Abstract
Purpose
To compare studies with and without oral contrast on performance of multidetector computed tomography (CT) and the order to CT examination turnaround time in cancer patients presenting to the emergency department (ED). To our knowledge, oral contrast utility has not previously been specifically assessed in cancer patients presenting to the emergency department.
Materials & Methods
Retrospective review of CT abdomen examinations performed in oncology patients presenting to the emergency department during one month. CT examinations performed with and without oral contrast were rated by two consensus readers for degree of confidence and diagnostic ability. Correlations were assessed for primary cancer type, age, sex, chief complaint/examination indication, body mass index, intravenous contrast status, repeat CT examination within 4 weeks, and disposition. Turnaround times from order to the start of the CT examination were calculated.
Results
The studied group consisted of 267 patients (127 men and 140 women) with a mean age of 56 years and a mean body mass index of 27.8 kg/m2. One hundred sixty CT examinations were performed without oral contrast, and 107 CT examinations were performed with oral contrast. There was no significant difference between cases with oral contrast and cases without oral contrast in the number of cases rated as “improved confidence” (odds ratio [OR] 0.54, 95% confidence interval [CI] 0.23–1.31, P=0.17), “improved diagnosis” (OR 0.58, 95% CI 0.20–1.64, P=0.3), “impaired confidence” (OR 3.92, 95% CI 0.46–33.06, P=0.21), or “impaired diagnosis” (OR 2.63, 95% CI 0.29–23.89, P=0.39). The turnaround time in the group receiving oral contrast (mean, 141 min; standard deviation, 49.8 min) was significantly longer than that in the group not receiving oral contrast (mean, 109.2 min; standard deviation, 64.8 min) with a mean difference of 31.8 minutes (P<0.0001).
Conclusion
On the basis of our findings and prior studies, targeted rather than default use of oral contrast shows acceptable diagnostic ability in the emergency setting for oncology patients. Benefit from oral contrast use is suggested in scenarios such as suspected fistula/bowel leak/abscess, hypoattenuating peritoneal disease, prior bowel surgery such as gastric bypass, and the absence of intravenous contrast administration. Improvement through the use of targeted oral contrast administration also supports the emergency department need for prompt diagnosis and disposition.
Keywords: oral contrast, emergency, abdominal, turnaround time, bowel
Introduction
Increasing emergency department (ED) patient volumes are an ongoing issue that can result in long waiting times and overcrowding. Increased patient volume has led to a concomitant increase in imaging utilization as imaging plays an essential role in triaging many of these patients to determine or exclude a diagnosis and allow for appropriate patient disposition. Abdominal complaints including pain, nausea, and vomiting represent common chief complaints in the ED that often lead to the use of computed tomography (CT) for further evaluation. This, typically necessary, imaging step inherently adds to the time a patient may spend in the ED. In an attempt to improve ED disposition times and throughput, oral contrast optimization has been an area of interest [1,2].
Historically, the administration of oral contrast has been recommended in most CT examinations of the abdomen, especially with the use of older-generation scanners [3]. Even recent data still supports the use of oral contrast. Kammerer et al. reported that with positive oral contrast, the odds of a diagnostic improvement are 4.66 times higher (95% confidence interval [CI] 1.36–15.97, P=0.0144) than with no oral contrast. The probability of a diagnostic improvement from the use of neutral oral contrast is increased by an odds ratio of 4.63 (95 % CI 1.35–15.87, P=0.7470) [4]. However, other studies have recently shown that diagnostic ability can be maintained without oral contrast in certain patient groups [5,6].
There is evidence for omitting oral contrast from CT protocols in many scenarios such as renal colic and major trauma to aid in the rapid diagnosis and treatment of patients [7,8]. Even long-standing assumptions that most bowel trauma evaluations benefit from oral contrast have been challenged [9–11]. Oral contrast extravasation is one of the least sensitive signs for bowel injury [12,13]. Strong evidence also supports the omission of oral contrast in other clinical scenarios such as appendicitis in which maintained diagnostic accuracy has been shown [14]. Importantly, a large retrospective study by Harieaswar et al. demonstrated equivalent diagnostic capability of multidetector CT performed without oral contrast compared with CT performed with positive oral contrast for routine oncologic CT follow-up [6]. In an ED study of patients with acute non-traumatic abdominal pain, CT without intravenous or oral contrast had an overall accuracy of 95.6% and a negative predictive value of 95.1% [15].
While many studies have demonstrated maintained CT performance without the use of oral contrast in specific clinical scenarios, there are also known benefits to neutral and/or no oral contrast. For example, assessing mucosal enhancement (hyper or hypoenhancement) can be useful in multiple settings for which positive oral contrast would often preclude this evaluation such as Crohn disease, bowel ischemia, and hyperenhancing tumors [16–19]. These benefits of not using oral contrast are amplified by the time savings.
The administration of oral contrast typically requires a 60- to 90-minute delay before imaging to allow for distal bowel opacification. Huynh et al. reported a difference in order-to-scan times of 68 minutes between CT protocols performed with and without oral contrast [1]. Studies have shown that eliminating oral contrast can increase patient throughput and improved overall patient experience, while maintaining diagnostic accuracy [8,5,20,1]. In rare instances, beyond the discomfort of drinking large quantities of liquid, there can even be safety concerns such as contrast allergy when using water-soluble iodinated contrast and aspiration [21,22].
Variable opacification of the gastrointestinal tract is another limitation when oral contrast is administered. The cause of this variation is multifactorial, with one main factor being poor tolerance of oral contrast resulting in varied degree of intake. In a satisfaction survey, the administration of oral contrast was rated to be the least favorite part of a CT examination, even below that of intravenous cannulation [6]. Importantly, there can also be marked differences in bowel motility between patients, which contribute to the variable opacification. Laituri et al. evaluated patients with appendicitis and revealed that oral contrast did not reach the region of interest, the terminal ileum, in 30% of patients [23].
In certain patient cohorts, the administration of oral contrast remains the preferred protocol. For example, thin patients with a paucity of intraabdominal fat are a known group for whom the administration of oral contrast is still suggested owing to less inherent separation of bowel loops [20]. Evaluations for possible anastomotic leak, fistula, extramural hematoma or abscess should typically include positive oral contrast [24,4]. Hypoattenuating peritoneal disease adjacent to bowel loops can also be difficult to evaluate without oral contrast. Lastly, cases imaged without intravenous contrast have been shown to generally benefit from oral contrast administration since the soft tissue delineation provided by intravenous contrast administration is not present [6].
At our institution, a quaternary oncologic center, we currently perform all ED abdominal imaging studies without oral contrast unless contrast is specifically requested by the ED clinician. To our knowledge, no prior evaluations have specifically assessed oral contrast utility in cancer patients presenting to the emergency department. The purpose of our study was to retrospectively compare between studies with and without oral contrast the performance of CT and the order to CT examination turnaround time in this specific patient group.
Materials and Methods
This retrospective study was approved by our institutional review board as Health Insurance Portability and Accountability Act compliant and the need for informed consent was waived.
Patient Selection
On the basis of a power analysis performed before the study, we reviewed a contiguous set of patients who underwent ED CT scanning of the abdomen/pelvis during April 2016. This was the first month of patient scanning after the implementation of a new oral contrast policy for CT imaging of the abdomen; the previous default administration of water-soluble, positive oral contrast was changed to no oral contrast unless requested otherwise by an ED clinician.
The patients’ primary cancer, age, sex, chief complaint/examination indication, body mass index and disposition from the ED were recorded. For each CT examination, the presence or absence of intravenous and oral contrast was noted and was compared with the original physician order. The official radiology reports were reviewed to identify any comments made as to perceived limitations of the study, particularly those related to the presence or absence of oral contrast. A record was made if a patient underwent additional CT scanning that included the abdomen/pelvis within 4 weeks of the incident scan.
Imaging technique
All patients underwent CT of the abdomen/pelvis with an identical imaging protocol performed on a 128-slice dual-source CT system (Somatom Definition Flash; Siemens Healthineers, Erlangen, Germany) with: gantry speed of 0.5 seconds, pitch of 0.6:1, table speed of 23 mm/rotation, beam collimation of 38.4 mm with detector configuration of 128×0.6 mm, and 120 kVp using tube current modulation. When intravenous contrast was ordered, 125 mL of Omnipaque 350 (GE Healthcare, Chicago, IL) was injected at 2.5–3 mL/sec with bolus tracking using a 100–Hounsfield unit trigger value in the abdominal aorta at the level of the celiac artery and a scan delay of 45 seconds. When oral contrast was ordered, 40 mL of Omnipaque 350 was mixed into water for a total administration of 800 mL with a target scan time of approximately 60 minutes after the start of administration.
Imaging Review
Three-millimeter axial image sets were used for primary review (2-mm coronal and sagittal reconstructions were available for correlation) using a picture archiving and communication system diagnostic reading station with two consensus readers in a non-blinded, randomized fashion over 10 sessions. Each reader was fellowship-trained in abdominal imaging with 5 subsequent years (C.J.) and 6 subsequent years (N.W.-B.) of experience reading abdominal CT. No time limit was set for review.
A five-point Likert scale was used by the readers to qualitatively grade the impact of enteric contrast status on confidence and on diagnosis as follows: 5, Definitely Improved; 4, Improved; 3, No Consequence; 2, Impaired; 1, Definitely Impaired. A score of 5 for a patient with oral contrast indicated that the readers felt that the presence of oral contrast definitely improved diagnosis or confidence. For a case without oral contrast, however, a score of 5 indicated that the absence of oral contrast definitely improved diagnosis or confidence. When assessing confidence, factors such as the degree with which oral contrast status affected mucosal detail, anatomic delineation such as separation of bowel loops from adjacent anatomy and delineation of pathology (when applicable) were used as criteria during scoring.
Readers utilized studies performed before and after the scan of interest and any available clinical notes in their assessment.
Turnaround time assessment
The electronic medical record was queried for CT order time and examination start time to calculate the turnaround time between the order and the oral contrast administration.
Statistical Analysis
Patient and imaging characteristics were summarized using mean, standard deviation (SD), and range. Pearson chi-square test, Fisher exact test, and two-sample t-test were used to assess the associations of oral contrast, patient disposition, and whether CT was repeated within 4 weeks status with other covariates. All tests were two-sided and p-values of 0.05 or less were considered statistically significant. Statistical analysis was carried out using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Patient characteristics
The studied group consisted of 267 patients (127 men and 140 women) with a mean age of 56 years (range, 19–57 years). Mean body mass index was 27.8 kg/m2 (range, 12.8–59.5 kg/m2). One hundred and sixty CT examinations were performed without oral contrast, and 107 CT examinations were performed with oral contrast. Forty-one CT examinations were performed without intravenous contrast and 226 CT examinations were performed with intravenous contrast. One hundred and ninety-seven of the patients were admitted to the inpatient service, and the other 70 were discharged home. The top three diagnoses were breast cancer, colon cancer, and leukemia, and the top three chief complaints were abdominal pain, nausea, and vomiting. No significant correlation was identified between age, sex, body mass index, chief complaint, cancer diagnosis, disposition status or repeat CT examination.
Turnaround Times
The turnaround time in the group receiving oral contrast (mean, 141 min; SD, 49.8 min) was significantly longer than in the group not receiving oral contrast (mean, 109.2 min; SD, 64.8 min), with a mean difference of 31.8 minutes (P<0.0001).
ED Physician Ordering
Of the 267 patients, the ED physician requested oral contrast for 88 patients, requested no oral contrast for 93 patients and made no comment in 86 patients; for cases without such a comment, our current default protocol is to use no oral contrast. Brief review of ED ordering in the subsequent 2 months revealed 88 and 69 requests, respectively, for oral contrast; 58 and 56 specific requests, respectively, for no oral contrast; and 104 and 129 cases, respectively, in which no specific comment was made in the order regarding oral contrast. Following the oral contrast protocol requested by the EC physician, only 6 patients were identified as inferior in reader confidence and/or diagnosis. In the 5 patients without oral contrast, limitations were related to cachexia, peritoneal implant mimicking small bowel, matted tumor in the pelvis, fistula and two cases that were performed without intravenous contrast, one of which had peritoneal disease from colon carcinoma. In the 1 patient with oral contrast that was rated to be inferior, there was peritoneal disease in a patient with endometrial carcinoma.
Imaging Review
For patients not receiving versus receiving oral contrast, there were more cases rated as impaired confidence (6 cases:1 case) (Odds ratio (OR) 3.92, 95% Confidence Interval (CI) 0.46–33.06, P=0.21) and impaired diagnosis (4:1) (OR 2.63, 95% CI 0.29–23.89, P=0.39) in relation to ratings of no consequence. Conversely, there were fewer cases rated as improved confidence (10:12) (OR 0.54, 95% CI 0.23–1.31, P=0.17) and improved diagnosis (7:8) (OR 0.58, 95% CI 0.20–1.64, P=0.3) in relation to ratings of no consequence. For patients not receiving versus receiving oral contrast in relation to impaired versus improved cases, there also was no statistical significance for confidence (OR 7.20, 95% CI 0.74–70.20, P=0.089) or diagnosis (OR 4.57, 95% CI 0.41–51.1, P=0.22) (Figure 1).
Figure 1.
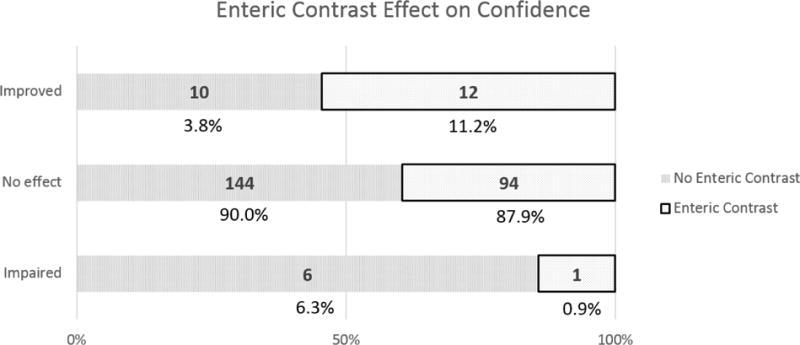
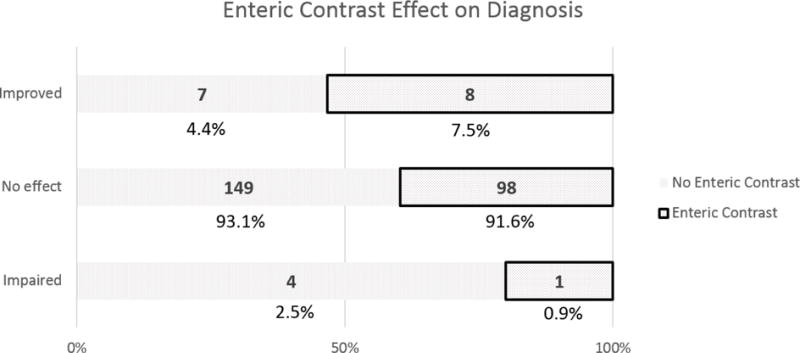
Reader scores for the impact of oral contrast status on confidence (a) and diagnosis (b) in CT scans. Enteric contrast status was of no consequence in the overwhelming majority of cases.
For patients not receiving intravenous contrast versus those receiving intravenous contrast, controlling for oral contrast status, there were more cases rated as impaired confidence (P=0.0169) and impaired diagnosis (P<0.0014) when no oral contrast was administered. No significant difference between intravenous contrast groups was identified when oral contrast was administered.
Cross-tabulations were performed on each cancer type with sample size greater than or equal to 5 with respect to readers scores; there were 18 cancer types that were assessed: bladder, endometrial, colon, lymphoma, leukemia, breast, carcinoma of unknown primary, cervical, cholangiocarcinoma, gastric, lung, melanoma, neuroendocrine, ovarian, pancreatic, prostate, renal and sarcoma. No significant correlations were identified through cross-tabulations.
Four scans were specifically described as suboptimal in the radiology report owing to lack of oral contrast; these cases were of typhlitis, obstructive uropathy, parastomal hernia with possible strangulation, and progression of peritoneal disease. Lack of oral contrast for these four studies was rated as “no consequence” except for the last case, which notably was performed without intravenous contrast. Readers rated that case as “likely impaired”. In the radiology reports of these 4 scans, only the case of typhlitis described a specific limitation which was due to the absence of positive oral contrast precluding fistula assessment.
Fifty-two patients underwent CT examination within 4 weeks after the incident scan. Every scan reviewed had a prior or subsequent study for comparison. No missed findings by the original reader related to the status of oral contrast were identified during imaging review.
Discussion
Our study evaluated an oncologic population presenting to the emergency department who underwent CT of the abdomen for abdominal complaints; no statistical difference in reader confidence or diagnosis was identified related to whether or not patients received oral contrast and there was a significant reduction in turnaround time for patients without oral contrast. This is in agreement with prior studies that have evaluated other patient populations [1,2,14,6].
Previous studies of CT oral contrast have been narrow in scope, focusing primarily on trauma, renal colic and appendicitis [20,1,4,18,2,17]. Some evaluations have studied outpatient and undifferentiated oncologic patient groups with mixed results [6,4]. Harieaswar et al. [6] audit of 447 patients presenting for oncologic follow-up revealed 94.9% of studies to be deemed adequate without oral contrast; when correlating the other cases with follow-up, only 1.6% of cases were ultimately classified as “bowel not clearly identified.”
In our study, while most cases of oral contrast status were rated as ‘no consequence’, similar benefits and limitations of using and withholding oral contrast were identified. For example, without oral contrast, readers noted that bowel enhancement, certain cases of bowel wall thickening, surgical clips, hemorrhage and hyperenhancing bowel/peritoneal tumors were better seen (Figures 2 and 3). It is possible that, had these cases been performed with oral water, improvement may have been seen relative to positive oral contrast, as described by Krammerer et al. [4]. Conversely, cases of matted tumor, post-operative cases involving the bowel, fistulas, leaks, certain duodenal stents, perforations, and hypoattenuating bowel/peritoneal tumors were limited in their evaluation by the lack of oral contrast and were generally better evaluated with oral contrast (Figure 4). Additionally, as described by Harieaswar et al., examinations in which intravenous contrast is otherwise contraindicated often benefit from the administration of oral contrast [6]. Some lesions were seen similarly in cases with and without oral contrast (Figure 5).
Figure 2.
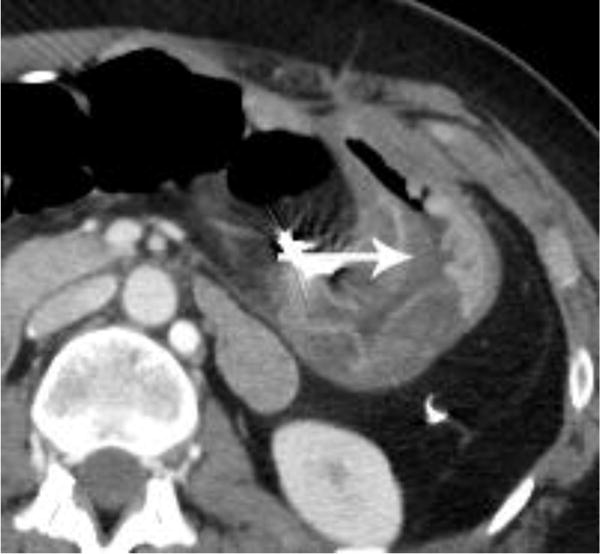
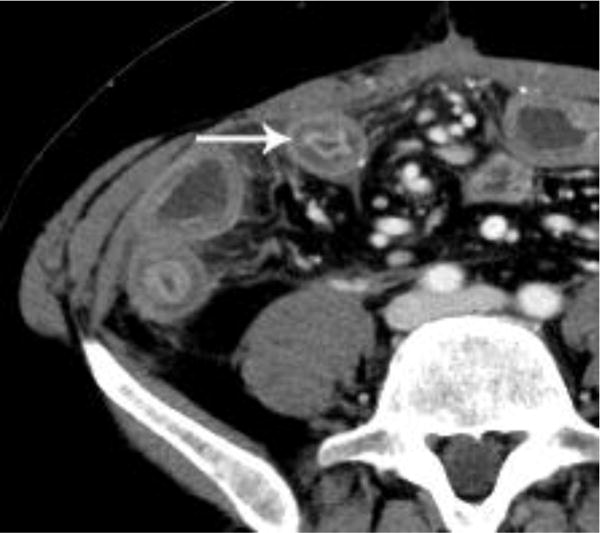
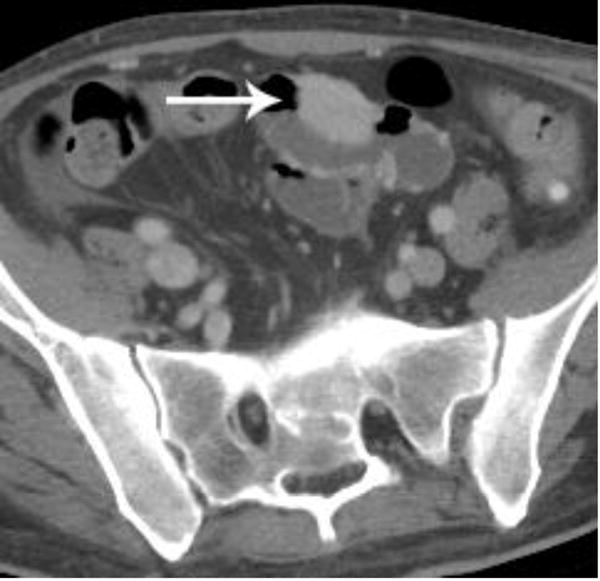
Intravenous contrast-enhanced CT examination examples from various patients in our study demonstrate the benefits of not having positive oral contrast. Gastric wall thickening with differential enhancement (a), small bowel thickening with mucosal hyperenhancement (b), hypervascular metastasis to the small bowel from renal cell carcinoma (c), and sarcoma metastases (d) are scenarios where no oral contrast or neutral contrast can be helpful (arrows).
Figure 3.
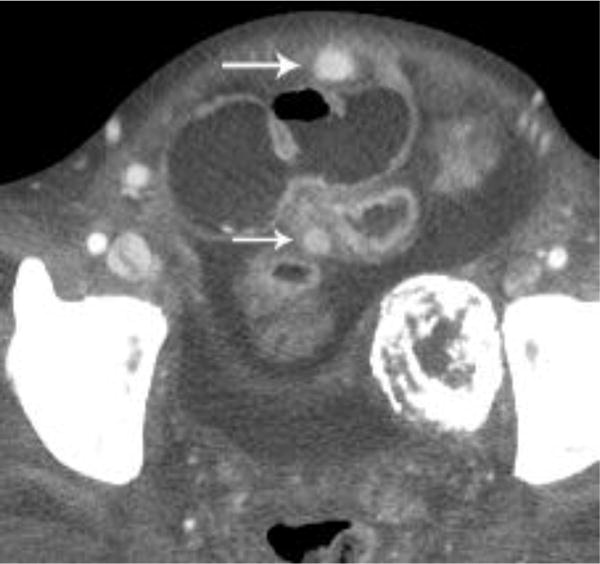
A metastatic implant in the midabdomen contained hyperdense active hemorrhage (arrow) and was associated with a hematocrit level (dashed arrow)(a). A CT examination performed 7 days earlier demonstrated multiple findings, but oral contrast obscured this lesion that was likely already bleeding, thus producing a hyperdense appearance (arrow)(b).
Figure 4.
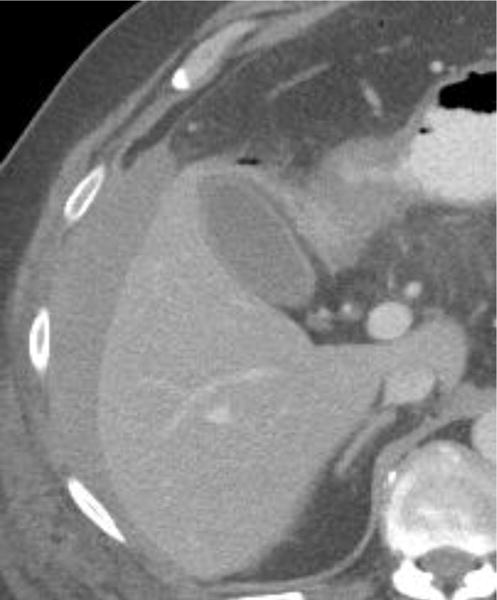
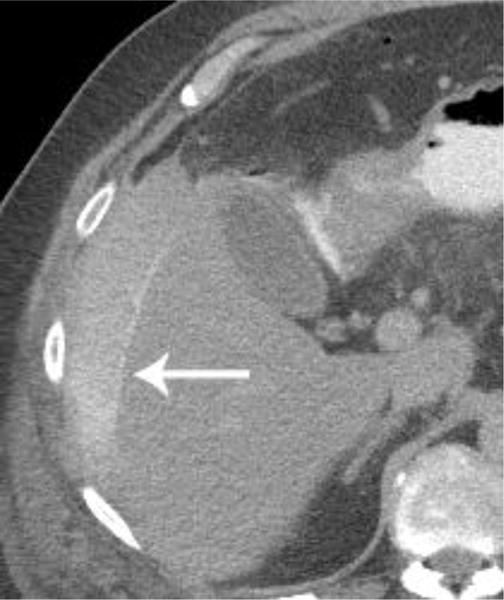
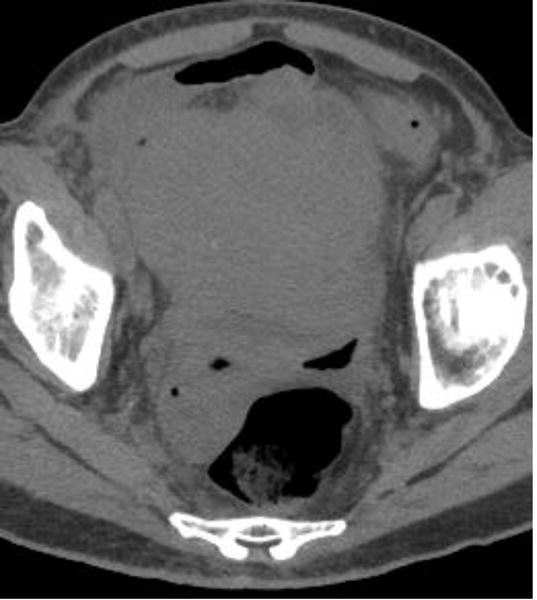
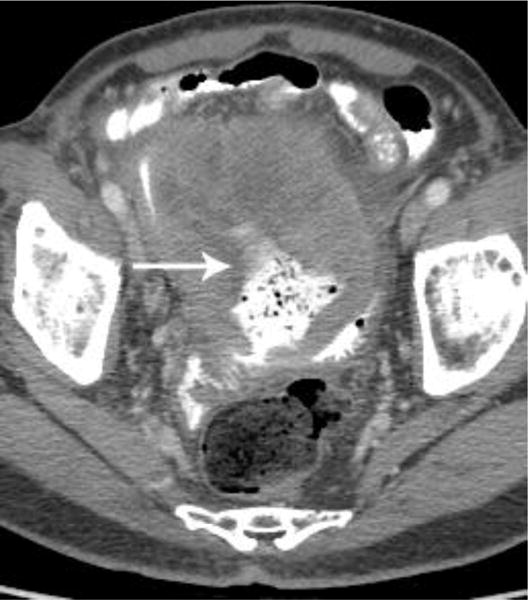
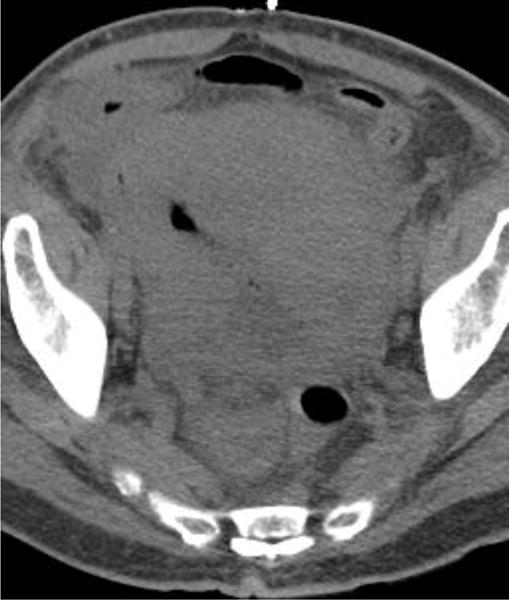
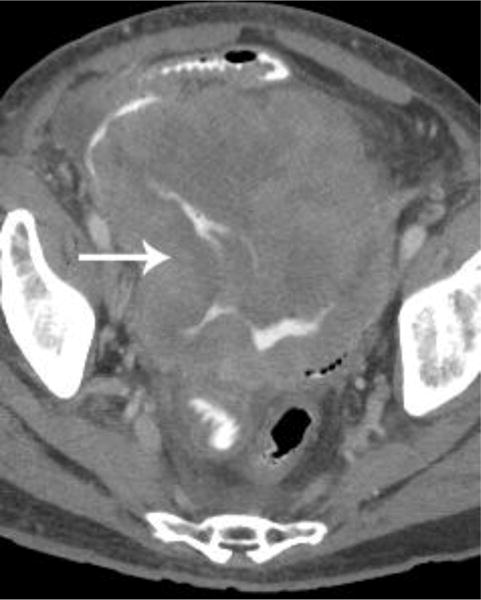
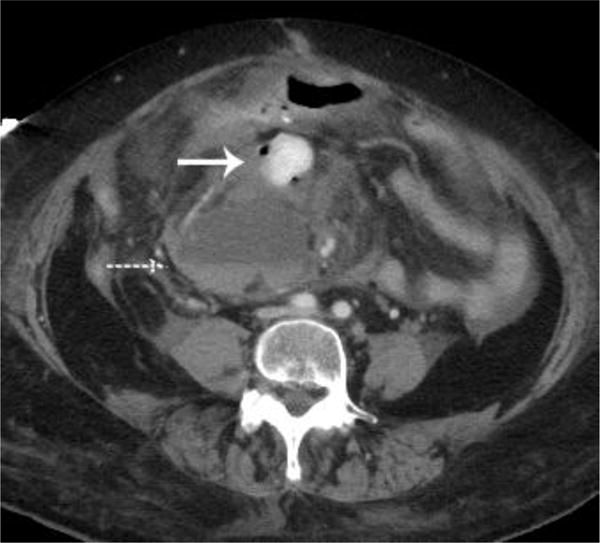
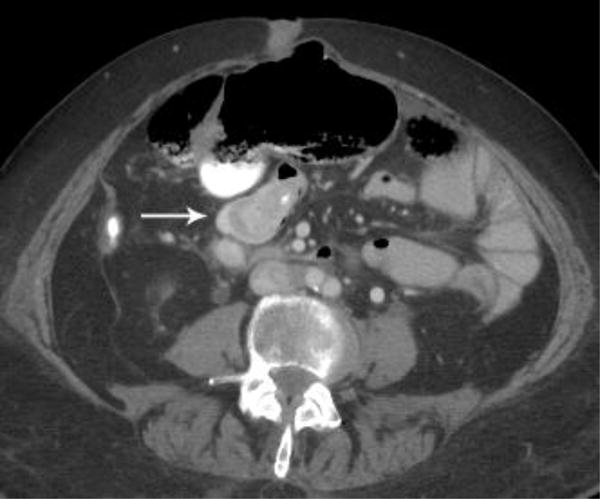
Examples of CT examinations from our study performed in three different patients demonstrate the benefits of oral contrast administration. In the first patient (a,b), a duodenal perforation was confirmed by the extravasation of oral contrast (arrow). Two other patients (c,d and e,f) underwent CT examinations that were performed within a short time period of one another, one without oral contrast and the other with oral contrast. In each case, a diagnosis of tumor fistula with bowel was directly identified only on the examinations with oral contrast (arrows).
Figure 5.
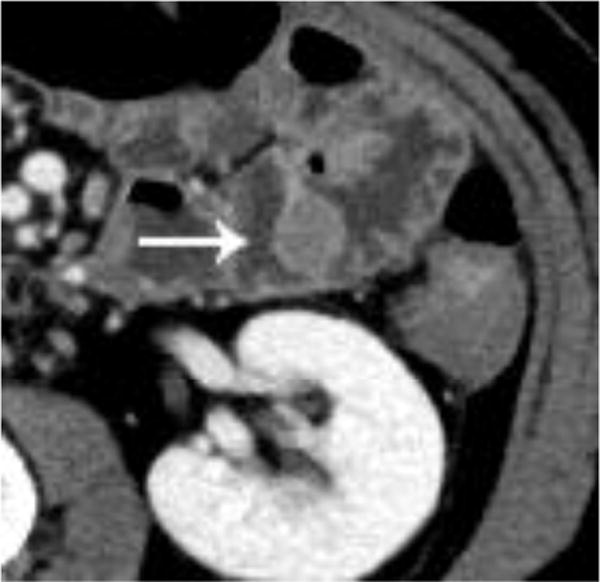
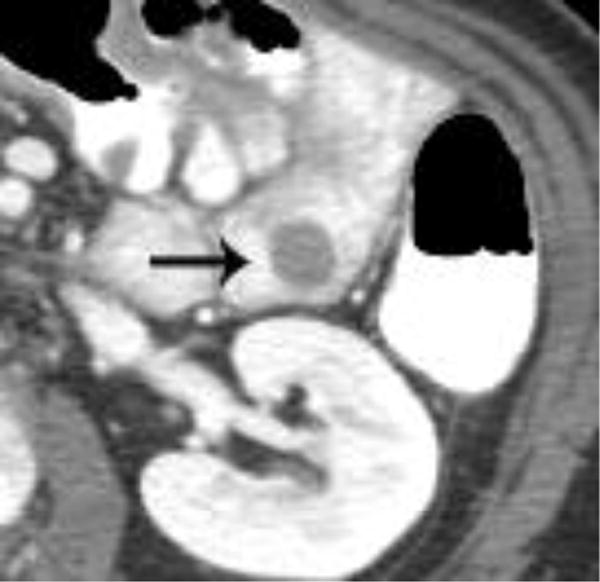
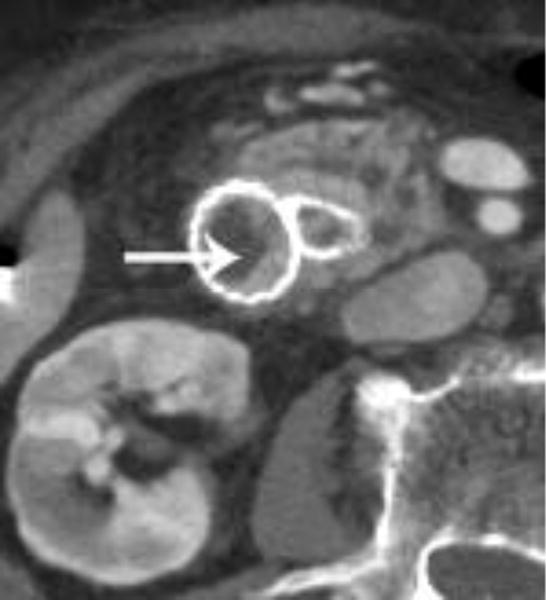
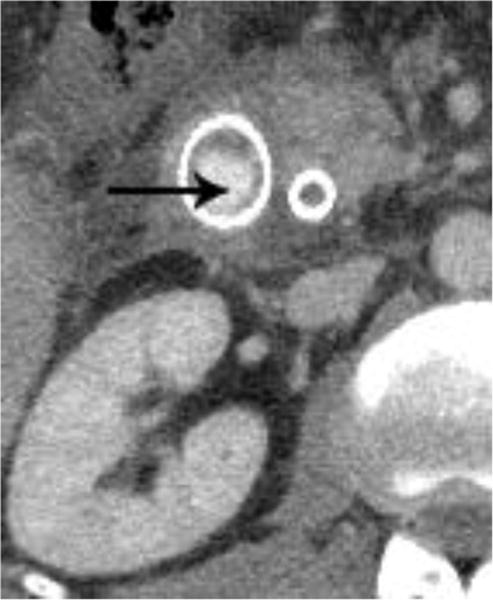
Intravenous contrast enhanced CT examinations in the same patient (a,b) show a polypoid jejunal lesion that is seen similarly irrespective of oral contrast status (arrows). Similarly, in two different patients (c,d), tumor within a duodenal stent can be seen to a similar degree in the patient without oral contrast (c) compared with the patient oral contrast (d).
Importantly, in addition to considerations of diagnostic accuracy and timeliness, the limitations of oral contrast should be understood. One of the most frequent limitations that has been reported and that we noted was the variable distribution of administered oral contrast; a patient in our study who waited over an hour during the administration of oral contrast still did not have oral contrast at the site of bowel fistula in the pelvis and another patient with a bowel leak did not have oral contrast reach the site of interest. Much of this variation likely relates to differences in bowel transit between patients and is at least partially influenced by patient tolerance of oral contrast [6,23]. In the literature and in our study, positive oral contrast has obscured lesions including primarily hyperenhancing bowel/peritoneal implants and hematomas, and it generally precludes the assessment of bowel wall enhancement [16].
Our study, similar to prior reports, showed a decreased order to examination turnaround time for patients not receiving oral contrast compared with those receiving contrast. CT examinations without oral contrast were performed a mean of 31.8 minutes earlier from time of order entry than those receiving oral contrast. This amount of time is less than our protocol-targeted delay of 60 minutes after start of oral contrast. Variation in this timing points to the complexity of the ED care pathway. Wang et al. detailed the complex process of length of stay and turnaround time assessments [2]. While the above-mentioned time savings are desired if the diagnostic performance is adequate, patients clearly have varied transit pathways through the ED, and a more detailed analysis would likely be useful.
Though not explored in this study, keeping radiation dose as low as reasonably achievable should be considered in relation to oral contrast. Wang et al. demonstrated that patients receiving positive oral contrast had a volume CT dose index that was 11% higher than that of patients receiving oral water alone [25]. While the significance of this dose savings will vary depending on patient age and clinical status, it remains important to note as another benefit of foregoing positive oral contrast.
The limitations of our study include the retrospective design, but importantly, our findings agree with most prior studies. Consensus reading has inherent limitations as well, but use of the official radiology report and prior/subsequent CT examinations were helpful to supplement evaluation. The goal of this study was to assess our new policy in which oral contrast administration is no longer the default in ED CT scanning of the abdomen/pelvis; thus, there was an inherent bias in the study population based on variation in ED physician requests for oral contrast. ED physician ordering is generally in agreement with our radiology department recommendations that patients with suspected bowel pathology including fistula, abscess, or leak receive positive oral contrast; however, there is certainly variation in these orders. Lastly, consensus evaluation infers significance, or lack thereof, based on knowledge of known benefits and limitations of oral contrast; for example, the authors may have rated a case with positive oral contrast as “no consequence”, but it is not possible to know whether underlying mucosal hyperenhancement was been present. This limitation was mitigated by detailed review of current and follow-up clinical notes, review of pertinent prior and subsequent scans, and the attempt to identify other features such as bowel wall thickening in the case of potentially obscured mucosal hyperenhancement.
On the basis of our findings and prior studies, targeted rather than default use of oral contrast shows acceptable diagnostic ability in the emergency setting for oncology patients. Benefit from oral contrast use is suggested in scenarios such as suspected fistula/bowel leak/abscess, hypo-attenuating peritoneal disease, prior bowel surgery such as gastric bypass, and the absence of intravenous contrast administration. Improvement through the use of targeted oral contrast administration also supports the emergency department need for prompt diagnosis and disposition. While some of the above conditions that may benefit from positive oral contrast may not always be suspected clinically during ordering and thus may not receive such contrast, it appears that more cases will benefit from discriminant oral contrast use. Certainly other nuances of protocols can be considered, such as shorter administration time of oral contrast. Further prospective evaluations of oral contrast protocols are needed.
Acknowledgments
Funding: Supported by institutional CCSG (cancer center support grant) from the NIH/National Cancer Institute under award number P30CA016672.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
References
- 1.Huynh LN, Coughlin BF, Wolfe J, Blank F, Lee SY, Smithline HA. Patient encounter time intervals in the evaluation of emergency department patients requiring abdominopelvic CT: oral contrast versus no contrast. Emerg Radiol. 2004;10(6):310–313. doi: 10.1007/s10140-004-0348-1. [DOI] [PubMed] [Google Scholar]
- 2.Wang DC, Parry CR, Feldman M, Tomlinson G, Sarrazin J, Glanc P. Acute Abdomen in the Emergency Department: Is CT a Time-Limiting Factor? AJR Am J Roentgenol. 2015;205(6):1222–1229. doi: 10.2214/AJR.14.14057. [DOI] [PubMed] [Google Scholar]
- 3.Hamlin DJ, Burgener FA. Positive and negative contrast agents in CT evaluation of the abdomen and pelvis. J Comput Tomogr. 1981;5(2):82–90. doi: 10.1016/0149-936x(81)90020-5. [DOI] [PubMed] [Google Scholar]
- 4.Kammerer S, Höink AJ, Wessling J, Heinzow H, Koch R, Schuelke C, Heindel W, Buerke B. Abdominal and pelvic CT: is positive enteric contrast still necessary? Results of a retrospective observational study. European Radiology. 2014;25(3):669–678. doi: 10.1007/s00330-014-3446-9. [DOI] [PubMed] [Google Scholar]
- 5.Ham H, McInnes MD, Woo M, Lemonde S. Negative predictive value of intravenous contrast-enhanced CT of the abdomen for patients presenting to the emergency department with undifferentiated upper abdominal pain. Emergency Radiology. 2012;19(1):19–26. doi: 10.1007/s10140-011-0996-x. [DOI] [PubMed] [Google Scholar]
- 6.Harieaswar S, Rajesh A, Griffin Y, Tyagi R, Morgan B. Routine use of positive oral contrast material is not required for oncology patients undergoing follow-up multidetector CT. Radiology. 2009;250(1):246–253. doi: 10.1148/radiol.2493080353. [DOI] [PubMed] [Google Scholar]
- 7.Richards JR, Derlet RW. Computed tomography for blunt abdominal trauma in the ED: a prospective study. Am J Emerg Med. 1998;16(4):338–342. doi: 10.1016/s0735-6757(98)90122-x. [DOI] [PubMed] [Google Scholar]
- 8.Abramson S, Walders N, Applegate KE, Gilkeson RC, Robbin MR. Impact in the emergency department of unenhanced CT on diagnostic confidence and therapeutic efficacy in patients with suspected renal colic: a prospective survey. 2000 ARRS President’s Award. American Roentgen Ray Society. AJR Am J Roentgenol. 2000;175(6):1689–1695. doi: 10.2214/ajr.175.6.1751689. [DOI] [PubMed] [Google Scholar]
- 9.Clancy TV, Ragozzino MW, Ramshaw D, Churchill MP, Covington DL, Maxwell JG. Oral contrast is not necessary in the evaluation of blunt abdominal trauma by computed tomography. Am J Surg. 1993;166(6):680–684. doi: 10.1016/s0002-9610(05)80679-8. discussion 684–685. [DOI] [PubMed] [Google Scholar]
- 10.Tsang BD, Panacek EA, Brant WE, Wisner DH. Effect of oral contrast administration for abdominal computed tomography in the evaluation of acute blunt trauma. Ann Emerg Med. 1997;30(1):7–13. doi: 10.1016/s0196-0644(97)70103-3. [DOI] [PubMed] [Google Scholar]
- 11.Stafford RE, McGonigal MD, Weigelt JA, Johnson TJ. Oral contrast solution and computed tomography for blunt abdominal trauma: a randomized study. Arch Surg. 1999;134(6):622–626. doi: 10.1001/archsurg.134.6.622. discussion 626–627. [DOI] [PubMed] [Google Scholar]
- 12.Butela ST, Federle MP, Chang PJ, Thaete FL, Peterson MS, Dorvault CJ, Hari AK, Soni S, Branstetter BF, Paisley KJ, Huang LF. Performance of CT in detection of bowel injury. AJR Am J Roentgenol. 2001;176(1):129–135. doi: 10.2214/ajr.176.1.1760129. [DOI] [PubMed] [Google Scholar]
- 13.Stuhlfaut JW, Soto JA, Lucey BC, Ulrich A, Rathlev NK, Burke PA, Hirsch EF. Blunt abdominal trauma: performance of CT without oral contrast material. Radiology. 2004;233(3):689–694. doi: 10.1148/radiol.2333031972. [DOI] [PubMed] [Google Scholar]
- 14.Anderson BA, Salem L, Flum DR. A systematic review of whether oral contrast is necessary for the computed tomography diagnosis of appendicitis in adults. Am J Surg. 2005;190(3):474–478. doi: 10.1016/j.amjsurg.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 15.MacKersie AB, Lane MJ, Gerhardt RT, Claypool HA, Keenan S, Katz DS, Tucker JE. Nontraumatic acute abdominal pain: unenhanced helical CT compared with three-view acute abdominal series. Radiology. 2005;237(1):114–122. doi: 10.1148/radiol.2371040066. [DOI] [PubMed] [Google Scholar]
- 16.Paulsen SR, Huprich JE, Fletcher JG, Booya F, Young BM, Fidler JL, Johnson CD, Barlow JM, Earnest Ft. CT enterography as a diagnostic tool in evaluating small bowel disorders: review of clinical experience with over 700 cases. Radiographics. 2006;26(3):641–657. doi: 10.1148/rg.263055162. discussion 657–662. [DOI] [PubMed] [Google Scholar]
- 17.Wiesner W, Hauser A, Steinbrich W. Accuracy of multidetector row computed tomography for the diagnosis of acute bowel ischemia in a non-selected study population. Eur Radiol. 2004;14(12):2347–2356. doi: 10.1007/s00330-004-2462-6. [DOI] [PubMed] [Google Scholar]
- 18.Megibow AJ, Babb JS, Hecht EM, Cho JJ, Houston C, Boruch MM, Williams AB. Evaluation of bowel distention and bowel wall appearance by using neutral oral contrast agent for multi-detector row CT. Radiology. 2006;238(1):87–95. doi: 10.1148/radiol.2381041985. [DOI] [PubMed] [Google Scholar]
- 19.Horton KM, Fishman EK. Multidetector-row computed tomography and 3-dimensional computed tomography imaging of small bowel neoplasms: current concept in diagnosis. J Comput Assist Tomogr. 2004;28(1):106–116. doi: 10.1097/00004728-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Harrison ML, Lizotte PE, Holmes TM, Kenney PJ, Buckner CB, Shah HR. Does high body mass index obviate the need for oral contrast in emergency department patients? West J Emerg Med. 2013;14(6):595–597. doi: 10.5811/westjem.2013.5.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laituri CA, Fraser JD, Aguayo P, Fike FB, Garey CL, Sharp SW, Ostlie DJ, St Peter SD. The lack of efficacy for oral contrast in the diagnosis of appendicitis by computed tomography. Journal of Surgical Research. 2011;170(1):100–103. doi: 10.1016/j.jss.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Kauv P, Benadjaoud S, Curis E, Boulay-Coletta I, Loriau J, Zins M. Anastomotic leakage after colorectal surgery: diagnostic accuracy of CT. Eur Radiol. 2015;25(12):3543–3551. doi: 10.1007/s00330-015-3795-z. [DOI] [PubMed] [Google Scholar]
- 23.Wang ZJ, Chen KS, Gould R, Coakley FV, Fu Y, Yeh BM. Positive enteric contrast material for abdominal and pelvic CT with automatic exposure control: what is the effect on patient radiation exposure? Eur J Radiol. 2011;79(2):e58–62. doi: 10.1016/j.ejrad.2011.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]


