Abstract
Purpose
Early detection is essential for treatment plans before onset of metastatic disease. Our purpose was to demonstrate feasibility to detect and monitor estrogen receptor 1 (ESR1) gene mutations at the single circulating tumor cell (CTC) level in metastatic breast cancer (MBC).
Experimental Design
We used a CTC molecular characterization approach to investigate heterogeneity of 14 hot spot mutations in ESR1 and their correlation with endocrine resistance. Combining the CellSearch® and DEPArray™ technologies allowed recovery of 71 single CTCs and 12 WBC from 3 ER-positive MBC patients. 40 CTCs and 12 WBC were subjected to whole genome amplification by MALBAC and Sanger sequencing.
Results
Among 3 selected patients, 2 had an ESR1 mutation (Y537). One showed two different ESR1 variants in a single CTC and another showed loss of heterozygosity. All mutations were detected in matched cell-free DNA (cfDNA). Furthermore, one had 2 serial blood samples analyzed and showed changes in both cfDNA and CTCs with emergence of mutations in ESR1 (Y537S and T570I), which has not been reported previously.
Conclusions
CTCs are easily accessible biomarkers to monitor and better personalize management of patients with previously demonstrated ER-MBC who are progressing on endocrine therapy. We showed that single CTC analysis can yield important information on clonal heterogeneity, and can be a source of discovery of novel and potential driver mutations. Finally, we also validate a workflow for liquid biopsy that will facilitate early detection of ESR1 mutations, the emergence of endocrine resistance and the choice of further target therapy.
Keywords: ESR1 mutation, single circulating tumor cell, metastatic breast cancer, endocrine resistance, tumor heterogeneity
INTRODUCTION
Breast cancer (BC) is the most frequent cancer and the leading cause of cancer death among women. Despite advances in prevention, diagnosis and adjuvant treatment, about 30% of BC patients develop metastatic disease [1]. Recent advances suggest that the presence of different tumor cell clones play an important role in metastatic progression and resistance to chemotherapy [2]. According to the clonal theory of tumor evolution, cancer is an evolving process [3] and the selective pressure exerted by multiple lines of treatment may lead to selection of much more aggressive sub-clone populations or even those with an acquired drug resistance [4].
About 75% of breast cancers express the estrogen receptor (ER); and, acting on this signaling pathway is a key treatment strategy. The main endocrine therapeutic approaches are: 1) selective ER modulators (SERMs); 2) inhibitor of aromatase (AIs); and, 3) selective ER down-regulators (SERDs) [5]. However, in 20–25% of metastatic breast cancer (MBC) patients endocrine therapy failure has been reported after several lines of treatment and new targeted therapies have been approved to be combined with hormone therapy [6,7,8]. Several molecular mechanisms of resistance may be involved, including down-regulation and post-translational modification of the ER encoded by the ESR1 gene [9]. Since ESR1 mutations are rare, occurring in only 1% of primary BC, their ability to confer endocrine resistance has been speculated for many years [10]. However, in metastatic tissues the incidence of such mutations is estimated at 20% [11]. In the last several years, 14 ESR1 point mutations have been reported, mainly localized in the ligand-binding domain (LBD) including 3-hot spot mutations in codons 380, 537 and 538. Functional study of these mutations showed an ER ligand-independent activity, highlighting their role in acquired endocrine resistance [12,13]. Therefore, genomic characterization of distant metastasis may provide clinically useful information for the selection of specific therapeutic treatments [14,15]. Even though genetic testing on repeated metastatic biopsies may not be representative of the whole tumor mass, and leads to an underestimation of tumor heterogeneity [16].
Liquid biopsy using either circulating tumor cells (CTCs) or cell-free tumor DNA (ctDNA) has become one of the most sensitive approaches to monitor tumor molecular evolution [17,18]. CTCs can be isolated non-invasively over time [19] and over the past decade, the prognostic value of CTCs has been shown in metastatic breast, colorectal and prostate cancer [20,21,22,23,24,25,26]. Significant advances in cancer diagnosis and in the evaluation of disease progression and treatment can be reached with single CTC analysis because of the improvements made in single cell genomics analysis [27]. Currently, there are few techniques available for single cell isolation, including micromanipulation, laser micro-dissection and high throughput fluorescence-activated cell sorting (FACS). These approaches have several disadvantages including inadequate detection sensitivity for CTC population, a required high number of cells as a starting population and are manual and laborious methods [28,29,30].
We decided to study the molecular features of CTCs in patients with hormone-receptor positive (HR+) MBC receiving endocrine therapy. We planned to validate a laboratory workflow for single CTC detection, isolation and molecular analysis by combining the sequential use of CellSearch® [20,21,22,23,24,25,26], DEPArray™ systems [31,32] and MALBAC techniques [33]. The purpose of this study was to investigate the incidence and heterogeneity of ER expression and to evaluate the detection of ESR1 mutations in individual CTCs. We also planned to compare our single CTC data with matched cfDNA.
MATERIALS AND METHODS
Patients and sample collection
Thirty MBCs patient were enrolled at the Department of Medical Oncology, Thomas Jefferson University in Philadelphia, between February and September 2015. Only patients with a primary ER-positive metastatic breast cancer were included. Clinical parameters included sex, age at surgery, differentiation grade, lymph node metastasis, distant metastasis, TNM stage and histology. Progesterone receptor (PR) and ER status of the primary tumor and of available metastases were recorded. All subjects gave informed consent and the study was approved by the Institutional Review Board. For each patient, 10 ml of blood was collected in two CellSave™ tubes (Veridex, LLC) for enrichment, enumeration and molecular characterization of CTCs using the FDA-approved CellSearch® System. All samples were taken at least 5 days after the last treatment. Matching primary tumor tissues were tested for presence of mutations in ER receptor before starting hormonal therapy.
Cell lines
Two human breast cancer cell lines (MCF-7 and FC-IBC-02), and a prostate cancer cell line (C4-2) were used to validate whole genome amplification experiments. MCF-7 cells were maintained in DMEM containing 10% (v/v) FBS, 100 units/ml penicillin and 100 mg/ml streptomycin. FC-IBC-02 primary cells were isolated from pleural effusion of IBC patients and cultured in Ham’s F12 with 10% (v/v) FBS, 5 ml Insulin and 100 µg/µl of hydrocortisone with antibiotic-antimicotic. C4-2 cell line was cultured in RPMI with 2.5–10% (v/v) FBS as previously described [34] All cell lines were maintained in T-25 or T-75 flasks using prescribed cell culture conditions [5% (v/v) CO2, 37 °C].
Quality control and experimental procedure validation
Single tumor cells were obtained from MCF-7 and FC-IBC-02 cell lines through micromanipulation and from C4-2 cell line by serial dilution. All collected cells were processed for MALBAC. Meanwhile, 30 pg of genomic DNA carrying the V600E mutation in the BRAF gene was used as MALBAC positive control for all WGA products. To validate the CellSearch® ability to capture CTC, 100 C4-2 cells were spiked into a healthy donor blood sample. Captured C4-2 cells together with WBC from the healthy donor were loaded on the DepArray cartridge to achieve single cell isolation and capture.
DEPArray™ system is a semiautomated system that allows the isolation of rare fluorescently labelled cells. An electric field is generated on the surface of a silicon chip directly interfaced to a microfluidic chamber containing the cell suspension and an array of electrodes. Each electrode can be programmed to achieve a cage of dielectrophoresis, inside of which single CTC can be trapped and then analyzed individually.
Individual C4-2 and WBC cells were subsequently MALBAC amplified and screened for 7 known mutations in the AR, CDH1, PIK3C3, NCOR2, ERBB2, CDK4 and ETV1 genes by Sanger sequencing. C4-2 and WBC genomic DNA from a healthy donor also were used to confirm the 7 variants by Sanger sequencing.
Enrichment, immune labeling and enumeration of CTCs
Standard CellSearch® protocol for CTCs enrichment and enumeration was employed according to the manufacturer’s instructions. Briefly, CTCs were enriched on the CellTracks Autoprep® using ferrofluid conjugated with EpCAM antibody. Cells were stained with fluorescently labeled monoclonal antibody for cytokeratin CK8-, CK18-, CK19-FITC as well as for leuckocyte common antigen CD45-APC and nuclear-stained with DAPI. Moreover, ER expression on MCF-7 cells and CTCs was assessed by staining the cells with a PE-conjugated anti-ER nuclear antibody.
Since the DEPArray™ system (Silicon Biosystem, San Diego, CA) provides the analysis of only 66% of the loaded volume, to optimize single CTC recovery rate, only patients exhibiting >20 CTCs (ER+ and ER-) were processed.
Single CTC isolation
Briefly, for each CellSearch enriched sample, 13 µl were loaded with 325 µl manipulation buffer (SB115, Silicon Biosiystem) into a A300K cartridge. Approximately 8.6 µl of the sample is de facto dielectrophoretically processed in which cells are individually trapped in cage. The cartridge is then scanned by an automated fluorescence microscope and cells detected by DAPI staining. Three different populations of cells were isolated: 1) ER+ CTCs, defined as ER-positive, CK-8, CK-18, CK-19 positive, CD45 negative; 2) ER- CTCs, defined as ER-negative, CK positive, CD45 negative; and, 3) WBCs, defined as CD45 positive, ER- and CK negative. Each cell was collected individually, washed two times in PBS and stored at −80°C or immediately lysed in accordance with MALBAC protocol [35]. To minimize DNA contamination in the same isolation cage containing the individual cell, an aliquot of the elution buffer from the single cage was MALBAC-amplified and subjected to DNA Sanger sequencing. No mutation was detected on all elution buffer reactions. Moreover, for each sequencing run, a no template control also was tested.
Whole Genome Amplification
Cell lysis and genome amplification was performed using the MALBAC kit (Yikon Genomics YK001A/B version 1302.1, Jiangsu, China) [33], following manufacturer’s instructions. A negative no template control (NTC), a blank control (SB115) and a MALBAC positive control were used for each MALBAC reaction. WGA products were then purified according to Agencourt AMPure XP bead kit (Beckman Coulter, Sharon Hill, PA) manufacturer’s protocol [36] and QC using Qubit® dsDNA High Sensitivity Assay kit (ThermoFisher, Waltham, MA). WGA products were run on 0.8% (v/v) agarose gel and checked for expected distribution in size (300 to 2000 bp).
Sanger sequencing
Sanger sequencing was performed to genotype all WGA products as well as 14 hot-spot mutations in the ESR1 gene found in MBC tissues and related controls on an AB 3730 following manufacturer’s protocol. Detailed PCR conditions (Tm) and primer sequences are available in supplementary methods (Table 1 supplementary methods). Sequences were analyzed and genotyped by SeqScape v3.0 analysis software (ThermoFisher, Waltham, MA).
RESULTS
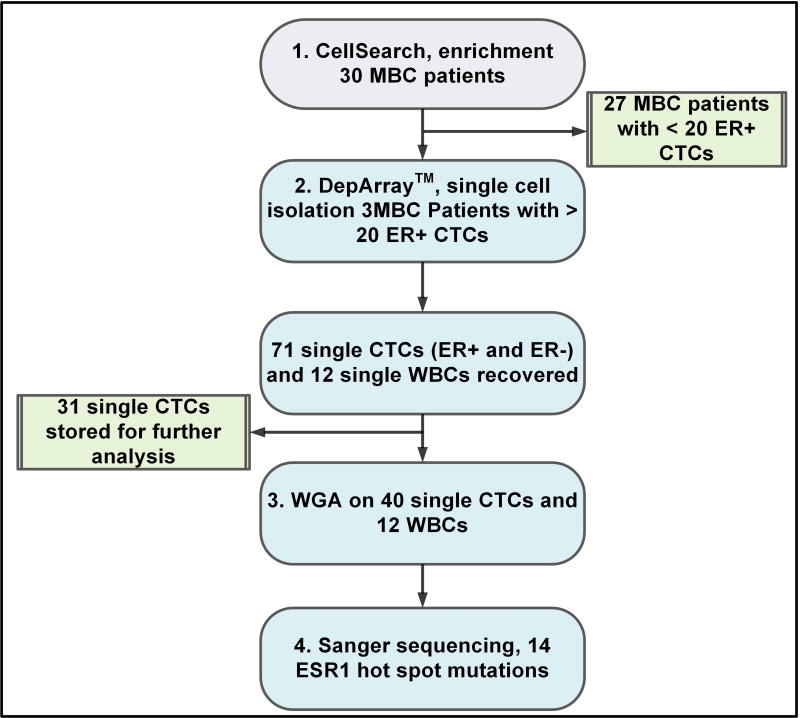
To investigate whether detection of ESR1 mutations in individual CTCs in MBC patients could be used as a tool to enable monitoring of the metastatic burden for clinical decision-making, a 4-step protocol was implemented with the following workflow: 1) CTC enrichment; 2) Single cell isolation; 3) Whole genome amplification; 4) Sanger sequencing (Figure 1).
Figure 1. Study workflow.
The total number of CTCs analyzed from 3 different patients which showed a number of ER+ CTCs > 20. For each patient 3 WBC were recovered as negative controls. One patient was tested twice because of disease progression
Patient and pathological features
A cohort of 30 metastatic breast cancer patients was characterized by a median age of 56 years. 36.6% of the patients showed evidence of one single metastatic lesion while 64% showed more than one at the time of the first draw. Clinical and pathological features of primary and metastatic tumor tissues are summarized in Table 1. Among the total number of patients, 28 had histologically confirmed ER positivity even at metastatic sites. At the time of surgery, primary tumor tissues were investigated for presence of ER mutations and all harbored a wild-type genotype.
Table 1.
Clinicopathologic features of 30 MBCs
| Clinicopathologic Features |
Detail | n |
|---|---|---|
| Age | Median | 56 |
| Minimum | 34 | |
| Maximum | 79 | |
| Histology | Ductal | 18 |
| Lobular | 7 | |
| Other | 2 | |
| Missing | 3 | |
| Type | IBC | 16 |
| No IBC | 14 | |
| ER | Positive | 30 |
| Negative | 0 | |
| PR | Positive | 20 |
| Negative | 10 | |
| HER2 | Positive | 2 |
| Negative | 24 | |
| Missing | 4 | |
| Metastasis sites | n=1 | 11 |
| n>1 | 19 | |
| ER metastatis* | Positive | 28 |
| Negative | 2 | |
| PR metastasis* | Positive | 20 |
| Negative | 10 |
IBC = inflammatory breast cancer;
ER and PR status immunohistochemistry on available metastatic lesions
Enrichment, isolation and genome amplification of individual CTCs
Enrichment and enumeration of CTCs performed on CellSearch® involved a total of 50 blood samples taken from the 30 patients enrolled. Number of CTCs based on ER expression (Figure 2) for each patient (ID) are shown in Table 2. Overall, the average of total CTCs enumerated was 80, with a maximum of 1375 cells. 22% of samples were negative (no CTCs) for presence of CTCs, defining a group of patients currently responsive to current treatment. The remaining 39 samples were divided into two groups depending on the established cut-off of 5 CTCs [37] used to identify patients with high risk of disease progression (12 samples <5 CTCs vs 27 samples ≥5 CTCs). Only 4 of the 50 samples analyzed showed a number of CTCs greater or equal to 20 and were processed on the DEPArray™. Seventy-one single CTCs and 12 white blood cells (WBCs) were retrieved. Forty of these CTCs and all the WBCs were subjected to WGA. The number of CTCs (subdivided in ER+ or ER-) isolated for each individual patient and the corresponding number of selected cells for WGA are summarized in Table 3. Samples processed on DEPArray™ showed between 21% and 30% of CTCs recovered.
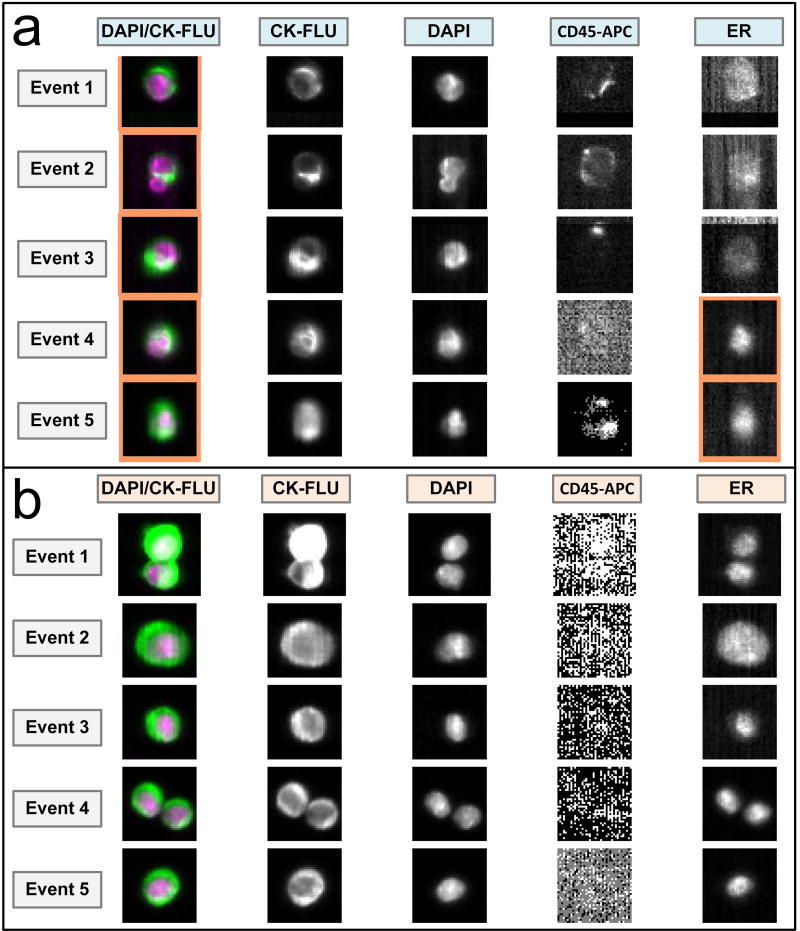
Figure 2. ER nuclear expression.
Representative CellSearch™ images for: a) ER expression in MCF-7 cell line (event 1, 2, 3 are ER-, event 4 and 5 are ER+); b) CTCs from patient sample (all event are ER+).
Table 2.
CTCs number assessed by CellSearch® for each patient, based on ER surface expression.
| Patient ID # |
CTC+/ER − |
CTC+/ER + |
Total |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 2* | 1 | 3 | 4 |
| 3 | 1 | 4 | |
| 3 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 |
| 4 | 1 | 0 | 1 |
| 5* | 2 | 2 | 4 |
| 0 | 2 | 2 | |
| 6 | 0 | 6 | |
| 6 | 3 | 0 | 3 |
| 7* | 3 | 0 | 3 |
| 2 | 2 | 4 | |
| 8 | 0 | 5 | 5 |
| 9 | 2 | 2 | 4 |
| 10* | 11 | 43 | 54 |
| 22 | 58 | 80 | |
| 10 | 13 | 31 | 44 |
| 11 | 0 | 0 | 0 |
| 12 | 5 | 0 | 5 |
| 13 | 1 | 0 | 1 |
| 14 | 2 | 3 | 5 |
| 15* | 9 | 18 | 27 |
| 0 | 1 | 1 | |
| 16* | 147 | 0 | 147 |
| 410 | 0 | 410 | |
| 17 | 0 | 2 | 2 |
| 18* | 13 | 15 | 28 |
| 5 | 14 | 19 | |
| 19 | 19 | 33 | 52 |
| 20* | 12 | 44 | 56 |
| 13 | 9 | 22 | |
| 21 | 0 | 0 | 0 |
| 22* | 1361 | 14 | 1375 |
| 931 | 0 | 931 | |
| 23 | 0 | 0 | 0 |
| 24 | 0 | 0 | 0 |
| 25 | 22 | 18 | 40 |
| 26 | 19 | 9 | 28 |
| 27 | 0 | 0 | 0 |
| 28 | 0 | 0 | 0 |
| 29 | 0 | 0 | 0 |
| 30 | 0 | 0 | 0 |
ID = identification number;
more than one draw was performed during the enrollment period.
Table 3.
Summary of the number of CTCs detected, isolated and selected to perform WGA
| Patient ID |
N CTCs on CellSearch® ER(+,−) |
N CTCs on DEPArray™ ER(+,−) |
% of CTCs recovered |
N WGA performed on ER(+,−) |
N WGA performed on WBCs |
|---|---|---|---|---|---|
| 19 | 56(44,12) | 17(8,9) | 30 | 7(5,2) | 3 |
| 20 | 52(33,19) | 11(6,5) | 21 | 5(3,2) | 3 |
| 10* | 54(43,11) | 15(11,4) | 28 | 12(8,4) | 3 |
| 10* | 80(28,52) | 19(8,11) | 23 | 16(8,8) | 3 |
Two draws were performed during the enrollment period; a third count on CellSearch® was performed and showed a decrease in the total number of CTCs (44), but an almost stable number of ER+ CTCs (23).
Pre-clinical validation of single cell genome amplification and analysis
Validation of single cell genome amplification was conducted on 30 individual single cells. DNA positive controls, after MALBAC amplification showed heterozygosity for the BRAF V600E (c.1860T>a) mutation (data not shown), as expected. No other BRAF mutations were found in all the wild-type single cells analyzed, demonstrating feasibility of the protocol.
Following spiking, enrichment and immune-labeling, 91 positive CTCs were detected by CellSearch®. 65% of these CTCs were identified on the DEPArray™ and finally 10 individual cells were recovered and subjected to whole genome amplification. In addition, 10 WBCs were recovered and subjected to WGA, as negative control. The sequences obtained from all the C4-2 cells (single or pooled) revealed all 7 carried known mutations (AR/T878A, CDH1/P94T, CDK4/P110L, ErbB2/E930D, ETV1/G207E, NCOR2/L167P, PIK3C3/F524C) (data not shown). Sequences obtained from the 10 WBCs from healthy donor’s buffy coat showed wild-type genotypes for all variants tested.
ESR1 mutational analysis in single CTCs
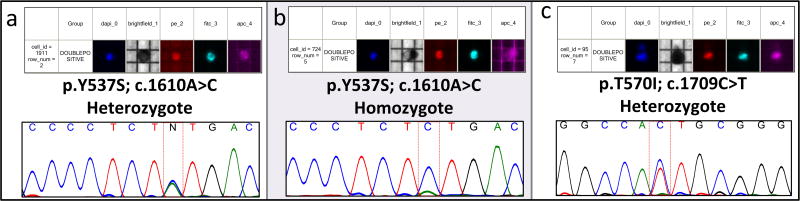
ESR1 mutation analysis was successfully performed on all single cells isolated. All mutations were located within the ligand-binding domain of the ESR1 gene in exons 4, 5, 6, 7 and 8 (Figure 3). All 12 WBCs, showed a wild type genotype confirming the absence of mutations in the germ line. Overall, we found ESR1 mutations in a total of 8 CTCs belonging to 2 MBC patients. High levels of intra- and inter-tumor genetic heterogeneity in ER positive CTCs populations also was revealed. The remaining 32 CTCs analyzed showed a wild-type ESR1 genotype. For patient ID20, a total of 5 single CTCs were analyzed and all showed wild type phenotype. Patient ID19 exhibited a heterogeneous ESR1 genotype in their CTC populations. Among the ER+ CTCs population we detected 3 different genotypes: a) one single wild type CTC; b) 3 CTCs heterozygous for a single mutation (Y537S) in exon 8 (Figure 3a); c) one single CTC homozygous for the same Y537S (LOH) (Figure 3b). Matching cfDNA was tested, confirming the Y537S mutation at 0.25% allele frequency. Patient ID10 was the only one who had 2 serial blood samples taken, 3 months apart. The first sample showed an ESR1 wild type genotype in all the 12 CTCs recovered. The second showed a wildtype genotype in all 8 ER- CTCs, and in 4 of the ER+ CTCs recovered. Three CTCs were heterozygous for the Y537S mutation, whereas the remaining ER+ CTC harbored 2 different mutations in exon 8. Other than the Y537S we found a new mutation, not reported until now, the T570I (Figure 3c). Also in this case data were compared with those obtained on matching cfDNA. No mutation was detected at the first draw, while Y537S was detected at the second sampling at a percentage of 0.18%.
Figure 3. DEPArray™’s imaging reports and Sanger Sequence result.
a) Example of Y537S heterozygous mutation; b) The only homozygous Y537S mutation found in patient ID10; c) T570I novel, unreported mutation found in patient ID10.
Correlation between ESR1 mutation and patients’ treatment
Patient ID19 with the Y537S mutation had only one sampling for CTC enumeration and circulating free tumor DNA (cfDNA) analysis. The first diagnosis of inflammatory ductal breast cancer was made in 2010, and the patient opted for holistic remedies. In 2011 the patient developed ascites and pleural effusions and started a chemotherapeutic treatment (Docetaxel-Cytoxan) for six months, followed by endocrine therapy with Aromasin. In 2014 the patient was subjected to 6 cycles of Doxil and Faslodex, but in January 2015, a liver metastasis was found. This progression to metastatic disease during treatment indicated a failure in the therapeutic approach. The analysis of CTCs and cfDNA confirmed this suspicion, with the finding of the Y537S activating mutation.
Patient ID10 reported two mutations, the Y537S and the newly reported T570I. This case report shows how the monitoring of ESR1 mutations can be crucial to monitor and predict disease evolution. First diagnosis was made in 2011, which was followed by a mastectomy. In 2012 she was irradiated and subsequently treated with Tamoxifen. Due to poor tolerability of the drug and to the occurrence of bone metastasis, the patient switched endocrine therapy, examestane followed by fulvetrant. At the time of the first draw, she was negative for ESR1 mutations, but a high increase in the number of CTCs was found compared to baseline CTCs count (80 vs. 54). The second sampling was taken only one month apart and was positive for the Y537S activating mutation. Furthermore, a third CTCs count was made after a further therapy switch to combination with palbociclib, showing a relative decrease in total number of CTCs (43 vs. 80), but an almost unchanged number of the ER+ population (23 vs 28). These prospective clinical evidences clearly indicate that the patient did not benefit from fulvestrant at time of ESR1 mutation detection. Instead, she benefited from the prompt switch to a combined therapy of palbociclib and fulvestrant, a highly effective regimen evaluated in the prospective, randomized, phase 3 study PALOMA-3, whose benefit appears irrespective of common genomic abnormalities such as ESR1 and PI3KCA [38, 39].
DISCUSSION
The frequency of ESR1 mutations in breast cancer is a matter of intense debate for the potential clinical utility of this information. Primary ESR1 mutations are relatively rare in primary tissue, up to 7% of specimens analyzed with very low allele frequencies (0.07–0.2%), when compared to a much higher detection in patients with metastatic disease [11]. The first study on the detection of ESR1 mutations in patients that were exposed to endocrine therapy was conducted on metastatic biopsies and matched cfDNA samples [37]. Two hot spot mutations in codon 537 and 538 of ESR1 gene were investigated by digital PCR (dPCR). Those findings showed monitoring ESR1 mutations by dPCR was feasible, but not all mutations found in the metastatic biopsies were detected also in matched cfDNA [40]. In our study we used the analysis on cfDNA as a validation of the results found at the CTC level. All mutations found in cfDNA were confirmed in the ER+ CTCs population. In CTCs we detected a new mutation in codon 8 (T570I) that was not detected in cfDNA. On the other hand, ER expression in CTCs showed a wide heterogeneous status. Most samples positive for CTCs showed a mixed population (ER+ and ER-), but 5 samples positive for CTCs were negative for ER expression. These results suggest that analysis of both CTCs and cfDNA can be a useful guide in clinical practice.
Our study is the first in which the combined systems (CellSearch and DEPArray) were applied to assess both ER expression and all 14 ESR1 hot spot mutations by MALBAC single cell amplification method. The combined approach, CellSearch® and DEPArray™, was tested already on cancer patients’ samples [41,42,43]. In a previous study, 510 CTCs were isolated from 66 MBCs. 37 CTCs were subjected to adaptor-ligation-mediated whole genome amplification and subsequently analyzed for the expression of the ErbB2 gene and for analysis of two hot spots in PIK3CA (exon 20 and 9) [41]. They demonstrated applicability of that workflow and also found some heterogeneity between the analyzed CTCs and primary tumor. Another group, studied the entire population of CTCs and white blood cells enriched from the CellSearch® system to genotype ESR1, PIK3CA, TP53, FGFR1 and FGFR2 genes, by a next generation sequencing (NGS) panel. Analysis of such markers also was done on cfDNA and 4 repeated samples over time during patient therapy monitoring. Two patients showed changes at the level of ESR1 gene mutations detected in cfDNA, only one in cfDNA and CTCs. This discordance can be explained by the fact that CTCs were analyzed as a pool with WBCs, where the predominant component is wild type [42]. Only recently the same group published a new study where the NGS panel was performed also on single CTC isolated by DEPArray™. The purpose of the study was to determine whether cfDNA can be compared to single CTC analysis to detect tumor mutation heterogeneity [44].
Our work is the first to evaluate detection of all activating ESR1 mutations among the LBD, at the single circulating tumor cell level in MBC patients. We also monitored the acquisition of endocrine resistance and validated data confirming mutations in matched cfDNA. Of the 4 samples processed and corresponding to 3 different patients, 2 had at least one mutation that was confirmed also in cfDNA, but not in primary tissue. In both samples, the mutation detected was Y537S, positioned in exon 8 of the LBD domain of ER, as well as one of the most common mutations found in metastatic lesions. This mutation was present only in some of the CTCs from the same patient, highlighting the importance of single cell analysis instead of the previously pooling strategy [40,42].
The role of wild type tyrosine 537 and the effects of a number of possible amino acid substitutions have been thoroughly investigated [13]. This site is located in domain E, ligand binding and recognition region, containing the functional transcription activating domain ligand dipendene-2 and involved in the regulation of ER transcriptional activity. Among all the substitutions tested, the Y537S was the only one that showed 100% activity of the receptor in the absence of ligand [45]. A recent study showed that such activity can be partially reduced by increasing the tamoxifen or fulvestrant doses, a possible strategy to avoid endocrine resistance [46]. This mutation could definitely be one of the main causes of poor or inadequate response to hormone therapy.
Finally, we also demonstrate how our workflow allows investigation of intra-tumor heterogeneity. A loss of heterozygosity (LOH) was detected in one single CTC. We cannot totally exclude that this may be due to a technical error deriving from WGA. Analyzing a higher number of CTCs for each patient has in part solved this issue. For the first time, in our work, MALBAC has been used in combination with the CellSearch® and the DEPArray™ systems. Among the various techniques, any cannot ensure the absence of errors like false positive/negative results or allelic dropout (ADO). Several comparison studies have shown that each WGA technique has its own benefits and drawbacks. The main advantages of MALBAC are associated with reduced ADO and PCR bias, high amplification efficiency even of GC rich regions and high genome coverage (up to 90%) [47]. Moreover, an improved ability of MALBAC in SNP variant identification has been recently reported, with better performance in uniformity and reproducibility [48]. Other methods, like SurePlex allows for a better copy number alterations (CNAs) detection, with a more uniformity amplification across the genome [49]. These observations led us to endorse MALBAC as the technique to be used in genotyping the ESR1 gene. MALBAC is therefore confirmed by us to be a reliable WGA method to address single CTC molecular profiling.
In conclusion, this study demonstrated the feasibility of our protocol to detect and monitor ESR1 gene mutations at the single CTC level in MBCs. Early detection is essential to set the correct treatment plan for patients, before onset of metastatic disease. In addition, analysis of individual CTCs could allow identification of new potentially driving mutations or even new genes involved in resistance. Further studies with larger numbers of patients are required to make this approach of use in the clinic.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Current treatment strategies including single agent endocrine agents or combinations with CDK4/6 inhibitors or mTOR inhibitors have increase capabilities of effective treatment of patients with hormone-receptor positive metastatic breast cancer. Primary or secondary endocrine-resistance is a major clinical challenge in the management of patients with advanced hormone-receptor positive breast cancer because is a dynamic phenomenon including development of estrogen-receptor (ESR1) mutations. The evaluation and longitudinal monitoring of endocrine resistance including enumeration of CTCs, measurement of heterogeneous estrogen-receptor expression in those cancer cells and detection of ESR1 mutations allows a real-time molecular monitoring allowing to adapt treatment modalities with potential impact on outcome.
Acknowledgments
We wish to thank Dr. Saul Surrey for helpful and valuable suggestions.
This work was supported in part by NIH-National Cancer Institute Core Grant P30CA56036 (PF), Thomas Jefferson University Institutional funds (PF), by the Catholic University of the Sacred Heart Institutional funds (CP).
Glossary
Abbreviations
- APC
allophycocyanin
- AR
androgen receptor
- CDH1
cadherin-1
- CDK4
cyclin-dependent kinase
- cfDNA
circulating free DNA
- CTC
circulating tumor cell
- DAPI
(4’,6-diamidino-2-phenylindole)
- EGFR2
human epidermal growth factor receptor 2
- ER
estrogen receptor
- ERBB2
erythroblastic leukemia viral oncogene homolog 2
- ESR1
estrogen receptor 1
- ETV1
ets-1 variant
- FITC
fluorescein-isothiocyanate
- IBC
inflammatory breast cancer
- MALBAC
multiple annealing and looping-based amplification cycles
- MBC
metastatic breast cancer
- NCOR2
nuclear receptor corepressor 2
- PE
phycoerythrin
- PIK3C3
phosphatidylinositol 3-kinase catalytic subunit type 3
- WGA
whole genome amplification
Footnotes
The authors declare no conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman Dl. Global cancer statistics. CA Cancer J Clin. 2011 Mar-Apr;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention therapy. J. Clin. Oncol. 2008;26:2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marjanovic ND, Weinberg RA, Chaffer CL. Cell plasticity and heterogeneity in cancer. Clin Chem. 2013 Jan;59(1):168–79. doi: 10.1373/clinchem.2012.184655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–13. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009 Sep;9(9):631–43. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 6.Sun B, Ding L, Wu S, Meng X, Song S. Combined treatment with everolimus and fulvestrant reversed anti-HER2 resistance in a patient with refractory advanced breast cancer: a case report. Onco Targets Ther. 2016 Jul;9:3997–4003. doi: 10.2147/OTT.S104398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016 Apr;17(4):425–39. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 8.Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R. ESR1 mutations—a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol. 2015 Oct;12(10):573–83. doi: 10.1038/nrclinonc.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer. 2002 Feb;2(2):101–12. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 10.Gu G, Fuqua SA. ESR1 Mutations in Breast Cancer: Proof-of-Concept Challenges Clinical Action. Clin Cancer Res. 2016 Mar 1;22(5):1034–6. doi: 10.1158/1078-0432.CCR-15-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segal CV, Dowsett M. Estrogen receptor mutations in breast cancer - new focus on an old target. Clin Cancer Res. 2014 Apr 1;20(7):1724–6. doi: 10.1158/1078-0432.CCR-14-0067. [DOI] [PubMed] [Google Scholar]
- 12.Angus L, Beije N, Jager A, Martens JW, Sleijfer S. ESR1 mutations: Moving towards guiding treatment decision-making in metastatic breast cancer patients. Cancer Treat Rev. 2017 Jan;52:33–40. doi: 10.1016/j.ctrv.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Jordan VC, Curpan R, Maximov PY. Estrogen receptor mutations found in breast cancer metastases integrated with the molecular pharmacology of selective ER modulators. J Natl Cancer Inst. 2015 Apr;107(6) doi: 10.1093/jnci/djv075. djv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012 Nov 1;367(18):1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roessler S, Lin G, Forgues M, Budhu A, Hoover S, Simpson RM, et al. Integrative genomic and transcriptomic characterization of matched primary and metastatic liver and colorectal carcinoma. Int J Biol Sci. 2015 Jan;11(1):88–98. doi: 10.7150/ijbs.10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012 Mar;366(10):883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchetti A, Del Grammastro M, Felicioni L, Malatesta S, Filice G, Centi I, et al. Assessment of EGFR mutations in circulating tumor cell preparations from MSCLC patients by next generation sequencing: toward a real-time liquid biopsy for treatment. PLoS One. 2014 Aug;9(8):e103883. doi: 10.1371/journal.pone.0103883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw JA, Page K, Blighe K, Hava N, Guttery D, Ward B, Brown J, et al. Genomic analysis of circulating cell-free DNA infers breast cancer dormancy. Genome Res. 2012 Feb;22(2):220–31. doi: 10.1101/gr.123497.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pantel K, Speicher MR. The biology of circulating tumor cells. Oncogene. 2016 Mar;35(10):1216–24. doi: 10.1038/onc.2015.192. [DOI] [PubMed] [Google Scholar]
- 20.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8(5):329–40. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 21.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–21. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 22.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004 Aug;351(8):781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 23.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–09. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 24.Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–24. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 25.Giordano A, Giuliano M, De Laurentiis M, Arpino G, Jackson S, Handy BC, et al. Circulating tumor cells in immunohistochemical subtypes of metastatic breast cancer: Lack of prediction in HER2-positive disease treated with targeted therapy. Ann Oncol. 2012;23:1144–50. doi: 10.1093/annonc/mdr434. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez JM, Fehm T, Orsini M, Cayrefourcq L, Maudelonde T, Pantel K, et al. Prognostic relevance of viable circulating tumor cells detected by EPISPOT in metastatic breast cancer patients. Clin Chem. 2014 Jan;60(1):214–21. doi: 10.1373/clinchem.2013.215079. [DOI] [PubMed] [Google Scholar]
- 27.Navin N, Hicks J. Future medical applications of single cell sequencing in cancer. Genome Med. 2011 May;3(5):31. doi: 10.1186/gm247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson SJ, Vachula M, Kennedy MJ, Kaizer H, Coon JS, Ghalie R, et al. Detection of tumor cells in the bone marrow, peripheral blood, and apheresis products of breast cancer patients using flow cytometry. Exp Hemato. 1995;23:1062–68. [PubMed] [Google Scholar]
- 29.Helzer KT, Barnes HE, Day L, Harvey J, Billings PR, Forsyth A. Circulating tumor cells are transcriptionally similar to the primary tumor in a murine prostate model. Cancer Res. 2009;69:7860–66. doi: 10.1158/0008-5472.CAN-09-0801. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Cho H, Han SI, Han KH. Single-Cell Isolation of Circulating Tumor Cells from Whole Blood by Lateral Magnetophoretic Microseparation and Microfluidic Dispensing. Anal Chem. 2016 May;88(9):4857–63. doi: 10.1021/acs.analchem.6b00570. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs AB, Romani A, Freida D, Medoro G, Abonnenc M, Altomare L, Chartier I, Guergour D, Villiers C, Marche PN, Tartagni M, Guerrieri R, Chatelain F, Manaresi N. Electronic sorting and recovery of single live cells from microlitre sized samples. Lab Chip. 2006 Jan;6(1):121–6. doi: 10.1039/b505884h. [DOI] [PubMed] [Google Scholar]
- 32.Peeters DJ, De Laere B, Van den Eynden GG, Van Laere SJ, Rothé F, Ignatiadis M, Sieuwerts AM, Lambrechts D, Rutten A, van Dam PA, Pauwels P, Peeters M, Vermeulen PB, Dirix LY. Semiautomated isolation and molecular characterisation of single or highly purified tumour cells from CellSearch enriched blood samples using dielectrophoretic cell sorting. Br J Cancer. 2013;108(6):1358–67. doi: 10.1038/bjc.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zong C, Lu S, Chapman AR, Xie XS. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science. 2012 Dec;338(6114):1622–6. doi: 10.1126/science.1229164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie BX, Zhang H, Wang J, Pang B, Wu RQ, Qian XL, et al. Analysis of differentially expressed genes in LNCaP prostate cancer progression model. J Andro. 2011 Mar-Apr;32(2):170–82. doi: 10.2164/jandrol.109.008748. [DOI] [PubMed] [Google Scholar]
- 35.Lu S1, Zong C, Fan W, Yang M, Li J, Chapman AR, et al. Probing meiotic recombination and aneuploidy of single sperm cells by whole-genome sequencing. Science. 2012 Dec;338(6114):1627–30. doi: 10.1126/science.1229112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke AC, Prost S, Stanton J-AL, White WTJ, Kaplan ME, Matisoo Smith EA, et al. From cheek swabs to consesus sequences: an A to Z protocol for high-throughput DNA sequencing of complete human mitochondrial genomes. BMC Genomincs. 2014 Jan 25;15:68. doi: 10.1186/1471-2164-15-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giuliano M, Giordano A, Jackson S, De Giorgi U, Mego M, Cohen EN, et al. Circulating tumor cells as early predictors of metastatic spread in breast cancer patients with limited metastatic dissemination. Breast Cancer Res. 2014 Sep 16;16(5):440. doi: 10.1186/s13058-014-0440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma S, Bartlett CH, Schnell P, DeMichele AM, Loi S, Ro J, et al. Palbociclib in Combination With Fulvestrant in Women With Hormone Receptor-Positive/HER2-Negative Advanced Metastatic Breast Cancer: Detailed Safety Analysis From a Multicenter, Randomized, Placebo-Controlled, Phase III Study (PALOMA-3) Oncologist. 2016 Oct;21(10):1165–75. doi: 10.1634/theoncologist.2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016 Nov;375(20):1925–36. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 40.Sefrioui D, Perdrix A, Sarafan-Vasseur N, Dolfus C, Dujon A, Picquenot JM, et al. Short report: Monitoring ESR1 mutations by circulating tumor DNA in aromatase inhibitor resistant metastatic breast cancer. Int J Cancer. 2015 Nov;137(10):2513–9. doi: 10.1002/ijc.29612. [DOI] [PubMed] [Google Scholar]
- 41.Polzer B, Medoro G, Pasch S, Fontana F, Zorzino L, Pestka A, et al. Molecular profiling of single circulating tumor cells with diagnostic intention. EMBO Mol Med. 2014 Nov;6(11):1371–86. doi: 10.15252/emmm.201404033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guttery DS, Page K, Hills A, Woodley L, Marchese SD, Rghebi B, et al. Noninvasive Detection of Activating Estrogen Receptor 1 (ESR1) Mutations in Estrogen Receptor–Positive Metastatic Breast Cancer. Clin Chem. 2015 Jul;61(7):974–82. doi: 10.1373/clinchem.2015.238717. [DOI] [PubMed] [Google Scholar]
- 43.Méhes G, Witt A, Kubista E, Ambros PF. Circulating breast cancer cells are frequently apoptotic. Am J Pathol. 2001 Jul;159(1):17–20. doi: 10.1016/S0002-9440(10)61667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Page K, Guttery DS, Fernandez-Garcia D, Hills A, Hastings RK, Luo J, et al. Next Generation Sequencing of Circulating Cell-Free DNA for Evaluating Mutations and Gene Amplification in Metastatic Breast Cancer. Clin Chem. 2017 Feb;63(2):532–41. doi: 10.1373/clinchem.2016.261834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weis KE, Ekena K, Thomas JA, Lazennec G, Katzenellenbogen BS. Constitutively active human estrogen receptors containing amino acid substitutions for tyrosine 537 in the receptor protein. Mol Endocrinol. 1996 Nov;10(11):1388–98. doi: 10.1210/mend.10.11.8923465. [DOI] [PubMed] [Google Scholar]
- 46.Jeselsohn R, Yelensky R, Buchwalter G, Frampton G, Meric-Bernstam F, Gonzalez-Angulo AM, et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. 2014 Apr;20(7):1757–67. doi: 10.1158/1078-0432.CCR-13-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang L, Ma F, Chapman A, Lu S, Xie XS. Single-Cell Whole-Genome Amplification and Sequencing: Methodology and Applications. Annu Rev Genomics Hum Genet. 2015;16:79–102. doi: 10.1146/annurev-genom-090413-025352. [DOI] [PubMed] [Google Scholar]
- 48.Chen M, Song P, Zou D, Hu X, Zhao S, Gao S, et al. Comparison of multiple displacement amplification (MDA) and multiple annealing and looping-based amplification cycles (MALBAC) in single-cell sequencing. PLoS One. 2014 Dec;9(12):e114520. doi: 10.1371/journal.pone.0114520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deleye L, De Coninck D, Christodoulou C, Sante T, Dheedene A, Heindryckx B, Van den Abbeel E, De Sutter P, Menten B, Deforce D, Van Nieuwerburgh F. Whole genome amplification with SurePlex results in better copy number alteration detection using sequencing data compared to the MALBAC method. Sci Rep. 2015;5:11711. doi: 10.1038/srep11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.