Abstract
Flavonoids, stilbenes, and chalcones are plant secondary metabolites that often possess diverse biological activities including anti-inflammatory, anti-cancer, and anti-viral activities. The wide range of bioactivities poses a challenge to identify their targets. Here, we studied a set of synthetically generated flavonoids and chalcones to evaluate for their biological activity, and compared similarly substituted flavonoids and chalcones. Substituted chalcones, but not flavonoids, showed inhibition of viral translation without significantly affecting viral replication in cells infected with hepatitis C virus (HCV). We suggest that the chalcones used in this study inhibit mammalian target of rapamycin (mTOR) pathway by ablating phosphorylation of ribosomal protein 6 (rps6), and also the kinase necessary for phosphorylating rps6 in Huh7.5 cells (pS6K1). In addition, selected chalcones showed inhibition of growth in Ishikawa, MCF7, and MDA-MB-231 cells resulting an IC50 of 1–6 μg/mL. When similarly substituted flavonoids were used against the same set of cancer cells, we did not observe any inhibitory effect. Together, we report that chalcones show potential for anti-viral and anti-cancer activities compared to similarly substituted flavonoids.
Keywords: Chalcone, HCV, Cancer, mTOR, rsp6
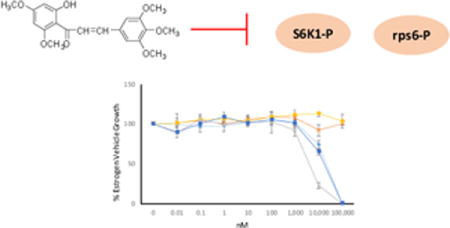
Graphical abstract
To create your abstract, type over the instructions in the template box below. Fonts or abstract dimensions should not be changed or altered.
Bioactive molecules, chalcones and flavonoids, present in plants are key components in therapeutic studies against infectious diseases, cancer, and metabolic disorders. The large plethora of available flavonoids and chalcones, studied so far indicate a wide spectrum of bioactivity, and sometimes promiscuous targets that resulted due to their effect on overlapping pathways. Many of these natural compounds possess anti-viral activity against several viruses including the hepatitis C virus (HCV). Flavonoids such as naringenin, quercetin, and leuteolin, and stilbenes such as tamoxifen – a chemotherapeutic drug for breast cancer – have been evaluated for its anti-HCV effect.1 In addition, several naturally occurring and synthetic flavonoids have been suggested to have anti-cancer activity in various cell types.2 However, the diverse bioactivity of these compounds pose a challenge to identify their individual targets, and study their structure function relationships. Prolonged HCV infection in the liver results in cirrhosis, hepatocellular carcinoma, and often leads to liver transplantation and other medical complications. In the absence of a vaccine, drugs are designed to target either viral proteins, or host factors necessary to support the viral life cycle inside the host cell.3–5 The current treatment involve oral intake of sofosbuvir.6, 7 But high expense for sofosbuvir treatment inspires a thorough look at available potential drug-like molecules that are easily extracted from plants, or their chemically synthesized derivatives (Table 1).7–9 HCV is a positive strand RNA virus.10 HCV encoded viral nonstructural protein 5A (NS5A) has been implicated in many steps of viral life cycle including RNA replication, RNA translation, and viral assembly.11
Table 1.
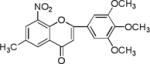
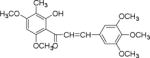
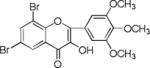
List of chalcones and flavonoids.
| NM_1 |
|
NM_10 |
|
| NM_2 |
|
NM_11 |
|
| NM_3 |
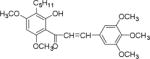
|
NM_12 |
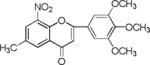
|
| NM_4 |
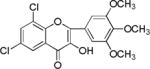
|
NM_13 |
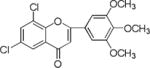
|
| NM_5 |
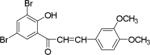
|
NM_14 |
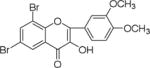
|
| NM_6 |
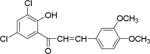
|
NM_15 |
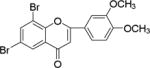
|
| NM_7 |
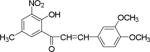
|
NM_16 |
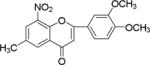
|
| NM_8 |
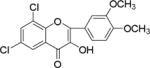
|
NM_17 |
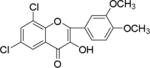
|
| NM_9 |
|
NM_18 |
|
During viral translation NS5A activates mammalian target of Rapamycin (mTOR) pathway by disrupting the interaction between FK506-binding protein 38 (FKB38) and mTOR.12, 13 Activated mTOR initiates phosphorylation of ribosomal protein S6 kinase beta-1 (S6K1) protein, which eventually phosphorylates ribosomal protein 6 (rps6).14 Expression of NS5A increases the level of phosphorylated pS6K1. Additionally, cells infected with genotype 2a HCV virus show significant increase in p-rps6 at Ser235/236.12 Both HCV infection and NS5A expression enhance the assembly of cap-dependent translational complex on the mRNA through mTOR activation.12 This enhanced translational complex increases protein expression from specific set of mRNAs, especially expression of ribosomal proteins, without changing the level of global translation.12, 13
Flavonoids and other plant metabolites, inhibited HCV propagation at different stages including virus entry, and replication.15 To our knowledge the study of chalcones as the HCV inhibitor is limited. On the contrary chalcones have long been studied for its potential in various diseases including cancer.16–19 Recent literature described mTOR as a potential target for hepatocellular carcinoma.20 Increased mTOR activity is altered in prostate cancer,19, 21 in breast and ovarian cancer,22 tumorigenesis,23 and in hepatocellular carcinoma.24 Several anticancer drugs have been identified as mTOR inhibitors,20, 25–28 including the breast cancer.26, 29 Since other mTOR inhibitors have been implicated in anti-cancer therapy, we compared substituted chalcones and flavonoids against hepatitis C virus, and in breast and ovarian cancer cells.
We screened eighteen synthetic flavonoids and chalcones (Table 1) against HCV infected cells to investigate any anti-HCV function. We infected Huh7.5 cells with genotype 2a strain (JHF1-FLAG), and co-treated with substituted flavonoids and chalcones at a concentration up to 50 μM.30–32 We analyzed both a viral protein (NS3) and a cellular protein (GAPDH), and evaluated the ratio of NS3 to GAPDH during the 72 hour incubation period. Although flavonoids naringenin, quercetin, and leuteolin are identified to interfere with the HCV life cycle at different stages, none of the flavonoids used in this study showed anti-HCV effect below 50 μM concentration in our assay.8 In contrast, most of the substituted chalcones (Table 1: NM1, NM2, NM5, NM6, NM7, NM8) exhibited varied degree of inhibition of HCV as measured by a reduction in the ratio of NS3 to GAPDH relative to cells treated with DMSO (Supplementary Figure S1). Our initial experiments verified that functionally active compounds do not inhibit an earlier step of viral life cycle including entry when viral infection was initiated with genomic RNA in the presence of active chalcones (Supplementary Figure S2). Since flavonoids and chalcones used in this study do not affect an early step in HCV life cycle, we investigated if these compounds are active in cells containing subgenomic replicon that are only able to replicate a partial viral genome followed by translation of several viral proteins required for replication.33, 34
We treated both genotype 2a (SGR2a) and 1b (GS5) containing cells with selected chalcones for 72 hours and analyzed the ratio of NS3 to GAPDH (Figs. 1a and 1b). Treatment of GS5 cells had a significantly greater inhibition than SGR2a. Furthermore, we also noticed that in both cell types bulky substituents on the benzene ring (–H, –CH3 vs –C5H11 in NM1, NM2 and NM3 respectively) caused significantly lower anti-viral activity in both genotypes (Figs 1a and 1b, right panel and S1) suggesting a structure-function relation between active chalcone and its target. NM1 and NM5 consistently showed significant inhibition of viral production, hence were selected for the rest of the study to understand the target of chalcone mediated inhibition of HCV. In order to understand concentration dependence of chalcone derivatives, we treated replicon cells with increasing concentrations of NM1, and NM5 for 72 hours (Figs. 2a and b). Since chalcones show inhibition of HCV in replicon cells, we extracted total RNA from cells and quantified viral RNA level by quantitative PCR using HCV specific primers (Supplementary Materials). To our surprise, HCV RNA level, although decreased modestly, remained unchanged over a concentration range that spans from 0.1 μM to 10 μM. Since the RNA level remained mostly unchanged, we analyzed the viral protein level by comparing NS3 to GAPDH ratio from both genotypes 2a and 1b cells. Unlike the RNA level, both genotypes showed 50% inhibition of NS3 protein at 5 μM concentration (Figs. 2c, d, and e). Surprisingly, at a concentration below 5 μM (at 0.5 and 1 μM), both NM1 and NM5 showed a consistent and significant increase in viral protein (150%) above the control treatment (DMSO, 100%) before reducing to 50% at 5 μM concentration (Figures 2c, d, and e). NM6 treatment also showed similar outcome (Supplementary figure S3). Therefore, viral translation, but not RNA replication is targeted by chalcones. To examine if all HCV inhibitors function by first stimulating HCV protein production before a significant inhibition, we used cyclosporine A (CsA), a well-studied inhibitor of HCV. SGR2a cells were treated with increasing concentrations of CsA until NS3 level was significantly reduced.35, 36 When the ratio of viral protein (NS3) was compared with β-Actin at different concentrations of CsA, a gradual decrease in the NS3 level was observed without ever reaching a value higher than DMSO treated cells (Supplementary figure S4), suggesting that the chalcones use a different mode of inhibition than CsA.
Figure 1.
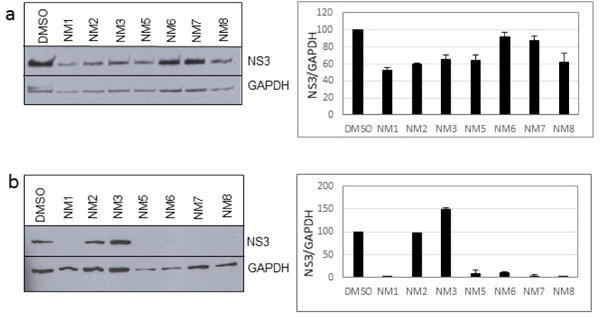
Inhibition of HCV replicon cell by chalcones. (a) SGR2a and (b) GS5 cells were treated for 72 hours with chalcones at 10 μM concentration. Cells were collected and extracted proteins were analyzed using Western Blot. Right side panels show quantitative analysis of the western blot on the left and repeats using Image J.
Figure 2.
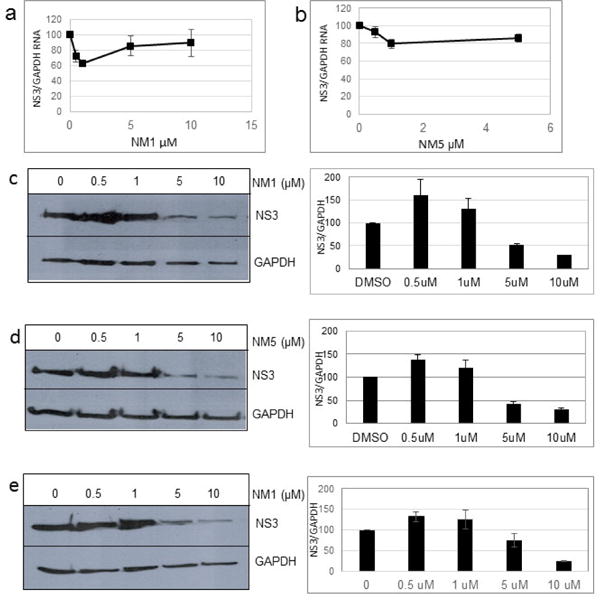
Concentration dependent inhibition of HCV. SGR2a cells were treated for 72 hours with (a) NM1 and (b) NM5 and GS5 cells were treated with (c) NM1, followed by isolation of RNA. HCV RNA and GAPDH mRNA were quantified using qPCR. Proteins from (a), (b) and (c) were analyzed by Western Blot. Right side panels for (c), (d) and (e) show quantitative analysis of the western blot on the left and their respective repeats using Image J.
A thorough look at the current literature suggests that HCV infected cells regulate the level of mTOR pathway to increase phosphorylation of ribosomal proteins.13 A previous study suggested that cells infected with 2a HCV virus in normal growth media (10% FBS) showed significant increase in phosphorylation of rps6 at Ser235/236 with modest increase in the basal level of rps6. Since chalcones effectively blocked viral translation, we asked if chalcones may block phosphorylation of rps6 protein which happens through the mTOR pathway. We analyzed the level of total and phosphorylated rps6 in SGR2a replicon cells treated with increasing concentration of NM5. We observed a modest decrease in the total rps6 but a significant drop in the level of the phosphorylated rps6 (Fig. 3a). A ratio of two showed a substantial decrease in the phosphorylated rps6 to below 50% relative to either GAPDH or total rps6 at the chalcone concentration of 0.5–1 μM (Fig. 3a, right panel). Similar effect was observed upon NM1 treatment (data not shown). Surprisingly, we observed an increase in the phosphorylated rsp6 under a low concentration of chalcone (0.5 and 1 μM), this result is parallel to increase in viral protein concentration under the same chalcone concentration suggesting phosphorylated rsp6 level may regulated viral NS3 level. mTOR function is defined by two separate signaling pathways. mTORC1 promotes protein synthesis through enhanced phosphorylation of S6K1 protein that serves as a kinase and is ultimately responsible for phosphorylating rps6. It is suggested that the mTORC2, a less studied pathway, influences cell growth and proliferation through phosphorylating AKT1 protein.37 To validate that the mTOR pathway is actually affected, we analyzed phosphorylated S6K1 protein in the treated cells.
Figure 3.
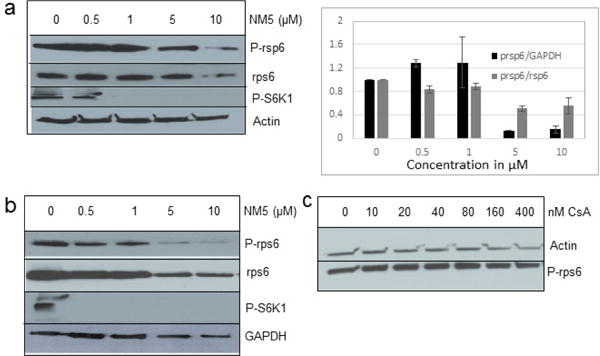
Chalcones repress phosphorylation of rsp6. (a) Left: SGR2a cells were incubated with NM5 for 72 hours followed by analysis of rps6, p-rsp6, and GAPDH. Right: Quantitative analysis of western blot of left panel and repeats using ImageJ. (b) Huh7.5 cells were incubated with NM5 followed by analysis of p-rps6, rps6, and actin. (c) SGR2a cells were incubated with increasing amount of CsA for 72 hours, followed by analysis of p-rsp6 and actin.
Results showed a sharp decrease in p-S6K1 at 0.1 μM concentration of chalcones (Fig. 3a). We examined if the effect of chalcones on S6K1 and rps6 is related to the presence of HCV (or NS5A), and incubated naïve Huh7.5 cells with increasing concentration of NM5. Results conclude that the effect of NM5 (Fig. 3b) on mTOR pathway is independent of the presence of HCV proteins. We tested if this is a unique property of NM5 and other chalcones, and analyzed the cells treated with CsA. Cell carrying subgenomic replicon type 2a (SGCR2a) was treated with an increasing concentration of CsA until NS3 level is diminished to 10% relative to the DMSO treated cells (Supplementary figure S4). p-rps6 remained constant throughout the concentration range (Fig. 3c). Chalcone mediated inhibition of HCV translation in cells with diminished level of phosphorylated rps6 suggest that HCV translation is severely affected by low level of phosphorylated rps6, while the cellular proteins including actin, GAPDH, and overall rps6 do not change significantly. Synthetic chalcones presented in this study inhibited viral protein translation without any significant inhibition of RNA replication. mTORC1 and mTORC2 complexes both share the same catalytic core that results kinase function.37 mTOR function is significantly enhanced in cells infected with HCV, and in cells expressing HCV encoded NS5A protein alone. High mTOR activity results phosphorylation of S6K1 protein, and subsequent phosphorylation of rps6.12, 13, 38 Presumably the phosphorylated rps6 is necessary for enhanced translation from HCV IRES. Similarly, increased level of mTOR is reported in many cancer cells, majority of these studies include breast cancer cells.25, 39 Recent work by Karthik et al. demonstrates reduction of downstream targets of mTOR including S6K1, rps6 which were also downregulated in our assay.22 In addition, authors reported significant reduction in mammosphere formation upon use of mTOR inhibitors alone or in combination. Disruption of kinase activity that shuts down mTORC2 leads to inhibition of PI3K-AKT pathway. Inhibition of these kinases have great impact on cancer cell viability, especially on breast cancer cells. Breast cancer and tumorigenesis is often linked to mutation in phosphoinositide 3-kinase (PI3K)/AKT/mTOR pathway, inspiring design and screening of drugs that target this pathway.40 We investigated if chalcones and flavonoids used in this study are also able to show anti-cancer properties in cancer cells. More specifically we asked if chalcones, but not flavonoids, inhibit growth of cancer cells due to their inhibitory effect on mTOR. In our assay, NM6 and NM7 were most effective inhibitor of growth in three different cancer cell line among other chalcones. Here we report a direct comparison of flavonoids NM17 and NM18 against their respective chalcones NM6 and NM7, carrying same substitution (−NO2, −Cl), in Ishikawa, MCF7, MDA-MB-231 cells by measuring estrogen vehicle growth (Supplementary material). Ishikawa cells are endometrial adenocarcinoma cells that produce both estrogen and progesterone receptors. MCF-7 and MDA-MB-231 are widely used breast cancer cells (Fig. 4).
Figure 4.
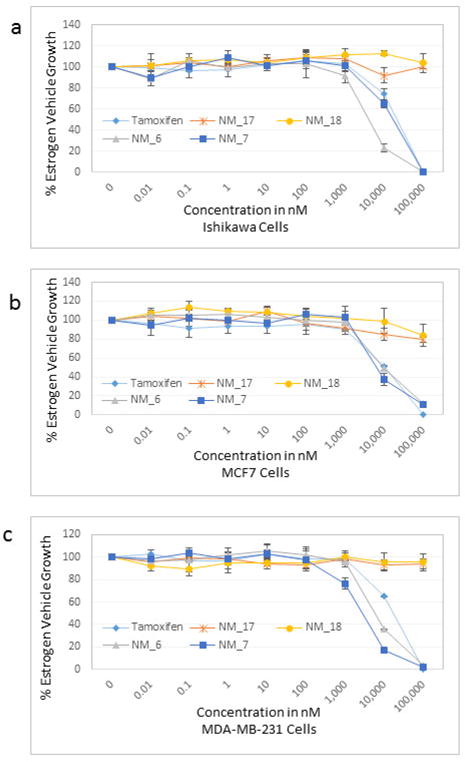
Analysis of estrogen mediated growth inhibition by chalcones and flavonoids in cancer cells. (a) Ishikawa, (b) MCF-7, and (c) MDA-MB-231 cells were plated in 96 well plates and were incubated with 10 nM estrogen and indicated amount of tamoxifen, NM6, NM7, NM17, and NM18 for 72 hrs. Cell were incubated with Cell-Glow-Titer for 12 minutes at room temperature before immunofluorescence measurement.
When compared the activity of chalcones against flavonoids containing same substitutions, we found chalcones were significantly better than corresponding flavonoids (IC50 1–6 μg/mL vs >35 μg/mL). In addition, when these chalcones, NM6 and NM7, were compared with widely established drug tamoxifen and its derivatives (Table 2 and supplementary materials), we found both compounds inhibited estrogen mediated growth of cancer cells effectively (IC50 of Tamoxifen = 3– 8 μg/mL). Our results suggest that chalcones are more effective inhibitor of both HCV translation, and growth of cancer cells. Since chalcones and flavonoids are structurally similar, we asked if chalcones exert better stabilization when bound to a potential target. Therefore, we performed molecular docking of NM6 and NM7 along with corresponding flavonoids (NM17 and NM18) against mTOR kinase, the protein responsible for triggering mTOR activity (PDB code: 4JSV). Our result suggests that both chalcones (Figs. 5a and 5b, left column) and flavonoids (Figs. 5c and 5d, right column) result similar free energy of binding when bound at the ATP binding site of mTOR kinase. However, only chalcones (NM6 and NM7) engage Asp2357, a key active site residue in the kinase reaction, while flavonoids bind to the active site without interacting with Asp2357 residue. Structural studies on mTOR suggest that Asp2357 is an active site residue that binds to the magnesium ion necessary for the kinase reaction.41 Given our results we suggest that Asp2357 may be a key residue that is blocked by chalcones effectively.
Table 2.
IC50 value comparing chalcones, flavonoids, and tamoxifen in cancer cells.
| IC50 (μg/mL) | |||
|---|---|---|---|
| Compound | Ishikawa | MCF-7 | MDA-MB-231 |
| NM_6 | 5.85 | 3.30 | 1.98 |
| NM_7 | 1.43 | 2.24 | 0.99 |
| NM_17 | >36.72 | >36.72 | >36.72 |
| NM_18 | >35.73 | >35.73 | >35.73 |
| Tamoxifen | 7.87 | 3.99 | 7.85 |
| Raloxifene-Hydrochloride | 10.53 | 0.98 | 11.21 |
| 4-Hydroxy Tamoxifen | 3.57 | 0.95 | 6.51 |
Figure 5.
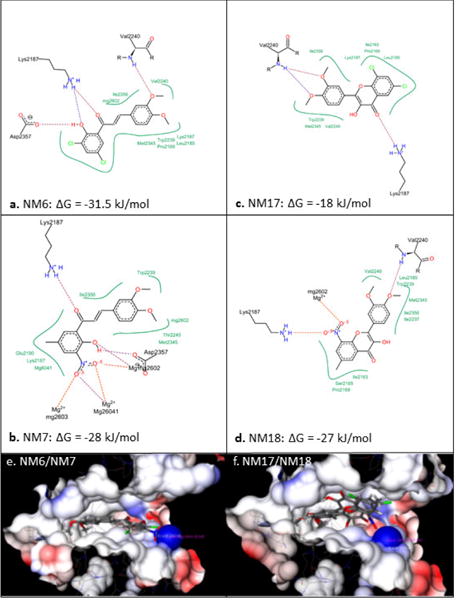
Top scoring bind poses of the ligands NM6-7, NM17-18 at the active site of mTOR protein kinase (PDB code: 4JSV). (a-d) Two dimensional docking (Posix View) of the ligands interactions in the active site of mTOR protein kinase. (e-f) flavonoids and chalcones are located in the ATP binding pocket of mTOR kinase.
Overall, our results have two significant outcomes. One, inhibition of phosphorylated rsp6 and S6K1 by chalcones dampen HCV translation. These results can be used to design and analyze drugs that are more effective. Second, compared to respective flavonoids, chalcones are more effective anti-HCV agents and anti-cancer compounds in Ishikawa, MCF7, and MDA-MB-231 cells. Chalcones block phosphorylation of rsp6 and S6K1 potentially through inhibition of mTOR (Fig. 3), and are effective anti-cancer agents that are comparable to Tamoxifen and its derivatives (Fig. 4). Although effective concentration of chalcones studied in this work are relatively high against HCV (5 μM), effective concentration necessary to inhibit growth in cancer cells are comparable to established cancer drugs. Chalcones and flavonoids differ by the closed ring structure present in the flavonoid but not in chalcones providing more flexibility in the latter during target binding. Interestingly, in our study all chalcones showed varied level of activity while flavonoids did not show any inhibition of HCV translation at the concentrations used in this study. Similarly, chacones, not flavonoids, effectively inhibited growth in cancer cells used in this study.
Supplementary Material
Acknowledgments
We thank Dr. Charles Rice (Rockefeller University) for providing Huh7.5 cells, Dr. Hengli Tang (Florida State University) for providing SGR2a and GS5 cells, technical support, and for his input in this project. We also thank Dr. Carl Goodman (RCMI, FAMU). A.N. was supported by Faculty Research Program Award (FRAP, 2014–2015). Research reported in this publication was supported by the National Institute on Minority Health and Health Disparities (NIMHD) of the National Institute of Health (NIH) under award number G12MD007582 to A. N.; U.O. and T.D.H were supported by Florida–Georgia Louis Strokes Alliance for Minority Participation (FGLSAMP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Watashi K, Inoue D, Hijikata M, Goto K, Aly HH, Shimotohno K. Anti-hepatitis C virus activity of tamoxifen reveals the functional association of estrogen receptor with viral RNA polymerase NS5B. J Biol Chem. 2007;282(45):32765–72. doi: 10.1074/jbc.M704418200. [DOI] [PubMed] [Google Scholar]
- 2.Kwee JK. Yin and yang of polyphenols in cancer prevention. A short review. Anticancer Agents Med Chem. 2015 doi: 10.2174/1871520616666151116124549. [DOI] [PubMed] [Google Scholar]
- 3.Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med. 2013;19(7):837–49. doi: 10.1038/nm.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Clercq E. Current race in the development of DAAs (direct-acting antivirals) against HCV. Biochem Pharmacol. 2014;89(4):441–52. doi: 10.1016/j.bcp.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Ortega-Prieto AM, Dorner M. The expanding toolbox for hepatitis C virus research. J Viral Hepat. 2016 doi: 10.1111/jvh.12500. [DOI] [PubMed] [Google Scholar]
- 6.Pawlotsky JM. New Hepatitis C Virus (HCV) Drugs and the Hope for a Cure: Concepts in Anti-HCV Drug Development. Semin Liver Dis. 2014;34(1):22–9. doi: 10.1055/s-0034-1371007. [DOI] [PubMed] [Google Scholar]
- 7.Abraham GM, Spooner LM. Sofosbuvir in the Treatment of Chronic Hepatitis C: New Dog, New Tricks. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu265. [DOI] [PubMed] [Google Scholar]
- 8.Calland N, Dubuisson J, Rouillé Y, Séron K. Hepatitis C virus and natural compounds: a new antiviral approach? Viruses. 2012;4(10):2197–217. doi: 10.3390/v4102197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology. 2014;60(1):37–45. doi: 10.1002/hep.27151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang H, Grisé H. Cellular and molecular biology of HCV infection and hepatitis. Clin Sci (Lond) 2009;117(2):49–65. doi: 10.1042/CS20080631. [DOI] [PubMed] [Google Scholar]
- 11.Ross-Thriepland D, Harris M. Hepatitis C virus NS5A: enigmatic but still promiscuous 10 years on! J Gen Virol. 2015;96(Pt 4):727–38. doi: 10.1099/jgv.0.000009. [DOI] [PubMed] [Google Scholar]
- 12.George A, Panda S, Kudmulwar D, Chhatbar SP, Nayak SC, Krishnan HH. Hepatitis C virus NS5A binds to the mRNA cap-binding eukaryotic translation initiation 4F (eIF4F) complex and up-regulates host translation initiation machinery through eIF4E-binding protein 1 inactivation. J Biol Chem. 2012;287(7):5042–58. doi: 10.1074/jbc.M111.308916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng L, Liang D, Tong W, Li J, Yuan Z. Hepatitis C virus NS5A activates the mammalian target of rapamycin (mTOR) pathway, contributing to cell survival by disrupting the interaction between FK506-binding protein 38 (FKBP38) and mTOR. J Biol Chem. 2010;285(27):20870–81. doi: 10.1074/jbc.M110.112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15(7):807–26. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 15.Khachatoorian R, Arumugaswami V, Raychaudhuri S, Yeh GK, Maloney EM, Wang J, Dasgupta A, French SW. Divergent antiviral effects of bioflavonoids on the hepatitis C virus life cycle. Virology. 2012;433(2):346–55. doi: 10.1016/j.virol.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.León-González AJ, Acero N, Muñoz-Mingarro D, Navarro I, Martín-Cordero C. Chalcones as Promising Lead Compounds on Cancer Therapy. Curr Med Chem. 2015;22(30):3407–25. doi: 10.2174/0929867322666150729114829. [DOI] [PubMed] [Google Scholar]
- 17.Das M, Manna K. Chalcone Scaffold in Anticancer Armamentarium: A Molecular Insight. J Toxicol. 2016;2016:7651047. doi: 10.1155/2016/7651047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mai CW, Yaeghoobi M, Abd-Rahman N, Kang YB, Pichika MR. Chalcones with electron-withdrawing and electron-donating substituents: anticancer activity against TRAIL resistant cancer cells, structure-activity relationship analysis and regulation of apoptotic proteins. Eur J Med Chem. 2014;77:378–87. doi: 10.1016/j.ejmech.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13(2):1886–918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokolowski KM, Koprowski S, Kunnimalaiyaan S, Balamurugan M, Gamblin TC, Kunnimalaiyaan M. Potential Molecular Targeted Therapeutics: Role of PI3-K/Akt/mTOR Inhibition in Cancer. Anticancer Agents Med Chem. 2015;16(1):29–37. doi: 10.2174/1871520615666150716104408. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, Wang S, Ren P, Martin M, Jessen K, Feldman ME, Weissman JS, Shokat KM, Rommel C, Ruggero D. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485(7396):55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karthik GM, Ma R, Lövrot J, Kis LL, Lindh C, Blomquist L, Fredriksson I, Bergh J, Hartman J. mTOR inhibitors counteract tamoxifen-induced activation of breast cancer stem cells. Cancer Lett. 2015;367(1):76–87. doi: 10.1016/j.canlet.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Bhardwaj A, Rosen D, Liu M, Liu Y, Hao Q, Ganesan N, Etzel CJ, Gullett A, Albarracin CT, Bedrosian I. Suppression of Akt-mTOR pathway-a novel component of oncogene induced DNA damage response barrier in breast tumorigenesis. PLoS One. 2014;9(5):e97076. doi: 10.1371/journal.pone.0097076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu J, Che L, Li L, Pilo MG, Cigliano A, Ribback S, Li X, Latte G, Mela M, Evert M, Dombrowski F, Zheng G, Chen X, Calvisi DF. Co-activation of AKT and c-Met triggers rapid hepatocellular carcinoma development via the mTORC1/FASN pathway in mice. Sci Rep. 2016;6:20484. doi: 10.1038/srep20484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moschetta M, Reale A, Marasco C, Vacca A, Carratù MR. Therapeutic targeting of the mTOR-signalling pathway in cancer: benefits and limitations. Br J Pharmacol. 2014;171(16):3801–13. doi: 10.1111/bph.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massacesi C, Di Tomaso E, Urban P, Germa C, Quadt C, Trandafir L, Aimone P, Fretault N, Dharan B, Tavorath R, Hirawat S. PI3K inhibitors as new cancer therapeutics: implications for clinical trial design. Onco Targets Ther. 2016;9:203–10. doi: 10.2147/OTT.S89967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asati V, Mahapatra DK, Bharti SK. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur J Med Chem. 2016;109:314–41. doi: 10.1016/j.ejmech.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Yousef BA, Hassan HM, Zhang LY, Jiang ZZ. Anticancer Potential and Molecular Targets of Pristimerin: A mini-review. Curr Cancer Drug Targets. 2016 doi: 10.2174/1568009616666160112105824. [DOI] [PubMed] [Google Scholar]
- 29.Lee YK, Chung HH, Kim JW, Song YS, Park NH. Expression of phosphorylated Akt and hTERT is associated with prognosis of epithelial ovarian carcinoma. Int J Clin Exp Pathol. 2015;8(11):14971–6. [PMC free article] [PubMed] [Google Scholar]
- 30.Mills CJ, Mateeva NN, Redda KK. Synthesis of Novel Substituted Flavonoids. J Heterocyclic Chem. 2006;43:59. [Google Scholar]
- 31.Mateeva NN, Kode RN, Redda KK. Synthesis of novel flavonoid derivatives as potential HIV-integrase inhibitor. Journal of Heterocyclic Chemistry. 2009;39(6):1251–1258. [Google Scholar]
- 32.Mateeva NN, Kode R, Redda KK. Synthesis of Novel Flavonoid Derivatives as Potential HIV-Integrase Inhibitors. J Heterocyclic Chem. 2002;39:1251. [Google Scholar]
- 33.Lohmann V, Körner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285(5424):110–3. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 34.Yang F, Robotham JM, Nelson HB, Irsigler A, Kenworthy R, Tang H. Cyclophilin A is an essential cofactor for hepatitis C virus infection and the principal mediator of cyclosporine resistance in vitro. J Virol. 2008;82(11):5269–78. doi: 10.1128/JVI.02614-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang F, Robotham JM, Grise H, Frausto S, Madan V, Zayas M, Bartenschlager R, Robinson M, Greenstein AE, Nag A, Logan TM, Bienkiewicz E, Tang H. A major determinant of cyclophilin dependence and cyclosporine susceptibility of hepatitis C virus identified by a genetic approach. PLoS Pathog. 2010;6(9):e1001118. doi: 10.1371/journal.ppat.1001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang H. Cyclophilin inhibitors as a novel HCV therapy. Viruses. 2010;2(8):1621–34. doi: 10.3390/v2081621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–94. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stohr S, Costa R, Sandmann L, Westhaus S, Pfaender S, Anggakusuma, Dazert E, Mauleman P, Vondran FWR, Manns MP, Steinmann E, von Hahn T, Ciesek S. Host cell mTORC1 is required for HCV RNA replication. Gut. 2015;0:1–12. doi: 10.1136/gutjnl-2014-308971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guerrero-Zotano A, Mayer IA, Arteaga CL. I3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016 doi: 10.1007/s10555-016-9637-x. [DOI] [PubMed] [Google Scholar]
- 41.Yang H, Rudge DG, Koos JD, Vaidialingam B, Yang HJ, Pavletich NP. mTOR kinase structure, mechanism and regulation. Nature. 2013;497(7448):217–23. doi: 10.1038/nature12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


