Abstract
Aim
Little is known about how toll-like receptor 4 (TLR4) influences the renal microvasculature. We hypothesized that acute TLR4 stimulation with lipopolysaccharide (LPS) impairs afferent arteriole autoregulatory behaviour, partially through reactive oxygen species (ROS).
Methods
We assessed afferent arteriole autoregulatory behaviour after LPS treatment (1 mg kg−1; i.p.) using the in vitro blood perfused juxtamedullary nephron preparation. Autoregulatory behaviour was assessed by measuring diameter responses to step-wise changes in renal perfusion pressure. TLR4 expression was assessed by immunofluorescence, immunohistochemistry and western blot analysis in the renal cortex and vasculature.
Results
Baseline arteriole diameter at 100 mmHg averaged 15.2 ± 1.2 μm and 12.2 ± 1.0 μm for control and LPS groups (P<0.05), respectively. When perfusion pressure was increased in 15 mmHg increments from 65 to 170 mmHg, arteriole diameter in control kidneys decreased significantly to 69 ± 6% of baseline diameter. In the LPS treated group, arteriole diameter remained essentially unchanged (103 ± 9% of baseline), indicating impaired autoregulatory behaviour. Pre-treatment with anti-TLR4 antibody or the TLR4 antagonist, LPS-RS, preserved autoregulatory behaviour during LPS treatment. P2 receptor reactivity was normal in control and LPS treated rats. Pre-treatment with Losartan (angiotensin type 1 receptor blocker; (AT1) 2 mg kg−1; i.p.), increased baseline afferent arteriole diameter but did not preserve autoregulatory behaviour in LPS treated rats. Acute exposure to Tempol (10−3 mol L−1), a superoxide dismutase mimetic, restored pressure-mediated vasoconstriction in kidneys from LPS-treated rats.
Conclusion
These data demonstrate that TLR4 activation impairs afferent arteriole autoregulatory behaviour, partially through ROS, but independently of P2 and AT1 receptor activation.
Keywords: endotoxemia, inflammation, oxidative stress, renal microcirculation, TLR4
INTRODUCTION
Renal autoregulation is essential for maintaining stable renal blood flow (RBF) and glomerular capillary pressure over a wide range of arterial pressure. Autoregulation of renal hemodynamics is accomplished by precise and automatic pressure-mediated resistance adjustments of the preglomerular vasculature 1, 2. Loss of autoregulatory efficiency can lead to inappropriate transmission of arterial pressure downstream to the glomerular capillaries. Sustained increases in glomerular capillary pressure lead to renal pathologies such as glomerular sclerosis, chronic kidney disease (CKD) and renal failure 2–4.
Acute kidney injury (AKI) during endotoxemia is characterized by increased renal vascular resistance and reduced RBF 5. In septic patients, the increased plasma Ang II levels reportedly correlate with microvascular dysfunction 6. In rodent models, endotoxemia reduces RBF and glomerular filtration rate (GFR), while increasing preglomerular vascular resistance 5, 7–10. Boffa et al. demonstrated that lipopolysaccharide (LPS) treatment decreased mean arterial pressure, RBF, and GFR, while increasing renal vascular resistance. Notably, renal vascular sensitivity to angiotensin II (Ang II), norepinephrine (NE), and L-N-Nitroarginine methyl ester (L-NAME), was normal compared to control mice 5. Endotoxemic rats exhibited increased afferent arteriole resistance with no change in efferent arteriole resistance 8. Furthermore, acute administration of the angiotensin type 1 (AT1) receptor blocker candesartan, after LPS treatment, significantly increased RBF 11. These data suggest that the sensitivity of the renal vasculature to vasoactive agonists is relatively normal in endotoxemia such that the elevated plasma Ang II levels in endotoxemia leads to renal vasoconstriction and reduction of RBF and GFR.
Toll-like receptors (TLRs) belong to a family of innate immune receptors that recognize pathogen-associated molecular patterns (PAMPs), and/or damage-associated molecular patterns (DAMPs) 12, 13. There are currently eleven human, and thirteen murine TLRs 13. The TLR family is separated into two distinct categories; 1) those that localize to the cell membrane and 2) those that localize to intracellular endosomes. PAMPs and DAMPs can activate TLRs, which in turn stimulate the innate immune system 12, 13. TLRs can signal through the MyD88-dependent pathway, using MyD88 as an adapter protein, and stimulate production of pro-inflammatory cytokines 14. Toll-like receptor 4 (TLR4), can also utilize a MyD88-indpendent signalling pathway using TRIF as an adapter protein 14. TLRs are expressed on immune cells, (monocytes/macrophages, B and T lymphocytes, dendritic cells), smooth muscle and endothelial cells 15, 16. Within the kidney, glomeruli and tubular epithelial cells express TLR1, TLR2, TLR3, TLR4 and TLR6 17, 18. LPS, a component of gram negative bacteria, is a well-accepted ligand for TLR4 19. Studies indicate that TLR4 mediates endotoxin-induced acute renal failure through rapid release of tumour necrosis factor (TNF), leading to renal neutrophil infiltration and apoptosis 20. In MyD88−/− mice, TLR4 is unresponsive to LPS as measured by improved survival rate and reduced pro-inflammatory mediators 14, 21, 22. Dear et al. employed a more severe sepsis model and found that acute renal failure involved MyD88-dependent signalling 23. Furthermore, recent studies revealed that TLR4 can also be activated by non-infectious DAMPs, such as high mobility group box-1, fibrinogen, and heat shock proteins in acute and chronic inflammatory pathologies 12, 24–27. Finally, TLR4 directly correlates with inflammatory markers and renal disease severity, suggesting a pathogenic role for TLR4 in CKD patients 28.
Stimulation of TLR4 impairs vascular function in peripheral vascular beds 26, 29–31. In hypertensive models, anti-TLR4 antibody treatment prevents vascular dysfunction in aorta and mesenteric arteries by supressing TNF-α, IL-6, and MCP-1 and reactive oxygen species (ROS) production 26, 27. Other studies in rodent models showed that impaired vascular function involves TLR4-dependent NADPH-oxidase generation of ROS 27, 30. Selective TLR4 blockade supressed MyD88 expression, NF-κB translocation, and ROS production in mesenteric arteries from STZ-induced diabetic rats 32. Thus, TLR4 activation can induce vascular dysfunction by increased oxidative stress in inflammatory pathologies such as atherosclerosis, diabetes, hypertension, and metabolic syndrome.
There is growing evidence that TLR4 stimulation facilitates progression of AKI and CKD 33, 34. Immune system activation and inflammation exert important influences on renal microvascular function. Both acute and chronic renal inflammation impair afferent arteriole autoregulatory behaviour in pathological conditions 35–37. Renal autoregulatory behaviour was preserved in Ang II hypertensive rats treated with the anti-inflammatories, pentosan polysulfate or mycophenolate mofetil, despite persistent hypertension 36, 38. Sharma et al. showed that acute exposure to TGF-β impairs afferent arteriole autoregulatory behaviour, and this impairment was reversed by acute Tempol treatment. These data suggest that inflammatory processes and oxidative stress, contribute to impaired renal autoregulatory efficiency in pathological conditions.
This study was designed to test the hypothesis that acute TLR4 stimulation impairs afferent arteriole autoregulatory behaviour in part by stimulating oxidative stress. To assess this, we acutely stimulated TLR4 with a low dose of a TLR4 selective LPS and determined its effect on afferent arteriole autoregulatory behaviour and reactivity. We also determined whether, or not, inhibition of TLR4 actions with either anti-TLR4 antibody, or a competitive antagonist of TLR4, LPS-RS, preserved autoregulatory behaviour. For this purpose, we determined the functionality of voltage-gated calcium channels, α-adrenergic receptors, P2 receptors, and AT1 receptors in untreated and acute LPS treated rats. P2 receptors, specifically P2X1 are involved in efficient afferent arteriole autoregulatory behaviour 39. The physiological role of TLR4 activation on afferent arteriole reactivity and overall autoregulatory behaviour has not been investigated. The results indicate that acute TLR4 activation impairs afferent arteriole autoregulatory behaviour through mechanisms that involve Tempol-sensitive mediators of oxidative stress, but are independent of P2 and AT1 receptor activation.
RESULTS
Acute LPS treatment significantly increases renal TLR4 expression
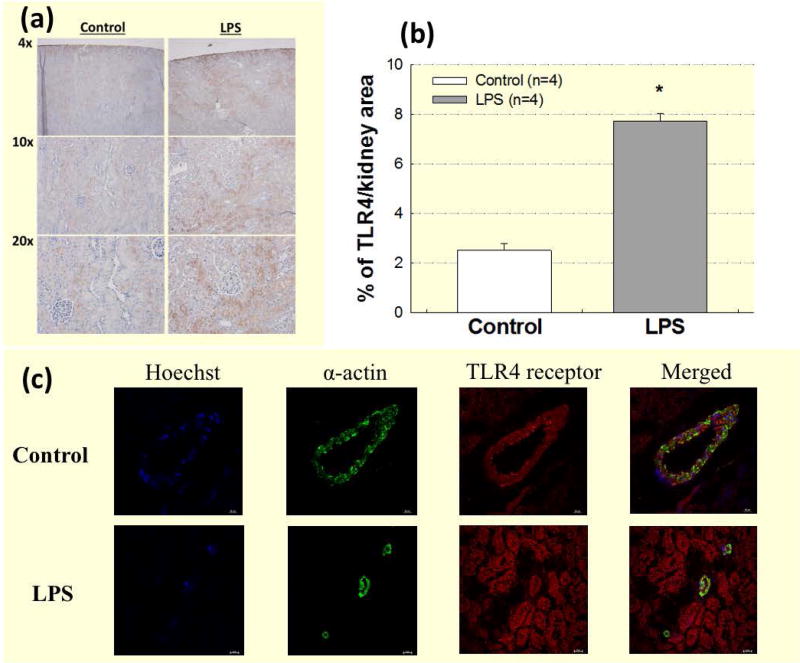
Renal TLR4 expression was assessed using DAB staining in whole kidney slices from control and LPS treated rats. In control kidneys, TLR4 was expressed in the cortex, with strong expression in the renal tubules (Fig. 1a, left panels). In the LPS group, TLR4 expression increased based on higher intensity brown staining (TLR4) compared to the control group (Fig. 1a, right panels). TLR4 expression increased significantly (Fig. 1b) in the LPS group (7.7 ± 0.3% of total kidney area; P<0.05 vs. control) compared to control kidneys (2.5 ± 0.3% of total kidney area).
Figure 1. TLR4 is expressed in renal vascular smooth muscle cells and LPS treatment increases renal TLR4 expression.
TLR4 is expressed in renal vascular smooth muscle cells, and LPS treatment increases renal TLR4 expression. (a): DAB (TLR4) staining of kidneys from control (left panels) and LPS (1 mg kg−1; i.p.; right panels) treated groups. (b): Data are expressed as a percentage of total kidney area stained for TLR4 from control (white bars) and LPS treatment (grey bars). Each bar represents the mean ± SE. n=4 per group. (c): Immunofluorescence staining of TLR4 in OCT frozen whole kidney sections. Representative images show nuclear staining (blue; Hoechst), vascular smooth muscle α-actin (green), TLR4 (red), and co-localization of TLR4 and vascular smooth α-actin (yellow). Images were taken at 40x magnification. Bar = 20 μm. * P<0.05 vs. control TLR4 expression.
TLR4 is expressed in renal vascular smooth muscle cells
To determine whether TLR4 protein is expressed in renal vascular smooth muscle cells, we looked for co-localization of immunofluorescence for TLR4 and α-actin in OCT frozen kidneys from control and acute 4-hour LPS treated rats using confocal immunofluorescence microscopy. TLR4 was strongly expressed in the renal tubules, and Figure 1c illustrates that TLR4 was also detected in the renal microvasculature by expression of TLR4 (red) in the same cells that expressed α-smooth muscle actin (green), in both the control (top panels) and LPS groups (bottom panels). These images reveal that TLR4 is expressed in renal vascular smooth muscle cells.
The effect of acute LPS treatment on SBP
Conscious baseline SBPs (n=12/group) were similar between control (136 ± 2 mmHg) and LPS (131 ± 1 mmHg) groups (Table 1). LPS treatment significantly decreased SBP within 60 minutes (113 ± 2 mmHg) and persisted throughout the 4-hour observation period.
Table 1.
The effect of acute LPS treatment on systolic blood pressure.
| Treatment Group |
Body Weight (g) |
Baseline SBP (mmHg) |
Hour 1 (mmHg) |
Hour 2 (mmHg) |
Hour 3 (mmHg) |
Hour 4 (mmHg) |
|---|---|---|---|---|---|---|
| Control | 372 ± 8 | 136 ± 2 | 131 ± 2 | 134 ± 2 | 134 ± 2 | 132 ± 2 |
| LPS | 365 ± 7 | 132 ± 1 | 113 ± 2* Ϯ | 102 ± 2* Ϯ | 103 ± 2* Ϯ | 109 ± 1* Ϯ |
| LPS | − | + | + | + | + |
P<0.05 vs. baseline SBP.
P<0.05 vs. control for same time point.
Acute TLR4 activation with LPS impaired afferent arteriole autoregulatory behaviour
Experiments were performed to assess the impact of TLR4 activation on afferent arteriole autoregulatory behaviour during acute LPS treatment (Fig. 2). Baseline diameters at 100 mmHg averaged 15.2 ± 1.2 and 12.2 ± 1.0 μm for control and LPS groups, respectively (P<0.05 vs. control; Fig. 2a). Reducing perfusion pressure from 100 to 65 mmHg increased afferent arteriole diameter to 112 ± 2% of baseline (P<0.05) in the control group (Fig. 2b), but to just 103 ± 9% of baseline in the LPS group (P<0.05 vs. control). When perfusion pressure was increased from 65 to 170 mmHg, in 15 mmHg increments, the control group exhibited stepwise reductions in diameter ultimately reaching 69 ± 6% of baseline at 170 mmHg (P<0.05). In contrast, arteriole diameter in the LPS treated group remained essentially unchanged at 170 mmHg, demonstrating impaired pressure-induced vasoconstriction. These data indicate that LPS treatment impairs afferent arteriole autoregulatory behaviour.
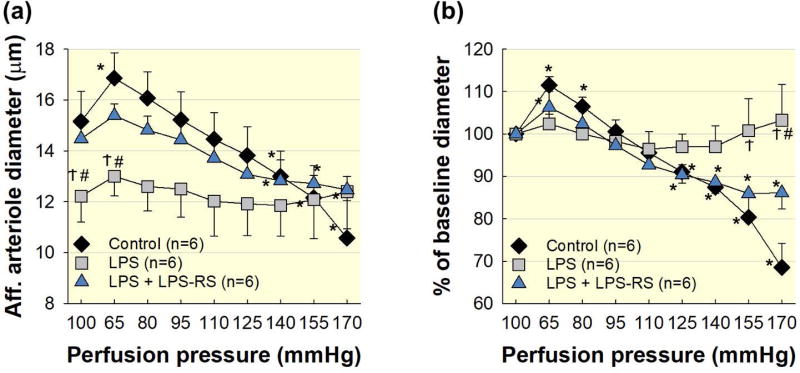
Figure 2. TLR4 blockade with LPS-RS preserves autoregulatory behaviour.
Co-treatment with the TLR4 antagonist, LPS-RS, preserves afferent arteriole autoregulatory behaviour in LPS treated kidneys. (a): Effect of renal perfusion pressure changes on afferent arteriole diameter in kidneys from control (black diamonds), LPS (1 mg kg−1; i.p.; grey squares), and LPS + LPS-RS (5 mg; i.p.; blue triangles) treated groups. (b): Data are expressed as a percent of the baseline diameter at 100 mmHg. Each data point represents the mean ± SE. n=6 per group. * P<0.05 vs. baseline diameter in the same group. Ϯ P<0.05 vs. control group for the same perfusion pressure. # P<0.05 vs. LPS-RS for the same perfusion pressure.
TLR4 blockade with LPS-RS preserved autoregulatory behaviour
Experiments were performed to assess the impact of TLR4 blockade on autoregulatory behaviour during acute LPS treatment. Rats were co-treated with LPS and a competitive TLR4 antagonist, LPS-RS, 4 hours prior to kidney harvesting. As shown in Figure 2a, baseline afferent arteriole diameter in the LPS + LPS-RS group at 100 mmHg averaged 14.5 ± 0.6 μm (P<0.05 LPS vs. LPS + LPS-RS). When perfusion pressure was decreased to 65 mmHg, arteriole diameter in the LPS + LPS-RS group increased to 106 ± 2% of baseline diameter (P<0.05, Fig. 2b). Increasing perfusion pressure from 65 to 170 mmHg, decreased arteriole diameter in the LPS + LPS-RS group to 86 ± 4% of the baseline diameter (P<0.05, LPS vs. LPS + LPS-RS), similar to the control group. These data demonstrate that TLR4 blockade preserves afferent arteriole autoregulatory behaviour during LPS treatment.
Pre-treatment with anti-TLR4 antibody preserved afferent arteriole autoregulatory behaviour during acute LPS treatment
Experiments were performed to assess the impact of TLR4 inhibition with anti-TLR4 antibody treatment on afferent arteriole autoregulatory behaviour during acute LPS treatment (Fig. 3). Rats received anti-TLR4 antibody treatment 12 hours prior to the bolus injection of LPS. Kidneys were harvested 4 hours later for evaluation of afferent arteriole autoregulatory behaviour. As shown in Figure 3a, baseline diameters averaged 15.6 ± 0.8 μm in the LPS + anti-TLR4 group. When perfusion pressure was reduced from 100 to 65 mmHg, afferent arteriole diameter in the LPS + anti-TLR4 group increased to 106 ± 3% of baseline diameter (Fig. 3b). Increasing perfusion pressure from 65 to 170 mmHg, the LPS + anti-TLR4 group demonstrated pressure-mediated vasoconstriction, virtually indistinguishable from control kidneys.
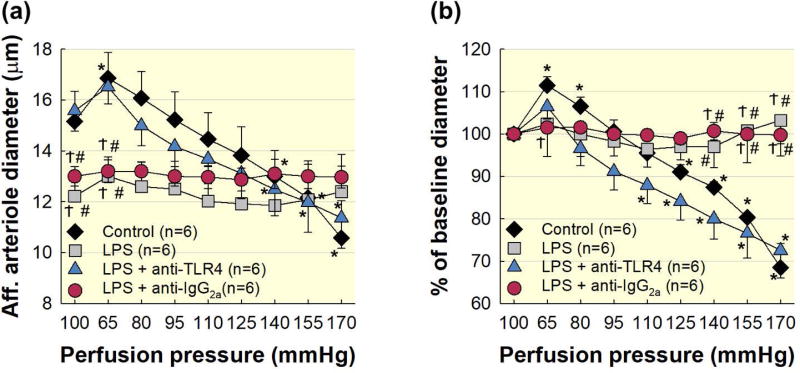
Figure 3. Pre-treatment with anti-TLR4 antibody preserved afferent arteriole autoregulatory behaviour during acute LPS treatment.
Pre-treatment with anti-TLR4 antibody preserves afferent arteriole autoregulatory behaviour in kidneys from LPS treated rats. (a): Effect of renal perfusion pressure changes on afferent arteriole in kidneys from control (black diamonds), LPS (1 mg kg−1; grey squares), LPS + anti-TLR4 antibody (1 μg; blue triangles), and LPS + anti-IgG2a antibody (red circles). (b): Data are expressed as a percent of the baseline diameter at 100 mmHg. Each data point represents the mean ± SE. n=6 per group. * P<0.05 vs. baseline diameter in the same group. Ϯ P<0.05 vs. control group for the same perfusion pressure. # P<0.05 vs. anti-TLR4 antibody for the same perfusion pressure.
To control for nonspecific antibody effects, we treated a separate group with LPS + IgG2a. Baseline diameter at 100 mmHg averaged 13.0 ± 0.4 μm (Fig. 3a) in the LPS + IgG2a group, which was similar to the LPS alone group but significantly smaller than the control or anti-TLR4 groups. When perfusion pressure was decreased to 65 mmHg, afferent arteriole diameter remained unchanged (Fig. 3b). Increasing perfusion pressure from 65 to 170 mmHg, produced no detectable change in diameter in the LPS + IgG2a group. These data demonstrate that anti-TLR4 antibody treatment preserves afferent arteriole autoregulatory behaviour despite a LPS challenge. Similar protection was not achieved with IgG2a indicating that the protective effect is TLR4 specific.
Afferent arteriole responses to KCl and NE were normal in acute LPS treated rats
Autoregulatory resistance adjustments require functional voltage-dependent calcium channels 40, 41. Since LPS treatment impaired autoregulatory behaviour, we assessed afferent arteriole responses to depolarization with KCl (55 mmol L−1), and α-adrenergic stimulation with NE (100 nmol L−1). Baseline arteriole diameters at 100 mmHg were similar between groups and averaged 15.9 ± 1.1 μm for control and 14.9 ± 1.2 μm in the LPS group. The response to KCl was nearly identical in both groups. Arteriole diameter decreased to 56 ± 4% of baseline diameter (P<0.05) in the control group and 56 ± 3% of baseline (P<0.05) in the LPS group.
Baseline diameters during assessment of NE (100 nmol L−1) averaged 11.9 ± 1.0 μm compared to the control group (16.3 ± 0.9 μm; P<0.05). Afferent arteriole responses to NE were essentially identical in the control (70 ± 2%) and LPS groups (72 ± 3%). Together, these data indicate that voltage-gated L-type calcium channels, and α-adrenergic receptors are functioning normally in rats receiving LPS treatment.
Afferent arteriole P2, P2X1, and P2Y2 receptor responses were normal during acute LPS treatment
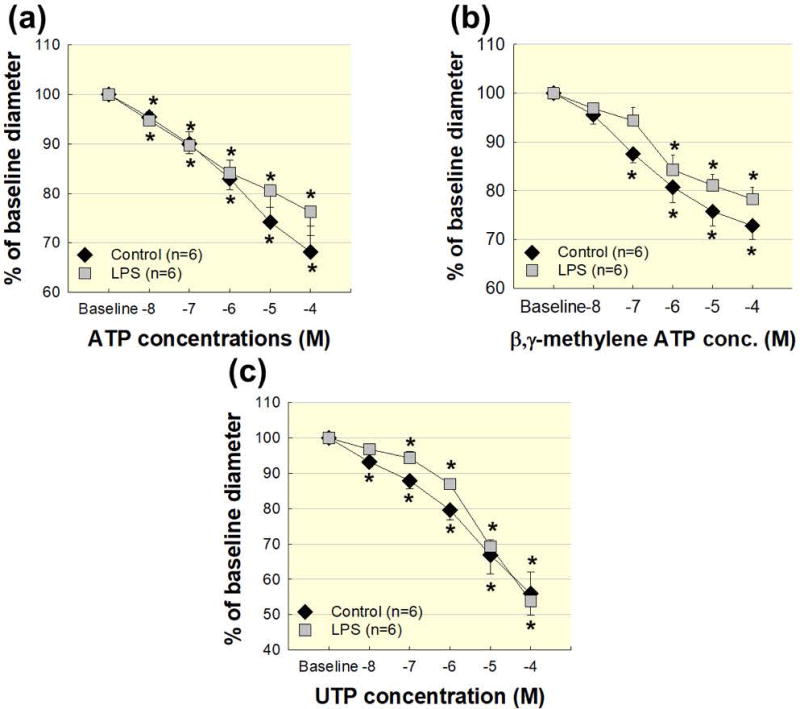
P2 receptors play an important role in afferent arteriole autoregulatory behaviour in normal conditions and in pathological settings, such as high salt diet and hypertension 42–45. Therefore, we examined afferent arteriole reactivity to P2, P2X1 and P2Y2 receptor activation using ATP, β,γ-methylene ATP (β,γ-mATP), and UTP, respectively. Figure 4a illustrates afferent arteriole reactivity to ATP (10−8–10−4 mol L−1). Baseline diameters at 100 mmHg averaged 15.1 ± 0.9 μm and 12.4 ± 0.8 μm in the control and LPS groups, respectively (P<0.05 vs. control). Increasing concentrations of ATP reduced arteriole diameter to 68 ± 5% (P<0.05 vs. baseline) and 76 ± 5% of baseline diameter (P<0.05 vs. baseline) in the control and LPS groups, respectively (Fig. 4a). The magnitude of the ATP-mediated responses were similar between the control and LPS groups, demonstrating that general P2 receptor function is not significantly changed in afferent arterioles of LPS treated rats.
Figure 4. Afferent arteriole reactivity to ATP, β,γ-mATP, and UTP are normal in acute LPS treated rats.
Afferent arteriole responses to ATP, β,γ-mATP, and UTP in acute LPS treated rats. (a): Afferent arteriole response to superfusion of ATP was assessed in control (black diamonds) and LPS (1 mg kg−1; i.p.; grey squares). (b): Afferent arteriole response to superfusion of β,γ-mATP was assessed in control (black diamonds) and LPS (grey squares). (c): Afferent arteriole response to superfusion of UTP was assessed in control (black diamonds) and LPS (grey squares). Data are expressed as a percent of the baseline diameter at 100 mmHg. Each data point represents the mean ± SE. n=6 per group. * P<0.05 vs. baseline diameter in the same group.
Inscho et al. reported that P2X1 receptor blockade or genetic deletion of P2X1 receptor (P2X1−/− mice) impairs afferent arteriole autoregulatory behaviour, indicating that P2X1 receptors are important for renal autoregulatory efficiency 39, 46, 47. P2X1 receptor function was assessed by determining arteriole responses to the selective P2X1 agonist, β,γ-mATP (10−8–10−4 mol L−1). β,γ-mATP evoked nearly identical concentration-dependent vasoconstriction in the control and LPS groups (Fig. 4b). The similarity of the responses to β,γ-mATP between the two groups, suggests that renal microvascular P2X1 receptor function is intact in LPS treated rats.
Afferent arterioles are also responsive to P2Y2 receptor stimulation with UTP 43, 48, so we determined the effect of LPS treatment on afferent arteriole reactivity to UTP (10−8–10−4 mol L−1). Baseline diameter at 100 mmHg was significantly smaller in the LPS group (P<0.05 vs. control). UTP decreased afferent arteriole diameter similarly in both the control and LPS groups indicating P2Y2 receptor function is not significantly altered by LPS treatment (Fig. 4c). Together with the KCl and NE findings, these data suggest that arterioles from the LPS treated group can vasoconstrict normally to some stimuli, but they do not respond appropriately to changes in perfusion pressure.
Effect of AT1 receptor blockade on SBP and impairment of autoregulatory behaviour in LPS treated rats
In the Losartan treated groups, baseline SBP’s were similar between control + Losartan (136 ± 1 mmHg) and LPS + Losartan (136 ± 1 mmHg) groups prior to Losartan and/or LPS treatment (Table 2). Losartan administration significantly decreased SBP in both groups in the first hour. In the control + Losartan group, SBP remained significantly lower over the 5-hour treatment period compared to baseline (Table 2). LPS treatment began after the first hour of Losartan treatment in the LPS + Losartan group. LPS treatment decreased SBP further (P<0.05 vs Losartan alone) in hour 2 (110 ± 1 mmHg; P<0.05), and this decrease was maintained throughout the combined Losartan + LPS treatment period (Table 2). These data indicate that AT1 receptor blockade decreases SBP in control and LPS treated rats and the effects of Losartan and LPS on SBP are additive.
Table 2.
The effect of Losartan treatment on systolic blood pressure in control and acute LPS treated rats
| Treatment group |
Body Weight (g) |
Baseline SBP (mmHg) |
Hour 1 (mmHg) |
Hour 2 (mmHg) |
Hour 3 (mmHg) |
Hour 4 (mmHg) |
Hour 5 (mmHg) |
|---|---|---|---|---|---|---|---|
| Control + Losartan | 348 ± 8 | 136 ± 1 | 124 ± 1* | 124 ± 1* | 127 ± 1* | 124 ± 1* | 124 ± 1* |
| LPS + Losartan | 356 ± 5 | 136 ± 1 | 126 ± 1* | 110 ± 1* Ϯ | 109 ± 1* Ϯ | 111 ± 1* Ϯ | 112 ± 1* Ϯ |
| Losartan | − | + | + | + | + | + | |
| LPS | − | − | + | + | + | + |
P<0.05 vs. baseline SBP.
P<0.05 vs. control + Losartan for the same time point.
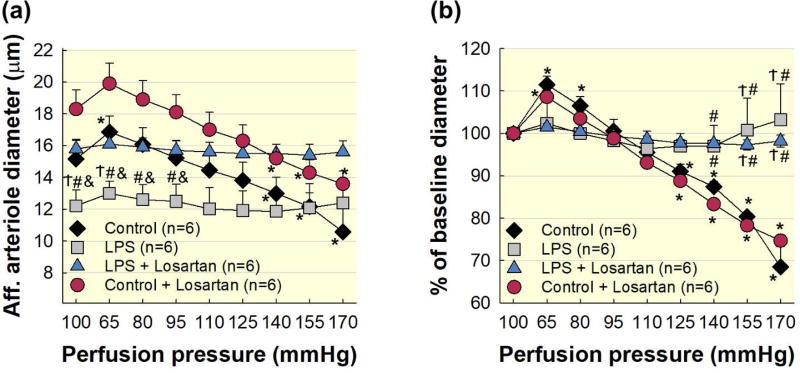
Plasma Ang II levels are increased in septic patients and correlate with microvascular dysfunction 6. Therefore, to control for the effects of Ang II on the renal microvasculature in our acute LPS treated model, we included two additional groups control + Losartan (2 mg kg−1; i.p.) and LPS + Losartan and assessed autoregulatory behaviour. Losartan treatment increased baseline diameter in kidneys from control + Losartan (18.3 ± 1.2 μm) and LPS + Losartan treated rats (15.8 ± 0.6 μm; P<0.05 vs. LPS; Fig. 5a). Reducing renal perfusion pressure from 100 to 65 mmHg increased arteriole diameter to 109 ± 1% of baseline diameter in the control + Losartan group (P<0.05), but the LPS + Losartan group remained essentially unchanged (Fig. 5b). When renal perfusion pressure was increased from 65 to 170 mmHg, the control + Losartan group exhibited pressure-mediated reductions in afferent arteriole diameter reaching 75 ± 2% of baseline at 170 mmHg (P<0.05; Fig. 5b). In contrast, arteriole diameter in the LPS + Losartan group remained essentially unchanged, reaching 98 ± 2% of baseline diameter at 170 mmHg (Fig. 5b). To verify AT1 receptor blockade, afferent arteriole diameter did not change when exposed to Ang II (10−9 mol L−1) in either the control + Losartan (98 ± 1% of baseline diameter) or the LPS + Losartan (98 ± 1% of baseline diameter) groups. These data demonstrate that AT1 receptor blockade decreases afferent arteriole vasoconstriction in LPS treated rats, but the impaired autoregulatory behaviour observed with this model of LPS treatment is independent of AT1 receptor activation.
Figure 5. Impairment of afferent arteriole autoregulatory behaviour is independent of AT1 receptor activation during acute LPS treatment.
(a): Effect of renal perfusion pressure changes on afferent arteriole diameter in control (black diamonds), LPS (1 mg kg−1; i.p.; grey squares), LPS + Losartan (2 mg kg−1; i.p.; blue triangles), and control + Losartan (red circles). (b): Data are expressed as a percent of the baseline diameter at 100 mmHg during the equilibration period. Control and LPS data are included from Figure 2 for reference. Each data point represents the mean ± SE. n=6 per group. * P<0.05 vs. baseline diameter in the same group. Ϯ P<0.05 vs. control for the same perfusion pressure. # P<0.05 vs. control + Losartan for the same perfusion pressure. & P<0.05 vs. LPS + Losartan for the same perfusion pressure.
Scavenging superoxide with Tempol restored afferent arteriole autoregulatory behaviour in LPS treated rats
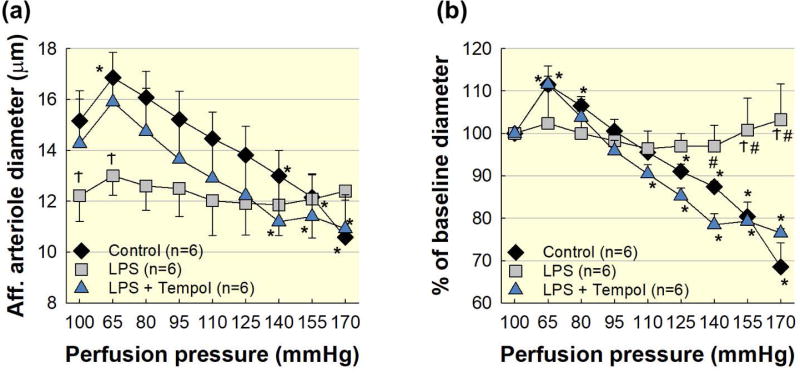
Increased ROS contribute to renal injury and impairment of renal autoregulatory behaviour in different models including normal kidneys, endotoxemia, high salt diet, and Ang II hypertension 11, 37, 49–53. Animals were treated with LPS 4 hours prior to experiments. Acute superfusion of Tempol, a superoxide dismutase mimetic, did not alter the baseline diameter in the LPS group (Fig. 6a). Under these conditions, arteriole diameter increased to 112 ± 4% of baseline in the LPS + Tempol group (P<0.05, LPS vs. LPS + Tempol; Fig. 6b) when perfusion pressure was decreased from 100 to 65 mmHg. Subsequent increases in perfusion pressure to 170 mmHg in the LPS + Tempol group demonstrated pressure-induced vasoconstriction similar to control kidney (P<0.05, LPS vs. LPS + Tempol; Fig. 6b). These data indicate that ROS contributes importantly to afferent arteriole autoregulatory impairment during LPS treatment.
Figure 6. Acute superfusion of Tempol restores afferent arteriole autoregulatory behaviour in LPS treated rats.
Acute superfusion of Tempol (10−3 mol L−1) restores afferent arteriole autoregulatory behaviour in LPS treated kidneys. Control and LPS groups are the same as shown in Figure 2. (a): Effect of renal perfusion pressure changes on afferent arteriole diameter in control (black diamonds), LPS (1 mg kg−1; i.p.; grey squares), and LPS + Tempol (blue triangles) kidneys. (b): Data are expressed as a percent of the baseline diameter at 100 mmHg. Each data point represents the mean ± SE. n=6 per group. * P<0.05 vs. baseline diameter in the same group. Ϯ P<0.05 vs. control group for the same perfusion pressure. # P<0.05 vs. Tempol for the same perfusion pressure.
Blockade of TLR4 during acute LPS treatment prevented upregulation of renal vascular TLR4 protein expression
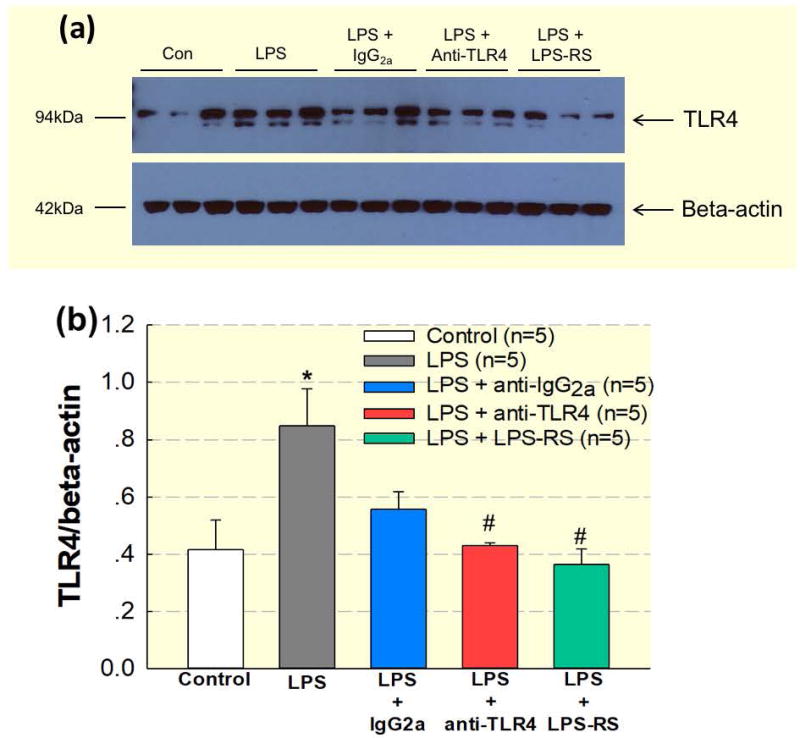
We examined TLR4 (≈100 kDa) protein expression in small intra-renal arteries by western blot analysis. Five groups (n=5/group) were prepared as described in the methods section, (control, LPS, LPS + IgG2a, LPS + anti-TLR4, and LPS + LPS-RS) and used for harvesting small intra-renal arteries. Figure 7 illustrates the ratio of renal vascular TLR4 protein expression over β-actin. In the LPS group, renal vascular TLR4 protein (0.84 ± 0.13) was significantly increased compared to control (0.41 ± 0.10; P<0.05). Antagonism of TLR4 with anti-TLR4 or LPS + LPS-RS prevented the increase of TLR4 protein in intra-renal arteries (0.43 ± 0.01 and 0.45 ± 0.16; P<0.05, respectively; Fig. 7b). Collectively, these data demonstrate that LPS increases renal vascular TLR4 protein expression and this increase can be prevented by inhibiting TLR4 activation during LPS treatment.
Figure 7. Blockade of TLR4 during LPS treatment prevents upregulation of renal vascular TLR4 protein expression.
Treatment with anti-TLR4 antibody (1 μg; i.p.) or LPS-RS (5 mg i.p.) decreases TLR4 protein expression in small intra-renal arteries in LPS (1 mg kg−1; i.p.) treated rats. (a): Representative image of typical gel. (b): Densitometry data normalized against β-actin. Each bar represents the mean ± SE. n=5 per group. * P<0.05 vs. control TLR4 expression. # P<0.05 vs. LPS TLR4 expression.
DISCUSSION
This study demonstrates that renal tubular and vascular smooth muscle cells express TLR4 in control and LPS treated animals and that tubular and vascular expression levels increase with acute LPS treatment. Both Losartan and/or LPS treatment significantly decrease SBP over the treatment period. Acute TLR4 activation with low-dose LPS treatment impairs afferent arteriole autoregulatory behaviour and this impairment is prevented by inhibition of TLR4 activation by either anti-TLR4 antibody or TLR4 blockade. Blockade of AT1 receptors with Losartan, relaxed afferent arterioles in control and LPS treated rats, but did not prevent autoregulatory impairment during LPS treatment. Scavenging superoxide with Tempol, a superoxide dismutase mimetic, restores autoregulatory behaviour in afferent arterioles from LPS treated rats. These novel findings suggest that acute TLR4 activation, with a low dose of LPS, impairs renal microvascular autoregulatory function partially through a ROS-dependent mechanism.
Autoregulation is an intrinsic property of afferent arterioles that allows regulation of stable RBF and glomerular capillary pressure over a wide range of arterial pressures 1, 2. Loss of renal autoregulatory efficiency can lead to inappropriate transmission of arterial pressure to the glomerular capillaries leading to glomerular sclerosis, CKD and renal failure. Renal autoregulatory behaviour is impaired in pathological conditions such as Ang II hypertension, high dietary salt, diabetes, DOCA-salt hypertension, and Dahl salt-sensitive hypertension 2, 4, 35, 36. In the present study, acute 4-hour LPS treatment impaired afferent arteriole autoregulatory behaviour, but vasoconstrictor responses to KCl, NE, and P2 receptor agonists were normal compared to control arterioles. This argues that the contractile apparatus is functional in LPS treated rats but the signalling pathways that transduce changes in transmural pressure into appropriate changes in afferent arteriolar resistance are impaired.
The kidney utilizes the myogenic (∼65%) and tubuloglomerular feedback (TGF; ∼35%) mechanisms to accomplish whole kidney autoregulation of RBF, though the existence of a third mechanism has also been proposed 2, 54, 55. Several studies determined the relative influence TGF plays on afferent arteriole autoregulatory behaviour 56–58. Moore and Casellas demonstrated that TGF-dependent resistance adjustments of afferent arterioles were greatest near (21 ± 2 μm, or last 4% of arteriole length) the glomerular attachment, and that it was diminished by ∼60% at a mid-afferent arteriole location (134 ± 6 μm from the glomeruli or last 27% of arteriole length) 56. In the present study, afferent arterioles studies averaged 420 ± 14 μm in total length and diameters were measured near the midpoint of arteriole length (239 ± 9 μm or a distance of ∼57% away from the glomeruli). Therefore, our measurements were made further away from the glomeruli than the Moore and Casellas report 56 at a location where the myogenic influence would exceed the contribution of TGF, but participation of TGF cannot be completely ruled out.
Growing evidence indicates that activation of the immune system, immune cells, and inflammation play an important role in renal vascular, tubular, and renal hemodynamic dysfunction 59–61. Renal inflammation contributes to reduced autoregulatory capability 35–37. A major mechanism by which inflammatory processes begin is by activation of pattern recognition receptors and the family of TLRs. TLRs are expressed in human renal tissue especially in glomerular capillaries and renal tubules 18. Tubular epithelia and mesangial cells express TLR1, TLR2, TLR3, TLR4, and TLR6 62, 63. TLR4 is found in the renal vasculature, glomeruli, and renal tubules 62. Recent studies show that renal TLR4 protein expression increases in models of AKI and CKD 34, 63, 64. For example, cortical TLR4 expression increases significantly 24 hours after cecal ligation and puncture, a murine model of sepsis 63. Kim et al. reported increases in mRNA and protein expression of TLR4 in renal tubules after ischemia-reperfusion injury 65. Although renal tubular TLR4 expression is upregulated in models of AKI, nothing is known about renal vascular TLR4 expression. We demonstrated that TLR4 is expressed in renal tubules by immunohistochemistry and immunofluorescence studies. TLR4 was also strongly expressed by renal vascular smooth muscle cells as shown by co-localization with α-smooth muscle actin, and expression levels increased following LPS treatment. These results demonstrate that TLR4 stimulation could enhance renal TLR4 expression initiating an immune response that contributes to reduced autoregulatory capability.
Evidence suggests that TLR4 contributes importantly to the progression of AKI and CKD 33, 66, 67, but the role of TLR4 on renal microvascular function is still unexplored. Dagher et al. showed that kidneys of TLR4−/− mice are protected against loss of renal function compared to WT controls in a model of AKI 68. Furthermore, Souza et al. demonstrated that TLR4 deficient mice are protected from renal fibrosis and albuminuria during 5/6 nephrectomy with Ang II infusion 34. Accordingly, we sought to determine the role of TLR4 in influencing renal microvascular function. Our studies revealed that inhibition of TLR4 with anti-TLR4 antibody, or competitive TLR4 antagonism with LPS-RS, preserved afferent arteriole autoregulatory behaviour during an LPS challenge. These data indicate that renal autoregulatory behaviour can be protected by prevention of TLR4 activation.
The mechanisms underlying the reduced blood pressure and increased renal vascular resistance during endotoxemia and sepsis are not completely understood. Studies have revealed the effects of endotoxemia and sepsis on the sympathetic nervous system 69, 70. Schaller et al. reported that endotoxin treated rats (lipopolysaccharide E. Coli 0111:B4; 2 mg rat−1; i.v.) exhibited a significant decrease in MAP compared to vehicle controls. Furthermore, plasma epinephrine, norepinephrine, renin activity, and vasopressin concentrations were significantly increased 90 minutes after endotoxin treatment, indicating an increase in sympathetic nervous system and renin angiotensin system activation during endotoxemia 69. In a severe LPS (20 mg kg−1; i.v; over 20 min) sepsis model, MAP decreased significantly (62 ± 7 mmHg), and was associated with elevated heart rate (+ 20 ± 6%) and renal sympathetic nerve activity (+ 455 ± 61%) three hours post LPS infusion 70. Boffa et al. demonstrated that some of the renal vasoconstriction observed in endotoxemic mice is mediated by thromboxane A2 receptors 9. Investigating whole kidney dynamic autoregulation using transfer function analyses, Nitescu et al. reported that endotoxemic rats (E. Coli 0127:B8; 7.5 mg kg−1; i.p.; for 16 hours) exhibited markedly reduced GFR and RBF and impaired dynamic autoregulation arising from reduced TGF function but that the myogenic component appeared intact. In our study, we used a lower LPS dose and a shorter incubation period but found markedly impaired autoregulatory behaviour in a region of the arteriole where autoregulatory responses are largely myogenic. The reason for this discrepancy with the work of Nitescu 11 is unclear but may relate to the type or dose of LPS used.
Nitescu 11, 71 also reported that inhibition of AT1 receptors with candesartan improved RBF and the TGF component of dynamic RBF autoregulation in the LPS rats, but did not normalize GFR 11, 71. These studies suggest that there is a mechanistic link between AT1 receptor activation and impaired renal microvascular dysfunction during endotoxemia. In the present study, we found that pre-treatment with Losartan, an AT1 receptor blocker, significantly decreased SBP and increased baseline afferent arteriole diameter in control and LPS treated rats, ameliorating the LPS-induced increase in afferent arteriole vascular resistance. Interestingly, the autoregulatory impairment in the LPS + Losartan group was nearly identical to that with LPS alone, indicating that AT1 receptor blockade did not restore autoregulatory capability. Prior studies from our laboratory have shown that Ang II (10−9 mol L−1) reduces afferent arteriole diameter by approximately 19% 37, 39, 72–76. In the present study, arterioles in Losartan treated kidneys were completely unresponsive to Ang II (10−9 mol L−1) confirming effective AT1 receptor blockade. Therefore, these data demonstrate that AT1 receptor activation contributes to the increased afferent arteriole resistance during exposure to LPS, but does not contribute to autoregulatory dysfunction in LPS treated rats.
TLR4 activation is often associated with accumulation of ROS 32, 77. Studies support interaction of TLR4 with NAD(P)H oxidase 4 in human embryonic kidney cells (HEK293T cells), in human aortic vascular smooth muscle cells, and in human mesangial cells 78–80. Thus, there appears to be a close mechanistic relationship between TLR4 activation, NADPH oxidase activation and ROS generation. We found that acute exposure to Tempol restored pressure-mediated vasoconstriction in afferent arterioles from LPS treated rats that typically exhibit marked autoregulatory impairment, whereas, it had no detectable effect on autoregulation in normal kidneys 37. These data suggest that accumulation of ROS, possibly superoxide, plays an important role in afferent arteriole autoregulatory impairment in the rat. Recent studies using isolated murine afferent arterioles by Lai et al. showed that increasing perfusion pressure led to increased superoxide production and enhanced myogenic reactivity, while treatment with Tempol, pegylated-SOD, or apocynin, all decreased ROS production 51. The apparent discrepancy in the role of ROS in autoregulatory signalling could be explained by the two different methods used to study afferent arterioles. Lai et al. conducted experiments in mouse isolated afferent arterioles, while this study employed Sprague Dawley rats and the juxtamedullary nephron preparation where the afferent arterioles are maintained in their microenvironment within the kidney 51. Alternatively, it could reflect differences in induction of oxidative signalling or the nature of the ROS induced. In any case, ROS play an important role in autoregulatory signalling but further work is needed for the specific mechanisms and conditions to be resolved.
P2 receptors play an important role in afferent arteriole autoregulatory behaviour in normal and pathological conditions, such as high salt diet and hypertension 36, 81, 82. P2 receptor function, specifically P2X1, is critical for appropriate afferent arteriole pressure-mediated resistance adjustments 39. In models of impaired afferent arteriole autoregulation, P2X1 receptor reactivity is also impaired 42, 43, 83. In the current study, we found that acute LPS treatment impairs afferent arteriole autoregulatory behaviour. Therefore, we hypothesized that P2X1 receptor reactivity would also be impaired in acute LPS treated rats. LPS treatment for 4 hours did not significantly alter afferent arteriole responses to P2 receptor activation. Even though P2 receptor reactivity was relatively normal, afferent arterioles from LPS treated rats still exhibited reduced autoregulatory capability. Impaired renal autoregulation with normal P2 receptor reactivity is unusual compared to what is observed with longer (14 days) term high salt and Ang II hypertensive settings and may reflect the duration of the “insult” imposed 36, 43. One study, reported normal afferent arteriole P2 receptor reactivity, with impaired autoregulatory behaviour during acute (∼15–20 min) topical exposure to TGF-β 37. Taken together, a potential explanation for the apparent disassociation between impaired autoregulation and P2 reactivity is that the 4-hour LPS exposure or short term exposure of TGF-β is too short to significantly impact P2 receptor trafficking and signalling. Another possible explanation is that during septic conditions where TLR4 would be stimulated, electron transport is compromised leading to a decrease in cellular ATP production 84, 85. In that setting, the reduced autoregulatory performance observed could reflect a decrease in ATP available for extracellular release and stimulation of renal microvascular P2 receptors. This reduced ATP release could blunt autoregulatory responsiveness.
In conclusion, this study provides novel evidence that acute stimulation of TLR4 causes afferent arteriole autoregulatory dysfunction, in part through a ROS-dependent mechanism, but independent of P2 and AT1 receptors. Inhibition of TLR4 signalling with anti-TLR4 antibody or LPS-RS, preserves renal autoregulatory behaviour. Overall, inhibition of TLR4 signalling and scavenging of ROS, during LPS treatment, confers significant protection of renal autoregulatory control. More work is needed to determine the mechanistic link between activation of TLR4, ROS accumulation, and impairment of renal microvascular function in renal pathologies.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (n=217; Charles River Laboratories, Raleigh, NC) weighing 345–420g were housed in institutional animal facilities in a 12–12 light-controlled room, with ad libitum access to drinking water and standard chow (PMI diet #: 5JL5; Lab Diets, St. Louis, Missouri, USA) All experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals using procedures approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Alabama at Birmingham and Georgia Regents University – currently named Augusta University.
Expression of renal vascular TLR4 by immunofluorescence
Rats were anesthetized with pentobarbital (50 mg kg−1; i.p.; Vortech Pharmaceuticals LTD, Dearborn, Michigan, USA) and the kidneys were perfused through the abdominal aorta. The kidneys were flushed with 5.2% bovine serum albumin (BSA) Tyrode’s solution. The kidneys were excised, decapsulated, and sectioned longitudinally, placed in optimized cutting temperature (OCT) gel and frozen over dry ice. OCT frozen kidneys were stored at −80°C. Briefly, kidney sections (6 μm) were cut in a cryostat and mounted to positively charged microscope slides (Fisher Scientific), fixed with pre-cooled acetone, and then washed in 1x phosphate buffered saline (PBS). The kidney sections were incubated first with primary antibody for TLR4 (ab22048; 1:500; Abcam), followed by primary antibody for α-smooth muscle actin (1:1000) overnight at 4°C. The slides were rinsed with PBS and incubated with both the TSA-Cy3 donkey anti-mouse horse-radish peroxidase (HRP) (1:400; Invitrogen) and TSA-FITC (donkey anti-rabbit; 1:400; Invitrogen) secondary antibodies for 60 minutes and then Hoechst nuclear stain for 5 minutes at room temperature. Slides were quenched with 3% H2O2 (10 minutes), washed in 1x PBS, and blocked (PBS blocking buffer) in between each step. After a final rinse with PBS, sections were covered with 50% glycerol and coverslips were applied. Immunofluorescence images were captured using a Nikon A1R Confocal microscope with the Nikon Plan Flour 40x DIC N2 oil immersion objective. Images were analysed using the Nis Element 4.50 Imaging Software (Nikon) in cooperation with the UAB High Resolution Imaging Facility.
Systolic blood pressure (SBP) monitoring
Conscious SBP was measured on the kidney and blood donor rats using tail cuff plethysmography (IITC Life Science, Woodland Hills, CA) at time 0 and again every hour (1–4 hour), covering the 4-hour LPS treatment, until the kidneys and blood were harvested for study. In both Losartan treated groups (control + Losartan and LPS + Losartan), SBP was measured at time 0 and again every hour (1–5 hours), covering the Losartan and/or LPS treatment period.
In vitro blood-perfused juxtamedullary nephron preparation
Kidneys were prepared for blood perfused juxtamedullary nephron experiments as previously described 39, 86. Briefly, two identically treated rats were anesthetized with pentobarbital (50 mg kg−1;i.p; Vortech Pharmaceuticals LTD, Dearborn, Michigan, USA) for each experiment. The right kidney from the kidney donor rat was cannulated via the superior mesenteric artery and continuously perfused with a Tyrode’s solution containing 5.2% BSA (Calbiochem, La Jolla, CA).
Blood from both the kidney and blood donor rats was collected into heparinized syringes (500 IU) via a carotid artery catheter and processed by centrifugation. The plasma fraction was collected and erythrocytes were washed twice with saline. The plasma and washed erythrocytes were mixed to form a reconstituted blood perfusate (hematocrit ≈ 33%).
The perfused kidney was harvested and prepared for videomicroscopy 39, 86. After completion of the dissection, the perfused kidney was visualized under a light microscope (Nikon Optiphot2-UD; Nikon, Tokyo, Japan) fitted with a Zeiss water-immersion objective (40x) and superfused with Tyrode’s buffer containing 1% BSA at 37°C. The perfusate was switched from Tyrode’s solution containing 5.2% BSA to the reconstituted blood delivered from a pressurized reservoir continuously gassed with 95%O2/5%CO2. Perfusion pressure was monitored using a pressure cannula positioned near the tip of the perfusion cannula and connected to a pressure transducer (Model TRN005; Kent Scientific Corporation, Torrington, CT). The focused image of the inner cortical surface and afferent arteriole was displayed on a video monitor and recorded on DVD for later analysis. Afferent arterioles averaged 420 ± 14 μm in total length and were monitored at a single site (239 ± 9 μm from the glomeruli or approximately 57% of total arteriole length; pooled data; n=84). Diameters were measured every 12 seconds using an image shearing monitor (Model 908; Vista Electronics, Valencia, CA). The mean arteriole diameter was calculated using all the diameter measurements obtained in the final 2 minutes of each treatment or pressure period.
Experimental protocols
After an equilibration period (10–15 minutes) with perfusion pressure held at 100 mmHg, the following experimental protocols were performed. Each protocol began with a 5-minute control period at 100 mmHg to establish a baseline afferent arteriole diameter.
Experiment 1: Effect of LPS on afferent arteriole autoregulatory behaviour
Eight groups were studied (n=6/group): control, LPS (1 mg kg−1; Lipopolysaccharide E. coli 055:B5; Sigma Aldrich, St. Louis, MO, USA), LPS (1 mg kg−1) + TLR4 antagonist, LPS-RS, (5 mg; Invivogen, San Diego, CA, USA) LPS (1 mg kg−1) + anti-TLR4 antibody (1 µg; sc-13591; Santa Cruz Biotechnology, Dallas, Texas, USA), LPS (1 mg kg−1) + IgG2a antibody (1 µg; sc-69917; Santa Cruz Biotechnology) and LPS (1 mg kg−1) + Tempol (10−3 mol L−1; Sigma Aldrich). The LPS group received an intraperitoneal injection of a low dose of LPS 4 hours prior to kidney harvesting. The LPS + LPS-RS group was co-treated (i.p.) with LPS and LPS-RS 4 hours prior to experiments. The LPS + anti-TLR4 antibody group received the antibody treatment (i.p.) 12 hours prior to LPS dosing and the kidney was collected 4 hours after LPS treatment. The LPS + Tempol group received LPS (i.p.) 4 hours prior to experiments, and Tempol (10−3 mol L−1) was added to the Tyrode’s buffer superfusion solution approximately 15 minutes before assessment of autoregulatory capability.
In cases of endotoxemia or sepsis, Ang II levels reportedly increase in concert with the hypotensive decline in blood pressure 6, 69, 87. To control for Ang II effects, we prepared two additional groups (control + Losartan (2 mg kg−1; i.p.; Sigma Aldrich), and LPS (1 mg kg−1; i.p.) + Losartan (2 mg kg−1; i.p.) to compare with those above. The LPS + Losartan group received Losartan 1 hour prior to LPS treatment and the kidney was collected 4 hours after the LPS. Losartan (10 μmol L−1) was added to the 5.2% BSA perfusion solution, 1% BSA superfusion solution, and the reconstituted blood perfusate. At the end of the autoregulatory protocol, Ang II (10−9 mol L−1) was applied to the inner cortical surface to verify effective AT1 receptor blockade.
Autoregulatory behaviour of afferent arterioles was assessed by measuring changes in lumenal diameter in response to step-wise changes in perfusion pressure. Autoregulatory behaviour was assessed by reducing perfusion pressure from 100 to 65 mmHg, and then increasing perfusion pressure from 65 to 170 mmHg in 15 mmHg increments at 5 minute intervals.
Experiment 2: Afferent arteriole responses to potassium chloride (KCl) and NE
Voltage dependent calcium channels are essential for efficient autoregulation 41. Therefore, the effect of LPS on the response to KCl was studied using afferent arterioles from control and LPS (1 mg kg−1; i.p.) treated rats (n=4). Baseline afferent arteriole diameters were measured at 100 mmHg before the superfusion solution was changed to a similar solution containing 55 mmol L−1 KCl (substituted for NaCl). After 5 minutes of KCl exposure the superfusion solution was returned to 1% BSA for a 5-minute recovery period. Lumenal diameters were continuously monitored before, during, and after KCl treatment.
Adrenergic influences are important regulators of renal vascular resistance 75. Accordingly, the effect of LPS on the response to NE, was studied in a separate group of (n=4) control and LPS (1 mg kg−1; i.p.) treated kidneys. Following a 5-minute control period, arterioles were exposed to NE (100 nmol L−1; Sigma Aldrich) for 5 minutes with renal perfusion pressure maintained at 100 mmHg. Five minutes later, the superfusion solution was returned to 1% BSA for a 5-minute recovery period.
Experiment 3: Afferent arteriole response to endogenous P2 receptor ligands
P2 receptors have been implicated in mediating autoregulatory changes in afferent arteriole diameter 42, 45. Afferent arteriole concentration-response relationships to adenosine triphosphate (ATP; P2 agonist; Sigma Aldrich; n=6) beta-gamma-methylene ATP (β,γ-mATP; Selective P2X agonist; Sigma Aldrich; n=6), and uridine triphosphate (UTP; P2Y2 agonist; Sigma Aldrich; n=6) were determined to establish P2 receptor reactivity in afferent arterioles from control and LPS treated kidneys 88. Arterioles were exposed to ATP, β,γ-mATP, or UTP at concentrations of 10−8 – 10−4 mol L−1 while perfusion pressure set at 100 mmHg.
Expression of TLR4 in intra-renal arteries by western blot
Five groups were studied (n=5/group): control, LPS (1 mg kg−1; i.p.), LPS + IgG2a (1 µg; i.p.), LPS + anti-TLR4 antibody (1 µg; i.p.), and LPS + LPS-RS (5 mg; i.p.). Rats were anesthetized with pentobarbital (50 mg kg−1; i.p.) and both kidneys were perfused through the aorta with 5.2% BSA Tyrode’s solution. The kidneys were removed, sectioned longitudinally, and placed in ice-cold physiologic salt solution (PSS). Intra-renal arteries were dissected from the inner cortex using a stereo microscope, snap frozen in liquid nitrogen, and stored at −80°C for protein isolation. The tissue was homogenized in RIPA lysis buffer with a protease inhibitor mini complete tablet (Roche Diagnostics, Indianapolis, IN USA) and 1mM phenylmethylsulfonyl fluoride (PMSF). The lysis buffer solution was centrifuged at 20,000xg for 12 minutes at 4°C and supernatant was aliquoted and stored at −80°C. The protein concentration was determined by the Bio-Rad protein assay (Bio-Rad Laboratories Hercules, CA). Equal amounts of protein for each sample were denatured in SDS sample buffer at 95–100°C for 5 minutes, separated by electrophoresis on Bolt™ 4–12% Bis-Tris gels (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membranes, (Amersham™ Hybond ECL GE Healthcare Life Science). Membranes were blocked in 5% nonfat dry milk in TBS-T 0.1% Tween 20 at room temperature for 60 minutes. Membranes were incubated for 16 hours with mouse anti-TLR-4 primary antibody (ab22048; 1:5000; Abcam) at 4°C. The blots were washed and incubated again with goat anti-mouse (1:5000; Cell Signalling, Danvers, MA) secondary antibody conjugated with horseradish peroxidase at room temperature for 60 minutes. The membrane was washed and visualized by enhanced chemiluminescence (Clarity Western ECL substrate), and images were developed after exposure to X-ray film. The same blots were stripped, washed and re-probed with monoclonal anti-β-actin (1:10,000, Sigma Aldrich) as a loading control.
Expression of TLR4 by immunohistochemistry
Rats were anesthetized with pentobarbital (50 mg kg−1; i.p.) and the kidneys were perfused through the abdominal aorta. The kidneys were flushed with 5.2% BSA Tyrode’s solution. The kidneys were excised, decapsulated, and fixed in 10% neutral buffered formalin for 24–48 hours at room temperature. The fixed kidneys were embedded in paraffin, cut into 5 μm sections and processed for immunohistochemistry. Briefly, the sections were deparaffinised in Citrisolv (Fisher Scientific), rehydrated, and placed in 10 mmol L−1 citrate buffer, and placed in a rice steamer for 30 minutes. After cooling (60 min.), sections were quenched in 3% H202 for 10 min and rinsed in PBS. The sections were blocked in PBS blocking buffer (0.1g BSA + 0.2% non-fat dry milk + 0.3 ml Triton X-100+100 ml 1X PBS) for 30 minutes at room temperature, and incubated with primary antibody for TLR4 (Abcam; ab22048; 1:2000) at 4°C in a humidity chamber overnight. Slides were washed in PBS and incubated with secondary antibody (SuperPicTure Polymer Detection; Invitrogen-879163) for 60 minutes at room temperature. 3,3′-Diaminobenzidine (DAB) was added to the sections and incubated for 6 minutes, rinsed in dH20, counterstained, and covered with Cytoseal60. Prepared kidney sections were evaluated for TLR4 staining using an Olympus BX43 microscope with an Olympus DP73 camera.
Statistical analysis
Data are expressed as means ± SEM. Within-group analysis was conducted using one-way ANOVA for repeated measures with post-hoc analysis using Dunnett’s multiple comparison test. Significant differences between groups were determined using one-way ANOVA and Dunnett’s multiple comparison test or Student’s t-test where appropriate. P values <0.05 were considered to indicate significant differences.
Physiological Relevance.
Immune system activation and inflammation are risk factors for multiple renal pathologies such as hypertension, high dietary salt, diabetes and ischemia-reperfusion injury. In these pathologies, damaged tissues can release damage-associated molecular patterns that in turn can activate TLR4. From this study, stimulation of TLR4 impairs autoregulatory efficiency that can lead to inappropriate transmission of arterial pressure downstream to the glomerulus. Activation of the immune system and inflammation, through tissue damage, could provide a mechanistic link between renal pathology and impaired kidney function. Therefore, resolving the mechanisms by which immune system activation and inflammation facilitate the loss of renal autoregulatory function could provide novel therapeutic targets for the prevention of or intervention in renal injury.
Acknowledgments
This study was supported by the American Heart Association (Pre-doctoral Fellowship Greater Southeast Affiliate: 14PRE20460061 to J.P.V.B.), the National Institutes of Health (Grants DK44628 and HL098135 to E.W.I.).
Histology was performed and analysed with support from Dr. Terry Lewis and the UAB Neuroscience Molecular Detection and Stereology Core (P30 NSO47466 to T.L.).
Confocal microscopy was performed and analysed with support from the UAB High Resolution Imaging Facility (HRIF) (P30 CA013148 and P30 AR048311).
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest in relation to the publication of the present study.
References
- 1.Navar LG. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol. 1978;234:F357–370. doi: 10.1152/ajprenal.1978.234.5.F357. [DOI] [PubMed] [Google Scholar]
- 2.Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95:405–511. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z, Woollard JR, Wang S, Korsmo MJ, Ebrahimi B, Grande JP, Textor SC, Lerman A, Lerman LO. Increased glomerular filtration rate in early metabolic syndrome is associated with renal adiposity and microvascular proliferation. Am J Physiol Renal Physiol. 2011;301:F1078–1087. doi: 10.1152/ajprenal.00333.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren Y, D’Ambrosio MA, Garvin JL, Peterson EL, Carretero OA. Mechanism of impaired afferent arteriole myogenic response in Dahl salt-sensitive rats: role of 20-HETE. Am J Physiol Renal Physiol. 2014;307:F533–538. doi: 10.1152/ajprenal.00283.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boffa JJ, Arendshorst WJ. Maintenance of renal vascular reactivity contributes to acute renal failure during endotoxemic shock. J Am Soc Nephrol. 2005;16:117–124. doi: 10.1681/ASN.2004060441. [DOI] [PubMed] [Google Scholar]
- 6.Doerschug KC, Delsing AS, Schmidt GA, Ashare A. Renin-angiotensin system activation correlates with microvascular dysfunction in a prospective cohort study of clinical sepsis. Crit Care. 2010;14:R24. doi: 10.1186/cc8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Jittikanont S, Falk SA, Li P, Feng L, Gengaro PE, Poole BD, Bowler RP, Day BJ, Crapo JD, Schrier RW. Interaction among nitric oxide reactive oxygen species, and antioxidants during endotoxemia-related acute renal failure. Am J Physiol Renal Physiol. 2003;284:F532–537. doi: 10.1152/ajprenal.00323.2002. [DOI] [PubMed] [Google Scholar]
- 8.Lugon JR, Boim MA, Ramos OL, Ajzen H, Schor N. Renal function and glomerular hemodynamics in male endotoxemic rats. Kidney Int. 1989;36:570–575. doi: 10.1038/ki.1989.232. [DOI] [PubMed] [Google Scholar]
- 9.Boffa JJ, Just A, Coffman TM, Arendshorst WJ. Thromboxane receptor mediates renal vasoconstriction and contributes to acute renal failure in endotoxemic mice. J Am Soc Nephrol. 2004;15:2358–2365. doi: 10.1097/01.ASN.0000136300.72480.86. [DOI] [PubMed] [Google Scholar]
- 10.Cryer HG, Bloom IT, Unger LS, Garrison RN. Factors affecting renal microvascular blood flow in rat hyperdynamic bacteremia. Am J Physiol. 1993;264:H1988–1997. doi: 10.1152/ajpheart.1993.264.6.H1988. [DOI] [PubMed] [Google Scholar]
- 11.Nitescu N, DiBona GF, Grimberg E, Guron G. Angiotensin II Type 1 Receptor Antagonism Attenuates Abnormalities in Dynamic Renal Blood Flow Autoregulation in Rats with Endotoxin-Induced Acute Kidney Injury. Kidney Blood Press Res. 2010;33:200–208. doi: 10.1159/000316705. [DOI] [PubMed] [Google Scholar]
- 12.Matzinger P. The danger model, a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 13.Matzinger P. and the extended family. Tolerance, danger, Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 14.Virtue A, Wang H, Yang XF. MicroRNAs and toll-like receptor/interleukin-1 receptor signaling. J Hematol Oncol. 2012;5:1–17. doi: 10.1186/1756-8722-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole JE, Georgiou E, Monaco C. The expression and functions of toll-like receptors in atherosclerosis. Mediators Inflamm. 2010;2010:1–18. doi: 10.1155/2010/393946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erridge C, Burdess A, Jackson AJ, Murray C, Riggio M, Lappin D, Milligan S, Spickett CM, Webb DJ. Vascular cell responsiveness to Toll-like receptor ligands in carotid atheroma. Eur J Clin Invest. 2008;38:713–720. doi: 10.1111/j.1365-2362.2008.02010.x. [DOI] [PubMed] [Google Scholar]
- 17.Anders HJ, Banas B, Schlondorff D. Signaling danger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol. 2004;15:854–867. doi: 10.1097/01.asn.0000121781.89599.16. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Gou SJ, Zhao MH, Chen M. The expression of Toll-like receptors 2, 4 and 9 in kidneys of patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Clin Exp Immunol. 2014;177:603–610. doi: 10.1111/cei.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham PN, Wang Y, Guo R, He G, Quigg RJ. Role of Toll-like receptor 4 in endotoxin-induced acute renal failure. J Immunol. 2004;172:2629–2635. doi: 10.4049/jimmunol.172.4.2629. [DOI] [PubMed] [Google Scholar]
- 21.Sato N, Takahashi N, Suda K, Nakamura M, Yamaki M, Ninomiya T, Kobayashi Y, Takada H, Shibata K, Yamamoto M, Takeda K, Akira S, Noguchi T, Udagawa N. MyD88 but not TRIF is essential for osteoclastogenesis induced by lipopolysaccharide diacyl lipopeptide, and IL-1alpha. J Exp Med. 2004;200:601–611. doi: 10.1084/jem.20040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dear JW, Yasuda H, Hu X, Hieny S, Yuen PS, Hewitt SM, Sher A, Star RA. Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int. 2006;69:832–836. doi: 10.1038/sj.ki.5000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goulopoulou S, McCarthy CG, Webb RC. Toll-like Receptors in the Vascular System: Sensing the Dangers Within. Pharmacol Rev. 2016;68:142–167. doi: 10.1124/pr.114.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy CG, Goulopoulou S, Wenceslau CF, Spitler K, Matsumoto T, Webb RC. Toll-like receptors and damage-associated molecular patterns: novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol. 2014;306:H184–196. doi: 10.1152/ajpheart.00328.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Batista PR, Palacios R, Martin A, Hernanz R, Medici CT, Silva MA, Rossi EM, Aguado A, Vassallo DV, Salaices M, Alonso MJ. Toll-like receptor 4 upregulation by angiotensin II contributes to hypertension and vascular dysfunction through reactive oxygen species production. PLoS One. 2014;9:e104020. doi: 10.1371/journal.pone.0104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernanz R, Martinez-Revelles S, Palacios R, Martin A, Cachofeiro V, Aguado A, Garcia-Redondo L, Barrus MT, de Batista PR, Briones AM, Salaices M, Alonso MJ. Toll-like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II-induced hypertension. Br J Pharmacol. 2015;172:3159–3176. doi: 10.1111/bph.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lepenies J, Eardley KS, Kienitz T, Hewison M, Ihl T, Stewart PM, Cockwell P, Quinkler M. Renal TLR4 mRNA expression correlates with inflammatory marker MCP-1 and profibrotic molecule TGF-beta(1) in patients with chronic kidney disease. Nephron Clin Pract. 2011;119:c97–c104. doi: 10.1159/000324765. [DOI] [PubMed] [Google Scholar]
- 29.Sollinger D, Eissler R, Lorenz S, Strand S, Chmielewski S, Aoqui C, Schmaderer C, Bluyssen H, Zicha J, Witzke O, Scherer E, Lutz J, Heemann U, Baumann M. Damage-associated molecular pattern activated Toll-like receptor 4 signalling modulates blood pressure in L-NAME-induced hypertension. Cardiovasc Res. 2014;101:464–472. doi: 10.1093/cvr/cvt265. [DOI] [PubMed] [Google Scholar]
- 30.Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, Kherada N, Brook RD, Reddy KM, Padture NP, Parthasarathy S, Chen LC, Moffatt-Bruce S, Sun Q, Morawietz H, Rajagopalan S. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res. 2011;108:716–726. doi: 10.1161/CIRCRESAHA.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MH. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond) 2012;122:535–543. doi: 10.1042/CS20110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrillo-Sepulveda MA, Spitler K, Pandey D, Berkowitz DE, Matsumoto T. Inhibition of TLR4 attenuates vascular dysfunction and oxidative stress in diabetic rats. J Mol Med (Berl) 2015;93:1341–1354. doi: 10.1007/s00109-015-1318-7. [DOI] [PubMed] [Google Scholar]
- 33.O’Neill S, Humphries D, Tse G, Marson LP, Dhaliwal K, Hughes J, Ross JA, Wigmore SJ, Harrison EM. Heat shock protein 90 inhibition abrogates TLR4-mediated NF-kappaB activity and reduces renal ischemia-reperfusion injury. Sci Rep. 2015;5:1–11. doi: 10.1038/srep12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souza AC, Tsuji T, Baranova IN, Bocharov AV, Wilkins KJ, Street JM, Alvarez-Prats A, Hu X, Eggerman T, Yuen PS, Star RA. TLR4 mutant mice are protected from renal fibrosis and chronic kidney disease progression. Physiol Rep. 2015;3:1–12. doi: 10.14814/phy2.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan Z, Singletary ST, Cha H, Van Beusecum JP, Cook AK, Pollock JS, Pollock DM, Inscho EW. Pentosan polysulfate preserves renal microvascular P2×1 receptor reactivity and autoregulatory behavior in DOCA-salt hypertensive rats. Am J Physiol Renal Physiol. 2016;310:F456–465. doi: 10.1152/ajprenal.00110.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guan Z, Giddens MI, Osmond DA, Cook AK, Hobbs JL, Zhang S, Yamamoto T, Pollock JS, Pollock DM, Inscho EW. Immunosuppression preserves renal autoregulatory function and microvascular P2X(1) receptor reactivity in ANG II-hypertensive rats. Am J Physiol Renal Physiol. 2013;304:F801–807. doi: 10.1152/ajprenal.00286.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma K, Cook A, Smith M, Valancius C, Inscho EW. TGF-beta impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol. 2005;288:F1069–1077. doi: 10.1152/ajprenal.00345.2004. [DOI] [PubMed] [Google Scholar]
- 38.Guan Z, Fuller BS, Yamamoto T, Cook AK, Pollock JS, Inscho EW. Pentosan polysulfate treatment preserves renal autoregulation in ANG II-infused hypertensive rats via normalization of P2×1 receptor activation. Am J Physiol Renal Physiol. 2010;298:F1276–1284. doi: 10.1152/ajprenal.00743.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2×1 receptors in renal microvascular autoregulatory behavior. J Clin Invest. 2003;112:1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inscho EW, Cook AK, Mui V, Imig JD. Calcium mobilization contributes to pressure-mediated afferent arteriolar vasoconstriction. Hypertension. 1998;31:421–428. doi: 10.1161/01.hyp.31.1.421. [DOI] [PubMed] [Google Scholar]
- 41.Inscho EW, Ohishi K, Cook AK, Belott TP, Navar LG. Calcium activation mechanisms in the renal microvascular response to extracellular ATP. Am J Physiol. 1995;268:F876–884. doi: 10.1152/ajprenal.1995.268.5.F876. [DOI] [PubMed] [Google Scholar]
- 42.Van Beusecum J, Inscho EW. Regulation of renal function and blood pressure control by P2 purinoceptors in the kidney. Curr Opin Pharmacol. 2015;21:82–88. doi: 10.1016/j.coph.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inscho EW, Cook AK, Clarke A, Zhang S, Guan Z. P2×1 receptor-mediated vasoconstriction of afferent arterioles in angiotensin II-infused hypertensive rats fed a high-salt diet. Hypertension. 2011;57:780–787. doi: 10.1161/HYPERTENSIONAHA.110.168955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inscho EW. ATP, P2 receptors and the renal microcirculation. Purinergic Signal. 2009;5:447–460. doi: 10.1007/s11302-009-9147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan Z, Fellner RC, Van Beusecum J, Inscho EW. P2 receptors in renal autoregulation. Curr Vasc Pharmacol. 2014;12:818–828. doi: 10.2174/15701611113116660152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Renal autoregulation in P2×1 knockout mice. Acta Physiol Scand. 2004;181:445–453. doi: 10.1111/j.1365-201X.2004.01317.x. [DOI] [PubMed] [Google Scholar]
- 47.Osmond DA, Inscho EW. P2X(1) receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo. Am J Physiol Renal Physiol. 2010;298:F1360–1368. doi: 10.1152/ajprenal.00016.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inscho EW, Cook AK. P2 receptor-mediated afferent arteriolar vasoconstriction during calcium blockade. Am J Physiol Renal Physiol. 2002;282:F245–255. doi: 10.1152/ajprenal.0038.2001. [DOI] [PubMed] [Google Scholar]
- 49.Lai EY, Onozato ML, Solis G, Aslam S, Welch WJ, Wilcox CS. Myogenic responses of mouse isolated perfused renal afferent arterioles: effects of salt intake and reduced renal mass. Hypertension. 2010;55:983–989. doi: 10.1161/HYPERTENSIONAHA.109.149120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai EY, Solis G, Luo Z, Carlstrom M, Sandberg K, Holland S, Wellstein A, Welch WJ, Wilcox CS. p47(phox) is required for afferent arteriolar contractile responses to angiotensin II and perfusion pressure in mice. Hypertension. 2012;59:415–420. doi: 10.1161/HYPERTENSIONAHA.111.184291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai EY, Wellstein A, Welch WJ, Wilcox CS. Superoxide modulates myogenic contractions of mouse afferent arterioles. Hypertension. 2011;58:650–656. doi: 10.1161/HYPERTENSIONAHA.111.170472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aksu U, Ergin B, Bezemer R, Kandil A, Milstein DM, Demirci-Tansel C, Ince C. Scavenging reactive oxygen species using tempol in the acute phase of renal ischemia/reperfusion and its effects on kidney oxygenation and nitric oxide levels. Intensive Care Med Exp. 2015;3:57. doi: 10.1186/s40635-015-0057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fellner RC, Cook AK, O’Connor PM, Zhang S, Pollock DM, Inscho EW. High-salt diet blunts renal autoregulation by a reactive oxygen species-dependent mechanism. Am J Physiol Renal Physiol. 2014;307:F33–40. doi: 10.1152/ajprenal.00040.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casellas D, Moore LC. Autoregulation and tubuloglomerular feedback in juxtamedullary glomerular arterioles. Am J Physiol. 1990;258:F660–669. doi: 10.1152/ajprenal.1990.258.3.F660. [DOI] [PubMed] [Google Scholar]
- 55.Arendshorst WJ. Autoregulation of renal blood flow in spontaneously hypertensive rats. Circ Res. 1979;44:344–349. doi: 10.1161/01.res.44.3.344. [DOI] [PubMed] [Google Scholar]
- 56.Moore LC, Casellas D. Tubuloglomerular feedback dependence of autoregulation in rat juxtamedullary afferent arterioles. Kidney Int. 1990;37:1402–1408. doi: 10.1038/ki.1990.129. [DOI] [PubMed] [Google Scholar]
- 57.Takenaka T, Harrison-Bernard LM, Inscho EW, Carmines PK, Navar LG. Autoregulation of afferent arteriolar blood flow in juxtamedullary nephrons. Am J Physiol. 1994;267:F879–887. doi: 10.1152/ajprenal.1994.267.5.F879. [DOI] [PubMed] [Google Scholar]
- 58.Guan Z, Pollock JS, Cook AK, Hobbs JL, Inscho EW. Effect of epithelial sodium channel blockade on the myogenic response of rat juxtamedullary afferent arterioles. Hypertension. 2009;54:1062–1069. doi: 10.1161/HYPERTENSIONAHA.109.137992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, Chen W, Mernaugh RL, Cai H, Bernstein KE, Goronzy JJ, Weyand CM, Curci JA, Barbaro NR, Moreno H, Davies SS, Roberts LJ, Madhur MS, 2nd, Harrison DG. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126:50–67. doi: 10.1172/JCI80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, Dale BL, Iwakura Y, Hoover RS, McDonough AA, Madhur MS. Interleukin-17A regulates renal sodium transporters and renal Injury in angiotensin II-induced hypertension. Hypertension. 2016;68:167–174. doi: 10.1161/HYPERTENSIONAHA.116.07493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity and hypertensive end-organ damage. Circ Res. 2015;116:1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami K, Nagai Y, Takeuchi O, Akira S, Matsuguchi T. Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol. 2002;169:2026–2033. doi: 10.4049/jimmunol.169.4.2026. [DOI] [PubMed] [Google Scholar]
- 63.El-Achkar TM, Huang X, Plotkin Z, Sandoval RM, Rhodes GJ, Dagher PC. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am J Physiol Renal Physiol. 2006;290:F1034–1043. doi: 10.1152/ajprenal.00414.2005. [DOI] [PubMed] [Google Scholar]
- 64.El-Achkar TM, Plotkin Z, Marcic B, Dagher PC. Sepsis induces an increase in thick ascending limb Cox-2 that is TLR4 dependent. Am J Physiol Renal Physiol. 2007;293:F1187–1196. doi: 10.1152/ajprenal.00217.2007. [DOI] [PubMed] [Google Scholar]
- 65.Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, Yang CW. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation. 2005;79:1370–1377. doi: 10.1097/01.tp.0000158355.83327.62. [DOI] [PubMed] [Google Scholar]
- 66.Smith JA, Stallons LJ, Collier JB, Chavin KD, Schnellmann RG. Suppression of mitochondrial biogenesis through toll-like receptor 4-dependent mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling in endotoxin-induced acute kidney injury. J Pharmacol Exp Ther. 2015;352:346–357. doi: 10.1124/jpet.114.221085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castoldi A, Braga TT, Correa-Costa M, Aguiar CF, Bassi EJ, Correa-Silva R, Elias RM, Salvador F, Moraes-Vieira PM, Cenedeze MA, Reis MA, Hiyane MI, Pacheco-Silva A, Goncalves GM. Saraiva Camara NO, TLR2, TLR4 and the MYD88 signaling pathway are crucial for neutrophil migration in acute kidney injury induced by sepsis. PLoS One. 2012;7:e37584. doi: 10.1371/journal.pone.0037584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dagher PC, Hato T, Mang HE, Plotkin Z, Richardson QV, Massad M, Mai E, Kuehl SE, Graham P, Kumar R, Sutton TA. Inhibition of toll-like receptor 4 signaling mitigates microvascular loss but not fibrosis in a model of ischemic acute kidney injury. Int J Mol Sci. 2016;17:1–10. doi: 10.3390/ijms17050647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schaller MD, Waeber B, Nussberger J, Brunner HR. Angiotensin II, vasopressin and sympathetic activity in conscious rats with endotoxemia. Am J Physiol. 1985;249:H1086–1092. doi: 10.1152/ajpheart.1985.249.6.H1086. [DOI] [PubMed] [Google Scholar]
- 70.Vayssettes-Courchay C, Bouysset F, Verbeuren TJ. Sympathetic activation and tachycardia in lipopolysaccharide treated rats are temporally correlated and unrelated to the baroreflex. Auton Neurosci. 2005;120:35–45. doi: 10.1016/j.autneu.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Nitescu N, Grimberg E, Guron G. Low-dose candesartan improves renal blood flow and kidney oxygen tension in rats with endotoxin-induced acute kidney dysfunction. Shock. 2008;30:166–172. doi: 10.1097/shk.0b013e31815dd780. [DOI] [PubMed] [Google Scholar]
- 72.Inscho EW, Cook AK, Webb RC, Jin LM. Rho-kinase inhibition reduces pressure-mediated autoregulatory adjustments in afferent arteriolar diameter. Am J Physiol Renal Physiol. 2009;296:F590–597. doi: 10.1152/ajprenal.90703.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Imig JD, Cook AK, Inscho EW. Postglomerular vasoconstriction to angiotensin II and norepinephrine depends on intracellular calcium release. Gen Pharmacol. 2000;34:409–415. doi: 10.1016/s0306-3623(01)00078-7. [DOI] [PubMed] [Google Scholar]
- 74.Ichihara A, Imig JD, Inscho EW, Navar LG. Interactive nitric oxide-angiotensin II influences on renal microcirculation in angiotensin II-induced hypertension. Hypertension. 1998;31:1255–1260. doi: 10.1161/01.hyp.31.6.1255. [DOI] [PubMed] [Google Scholar]
- 75.Inscho EW, Imig JD, Cook AK. Afferent and efferent arteriolar vasoconstriction to angiotensin II and norepinephrine involves release of Ca2+ from intracellular stores. Hypertension. 1997;29:222–227. doi: 10.1161/01.hyp.29.1.222. [DOI] [PubMed] [Google Scholar]
- 76.Ichihara A, Inscho EW, Imig JD, Michel RE, Navar LG. Role of renal nerves in afferent arteriolar reactivity in angiotensin-induced hypertension. Hypertension. 1997;29:442–449. doi: 10.1161/01.hyp.29.1.442. [DOI] [PubMed] [Google Scholar]
- 77.Pi Y, Zhang LL, Li BH, Guo L, Cao XJ, Gao CY, Li JC. Inhibition of reactive oxygen species generation attenuates TLR4-mediated proinflammatory and proliferative phenotype of vascular smooth muscle cells. Lab Invest. 2013;93:880–887. doi: 10.1038/labinvest.2013.79. [DOI] [PubMed] [Google Scholar]
- 78.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 79.Patel DN, Bailey SR, Gresham JK, Schuchman DB, Shelhamer JH, Goldstein BJ, Foxwell BM, Stemerman MB, Maranchie JK, Valente AJ, Mummidi S, Chandrasekar B. TLR4-NOX4-AP-1 signaling mediates lipopolysaccharide-induced CXCR6 expression in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2006;347:1113–1120. doi: 10.1016/j.bbrc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 80.Lee IT, Shih RH, Lin CC, Chen JT, Yang CM. Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1 expression induced by lipopolysaccharide in human renal mesangial cells. Cell Commun Signal. 2012;10:33. doi: 10.1186/1478-811X-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schneider MP, Inscho EW, Pollock DM. Attenuated vasoconstrictor responses to endothelin in afferent arterioles during a high-salt diet. Am J Physiol Renal Physiol. 2007;292:F1208–1214. doi: 10.1152/ajprenal.00280.2006. [DOI] [PubMed] [Google Scholar]
- 82.Howarth AR, Conway BR, Bailey MA. Vascular and inflammatory actions of P2X receptors in renal injury. Auton Neurosci. 2015;191:135–140. doi: 10.1016/j.autneu.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Guan Z, Osmond DA, Inscho EW. P2X receptors as regulators of the renal microvasculature. Trends Pharmacol Sci. 2007;28:646–652. doi: 10.1016/j.tips.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 84.Parikh SM. Therapeutic targeting of the mitochondrial dysfunction in septic acute kidney injury. Curr Opin Crit Care. 2013;19:554–559. doi: 10.1097/MCC.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364:545–548. doi: 10.1016/S0140-6736(04)16815-3. [DOI] [PubMed] [Google Scholar]
- 86.Casellas D, Carmines PK, Navar LG. Microvascular reactivity of in vitro blood perfused juxtamedullary nephrons from rats. Kidney Int. 1985;28:752–759. doi: 10.1038/ki.1985.194. [DOI] [PubMed] [Google Scholar]
- 87.Correa TD, Takala J, Jakob SM. Angiotensin II in septic shock. Crit Care. 2015;19:98. doi: 10.1186/s13054-015-0802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Inscho EW, Cook AK, Mui V, Miller J. Direct assessment of renal microvascular responses to P2-purinoceptor agonists. Am J Physiol. 1998;274:F718–727. doi: 10.1152/ajprenal.1998.274.4.F718. [DOI] [PubMed] [Google Scholar]