Abstract
Background/Objectives
Polypharmacy and prescribing potentially inappropriate medications (PIMs) are common among older persons. Appropriate prescribing requires robust communication and shared decision making about medications. This study examines the effect of TRIM (Tool to Reduce Inappropriate Medications), a web tool linking the electronic health record (EHR) to a clinical decision support system, on medication communication and prescribing.
Design
Randomized clinical trial
Setting
Primary care clinics at a VA Medical Center
Participants
128 Veterans age 65 years and older prescribed ≥ 7 medications, randomized to receipt of TRIM or usual care.
Intervention
TRIM extracts medications and chronic conditions from the EHR and contains data entry screens for information obtained from brief chart review and telephonic patient assessment. These data serve as input for automated algorithms identifying medication reconciliation discrepancies, PIMs, and potentially inappropriate regimens. Clinician feedback reports summarize discrepancies and provide recommendations for deprescribing. Patient feedback reports summarize discrepancies and self-reported medication problems.
Measurements
Primary: subscales of the Patient Assessment of Care for Chronic Conditions (PACIC) related to shared decision making, clinician and patient communication; secondary: changes in medications.
Results
While 29.7% of TRIM participants versus 15.6% of control participants provided the highest PACIC ratings, the difference was nonsignificant. Adjusting for covariates and clustering of patients within clinicians, TRIM was associated with significantly more active patient communication and facilitative clinician communication, and with more medication-related communication among both. TRIM was significantly associated with correction of medication discrepancies, but had no effect on number of medications or reduction in PIMs.
Conclusions
TRIM improved communication around medications and accuracy of documentation. While there was no association with prescribing, the small sample size provided limited power to examine medication-related outcomes.
Keywords: Polypharmacy, communication, medication prescribing
INTRODUCTION
Polypharmacy, the receipt of multiple medications and variably defined according to different cutpoints,1,2 is common among older persons. Among Medicare Part D enrollees in 2012, 48% filled four or more prescriptions per month and 19% filled eight or more.3 Although the data are mixed, the majority of studies examining polypharmacy have demonstrated associations with a range of undesirable outcomes, including adverse drug events, falls, hospitalization, physical and cognitive disability, and hospitalization.4 The greater the number of total prescribed medications, the greater the likelihood of prescription of a medication individually associated with risk of harm, otherwise known as a potentially inappropriate medication (PIM).5
A recent review of deprescribing trials to reduce polypharmacy and/or PIMs concluded that the most effective trials involved resource-intensive interventions, such as multidisciplinary team medication review or academic detailing.6 These interventions have the advantage of identifying a wide range of PIMs based on implicit review and clinical expertise. However, a second review of studies utilizing less resource-intensive e-prescribing and computerized decision support system (CDSS) technologies found that many were successful in reducing the use of certain medications or classes of medications.7 While these interventions have the potential for more widespread dissemination, they generally target a narrow range of PIMs.
TRIM, the Tool to Reduce Inappropriate Medication, was designed to bridge the gap between these two types of interventions.8 TRIM links a CDSS to the Veterans’ Affairs electronic health record (EHR) and evaluates the appropriateness of the medication regimen, providing feedback to the patient and clinician. It supplements EHR data with components of patient assessment necessary to perform a comprehensive medication reconciliation and to facilitate an assessment of appropriateness in the context of individual patient characteristics not reliably found in the medical record. Decisions about medication appropriateness in older patients involve a consideration of benefits and harms and how these relate to patient goals9 in a process of shared decision making.10 By alerting clinicians to potentially inappropriate medications and regimens, the feedback generated by TRIM is designed to prompt clinicians to ask patients about adverse effects specifically, and their experience with medications more generally. TRIM also provides simplified feedback to patients, focusing on patient-reported discrepancies and problems, in order to enhance patient self-efficacy in discussing these issues with their clinicians. The purpose of the current study was to examine the effects of TRIM on shared decision making about medications. The primary outcomes were patients’ perceptions about participation in their care and patient-clinician medication-related communication. The secondary outcomes were changes in the medication regimen.
METHODS
Participants
The sample size of 128 was calculated to provide power of 0.80 for a two-sided test with Type 1 error of 0.05 to detect an effect size (Cohen’s d) of 0.5 for continuous outcomes. Participants were community-dwelling Veterans age 65 years and older with an upcoming primary care appointment at VA Connecticut Healthcare System who were prescribed ≥ 7 medications including at least one each for hypertension (HTN) and diabetes mellitus (DM). From December 2014 through January 2016, consecutively eligible Veterans were mailed an opt-out letter followed by a telephone call to screen for exclusion criteria. These included severe hearing loss, prescription of medications by clinician(s) outside of the VA, medication management by someone other than the Veteran, and severe acute illness. Eligible participants were assigned to receive the TRIM intervention or to usual care, with one-half of those in the usual care group receiving the TRIM telephone assessment, as described further below. To avoid clinicians seeing control and intervention patients simultaneously, assignments were made in blocks of time, such that, for each period of time, all eligible patients were assigned to a given group. Written informed consent was obtained from all the participants, and the protocol was approved by the Human Subjects Subcommittee of VA Connecticut Healthcare System. The trial was registered at Clinical Trials.gov: NCT02501967. A flow diagram is provided in Figure 1.
Figure 1.
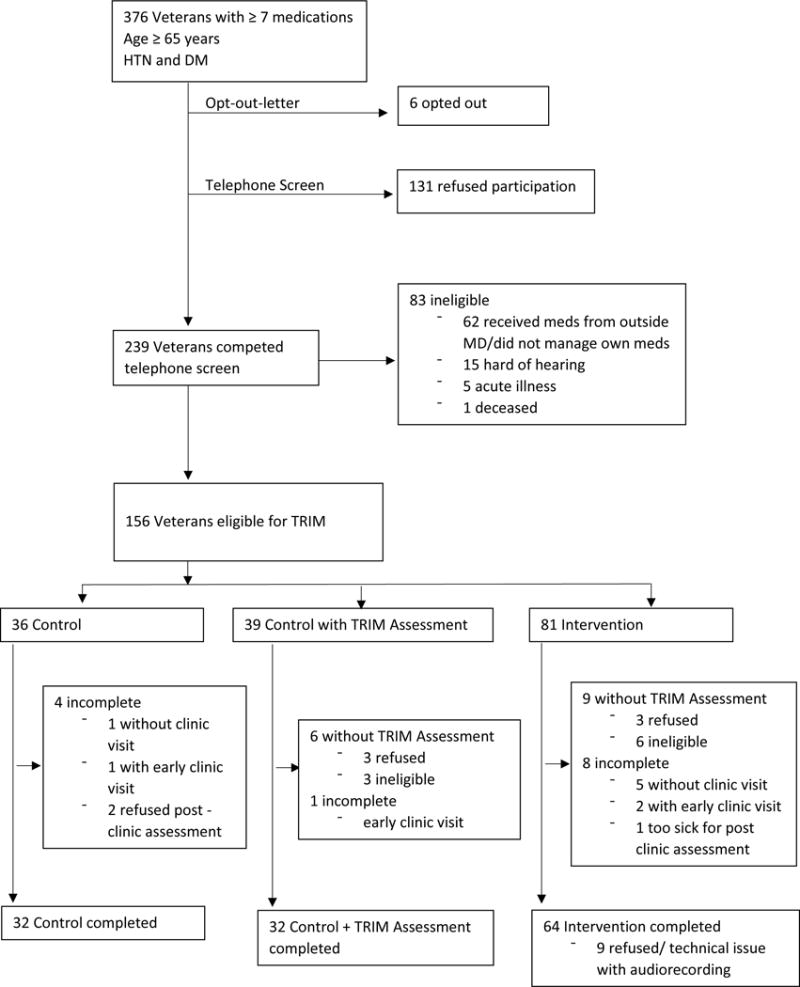
Flow diagram for study
Intervention
The development of TRIM has been previously described.8 TRIM consists of two web applications. The first application extracts medications and chronic conditions from the EHR. The second application consists of three components. The first is an interface for chart review and telephonic patient assessment. These data, along with the extracted EHR data, serve as inputs for the second component, a set of automated algorithms evaluating medication appropriateness. TRIM evaluates medication appropriateness based on a range of criteria, including feasibility in the context of the patient’s cognition and social support, potential overtreatment of DM and/or HTN, “traditional” PIMs according to Beers and STOPP criteria, inappropriate renal dosing, and patient report of adverse medication effects. The algorithms generate the third component, a patient-specific medication management feedback report for the clinician. This report includes a complete medication reconciliation, recommendations for discontinuation or dosage changes for inappropriate medications, and a recommendation regarding the need to simplify the regimen of patients with problems with adherence and poor social support. The report was e-mailed to the clinician 24 hours prior to the primary care appointment and handed to the clinician just before the appointment. The algorithms also generate a simple, short report for the patient, consisting of a listing of medication reconciliation discrepancies and reported problems with medications. The report is given to the patient just prior to the appointment with brief coaching on using the report to discuss medication concerns with their clinician. The telephone assessments occurred within three days prior to their primary care appointment.
Control
The control group received usual care. Performance of the TRIM telephone assessment was necessary to identify potential medication problems in order to compare changes in the medication regimen according to intervention assignment. There was concern that this assessment, even without provision of feedback reports, could influence medication decision making. We balanced the need for assessment with the concern about its effects on prescribing by performing the TRIM telephone assessment on only one-half of the control group. These participants (control + assessment) received the assessment within three days prior to their primary care appointment. However, neither they nor their physician received a feedback report.
Data collection and measures
The primary care visits were audio recorded in order to analyze the communication between patient and clinician; however for nine visits the patient or clinician refused to be recorded or the audio file was of insufficient quality to be analyzed. Patients were interviewed directly following their visit, and a chart review was performed ninety days following the primary care visit by a researcher who was not blinded to study assignment. The primary outcome measures were patient involvement in their care and patient-clinician communication. Patient involvement was measured using a sum of the scores of three subscales from the Patient Assessment of Care for Chronic Conditions (PACIC).11 The PACIC was developed as a patient self-report measure on the receipt of patient-centered care. As supported by the scale’s developers,11 we utilized the patient activation, goal setting, and problem-solving/contextual counseling subscales as the most relevant to shared decision making. The original PACIC asks patients to report on the frequency with which they engaged in certain behaviors in their chronic illness care over the last six months. Because we used the PACIC to evaluate a single clinic visit, we modified the response choices to ask the patient whether or not they engaged in the behavior during the single visit, resulting in a range of scores from 0 to 12.
The clinician-patient communication variables consisted of active patient participation, clinician facilitation of patient participation, and communication about medication. Active patient participation was coded using the Active Patient Participation Coding Scheme. This scheme identified the presence of three types of patient speech: questions, assertive responses (e.g., stating preferences, making requests, introducing topics to discuss), and expressions of concern,12 each of which influences a clinician’s behavior and treatment.12,13 The scheme also identified the presence of two types of clinician communication aimed at facilitating active participation. These included partnership-building responses (e.g., soliciting patient opinions, concerns, or questions) and supportive talk (e.g, reassurance, encouragement).14 Clinician and patient communication related to medication management was coded using a scheme adapted from earlier studies.15,16 The medication-related communication was coded by identifying utterances pertaining to medication adverse effects, medication instructions and assistance with medication administration, necessity of/indication for medications, complexity of the regimen, and new or changes in medications.
A total of 15 audio files were coded by two trained raters blinded to study assignment who participated in three 2-hour training sessions. Intraclass correlation coefficients (ICCs) comparing the coding for these 15 indicated adequate reliability of active patient involvement (ICC = .82), physician facilitative communication (ICC = .78), and medical-related utterances (ICC = .79). The remaining audio files were coded by a single rater. Scores for active patient communication, facilitative clinician communication, physician recommendations, patient medication-related communication, and clinician medication-related communication were created by summing the total number of utterances in each of the relevant categories.
Secondary outcomes were changes in the patients’ medication regimens. The change in total number of medications from baseline to ninety days was calculated. For patients in the intervention and control + assessment groups, the medication list was examined to evaluate the number of TRIM recommendations that were implemented and the number of medication discrepancies corrected.
Data regarding characteristics used to describe the study population were obtained from interview and chart review. Patients provided self-report of education, ethnicity, sufficiency of monthly income, employment status, quality of life, and self-rated health. Age and chronic conditions were obtained from chart review.
Analysis
Proportions were used to describe the participants in the intervention and control groups. The PACIC was dichotomized as a score of 11 or 12 versus 10 or less to evaluate the proportion of individuals who reported participation in all or nearly all of the activities related to patient-centered care. As a sensitivity analysis, we examined the PACIC with a cut-point of ≥ 10 versus <10. The communication variables were examined as continuous outcomes except for clinician recommendation, which was dichotomized as 1 or more recommendation(s) versus none. The significance of bivariate associations was analyzed using chi-square tests for categorical outcomes and non-parametric Kruskal-Wallis tests for continuous outcomes, although means are also presented for ease of interpretation. Multivariable analyses were conducted using Generalized Estimating Equations and mixed models in order to account for the clustering of patients receiving care from the same clinician. These analyses also adjusted for imbalances in the intervention and control groups and for variables associated with each of the outcomes at p < .10. Further analyses of the communication variables examined the relationship between patient and clinician communication, since the two can influence each other.17,18 Clinician facilitative communication was added to the model examining the outcome of patient active communication, and patient active communication was added to the model examining the outcome of clinician facilitative communication. In the same way, clinician and patient medication communication variables were added to the reciprocal models.
RESULTS
A total of 128 patients were assigned to the intervention and control groups (Table 1). Few women participated in the study, and the majority was white. Fewer patients in the intervention group were married, had a college education, or money left over at the end of the month, and more were employed and rated their health as excellent or very good.
Table 1.
Description of participants and of medication reconciliation discrepancies and recommendations provided by TRIM assessment
| Variable | Intervention N=64 | Control N=64 |
|---|---|---|
| Female, n (%) | 1 (1.6) | 1 (1.6) |
| Age, n (%) | ||
| < 70 | 27 (42.2) | 25 (39.1) |
| 70–79 | 31 (48.4) | 26 (40.6) |
| 80+ | 6 (9.4) | 13 (20.3) |
| Nonwhite, n (%) | 17 (26.6) | 14 (21.9) |
| Married, n (%) | 30 (46.9) | 36 (57.1) |
| Educational level, n (%) | ||
| High school or less | 9 (14.1) | 6 (9.4) |
| Some college | 38 (59.4) | 34 (53.1) |
| College or more | 17 (26.6) | 24 (37.5) |
| Income, n (%) | ||
| Some money left over | 17 (26.6) | 33 (36.5) |
| Just enough | 35 (54.7) | 32 (50.8) |
| Not enough | 12 (18.8) | 5 (12.7) |
| Employment status, n (%) | ||
| Full time | 5 (7.8) | 3 (4.7) |
| Part time | 11 (17.2) | 6 (9.4) |
| Quality of life best possible or good, n (%) | 48 (75.0) | 49 (76.6) |
| Self-rated health, n (%) | ||
| Excellent or very good | 21 (32.8) | 14 (21.9) |
| Good | 25 (39.1) | 30 (46.9) |
| Fair or poor | ||
| >5 chronic conditions, n (%) | 22 (34.4) | 24 (37.5) |
| Number of medications, mean (SD) | 13.4 (5.2) | 13.8 (4.8) |
| Medication reconciliation | ||
| 1+ discrepancies, n (%) | 63 (98) | 31 (97) |
| Number of discrepancies, mean (SD) | 4.25 (2.4) | 5.9 (2.9) |
| Recommendations | ||
| 1+ recommendation(s), n (%) | 60 (93) | 64 (100) |
| 1+ potentially inappropriate medications, n (%) | 34 (53) | 16 (50) |
| Overtreatment of DM, n (%) | 28 (44) | 11 (34) |
| Overtreatment of HTN, n (%) | 49 (77) | 26 (81) |
| Incorrect dosing for renal function, n (%) | 11 (17) | 5 (16) |
| Low adherence and/or cognitive impairment, n (%) | 20 (31) | 15 (47) |
TRIM found potential problems with virtually all medication regimens (Table 2). The feedback report for 98% of intervention and 97% of control participants noted discrepancies in the medications they reported taking at home and the medications in their record. The clinician feedback report included at least one recommendation for 93% of intervention and 100% of control participants to discontinue or decrease a potentially inappropriate medication and/or to simplify the medication regimen. Approximately one-half of participants had one or more potentially inappropriate medications as identified using Beers and STOPP criteria, > 75% had potential overtreatment of HTN, and > 30% had potential overtreatment of DM. Over 30% also reported low adherence and/or had cognitive impairment, as indicators of the need to simplify the regimen.
Table 2.
Bivariate associations of intervention with patient-centered care, communication, and medication changes
| Outcome | Intervention N=64a | Control N=64b | p-value |
|---|---|---|---|
| Patient Outcomes | |||
| PACIC >10, % | 29.7 | 15.6 | .057 |
| Active participation, meana | 5.6 | 2.7 | .0011 |
| Medication-related communication, meana | 7.5 | 3.6 | .0003 |
| Number of medications at 90 days, mean | 13.3 | 13.8 | .65 |
| Proportion of medication reconciliation errors corrected, %b | 48.4 | 14.3 | <.001 |
| 1+ TRIM recommendations implemented, %b | 29.7 | 21.9 | .42 |
| Clinician Outcomes | |||
| Facilitative communication, meana | 1.53 | 0.67 | .023 |
| Medication-related communication, (mean)a | 7.3 | 4.6 | .0025 |
| Recommendation(s), %a | 63.6 | 32.8 | .0008 |
Because 9 audiofiles were of insufficient quality for analysis, N=55 for outcomes of active participation, medication-related communication, and facilitative communication.
Because only one-half of the control group had medication appropriateness examined by TRIM, N=32 for outcomes of proportion of medication reconciliation errors corrected and 1+ TRIM recommendations implemented.
In bivariate analysis, a greater proportion of patients who received TRIM than control patients reported a PACIC score of 11 or 12, but this difference was nonsignficant (29.7% versus 15.6%, p=.057). (Table 3). In sensitivity analysis, using a cut-point for the PACIC of ≥ 10 versus < 10, 54.6% of intervention patients compared to 34.4% of control patients reported a high PACIC score, p = .02. In adjusted analysis, controlling for clustering and potential confounders, participants who received TRIM were 2.8 times more likely to report a score of 11 or 12 than control participants, p=.10.
Table 3.
Association of intervention with patient-centered care and communication, adjusted for covariatesa and accounting for clustering of patients by clinician
| Outcome | Odds ratiob | 95% Confidence Interval | P-value |
|---|---|---|---|
| PACIC >10 | 2.73 | 0.82, 9.08 | 0.1018 |
| 1+ clinician recommendation(s) | 3.33 | 1.37, 8.04 | 0.0076 |
| Intervention parameterc | Standard error | P-value | |
| Patient active participation | 2.89 | 0.78 | 0.0003 |
| Patient medication communication | 3.68 | 0.92 | 0.0001 |
| Clinician facilitative communication | 0.89 | 0.30 | 0.0036 |
| Clinician medication communication | 2.45 | 1.11 | 0.0288 |
| Association of intervention with communication, controlling for communication of other member of the clinician-patient dyad | |||
| Outcome | Intervention parameterc | Standard error | P-value |
| Patient active participation | 1.48 | 0.65 | 0.0257 |
| Patient medication communication | 2.58 | 0.79 | 0.0014 |
| Clinician facilitative communication | 0.22 | 0.26 | 0.3872 |
| Clinician medication communication | 0.01 | 1.01 | 0.9923 |
All models included the covariates of age, marital status, income, education, employment, self-rated health, and count of chronic conditions. Models examining patient active participation, also included quality of life, and models examining patient and clinician medication communication included quality of life and race/ethnicity.
Obtained from GEE models
Obtained from mixed models
There was significantly more active participation and medication-related communication among patients who received TRIM compared to controls. The clinicians of patients who received TRIM demonstrated significantly more facilitative and medication-related communication than clinicians of control patients, and a significantly larger proportion made a medication-related recommendation (Table 3). These differences were maintained after adjustment for clustering and potential confounding (Table 4). There were no differences between intervention and control patients in the number of medications prescribed at ninety days or in the number of TRIM-related recommendations implemented, although over three times as many patients who received TRIM had correction of medication reconciliation errors as those who did not (48.4% versus 14.3%, p < .001) (Table 3).
When facilitative clinician communication was included in the model, the relationship between receipt of TRIM and active patient communication remained significant (p<0.03). In contrast, when active patient communication was included in the model, the relationship between receipt of TRIM and clinician facilitative communication was no longer significant (p=0.39). This same pattern held true for patient and clinician medication-related communication (Table 4).
DISCUSSION
In this randomized controlled trial conducted among older Veterans prescribed 7 or more medications, including for DM and HTN, the use of TRIM significantly improved medication-related communication and was associated with a nonsignificant increase in the proportion of Veterans providing the highest ratings of patient-centered care most relevant to medication management. Its use also significantly improved the accuracy of the medication list. There was no association between the use of TRIM and medication prescribing; however, the small sample size did not provide adequate power to examine this outcome.
Appropriate prescribing in older patients requires a process of shared decision-making. The results of this study suggest that TRIM was successful in promoting this process. Because there is no medication-specific tool for shared decision-making, we adapted the PACIC, a general measure of patient involvement in the care of chronic conditions, using the subscales most relevant to medications. These subscales nonetheless referred to behaviors and qualities of all aspects of the care the patient received. Therefore, while the difference did not reach significance, the finding that a larger proportion of participants who received TRIM reported that these behaviors had occurred suggests that TRIM had an effect on the process of care.
The use of TRIM was more definitively associated with medication-related communication among both patients and their clinicians. When controlling for clinician facilitative communication, TRIM remained associated with more active patient participation, but TRIM was no longer associated with clinician communication after controlling for patient active communication. This finding suggests that TRIM had a direct effect on patients but an indirect effect on clinicians, with the patients’ active communication style promoting a more participatory communication style among their clinicians. This was an unexpected finding, given that the clinicians received more extensive and detailed feedback than did patients. However, patients frequently have medication-related symptoms19 and other concerns about medications20 that they do not discuss with their physicians and desire more information about medications,21 suggesting that only modest prompting is necessary to encourage patient communication.
The lack of a direct effect on clinician communication may help to explain the finding that the use of TRIM was not associated with changes in the patients’ medication regimens. While clinicians in the intervention group responded to patients’ questions and concerns about their medications, they were no more likely than those in the control group to implement the specific recommendations provided in the TRIM feedback. One study of blood pressure management among Veterans with DM demonstrated that facilities with higher rates of achieving the threshold measure of <140/90 also had higher rates of potential overtreatment of HTN.22 This study highlighted the emphasis placed on performance improvement over the past decade without concomitant recognition of the potential for harm associated with overtreatment. These performance improvement efforts were necessary to overcome the phenomenon of clinical inertia.23 It is likely that clinical inertia is also relevant to de-intensifying therapy and/or deprescribing. In one trial to reduce inappropriate prescribing, a CDSS provided primary care physicians with alerts about potential problems with prescribing. While the CDSS reduced the number of new potentially inappropriate prescriptions it did not have an effect on the discontinuation of pre-existing inappropriate medications.24 Patients with multiple chronic conditions can have many concerns for the clinician to address in a brief clinical encounter, and deprescribing may not be a priority. While TRIM was designed to provide feedback about medications without the use of expensive clinical resources, it may not be possible to overcome inertia and other barriers to improved medication prescribing without more intensive interventions such as academic detailing6,25 or interdisciplinary teams with the inclusion of pharmacist care.
The study lacked sufficient power for the outcome of deprescribing. A total of 224 participants would have been required to demonstrate a difference in two medications between the intervention and control groups. Only one-half of the control patients could be used in the analysis examining the number of TRIM recommendations that were implemented. Therefore, for the medication outcomes, this needs to be considered a pilot study, and a larger study will be necessary for more definite results regarding the effect of TRIM on prescribing.
In conclusion, the use of TRIM, an EHR-linked CDSS with supplementary patient assessment that delivers feedback regarding potentially inappropriate medications and medical regimens to primary care patients and their clinicians, improved shared decision-making and reduced medication reconciliation errors but did not change prescribing. The challenges of accomplishing deprescribing may require more intensive interactions with clinicians.
Acknowledgments
Sponsor’s role:
The funding organizations had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of the paper.
This material is the result of work supported with resources and the use of facilities at the VA Connecticut Healthcare System.
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
This work was supported by grant DF11-303 from the Donaghue Foundation and by the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (#P30AG21342 NIH/NIA), and by NIH Grant UL1 RR024139.
Footnotes
Authors contributions:
TRF: Conception and design; obtaining funding; acquisition of data, analysis, and interpretation of data; drafting of the manuscript. KMN: Acquisition of subjects and data, analysis, and interpretation of data; critical revision of the manuscript; RLS: Analysis and interpretation of data; critical revision of the manuscript. PAC, NR, PLM: Acquisition and interpretation of data; critical revision of the manuscript; technical support in design of intervention. MKG: Conception and design; analysis and interpretation of data; critical revision of the manuscript; JRO, BTF: Analysis and interpretation of data; critical revision of the manuscript.
Conflict of interest:
The authors have no conflicts of interest to disclose.
References
- 1.Viktil KK, Blix HS, Moger TA, et al. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol. 2007;63:187–95. doi: 10.1111/j.1365-2125.2006.02744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried TR, Niehoff K, Tjia J, et al. A Delphi process to address medication appropriateness for older persons with multiple chronic conditions. BMC Geriatrics. 2016;16:1–8. doi: 10.1186/s12877-016-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medicare Payment Advisory Commission (US) Report to the Congress: Medicare and the Health Care Delivery System. Medicare Payment Advisory Commission; 2015. [Google Scholar]
- 4.Fried TR, O’Leary J, Towle V, et al. Health outcomes associated with polypharmacy in community-dwelling older adults: A systematic review. J Am Geriatr Soc. 2014;62:2261–72. doi: 10.1111/jgs.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman MA, Seth Landefeld C, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516–23. doi: 10.1111/j.1532-5415.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 6.Gnjidic D, Le Couteur DG, Kouladjian L, et al. Deprescribing trials: Methods to reduce polypharmacy and the impact on prescribing and clinical outcomes. Clin Geriatr Med. 2012;28:237–53. doi: 10.1016/j.cger.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Clyne B, Bradley MC, Hughes C, et al. Electronic prescribing and other forms of technology to reduce inappropriate medication use and polypharmacy in older people: A review of current evidence. Clin Geriatr Med. 2012;28:301–22. doi: 10.1016/j.cger.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Niehoff KM, Rajeevan N, Charpentier PA, et al. Development of the Tool to Reduce Inappropriate Medications (TRIM): A clinical decision support system to improve medication prescribing for older adults. Pharmacotherapy. 2016;36:694–701. doi: 10.1002/phar.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried TR, Tinetti ME, Towle V, et al. Effects of benefits and harms on older persons’ willingness to take medication for primary cardiovascular prevention. Arch Intern Med. 2011;171:923–8. doi: 10.1001/archinternmed.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jansen J, Naganathan V, Carter SM, et al. Too much medicine in older people? Deprescribing through shared decision making. BMJ. 2016;353 doi: 10.1136/bmj.i2893. [DOI] [PubMed] [Google Scholar]
- 11.Glasgow RE, Wagner EH, Schaefer J, et al. Development and validation of the patient assessment of chronic illness care (PACIC) Med Care. 2005;43:436–44. doi: 10.1097/01.mlr.0000160375.47920.8c. [DOI] [PubMed] [Google Scholar]
- 12.Street RL, Jr, Millay B. Analyzing patient participation in medical encounters. Health Communication. 2001;13:61–73. doi: 10.1207/S15327027HC1301_06. [DOI] [PubMed] [Google Scholar]
- 13.Street RL, Jr, Gordon HS, Ward MM, et al. Patient participation in medical consultations: why some patients are more involved than others. Med Care. 2005;43:960–9. doi: 10.1097/01.mlr.0000178172.40344.70. [DOI] [PubMed] [Google Scholar]
- 14.Street RL, Jr, Gordon H, Haidet P. Physicians’ communication and perceptions of patients: is it how they look, how they talk, or is it just the doctor? Soc Sci Med. 2007;65:586–98. doi: 10.1016/j.socscimed.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon HS, Street RL, Kelly PA, et al. Physician–patient communication following invasive procedures: an analysis of post-angiogram consultations. Soc Sci Med. 2005;61:1015–25. doi: 10.1016/j.socscimed.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Gordon HS, Street RL, Sharf BF, et al. Racial differences in doctors’ information-giving and patients’ participation. Cancer. 2006;107:1313–20. doi: 10.1002/cncr.22122. [DOI] [PubMed] [Google Scholar]
- 17.Street RL, Jr, Krupat E, Bell RA, et al. Beliefs about control in the physician-patient relationship: effect on communication in medical encounters. J Gen Intern Med. 2003;18:609–16. doi: 10.1046/j.1525-1497.2003.20749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zandbelt LC, Smets EM, Oort FJ, et al. Patient participation in the medical specialist encounter: does physicians’ patient-centred communication matter? Patient Educ Couns. 2007;65:396–406. doi: 10.1016/j.pec.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Weingart SN, Gandhi TK, Seger AC, et al. Patient-reported medication symptoms in primary care. Arch Intern Med. 2005;165:234–40. doi: 10.1001/archinte.165.2.234. [DOI] [PubMed] [Google Scholar]
- 20.Barry CA, Bradley CP, Britten N, et al. Patients’ unvoiced agendas in general practice consultations: qualitative study. BMJ. 2000;320:1246–50. doi: 10.1136/bmj.320.7244.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber N, Parsons J, Clifford S, et al. Patients’ problems with new medication for chronic conditions. Qual Saf Health Care. 2004;13:172–5. doi: 10.1136/qshc.2003.005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerr E, Lucatorto MA, Holleman R, et al. Monitoring performance for blood pressure management among patients with diabetes mellitus: Too much of a good thing? Arch Intern Med. 2012;172:938–45. doi: 10.1001/archinternmed.2012.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connor PJ, Sperl-Hillen JM, Johnson PE, Rush WA, Biltz G. Clinical inertia and outpatient medical errors. In: Henriksen K, Battles J, Marks E, DI L, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology) Rockville, MD: Agency for Healthcare Research and Quality (US); 2005. [PubMed] [Google Scholar]
- 24.Tamblyn R, Huang A, Perreault R, et al. The medical office of the 21st century (MOXXI): effectiveness of computerized decision-making support in reducing inappropriate prescribing in primary care. Can Med Assoc J. 2003;169:549–56. [PMC free article] [PubMed] [Google Scholar]
- 25.Majumdar SR, Soumerai SB. Why most interventions to improve physician prescribing do not seem to work. Can Med Assoc J. 2003;169:30–1. [PMC free article] [PubMed] [Google Scholar]


