Abstract
BACKGROUND
Patients with chronic conditions are often responsible for self-managing complex, multi-drug regimens with minimal professional clinical support. While numerous interventions to promote and support medication adherence have been tested, most have had limited success or have been too resource-intensive for real-world implementation.
OBJECTIVE
To compare the effectiveness of multiple low-cost, technology-enabled strategies, alone and in combination, for promoting medication regimen adherence among older adults.
METHODS
Older, English or Spanish-speaking patients on complex drug regimens (N=1,505) will be recruited from a community health system in Chicago, IL. Enrolled patients will be randomized to one of four study arms, receiving either: 1) enhanced usual care alone; 2) daily medication reminders via SMS text messages; 3) medication monitoring via a patient portal-based assessment; or 4) both SMS text message reminders and portal-based medication monitoring. The primary outcome of the study is medication adherence, which will be assessed via multiple measures at baseline, 2 months, and 6 months. The effect of intervention strategies on clinical markers (hemoglobin A1c, blood pressure, cholesterol level), as well as intervention fidelity and the barriers and costs of implementation will also be evaluated.
CONCLUSIONS
This randomized controlled trial will evaluate the impact of various low-cost intervention strategies on adherence to complex medication regimens and will explore barriers to implementation. If the studied intervention strategies are shown to be effective, then these approaches could be effectively deployed across a diverse range of clinical settings and patient populations.
Keywords: health literacy, medication adherence, chronic conditions
INTRODUCTION
In outpatient settings, patients with chronic conditions are primarily responsible for managing their own prescription (Rx) medication regimen, usually with limited professional clinical support and oversight. This can be a complex task, requiring patients to have a functional understanding of multiple medication instructions, the ability to integrate an Rx regimen into their daily schedule, and the skills needed to problem-solve medication challenges as regimen changes and life events occur [1]. Not surprisingly, studies have repeatedly shown that many patients, particularly those with low health literacy, have problems performing these tasks [2–5]. As the number of patients with multiple chronic conditions continues to rise, along with the percentage of US adults taking complex drug regimens, effective and practical interventions are needed to provide better adherence support for chronic disease self-management [6, 7].
While numerous interventions have been implemented to date to promote medication adherence, most have had substantial limitations [8, 9]. A 2014 Cochrane review of interventions to enhance medication adherence included 182 RCTs, only 5 of which were considered methodologically rigorous and reported an impact on both adherence and clinical outcomes [8]. An examination of these RCTs found that most tested multi-faceted, resource-intensive interventions that were unlikely to be practical for real-world implementation. Authors therefore called for the testing of more feasible, sustainable interventions that minimized impact on clinic workflow and costs. This review also highlighted the need for future studies to evaluate process outcomes and costs to better understand the costs and benefits of various approaches [8].
In response, we are conducting a randomized controlled trial (RCT) to compare the effectiveness of multiple low-cost, technology-enabled strategies, alone and in combination, for promoting medication regimen adherence in older adults. This trial will also evaluate the ‘fidelity’ of each tested strategy, or how well each strategy was implemented and delivered as intended. It will also explore patient, provider and health system factors influencing intervention implementation, and assess and compare the costs required to implement each strategy from a health system perspective. Herein we provide an overview of this four-year trial, funded by the National Institute on Aging, and describe the methods and rationale for our approach.
METHODS
Our four-arm RCT will evaluate the use of text messaging and the patient portal, alone and in combination, to promote older patients’ regimen adherence in outpatient settings. The Institutional Review Board (IRB) of Northwestern University approved study procedures. The RCT is registered on clinicaltrials.gov [NCT02820753].
Study Site
This trial is being conducted at Erie Family Health Center, a Federally Qualified Health Center (FQHC) in metropolitan Chicago. This community health center is comprised of 12 individual clinic sites throughout the city and suburbs that provide medical care for nearly 70,000 patients [10]. Patients at the community health center are racially, ethnically and linguistically diverse and 83% live in low-income households [10]. Erie Family Health Center is a member of AllianceChicago, a health information technology user-community composed of safety net providers who utilize a common EHR platform (GE Centricity) and other technology-related resources [11].
Participants
We will recruit 1,505 primary care patients from eight Erie Family Health Center clinics into the study; recruitment began in April 2017 and will continue for approximately three years. Eligibility criteria for the study are: 1) age 50 years and older, 2) English or Spanish speaking, 3) currently prescribed three or more medications used on a regular basis, 4) primary responsibility for administering one’s own medication, 5) a private cell phone, 6) internet access at home, 7) a personal email account, and 8) basic familiarity with text messaging and using the internet for email. Adults with severe, uncorrectable vision, hearing, or cognitive impairments, or who are unable to consent, are excluded from this research.
Recruitment
To recruit participants, the study was described to providers at participating clinics and their approval obtained to generate lists of potentially eligible patients (by age, English/Spanish language, and number of medications). Patient lists will be generated on a rolling basis and will include patients seen at clinic sites within the last three months. Providers will be alerted to review these patient lists by an email within the EHR; they will indicate if any patients should not be contacted for medical or personal reasons. A letter will then be mailed to patients remaining on the list, notifying them that a research assistant (RA) will be telephoning to invite them to participate in a study. If patients would prefer not to be contacted, they may call a phone number and leave a message to opt-out of receiving any further communication.
Approximately two weeks after the initial mailing, the RA will contact patients who have not opted out to ask whether they would be interested in participating. If the patient is interested, the RA will ask questions to determine eligibility (i.e. patient responsible for administering their medications; have cell phone and internet access, no severe cognitive, hearing or visual impairments). If eligible, the RA will schedule a time to meet in-person to obtain written informed consent and enroll the patient, then conduct the baseline interview. Patients will be asked to bring all their medications with them to the baseline visit.
Randomization
Patients will be randomized to one of the four study arms (1:2:2:2) after enrollment. To achieve group comparability and balance, the randomization will be stratified by clinic and language spoken (English/Spanish) with random block sizes of 7 and 14.
Study Arms
The four study arms are the result of a 2×2 factorial design, where SMS text message reminders and a patient portal questionnaire are the intervention factors, with all four arms receiving enhanced usual care (see Figure 1). Each arm is described in detail below:
Figure 1.
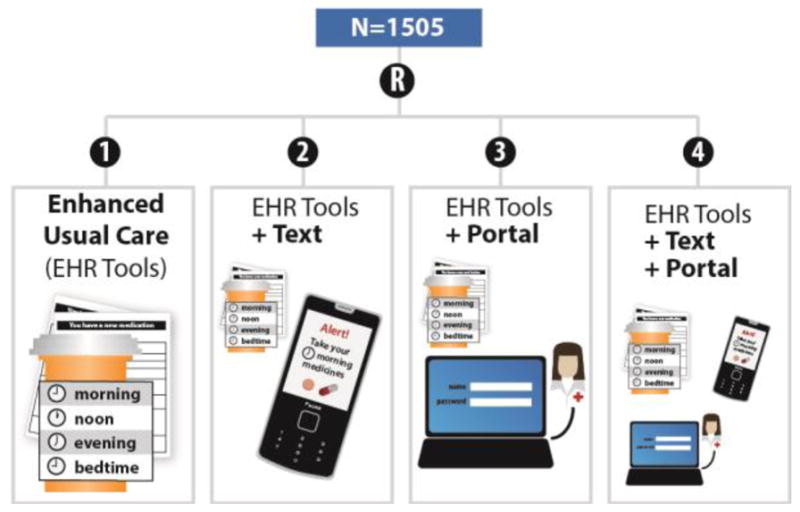
Study Design
Enhanced Usual Care. Patients in the enhanced usual care arm will receive standard care at the study site. However, this community health center is unique in that its ‘usual care’ includes the use of EHR-based tools that support patient understanding and use of medications. Specifically, the EHR has been programmed to print low-literacy medication information summaries and a medication list along with the After Visit Summary. Both were developed by our research team and a previous study among 1003 patients found that the summaries significantly improved patients’ ability to retrieve and apply medication information [12]. These tools now serve as standard of care for Erie Family Health clinics providing primary care for adult patients.
SMS Text-Messaging (Text): In addition to the EHR-based tools described in Enhanced Usual Care, patients randomized to the SMS text-messaging arm will be sent daily text messages, at morning and at night, to remind them to take their medicine. Text messages will consist of a uniform, generic message (e.g. “Good morning! Please remember to take your medicine today”) to support adherence. These text messages will be programmed to send for six weeks. However, patients may opt out of receiving the reminders at any time or can also elect to continue receiving reminders after six weeks, if desired.
Patient Portal Questionnaire (Portal): In addition to the EHR-based tools described in Enhanced Usual Care, patients randomized to the patient portal questionnaire arm will be emailed a request to log on to their patient portal to complete a brief survey regarding their medication use. The questionnaire consists of 10 items, asking patients to report problems understanding medication instructions, managing and consolidating daily regimens, and paying for medicines. Items also assess if patients have decided to discontinue a medication or have had difficulties remembering to take medications during the past three days. Results from the questionnaire are recorded in the patients’ EHR and are sent as an inbox message to a common user desktop in the clinic. A nurse or other clinical staff on the clinic’s care management team will monitor reports and respond to any identified concerns according to their established clinical care protocols. Patients in this arm will be asked to complete this survey at 2 weeks, 1-, 2-, 3-, 4-, and 5-months post-enrollment.
SMS Text-Messaging + Patient Portal Questionnaire (Text + Portal). The final arm is a combination of the SMS Text-Messaging and Patient Portal Questionnaire arms. Participants randomized to this arm will receive both the daily SMS text reminders and requests to complete the patient portal assessment along with the EHR tools, as described above.
Study Aims and Hypotheses
The three specific aims of this study are to: 1) compare the effectiveness of the SMS text messaging and patient portal questionnaire interventions, alone and in combination, to enhanced usual care; 2) evaluate the ‘fidelity’ of each strategy and explore patient, staff, physician, and health system factors influencing the delivery of the interventions; and 3) assess the costs required to deliver each of the interventions from a health system perspective. Our hypotheses are that, compared to enhanced usual care, patients receiving text messages or the portal questionnaire will have greater adherence to their Rx regimen. We also hypothesize that patients receiving both the portal questionnaire and the text messaging will have better adherence than those receiving usual care or one intervention strategy alone.
Data Collection
Enrolled patients will participate in three interviews: a baseline in-person interview, a two-month telephone interview and a 6-month in-person interview. Interviews will be administered by trained, bilingual RAs and study data collected and managed using REDCap data capture tools hosted at Northwestern University [13]. Additionally, relevant patient clinical measures and fidelity outcomes will be abstracted from electronic medical records. Patients will also sign a release allowing us to obtain fill information from pharmacies.
Patient Outcomes
The main study outcome is medication adherence, which will be assessed via multiple methods [14, 15]. First, trained RAs will conduct a pill count for all chronic, pill form medications. This will be done using an electronic pill counter at baseline and 6 months. The proportion of pills taken over pills prescribed (PT/PP) will be calculated for each medication at each time point. Pills taken will be calculated by subtracting the number of pills from the total quantity prescribed. Pills prescribed will be calculated by multiplying the number of pills prescribed each day by the number of days since the medication was filled. Second, proportion days covered (PDC) will be calculated for each medication by summing the number of days’ supply obtained by a patient during a given time period and dividing that by the number of days for which the medication was prescribed [14]. For each of these measures, we will assess adherence within drug class per patient. If a patient fills a prescription and switches to another drug within the same class, all prescriptions will be summed in the numerator. Adherence will be treated both continuously (PDC, PT/PP) and dichotomously (yes/no; PDC≥80%, PT/PP≥80%).
To get a more detailed understanding of medication taking behaviors, we will administer the ASK-12 questionnaire, a brief measure of adherence that covers three key domains: inconvenience/forgetfulness, treatment beliefs and behavior at all interviews [15]. The ASK-12 is scored by summing the selected responses (with scores ranging from 12–60) with higher scores indicating greater barriers to adherence. We will also ask patients to participate in a 24 hour recall of medication use. Specifically, RAs will review patients’ medications and ask patients to report, one by one, how they took each of their medications over the past 24 hours. Patients will be asked to specify the amount taken (i.e. dose) and when taken (to determine frequency and interval between doses). Adherence to each medication (yes/no) will be determined by examining whether the patient took the medication as prescribed. Patients who are non-adherent (either not taking a medication or taking incorrectly) will be questioned to determine the reason (e.g. forgetfulness, inconvenience, misunderstanding of instructions). Data from this exercise will also be used to calculate regimen consolidation, or the total number of time points throughout the 24-hour period that a patient reported taking one or more medicines (count variable, range: 1–24) and patient understanding of medication instructions for use (yes/no).
Clinical measures will also be collected during in-person interviews and from EHR abstractions. Trained RAs will follow established procedures to measure patients’ systolic and diastolic blood pressure during the baseline and 6 month interviews. Multiple measurements will be taken during each interview and averaged to account for fluctuations. Additionally, other relevant clinical measures, such as Hemoglobin A1C (HbA1c) for patients with diabetes or LDL cholesterol for patients with high cholesterol, will be obtained from patients’ EHR for exploratory analyses.
Covariates
We will collect data on a number of relevant covariates via the use of validated measures and items utilized in prior research by this study team. This includes, but is not limited to, social support, [16] technology use, cognition, [17, 18] consumer health activation, health literacy, [19–22] socio-demographic characteristics, medication regimen complexity, [23] depression and anxiety [24].
Fidelity Outcomes
In addition to investigating the effect of the various intervention strategies on patient outcomes, we will evaluate the fidelity of the approaches and their implementation. Patients in intervention arms will be asked to report (yes/no) receipt of intervention components (e.g. the patient portal questionnaire, SMS text messages) and to qualitatively describe any barriers to their use (e.g. difficulty logging on to the portal, challenges receiving/viewing text messages). We will record data on how many patients opted out of receiving text messages from the text message provider as well as how often the patient portal questionnaire was completed via EHR review.
Patients who receive the portal questionnaire will be asked to report how often clinic staff contacted them regarding survey responses and the quality of this follow-up. The EHR notes will be analyzed as needed to verify information and provide further context. Finally, patients will be asked to report on their satisfaction with the intervention strategies and perceptions of their usefulness based on a scale of 1–10.
Post-trial Investigations
To investigate patient, healthcare provider, and/or healthcare system-level barriers to implementation, we will conduct semi-structured interviews with a subsample of patients from each intervention arm. The interview will explore patients’ personal challenges with regimen adherence, perceived value of the relevant intervention strategy/ies, and unmet needs and acceptability of other tools and approaches to support medication use. We will also conduct brief interviews and/or focus groups (n~25) with clinic providers and staff to explore barriers and facilitators to implementing the various intervention strategies.
Finally, we will compare the cost-effectiveness of each strategy. Specifically, we will estimate the incremental cost of interventions over the cost of usual care from the perspective of the Alliance and Erie. The primary operational costs of the intervention strategies include texting related costs and the programming and maintenance costs of the GE Centricity EHR and the patient portal. We will separately track development costs of the interventions, conditional on having an EHR and portal, which are primarily comprised of software generation and other programming requirements based on programmer hours. Staff/programmer costs will be measured using tracked time spent on developing and running the intervention and converted to dollars using current wages.
Data Analysis Plan
The proposed trial uses patient 1:2:2:2 randomization with random block size to achieve group comparability and balance. Stratified by clinic and language spoken, each participant will be randomized to one of the four arms described above. We conservatively anticipate at least 80% retention for follow-up at six months. This will result in 1,505 participants recruited to the study with an anticipated minimum of 1,204 patients (172 participants in enhanced usual care and 344 per the other arms) available after six months, available for primary data analysis.
To assess adequate balance across treatment arms, baseline outcomes and potential confounders including socio-demographic characteristics, comorbidities, regimen complexity, health literacy, and language will be examined across groups using ANOVA models and χ2 tests, as appropriate. Variables found to have prognostic strength, or which are noted to have chance imbalances across treatment groups, will be entered as covariates in the generalized linear mixed models (GLMMs) used for formal analyses as described below.
Prescription medication adherence at 6 months is the primary outcome of interest. We will use generalized linear mixed-effects models (GLMMs) to test for the effects of SMS text reminders and the portal questionnaire, specifying the proper link functions based on the distribution of the outcome variable. Analyses will be performed using PROC GENMOD in SAS (v.9.4). Binary variables indicating the two intervention factors (SMS text messaging (yes/no) and the patient portal questionnaire (yes/no) along with an interaction term will be included in the model to denote the four study arms. Since adherence will be assessed for each medication, 2-level GLMM will be used with medications nested in participants. We will test for differences in the adherence between each of the intervention groups and the enhanced usual care arm to determine benefits of SMS texting or the portal questionnaire, or both by constructing contrasts.
We will determine the fidelity of the intervention strategies, or the extent to which the interventions were implemented as planned (a process evaluation), across each site and for all four study arms. We will also compare the costs of developing and running each intervention, alone and in combination. Specifically, we will estimate the incremental cost of the interventions relative to usual care and to each other. These costs will include the monthly costs of SMS texting, EHR programming and maintenance and clinical staff effort. Staff/programmer costs will be measured using tracked time spent on the intervention and current wage estimates. We will test the sensitivity of operational costs to different assumptions about the potential use of variable staff using different internal salaries but assuming the same proficiency in terms of time required. Further, we will assess the sensitivity of the estimates to different estimated proficiency levels that could arise from learning by doing.
Exploratory Analyses
We will repeat all GLMM analyses described above to explore whether differences in interventions vary by relevant covariates representing patient and regimen characteristics known to impact adherence. Interaction terms will be included in models accordingly. Statistical significance for a tested interaction (p<0.05) will indicate that intervention groups differ in outcomes by the studied variable (i.e. age, literacy level, regimen complexity, drug class, language, etc.). Exploratory analyses will examine HbA1c and blood pressure from baseline to 6 months, although power will be limited.
Sample Size and Power
Study sample size was based on logistic regression models of the primary outcome of medication adherence at six months with an interaction of text and portal, and assuming 1:2:2:2 randomization to the Enhanced Usual Care, Text, Portal, and Text + Portal arms. We expect 69% of enhanced usual care participants to be adherent at six months. Enrolling 1,505 participants and conservatively estimating 80% retention at six months (n=1,204; 172 enhanced usual care and 344 per other arms), we will have 80% power to detect a minimum absolute difference between study arms of 12% (increase from 69% to 81%) using a Type 1 error rate of 2.5% to adjust for multiple comparisons. With this sample size, if there were an interaction such that both interventions increased adherence 14% compared to each alone, then we would have 83% power to detect that interaction at a type I error rate of 5% (assuming responses of 69% for Enhanced Usual Care, 81% for Text, 69% for Portal and 95% for Text + Portal arms). The rationale for unequal randomization (1:2:2:2) is that we strongly believe that the text messaging or patient portal interventions compared to usual care will significantly impact outcomes, so the impact on power for those comparisons will be negligible. However, by increasing sample sizes in the intervention arms we have more additional power to detect any potential interactions as well as detect any potential differences between the interventions.
DISCUSSION
Our four-arm, randomized controlled trial will evaluate the use of text messaging and the patient portal, alone and in combination, to promote older patients’ regimen adherence in outpatient settings. Prior studies evaluating these technologies independently indicate that they might support medication adherence [8, 25, 26]. Thakkar and colleagues’ meta-analysis of data from 16 randomized controlled trials concluded that text messaging nearly doubled the odds of medication adherence among patients with a chronic disease [25]. However, many of the studies included in the review relied upon self-reported adherence and minimal evidence was provided on the effects of text messaging on clinical outcomes.
While unidirectional text messages may support adherence by helping patients remember when to take a medication, it is unlikely to help with other common medication concerns, such as difficulty understanding medication instructions, problems paying for a medication or challenges organizing a multi-drug regimen. To address these more complex issues, patients are likely to need direct assistance from the clinical care team. Using a portal-based questionnaire like the one tested in this trial could help identify those patients in need of further resources and provide a clear target for clinical intervention. While prior studies have shown that many patients are willing to use the patient portal and that certain portal functions (e.g. prescription refill, provider messaging) can positively impact patient behaviors, [26–28] to our knowledge, no studies have developed and evaluated a portal-based strategy for identifying and intervening with older patients to improve medication adherence.
This randomized controlled trial will address many of the criticisms posed by prior systematic reviews of interventions to promote medication adherence [8, 9]. Specifically, this study will include multiple measures of adherence and will not rely solely upon self-report; it will also examine adherence longitudinally and will assess the impact of the various intervention strategies on key clinical measures (e.g. hemoglobin A1c, blood pressure). Finally, the trial will evaluate multiple low cost, practical approaches that would be feasible to implement across diverse clinic settings; the cost-effectiveness analyses will also provide further information on the utility of the various strategies.
Despite these strengths, there are limitations to this study that should be noted. First, this trial is being conducted among older English and Spanish-speaking patients affiliated with a community health center in Chicago, IL. Results may not be generalizable to younger patients or those living outside a metropolitan area such as Chicago. Secondly, the intervention strategies tested may not be suitable for all patients, as enrollment requires access to a personal cell phone and the internet. While recent national survey data indicates that the vast majority of US adults owns a cell phone (95%) and use the internet (88%), age-related disparities persist and patients are likely to vary in terms of their computer skills and comfort with technology [29, 30].
Overall, this randomized controlled trial should offer valuable insight on the utility and effectiveness of various low-cost strategies to promote medication adherence and safe use of prescribed drugs among older adults. As the number of patients managing complex drug regimens in the United States continues to rise, developing a better understanding of the tools and methods available to help support safe and consistent prescription medication use will become increasingly essential [6, 7]. Findings from this trial will hopefully deepen our understanding of the problem of medication non-adherence and identify potential solutions to address this public health and patient safety concern.
Acknowledgments
Funding Sources: This study was funded through NIA 1R01AG046352.
Footnotes
Clinical Trial Registration: This trial is registered on clinicaltrials.gov NCT02820753.
Conflicts of Interest and Disclosures: Stacy Bailey has served as a consultant to Merck, Sharp & Dohme Corp and Luto LLC for work unrelated to this manuscript. She has also received grant support via her institution from Merck, Sharp & Dohme Corp and Eli Lilly and Company. Michael Wolf has served as a consultant to Merck, Sharp & Dohme Corp, Abbvie, Vivus, Inc., Luto LLC, Anheuser Busch Imbev, DenverHealth, and Teva Pharmaceuticals for work unrelated to this manuscript. He also has received grant support via his institution from Merck, Sharp & Dohme Corp, Eli Lilly and Company, Abbvie, and UnitedHealthcare. Ruth Parker has received grant support from Merck, Sharp & Dohme. Surrey Walton has served as a consultant to Merck, Sharp & Dohme, Abbott and Abbvie, and Baxter for work unrelated to this manuscript. Amisha Wallia has received grant support via her institution from Merck, Sharp & Dohme Corp and Eli Lilly and company. Dr. Wallia also serves as an adjudicator for Lexicon Therapeutics and a consultant for GLytec. Alastair J.J. Wood has served as a consultant to various drug companies and is a partner at Symphony Capital LLC. None of his consulting or work at Symphony Capital was related to the content or topic of this manuscript. Other study authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bailey SC, Oramasionwu CU, Wolf MS. Rethinking adherence: a health literacy-informed model of medication self-management. J Health Commun. 2013;18(Suppl 1):20–30. doi: 10.1080/10810730.2013.825672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf MS, Curtis LM, Waite K, Bailey SC, Hedlund LA, Davis TC, Shrank WH, Parker RM, Wood AJ. Helping patients simplify and safely use complex prescription regimens. Arch Intern Med. 2011;171(4):300–5. doi: 10.1001/archinternmed.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis TC, Wolf MS, Bass PF, 3rd, Thompson JA, Tilson HH, Neuberger M, Parker RM. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887–94. doi: 10.7326/0003-4819-145-12-200612190-00144. [DOI] [PubMed] [Google Scholar]
- 4.Wolf MS, Davis TC, Tilson HH, Bass PF, 3rd, Parker RM. Misunderstanding of prescription drug warning labels among patients with low literacy. Am J Health Syst Pharm. 2006;63(11):1048–55. doi: 10.2146/ajhp050469. [DOI] [PubMed] [Google Scholar]
- 5.Farber HJ, Capra AM, Finkelstein JA, Lozano P, Quesenberry CP, Jensvold NG, Chi FW, Lieu TA. Misunderstanding of asthma controller medications: association with nonadherence. J Asthma. 2003;40(1):17–25. doi: 10.1081/jas-120017203. [DOI] [PubMed] [Google Scholar]
- 6.Gu Q, Dillon CF, Burt VL. Prescription drug use continues to increase: U.S. prescription drug data for 2007–2008. NCHS data brief. 2010;(42):1–8. [PubMed] [Google Scholar]
- 7.Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB, Blumenthal D. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(Suppl 3):391–5. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, Agoritsas T, Mistry N, Iorio A, Jack S, Sivaramalingam B, Iserman E, Mustafa RA, Jedraszewski D, Cotoi C, Haynes RB. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;(11):CD000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haynes RB, Yao X, Degani A, Kripalani S, Garg A, McDonald HP. Interventions for enhancing medication adherence (Review) Cochrane Database of Syst Rev. 2005;4 doi: 10.1002/14651858.CD000011.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Erie Family Health Center. [Accessed 03/17]; Available from: http://www.eriefamilyhealth.org/about-erie/
- 11.Alliance of Chicago. [Accessed 03/17]; Avalailable from: http://alliancechicago.org/
- 12.Wolf MS, Bailey SC, Serper M, Smith M, Davis TC, Russell AL, Manzoor BS, Belter L, Parker RM, Lambert B. Comparative effectiveness of patient-centered strategies to improve FDA medication guides. Med Care. 2014;52(9):781–9. doi: 10.1097/MLR.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choudhry NK, Shrank WH, Levin RL, Lee JL, Jan SA, Brookhart MA, Solomon DH. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15(7):457–64. [PMC free article] [PubMed] [Google Scholar]
- 15.Matza LS, Park J, Coyne KS, Skinner EP, Malley KG, Wolever RQ. Derivation and validation of the ASK-12 adherence barrier survey. Ann Pharmacother. 2009;43(10):1621. doi: 10.1345/aph.1M174. [DOI] [PubMed] [Google Scholar]
- 16.Woloshin S, Schwartz LM, Tosteson AN, Chang CH, Wright B, Plohman J, Fisher ES. Perceived adequacy of tangible social support and health outcomes in patients with coronary artery disease. J Gen Intern Med. 1997;12(10):613–8. doi: 10.1046/j.1525-1497.1997.07121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc. 2006;1(5):2277–81. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- 18.Salthouse TA, Babcock RL, Shaw RJ. Effects of adult age on structural and operational capacities in working memory. Psychol Aging. 1991;6(1):118–27. doi: 10.1037//0882-7974.6.1.118. [DOI] [PubMed] [Google Scholar]
- 19.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588–94. [PubMed] [Google Scholar]
- 20.Chew LD, Griffin JM, Partin MR, Noorbaloochi S, Grill JP, Snyder A, Bradley KA, Nugent SM, Baines AD, VanRyn M. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Inter Med. 2008:561–566. doi: 10.1007/s11606-008-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar U, Schillinger D, Lopez A, Sudore R. Validation of self-reported health literacy questions among diverse English and Spanish-speaking populations. J Gen Intern Med. 2011;26(3):265–71. doi: 10.1007/s11606-010-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, Mockbee J, Hale FA. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3(6):514–22. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George J, Phun YT, Bailey MJ, Kong DC, Stewart K. Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004;38(9):1369–76. doi: 10.1345/aph.1D479. [DOI] [PubMed] [Google Scholar]
- 24.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R, Group PC. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thakkar J, Kurup R, Laba TL, Santo K, Thiagalingam A, Rodgers A, Woodward M, Redfern J, Chow CK. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med. 2016;176(3):340–9. doi: 10.1001/jamainternmed.2015.7667. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar U, Schillinger D, Ralston JD, Allen JY, Nguyen R, Karter AJ. Refilling medications through an online patient portal: consistent improvements in adherence across racial/ethnic groups. J Am Med Inform Assoc. 2016;23(e1):e28–e33. doi: 10.1093/jamia/ocv126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruse CS, Argueta DA, Lopez L, Nair A. Patient and provider attitudes toward the use of patient portals for the management of chronic disease: a systematic review. J Med Internet Res. 2015;17(2):e40. doi: 10.2196/jmir.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkar U, Schillinger D, Ralston JD, Ratanawongsa N, Pasick R, Lyles CR. Barriers and facilitators to online portal use among patients and caregivers in a safety net health care system: a qualitative study. J Med Internet Res. 2015;17(12):1–11. doi: 10.2196/jmir.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey SC, O’Conor R, Bojarski EA, Mullen R, Patzer RE, Vicencio D, Jacobson KL, Parker RM, Wolf MS. Literacy disparities in patient access and health-related use of Internet and mobile technologies. Health Expect. 2014 doi: 10.1111/hex.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mobile Technology Fact Sheet. Pew Research Center; Washington, D.C: 2014. [Accessed 03/17]. Available from: http://www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/ [Google Scholar]


