Abstract
Characterization of newly isolated mycoviruses may contribute to understanding of the evolution and diversity of viruses. Here, a deep sequencing approach was used to analyze the double-stranded RNA (dsRNA) mycoviruses isolated from field-collected P. striiformis samples in China. Database searches showed the presence of at least four totivirus-like sequences, termed Puccinia striiformis virus 1 to 4 (PsV1 to 4). All of these identified sequences contained two overlapping open reading frames (ORFs) which encode a putative coat protein (CP) and an RNA-dependent RNA polymerase (RdRp) showing similar structures to members of the genus Totivirus. Each PsV contained a -1 ribosomal frameshifting region with a slippery site and a pseudoknot structure in the overlapped regions of these ORFs, indicating that the RdRp is translated as a CP-RdRp fusion. Phylogenetic analyses based on RdRp and CP suggested that these novel viruses belong to the genus Totivirus in the family Totiviridae. The presences of these PsVs were further validated by transmission electron microscope (TEM) and RT-PCR. Taken together, our results demonstrate the presence of diverse, novel totiviruses in the P. striiformis field populations.
Keywords: wheat stripe rust, Puccinia striiformis, deep sequencing, mycovirus, Totivirus
Introduction
Mycoviruses (fungal viruses) are of common occurrence in all major taxonomic groups of filamentous fungi, yeasts and oomycetes (Pearson et al., 2009; Ghabrial et al., 2015). Although most mycoviruses infect their fungal hosts symptomlessly (cryptic infections), some of them cause phenotypic alterations including hypovirulence and debilitation, and thus can be used as biological control agents against fungal diseases, such as the +ssRNA mycovirus Cryphonectria hypovirus 1 (CHV1) against Cryphonectia parasitica (Nuss, 2005) and the ssDNA mycovirus Sclerotinia sclerotiorum hypovirulence-associated DNA virus 1 (SsHADV-1) against S. sclerotiorum (Yu et al., 2013). Most mycoviruses have double-stranded RNA (dsRNA) genomes and form typical virus particles (Lin et al., 2012; Zheng et al., 2014). DsRNA mycoviruses are now classified into six families, including Totiviridae, Partitiviridae, Megabirnaviridae, Reoviridae, Quadriviridae, Chrysoviridae (Kondo et al., 2016). Among them, the families Totiviridae and Partitiviridae are the largest.
The Basidiomycota rust fungi cause disease on a variety of host crop species including soybean, coffee, groundnuts, wheat, and tree species such as conifers and poplars. Rust fungi belong to the order Pucciniales which consists of over 5,000 species and over 100 genera (Zhang et al., 1994). Rusts are obligate biotrophs, which can only absorb nutrients from alive host tissue. Wheat stripe rust, caused by Puccinia striiformis f. sp. tritici, is one of the most important diseases of wheat worldwide (Chen, 2005; Wellings, 2011). In China, the annual yield loss was estimated to be approximately 1.0 million metric tons (Chen et al., 2009). The presence of dsRNA in rusts was first reported in Newton et al. (1985). Subsequently, indirect evidence suggested the presence of mycovirus-like RNA molecules in Phakopsora pachyrhizi, the causal agent of Asian soybean rust (Link et al., 2014). Recently, a dsRNA mycovirus was identified from the fungus P. pachyrhizi (Cooper et al., 2016). Although some progress has been made in obtaining dsRNAs from rust mycoviruses, little is known about their genome organization, which is due in a large extent to their asymptomatic infections (Zhang et al., 1994). The cryptic dsRNAs are mostly reported in the families Totiviridae and Partitiviridae (Ghabrial, 1998; Zheng et al., 2014). The family Totiviridae currently comprises five approved genera, of which totiviruses and victoriviruses infect only fungi, while giardiaviruses, trichomonasviruses and leishmaniaviruses infect mainly protozoa (Goodman et al., 2011; Kondo et al., 2016). Members of this family have non-segmented dsRNA genomes being 4.6–7.0 kbp in length and usually contain two large, partially overlapping open reading frames (ORFs) which encode a capsid protein (CP) and an RNA-dependent RNA polymerase (RdRp) respectively (Ghabrial et al., 2015).
Here we report four new complete totivirus-like genome sequences based on deep sequencing of dsRNAs isolated from field-collected P. striiformis samples in China. Based on viral genome organization, phylogeny and particle morphology, these novel viruses were identified to belong to the genus Totivirus in the family Totiviridae.
Materials and Methods
P. striiformis Fungal Samples
Puccinia striiformis urediniospores were obtained from the susceptible wheat cultivar Mingxian 169 in an experimental field (approximately 3 m × 6 m area) at the Northwest A&F University, Yangling, Shaanxi, China in the summer of 2015. Samples were collected and stored in a desiccator at 4°C. The Puccinia striiformis species identity of these field samples were validated based on the complete internal transcribed spacer (ITS) sequence of ribosomal DNA (rDNA). Total genomic DNA was extracted from uredinospores of P. striiformis with CTAB method as described by Justesen et al. (2002). The ribosomal rDNA-ITS primers, ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′), were synthesized by Sangon Bio-Tech Co., Ltd. Polymerase chain reaction (PCR) amplification was done following standard methods according to Zheng et al. (2017).
Extraction of dsRNA
DsRNA was extracted from 1.0 g of P. striiformis urediniospores according a described method with minor modifications (Zheng et al., 2014), and absorption column made up of cellulose powder CF-11 (Whatman, United Kingdom) was used. Extracted dsRNAs were treated with DNase I and S1 nuclease (TaKaRa Bio Inc) to remove genomic DNA and single-stranded RNA (ssRNA) contaminations, the qualities of which were then analyzed based on 1.0% (w/v) agarose gel electrophoresis.
cDNA Library Construction and Illumina Sequencing
The dsRNA sample (1.0 μg) was used for cDNA library construction using the NEBNext®UltraTM RNA Library Prep Kit (Illumina, United States) following manufacturer’s instructions. The cDNA is end-repaired and adenylated prior to adaptor ligation, library construction and amplification under this method. Then the sequenced-ready library was subjected to 5 million of 150 nucleotide (nt) paired-end reads using Illumina HiSeq 4000 technology. The cDNA library construction and deep sequencing analysis were carried out by Shanghai Hanyu Bio-Tech Co., Ltd. After deep sequencing (>40,000 × coverage), raw reads were cleaned by removing adapter sequences and low-quality bases (PHRED quality scores ≤ 5), and truncated reads smaller than 35 bp were discard. 29 contigs were obtained and the N50 length is 1,489 nt. The total length of sequencing is about 37 kb with a GC content of 42.06%. De novo assembly of contiguous sequences was conducted using the Velvet de novo assembly algorithm with k-mer: 69. Minimum contig length was 500 bp as well as minimum coverage was 18. The ends and the other parts of the sequences were all confirmed by Sanger sequencing. To obtain the termini of the dsRNAs, rapid amplification of cDNA ends (RACE) was performed (Zheng et al., 2013).
Database Search and Sequence Analysis
Open reading frames (ORFs) were identified using the National Center for Biotechnology Information (NCBI) ORF Finder program1. Motif searches were performed in three databases, including PROSITE2, Pfam3 and CDD4. RNA pseudoknot structure was predicted using the DotKnot program and drawn with PseudoViewer3 (Byun and Han, 2009; Sperschneider and Datta, 2010).
Phylogenetic Analysis
Phylogenetic trees were constructed based on the deduced amino acid sequences of the putative RdRp and CP regions using the maximum-likelihood (ML) method of the MEGA program (version 6.0)with 1,000 bootstrap replicates as described previously with minor modifications (Kondo et al., 2016). Multiple alignments of the sequences of RdRp and CP were conducted with Clustal-X program (Thompson et al., 1997).
Purification of Viral Particles and Electron Microscopy
Virus particles were purified using the method as described previously with minor modifications (Sanderlin and Ghabrial, 1978). Three gram P. striiformis urediniospores were grounded into fine powder in the presence of liquid nitrogen with a sterilized mortar and pestle. The powder was then mixed with 200 ml extraction buffer made up of 0.1 M sodium phosphate, and pH 7.0 containing 3% Triton X-100. The suspension was centrifuged at 10,000 × g for 20 min to remove the spore cell debris. Subsequently, the supernatant was subject to a 1.5 h ultracentrifugation at 120,000 × g for viral particle precipitation. The pellets were then suspended in 0.1 M sodium phosphate buffer and centrifuged at 15,000 × g for 30 min. Then the suspension containing the virus particles was fractionated via a 10–40% (w/v) sucrose gradient by centrifugation at 60,000 × g for 3.5 h. Fractions in the middle portion of the tube were collected, and stained with 2% phosphotungstic acid (pH 7.4) and observed under a transmission electron microscope (TEM) (HT7700, Japan).
Validation of the Presence of Mycovirus-Like dsRNAs in Isolated Viral Particles
The dsRNAs from virus particles was extracted using phenol, chloroform and isoamyl alcohol, and then subjected to electrophoresis in 1% (w/v) agarose gel. Using the dsRNAs from virus particles as templates, complementary DNAs were synthesized as described by Xie et al. (2011) with tagged random dN6 primers (Rong et al., 2002; Zheng et al., 2013). Reverse transcription polymerase chain reaction (RT-PCR) was then performed with specific primers (Supplementary Table S1) which target the four PsVs respectively according to the method of Vainio et al. (2012) with minor modifications.
Results
P. striiformis Urediniospores Contain Putative Totiviruses-Like dsRNAs
To detect dsRNA virus, P. striiformis urediniospores were collected from heavily infected wheat leaves growing in the field (Figure 1A). Urediniospores were round-shaped and approximately 30 μm in diameter (Figure 1B). The sequence length of ITS is 663 bp and it has 99% sequence identity to the P. striiformis strain CYR32. The result confirmed the fungus belonged to P. striiformis species. Nucleic acid extraction obtained nucleic acid bands of approximately 5.0 kb in size (Figure 1C) that resisted DNase I and S1 nuclease digestions, indicating the presence of dsRNA-like nucleotides.
FIGURE 1.
Puccinia striiformis f. sp. tritici urediniospores contain dsRNA-like nucleic acids elements. (A) Symptoms of stripe rust disease on wheat leaves. (B) P. striiformis urediniospores collected from wheat leaves. (C) Agarose gel electrophoresis of dsRNA extracted from the urediniospores of P. striiformis. M indicates molecular markers of aaaDNA digested with Hind III.
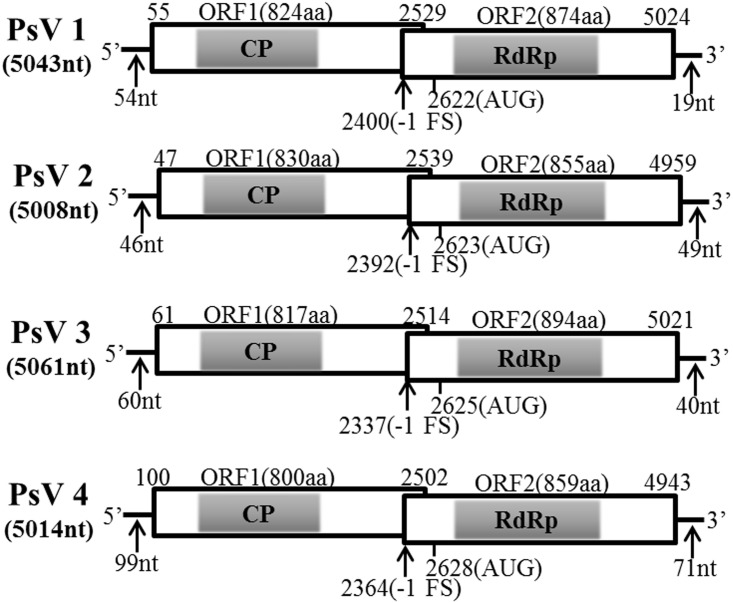
Four P. striiformis-derived totivirus-like sequences, termed as Puccinia striiformis virus 1 to 4 (PsV1 to 4) (Supplementary Table S2), were identified from the post-assembly contigs by deep sequencing. The 5′- and 3′- untranslated regions (UTRs) of all PsVs were obtained. The complete nucleotide sequences of PsVs (1 to 4), ranging from 5,008 to 5,061 nt, were all deposited in GenBank under accession numbers KY207361-KY207364, respectively.
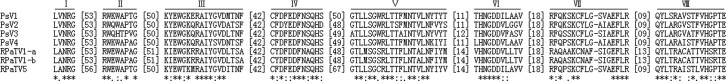
Pair-wise comparisons among proteins encoded by PsV1 to 4 revealed moderate levels (37–42%) of amino acid sequence identity of RdRp, but low-level similarities (31–33%) among CP. It is notable that the 5′-end sequences of PsVs 1 to 4 (5′-AUAAAUCCCC…-3′, 5′-AUACAAUCCCC…-3′, 5′-AUAUAACUCCC…-3′ and 5′-AUAAAACCCCC…-3′, respectively) appeared to be partially conserved. Likewise, the 3′-end sequences were not conserved (data not shown). All four genomes contained two ORFs encoding CP and RdRp respectively (ORF1 and ORF2) (Figure 2), such organization is typical of totiviruses. The predicted CP and RdRp proteins showed moderate levels of amino acid sequence identities (32–34% for CP and 39–41% for RdRp, respectively) with Phakopsora pachyrhizi mycovirus (PpV) and red clover powdery mildew-associated totivirus 5 (RPaTV5) (Supplementary Table S3). A search of conserved domain database (CDD) and multiple protein alignment confirmed that the predicted RdRp domains contain eight conserved motifs (I to VIII), including the GDD motif, which are the typical characteristics of mycovirual RdRps (Figure 3) (Routhier and Bruenn, 1998).
FIGURE 2.
Genomic organizations of PsVs sequences. The two overlapping ORFs and the untranslated regions (UTRs) are shown by open boxes and a single line, respectively. The conserved CPs and RdRps domain are indicated by shadows. Nucleotide positions of ORFs and the putative slippery site for -1 frameshifting are shown with black arrows.
FIGURE 3.
Sequence alignment of PsV RdRp motifs with those of selected viruses in the genus Totivirus. Horizontal lines above the alignment indicate the eight motifs, numbers in brackets suggest the amino acid positions, shadow area and asterisks indicate identical amino acid residues, colons indicate the similar residues.
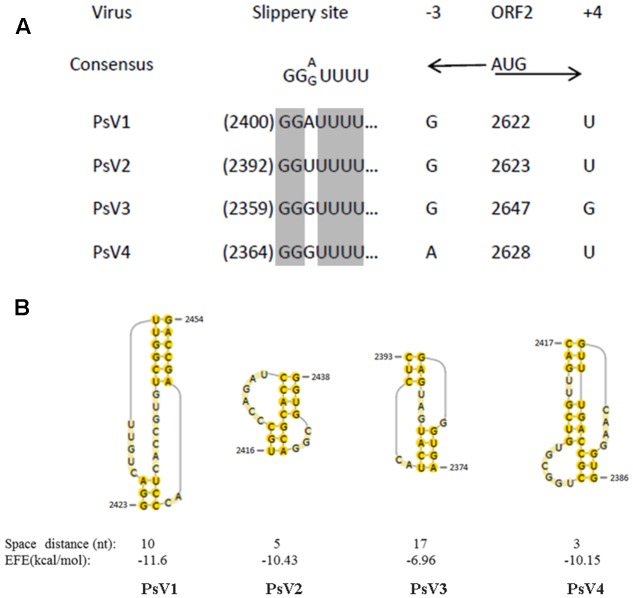
The PsV sequences between position -3 and +4 relative to the ORF2 AUG start codons (Figure 4A) are similar to the translation initiation sites of the viruses of plants and yeasts (Lütcke et al., 1987; Hamilton et al., 1987). Sequence analysis of PsVs indicated that there is an overlap region between ORFs 1 and 2 (Figure 2). It is therefore possible that ORFs 2 of PsVs is translated as a fusion protein with ORFs 1 through a -1 ribosomal frameshift which is a canonical slippery sites ‘XXXYYYZ’ (where X is A/C/G/U, Y is A/U, and Z is A/C/U) within the overlapping region (Bekaert and Rousset, 2005). The slippery site ‘GGA/GUUU’ sequence in PsV1 to 4 sequences (Figure 4B) is similar to these of the RPaTVs (Kondo et al., 2016). Using the DotKnot program, a pseudoknot structure was predicted in the downstream of each putative slippery site (Figure 4B). RNA pseudoknots are known to help pausing the translating ribosome and increasing the frequency of frameshifting (Plant et al., 2003; Zhai et al., 2008; Kondo et al., 2016).
FIGURE 4.
Putative slippery sites and pseudoknot structures predicted in PsVs. (A) The putative slippery sequences (XXXYYYZ) involved in -1 translational frameshift. The PsVs nucleotide sequences at –3 and +4 relative to the AUG start codon are indicated by black arrows. (B) The predicted pseudoknot structures downstream of the potential slippery sequences for –1 frameshifting are shown by yellow shadow. Spacer distance represents the number of nucleotides between the slippery site and the pseudoknot. EFE (kcal/mol) indicates the minimal free energy.
Phylogenetic Analyses
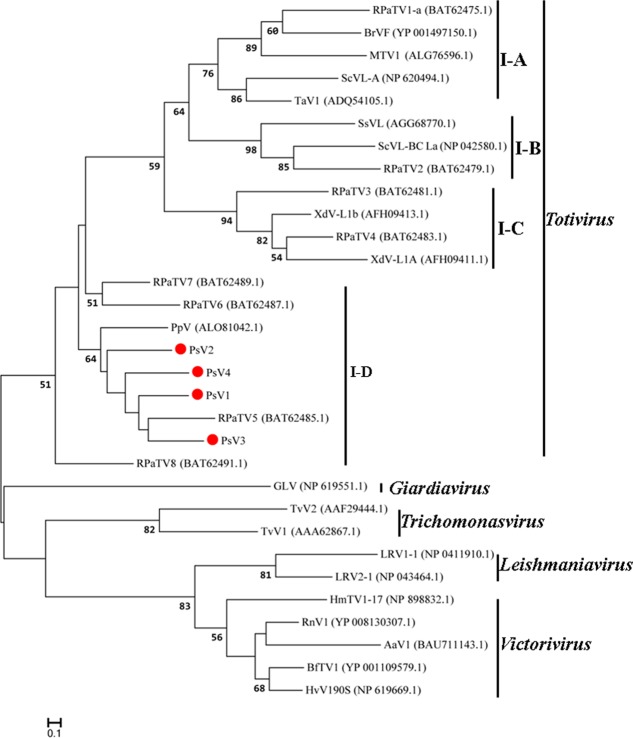
The maximum likelihood (ML) tree for RdRP is shown in Figure 5, Totivirus contained four subgroups, I-A, I-B, I-C and I-D. PsV1 to 4 clustered with RPaTVs 5 to 8 and PpV in the subgroup I-D. The ML phylogeny based on CPs is shown in Figure 6, which had similar topology as the one based on RdRPs.
FIGURE 5.
Phylogenetic analysis based on the deduced amino acid sequences of putative RdRps using the maximum likelihood (ML) method with 1,000 bootstrap replicates. The scale bar represents a genetic distance of 0.1 amino acid substitutions per site. Red circles indicate the novel mycoviruses PsVs in the present study.
FIGURE 6.
Phylogenetic analysis based on the deduced amino acid sequences of putative CPs using the maximum likelihood (ML) method with 1,000 bootstrap replicates. The scale bar represents a genetic distance of 0.1 amino acid substitutions per site. Red circles indicate the novel mycoviruses PsVs in the present study.
Taken together, genome organizations, amino acid sequence alignments and phylogenetic analyses all support that PsV1 to 4 are new members of the genus Totivirus within the family Totiviridae.
Observation of Viral Particle and Validation of the Viral Genome Sequences
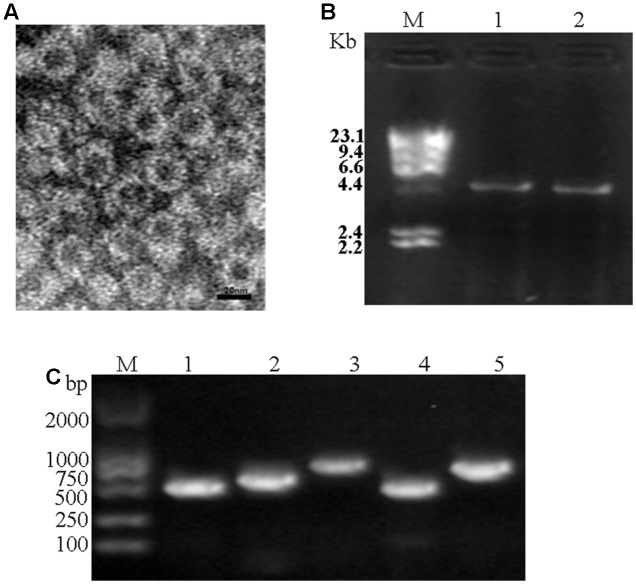
Under TEM, the viral particles purified from P. striiformis urediniospores were isometric with an average diameter of 35 nm (Figure 7A). The sizes of dsRNAs from viral particles were similar with the dsRNAs extracted directly from P. striiformis urediniospores (Figure 7B). Using the dsRNAs extracted from viral particles as templates, we performed RT-PCR based on PsV-specific primers targeting RdRps, which successfully amplified products and in all cases the sizes were identical to ones expected based on the PsV genomes (Figure 7C).
FIGURE 7.
Electron microscopy and RT-PCR. (A) Viral particles purified from field-collected P. striiformis urediniospores. (B) Agarose gel electrophoresis of dsRNAs extracted from purified virus particles of PsVs (lane 1) and the urediospores of P. striiformis from field-collected samples (lane 2). (C) Confirmation of identity of dsRNAs extracted from purified virus particles using PsVs 1-4 specific primers (lanes 1-4, respectively) (Supplementary Table S1). M indicates molecular markers of aaaDNA digested with Hind III.
Discussion
Characterization of newly isolated fungal viruses may contribute to understanding of the evolution and diversity of viruses (Chiba et al., 2009; Nibert et al., 2013; Zheng et al., 2014). Although mycoviruses have been identified from all major groups of filamentous fungi (Ghabrial and Suzuki, 2009), it is rarely reported in obligate biotrophs, such as rust fungi and powdery mildews most likely due to their unculturablity (Kondo et al., 2016). Recently, with the development of next generation sequencing technologies, new mycoviruses have been identified directly from field-collected fungal samples (Feldman et al., 2012; Kondo et al., 2016; Marzano and Domier, 2016). The present study revealed four complete (PsV1 to 4) totiviral sequences. The presence of these PsVs was further validated by TEM and RT-PCR. To the best of our knowledge, this study provides the first evidence of dsRNA mycoviruses infections in the P. striiformis f. sp. tritici, the causal agent of wheat stripe rust.
Each of the four PsV genomes contained two overlapping ORFs which encode the conserved domains of CP and RdRp, respectively. Moreover, the four PsVs were deduced to universally contain the -1 ribosomal frameshifting at the overlapping regions (Figure 2), which could facilitate translation of ORF1 and ORF2 as a fusion polyprotein. The predicted ORF2 coding strategy of PsVs were in line with members of genus Totivirus in the family Totiviridae, such as Saccharomyces cerevisiae virus L-A (ScVL-A) (Dinman et al., 1991) and red clover powdery mildew-associated totiviruses (RPaTVs) (Kondo et al., 2016).
Phylogenetic analysis with RdRp and CP sequences placed PsVs in a distinctive clade with members of Totivirus in the family Totiviridae (Figures 5, 6). Interestingly, both RdRp and CP phylogeny placed PsV1 to 4 together with RPaTVs 5 to 8 and PpV in the subgroup I-D. Interestingly, all the virus hosts in subgroup I-D are obligate biotrophic fungi, such as P. striiformis, P. pachyrhizi, and powdery mildew fungi, indicating an unknown interaction between obligate biotrophic fungi and totiviruses in this subgroup.
The current criteria for species demarcations of the genus Totivirus require less than 50% sequence identity of CP/RdRp proteins (Wickner et al., 2011). In the present study, proteins encoded by PsV1 to 4 shared moderate levels (RdRp, 37–42%; CP, 31–33%) of amino acid sequence identities to known totivirus species and among themselves, based on which they should represent novel totivirus species.
Taken together, this study characterized the molecular features of PsVs present in field-collected samples of wheat stripe rust fungus. The four novel viruses PsVs belong to the genus Totivirus in the family Totiviridae. This study demonstrates the presence of diverse, novel totiviruses in the P. striiformis f. sp. tritici populations, characterizing their interactions with the P. striiformis host will potentially allow for developing novel rust disease control strategies.
Author Contributions
ZK designed experiments; LZ performed the experiments; LZ, XL, XfL, and SJ analyzed the data; JZ, GZ, PL, and JW joined the discussion and gave the original ideas; LZ wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Key Basic Research Program of China (No. 2013CB127700), the China Postdoctoral Science Foundation (No.2016M592845), the Shaanxi Postdoctoral Science Foundation (No.2016BSHEDZZ116) and the National Natural Science Foundation of China (No. 31701732).
Footnotes
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2017.01960/full#supplementary-material
References
- Bekaert M., Rousset J. P. (2005). An extended signal involved in eukaryotic -1 frameshifting operates through modification of the E site tRNA. Mol. Cell 17 61–68. 10.1016/j.molcel.2004.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun Y., Han K. (2009). Pseudoviewer 3: generating planar drawings of large-scale RNA structures with pseudoknots. Bioinformatics 25 1435–1437. 10.1093/bioinformatics/btp252 [DOI] [PubMed] [Google Scholar]
- Chen W. Q., Wu L. R., Liu T. G., Xu S. C., Jin S. L., Peng Y. L., et al. (2009). Race dynamics, diversity, and virulence evolution in Puccinia striiformis f. sp. tritici, the causal agent of wheat stripe rust in China from 2003 to 2007. Plant Dis. 93 1093–1101. 10.1094/PDIS-93-11-1093 [DOI] [PubMed] [Google Scholar]
- Chen X. M. (2005). Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Can. J. Plant Pathol. 27 314–337. 10.1080/07060660509507230 25512677 [DOI] [Google Scholar]
- Chiba S., Salaipeth L., Lin Y. H., Sasaki A., Kanematsu S., Suzuki N. (2009). A novel bipartite double-stranded RNA mycovirus from the white root rot fungus Rosellinia necatrix: molecular and biological characterization, taxonomic considerations, and potential for biological control. J. Virol. 83 12801–12812. 10.1128/JVI.01830-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper B., Campbell K. B., Garrett W. M. (2016). Expression of a synthetic rust fungal virus cDNA in yeast. Arch. Virol. 161 111–123. 10.1007/s00705-015-2639-0 [DOI] [PubMed] [Google Scholar]
- Dinman J. D., Icho T., Wickner R. B. (1991). A -1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc. Natl. Acad. Sci. U.S.A. 88 174–178. 10.1073/pnas.88.1.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman T. S., Morsy M. R., Roossinck M. J. (2012). Are communities of microbial symbionts more diverse than communities of macrobial hosts? Fungal Biol. 116 465–477. 10.1016/j.funbio.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Ghabrial S. A. (1998). Origin, adaptation and evolutionary pathways of fungal viruses. Virus Genes 16 119–131. 10.1023/A:1007966229595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabrial S. A., Jiang D., Nibert M. L., Suzuki N. (2015). 50-plus years of fungal viruses. Virology 479 356–368. 10.1016/j.virol.2015.02.034 [DOI] [PubMed] [Google Scholar]
- Ghabrial S. A., Suzuki N. (2009). Viruses of plant pathogenic fungi. Annu. Rev. Phytopathol. 47 353–384. 10.1146/annurev-phyto-080508-081932 [DOI] [PubMed] [Google Scholar]
- Goodman R. P., Ghabrial S. A., Fichorova R. N., Nibert M. L. (2011). Trichomonasvirus: a new genus of protozoan viruses in the family Totiviridae. Arch. Virol. 156 171–179. 10.1007/s00705-010-0832-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R., Watanabe C. K., de Boer H. A. (1987). Compilation and comparison of the sequence context around the AUG start codons in Saccharomyces cerevisiae mRNAs. Nucleic Acids Res. 15 3581–3593. 10.1093/nar/15.8.3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justesen A. F., Ridout C. J., Hovmøller M. S. (2002). The recent history of Puccinia striiformis f. sp. tritici in Denmark as revealed by disease incidence and AFLP markers. Plant Pathol. 51 13–23. 10.1046/j.0032-0862.2001.00651.x 21774816 [DOI] [Google Scholar]
- Kondo H., Hisano S., Chiba S., Maruyama K., Andika I. B., Toyoda K., et al. (2016). Sequence and phylogenetic analyses of novel totivirus-like double-stranded RNAs from field-collected powdery mildew fungi. Virus Res. 213 353–364. 10.1016/j.virusres.2015.11.015 [DOI] [PubMed] [Google Scholar]
- Lin Y. H., Chiba S., Tani A., Kondo H., Sasaki A., Kanematsu S., et al. (2012). A novel quadripartite dsRNA virus isolated from a phytopathogenic filamentous fungus, Rosellinia necatrix. Virology 426 42–50. 10.1016/j.virol.2012.01.013 [DOI] [PubMed] [Google Scholar]
- Link T. I., Lang P., Scheffler B. E., Duke M. V., Graham M. A., Cooper B., et al. (2014). The haustorial transcriptomes of Uromyces appendiculatus and Phakopsora pachyrhizi and their candidate effector families. Mol. Plant Pathol. 15 379–393. 10.1111/mpp.12099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. (1987). Selection of AUG initiation codons differs in plants and animals. EMBO J. 6 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzano S. Y., Domier L. L. (2016). Novel mycoviruses discovered from metatranscriptomics survey of soybean phyllosphere phytobiomes. Virus Res. 213 332–342. 10.1016/j.virusres.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Newton A. C., Caten C. E., Johnson R. (1985). Variation for isozymes and double-stranded RNA among isolates of Puccinia striiformis and two other cereal rusts. Plant Pathol. 34 235–247. 10.1111/j.1365-3059.1985.tb01355.x [DOI] [Google Scholar]
- Nibert M. L., Tang J., Xie J., Collier A. R., Ghabrial S. A., Baker T. S., et al. (2013). 3D structures of fungal partitiviruses. Adv. Virus Res. 86 59–85. 10.1016/B978-0-12-394315-6.00003-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L. (2005). Mycoviruses at the fungal-plant interface. Nat. Rev. Microbiol. 3 632–642. 10.1038/nrmicro1206 [DOI] [PubMed] [Google Scholar]
- Pearson M. N., Beever R. E., Boine B., Arthur K. (2009). Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 10 115–128. 10.1111/j.1364-3703.2008.00503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant E. P., Jacobs K. L., Harger J. W., Meskauskas A., Jacobs J. L., Baxter J. L., et al. (2003). The 9-A solution: how mRNA pseudoknots promote efficient programmed -1 ribosomal frameshifting. RNA 9 168–174. 10.1261/rna.2132503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R., Rao S., Scott S. W., Carner G. R., Tainter F. H. (2002). Complete sequence of the genome of two dsRNA viruses from Discula destructive. Virus Res. 90 217–224. 10.1016/S0168-1702(02)00178-8 [DOI] [PubMed] [Google Scholar]
- Routhier E., Bruenn J. A. (1998). Functions of conserved motifs in the RNA-dependent RNA polymerase of a yeast double-stranded RNA virus. J. Virol. 72 4427–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderlin R. S., Ghabrial S. A. (1978). Physicochemical properties of two distinct types of virus-like particles from Helminthosporium victoriae. Virology 87 142–151. 10.1016/0042-6822(78)90166-6 [DOI] [PubMed] [Google Scholar]
- Sperschneider J., Datta D. (2010). Dotknot: pseudoknot prediction using the probability dot plot under a refined energy model. Nucleic Acids Res. 38:e103. 10.1093/nar/gkq021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. (1997). The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25 4876–4882. 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio E. J., Hyder R., Aday G., Hansen E., Piri T., Doğmus-Lehtijärvi T., et al. (2012). Population structure of a novel putative mycovirus infecting the conifer root-rot fungus Heterobasidion annosum sensu lato. Virology 422 366–376. 10.1016/j.virol.2011.10.032 [DOI] [PubMed] [Google Scholar]
- Wellings C. R. (2011). Global status of stripe rust: a review of historical and current threats. Euphytica 179 129–141. 10.1007/s10681-011-0360-y [DOI] [Google Scholar]
- Wickner R. B., Ghabrial S. A., Nibert M. L., Patterson J. L., Wang C. C. (2011). “Family Totiviridae,” in Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses, eds King A. M. Q., Adams M. J., Carstens E. B., Lefkowits E. J. (Tokyo: Elsevier Academic Press; ), 639–650. [Google Scholar]
- Xie J., Xiao X., Fu Y., Liu H., Cheng J., Ghabrial S. A., et al. (2011). A novel mycovirus closely related to hypoviruses that infects the plant pathogenic fungus Sclerotinia sclerotiorum. Virology 418 49–56. 10.1016/j.virol.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Yu X., Li B., Fu Y. P., Xie J. T., Cheng J. S., Ghabrial S. A., et al. (2013). Extracellular transmission of a DNA mycovirus and its use as a nature fungicide. Proc. Natl. Acad. Sci. U.S.A. 110 1452–1457. 10.1073/pnas.1213755110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y. G., Lv X. J., Sun X. H., Fu S. H., Gong Z. D., Fen Y., et al. (2008). Isolation and characterization of the full coding sequence of a novel densovirus from the mosquito Culex pipiens pallens. J. Gen. Virol. 89 195–199. 10.1099/vir.0.83221-0 [DOI] [PubMed] [Google Scholar]
- Zhang R., Qiu B., Tien P., Pryor A. (1994). Presence of double-stranded RNA in the virus-like particles of Uromyces vignae Barcl. Acta Mycol. Sin. 13 48–51. [Google Scholar]
- Zheng L., Liu H. Q., Zhang M. L., Cao X., Zhou E. X. (2013). The complete genomic sequence of a novel mycovirus from Rhizoctonia solani, AG-1 IA strain B275. Arch. Virol. 158 1609–1612. 10.1007/s00705-013-1637-3 [DOI] [PubMed] [Google Scholar]
- Zheng L., Zhang M. L., Chen Q. G., Zhu M. H., Zhou E. X. (2014). A novel mycovirus closely related to viruses in the genus Alphapartitivirus, confers hypovirulence in the phytopathogenic fungus Rhizoctonia solani. Virology 456 220–226. 10.1016/j.virol.2014.03.029 [DOI] [PubMed] [Google Scholar]
- Zheng L., Zhao J., Liang X. F., Zhan G. M., Jiang S. C., Kang Z. S. (2017). Identification of a novel Alternaria alternata strain able to hyperparasitize Puccinia striiformis f. sp. tritici, the causal agent of wheat stripe rust. Front. Microbiol. 8:71. 10.3389/fmicb.2017.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.