Abstract
Background
Atrial Fibrillation (AF) is a common cardiac arrhythmia that is challenging for patients and adversely impacts health-related quality of life (HRQoL). Long-term management of AF requires that patients adhere to complex therapies, understand difficult terminology, navigate subspecialty care, and have continued symptom monitoring with the goal of preventing adverse outcomes. Continued interventions to ameliorate the patient experience of AF are essential.
Design
The Atrial Fibrillation health Literacy Information Technology Trial (AF-LITT; NCT03093558) is an investigator-initiated, 2-arm randomized clinical trial (RCT). This RCT is a pilot in order to implement a novel, smartphone-based intervention to address the patient experience of AF. This pilot RCT will compare a combination of the embodied conversational agent (ECA) and the Alive Cor Kardia Mobile heart rhythm monitor to the current standard of care. The study will enroll 180 adults with non-valvular AF who are receiving anticoagulation for stroke prevention and randomize them to receive a 30-day intervention (smartphone-based ECA/Kardia) or standard of care, which will include a symptom and adherence journal. The primary end-points are improvement in HRQoL and self-reported adherence to anticoagulation. The secondary end-points are the acceptability of the intervention to participants, its use by participants, and acceptability to referring physicians.
Conclusions
The AF-LITT pilot aims to evaluate the efficacy of the ECA/Kardia to improve HRQoL and anticoagulant adherence, and to guide its implementation in a larger, multicenter clinical trial. The intervention has potential to improve HRQoL, adherence, and health care utilization in individuals with chronic AF.
Keywords: randomized clinical trial, atrial fibrillation, health-related quality of life, adherence, relational agents
Background
Atrial fibrillation (AF) is a common and complex cardiac arrhythmia for patients that requires adherence to anticoagulation for stroke prevention, symptom assessment and monitoring, and subspecialty care. AF has an adverse impact on health-related quality of life (HRQoL) in addition to multiple adverse clinical outcomes such as increased risks of stroke, heart failure, and death.[1, 2] The symptoms, treatment burden, prognostic uncertainty, impact on functional status, and cost of AF impact HRQoL. The disease is complicated by individuals being unaware of having the condition or concern for when they may be in AF. As is typical for a chronic disease with complicated and long-term therapies, adherence to medications may be challenging.[3]
The challenges summarized here may be amplified by limited health literacy.[4] Limited health literacy has been associated with worse anticoagulation as measured by time in therapeutic range for warfarin, decreased understanding of stroke prevention, and symptom severity.[3, 5–8] Like other chronic diseases, individuals with limited health literacy may have further difficulty managing self-care, negotiating specialty clinics, adhering to treatments, and accessing resources. Such challenges may be worsened by social determinants of health related to health literacy, such as socioeconomic status and access to care.[9] Implementation of mobile devices may provide an opportunity to address the challenges described here and aim to improve outcomes in vulnerable individuals with AF. While smartphone-based technologies are effective for screening and monitoring AF,[10, 11] the data regarding their use to address adherence and HRQoL in AF remain sparse.
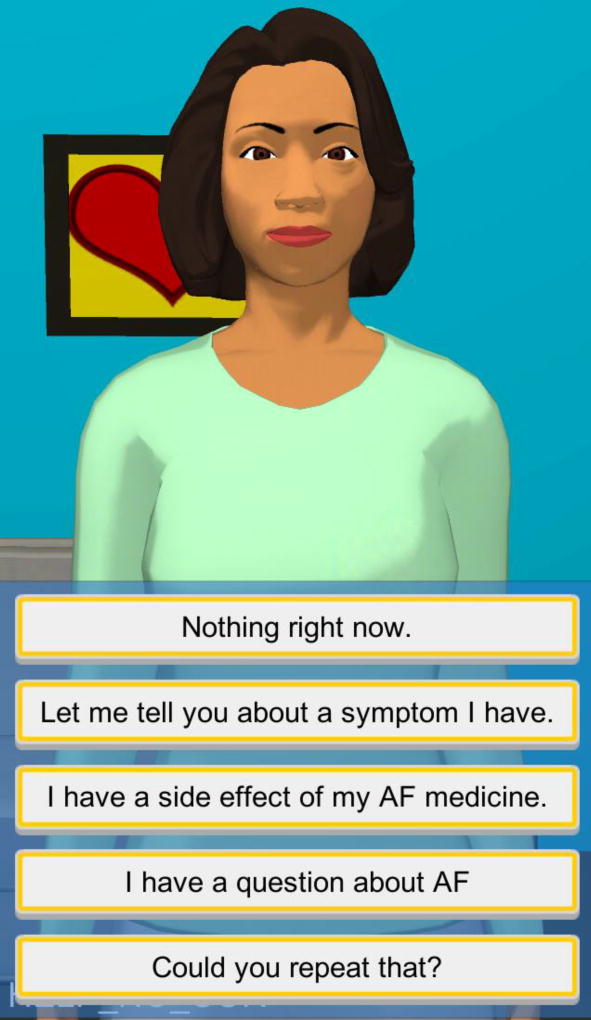
The Embodied Conversational Agent (ECA) – the central intervention piloted in this RCT – is delivered by smartphone and has the potential to improve patient-centered care in AF. The ECA functions by simulating a face-to-face conversation with a health counselor using synthetic speech and computer-animated counselor that uses nonverbal conversational behaviors such as hand gestures. A screenshot of the ECA is shown in Figure 1. The ECA is able to tailor interactions with the user using the user’s name and other personal information, as well as responding to conversational input from the user during the current and prior conversations. The ECA has been used in multiple contexts for health intervention to foster a therapeutic alliance and assist self-care in individuals with chronic medical conditions, particularly those with inadequate health literacy.[12–15] This pilot RCT will implement a novel ECA designed to improve HRQoL and anticoagulation adherence in individuals with AF.
Figure 1.
Screenshot of the embodied conversational agent, or ECA, with responses for content related to atrial fibrillation.
In conjunction with the ECA, we will use AliveCor’s Kardia Mobile heart rhythm monitor (hereafter Kardia, for simplicity). The Kardia is a smartphone-based app that allows users to obtain heart rhythm and rates and is FDA-approved for enhanced detection and management of AF.[10, 11, 16, 17] As a preliminary assessment, we combined the ECA and the Kardia to test their utility in 31 individuals with chronic AF. We developed ECA content to prompt use of the Kardia for symptoms, intending to provide valuable correlation between symptoms and heart rate and rhythm. Our ECA content included education of AF, and we recognized that patients’ understanding of their heart rates and rhythms has potential to enhance self-management and engagement in AF. We now apply the ECA and Kardia as an RCT to assess efficacy of this highly synergistic mobile health (mHealth) intervention. We expect this novel mHealth intervention to enhance clinical monitoring and to improve the patient experience of this chronic condition.
Objectives
The study has complementary objectives that consist in the assessment of efficacy to improve HRQoL and anticoagulant adherence and to determine the acceptability of the intervention. As our primary objective, we will establish the efficacy of the intervention to improve (a) HRQoL and (b) self-reported adherence to anticoagulation at 30 days using validated instruments. We hypothesize that intervention participants will have both greater improvement in HRQoL and better anticoagulant adherence when compared to participants randomized to receive standard of care. As our secondary objective, we will determine the 30-day utility and acceptability of the combined ECA/Kardia smartphone-based intervention. Utility and acceptability will be quantified with extensive usage statistics for the ECA/Kardia applications and administering validated instruments to study participants. As an exploratory aim, we will evaluate the difference in events (hospitalization, vital status) between the intervention and control arms at 90 days. We expect that the pilot use of the ECA/Kardia will establish the efficacy to guide a larger, pragmatic clinical trial of more sustained duration.
Trial Oversight
The study is an unblinded two-arm, randomized clinical trial (RCT) that compares usual care plus a smartphone-based intervention to usual care alone. The study will be conducted at the University of Pittsburgh, Pittsburgh, PA. The intervention consists of daily use of the ECA and Kardia heart monitor for 30 consecutive days. The objectives of the study are to demonstrate the utility and acceptability of the intervention and to evaluate the impact of the intervention on (a) HRQoL and (b) self-reported adherence to anticoagulation in comparison with standard of care.
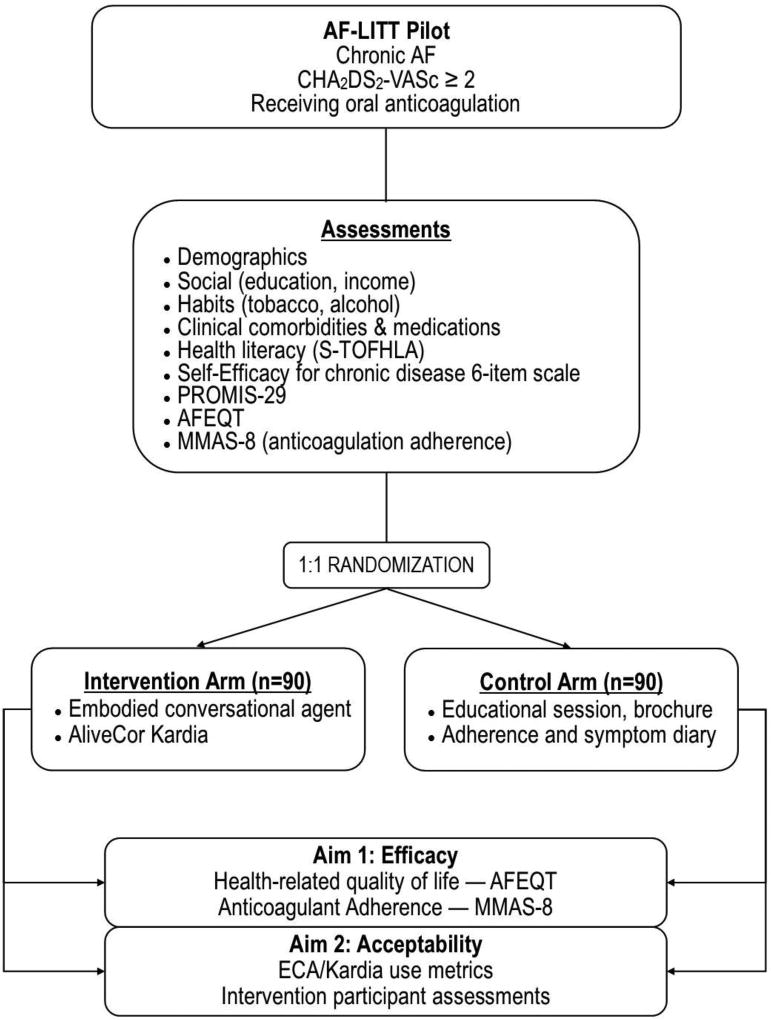
The study protocol and informed consent have been reviewed and approved by the University of Pittsburgh Institutional Review Board. The study is deemed to be low-risk and the study team will document and classify all adverse experiences that occur and will conduct data safety monitoring by reviewing Kardia results as available. The trial is registered at ClinicalTrials.gov (NCT03093558). A trial overview is presented in Figure 2.
Figure 2.
Flow diagram summarizing the Atrial Fibrillation health Literacy Information Technology Trial (AF-LITT). AF indicates Atrial Fibrillation; ECA, Embodied Conversational Agent; Kardia, Alive Cor’s Kardia Mobile; HRQoL, Health-Related Quality of Life, AFEQT, Atrial Fibrillation Effect on Quality of Life; MMAS-8 indicates Morisky Medication Adherence Score. Other abbreviations as per text.
Preliminary data using the ECA/Kardia
The study team conducted a 30-day preliminary assessment of the ECA/Kardia in 31 individuals (mean age 68±11, 39% women) with a history of chronic AF, receiving anticoagulation, and limited health literacy or poor HRQoL. Participants used the ECA a median of 17 days, averaging 18.0±10.0 days of use. Median Kardia use was 29 days, ranging from 5–30, and averaging 26.5±5.9 uses over the 30-day observation. As proposed in the pilot RCT described here, we used the Atrial Fibrillation Effect on QualiTy of life (or AFEQT),[18] an instrument specific to AF, for measurement of HRQoL. AFEQT scores range from 0 to 100, where higher scores indicated greater HRQoL in AF. We observed baseline AFEQT scores of 64.5±22.9 and 30-day follow-up scores of 76.3±19.4. We used the Morisky 8-item Medication Adherence Scale (MMAS-8)[19] for self-reported anticoagulation adherence (range 0–8). The MMAS-8 was 7.3±0.9 at baseline and 7.7±0.5 at 30 days. Results of the preliminary use of the ECA/Kardia guided the development of the pilot RCT described here. Participants had variable experience with smartphones and those who did not have a smartphone were provided with one and instruction on its use for this preliminary assessment. In this limited-sized preliminary cohort we did not observe differences in use of the ECA/Kardia by prior smartphone use.
Participant Selection
The inclusion criteria are designed to enroll 180 participants with non-valvular AF, a, and receiving oral anticoagulation for prevention of thromboembolism in AF.[20] Exclusions consist in AF due to non-cardiac causes; AF within 30 days of cardiothoracic or thoracic surgery; inability to use the ECA/Kardia applications after teaching; non-English speaking (and hence likely inability to use the ECA); unstable medical condition as indicated by history, physical, and/or laboratory findings; or presence of non-cardiovascular conditions likely to be fatal within 12 months (such as cancer). To avoid bias, prior experience with a smartphone is not a criterion for participation. As in our preliminary assessment, individuals randomized to the intervention who do not have a smartphone will receive one for use during the study.
Participants will be identified by reviewing the electronic medical record, referral to the study using University of Pittsburgh’s Center for Assistance in Research eRecord (CARE) protocol, referral by clinical providers or participant-initiated self-referral.
Assessments
All participants will undergo baseline assessments following informed consent, as summarized by Table 1. Assessments will consist in (a) demographics (age, sex, race) and primary and alternative contact numbers; (b) social factors, such as highest level of education completed and finances; (c) habits, including tobacco and alcohol use; (d) clinical conditions, including those that define the CHA2DS2-VASc (heart failure, hypertension, age, diabetes, prior stroke/TIA, cardiovascular disease, female sex)[20]; (e) Patient Health Questionnaire (PHQ-9) for depression[21]; (f) medications; (g) prior treatments for AF, such as cardioversion and pulmonary vein isolation; (h) Short-Test Of Functional Health Literacy in Adults[22]; (i) Self-Efficacy for Managing Chronic Disease 6-Item Scale[23]; (j) symptom and HRQoL assessment with the 29-item Patient-Reported Outcomes Measurement Information System (PROMIS)-29 Profile v2.0[24] and the 20-item AFEQT,[18] and (k) self-reported anticoagulation adherence with the MMAS-8.[19]
Table 1.
Summary of baseline and follow-up assessments for the Pilot Atrial Fibrillation health Literacy Information Technology Trial (Pilot AF-LITT).
| Demographics | Age, sex, race |
| Habits | Smoking (never/former, current); alcohol use |
| Anthropometry | Body mass index; SBP, DBP |
| Social, economic factors | Marital status; income, assets, insurance, education |
| AF assessments, treatment | Date of diagnosis, history of cardioversion/pulmonary vein isolation; anticoagulation history |
| Prevalent Conditions (definitions) | CHD (prior MI, catheterization, positive stress test) |
| History of stroke or transient ischemic attack | |
| CHF (LVEF<40% or ≥ NYHA II) | |
| DM (HgbA1c ≥ 7%, medications, history) | |
| HTN (SBP ≥ 140 mm Hg, DBP ≥ 90 mm Hg, medications) | |
| Medications | Antiarrhythmics, beta and calcium channel blockers, digoxin, anticoagulants (VKA, NOAC, aspirin) |
| Health-related Quality of Life* | Atrial Fibrillation Effect on QualiTy of life (AFEQT)[19] |
| Adherence* | Morisky Medication Adherence Scale, 8 items (MMAS-8)[20] |
| Patient Activation* | Patient Activation Measure[26] |
| Hospitalization events, Vital status† | Electronic health record, telephone contact |
SBP indicates systolic blood pressure; DBP, diastolic blood pressure; CHD, coronary disease; MI, myocardial infarction; CHF, congestive heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association class; DM, diabetes; HTN, hypertension; VKA, Vitamin K antagonist; NOAC, novel oral anti-coagulant.
Indicates assessed at baseline and 30-day follow-up.
Indicates assessed at 90 days.
At 30 days, all participants will have repeat assessments of HRQoL and self-reported adherence to anticoagulation. The AFEQT instrument is selected for HRQoL in this RCT because of its specific utility in AF. The instrument is a well-validated 20-item questionnaire, which measures the effect of AF on HRQoL across 4 conceptual domains: symptoms, daily activities, treatment concern, and treatment satisfaction. We employ the MMAS-8 for self-reported anticoagulation adherence at 30 days. The MMAS-8 has been validated for use in individuals with diverse conditions and with limited health literacy.[25, 26] Our use of the MMAS-8 is specific to anticoagulation, and not the multiple other therapies that participants with chronic AF may be taking for comorbid conditions. We focused on anticoagulation adherence because of the prominent importance of adherence to therapies for stroke prevention in AF.
ECA/Kardia use will be downloaded remotely. ECA data are stored locally on the smartphone and then synchronized with a secure web server. This design facilitates capturing ECA use including date and time of use, duration, extent of use (days, duration, and topics accessed) and the content (response to content about adherence and symptoms). ECA utilization will be quantified with descriptive statistics to describe the total number of uses, duration, days of consecutive use, and content accessed. For acceptability, intervention participants will complete a 22-item instrument with 7-point scale evaluating the experience of using the ECA, used by us in prior ECA health-services applications. Intervention participants will additionally have open-ended questions about their experiences with the ECA. Acceptability of the Kardia will likewise be determined by review of use statistics. Participants will be assigned an email and have results of use sent to a central server for review. Data available from the Kardia consist in dates of use and heart rate and rhythm.
Randomization
After providing informed consent, eligible individuals will be randomly assigned to receive the ECA/Kardia or usual care in a 1:1 ratio with a computer-generated randomization scheme.
Procedures
a. Intervention - Embodied Conversational Agent and Kardia
ECA dialogue content for AF-LITT consists of distinct modules focused on AF education, symptoms and adverse events, and adherence, and is summarized in Table 2. The educational content is arranged in discrete topics that contain definitions and causes of AF, treatment, and stroke prevention. The symptom and adverse event modules covers symptoms, rated 0–4 for severity. The ECA is able to “recall” prior reported symptoms and query users about the status of those symptoms. The ECA aims to address challenges as immediately as possible and accommodate for changes to anticoagulation adherence and barriers. Scripts used in the ECA emphasize the self-efficacy to maintain a long-term commitment towards adherence. Such an approach contrasts with other mHealth platforms by providing empathic and interactive support, absent in approaches that use electronic reminders, financial incentives, app dashboards, or texting. The ECA refers to the Kardia regularly, provide teaching in its use, and direct users to check their heart rates and rhythm as symptoms are reported. Participants in the treatment group will have the ECA and Kardia applications downloaded to a smartphone and will be provided a Kardia mobile heart rhythm monitor that attaches to the phone. Participants who do not own a smartphone will be provided one for use in the study; payment for the study will be contingent on returning the phone. Participants will be instructed to use the ECA and Kardia applications daily for 30 days.
Table 2.
Summary of domains reviewed by the Embodied Conversational Agent and domain content in the Pilot Atrial Fibrillation health Literacy Information Technology Trial (Pilot AF-LITT).
| Domain | Module content |
| Definition of AF | |
| Causes of AF | |
| Education | AF treatment |
| Stroke prevention for AF | |
| AliveCor Kardia Mobile use and trouble-shooting | |
|
| |
| Symptom overview | |
| Seeking help | |
| Symptom, self-assessment and monitoring | Chest pain or pressure |
| Palpitations or heart racing | |
| Shortness of breath with activities | |
| Fatigue | |
|
| |
| Adherence overview | |
| Daily assessment of medication adherence | |
| Adherence | Appointment adherence |
| Barriers to adherence | |
| Addressing adherence challenges | |
| Extended adherence and relevance to AF | |
AF indicates atrial fibrillation.
b. Coaching
A team member will provide telephone contact at days 7, 14, and 21 to the study participants for formal coaching on maintaining adherence to the ECA/Kardia or the study journal. Telephone-based coaching will be guided by a protocol and focus on trouble-shooting the ECA/Kardia, participant concerns, how often the applications are being used, adherence to the journal, and strengths/weaknesses of the content. We will also track use of the Kardia in real-time and contact participants without use for ≥3 days.
c. Control arm – Journal of symptoms and adherence
Journals will be provided for the participants comprised of a 30-day calendar with open- and close-ended questions. These items will inquire whether participants took their medications along with space to record why they did not, symptoms, and questions about their experience of AF.
d. Efficacy
After 30 days, participants in both the intervention and control arm will repeat the AFEQT and MMAS-8.
e. Acceptability
Participants receiving the ECA/Kardia will complete an interview to provide feedback and assess acceptability of the ECA intervention. The interview will consist of 20 items to be answered using a Likert scare and three open-ended questions, provided in Table 3. Responses will be analyzed for qualitative analysis, inform the acceptability of the intervention, and guide development of the next iteration of the ECA.
Table 3.
ECA Acceptability Assessment
| Closed-ended Questions (Likert-scale, scored from 1–7) |
| How satisfied were you with Tanya? |
| Was Tanya repetitive? |
| How easy was talking to Tanya? |
| How much would you like to continue working with Tanya? |
| How would you characterize your relationship with Tanya? |
| How much do you trust Tanya? |
| How much do you feel that Tanya cares about you? |
| How much do you feel that you and Tanya understand each other? |
| I feel comfortable with Tanya. |
| Tanya and I understand each other. |
| I believe Tanya likes me. |
| I believe Tanya is genuinely concerned about my welfare. |
| Tanya and I respect each other. |
| I feel that Tanya is not totally honest about her feelings towards me. |
| I am confident in Tanya’s ability to help me. |
| I feel that Tanya appreciates me. |
| Tanya and I trust one another. |
| My relationship with Tanya is very important to me. |
| I have the feeling that if I say or do the wrong things, Tanya will stop working with me. |
| I feel Tanya cares about me even when I do things she does not approve of. |
| Open Ended Questions |
| What are your thoughts about using the agent? |
| How has this project helped you with having atrial fibrillation? |
| What are your suggestions for making this project better? |
f. Reimbursement
Participants will be given $20 for the initial visit and $30 for the final visit.
g. Exploratory follow-up at 90 days
At 90 days, a team member will review the electronic health record to assess for events. Participants without interim clinical evaluations will be contacted by telephone to verify vital status and absence of hospitalization events.
Statistical Analysis
The study will use an intention-to-treat analysis including all subjects according to their randomized assignment. The baseline variables will be assessed to examine for differences between the treatment and control arm. Bivariate relationships will be examined using chi-square or Fisher’s exact tests for categorical variables and two-sample t-tests or Wilcoxon rank sum tests. The primary analysis will evaluate the difference in AFEQT scores at 30 days between arms using generalized linear mixed effects models including subject-specific random intercepts to account for correlation due to having repeated observations for each subject. We will also compare self-reported adherence to anticoagulation between the two groups using MMS-8 score as a continuous score. We will summarize use of the ECA and Kardia by number of days used, frequency, and duration of use. We will summarize content and symptoms reported in descriptive analyses. All analyses will be multivariable adjusted in progressive models that include age and sex initially; then habits, clinical conditions, medications, and prior AF treatments; then social and economic factors, including access to transportation; then PHQ-9, self-efficacy, and health literacy. For the exploratory analysis we will compare events between the 2 arms at 90 days. Missing data patterns will be evaluated including the frequency and percentage of subjects missing for each variable and the distribution of the number of variables missing for subjects. Data collected to the point of lost to follow-up will be compared to the data of those who complete the study to examine missing data mechanisms, e.g., missing completely at random (MCAR), missing at random (MAR), or not missing at random (NMAR). We will consider various approaches to account for missing data such as multiple imputation methods and likelihood-based approaches, if indicated.[27–30] When missing data occurs, we will document reasons when possible.
Power and sample size determination
For statistical power we estimated our ability to detect differences in AFEQT scores between the intervention and control arms at 30 days using the results of the modest (n=31) ECA/Kardia preliminary assessment. Our power calculations assume an alpha=0.05, statistical power=0.80 and a global AFEQT score standard deviation of 20, consistent with the results of the preliminary assessment. Given these assumptions, we estimate that we will have >80% statistical power to detect a 10-point difference in AFEQT scores at 30 days between the intervention and control arms by enrolling 90 participants in each study arm.
Limitations
Our trial has distinct limitations. The lack of blinding to the intervention introduces potential biases. Participants in the intervention and standard care arms receive different levels of attention. We do not expect that the journal provided to standard care participants is equivalent in attention to that provided to those receiving the ECA/Kardia intervention and the accompanying coaching. However, we consider that a control that matches on attention would underestimate the effect of the combined ECA/Kardia intervention in a pilot RCT. Second, assessments at 30-days are not blinded to randomization arm. We expect this pilot study to provide the essential preliminary data for a larger trial that will conduct blinded assessments in order to prevent contamination. Third, there is possible selection bias against individuals who are not comfortable with using a smartphone as part of the intervention. While participants randomized to the intervention receive detailed instruction on smartphone use, it is possible that some potential participants may be intimidated from enrolling in a study that incorporates a smartphone. Our anecdotal experience with our pilot, however, is that even those not familiar with the smartphone were enthusiastic about the possibility of engaging with the ECA and Kardia. Fourth, we employ the MMAS-8, which measures self-reported medication adherence, and focus on anticoagulation adherence. Self-report may have challenges to reliability. Assessing adherence objectively and for all medications is not feasible in this limited duration pilot. Finally, results from this pilot RCT are not entering the electronic health record or being used to coordinate care. We will communicate summaries of participant data regarding health literacy, symptoms, HRQoL, and results from the ECA and Kardia. However, we recognize the relevance of bringing the intervention results into the healthcare context for real-time management. We consider this pilot as limited duration and sample size, and will use the pilot results to develop a more robust RCT that integrates ECA/Kardia results in the electronic record. Such a collaborative model has strong potential to improve clinical management for a complex disease like AF.
Conclusions/Summary
This study will evaluate a novel AF-focused ECA in concert with the Kardia heart rhythm monitor to examine differences in HRQoL and adherence to anticoagulation across the 2 arms. As a secondary objective, this pilot will assess the acceptability and use of the smartphone-based application. We expect the results of this limited-sized trial to provide the foundation for a larger clinical trial that is guided by the efficacy and acceptability data generated by this pilot iteration. We expect that the ECA/Kardia has potential to improve patient-centered care in AF and provide a low-cost, effective means of reducing the social and medical morbidity associated with this condition. Further efforts will build on this pilot RCT to integrate ECA/Kardia results into the electronic health record and on-going patient management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
This work was supported by Grant 2015084 from the Doris Duke Charitable Foundation. The authors are solely responsible for the design and conduct of this study and all study analyses, the drafting and editing of the manuscript and its final contents.
References
- 1.Magnani JW, Norby FL, Agarwal SK, Soliman EZ, Chen LY, Loehr LR, et al. Racial differences in atrial fibrillation-related cardiovascular disease and mortality: The atherosclerosis risk in communities (aric) study. JAMA Cardiol. 2016 Jul 01;1:433–41. doi: 10.1001/jamacardio.2016.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L, Gallagher R, Neubeck L. Health-related quality of life in atrial fibrillation patients over 65 years: A review. Eur J Prev Cardiol. 2015 Aug;22:987–1002. doi: 10.1177/2047487314538855. [DOI] [PubMed] [Google Scholar]
- 3.Fang MC, Panguluri P, Machtinger EL, Schillinger D. Language, literacy, and characterization of stroke among patients taking warfarin for stroke prevention: Implications for health communication. Patient Education and Counseling. 2009 Jun;75:403–10. doi: 10.1016/j.pec.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichler K, Wieser S, Brugger U. The costs of limited health literacy: A systematic review. Int J Public Health. 2009;54:313–24. doi: 10.1007/s00038-009-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diug B, Evans S, Lowthian J, Maxwell E, Dooley M, Street A, et al. The unrecognized psychosocial factors contributing to bleeding risk in warfarin therapy. Stroke. 2011 Oct;42:2866–71. doi: 10.1161/strokeaha.111.615674. [DOI] [PubMed] [Google Scholar]
- 6.Fang MC, Machtinger EL, Wang F, Schillinger D. Health literacy and anticoagulation-related outcomes among patients taking warfarin. Journal of General Internal Medicine. 2006 Aug;21:841–6. doi: 10.1111/j.1525-1497.2006.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oramasionwu CU, Bailey SC, Duffey KE, Shilliday BB, Brown LC, Denslow SA, et al. The association of health literacy with time in therapeutic range for patients on warfarin therapy. J Health Commun. 2014;19(Suppl 2):19–28. doi: 10.1080/10810730.2014.934934. [DOI] [PubMed] [Google Scholar]
- 8.Goli NM, Thompson T, Sears SF, Mounsey JP, Chung E, Schwartz J, et al. Educational attainment is associated with atrial fibrillation symptom severity. Pacing and Clinical Electrophysiology. 2012 Sep;35:1090–6. doi: 10.1111/j.1540-8159.2012.03482.x. [DOI] [PubMed] [Google Scholar]
- 9.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, et al. Social determinants of risk and outcomes for cardiovascular disease: A scientific statement from the american heart association. Circulation. 2015 Sep 1;132:873–98. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 10.Hickey KT, Hauser NR, Valente LE, Riga TC, Frulla AP, Masterson Creber R, et al. A single-center randomized, controlled trial investigating the efficacy of a mhealth ecg technology intervention to improve the detection of atrial fibrillation: The iheart study protocol. BMC Cardiovasc Disord. 2016;16:152. doi: 10.1186/s12872-016-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desteghe L, Raymaekers Z, Lutin M, Vijgen J, Dilling-Boer D, Koopman P, et al. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace. 2016 Feb 17; doi: 10.1093/europace/euw025. [DOI] [PubMed] [Google Scholar]
- 12.Bickmore TW, Pfeifer LM, Byron D, Forsythe S, Henault LE, Jack BW, et al. Usability of conversational agents by patients with inadequate health literacy: Evidence from two clinical trials. Journal of health communication. 2010;15(Suppl 2):197–210. doi: 10.1080/10810730.2010.499991. [DOI] [PubMed] [Google Scholar]
- 13.Bickmore TW, Silliman RA, Nelson K, Cheng DM, Winter M, Henault L, et al. A randomized controlled trial of an automated exercise coach for older adults. Journal of the American Geriatrics Society. 2013 Oct;61:1676–83. doi: 10.1111/jgs.12449. [DOI] [PubMed] [Google Scholar]
- 14.Gardiner P, Hempstead MB, Ring L, Bickmore T, Yinusa-Nyahkoon L, Tran H, et al. Reaching women through health information technology: The gabby preconception care system. American journal of health promotion : AJHP. 2013 Jan-Feb;27:eS11–20. doi: 10.4278/ajhp.1200113-QUAN-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Bickmore T, Bowen DJ, Norkunas T, Campion M, Cabral H, et al. Acceptability and feasibility of a virtual counselor (vicky) to collect family health histories. Genetics in medicine : official journal of the American College of Medical Genetics. 2015 Jan 15; doi: 10.1038/gim.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan PH, Wong CK, Poh YC, Pun L, Leung WW, Wong YF, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc. 2016 Jul;5 doi: 10.1161/jaha.116.003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarakji KG, Wazni OM, Callahan T, Kanj M, Hakim AH, Wolski K, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: The itransmit study. Heart Rhythm. 2015 Mar;12:554–9. doi: 10.1016/j.hrthm.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, et al. Development and validation of the atrial fibrillation effect on quality-of-life (afeqt) questionnaire in patients with atrial fibrillation. Circulation. Arrhythmia and electrophysiology. 2011 Feb;4:15–25. doi: 10.1161/CIRCEP.110.958033. [DOI] [PubMed] [Google Scholar]
- 19.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. Journal of Clinical Hypertension. 2008 May;10:348–54. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010 Feb;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The phq-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient Educ Couns. 1999 Sep;38:33–42. doi: 10.1016/s0738-3991(98)00116-5. [DOI] [PubMed] [Google Scholar]
- 23.Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self - management program on patients with chronic disease. Eff Clin Pract. 2001 Nov-Dec;4:256–62. [PubMed] [Google Scholar]
- 24.D. o. H. H. Services. U.S. Promis: Dynamic tools to measure health outcomes from the patient perspective. 2016 May 21; Available: http://www.healthmeasures.net/explore-measurement-systems/promis.
- 25.Krousel-Wood M, Joyce C, Holt EW, Levitan EB, Dornelles A, Webber LS, et al. Development and evaluation of a self-report tool to predict low pharmacy refill adherence in elderly patients with uncontrolled hypertension. Pharmacotherapy. 2013 Aug;33:798–811. doi: 10.1002/phar.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thurston MM, Bourg CA, Phillips BB, Huston SA. Impact of health literacy level on aspects of medication nonadherence reported by underserved patients with type 2 diabetes. Diabetes Technol Ther. 2015 Mar;17:187–93. doi: 10.1089/dia.2014.0220. [DOI] [PubMed] [Google Scholar]
- 27.Rubin D. Multiple imputation for nonresponse surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 28.Little RJA, Rubin DB. Statistical analysis with missing data. Hoboken, NJ: Jon Wiley & Sons, Inc.; 2002. [Google Scholar]
- 29.Ibrahim JG. Incomplete data in generalized linear models. J Am Stat Assoc. 1990;85:765–769. [Google Scholar]
- 30.Horton NJ, Kleinman KP. Much ado about nothing: A comparison of missing data methods and software to fit incomplete data regression models. The American statistician. 2007 Feb;61:79–90. doi: 10.1198/000313007X172556. [DOI] [PMC free article] [PubMed] [Google Scholar]