Abstract
Cancer is the leading cause of disease-related death for adolescents and young adults (AYAs) in the United States. Parents of AYAs with life-threatening illnesses have expressed the desire to talk to their children about end of life (EOL) care, yet, like caregivers of adult patients, struggle to initiate this conversation. Building Evidence for Effective Palliative/End of Life Care for Teens with Cancer is a longitudinal, randomized, controlled, single-blinded clinical trial aimed at evaluating the efficacy of FAmily CEntered disease-specific advance care planning (ACP) for teens with cancer (FACE-TC). A total of 130 dyads (260 subjects) composed of AYAs 14–20 years old with cancer and their family decision maker (>18 years old) will be recruited from pediatric oncology programs at Akron Children’s Hospital and St. Jude Children’s Research Hospital. Dyads will be randomized to either the FACE-TC intervention or Treatment as Usual (TAU) control. FACE-TC intervention dyads will complete three 60-minute ACP sessions held at weekly intervals. Follow-up data will be collected at 3, 6, 12, and 18 months post-intervention by a blinded research assistant (RA). The effects of FACE-TC on patient-family congruence in treatment preferences, quality of life (QOL), and advance directive completion will be analyzed. FACE-TC is an evidenced-based and patient-centered intervention that considers QOL and EOL care according to the AYA’s representation of illness. The family is involved in the ACP process to facilitate shared decision making, increase understanding of the AYA’s preferences, and make a commitment to honor the AYA’s wishes.
Keywords: Pediatric advance care planning, cancer, end of life care, medical decision-making, advance directive
Introduction
ACP is the process of preparing for future medical decision-making using a series of conversations about goals of care between an individual and their surrogate decision-maker [1]. Ideally, it is initiated early in the course of a serious illness and is ongoing to reflect any updates about the patient’s wishes. ACP provides an extra level of support for patients, their families, and their treating physicians. ACP involves (1) designation of a surrogate decision maker, heretofore referred to as family (a person who will make medical decisions for a patient if the patient cannot express their own preferences or cannot do so by law because they are too young); (2) discussions of goals of care in the context of disease specific hypothetical situations that might occur in the future if disease progresses; and (3) documentation of goals of care, especially for situations involving diagnostic uncertainty.
Failure to engage in ACP conversations with adult patients is associated with aggressive EOL care, which may be unwanted; decreased use of hospice and palliative care services; increased hospitalizations; decreased patient-family congruence regarding treatment preferences, decreased compliance with patient’s wishes for EOL care, and decreased quality of EOL care [2–5]. Completing ACP discussions, on the other hand, results in less conflict, anxiety, depression and distress for patients, families and medical staff [6–11].
Parents of AYAs with life-threatening and life-limiting illnesses have expressed the desire to talk to their children about EOL care, yet, like caregivers of adult patients, struggle to initiate this conversation [12, 13]. Pediatric ACP (pACP) is sometimes avoided because it is thought that only a physician should initiate such conversations and that these discussions would be especially distressing to younger patients [14, 15]. However, a recent two-arm randomized controlled trial conducted at five urban hospitals has shown that ACP with AYAs is feasible and not psychologically harmful in the HIV/AIDS population [10]. A second two-arm randomized controlled pilot study has shown similar results in a cancer population [5]. Given that cancer is the leading cause of disease-related death for AYAs in the United States, AYAs with cancer represent an important population with whom to extend this research [9, 12, 13, 16–18].
Objectives of the FACE-TC ACP Study
The FACE-TC project involves a dyadic interview with both AYAs and a family decision maker, where participants will complete a facilitated conversation about values and goals of care, as well as concerns, fears and hopes for the future. The primary purposes of the study are to give AYAs with cancer a voice in the present if they cannot speak for themselves in the future, to ensure that families know what AYAs would want in a bad outcome situation, and to explore if the care desired is the care received for those AYAs who die during the study.
Study design
Overview
This study is a prospective, longitudinal, two-arm, randomized, controlled, single-blinded, clinical trial (RCCT) with an intent-to-treat design. Data will be collected during baseline and study intervention, as well as 3, 6, 12, and 18 months post-intervention. Dyads (N=130 dyads; 260 subjects), composed of AYAs with cancer and their family decision maker, will be enrolled and randomized to either the FACE-TC intervention (N=87 dyads) or TAU control (N=43 dyads) at a 2:1 ratio, because of the demonstrated benefits of pACP in our previous studies [4, 5, 8–10]. Estimating there will be up to 30% attrition, our goal is to have complete data through the 18 month visit for 91 dyads (N=182 subjects). A visualization of the study visits (Figure 1) and the Consort Diagram (Figure 2) provide details of enrollment, randomization, arm allocation, and follow-up. We estimate that approximately 40% of the sample will be minorities, including African-American, Hispanic-Latino, or Asian participants, and approximately 50% of the sample will be female.
Figure 1.
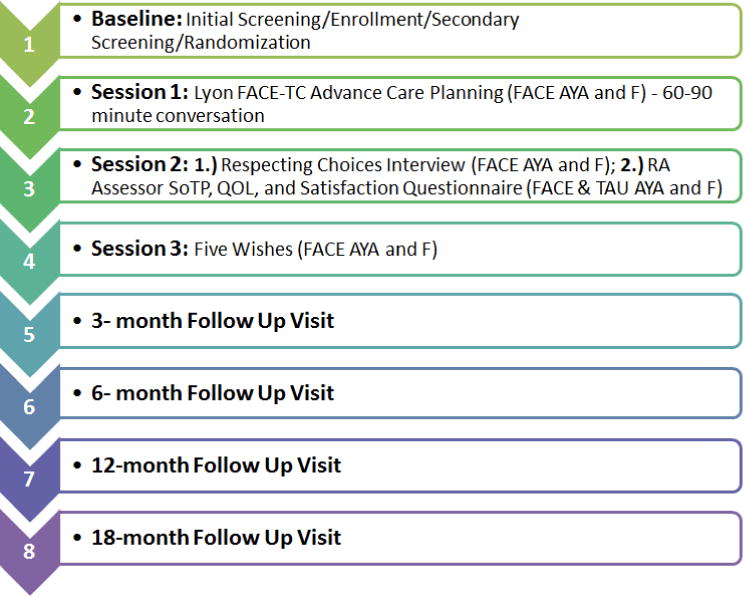
FACE-TC Study Visit Visualization
Key: FACE AYA= adolescent/young adult patient in intervention; TAU AYA=adolescent/young adult in control; F=family/surrogate decision maker; SoTP=Statement of Treatment Preferences; QOL=Quality of Life Questionnaire
Figure 2.
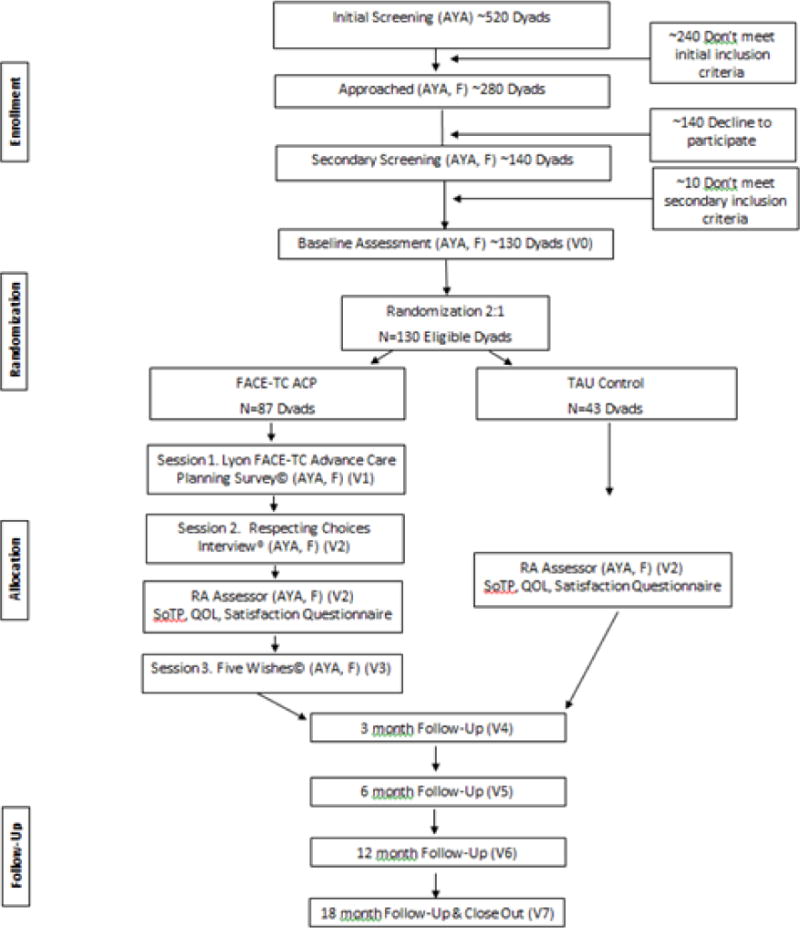
Consort Diagram
*Key: AYA = Adolescents and Young Adults; F = Family/Surrogate Decision Maker
Sites
Participants will be recruited from the established pediatric oncology and palliative care programs at Akron Children’s Hospital and St. Jude Children’s Research Hospital, from which we have created our interdisciplinary study team. Approximately 520 AYAs ages 14–20 years utilize these oncology programs per year, so we anticipate meeting our enrollment goal of 130 dyads. Given this goal, we will have adequate statistical power to conduct our analyses and generalize our findings (see Sample Size and Power section for details). Block randomization by study site will control for site-specific effects. Children’s National Health System will serve as the coordinating center only, as FACE-TC was pilot tested at this location thereby creating potential contamination effects at the site.
Research Team
Each site has a Co-Investigator, blinded RA-Assessor, RA-Interventionist, and a Clinical Coordinator. Site Co-Investigators will provide weekly supervision to study staff during scheduled meetings and help maintain recruitment goals, participant retention, safety of subjects, and fidelity to the protocol. A three-day training will be conducted for study staff prior to opening enrollment. After training in the research protocol, the RA-Interventionists and RA-Assessors will be responsible for participant recruitment, enrollment, and baseline data collection. The RA-Interventionists have been certified in the Respecting Choices ACP program as facilitators and will administer the intervention sessions. Only the blinded RA-Assessor will administer post-randomization outcomes assessments.
Booster training sessions will be held for study staff as needed. As the Coordinating Center, Children’s National staff designed and will maintain the database using the Research Electronic Data Capture System (REDCap) (https://catalyst.harvard.edu/services/redcap/), perform data quality checks, and be responsible for the statistical analyses. Staff at Children’s National will also help ensure the two sites maintain fidelity to the protocol and regulatory compliance through monthly conference calls and twice yearly site monitoring visits during study intervention implementation.
Enrollment of Participant Dyads
For AYAs <18 years, the enrolled family member will be their parent or legal guardian. For AYAs 18–20 years, the enrolled family member will be an adult, 18 years or older, of their choosing. We ask AYAs 18–20 years to consider these criteria in choosing a family decision maker: (1) Is the person willing to be a surrogate? Sometimes even the most trusting and loving people would find this role difficult; (2) Do you trust the person to know your views and be willing to talk with you?; (3) Is the person able to follow through and honor your wishes, even if they might not agree with your choices?; and (4) Can the person make decisions under sometimes stressful and difficult situations? Participants will commit to stay in the study regardless of the group to which they are randomly assigned.
Safety of Minors, AYAs and Families
The study has been approved by the three participating sites’ Institutional Review Boards and will be reviewed bi-annually by a study-specific Safety Monitoring Committee, in keeping with the guidelines of the funding agency, the National Institute of Nursing Research at the National Institutes of Health. Additional policies and procedures have been put into place to ensure the safety of the minors and AYAs involved in this study. Inclusion and exclusion criteria have been created to protect and exclude those AYAs or families with cognitive or developmental impairment, clinically significant depression, suicidal or homicidal thoughts, psychosis, or who are in the foster system. AYAs 18 years or older will provide informed, written consent. AYAs under the age of 18 will be required to provide informed, written assent along with obtaining their legal guardian’s consent to participate. The definitions of an adverse event and serious adverse event have been operationalized for this study using guidance from the Coordinating Center’s Institutional Review Board, the Safety Monitoring Committee, and our pilot study experience (Table 1).
Table 1.
Operationalized Definition of Adverse Event
| Form | Item | Question | Answer |
|---|---|---|---|
| Satisfaction Questionnaire | 5 | It was too much to handle | Agree/Strongly Agree |
| Satisfaction Questionnaire | 7 | It was harmful | Agree/Strongly Agree |
| Satisfaction Questionnaire | 14 | Qualitative statement | Similar negative statement as above |
| Satisfaction Questionnaire | 1 | It was useful | Disagree/Strongly Disagree |
| Satisfaction Questionnaire | 2 | It was helpful | Disagree/Strongly Disagree |
| Satisfaction Questionnaire | 6 | I feel satisfied | Disagree/Strongly Disagree |
| Satisfaction Questionnaire | 11 | I felt courageous | Disagree/Strongly Disagree |
| Satisfaction Questionnaire | 13 | It was worthwhile | Disagree/Strongly Disagree |
Note: An adverse event occurs when a participant disagrees or strongly disagrees with items 1, 2, 6, 11, and 13 AND agrees or strongly agrees with either item 5, 7 or remarks a similar sentiment in the qualitative section.
Eligibility, Recruitment, Consent and Randomization
After consulting with a patient’s primary oncology provider, RAs will approach potentially eligible patients in the oncology clinic. Primary providers are not present during the approach, to prevent the perception of coercion. AYAs and family decision makers who are interested in participating will complete written informed consent and/or assent forms with the RAs, either at the time of approach or at the next medical visit if they want additional time to consider participation. Eligibility is verified during a screening session using the inclusion and exclusion criteria outlined below (Table 2). Consent and screening take approximately 45 minutes in total per person (AYA and family decision maker).
Table 2.
Inclusion and Exclusion Criteria for Participants
| Adolescents and Young Adults (AYA) | |||
|---|---|---|---|
| Inclusion | Exclusion | ||
|
| |||
| 1. | Ever diagnosed with cancer | 1. | Actively homicidal or suicidal |
| 2. | Knows his/her cancer status | 2. | Severely depressed (≥29 BDI-II) |
| 3. | Ages ≥14 up to <21 years | 3. | Developmentally delayed |
| 4. | Ability to speak English | 4. | In foster care |
| 5. | Legal guardian consent if AYA <18 | ||
| 6. | Surrogate consent if AYA ≥18 | ||
| 7. | Assent from AYA <18 | ||
| 8. | Consent from AYA ≥18 | ||
|
Legal Guardian for AYA 14–17 | |||
| Inclusion | Exclusion | ||
|
| |||
| 1. | Legal guardian of AYA | 1. | Actively homicidal or suicidal |
| 2. | Knows cancer status of AYA | 2. | Severely depressed (≥29 BDI-II) |
| 3. | Ages ≥18 years | 3. | Developmentally delayed |
| 4. | Ability to speak English | ||
| 5. | Willing to complete ACP with AYA | ||
| 6. | Legal guardian consent for AYA <18 | ||
| 7. | Consent to participate | ||
|
Chosen Surrogate for AYA 18–20 | |||
| Inclusion | Exclusion | ||
|
| |||
| 1. | Selected by AYA | 1. | Actively homicidal or suicidal |
| 2. | Knows cancer status of AYA | 2. | Severely depressed (≥29 BDI-II) |
| 3. | Ages ≥18 years | 3. | Developmentally delayed |
| 4. | Ability to speak English | ||
| 5. | Willing to complete ACP with AYA | ||
| 6. | Consent to participate | ||
Once the two members of the dyad have consented and are deemed eligible, the RA administers baseline questionnaires to the AYA and the family decision maker separately. Baseline questionnaires may be administered on the same day as screening and consent if this is convenient for the AYA and family decision maker, but may also be scheduled the following week. Baseline takes approximately 60 minutes per person. At this time, all study participants will receive ACP information in the form of the booklet, Caring Decisions [19].
After completion of the baseline assessment, randomization will occur in REDCap. Dyads will be randomized to the FACE-TC ACP intervention or TAU control group using a 2:1 ratio, blocking by study site. If randomized to the intervention group, RAs assigned to conduct the intervention will schedule three 60–90 minute sessions with the participants held one week apart. If randomized to the TAU control group, participants will not complete these sessions.
FACE-TC ACP Intervention
The FACE-TC intervention is a theoretically informed dyadic interview involving both the AYA and family surrogate decision maker. Using community-based participatory research, we adapted and developed FACE-TC in collaboration with AYAs patients living with cancer, their families, their oncologists, neurologists, and a palliative care specialist. FACE-TC Session Two was developed in collaboration with Linda Briggs and colleagues, who create disease-specific ACP models for adult patients with serious illnesses such as cancer and kidney failure called Next Steps: Respecting Choices® [11, 20, 21].
FACE-TC is based on Donovan and Ward’s Representational Approach to Patient Education [22], as well as Leventhal’s theory of Illness Representation [23] and Lazarus and Folkman’s Stress and Coping Theory [24] (Figure 3). According to Levanthal, an illness representation is a set of thoughts that a person has about a health problem (whether medically accurate or not) [23]. The illness representation has five dimensions - identity, cause, time-line, consequences and cure/control - which are incorporated into the Respecting Choices interview. Illness representation (resulting from patient or caregiver appraisal of the patient’s disease condition) elicits either emotion-focused or problem-focused coping, which ultimately influences appraisal [24]. Conducted in a dyadic format with a certified facilitator, this developmentally and culturally appropriate intervention provides AYAs with a sense of control in a low control situation.
Figure 3.
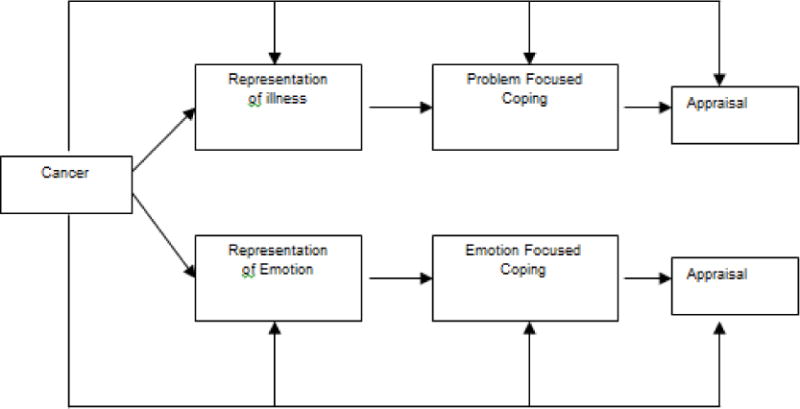
Leventhal’s Common Sense Model of Self-Regulation of Health and Illness
The FACE-TC ACP intervention involves three sessions (Table 3). In Session One, AYAs and family decision makers separately complete the Lyon FAmily CEntered Advance Care Planning Survey©. The RA-Interventionist reads the survey questions aloud and records the participant’s responses directly into the REDCap data base by laptop computer. This approach, compared to a paper or electronic survey completed by the participants on their own, eliminates any concerns about literacy or visual impairment, serves as an effective engagement strategy, and allows for monitoring of the participant’s emotional reaction. The purpose of the survey is to introduce the intervention dyads to ACP topics that will be discussed during Session Two (i.e., “breaks the ice”), and to initially assess the degree to which the family knows the AYA’s values, beliefs and attitudes about death, dying and EOL care treatment preferences.
Table 3.
FAmily CEntered Teens with Cancer Advance Care Planning Intervention
| Session 1 | ||
|---|---|---|
| Foundation | Goals | Process |
| Lyon Family Centered Advance Care Planning (ACP) Survey – AYA and Surrogate Versions© to set stage for EOL conversation |
|
|
| Session 2 | ||
| Foundation | Goals | Process |
| Next Steps: Advance Care Planning Respecting Choices Interview© [41] |
|
|
| Session 3 | ||
| Foundation | Goals | Process |
| The Five Wishes© is a legal document that helps a person express how they want to be treated if they are seriously ill and unable to speak for him/herself. Unique among living will and health agent forms-it looks to all of a person’s needs: medical, personal, emotional, spiritual |
|
For AYAs under the age of 18, The Five Wishes© must be signed by their legal guardian. Processes, such as labeling feelings and concerns, as well as finding solutions to any identified problem, are facilitated. Appropriate referrals are made. This session may include other family members or loved ones (ex-both parents). |
The following week the dyad meets with the RA-Interventionist to complete Session Two together, the Next Steps: Advance Care Planning Respecting Choices Interview Tool for Teens©. The RA-Interventionist facilitates a conversation about EOL decision making between the AYA and family decision maker, where the AYA can express their goals and values for treatment as well as fears and concerns, and hopes for the future. The purpose of this session is to understand the AYA’s wishes for future medical care and to facilitate discussion between the AYA and their family, so that the family knows what the AYA would want and so they have an opportunity to discuss the benefits and burdens of the different treatment choices. In addition, the family decision maker is asked if they will agree to honor the AYA’s treatment preferences. If there is disagreement between the AYA and the family that cannot be resolved during the session, they are referred to either the study site’s chaplain or ethicist, where they can engage in further discussions to help increase congruence in treatment preferences. With participant permission, Session Two is audio or video-taped so the Principal Investigator (Lyon) and Co-Investigator (Briggs) can maintain fidelity of the intervention protocol by reviewing the session. Immediately following Session Two, the dyad separately completes a Statement of Treatment Preferences (SoTP) tool with the blinded RA-Assessor. The SoTP reviews 4 different cancer-related EOL situations with varying prognostic certainty and QOL outcomes. Participants are then asked to select options related to wishes for pursuing all treatments, allowing natural death, or letting others decide. There is also a “Don’t Know” option. At this time, a questionnaire assessing satisfaction with study participation is filled out with the RA-Assessor by each member of the dyad.
One week later, the dyad meets with the RA-Interventionist to complete Session Three together, filling out the Five Wishes© document. Five Wishes is an advance directive that is considered a legal document in 42 states and the District of Columbia. The AYA can express how they want to be treated if they are seriously ill and cannot communicate. In addition to medical needs, Five Wishes addresses personal, emotional and spiritual domains, and establishes the identity of the family decision maker.
After completion of Session Three, the RA-Interventionist places the Five Wishes and SoTP in the patient’s Electronic Health Record (EHR) to provide legal documentation for medical preferences. The RA-Interventionist also notifies the patient’s primary oncology provider via a Health Insurance Privacy and Portability Act compliant email that includes a brief summary of the Respecting Choices Interview, whether or not there was any conflict or questions the patient had, and a copy of the SoTP and Five Wishes document for their record.
TAU Control
The TAU Control also follows a dyadic approach, with the AYA’s parent/guardian (if AYA is <18 years) or an adult of the AYA’s choosing (if AYA is 18–20 years) participating as the family decision maker. TAU Control participants do not receive the ACP intervention, but they do receive the Caring Decisions information booklet given to all study participants at baseline [19]. They do not have a Session One, Two or Three as in the FACE-TC group; however they do meet separately with the blinded RA-Assessor two weeks post-baseline to complete the SoTP and the Satisfaction Questionnaire.
Follow Up Visits
All participants, regardless of randomization, complete follow-up assessments 3, 6, 12, and 18 months post-baseline, scheduled with the blinded RA (Table 4). These visits take approximately 60 minutes.
Table 4.
Schedule of Study Assessments and Measures
| Measure | Completed By | Time Point | Descriptions | Psychometrics |
|---|---|---|---|---|
| Demographic Data Interview | AYA/F | 0 | Demographic data collection | N/A |
| Psychological Interview (Inclusion/Exclusion Criteria) | AYA/F | 0 | Standardized questions to screen for sucidality, homicidality and psychosis | N/A |
| Beck Depression Inventory-II (BDI-II) | AYA/F | 0 | Used as a screening tool for both AYA and family. Used to assess depressed mood. 21 items. | Internal reliability, internal consistency (α=0.91), factorial validity, concurrent, convergent and discriminant validity [44–46] |
| Medical Chart Abstraction (EHR) | SS | 0, 7 | AYA diagnosis, time since diagnosis, hospitalization, ER visit or other treatments since last study visit, care coordination | N/A |
| Medical History | SS | 0 | Assesses date of AYA diagnosis, hospitalization, ER visits, treatments, prognosis for survival. | N/A |
| Patient-Reported Outcomes Measurement Information System Short Forms (PROMIS-SF) | AYA | 0, 4, 5, 6, 7 | Valid and reliable measure of patient’s Emotional distress-Anxiety, Emotional Distress-Depressive Symptoms; Fatigue; and Pain Interference. 34 items total. | Internal reliability, internal consistency (α=0.90), convergent and discriminant validity [47] |
| Brief Multidimensional Measurement of Religiousness/Spirituality (BMMRS-adapted) | AYA/F | 0, 4, 5, 6, 7 | Assess the construct of spiritual functioning and religious practices, e.g. religious preferences and practices, feeling God’s presence. Nonreligious participants can pass on these items. 41 items. | Internal reliability, internal consistency (α =≥0.70); Factorial, construct, convergent, discriminant validity [48–50] |
| Functional Assessment of Chronic Illness Therapy of Spiritual Well Being Scale-IV (FACIT-Sp IV) | AYA/F | 0, 4, 5, 6, 7 | Assesses spiritual well-being and its relation to illness. 23 items. | Internal reliability, internal consistency (α≥0.80), factorial, convergent, and concurrent validity [51–53] |
| Family Appraisal of Caregiving Questionnaire (FACQ) | F | 0, 4, 5, 6, 7 | Assesses experiences of caring for the patient in the past two weeks. 25 items. | Internal consistency (α≥0.70), convergent and discriminant validity [54] |
| Statement of Treatment Preferences (SoTP) | AYA/F | 2, 4,5, 6, 7 | Tool to express values and goals related to future decision-making re: frequently occurring situations. 6 situations. | N/A |
| Satisfaction Questionnaire | AYA/F | 2 | Process measure assessing participant satisfaction. 5 items. | N/A |
| Longitudinal Satisfaction Questionnaire | AYA/F | 4, 5, 6, 7 | Process measure assessing participant satisfaction. 5 items. | N/A |
Key: AYA = adolescents and young adults; F = family/surrogate decision maker; SS = study staff; 0 = Screening/Baseline; 1 = Session 1; 2 = Session 2; 3 = Session 3; 4 = 3-month visit; 5 = 6-month visit; 6 = 12-month visit; 7 = 18-month visit
Death of Study Subject
If a participant passes away during the course of the study, the study team is notified. Follow up visits with the family will not continue. For these participants, data will be extracted from the EHR for the 30 days prior to death by a trained blinded data abstractor. The data will be analyzed to determine if medical care at EOL was consistent with patient wishes for goals of care as documented in the medical record.
Aims and Hypotheses
Aim 1: To evaluate the efficacy of FACE-TC on patient-family congruence in treatment preferences
Congruence between AYAs and their family decision maker will be assessed across 18 months. The aim of congruence is to ensure that AYAs have a voice in their medical treatment and the family, should the AYAs no longer be able to speak for themselves, knows what the patient wants and has made a commitment to honor the patient’s wishes. We hypothesize that FACE-TC participants will maintain better congruence over time, as compared to controls. Development of congruence may not be homogenous, but FACE-TC may influence the pattern of congruence development. We will use innovative statistical methods to determine whether there are subpopulations of AYA/family dyads with respect to longitudinal congruence in treatment preferences, rather than assume homogeneity across time among study AYA/family dyads, which has been the traditional approach.
Aim 2: To evaluate efficacy of FACE-TC on AYA patient QOL (pain/fatigue, psychological, spiritual/existential, meaning/purpose, and peace) and family QOL (psychological, spiritual/existential)
We hypothesize that FACE-TC participants will report higher or greater improvement in QOL, compared to controls.
Aim 3: To evaluate the efficacy of FACE-TC on early completion of pACP goals of care and advance directives such as advance planning of EOL care
We hypothesize that FACE-TC participants will be more likely to have completed goals of care and advance directive documents in their medical records at 18 month follow up compared to controls.
Exploratory Aim: Among the AYAs who die during the course of this study, we will explore if FACE-TC improved the match between patients’ goals of care and the medical care received at the EOL
If AYA participants die during the course of the study, we will retrieve information from the EHR to determine the medical care received at EOL. We hypothesize that participants in the FACE-TC group will have greater congruence between documented patient wishes for goals of care in the EHR and actual medical care received at EOL.
Data Analysis
Statistical analyses will use the following primary outcomes: SoTP, Patient-Reported Outcomes Measurement Information System Short Forms (PROMIS-SF measures), Beck Depression Inventory-II (BDI-II), Functional Assessment of Chronic Illness Therapy-Spiritual Well-being Scale IV (FACIT-Sp IV), Family Appraisal of Caregiving Questionnaire (FACQ); and time-varying covariates: Brief Multidimensional Measure of Religiousness/Spirituality-adapted (BMMRS-adapted). We will also assess the following time-invariant covariates: age, gender, race, ethnicity, time passed since initial diagnosis, education level, socioeconomic position, and study site. A variety of statistical methods, including descriptive statistics, logistic regression, latent growth model (LGM), and growth mixture model (GMM) will be used for data analysis in order to achieve the proposed analytical aims. Prior to parametric testing, scale reliabilities for multi-item measures (e.g., pain/fatigue, child and parent psychological well-being, spiritual/religious measures) will be assessed using Cronbach’s α and their composite scores will be used for data analyses. All hypotheses will be tested at an alpha level of 0.05.
Analytical Plans
Aim 1
Congruence in decision-making for medical treatment will be tested based on agreement (i.e., both patient and his/her family choose the same option) on the responses from SoTP. Kappa coefficients will be applied to assess chance-adjusted agreement between patient and family responses. Change in Kappa coefficient (congruence improvement) from baseline to each follow-up time point during the study period will be tested using bootstrapping technique [25, 26, 27]. The LGM with categorical outcomes will be used to test Aim 1, i.e., FACE-TC participants will have a higher congruence rate over time [28–30]. In the LGM, we will set time scores, except those for identification purpose, as free parameters to let the shape of growth trajectory be determined by data [31]. As such, the congruence development trajectory would have an empirically based nonlinear shape, instead of assuming a linear or nonlinear polynomial function (e.g., quadratic or cubic). We will apply the GMM to test heterogeneity of congruence development trajectories and identify possible patterns of congruence in development trajectories in the full sample [32–34]. Bayesian Information Criterion (BIC), Akaike Information Criterion (AIC), Lo–Mendel–Rubin likelihood ratio (LMR LR), the adjusted LMR LR test, and the bootstrap likelihood ratio test (BLRT) will be used for model comparison and identification of the optimal number of trajectory groups in the GMM. The latent class variable estimated from the GMM captures the pattern of congruence development trajectories. Time-invariant covariates (e.g., demographics including gender, race, ethnicity) will be used to predict the memberships of the latent trajectory groups; and time-varying covariates (e.g. religion/religious beliefs using BMMRS-adapted) will be included to predict the level of congruence at different time points. To further explore Aim 1 we will regress the latent growth slope factor and the latent class variable on FACE-TC intervention data, controlling for covariates in the GMM to assess (1) how FACE-TC would affect the membership of the latent classes of congruence development; and (2) how the effect of FACE-TC on congruence changes over time vary across the latent trajectory classes.
Aim 2
The LGM and GMM models proposed for evaluating Aim 1 can be readily applied to evaluate Aim 2 where the outcome measures are continuous variables. When examining the effects of FACE-TC on QOL for AYAs with cancer (PROMIS-SF measures of pain/fatigue, anxiety depressive symptoms, FACIT-Sp IV) and their families (BDI-II, FACIT-Sp IV), socio-demographics will be controlled as time-invariant covariates, while time-varying covariates (e.g., religion/religious beliefs using BMMRS-adapted) will be included in the model to predict measures of QOL at different time points. In addition, family anxiety/depression measured at the end of the study period (i.e., 18 months post-intervention) will be included as a distal outcome in the GMM models, and how this distal outcome is associated with the patterns of the developmental trajectories of AYA QOL will be assessed. The GMM model will be used to examine growth trajectories of multiple QOL outcomes, respectively. When multiple hypotheses of intervention effects on the growth trajectories of different QOL measures are tested, Bonferroni correction will be applied to exert a stringent control over false discovery [35].
The LGM and GMM models will be implemented in Mplus [36]. When running GMM, we will check the class allocation consistency in the models to ensure that class membership remains basically unchanged after covariates and/or distal outcome are included. Otherwise, the 3-step procedure will be employed to take into account the measurement error in most likely class membership [37]. Further, we will impute the plausible values of each latent variable (e.g., latent growth factors in LGM, latent class variable in GMM) using Bayesian approach and save them as “observed” variables for further statistical analysis [38]. Ten plausible value data sets will be imputed and each of them will be merged with the original data set to generate ten new data sets that will be analyzed in subsequent models just like multiple imputations data sets using Rubin’s method [39].
As attrition is inevitable in longitudinal studies, a robust model estimator (e.g., MLR) using full information maximum likelihood will be used for model estimation. Importantly, missing at random, instead of missing completely at random, can be assumed in MLR. Missing at random is a plausible assumption that allows missingness to be dependent on observed measures like intervention assignment (e.g., participants in the control group may be more likely to drop out).
Aim 3
First, we will use the two-proportion z-test to test the differences in proportions of completion of an advance directive (e.g., Five Wishes) between FACE-TC and control groups. Then logistic regressions will be used to control for socio-demographics. Interaction between intervention and ethnicity will be included in the models to test ethnic disparity in regard to intervention efficacy.
Exploratory Aim
We will explore if FACE-TC improves the match between patients’ goals of care and the medical care received at the EOL among the AYAs who may die (about 25% of the sample). Descriptive statistics will be used to estimate the frequencies of the study variables. Chi-square statistics with Fisher Exact tests will be used to assess the difference in the match between FACE-TC and controls; and exact logistic regression will be applied to examine the effect of FACE-TC on such a match, controlling for covariates.
Sample Size and Power
Usually, the power of a statistical test depends upon significance level, sample size, and effect size of the exposure variable [40]. However, in longitudinal studies, the number of repeated measurements plays an important role in statistical power because there is a tradeoff between the sample size and the number of repeated measurements [41]. The statistical power of longitudinal data analyses is, therefore, strengthened by the presence of up to five repeated measures in our proposed study. For continuous outcomes with a modest observation autocorrelation (ρ =0.20) and moderate effect size (Δ=0.35), the estimated sample size to achieve a power of 0.80 at α=0.05 level and detect a moderate effect size (Δ=0.35) is about N=76 individuals at each of the five observation time points. For binary outcomes, a sample size of N=70 can achieve a power of 0.80 to detect a moderate response probability difference of d=0.17 given ρ=0.20.
In our pilot study, the average difference in rate of congruence between the intervention and control groups was 27.2% across all situations of the SoTP among AYAs with cancer. As such, assuming up to 30% attrition, our proposed sample of N=130 dyads will ensure a large enough statistical power for our proposed longitudinal analyses on patient data and family data, respectively. For the cross-sectional logistic regression model proposed to evaluate Aim Three, a sample size of N=100 would achieve a power of 0.83 at α=0.05 level to detect an odds ratio of four in regard to having a SoTP or completion of an advance directive (e.g., Five Wishes). The results of our pilot study show that everyone in the intervention group had completed Five Wishes, while almost none in the control group had completed any advance directive document, indicating that the corresponding odds ratio is much larger than four. Therefore, our proposed sample size of 130 AYAs and 130 family decision makers will ensure a large enough power for evaluating Aim 3.
Discussion
FACE-TC has advantages over the current treatment as usual, which offers little to no support for facilitating EOL conversations with AYAs and can lead to deficits in the delivery of quality EOL care and lack of documentation concerning treatment preferences and goals of care. FACE-TC provides the opportunity for families and AYAs to participate in: (1) shared decision making in a safe environment [42]; (2) evidence-based ACP [43]; (3) disease-specific ACP [43]; (4) ACP rooted in theories of self-regulation and illness representation [22–24]; and (5) alerts the patient’s health care provider of advance directive completion.
FACE-TC is a patient-centered approach about goals of care, where the facilitator is trained to respect and honor patient values, even if they are different than their own. To ensure the most favorable outcomes with regard to recruitment, and that study staff are comfortable approaching and working with participants, training for the RA-Interventionists involved multiple opportunities for role play and practice. In addition, fidelity to the protocol is monitored through the use of video-taped and Principal Investigator-reviewed intervention sessions. For those individuals who are approached but decide not to participate, we will collect demographic data and information about the reason for declining to participate in order to check for selection bias during recruitment. Lastly, since this is a dyadic study, it is possible that an AYA may want to participate but their family member is not willing or vice-versa. All instances of individuals who decline to participate are documented, so at the conclusion of the study we will determine if any cases of failure to participate were due to one party in the dyad being unwilling.
Conclusion
This study will produce replicable, evidence-based information about pACP. The FACE-TC intervention is patient and family-centered. It considers QOL and EOL care according to the patient’s representation of illness, dying, and death. The family is involved in the ACP process to facilitate shared decision making, increase understanding of the AYA’s preferences, and make a commitment to honor the AYA’s wishes.
Acknowledgments
This research was supported by grant number 1 R01 NR015458-02 from the National Institute of Nursing Research and the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of funding agency.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
There are no conflicts of interest to report.
References
- 1.Kelley AS, Morrison RS. Palliative care for the seriously ill: Review article. N Engl J Med. 2015;373(8):747–755. doi: 10.1056/NEJMra1404684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mack JW, Chen K, Boscoe FP, Gesten FC, Roohan PJ, Schymura MJ, Schrag D. High intensity of end-of-life care among adolescent and young adult cancer patients in the New York State Medicaid Program. Med Care. 2015;53(12):1018–1026. doi: 10.1097/MLR.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkman-Stoppelenburg A, Rietjens JAC, van der Heide A. The effects of advance care planning on end-of-life care: A systematic review. Palliat Med. 2014;28(8):1000–1025. doi: 10.1177/0269216314526272. [DOI] [PubMed] [Google Scholar]
- 4.Lyon ME, Jacobs S, Briggs L, Cheng YI, Wang J. A longitudinal randomized controlled trial of advance care planning for teens with cancer: Anxiety, depression, quality of life, advance directives, spirituality. J Adolesc Health. 2014;54(6):701–717. doi: 10.1016/j.jadohealth.2013.10.206. [DOI] [PubMed] [Google Scholar]
- 5.Lyon ME, Jacobs S, Briggs L, Cheng YI, Wang J. Family-centered advance care planning for teens with cancer. JAMA Pediatr. 2013;167(5):460–467. doi: 10.1001/jamapediatrics.2013.943. [DOI] [PubMed] [Google Scholar]
- 6.Lynn J, Goldstein NE. Advance care planning for fatal chronic illness: avoiding commonplace errors and unwarranted suffering. Ann Intern Med. 2003;138(10):812–818. doi: 10.7326/0003-4819-138-10-200305200-00009. [DOI] [PubMed] [Google Scholar]
- 7.Wenger NS, Kanouse DE, Collins RL. End-of-life discussions and preferences among persons with HIV. JAMA. 2001;285(22):2880–2887. doi: 10.1001/jama.285.22.2880. [DOI] [PubMed] [Google Scholar]
- 8.Lyon ME, Garvie PA, Briggs L, He J, McCarter R, D’Angelo LJ. Development, feasibility, and acceptability of the Family/Adolescent-Centered (FACE) advance care planning intervention for adolescents with HIV. J Palliat Med. 2009;12(4):363–372. doi: 10.1089/jpm.2008.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyon ME, Garvie PA, McCarter R, Briggs L, He J, D’Angelo LJ. Who will speak for me? Improving end-of-life decision-making for adolescents with HIV and their families. Pediatr. 2009;123(2):e199–e206. doi: 10.1542/peds.2008-2379. [DOI] [PubMed] [Google Scholar]
- 10.Lyon ME, Garvie PA, Briggs L. Is it safe? Talking to teens with HIV/AIDS about death and dying: a 3-month evaluation of Family Centered Advance Care (FACE) planning — anxiety, depression, quality of life. HIV/AIDS Res Palliat Care. 2010;2:27–37. doi: 10.2147/hiv.s7507. (Auckl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briggs LA, Kirchhoff KT, Hammes BJ, Song MK, Colvin ER. Patient-centered advance care planning in special patient populations: a pilot study. J Prof Nurs. 2004;20(1):47–58. doi: 10.1016/j.profnurs.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Hinds PS, Oakes LL, Hicks J. “Trying to be a good parent” as defined by interviews with parents who made Phase I, terminal care, and resuscitation decisions for their children. J Clin Oncol. 2009;27(35):5979–5985. doi: 10.1200/JCO.2008.20.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter JK, Rosenberg AR, Feudtner C. Tackling taboo topics: how to have effective advanced care planning discussions with adolescents and young adults with cancer. JAMA Pediatr. 2013;167(5):489–490. doi: 10.1001/jamapediatrics.2013.1323. [DOI] [PubMed] [Google Scholar]
- 14.Singer PA, Martin DK, Lavery JV. Reconceptualizing advance care planning from the patient’s perspective. Arch Intern Med. 1998;158(8):879–884. doi: 10.1001/archinte.158.8.879. [DOI] [PubMed] [Google Scholar]
- 15.Tierney WM, Dexter PR, Gramelspacher GP, Perkins AJ, Zhou XH, Wolinsky FD. The effect of discussions about advance directives on patients’ satisfaction with primary care. J Gen Intern Med. 2001;16(1):32–40. doi: 10.1111/j.1525-1497.2001.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Kochanek KD, Murphy SL, Tejada-Vera B. National vital statistics reports. 2010;58(19):1–135. [PubMed] [Google Scholar]
- 17.Vinchon M, Baroncini M, Leblond P, Delestret I. Morbidity and tumor-related mortality among adult survivors of pediatric brain tumors: a review. Childs Nerv Syst. 2011;27(5):697–704. doi: 10.1007/s00381-010-1385-6. [DOI] [PubMed] [Google Scholar]
- 18.Robison LL. Late effects of acute lymphoblastic leukemia therapy in patients diagnosed at 0–20 years of age. Hematol. 2011;2011(1):238–242. doi: 10.1182/asheducation-2011.1.238. [DOI] [PubMed] [Google Scholar]
- 19.Xafis V, Gillam L, Hynson J, Sullivan J, Cossich M, Wilkinson D. Caring decisions: The development of a written resource for parents facing end-of-life decisions. J Palliat Med. 2015;18(11):945–955. doi: 10.1089/jpm.2015.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. Bmj Mar. 2010;340:1–9. doi: 10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song M, Ward SE, Connolly MC. Randomized controlled trial of SPIRIT: an effective approach to preparing African-American dialysis patients and families for end of life. Res Nurs Health. 2009;32(3):260–273. doi: 10.1002/nur.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donovan HS, Ward S. A representational approach to patient education. J Nurs Scholarsh. 2001;33(3):211–216. doi: 10.1111/j.1547-5069.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- 23.Leventhal HH, Nerenz DR, Steele DJ. Illness representations and coping with health threats. In: Baum A, Taylor SE, Singer JE, editors. Handbook of Psychology and Health: Social Psychological Aspects of Health Hillsdale. New Jersey: Erlbaum; 1984. pp. 219–252. [Google Scholar]
- 24.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer Publishing Company; 1984. [Google Scholar]
- 25.Efron B. Bootstrap methods: another look at the Jackknife. Ann Stat. 1979 Jan;1:1–26. [Google Scholar]
- 26.Mooney CZ, Duval BD. Bootstrapping: A Nonparametric Approach to Statistical Inference. Newbury Park, CA: Sage Publications; 1993. [Google Scholar]
- 27.Lyon ME, D’Angelo LJ, Dallas R, Hinds P, Garvie PA, Wilkins ML, Garcia A, Briggs L, Flynn PM, Rana SR, Cheng YI, Wang J, For the Adolescent Palliative Care Consortium A randomized controlled clinical trial of adolescents with HIV/AIDS: pediatric advance care planning. AIDS Care. doi: 10.1080/09540121.2017.1308463. In Press. http://dx.doi.org/10.1080/09540121.2017.1308463. [DOI] [PMC free article] [PubMed]
- 28.Muthén B, Curran PJ. General longitudinal modeling of individual differences in experimental designs: A latent variable framework for analysis and power estimation. Psychol Methods. 1997;2(4):371–402. [Google Scholar]
- 29.Duncan SC, Duncan TE. Modeling the processes of development via latent variable growth curve methodology. Struct Equ Modeling. 1995;2(3):187–213. [Google Scholar]
- 30.Meredith W, Tisak J. Latent curve analysis. Psychometrika. 1990;55(1):107–122. [Google Scholar]
- 31.Wang J. Significance testing for outcome changes via latent growth modeling. Struct Equ Modeling. 2004;11(3):375–400. [Google Scholar]
- 32.Wang J, Wang X. Structural Equation Modeling: Applications Using Mplus. New York, NY: John Wiley; 2012. [Google Scholar]
- 33.Muthén BO, Beyond SEM. General latent variable modeling. Behaviormetrikam. 2002;29(1):81–117. [Google Scholar]
- 34.Muthén BO. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of Quantitative Methodology for the Social Sciences. Newbury Park, CA: Sage; 2004. pp. 345–368. [Google Scholar]
- 35.Shaffer JP. Multiple hypothesis testing. Annu Rev Psychol. 1995;46(1):561–584. [Google Scholar]
- 36.Muthén L, Muthén BO. Mplus User’s Guide. Los Angeles, CA: Muthén &Muthén; 1998. [Google Scholar]
- 37.Asparouhov T, Muthén BO. Auxiliary variables in mixture modeling: Three-step approaches using M plus. 2014;21(3):329–341. [Google Scholar]
- 38.Asparouhov T, Muthén BO. Technical Report. Los Angeles, CA: Muthén & Muthén; 2010. Plausible Values for Latent Variables using Mplus. [Google Scholar]
- 39.Rubin D. Multiple Imputation for Nonresponse in Survey. New York, NY: Wiley; 1987. [Google Scholar]
- 40.Cohen J. Statistical Power Analysis for the Behavioral Sciences Hillside. New Jersey: Erlbaum; 1988. [Google Scholar]
- 41.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 42.Solomon MZ, Sellers DE, Heller KS. New and lingering controversies in pediatric end-of-life care. Pediatr. 2005;116(4):872–883. doi: 10.1542/peds.2004-0905. [DOI] [PubMed] [Google Scholar]
- 43.Briggs LA, Hammes BJ. Disease-Specific Patient Centered Advance Care Planning (DS-ACP) Manual. La Crosse, WI: Gundersen Lutheran Medical Foundation, Inc; 2011. [Google Scholar]
- 44.Storch EA, Roberti JW, Roth DA. Factor structure, concurrent validity, and internal consistency of the beck depression inventory—second edition in a sample of college students. Depress anxiety. 2004;19(3):187–189. doi: 10.1002/da.20002. [DOI] [PubMed] [Google Scholar]
- 45.Dozois DJ, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory–II. Psychol Assess. 1998;10(2):83–89. [Google Scholar]
- 46.Osman A, Kopper BA, Barrios F, Gutierrez PM, Bagge CL. Reliability and validity of the Beck depression inventory–II with adolescent psychiatric inpatients. Psychol Assess. 2004;16(2):120–132. doi: 10.1037/1040-3590.16.2.120. [DOI] [PubMed] [Google Scholar]
- 47.Bjorner JB, Rose M, Gandek B, Stone AA, Junghaenel DU, Ware JE. Method of administration of PROMIS scales did not significantly impact score level, reliability, or validity. J Clin Epidemiol. 2014;67(1):108–113. doi: 10.1016/j.jclinepi.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masters KS, Carey KB, Maisto SA, Caldwell PE, Wolfe TV, Hackney HL, Himawan L. Psychometric examination of the brief multidimensional measure of religiousness/spirituality among college students. Int J Psychol Relig. 2009;19(2):106–120. [Google Scholar]
- 49.Stewart C, Koeske GF. A preliminary construct validation of the Multidimensional Measurement of Religiousness/Spirituality instrument: A study of Southern USA samples. Int J Psychol Relig. 2006;16(3):181–196. [Google Scholar]
- 50.Harris SK, Sherritt LR, Holder DW, Kulig J, Shrier LA, Knight JR. Reliability and validity of the brief multidimensional measure of religiousness/spirituality among adolescents. J Relig Health. 2008;47(4):438–457. doi: 10.1007/s10943-007-9154-x. [DOI] [PubMed] [Google Scholar]
- 51.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy—Spiritual Well-being Scale (FACIT-Sp) Ann Behav Med. 2002;24(1):49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi K, Green J, Shimonagayoshi M, Kanemoto N, Kasai R, Itoh Y, Kudoh S. Validation of the care notebook for measuring physical, mental and life well-being of patients with cancer. Qual Life Res. 2005;14(4):1035–1043. doi: 10.1007/s11136-004-2958-1. [DOI] [PubMed] [Google Scholar]
- 53.Canada AL, Murphy PE, Fitchett G, Peterman AH, Schover LR. A 3-factor model for the FACIT-Sp. Psycho-oncology. 2008;17(9):908–916. doi: 10.1002/pon.1307. [DOI] [PubMed] [Google Scholar]
- 54.Cooper B, Kinsella GJ, Picton C. Development and initial validation of a family appraisal of caregiving questionnaire for palliative care. Psycho-oncology. 2006;15(7):613–622. doi: 10.1002/pon.1001. [DOI] [PubMed] [Google Scholar]


