10.1038/248588a0. [DOI] [PubMed] [Google Scholar]
- 22.Suara RO, Crowe JE Jr. 2004. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob Agents Chemother 48:783–790. doi: 10.1128/AAC.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alirezaei M, Nairn AC, Glowinski J, Premont J, Marin P. 1999. Zinc inhibits protein synthesis in neurons. Potential role of phosphorylation of translation initiation factor-2α. J Biol Chem 274:32433–32438. [DOI] [PubMed] [Google Scholar]
- 24.Nair VP, Anang S, Subramani C, Madhvi A, Bakshi K, Srivastava A, Shalimar, Nayak B, Ranjith-Kumar CT, Surjit M. 2016. Endoplasmic reticulum stress induced synthesis of a novel viral factor mediates efficient replication of genotype-1 hepatitis E virus. PLoS Pathog 12:e1005521. doi: 10.1371/journal.ppat.1005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair V, Anang S, Srivastava A, Surjit M. 2017. RNA-dependent RNA polymerase assay for hepatitis E virus. Bio Protoc 7:e2199. doi: 10.21769/BioProtoc.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debing Y, Emerson SU, Wang Y, Pan Q, Balzarini J, Dallmeier K, Neyts J. 2014. Ribavirin inhibits in vitro hepatitis E virus replication through depletion of cellular GTP pools and is moderately synergistic with alpha interferon. Antimicrob Agents Chemother 58:267–273. doi: 10.1128/AAC.01795-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasad AS, Bao B, Beck FWJ, Kucuk O, Sarkar FH. 2004. Antioxidant effect of zinc in humans. Free Radic Biol Med 37:1182–1190. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Haase H, Overbeck S, Rink L. 2008. Zinc supplementation for the treatment or prevention of disease: current status and future perspectives. Exp Gerontol 43:394–408. doi: 10.1016/j.exger.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Berg K, Bolt G, Andersen H, Owen TC. 2001. Zinc potentiates the antiviral action of human IFN-α tenfold. J Interferon Cytokine Res 21:471–474. doi: 10.1089/10799900152434330. [DOI] [PubMed] [Google Scholar]
- 30.Eby GA, Davis DR, Halcomb WW. 1984. Reduction in duration of common colds by zinc gluconate lozenges in a double-blind study. Antimicrob Agents Chemother 25:20–24. doi: 10.1128/AAC.25.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grungreiff K, Reinhold D. 2010. Zinc: a complementary factor in the treatment of chronic hepatitis C? (Review). Mol Med Rep 3:371–375. doi: 10.3892/mmr_00000267. [DOI] [PubMed] [Google Scholar]
- 32.Krenn BM, Gaudernak E, Holzer B, Lanke K, Van Kuppeveld FJM, Seipelt J. 2009. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J Virol 83:58–64. doi: 10.1128/JVI.01543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung M, Gibbs CS, Tsiang M. 2002. Biochemical characterization of rhinovirus RNA-dependent RNA polymerase. Antiviral Res 56:99–114. doi: 10.1016/S0166-3542(02)00101-8. [DOI] [PubMed] [Google Scholar]
- 34.Sugarman B. 1983. Zinc and infection. Rev Infect Dis 5:137–147. doi: 10.1093/clinids/5.1.137. [DOI] [PubMed] [Google Scholar]
- 35.Maret W, Sandstead HH. 2006. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol 20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Gibson RS. 1994. Zinc nutrition in developing countries. Nutr Res Rev 7:151–173. doi: 10.1079/NRR19940010. [DOI] [PubMed] [Google Scholar]
- 37.Brown KH, Wuehler SE, Peerson JM. 2001. The importance of zinc in human nutrition and estimation of the global prevalence of zinc deficiency. Food Nutr Bull 22:113–125. doi: 10.1177/156482650102200201. [DOI] [Google Scholar]
- 38.Emerson SU, Nguyen H, Graff J, Stephany DA, Brockington A, Purcell RH. 2004. In vitro replication of hepatitis E virus (HEV) genomes and of an HEV replicon expressing green fluorescent protein. J Virol 78:4838–4846. doi: 10.1128/JVI.78.9.4838-4846.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shukla P, Nguyen HT, Faulk K, Mather K, Torian U, Engle RE, Emerson SU. 2012. Adaptation of a genotype 3 hepatitis E virus to efficient growth in cell culture depends on an inserted human gene segment acquired by recombination. J Virol 86:5697–5707. doi: 10.1128/JVI.00146-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blight KJ, Kolykhalov AA, Rice CM. 2000. Efficient Initiation of HCV RNA replication in cell culture. Science 290:1972–1974. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- 41.Li TC, Yamakawa Y, Suzuki K, Tatsumi M, Razak MA, Uchida T, Takeda N, Miyamura T. 1997. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J Virol 71:7207–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi M, Tanaka T, Takahashi H, Hoshino Y, Nagashima S, Jirintai Mizuo H, Yazaki Y, Takagi T, Azuma M, Kusano E, Isoda N, Sugano K, Okamoto H. 2010. Hepatitis E virus (HEV) strains in serum samples can replicate efficiently in cultured cells despite the coexistence of HEV antibodies: characterization of HEV virions in blood circulation. J Clin Microbiol 48:1112–1125. doi: 10.1128/JCM.02002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]