Abstract
Purpose
Receptor tyrosine kinases (RTKs) are frequently deregulated in leukemia, yet the biological consequences of this deregulation remain elusive. The mechanisms underlying aberrant methylation, a hallmark of leukemia, are not fully understood. Here we investigated the role of RTKs in methylation abnormalities and characterized the hypomethylating activities of RTK inhibitors.
Experimental Design
Whether and how RTKs regulate expression of DNA methyltransferases (DNMTs), tumor suppressor genes (TSGs) as well as global and gene specific DNA methylation were examined. The pharmacologic activities and mechanisms of actions of RTK inhibitors in vitro, ex vivo, in mice and in nilotinib-treated leukemia patients were determined.
Results
Upregulation of RTKs paralleled DNMT overexpression in leukemia cell lines and patient blasts. Knockdown of RTKs disrupted, whereas enforced expression increased, DNMT expression and DNA methylation. Treatment with the RTK inhibitor, nilotinib, resulted in a reduction of Sp1-dependent DNMT1 expression, the diminution of global DNA methylation and the upregulation of the p15INK4B gene through promoter hypomethylation in AML cell lines and patient blasts. This led to disruption of AML cell clonogenicity and promotion of cellular apoptosis without obvious changes in cell cycle. Importantly, nilotinib administration in mice and human patients with AML impaired expression of DNMTs followed by DNA hypomethylation, TSG re-expression, and leukemia regression.
Conclusions
Our findings demonstrate RTKs as novel regulators of DNMT-dependent DNA methylation and define DNA methylation status in AML cells as a pharmacodynamic marker for their response to RTK-based therapy, providing new therapeutic avenues for RTK inhibitors in overcoming epigenetic abnormalities in leukemia.
Keywords: Acute myeloid leukemia, Receptor tyrosine kinases, DNA methyltransferase, DNA methylation, Nilotinib
Introduction
Receptor tyrosine kinases (RTKs) are membrane-spanning proteins that exhibit intrinsic phosphotyrosine kinase activity. While ligand binding triggers the dimerization and sequential activation of these kinases, studies also show that the ligand-mediated dimerization of kinases can be bypassed through kinase overexpression or/and gene mutations. Because hyperactive RTKs, such as KIT, JAK2, FLT3 and BCR/ABL (1-4), crucially contribute to leukemia pathogenesis, they are promising targets for therapeutic intervention. For instance, BCR/ABL is a chimeric oncogene with constitutive kinase activity (5) that has a fundamental role in the chronic myelogenous leukemia (CML) (4,6). Thus, imatinib was rationally designed to override BCR/ABL activity and resulted in excellent outcomes in CML patients. However, acquisition of imatinib resistance, due to point mutations in the BCR/ABL kinase domain (7), has limited its therapeutic application. To this end, nilotinib (Tasigna®), an analog of imatinib, was developed to selectively antagonize BCR/ABL kinase in imatinib-resistant cells (8-10). However, in addition to targeting BCR/ABL, the effectiveness of nilotinib in suppressing proliferation has been extended to KIT, RAF, PDGFR and colony-stimulating factor receptor-1 (11-14). This is consistent with the idea that RTK inhibitors (RTKi) have multiple kinase targets and can induce apoptosis and block cell expansion in cancer cells in an “off-target” fashion. In fact, imatinib impairs proliferation in cells without BCR/ABL, KIT or PDGFR; and PKC412 (Midostaurin) is known to promote apoptosis in both KIT mutation-positive or -negative cells. These findings indicate that the molecular basis of RTKi's activity against leukemia is incompletely understood. Notably, compared to RTK mutations, abnormal RTK expression is more prevalent in cancer patients (1). This is consistent with the finding that >50% of RTK genes identified in the human genome are deregulated in human malignancies (15). Additionally, aberrant degradation of RTKs is associated with numerous cancers (16,17), which underscores the importance of RTK deregulation in cancer pathogenesis. However, the biological consequences of RTK deregulation remain elusive.
DNA hypermethylation and the subsequent inactivation of tumor suppressor genes (TSGs) are hallmarks of leukemia (18,19). This aberrant DNA methylation in leukemia cells is attributed to hyperactive DNMTs resulting from DNMT overexpression (20-22). Although methylation of CpG dinucleotides is cooperatively regulated by DNMT1, DNMT3a and DNMT3b (23), which are highly elevated in leukemia, only DNMT1 was found to be overexpressed in leukemia stem cells (LSCs). DNMT1 dysfunction was sufficient to delay progression of leukemogenesis and impair the self-renewal of LSCs (24), which underlie disease relapse and are chemotherapy-resistant in vivo (25). Further, DNMT1 is one of the most abundant cellular enzymes and possesses both maintenance activity, which targets replicative or nascent form of DNA, and de novo DNMT functions that initiate promoter DNA methylation without the requirement of cell cycle progression and DNA replication. While Sp1/NFκB/miR-29b (26,27) and nucleolin (22) are important regulators for DNMT1 gene expression, the protein kinase AKT was found to phosphorylate and stabilize the DNMT1 protein resulting in enhanced DNMT activity (28). Because NFκB function is also modulated by phosphorylation (22), RTKs might govern DNMT1 abundance and subsequent DNA methylation. Here, we show that DNMT expression is positively correlated with RTK expression in leukemia cell lines and patient primary cells, and that RTKs are positive regulators of DNMT1-dependent DNA methylation. Dysfunction of RTKs impairs DNMT expression, reduces DNA methylation and activates epigenetically-silenced p15INK4B. This leads to suppression of leukemia growth in vitro, and importantly, in vivo in AML mice and in AML patients receiving nilotinib. These studies identify DNA methylation machinery as a previously unrecognized functional element that plays a role in RTK-associated leukemia and the response of leukemia cells to RTKi exposure.
Materials and Methods
Cell lines, reagents and transfection
The MV4-11, Kasumi-1, K562, KU812, THP-1, U937, HEL and C1498 cell lines were purchased from American Type Culture Collection, and the NB4 cell line was from Deutsche Sammlung von Mikroorganismen und Zellkulturen. The SKNO-1 cells were kindly provided by Dr. Clara Nervi (University of Rome, Italy). Cell lines were newly obtained with no further authentication and no further testing for mycoplasma. We froze initial cell line stocks and used early passages of cells (< 3 months in culture) in all experiments. Nilotinib was obtained from LC Laboratories. On-targetplus Smart pool siRNAs containing a mixture of 4 oligonucleotides against Sp1 (L-026959), DNMT1 (L-004605), KIT (L-003150), BCR (L-003875), mTOR (L-003008) and their scrambled oligos (D-001810-10) were obtained from Thermo Scientific. Cells (1×106) were seeded into 6-well plates overnight before transfection. The siRNA and scrambled oligos (100 nM) were introduced into cells using Lipofectamine™ RNAiMAX (Invitrogen) as previously described (22,29).
Clonogenic and flow cytometry analysis
Methylcellulose colony-forming assays were performed in the MethoCult® mixture (Stem Cell Technologies) as previously described (22,29,30). Colonies were scored at 7-10 days. For flow cytometry analysis (29), the treated cells were fixed in ethanol and stained with propidium iodide and Annexin V-FITC (BD Biosciences PharMingen). The FlowJo software program (Version 7.6.1, Treestar) was used for subsequent analysis.
Western blot analysis
Total cellular lysates were subjected to Western blot analysis as previously described (1,27). The antibodies were obtained from Santa Cruz Biotechnology: DNMT3a (sc-20703), Sp1 (sc-59), Bid (sc-11423), Noxa (sc-30209), Bcl-xL (sc-7195) and β-actin (sc-1616); Cell Signaling Technology: PCNA (2586S), phospho-KIT (Tyr719, 3391L), phospho-FLT3 (Tyr589/591, 3464S), phospho-STAT5 (Tyr694, 9351S), phospho-AKT (9272), total KIT (3392S), total FLT3 (3462S) and total STAT5 (9352); New England Biolabs: DNMT1 (M0231L); and Abcam: total AKT (ab126811) and DNMT3b (ab16049).
Dotblot analysis
Genomic DNA was extracted using the DNA Blood/Tissue Kit (QIAGEN) and dotblotting for the quantification of DNA methylation was performed as previously described (22,29,31). Briefly, 2 μg DNA was denatured at 100°C and loaded onto a pre-wet nitrocellulose membrane. The DNA-spotted membrane was baked at 80°C, blocked with 5% nonfat milk and incubated with a mouse anti-5mC antibody (39649, Active Motif). The signal was detected by a HRP-conjugated secondary antibody and enhanced chemiluminescence. The membrane was stained with 0.02% methylene blue in 0.5 M sodium acetate (pH 5.0) as a loading control.
Bisulfite sequencing
The bisulfite sequencing for promoter DNA methylation was performed as previously described (22,29,31). Briefly, the genomic DNA was bisulfite-conversed using the EpiTect Bisulfite Kit (QIAGEN), amplified by PCR and subcloned using the TA cloning Kit (Invitrogen). The individual clones were subjected to sequencing with M13R primer by Genewiz. The tested regions were located between -4 and +247 in the p15INK4B promoter encompassing 27 CpG sites as well as -251 and +139 in the CDH1 promoter. The primers are listed in Supplementary Table.
RNA isolation, cDNA preparation and quantitative PCR (qPCR)
RNA was isolated using the miRNeasy Kit (QIAGEN) and reverse transcription for synthesizing cDNA was performed using SuperScript® III First-Strand Synthesis System (Invitrogen). The expression of KIT, Sp1, DNMT1, DNMT3a and DNMT3b was determined by TaqMan qPCR (Applied Biosystems), but the changes in FLT3, p15INK4B, p16, p18, p21 and CDH1 were determined using SYBR Green (Applied Biosystems). The 18S RNA levels were used for normalization. Expression of target genes was measured using the ΔCT approach. The primers are listed in Supplemental Table.
AML patients
The current study was approved by the Mayo Clinic Institutional Review Board and conducted in accordance with the Declaration of Helsinki. The diagnosis of AML was made according to the criteria of the World Health Organization. All patients signed informed consent to store and use their leukemia tissue for discovery studies. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using Ficoll-Paque™ and recovered in RPMI1640 buffer with 20% FBS for 6 hours prior to treatment. The PBMCs from AML patients receiving nilotinib therapy were analyzed directly without further culture.
In vivo leukemogenesis assays
C57BL/6 mice (female, 4-6 weeks old) were purchased from The Jackson Laboratory. All animal experiments were approved by the University of Minnesota Institutional Animal Care and Use Committee. For the leukemia mouse model, C1498 cells (0.1×106) were injected into C57BL/6 mice through their tail vein. The development of leukemic disease was monitored by white blood cell (WBC) counts. When the WBC counts showed the illness, the leukemic mice (n = 3) were sequentially treated with 5, 10 or 15 mg/kg of nilotinib in PEG400 and saline (ratio 15:38:47) by tail-vein injection twice a week for a total of 6 doses. Mice injected with only vehicle (n = 3) served as controls. The experiments were terminated at 3 weeks after drug administration. Following euthanasia, the body, liver and spleen weights were recorded, and single spleen mononuclear cells (MNC) were obtained by grinding and after red blood cell disruption. The spleen and bone marrow cells (BM) were utilized for Western blot, qPCR or dotblotting assays (1,22). For histopathologic analysis, tissue sections from livers, lungs and spleens were preserved in 10% neutral-buffered formalin, embedded in paraffin blocks and stained with Haemotoxylin and Eosin (Thermo-Scientific).
Statistical analysis
The statistical analysis, including the quantifications for Western blot, dotblot, apoptosis and spleen/liver weight, was conducted and all graphs were generated using the Student's t test. All analyses were performed using the GraphPad Prism 5 software program. Correlation data were acquired using Pearson correlation coefficients. A P value of < 0.05 was considered statistically significant. All P values were two-tailed.
Additional methods are provided in the Supplementary Materials and Methods.
Results
RTKs govern DNMT expression and DNA methylation in leukemia cells
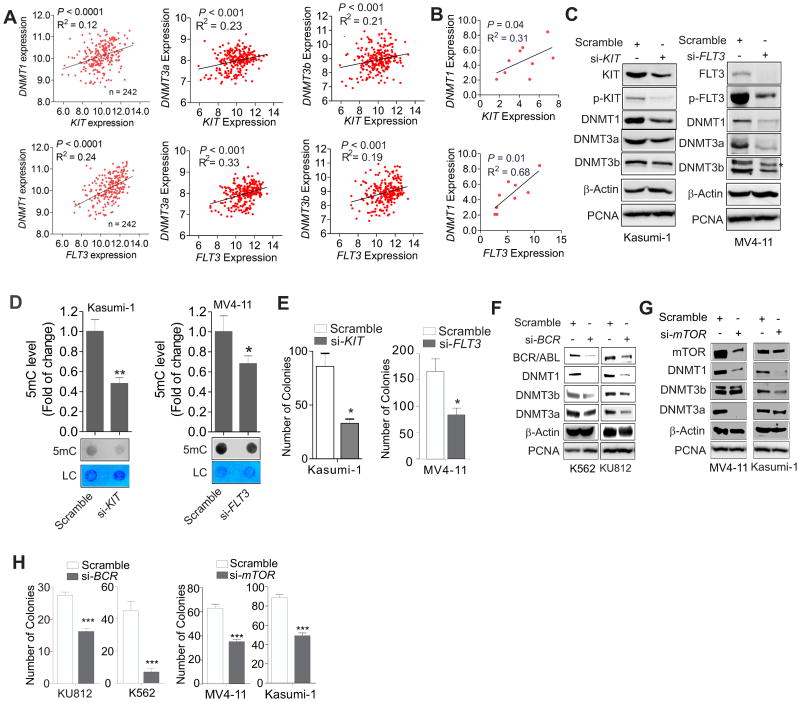
Because upregulation of RTKs (e.g., KIT and FLT3) and DNMTs is frequently observed in blasts from multiple AML subtypes (1,20,22), we first addressed the question of whether the expression of these deregulated genes is correlated. We analyzed GEO dataset GSE12417, which contain 242 patients with AML (≤ 60 years old), for the expression of KIT, FLT3 and DNMTs. We found that higher levels of DNMT1, DNMT3a or DNMT3b were accompanied by overexpression of KIT or FLT3, whereas a lower expression was observed in AML patients exhibiting lower KIT or FLT3 expression (Fig. 1A). In agreement with this finding, qPCR results revealed a positive correlation between DNMTs and KIT or FLT3 in 9 leukemia cell lines (Fig. 1B; Supplementary Fig. 1A and B). Thus, DNMTs, KIT and FLT3 might have regulatory interactions in leukemia cells.
Figure 1.
KIT and FLT3 upregulate DNMT expression in AML cells. A and B, The analysis of the GEO dataset GSE12417 series, platform GPL570 and GPL96, AML, n = 242 (A) and qPCR results of 9 leukemia cell lines (B) showing the correlation between FLT3 or KIT and DNMT gene expression, which was assessed by Spearman correlation. C, Kasumi-1 or MV4-11 cells were transfected for 48 h with FLT3 or KIT siRNA (si-) or scrambled control and the expression of targeted genes was detected by Western blot analysis. D, MV4-11 or Kasumi-1 cells were transfected for 48 h with FLT3 or KIT siRNA, respectively, or scrambled control. The genomic DNA was subjected to dotblot analysis. Graphs are the quantification of dot intensity. E, Quantitative analysis for colony-forming assays of Kasumi-1 or MV4-11 cells transfected with FLT3 or KIT siRNA or scrambled control. F, K562 or KU812 cells were transfected for 48 h with BCR siRNA and the cell lysates were subjected to Western blot analysis. G, Kasumi-1 or MV4-11 cells were transfected for 48 h with mTOR siRNA or scrambled control. The cell lysates were subjected to Western blot analysis. H, Quantitative analysis for colony-forming assays of K562 and KU812 cells transfected with BCR siRNA (left) or MV4-11 and Kasumi-1 cells with mTOR siRNA (right). In C, D, F and G, data represent 3 independent experiments; In E and H, the assays were performed in triplicate; In D, E and H, data are shown as mean values ± S.D; *P < 0.05, **P < 0.01, ***P < 0.001.
Because Kasumi-1 and MV4-11 cells carry KIT or FLT3 mutations, respectively, we transfected each cell line with two pools of siRNAs, each containing 4 oligos targeting different regions of either the KIT or FLT3 gene. The siRNA-triggered KIT and FLT3 depletion resulted in downregulation of DNMT1, DNMT3a and DNMT3b RNA (not shown) and protein expression (Fig. 1C). This was accompanied by a concurrent decrease in global DNA methylation (Fig. 1D) coupled with the impaired clonogenic potential of MV4-11 and Kasumi-1 cells (Fig. 1E). To explore whether kinase-modified DNA methylation could represent a common mechanism in leukemia, we examined the association of DNMTs with BCR/ABL or mTOR, and identified a positive correlation in the expression of these genes in leukemia patients (Supplementary Fig. 1C and D). In addition, abrogation of BCR/ABL in K562 and KU812 cells or mTOR in MV4-11 and Kasumi-1 cells decreased the levels of DNMT1, DNMT3a, DNMT3b (Fig. 1F and G) and DNA methylation (Supplementary Fig. 1E and F) and also inhibited colony formation (Fig. 1H). Notably, knockdown of KIT, FLT3, BCR/ABL or mTOR had no effect on the levels of PCNA, a DNA replication parameter. This suggested that RTK-mediated DNA methylation occurs in a cell cycle-independent manner, which was further verified by cell cycle analysis in KIT-depleted Kasumi-1 cells (Supplementary Fig. 1G). Collectively, these findings support the idea that RTKs are underappreciated epigenetic modulators in leukemia cells.
Nilotinib treatment impairs DNMT1 expression through Sp1 dysfunction
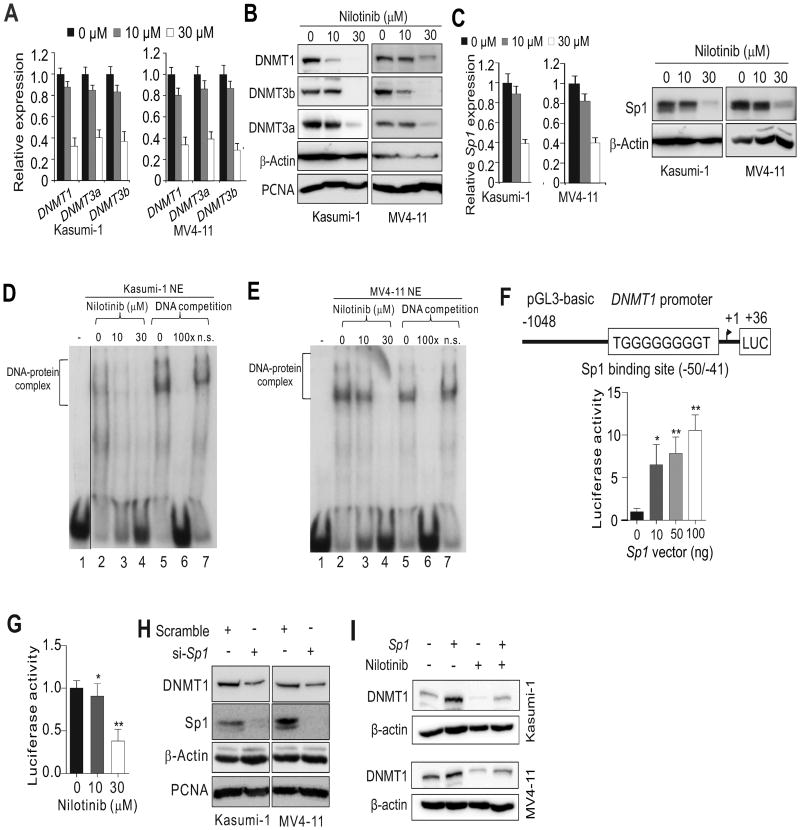
If RTKs are positive regulators for DNMT genes, RTK inhibitors could possibly suppress DNMT expression. As a proof of concept, we chose nilotinib, a second-generation RTK inhibitor that has been in clinical use for newly diagnosed, imatinib-resistant or -intolerant CML (32). Exposure to nilotinib significantly reduced the expression and phosphorylation of KIT in Kasumi-1 cells or FLT3 in MV4-11 cells (Supplementary Fig. 2A). This led to a downregulation and dephosphorylation of STAT5 and AKT, which are well-characterized downstream effectors of KIT and FLT3 (Supplementary Fig. 2B). These results not only validated KIT signaling as a nilotinib target (33), but also unraveled FLT3 as a new hit underlying the antileukemia activity of nilotinib. Consistent with the results of KIT and FLT3 deactivation, exposure of Kasumi-1 and MV4-11 cells to nilotinib downregulated DNMT1, DNMT3a and DNMT3b in a dose-dependent fashion at both RNA and protein levels (Fig. 2A and B). Because nilotinib has been approved to treat BCR/ABL-positive leukemia patients, we examined the response of K562 and KU812 cells to nilotinib (3, 5, 10 or 30 μM) and, similarly, observed a significant downregulation of DNMT1, DNMT3a and DNMT3b (not shown). These findings support DNMTs as unconventional targets for nilotinib's anticancer activities.
Figure 2.
Sp1 activity accounts for the suppression of DNMT1 expression in AML cell lines treated with nilotinib. A-C, Kasumi-1 or MV4-11 cells were treated for 24 h with the indicated doses of nilotinib and subjected to qPCR or Western blot analysis to detect the expression levels of DNMT1, DNMT3a, DNMT3b (A,B) and Sp1 (C). D and E, EMSA assays were used to detect Sp1 binding to the DNMT1 promoter in Kasumi-1 (D) or MV4-11 (E) cells treated for 24 h with nilotinib. In D a vertical line was inserted to indicate a repositioned gel lane. F and G, Reporter gene assays were used to determine DNMT1 gene promoter activity in 293T cells transfected with the DNMT1 promoter-luciferase plasmids together with the Sp1 expression vectors (F) or treated for 24 h with nilotinib (G). H, Western blot analysis of Kasumi-1 and MV4-11 cells transfected for 48 h with Sp1 siRNA (si-) or scramble control. I, Kasumi-1 and MV4-11 cells were transfected with Sp1 expression or control vectors for 12 h and then treated with 10 μM nilotinib for another 24 h. Gene expression was assessed by Western blot analysis. Data represent 3 independent experiments.
Although all three DNMT isoforms are targeted by nilotinib in our cell models, we mainly focused on DNMT1 to delineate the underlying mechanisms because DNMT1 is the most abundant DNMT with both de novo and maintenance DNA methylation activities (22,31). Importantly, although the changes of DNMT3a and DNMT3b displayed variations (not shown), analysis of GEO datasets (i.e., GSE51083, GDS3518, GDS838, GSE19567, GDS4177, GDS4175, GDS2706) supported the idea that DNMT1 downregulation represents one common mechanism behind the anti-leukemia activities of RTK inhibitors (Supplementary Fig. 3A-G). Given that Sp1 is a positive DNMT1 regulator (27), because KIT or FLT3 knockdown decreased Sp1 expression (Supplementary Fig. 4A), Sp1 could link RTK to DNMT pathways (Supplementary Fig. 4B). To test this, we first measured Sp1 levels and found a significant decrease in Sp1 expression in nilotinib-treated Kasumi-1 and MV4-11 cells (Fig. 2C). We then performed an EMSA assay (1,27) to examine Sp1 binding affinity using probes spanning the Sp1 binding sites in the human DNMT1 promoter. Nuclear extracts (NE) were prepared from Kasumi-1 or MV4-11 cells and incubated with 32p-labeled DNMT1 promoter probes. Results indicated slower migrating DNA-protein complexes (Fig. 2D and E). The binding specificity was demonstrated by competition assays using 100× unlabeled probes containing the consensus Sp1-binding site and non-specific probes without the Sp1-binding site. The unlabeled DNA oligos efficiently reduced the protein binding to DNMT1-Sp1 probes (Fig. 2D, lane 6; Fig. 2E, lane 6). The non-specific probes had no effect on the binding (Fig. 2D, lane 7; Fig. 2E, lane 7), which supports the specific interaction of the DNMT1 promoter with the Sp1 protein. Importantly, nilotinib treatment markedly reduced the amount of DNA-protein complexes (Fig. 2D, lane 3, 4; Fig. 2E, lane 3, 4), indicating that nilotinib could disrupt Sp1 binding in the DNMT1 promoter. We then cloned the DNMT1 promoter region harboring the Sp1 binding element into a luciferase reporter (pGL3-DNMT1) and co-transfected pGL3-DNMT1 with Sp1 expression or control vectors in 293T cells. The reporter assays showed that the luciferase activity driven by the DNMT1 promoter containing the Sp1 binding sites was increased by 10.6 folds after Sp1 overexpression (100 ng; Fig. 2F), but was decreased by 2.6 folds in the presence of 30 μM nilotinib (Fig. 2G). Because Sp1 depletion reduced endogenous DNMT1 expression (Fig. 2H) and Sp1 overexpression attenuated nilotinib-mediated DNMT1 downregulation (Fig. 2I), these results support the idea that nilotinib inhibits DNMT1 expression through the abrogation of Sp1 transcriptional activity.
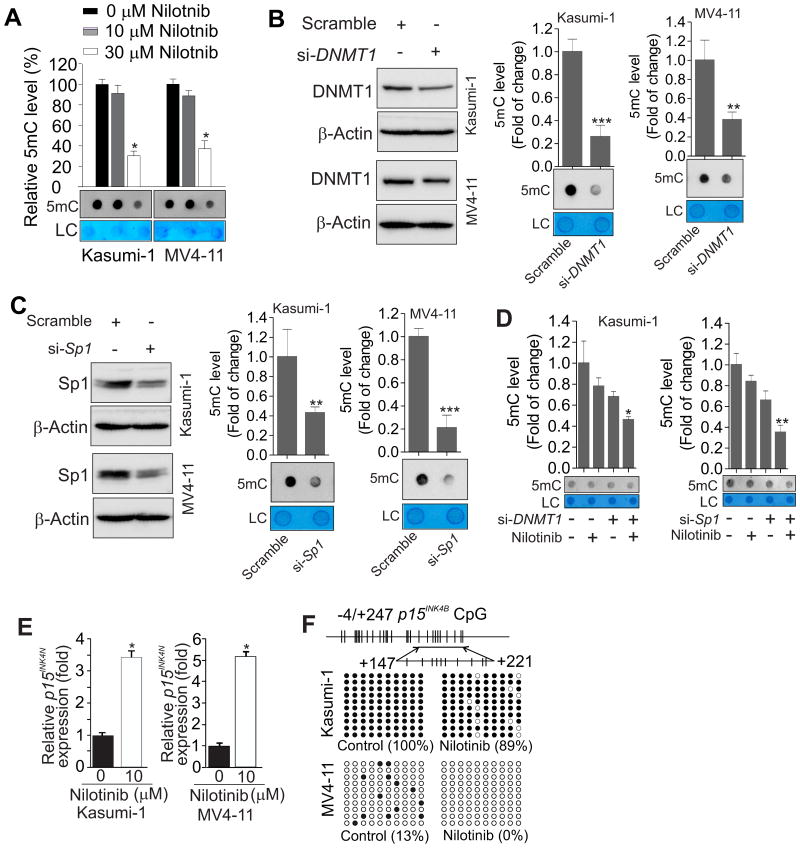
Nilotinib reduces global DNA methylation and restores TSG expression through promoter DNA hypomethylation
To examine whether nilotinib could change DNA methylation levels, Kasumi-1, MV4-11, K562 and KU812 cells were exposed to nilotinib and the genomic DNA was subjected to dotblot analysis, which has been specifically established for assessing methylated CpG (22,29,31). As expected, nilotinib incubation significantly decreased the levels of global DNA methylation in these cell lines (Fig. 3A, Supplementary Fig. 5A). When Sp1 and DNMT1 were specifically depleted in Kasumi-1 and MV4-11 cells, we observed decreased DNA methylation levels (Fig. 3B and C), and importantly, synergy with nilotinib to cause more DNA demethylation (Fig. 3D). In addition, we found that Sp1-associated DNA hypermethylation was significantly attenuated by DNMT1 knockdown (Supplementary Fig. 5B). Collectively, these results suggest that the Sp1-DNMT1 axis mediates the DNA hypomethylating activity of nilotinib.
Figure 3.
Nilotinib induces global and gene promoter DNA hypomethylation. A, Dotblot analysis using anti-5mC to evaluate changes in global DNA methylation in Kasumi-1 or MV4-11 cells treated for 24 h with nilotinib. B and C, Kasumi-1 or MV4-11 cells were transfected for 48 h with DNMT1 (B) or Sp1 siRNA (C) or scrambled control. The protein expression levels were assessed by Western blot (upper) and the levels of global DNA methylation were determined by dotblot analysis (lower). D, Kasumi-1 cells were transfected for 12 h with DNMT1 or Sp1 siRNA or scrambled control and then treated with 10 μM nilotinib for another 24 h. Global DNA methylation was determined by dotblot analysis. E, qPCR analysis of p15INK4B expression in Kasumi-1 or MV4-11 cells treated for 48 h with 10 μM nilotinib. Values are shown as a fold change of gene expression normalized to 18S RNA and compared to vehicle. F, Upper: diagram of the p15INK4B promoter indicating the location of CpG nucleotides; lower: bisulfite sequencing analysis of changes in p15INK4B promoter methylation (transcription start site +147 to +221) in Kasumi-1 or MV4-11 cells treated for 48 h with 10 μM nilotinib. Results of 10 clones are presented. Methylated CpG sites are shown as solid circles and open circles indicate non-methylated CpG sites. In A-D, the graphs show the quantification of dot intensity as mean values ± S.D. from 3 independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001; si- = siRNA; LC = loading control.
Frequent silencing of the TSG, p15INK4B, a cyclin-dependent kinase inhibitor, is associated with promoter hypermethylation at multiple sites in CpG islands in leukemia (34-36). Because nilotinib suppressed DNMT1-dependent DNA methylation, p15INK4B could possibly be epigenetically reactivated by nilotinib. To test this idea, we assessed p15INK4B expression in Kasumi-1 and MV4-11 cells treated with nilotinib for 24 or 48 h, and observed a marked p15INK4B upregulation at 48 h in response to 10 μM nilotinib. The increase at 48 h was 3.4 folds in Kasumi-1 and 5.2 folds in MV4-11 cells (Fig. 3E), although no significant change was seen at 24 h (not shown). Consistently, KIT or FLT3 knockdown increased p15INK4B expression (Supplementary Fig. 6A). We also examined the effects of nilotinib on other CDKIs, such as p18, p21 and p16, which are important for leukemia cell growth. As shown in Supplementary Fig. 6B and C, p21 and p16 were upregulated in both cell lines, but expression of p18 exhibited difference with downregulation in Kasumi-1 and upregulation in MV4-11 cells. To elucidate the mechanisms of p15INK4B re-expression, we examined CpG methylation status in the p15INK4B promoter region (-4 and +247) containing 27 CpG sites. Bisulfite sequencing revealed that the CpG islands examined in the control group were fully methylated in Kasumi-1 cells and partially methylated in MV4-11 cells. However, nilotinib incubation caused a significant decrease of the methylated CpG sites in this region, from 100 to 89% in Kasumi-1 or from 13% to a complete loss in MV4-11 cells (Fig. 3F). To substantiate the DNA hypomethylating effects of nilotinib, we examined the CDH1 gene, whose DNA hypermethylation is associated with an adverse prognosis (37,38). Similarly, nilotinib exposure resulted in upregulation of CDH1 expression and demethylation of the CDH1 promoter (Supplementary Fig. 6D and E). Interestingly, Kasumi-1 and MV4-11 cells resistant to nilotinib showed upregulation of DNMT1 and increase of DNA methylation (Supplementary Fig. 7), which is in agreement with our previous report in lung cancer cells resistant to multiple RTK inhibitor Midostaurin (39), further underscoring the epigenetic role of RTKs. Together, these data support the idea that nilotinib is a potent DNA hypomethylating agent in leukemia.
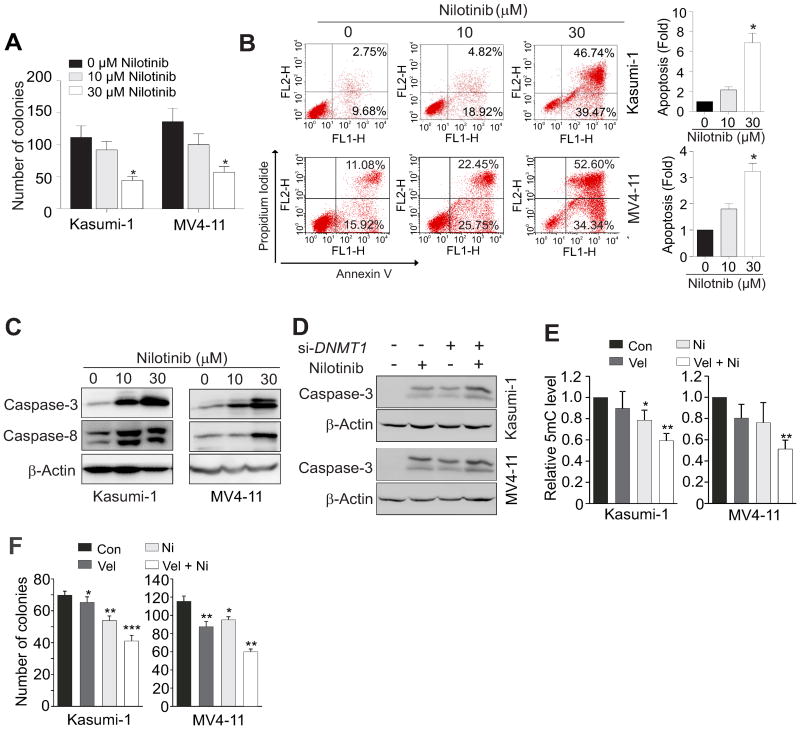
Nilotinib blocks cell proliferation and promotes cell apoptosis in vitro
Because epigenetic-silencing of TSGs is frequently associated with poor prognosis, nilotinib-restored p15INK4B and CDH1 expression could lead to a blockage of leukemia cell expansion. To examine this idea, Kasumi-1 and MV4-11 cells were exposed to nilotinib (10, 30 μM). After 6 h (prior to the onset of apoptosis), ∼500 treated-cells were subjected to methylcellulose assays (22,29). Compared to control cells, exposure to 30 μM nilotinib resulted in a significant 60 and 58% reduction of colony number in Kasumi-1 and in MV4-11 cells, respectively (Fig. 4A). This suggests that even short periods of drug exposure can cause irreversible damage to the ability of cells to proliferate. Notably, Sp1 overexpression attenuated the inhibition of cellular proliferation mediated by nilotinib, strengthening the role of Sp1 in DNMT1 regulation (Supplementary Fig. 8). Annexin V-PI staining assays detected more Annexin V-positive apoptotic cells in 30 μM nilotinib-treated Kasumi-1 (6.9 fold) or MV4-11 (3.2 fold) cells than corresponding untreated controls (Fig. 4B). Because caspase activities are hallmarks of apoptosis, we examined caspase activation and found that nilotinib greatly increased the protein levels of cleaved caspase-3 and caspase-8 (Fig. 4C). We also examined the expression of several pro- and anti-apoptotic members in Bcl-2 family. As shown in Supplementary Fig. 9, expression of Bcl-XL was downregulated, Bid was cleaved that is likely by the activated caspase-8, and Noxa was upregulated. In addition, exposure to nilotinib did not result in significant changes in cell cycle (Supplementary Fig. 10). Collectively, these results support the notion that nilotinib promotes cell apoptosis, at least partially, through suppression of anti-apoptotic or/and upregulation of pro-apoptotic genes. Moreover, if nilotinib abrogates leukemia growth through DNMT1 downregulation, then hypothesizing that cells lacking DNMT1 could display increased sensitivity to nilotinib is reasonable. Thus, Kasumi-1 and MV4-11 cells were transfected with a scrambled control or DNMT1 siRNA for 12 h and then treated with 10 μM nilotinib for another 24 h. As expected, DNMT1-depleted cells displayed higher protein levels of cleaved caspase-3 (Fig. 4D). In agreement with this finding, treatment of cells with a suboptimal dose of nilotinib enhanced the effects of bortezomib, a distinct DNA hypomethylating agent (27). Specifically, the enhanced effects of bortezomib included more downregulation of Sp1 and DNMT expression (Supplementary Fig. 11A), further reduction of DNA methylation (Fig. 4E), increased suppression of AML cell colony-formation (Fig. 4F) and increased promotion of apoptosis (Supplementary Fig. 11B). These findings were substantiated by a lower colony number and more apoptosis upon exposure to a combination of decitabine and nilotinib compared to the single agent (Supplementary Fig. 11C and D). Together, nilotinib treatment disrupts leukemia cell propagation through its DNA hypomethylating activity.
Figure 4.
Nilotinib suppresses growth and promotes apoptosis in AML leukemia cell lines. A-C, Kasumi-1 and MV4-11 cells were treated with nilotinib at concentrations of 0, 10 or 30 μM. (A) Colony-forming assays show the colony number as mean values ± S.D. from 3 independent experiments. (B) Flow cytometry was used for analysis of apoptosis and data are shown as a fold change in apoptotic cells compared to untreated control. (C) Western blot analysis was used to assess the levels of cleaved caspase forms. D, Kasumi-1 and MV4-11 cells were transfected for 12 h with DNMT1 siRNA (si-) or scrambled control and then treated with 10 μM nilotinib for another 24 h. Western blot detected cleaved caspase isoforms. E and F, Kasumi-1 and MV4-11 cells were treated with 5 nM velcade (Vel) or/and 3 μM nilotinib (Ni) and subjected to dotblot analysis (E) or colony assay (F). Data are shown as mean values ± S.D. from 3 independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001.
Nilotinib induces DNA hypomethylation and suppresses leukemia growth in mice
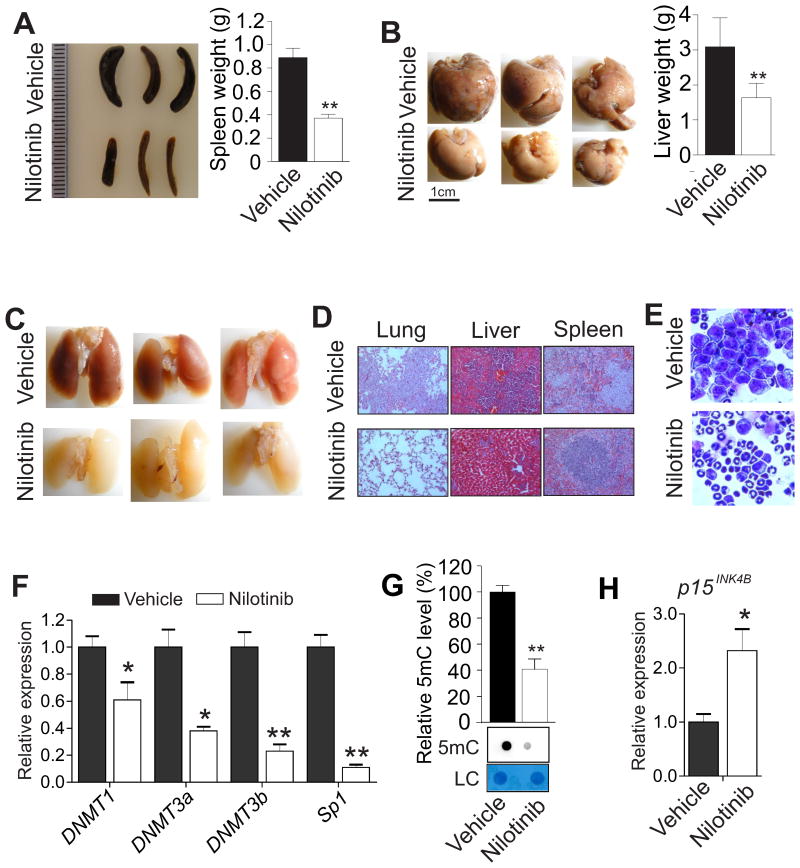
To explore the DNA hypomethylating activity of nilotinib in vivo, we used the C57BL/6 mouse with a competent immune system and the murine AML cell line, C1498, which is syngeneic to the C57BL/6 mouse (29), to mimic leukemic disease. First, similar to the results from human MV4-11 and Kasumi-1 cells, exposure of C1498 cells to nilotinib significantly downregulated DNMT1, DNMT3a, DNMT3b and Sp1 (Supplementary Fig. 12A) and decreased global DNA methylation (Supplementary Fig. 12B), leading to cell apoptosis (Supplementary Fig. 12C) through the activated caspase-3 and caspase-8 (Supplementary Fig. 12D). Second, we injected C1498 cells (0.1×106) through the tail vein into the C57BL/6 mice. When the white blood cell counts indicated illness, these leukemia-bearing mice (n = 3 mice/group) were sequentially given 5, 10 or 15 mg/kg of nilotinib in PEG400 and saline (ratio 15:38:47) intraperitoneally twice a week for a total of 6 doses. The leukemic mice (n = 3 mice/group) injected with only vehicle served as controls. Compared to the untreated controls, mice treated with nilotinib had a favorable prognosis, because the treated mice had a decrease in spleen weight (890 ± 81 vs. 370 ± 33 mg; Fig. 5A), and a diminished metastatic growth in liver (Fig. 5B) and in lung (Fig. 5C). H&E staining showed that, compared to the nilotinib-treated mice, the untreated control group displayed an increased infiltration of leukemic cells into the spleens, lungs and livers of recipients, leading to considerable damage to these organs (Fig. 5D). The bone marrow histopathology from nilotinib-treated mice identified more differentiated cells containing metamyelocytes, bands and segmented neutrophils compared to untreated control mice (Fig. 5E). No toxicity was observed for the tested drug dosage and schedule because we did not see any evident change in body weight. Mechanistically, nilotinib administration in mice remarkably diminished the levels of DNMT1, DNMT3a, DNMT3b and Sp1 gene expression (Fig. 5F) with a significant decrease (60%) in DNA methylation (Fig. 5G) and upregulation of the p15INK4B gene (Fig. 5H). Based on these results, we conclude that nilotinib induces leukemia regression in mice by suppressing the DNA methylation machinery.
Figure 5.
Nilotinib administration induces leukemia regression in mice. A, Approximately 0.1×106 C1498 cells were injected into C57BL/6 mice through the tail vein. When the white blood cell counts showed the illness, the leukemic mice were treated with vehicle or nilotinib for 3 weeks. External view (left) and quantification of spleen weight (right) from leukemia-bearing mice are shown. Data are presented as mean values ± S.D. B, Photographs (left) are representative external views of livers from leukemia-bearing mice and the graph (right) shows the quantification of liver weight. Data are shown as mean values ± S.D. C, Pictured are images of lung from leukemia-bearing mice. D, Representative images of H&E stained sections of lungs, livers and spleens from leukemia-bearing mice are shown (magnification × 200). E, Wright-Giemsa stained BM cells from leukemia-bearing mice are shown (magnification × 400). F, qPCR was used to determine the expression of DNMT1, DNMT3a, DNMT3b and Sp1 in BM cells from leukemia-bearing mice. G, The genomic DNA in BM cells from leukemia-bearing mice was isolated and subjected to dotblot analysis. H, qPCR was used to determine the expression of p15INK4B in BM cells from leukemia-bearing mice. Graph shows the quantification of dot intensities. Note, n = 3 mice/group; data are presented as mean values ± S.D; *P < 0.05, **P < 0.01.
Nilotinib therapy causes DNA hypomethylation in AML patients
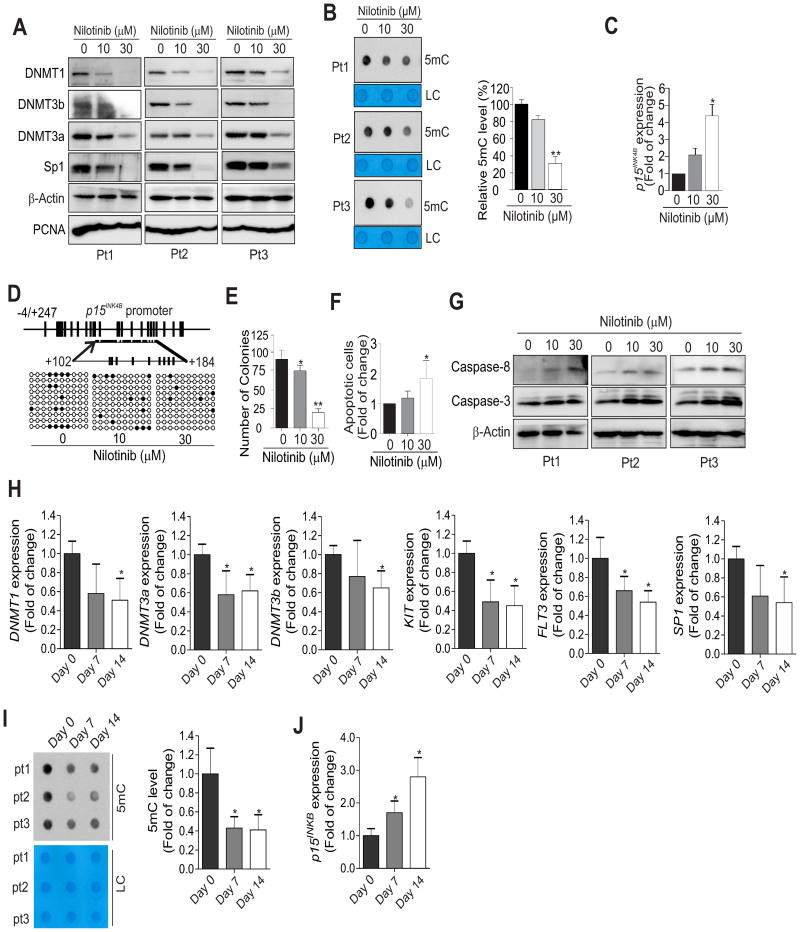
To establish the relevance of our findings in human AML patients, we treated the PBMCs of treatment-naive AML patients (n = 3) with nilotinib for 24 h. Consistent with the results acquired from leukemia cell lines and mice, exposure of PBMCs to nilotinib decreased the expression of KIT, DNMT1, DNMT3a, DNMT3b and Sp1 (Fig. 6A, Supplementary Fig. 13A). This was followed by global DNA hypomethylation (Fig. 6B), p15INK4B upregulation (∼2.1 or ∼4.4 folds) (Fig. 6C) and decreased DNA methylation in the p15INK4B promoter region (+102 to +184) from 14 to 12 or 6% (Fig. 6D). Functional investigations revealed that nilotinib treatment greatly reduced the colony number (Fig. 6E) with a remarkable increase in apoptosis (Fig. 6F, Supplementary Fig. 13B) accompanied by increases in cleaved caspase-3 and caspase-8 (Fig. 6G). Importantly, we found that the levels of DNMT1, DNMT3a, DNMT3b and Sp1 gene expression were significantly lowered in AML patients (n = 14) receiving nilotinib therapy (Fig. 6H). The downregulation of DNMTs was further confirmed in GSE33075 (Supplementary Fig. 14A), where CML patients (n = 9) received imatinib therapy. In line with these, DNA methylation was decreased and p15INK4B expression was upregulated (Fig. 6I and J). We conducted functional annotations using DAVID bioinformatics resources 6.7 for the genes listed in GSE33075 that were changed. The activated pathways on the top lists included methylation, tumor suppressor, S-adenosyl-L-methionine and methyltransferase (Supplementary Fig. 14B). This result supports DNA methylation as an important mechanism underlying the anti-leukemia activities of RTK inhibitors. Consequently, out of 14 patients evaluable for response, 12 (80%) achieved complete remission (CR) or CR with incomplete platelet recovery. These results suggest that DNA hypomethylation, p15INK4B re-expression and caspase pathway activation are among the molecular events associated with nilotinib-induced growth arrest of leukemia cells in AML patients. Notably, FLT3 testing was positive in 27%, but KIT gene sequencing (exon 8, 9, 10, 11, 17) revealed no pathogenic mutations (not shown). The impaired KIT and FLT3 gene expression (see Fig. 6H) supports the idea that nilotinib treatment could possibly benefit patients with KIT and FLT3 overexpression, although nilotinib was originally designed to block ABL kinase activities through competitive inhibition at the ATP-binding site of BCR/ABL.
Figure 6.
Nilotinib suppresses DNMT expression, induces DNA hypomethylation and impairs AML patient cell expansion ex vivo and in vivo. A-G, AML patient (Pt) primary cells (n = 3) were treated for 24 h with 0, 10 or 30 μM nilotinib. (A) Cell lysates were subjected to Western blot analysis with the indicated antibodies. (B) The genomic DNA was subjected to dotblot analysis to assess changes in global DNA methylation. (C) qPCR analysis was used to determine p15INK4B gene expression. (D) Bisulfite sequencing was used to examine p15INK4B promoter DNA methylation. Methylated CpG sites are shown as solid circles and open circles indicate non-methylated CpG sites. A representative 10 clones are shown in the dot plot. (E) Colony-forming assays show cell proliferation. (F) Cellular apoptosis was determined by flow cytometry. (G) Western blot analysis was used to detect the activated forms of caspases. H-J, The PBMCs from AML patients (n = 14) receiving nilotinib therapy (day 0, day 7 or day 14) were subjected to qPCR for gene expression (H, J) or dotblot analysis for changes in global DNA methylation (I). In B, C, E, F, H, I and J, data are shown as mean values ± S.D; *P < 0.05, **P < 0.01.
Discussion
Methylation of cytosine in DNA at C-5 of CpG catalyzed by DNMTs is the most abundant epigenetic modification that usually leads to the altered expression of genes, like TSGs (22,30). RTKs, the most abundant enzyme-linked receptors, phosphorylate specific tyrosine on intracellular signaling proteins, thus controlling cell survival and proliferation (1). Deregulation of both RTKs and DNMTs frequently occurs and is a poor prognostic indicator in leukemia patients. However, whether RTKs and the DNA methylation machinery communicate in promoting leukemia progression remains puzzling. We present convincing evidence showing that RTKs significantly regulate DNMT-driven DNA methylation in leukemia cells. Genetic and pharmacological inactivation of RTKs abolished aberrant DNMT activity resulting in DNA demethylation and AML regression. These findings identify RTKs as previously unknown epigenetic modulators and shed light on the mechanistic aspects of RTKs and their inhibitors in leukemia pathogenesis and therapies.
In the present study, we focused on the deregulation of RTKs and DNMTs in leukemia, because 1) RTK overexpression occurs in the majority of patients (1); 2) ligand-dependent activation of wild-type RTKs can be bypassed by their overexpression that results in the increased dynamics of homo/heterodimerization (40,41); and 3) in the absence of mutations, RTK overexpression is a mechanism for kinase activation (1,42). Additionally, DNMT1 gene abundance has been linked to carcinogenesis in vitro and in vivo, supported by findings illustrating that DNMT1 upregulation 1) induces NIH 3T3 cell transformation (43); 2) causes CpG island hypermethylation in human fibroblasts (44); and 3) is essential in c-Fos mediated transformation of rodent fibroblasts (45). In contrast, DNMT1 depletion in vivo reduces tumor formation (46). These findings are in agreement with our observation that abrogation of RTK and DNMT expression markedly disrupted the clonogenic potential of leukemia cells. We present evidence showing that the expression of RTKs, including KIT, FLT3, BCR/ABL and mTOR, parallels the expression of DNMTs (DNMT1, DNMT3a and DNMT3b) in leukemia patients and cell lines. The siRNA-triggered depletion of each of these tested RTKs greatly decreased DNMT expression at the levels of RNA and protein, leading to DNA demethylation and reduction of cell colony number. These discoveries demonstrate that RTKs are a new class of epigenetic regulators in leukemia cells and support the interplay of RTKs and DNMTs as an additional mechanism underlying leukemia pathogenesis. While meriting further elucidation, the possibility that RTKs regulate DNMT1 abundance/activity at the post-transcriptional level or transcriptional level exists. At the post-transcriptional level RTKs could act by controlling AKT phosphorylation, which is found to enhance DNMT1 protein stabilization (28). The altered phosphorylation of DNMT1 could change its physical interaction with EZH2 or PCNA, which determine its DNA binding affinity, thus regulating DNMT1-associated DNA methylation. At the transcriptional level RTKs could act by modulating the activities of DNMT1 transcription factors, such as Sp1/NFκB, nucleolin and STAT3 (22,27,47). DNMT1 upregulation by RTKs could enhance its capability to bind DNA and other factors (EZH2 or PCNA) resulting in DNA hypermethylation.
Although many patients with imatinib-resistant CML and Philadelphia chromosome-positive ALL are successfully treated with nilotinib, other patients remain ineligible due to a lack of knowledge regarding the specific pathways underlying nilotinib's anti-leukemia actions. Given our finding that RTKs positively regulates DNA methylation, we examined the DNA hypomethylating activities of nilotinib in leukemia cells. We demonstrate that nilotinib ablated DNMT expression, reduced DNA methylation and restored p15INK4B and CDH1 expression through promoter DNA demethylation in vitro, ex vivo and in vivo. When DNMT1 was depleted, AML cells became more sensitive to nilotinib treatment. These results reveal the DNMTs and the subsequent DNA methylation as non-RTK targets underlying the anti-leukemic actions of nilotinib. Although we demonstrated that nilotinib abolished DNMT1 expression through the dysfunction of Sp1, a DNMT1 activator (27), nilotinib could also dephosphorylate DNMT1 through inactivation of KIT/FLT3-AKT axis. This could lead to the destabilization of the DNMT1 protein or the disruption of the DNMT1 and EZH2 complex, eventually reducing DNA methylation levels. Furthermore, because the expression of DNMT3a and DNMT3b was also impaired in the presence of nilotinb, and given that Sp1 is a transcriptional regulator of the DNMT3a and DNMT3b genes (48), nilotinib-induced DNA hypomethylation is, at least partially, attributed to the abrogation of Sp1-associated DNMT3a and DNMT3b expression. Notably, although upregulation of DNMTs is prevalent in AML (1,20,22), that all AML patients carry such overexpression is unlikely. Thus, nilotinib, when used as a DNA hypomethylating agent, remains an additional therapeutic option only for a patient subgroup characterized by deregulation of RTK/DNMT axis or who are unresponsive to decitabine therapies. Collectively, given that other RTK inhibitors, such as PKC412 and imatinib, also induced DNA demethylation in leukemia cells (unpublished data/Liu), the findings from this study support the hypothesis that RTKs are additional upstream activators of the DNA methylation program and RTK inhibitors could be considered as a new class of DNA methylation inhibitors by distinct mechanisms compared to classical DNA hypomethylating agents.
Supplementary Material
Translational Relevance.
This study provides the first evidence that RTKs are modulators of DNMT1-dependent DNA methylation in leukemia cells. Our study has for the first time documented that RTK inhibitors (RTKi) impair DNMT1 expression resulting in global and gene specific DNA hypomethylation. These findings demonstrate RTKs as new types of epigenetic regulators and unravel a signaling interaction between RTKs and DNMTs in leukemia pathogenesis, shedding light on leukemia molecular biology. Our data identify the DNA hypomethylating activities of RTKi, thus significantly expanding the pool of DNA methylation inhibitors. Our discoveries provide a mechanistic explanation why RTKi show therapeutic efficacy in patients without target mutations, and suggest that altered DNA methylation profile might be alternative predictors of responses in patients without RTK mutations. Altogether, our work provides the preclinical rationale for using RTKi to benefit patient subpopulations characterized by aberrant DNA methylation including those who relapse from current epigenetic therapy.
Acknowledgments
The authors wish to thank the Novartis Pharmaceuticals Corporation for providing financial support and drug (Nilotinib) supply for the clinical trial.
Grant Support: This work was supported in part by Hormel Foundation and National Cancer Institute (Bethesda, MD) grants R01CA149623 and R21CA155915
Footnotes
Authors' Contributions: Conception and design: S. Liu
Development of methodology: N. Shen, F. Yan, J. Pang, N. Zhao, L. Wu
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): S. Liu, M. Litzow, A. Al-Kali, N. Gangat
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): S. Liu, N. Shen, F. Yan
Writing, review, and/or revision of the manuscript: S. Liu, M. Litzow, A. Al-Kali, A. Bode, N. Shen, F. Yan, N. Gangat
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databased): S. Liu, M. Litzow, A. Al-Kali
Study supervision: S. Liu
References
- 1.Liu S, Wu LC, Pang J, Santhanam R, Schwind S, Wu YZ, et al. Sp1/NFkappaB/HDAC/miR-29b regulatory network in KIT-driven myeloid leukemia. Cancer cell. 2010;17(4):333–47. doi: 10.1016/j.ccr.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vicente C, Vazquez I, Marcotegui N, Conchillo A, Carranza C, Rivell G, et al. JAK2-V617F activating mutation in acute myeloid leukemia: prognostic impact and association with other molecular markers. Leukemia. 2007;21(11):2386–90. doi: 10.1038/sj.leu.2404812. [DOI] [PubMed] [Google Scholar]
- 3.Whitman SP, Ruppert AS, Marcucci G, Mrozek K, Paschka P, Langer C, et al. Long-term disease-free survivors with cytogenetically normal acute myeloid leukemia and MLL partial tandem duplication: a Cancer and Leukemia Group B study. Blood. 2007;109(12):5164–7. doi: 10.1182/blood-2007-01-069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Advani AS, Pendergast AM. Bcr-Abl variants: biological and clinical aspects. Leukemia research. 2002;26(8):713–20. doi: 10.1016/s0145-2126(01)00197-7. [DOI] [PubMed] [Google Scholar]
- 5.Quintas-Cardama A, Cortes JE. Chronic myeloid leukemia: diagnosis and treatment. Mayo Clinic proceedings. 2006;81(7):973–88. doi: 10.4065/81.7.973. [DOI] [PubMed] [Google Scholar]
- 6.Quintas-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood. 2009;113(8):1619–30. doi: 10.1182/blood-2008-03-144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer cell. 2002;2(2):117–25. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 8.Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer cell. 2005;7(2):129–41. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. The New England journal of medicine. 2006;354(24):2542–51. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 10.Giles FJ, le Coutre PD, Pinilla-Ibarz J, Larson RA, Gattermann N, Ottmann OG, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27(1):107–12. doi: 10.1038/leu.2012.181. [DOI] [PubMed] [Google Scholar]
- 11.Packer LM, Rana S, Hayward R, O'Hare T, Eide CA, Rebocho A, et al. Nilotinib and MEK inhibitors induce synthetic lethality through paradoxical activation of RAF in drug-resistant chronic myeloid leukemia. Cancer cell. 2011;20(6):715–27. doi: 10.1016/j.ccr.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow KU, Nowak D, Trepohl B, Hochmuth S, Schneider B, Hoelzer D, et al. The tyrosine kinase inhibitor AMN107 (Nilotinib) exhibits off-target effects in lymphoblastic cell lines. Leukemia & lymphoma. 2007;48(7):1379–88. doi: 10.1080/10428190701385181. [DOI] [PubMed] [Google Scholar]
- 13.Verstovsek S, Golemovic M, Kantarjian H, Manshouri T, Estrov Z, Manley P, et al. AMN107, a novel aminopyrimidine inhibitor of p190 Bcr-Abl activation and of in vitro proliferation of Philadelphia-positive acute lymphoblastic leukemia cells. Cancer. 2005;104(6):1230–6. doi: 10.1002/cncr.21299. [DOI] [PubMed] [Google Scholar]
- 14.Manley PW, Drueckes P, Fendrich G, Furet P, Liebetanz J, Martiny-Baron G, et al. Extended kinase profile and properties of the protein kinase inhibitor nilotinib. Biochimica et biophysica acta. 2010;1804(3):445–53. doi: 10.1016/j.bbapap.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 16.Peschard P, Park M. Escape from Cbl-mediated downregulation: a recurrent theme for oncogenic deregulation of receptor tyrosine kinases. Cancer cell. 2003;3(6):519–23. doi: 10.1016/s1535-6108(03)00136-3. [DOI] [PubMed] [Google Scholar]
- 17.Bache KG, Slagsvold T, Stenmark H. Defective downregulation of receptor tyrosine kinases in cancer. The EMBO journal. 2004;23(14):2707–12. doi: 10.1038/sj.emboj.7600292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plass C, Yu F, Yu L, Strout MP, El-Rifai W, Elonen E, et al. Restriction landmark genome scanning for aberrant methylation in primary refractory and relapsed acute myeloid leukemia; involvement of the WIT-1 gene. Oncogene. 1999;18(20):3159–65. doi: 10.1038/sj.onc.1202651. [DOI] [PubMed] [Google Scholar]
- 19.Melki JR, Vincent PC, Clark SJ. Concurrent DNA hypermethylation of multiple genes in acute myeloid leukemia. Cancer research. 1999;59(15):3730–40. [PubMed] [Google Scholar]
- 20.Mizuno S, Chijiwa T, Okamura T, Akashi K, Fukumaki Y, Niho Y, et al. Expression of DNA methyltransferases DNMT1, 3A, and 3B in normal hematopoiesis and in acute and chronic myelogenous leukemia. Blood. 2001;97(5):1172–9. doi: 10.1182/blood.v97.5.1172. [DOI] [PubMed] [Google Scholar]
- 21.Hayette S, Thomas X, Jallades L, Chabane K, Charlot C, Tigaud I, et al. High DNA methyltransferase DNMT3B levels: a poor prognostic marker in acute myeloid leukemia. PloS one. 2012;7(12):e51527. doi: 10.1371/journal.pone.0051527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen N, Yan F, Pang J, Wu LC, Al-Kali A, Litzow MR, et al. A nucleolin-DNMT1 regulatory axis in acute myeloid leukemogenesis. Oncotarget. 2014;5(14):5494–509. doi: 10.18632/oncotarget.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsumura A, Hayakawa T, Kumaki Y, Takebayashi S, Sakaue M, Matsuoka C, et al. Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes to cells : devoted to molecular & cellular mechanisms. 2006;11(7):805–14. doi: 10.1111/j.1365-2443.2006.00984.x. [DOI] [PubMed] [Google Scholar]
- 24.Trowbridge JJ, Sinha AU, Zhu N, Li M, Armstrong SA, Orkin SH. Haploinsufficiency of Dnmt1 impairs leukemia stem cell function through derepression of bivalent chromatin domains. Genes & development. 2012;26(4):344–9. doi: 10.1101/gad.184341.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito Y, Kitamura H, Hijikata A, Tomizawa-Murasawa M, Tanaka S, Takagi S, et al. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Science translational medicine. 2010;2(17) doi: 10.1126/scitranslmed.3000349. 17ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garzon R, Liu S, Fabbri M, Liu Z, Heaphy CE, Callegari E, et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009;113(25):6411–8. doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S, Liu Z, Xie Z, Pang J, Yu J, Lehmann E, et al. Bortezomib induces DNA hypomethylation and silenced gene transcription by interfering with Sp1/NF-kappaB-dependent DNA methyltransferase activity in acute myeloid leukemia. Blood. 2008;111(4):2364–73. doi: 10.1182/blood-2007-08-110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esteve PO, Chang Y, Samaranayake M, Upadhyay AK, Horton JR, Feehery GR, et al. A methylation and phosphorylation switch between an adjacent lysine and serine determines human DNMT1 stability. Nature structural & molecular biology. 2011;18(1):42–8. doi: 10.1038/nsmb.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao XN, Yan F, Lin J, Gao L, Lu XL, Wei SC, et al. AML1/ETO cooperates with HIF1alpha to promote leukemogenesis through DNMT3a transactivation. Leukemia. 2015;29(8):1730–40. doi: 10.1038/leu.2015.56. [DOI] [PubMed] [Google Scholar]
- 30.Yan F, Shen N, Pang J, Xie D, Deng B, Molina JR, et al. Restoration of miR-101 suppresses lung tumorigenesis through inhibition of DNMT3a-dependent DNA methylation. Cell death & disease. 2014;5:e1413. doi: 10.1038/cddis.2014.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan F, Shen N, Pang JX, Zhang YW, Rao EY, Bode AM, et al. Fatty acid-binding protein FABP4 mechanistically links obesity with aggressive AML by enhancing aberrant DNA methylation in AML cells. Leukemia. 2016;31(6):1434–1442. doi: 10.1038/leu.2016.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ottmann OG, Larson RA, Kantarjian HM, le Coutre PD, Baccarani M, Hochhaus A, et al. Phase II study of nilotinib in patients with relapsed or refractory Philadelphia chromosome--positive acute lymphoblastic leukemia. Leukemia. 2013;27(6):1411–3. doi: 10.1038/leu.2012.324. [DOI] [PubMed] [Google Scholar]
- 33.Cullinane C, Natoli A, Hui Y, Conus N, Jackson S, Bruggen J, et al. Preclinical evaluation of nilotinib efficacy in an imatinib-resistant KIT-driven tumor model. Molecular cancer therapeutics. 2010;9(5):1461–8. doi: 10.1158/1535-7163.MCT-09-1181. [DOI] [PubMed] [Google Scholar]
- 34.Jelinek J, Gharibyan V, Estecio MR, Kondo K, He R, Chung W, et al. Aberrant DNA methylation is associated with disease progression, resistance to imatinib and shortened survival in chronic myelogenous leukemia. PloS one. 2011;6(7):e22110. doi: 10.1371/journal.pone.0022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herman JG, Jen J, Merlo A, Baylin SB. Hypermethylation-associated inactivation indicates a tumor suppressor role for p15INK4B. Cancer research. 1996;56(4):722–7. [PubMed] [Google Scholar]
- 36.Quesnel B, Guillerm G, Vereecque R, Wattel E, Preudhomme C, Bauters F, et al. Methylation of the p15(INK4b) gene in myelodysplastic syndromes is frequent and acquired during disease progression. Blood. 1998;91(8):2985–90. [PubMed] [Google Scholar]
- 37.Zhang TJ, Zhou JD, Ma JC, Deng ZQ, Qian Z, Yao DM, et al. CDH1 (E-cadherin) expression independently affects clinical outcome in acute myeloid leukemia with normal cytogenetics. Clinical chemistry and laboratory medicine. 2017;55(1):123–131. doi: 10.1515/cclm-2016-0205. [DOI] [PubMed] [Google Scholar]
- 38.Liersch R, Muller-Tidow C, Berdel WE, Krug U. Prognostic factors for acute myeloid leukaemia in adults--biological significance and clinical use. British journal of haematology. 2014;165(1):17–38. doi: 10.1111/bjh.12750. [DOI] [PubMed] [Google Scholar]
- 39.Yan F, Shen N, Pang J, Molina JR, Yang P, Liu S. The DNA Methyltransferase DNMT1 and Tyrosine-Protein Kinase KIT Cooperatively Promote Resistance to 5-Aza-2′-deoxycytidine (Decitabine) and Midostaurin (PKC412) in Lung Cancer Cells. The Journal of biological chemistry. 2015;290(30):18480–94. doi: 10.1074/jbc.M114.633693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R, Kobayashi R, Bishop JM. Cellular adherence elicits ligand-independent activation of the Met cell-surface receptor. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(16):8425–30. doi: 10.1073/pnas.93.16.8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giordano S, Ponzetto C, Di Renzo MF, Cooper CS, Comoglio PM. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature. 1989;339(6220):155–6. doi: 10.1038/339155a0. [DOI] [PubMed] [Google Scholar]
- 42.Dicker F, Haferlach C, Kern W, Haferlach T, Schnittger S. Trisomy 13 is strongly associated with AML1/RUNX1 mutations and increased FLT3 expression in acute myeloid leukemia. Blood. 2007;110(4):1308–16. doi: 10.1182/blood-2007-02-072595. [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Issa JP, Herman J, Bassett DE, Jr, Nelkin BD, Baylin SB. Expression of an exogenous eukaryotic DNA methyltransferase gene induces transformation of NIH 3T3 cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(19):8891–5. doi: 10.1073/pnas.90.19.8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vertino PM, Yen RW, Gao J, Baylin SB. De novo methylation of CpG island sequences in human fibroblasts overexpressing DNA (cytosine-5-)-methyltransferase. Molecular and cellular biology. 1996;16(8):4555–65. doi: 10.1128/mcb.16.8.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakin AV, Curran T. Role of DNA 5-methylcytosine transferase in cell transformation by fos. Science. 1999;283(5400):387–90. doi: 10.1126/science.283.5400.387. [DOI] [PubMed] [Google Scholar]
- 46.Belinsky SA, Klinge DM, Stidley CA, Issa JP, Herman JG, March TH, et al. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer research. 2003;63(21):7089–93. [PubMed] [Google Scholar]
- 47.Zhang Q, Wang HY, Woetmann A, Raghunath PN, Odum N, Wasik MA. STAT3 induces transcription of the DNA methyltransferase 1 gene (DNMT1) in malignant T lymphocytes. Blood. 2006;108(3):1058–64. doi: 10.1182/blood-2005-08-007377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jinawath A, Miyake S, Yanagisawa Y, Akiyama Y, Yuasa Y. Transcriptional regulation of the human DNA methyltransferase 3A and 3B genes by Sp3 and Sp1 zinc finger proteins. The Biochemical journal. 2005;385(Pt 2):557–64. doi: 10.1042/BJ20040684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.