Abstract
New therapeutics to manage post-surgical pain are needed to mitigate the liabilities of opioid and other analgesics. Our previous work shows that key modulators of excitability in peripheral nociceptors, such as extracellular signal-regulated kinases (ERK) are inhibited by activation of adenosine monophosphate activated protein kinase (AMPK). We hypothesized that AMPK activation would attenuate acute incision-evoked mechanical hypersensitivity and the development of hyperalgesic priming caused by surgery in mice. Here we have used a variety of administration routes and combinations of AMPK activators to test this hypothesis. Topical administration of a resveratrol-based cream inhibited acute mechanical hypersensitivity evoked by incision and blocked the development of hyperalgesic priming. We also observed that systemic administration of metformin dose-dependently inhibited incision-evoked mechanical hypersensitivity and hyperalgesic priming. Interestingly, low doses of systemic metformin and local resveratrol that had no acute effect were able to mitigate development of hyperalgesic priming. Combined treatment with doses of systemic metformin and local resveratrol that were not effective on their own enhanced the acute efficacy of the individual AMPK activators for post-surgical mechanical pain alleviation and blocked the development of hyperalgesic priming. Finally, we used dorsal root ganglion (DRG) neurons in culture to show that resveratrol and metformin given in combination shift the concentration-response curve for AMPK activation to the left and increase the magnitude of AMPK activation. Therefore, we find that topical administration is an effective treatment route of administration and combining systemic and local treatments led to anti-nociceptive efficacy in acute mechanical hypersensitivity at doses that were not effective alone. Collectively our work demonstrates a specific effect of AMPK activators on post-surgical pain and points to novel therapeutic opportunities with potential immediate impact in the clinical setting.
Keywords: AMPK, metformin, resveratrol, plantar incision, & topical therapeutics
Graphical abstract
INTRODUCTION
One out of four chronic pain patients suffer from persistent pain because of prior surgery (Crombie et al., 1998) and up to 50% of patients who undergo common surgical procedures develop chronic pain (Kehlet et al., 2006, Johansen et al., 2012). Surgery remains a major cause of persistent pain despite the fact that analgesics for the treatment of acute post-surgical pain are widely available. This suggests that treatments targeting the molecular pathology to prevent or block the transition to chronic post-surgical pain are needed. New ways to interfere with acute post-surgical pain are also needed given the growing negative impact of opioids on society (Skolnick and Volkow, 2016, Volkow and Collins, 2017). While there have been advances in our understanding of signaling pathways and mediators that play an important role in driving post-surgical pain (Velnar et al., 2009, Ling et al., 2017), treatment approaches that target these pathways or mediators are currently not clinically utilized.
In animal models of post-surgical pain, there is an increase in interleukin 6 (IL-6) and nerve growth factor (NGF) levels in serum and skin around the incision site, which signal through the ERK and mechanistic target of rapamycin (mTOR) pathways inducing nociceptive sensitization (Matsuda et al., 1998, Sato and Ohshima, 2000, Banik et al., 2005, Bryan et al., 2005). We have shown that both of these pathways are negatively regulated by adenosine monophosphate (AMP)-activated protein kinase (AMPK) in nociceptors (Melemedjian et al., 2010, Tillu et al., 2012b, Mejia et al., 2016). AMPK is a ubiquitous energy sensing kinase, activated by an increase in intracellular AMP/ATP ratio during energy deprivation. The anti-diabetic drug metformin activates AMPK indirectly via inhibition of mitochondrial complex I (Owen et al., 2000, Shaw et al., 2005) or via inhibition of AMP deaminase (Ouyang et al., 2011). Both of these mechanisms lead to increased AMP levels in cells which allosterically activates AMPK (Hardie, 2015). Resveratrol activates AMPK through multiple mechanisms, but its mechanism of action in neurons appears to be distinct. In neuronal-like cell lines, and cortical and DRG neurons resveratrol does not alter AMP/ATP cellular ratios but activates AMPK via a mechanism that requires the upstream kinase liver kinase B1 (LKB1) (Dasgupta and Milbrandt, 2007), which phosphorylates the α subunit of AMPK to increase kinase activity (Shaw et al., 2005). We have demonstrated that the AMPK activators, metformin, A769662, and R419 inhibited translation regulation signaling pathways and nascent protein synthesis in DRG neurons resulting in a resolution of neuropathic allodynia induced by peripheral nerve injury, in the case of metformin and A769662 (Melemedjian et al., 2011), or incision evoked mechanical pain in the case of R419 (Mejia et al., 2016). Additionally, we demonstrated that resveratrol profoundly inhibited ERK and mTOR signaling in sensory neurons in a time- and dose-dependent fashion and local injection of resveratrol around the surgery site attenuated surgery-induced mechanical hypersensitivity in a model of post-surgical pain (Tillu et al., 2012b).
The aim of the present study was to further establish AMPK activation as a mechanism for the prevention of post-surgical persistent pain states and to introduce novel therapeutics and therapeutic strategies that employ this mechanism of action for use in humans. To do this, we utilized multiple AMPK activators and administration routes, including topical and local resveratrol, as well as systemic metformin. Our experimental endpoint was incision-induced mechanical hypersensitivity and hyperalgesic priming in mice where we observed efficacy for the attenuation of acute mechanical hypersensitivity and prevention of development of hyperalgesic priming.
EXPERIMENTAL PROCEDURES
Experimental animals
Male ICR mice (Envigo, 20–25 grams) were used for the study. All animal procedures were approved by the Institutional Animal Care and Use Committee of The University Texas at Dallas and University of Arizona and were in accordance with International Association for the Study of Pain guidelines. Mice were used in behavioral experiments starting one week after arrival at the animal facility at University of Texas at Dallas. Animals were housed with a 12 hour light/dark cycle and had food and water available ad libitum.
Behavior testing conditions
For behavioral testing, animals were placed in acrylic boxes with wire mesh floors and allowed to habituate for approximately 1 hour on all testing days. Paw withdrawal thresholds were measured using calibrated von Frey filaments (Stoelting) by stimulating the plantar aspect of left hind paw using the up-down method (Chaplan et al., 1994).
Plantar incision, behavioral testing, and treatments
Prior to surgery all animals were assessed for baseline paw withdrawal thresholds. A mouse model of plantar incisional pain was used for this study (Banik et al., 2006). A 5 mm longitudinal incision was made with a number 11 blade through skin, fascia, and muscle of the plantar aspect of the left hindpaw in anesthetized animals (1% isoflurane). The skin was closed with 2 sutures of 5 mm silk. The sutures were removed 48 hours later. Animals were allowed to recover for 24 hours and then paw withdrawal thresholds were measured at several time-points (1, 2, 3, 4, 5, 6, 10, 11 and 14 days) post-surgery. For hyperalgesic priming experiments, the animals received an intraplantar injection of prostaglandin E2 (PGE2) (100 ng/25 μl) 14 or more days following incision when they had completely returned to baseline mechanical thersholds (Asiedu et al., 2011). The paw withdrawal thresholds were again measured at 3 hours and 24 hours following the PGE2 injection. For topical cream preparation with resveratrol (Cayman chemical), lyophilized resveratrol was serially dissolved in polyethylene glycol 400 (PEG 400) and solid PEG ointment to achieve a final concentration of 2 mg/ml. For experiments with topical resveratrol, mice were maintained under anesthesia (1% isoflurane) for 1 hour to allow for absorption of the topically applied cream into the skin at times indicated immediately after the incision and 1-day post incision. For local injection treatments, animals received a 25 μL intraplantar injection of resveratrol (Cayman chemical) (1 or 3 μg) or vehicle immediately after surgery and 1-day post incision. Mice were not anesthetized for these injection and were only anesthetized briefly for incision, again with isoflurane. For systemic treatments, animals received an intraperitoneal (IP) injection of freshly prepared metformin (LKT laboratories, 30, 100, 150, or 200 mg/kg) for 4 days; 2 days before surgery, the day of surgery, and 1-day post surgery. The experimenters measuring mechanical withdrawal thresholds were always blinded to the experimental conditions. Mice were randomized to groups by a blinded experimenter and mice of individual groups were never housed together (e.g. home cages were always mixed between experimental groups).
Tissue Culture for Cellular Imaging
Dorsal root ganglia (DRG) were extracted aseptically from 5–12 4-week old male ICR mice per cell culture plate for each cellular imaging experiment and placed in Hank’s Buffered Salt Solution (HBSS, Invitrogen) on ice. The ganglia were dissociated enzymatically at 37° C; first with collagenase A (1 mg/ml, Roche) for 25 minutes, then collagenase D (1 mg/ml, Roche) that included papain (30 μg/ml, Roche) for 20 minutes. Afterwards, a trypsin inhibitor (1 mg/ml, Roche) that contained bovine serum albumin (BSA, Fisher, 1 mg/ml) was applied and the ganglia were mixed to allow for further dissociation with a polished Pasteur pipette. The tissue was then filtered through 70 μm nylon cell strainer (Falcon) and re-suspended in DMEM F-12 GlutaMax media (Invitrogen) that contained 10% fetal bovine serum (FBS, Hyclone) and 1x penicillin streptomycin (Pen-Strep). The media also contained NGF (10 ng/ml, Millipore) and 5-fluoro-2′-deoxyuridine + uridine (FRDU-U, 3.0ug/ml + 7.0ug/ml, Sigma) to reduce proliferation of glia and fibroblasts. Neurons were cultured for three-five days on 12 mm glass coverslips (#1 thickness, Chemglass) in a 24-well tissue culture plate (Falcon) coated with poly-D-Lysine (Sigma) at 37°C with 95% air and 5% CO2. The day before the experiment, FRDU-U was removed from the media and excluded. On the day of the experiment, drugs were diluted into DMEM F-12 plus GlutaMax media and added directly onto the neurons at a 10X concentration without any wash.
Immunocytochemistry (ICC) and Image Analysis
3 to 5 days days after establishment of DRG cultures, AMPK activator treatments were administered to assess acetyl-CoA carboxylase (ACC) phosphorylation. Cells were treated with drug concentrations applied from 10× stocks in DMEM F-12 plus GlutaMax, including FBS and Pen-Strep. Following treatment, cells were fixed in ice cold 10% formalin in phosphate buffered saline (PBS) for 1 hour. Cells were then washed with PBS and permeabilized in PBS containing 10% heat inactivated normal goat serum (NGS, Atlanta Biologicals,) and 0.02% Triton X-100 (Sigma) in PBS for 30 minutes and then blocked in 10% NGS in PBS for at least 1 hour. Following additional washes, primary antibody (p-ACC, Cell Signaling Technologies) was applied overnight at 4°C and the next day appropriate secondary antibodies (goat anti rabbit – alexa fluor 488, Invitrogen) were applied for 1 hour. After additional PBS washes, coverslips were mounted on slides with ProLong Gold anti-fade with DAPI (Invitrogen). Images were taken on an Olympus Fluoview FV1200 laser scanning confocal microscope and analyzed using the co-localization tool within Olympus’ FV software. The intensity of each channel was adjusted so that only areas that contained a strong signal of 488nm and 405nm were visible. This adjusted imaged contained distinct puncta that could then be counted and analyzed using Graphpad prism 7.01.
Tissue histology
To determine re-epithelialization, plantar skin was excised from the left hind paw of mice either 3 days or 7 days following plantar incision surgery. The skin was immediately cryoprotected and frozen in O.C.T compound (Fisher Scientific) and sectioned (20 μm) on cryostat. The sections were then fixed in formalin and immersed in 0.1% Hematoxylin Gill 2X (Fisher Scientific) for 3 minutes, washed in tap water, then immersed in 0.1% eosin for 3 minutes, and dehydrated through graded ethanol (Protocol from University of Michigan Center for Organogenesis). Finally sections were cover slipped with Permount (Fisher Scientific). The sections were imaged and the width of the gap between 2 epithelial edges was measured using an Olympus BX51 microscope.
Statistical Analysis
Data are shown as means and the standard error of the mean (± SEM) of 6 animals per group for behavioral studies. EC50’s ± SEM were calculated for in vitro DRG experiments. Graph plotting and statistical analysis used Graphpad Prism Version 7.01 (Graph Pad Software, Inc.). Statistical evaluation was performed by one- or two-way analysis of variance (ANOVA), followed by appropriate post-hoc tests. The a priori level of significance at 95% confidence level was considered at p < 0.05.
RESULTS
Topical Resveratrol inhibits acute mechanical hypersensitivity and hyperalgesic priming induced by plantar incision
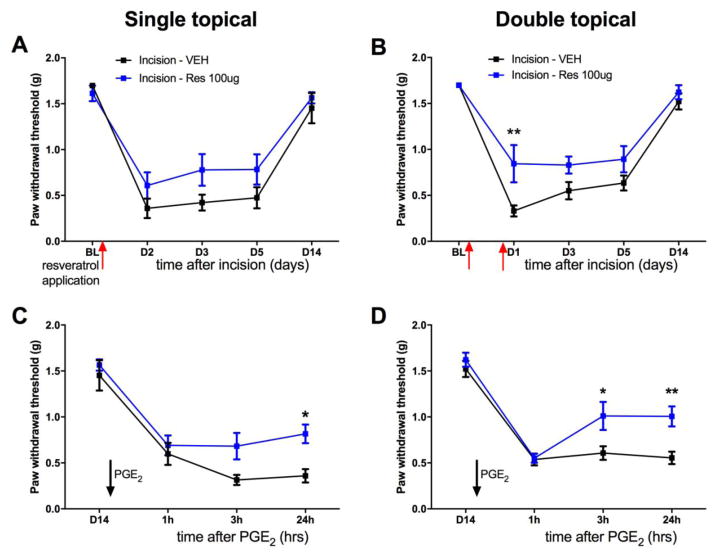
We have previously demonstrated that a 10 μg local injection of resveratrol into the hindpaw following plantar incision inhibits incision-mediated mechanical hypersensitivity as well as hyperalgesic priming induced by incision. Though local resveratrol injections efficaciously blocked incision-induced allodynia, clinical translatability necessitates a route of administration which is convenient and causes minimal discomfort. To address this issue, we generated a resveratrol cream preparation to be applied topically. Lyophilized resveratrol was serially dissolved in polyethylene glycol 400 (PEG 400) and solid PEG ointment to achieve a final concentration of 2 mg/ml. Utilizing a mouse model of incision pain, we assessed if topical application of resveratrol can prevent development of mechanical hypersensitivity following surgery (Brennan et al., 1996, Pogatzki and Raja, 2003). All animals received a plantar incision on the left hindpaw. Resveratrol (100 μg in 50 μL) or vehicle was applied on the paw of the incision once immediately following incision; or twice, immediately following incision and 1-day following incision. All mice were maintained under anesthesia for 1 hour during the application of the topical treatment. Mice with plantar incision that received vehicle displayed mechanical hypersensitivity lasting for at least 10 days. While a single application of topical resveratrol did not have a significant effect on acute mechanical hypersensitivity (Figure 1A), applying resveratrol twice significantly attenuated mechanical hypersensitivity induced by incision (Figure 1B). In this model, hyperalgesic priming can be revealed by an intraplantar injection of inflammatory mediator PGE2 (100 ng/25 μL) in the left hindpaw, after the resolution of initial allodynia (Asiedu et al., 2011) following plantar incision. Topical application of resveratrol once, or twice, inhibited the development of hyperalgesic priming precipitated by hindpaw injection of PGE2 following resolution of incision-induced mechanical hypersensitivity (Figure 1C and 1D). Thus, topically-applied resveratrol is capable of not only blocking acute mechanical hypersensivity induced by plantar incision but it also attenuates the development of hyperalgesic priming. These results suggest that topical application of resveratrol can be an efficacious treatment for post-surgical mechanical hypersensitivity.
Figure 1. Topical Resveratrol inhibits acute mechanical hypersensitivity and hyperalgesic priming induced by plantar incision.
All animals received a plantar incision on the left hindpaw. Resveratrol-based cream (100 μg/50 μL) or vehicle cream was applied directly on the incision site immediately following incision surgery (A) or twice (B), immediately following incision then 1-day post surgery and animals were assessed for mechanical sensitivity by von Frey hair stimulation at the indicated time points. A) A single application of topical resveratrol (100 μg/50 μL) immediately following incision did not change incision-induced acute hypersensitivity C) but inhibited plantar incision-induced hyperalgesic priming precipitated by PGE2 injection on day 14 after incision surgery. B) Two applications of topical resveratrol (100 μg) immediately following incision and 1-day post incision significantly inhibited plantar incision-induced acute hypersensitivity D) and plantar incision-induced hyperalgesic priming precipitated by PGE2 injection on day 14 after incision. Behavioral data was analyzed by two-way ANOVA with Bonferroni post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001 (n = 6 per group).
Low doses of resveratrol inhibit hyperalgesic priming in a model of post-surgical pain
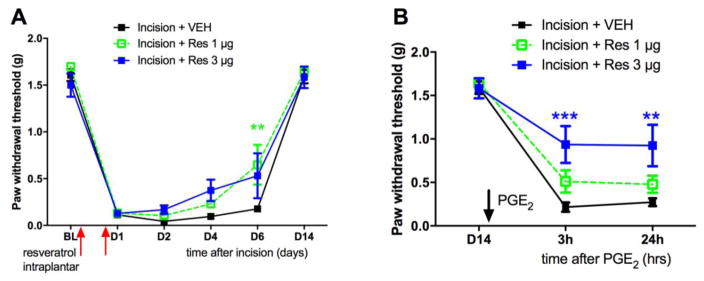
A single application of topical resveratrol does not influence acute mechanical hypersensitivity to incision but blocks hyperalgesic priming. This suggests that AMPK activation at the time of injury is capable of inhibiting the development of persistent nociceptor plasticity even if it is not effective for pain in the acute phase. To test this in more detail we used local injections of resveratrol so we could more precisely control the dose of compound. We gave two injections, spaced by 24 hrs, of 1 or 3 ug of resveratrol around the surgery site. While both doses had no effect on acute mechanical hypersensitivity until at least 6 days after incision, we observed significant inhibition of hyperalgesic priming with the 3 μg dose of intraplantar resveratrol (Figure 2A and 2B).
Figure 2. Low dose resveratrol inhibits hyperalgesic priming induced by plantar incision.
All animals received a plantar incision on the left hindpaw. Animals received 2 intraplantar injections of resveratrol (1 or 3 μg) or vehicle immediately following incision and 1-day post surgery and animals were assessed for mechanical sensitivity by von Frey hair stimulation at the indicated time points. A) Low dose resveratrol (1 or 3 μg) injection had little effect on incision-induced acute hypersensitivity (B) while just the 3 μg intraplantar injection of resveratrol immediately following incision surgery significantly attenuated development of plantar incision-induced hyperalgesic priming precipitated by PGE2 injection on day 14 after incision. Behavioral data was analyzed by two-way ANOVA with Bonferroni post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001 (n = 6, vehicle and 1 μg resveratrol; n = 5, 3 μg resveratrol).
Metformin inhibits acute mechanical hypersensitivity and hyperalgesic priming induced by plantar incision
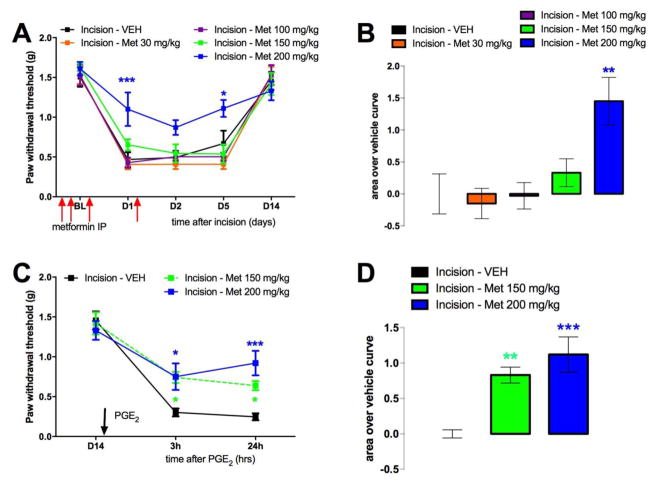
Another therapeutic opportunity for activating AMPK is metformin. Metformin is already clinically available, safe, inexpensive and a well-tolerated drug. Moreover, metformin has a different mechanism of action in activating AMPK than resveratrol and may have differential efficacy in modulation of downstream targets. Metformin is known to activate AMPK through multiple indirect mechanisms including inhibition of mitochondrial complex I, LKB1 stimulation (Shaw et al., 2005) and inhibition of AMP deaminase (Ouyang et al., 2011). Additionally, in contrast to resveratrol, metformin has an improved bioavailability and thus can be given systemically, which is the preferred route of administration in humans. Hence, we investigated if systemic application of metformin can prevent development of incision-induced mechanical hypersensitivity and hyperalgesic priming following incision. Animals received an incision on the left hindpaw. Metformin (30, 100, 150 or 200 mg/kg) or vehicle was injected IP for 4 days; starting 2 days prior to the surgery, the day of, and 1-day post surgery. Mice with incision that received vehicle displayed long-lasting acute mechanical hypersensitivity as well as hyperalgesic priming following PGE2 lasting at least 24 hours. In contrast, IP injection of a 200 mg/kg dose of metformin prevented both acute incision-induced mechanical hypersensitivity (Figure 3A and B) and the development of hyperalgesic priming following PGE2 injection on day 14 (Figure 3C and D). Interestingly, the 150 mg/kg dose was not effective in blocking acute incision-induced mechanical hypersensitivity (Figure 3A and B); but did significantly block hyperalgesic priming (Figure 3C and D) similar to observations with resveratrol local dosing where doses that had little effect on acute mechanical hypersensitivity were effective in blocking hyperalgesic priming.
Figure 3. Metformin inhibits acute mechanical hypersensitivity and hyperalgesic priming induced by plantar incision.
All animals received a plantar incision on the left hindpaw. Metformin (30, 100, 150 or 200 mg/kg) or vehicle was injected IP for 4 consecutive days, starting 2 days prior to surgery, the day of surgery, and 1 day post-incision surgery and animals assessed for mechanical sensitivity by von Frey hair stimulation at the indicated time points. A) Metformin (30, 100, 150 or 200 mg/kg) injection dose-dependently inhibited plantar incision-induced acute mechanical hypersensitivity (area over vehicle curve shown in B) and (C) hyperalgesic priming precipitated by PGE2 (100 ng) in the left hindpaw on day 14 after incision (area over vehicle curve shown in D). Behavioral data was analyzed by two-way ANOVA with Bonferroni post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001 (n = 6 per group except metformin 150 mg/kg, n = 8).
Co-treatment with low doses of systemic metformin and local resveratrol inhibits acute mechanical hypersensitivity and hyperalgesic priming in a model of post-surgical pain
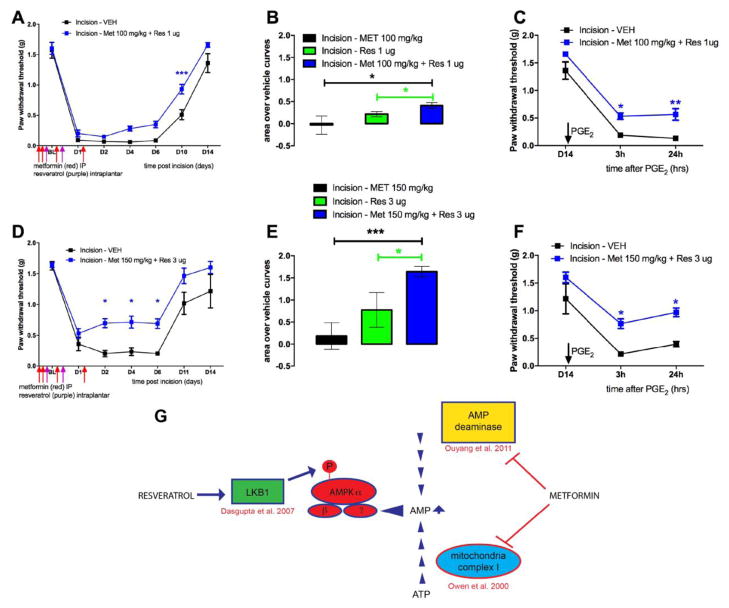
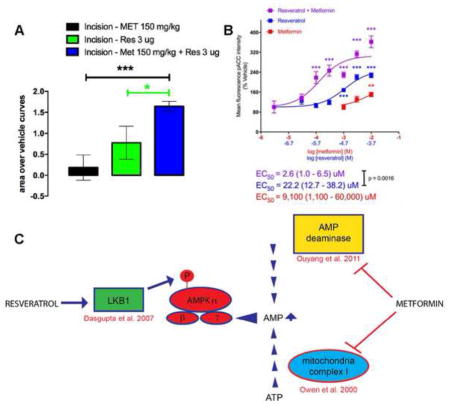
Although, various AMPK activators can independently attenuate the development of the incision-induced hypersensitivity and hyperalgesic priming at higher doses, an effective treatment strategy could be to utilize sub-efficacious doses of AMPK activators and investigate if the co-treatment is equally efficacious in attenuating acute hypersensitivity and blocking the development of hyperalgesic priming following plantar incision. This strategy could minimize unwanted side-effects of metformin which could occur at higher doses. The primary side effect of metformin is gastrointestinal disturbance and diarrhea but lactic acidosis can occur in rare instances. This strategy might also allow for lowering doses into ranges that are more commonly given to humans. For instance, metformin doses in humans are typically 1/10th of what is given to mice (e.g. 25 mg/kg) (Gong et al., 2012). Hence, we investigated if co-treatment of sub-efficacious doses of metformin and resveratrol (100 mg/kg and 1 μg or 150 mg/kg and 3 μg) could have additive effects on attenuating incision-induced mechanical hypersensitivity and development of hyperalgesic priming. Animals received a plantar incision on the left hindpaw and received treatment of vehicle, resveratrol or metformin alone or a co-treatment of metformin and resveratrol. Metformin (100 or 150 mg/kg) was injected IP for 4 days; starting 2 days prior to the incision and resveratrol (1 or 3 μg) was injected in the left hindpaw around the incision on the day of the incision and 24 hrs later. Mice with incision that received vehicle again displayed long-lasting acute mechanical hypersensitivity as well as hyperalgesic priming following PGE2. Mice which received co-treatments of individually sub-efficacious doses of metformin and resveratrol (100 mg/kg and 1μg) displayed a significant improvement in recovery on day 10 (Figure 4A) and the cumulative effect of the combined doses was significantly greater than the individual doses alone compared to their respective vehicle treatment experiments (Figure 4B). Moreover, this combined dose effectively inhibited hyperalgesic priming compared to vehicle treated mice (Figure 4C). Metformin and resveratrol at 150 mg/kg and 3 μg, respectively, significantly reduced acute mechanical hypersensitivity starting at day 2 (Figure 4D) and produced a large enhancement of effect versus single treatment with either compound (Fig 4E). This combined treatment inhibited hyperalgesic priming (Figure 4F), displaying a strong additive effect compared to giving the compounds alone. A summary of the mechanism of action of metformin and resveratrol to activate AMPK is shown in Figure 4G (Owen et al., 2000, Dasgupta and Milbrandt, 2007, Ouyang et al., 2011). Importantly, these compounds are thought to have distinct mechanisms of action in activating AMPK in neurons.
Figure 4. Co-treatment with low doses of systemic metformin and local resveratrol inhibit acute mechanical hypersensitivity and hyperalgesic priming induced by plantar incision.
All animals received a plantar incision on the left hind paw. Metformin (100 or 150 mg/kg) was injected systemically for 4 consecutive days, starting 2 days prior to surgery, the day of surgery, and 1-day post surgery (red arrows) and resveratrol (1 or 3 μg) was injected in the left hind paw on the day of the surgery and 1-day post-surgery (purple arrows) and animals assessed for mechanical sensitivity by von Frey hair stimulation at the indicated time points. A) Co-treatment with systemic metformin (100 mg/kg) and local resveratrol (1 μg) significantly improved recovery from plantar incision-induced acute hypersensitivity. B) Cumulative treatment effect size of individual doses versus vehicle or combined doses versus vehicle is shown as area over the vehicle curve. C) Co-treatment with systemic metformin (150 mg/kg) and local resveratrol (3μg) significantly inhibited plantar incision-induced acute hypersensitivity. D) Co-treatment with systemic metformin (100 mg/kg) and local resveratrol (1 μg) significantly interfered with development of plantar incision-induced hyperalgesic priming precipitated by PGE2 injection on day 14 after incision. D) Cumulative treatment effect size of individual doses versus vehicle or combined doses versus vehicle is shown as area over the vehicle curve. F) Co-treatment with systemic metformin (150 mg/kg) and local resveratrol (3 μg) significantly inhibited plantar incision-induced hyperalgesic priming precipitated by PGE2 injection on day 14 after incision. G) Summary diagram of mechanisms underlying metformin and resveratrol activation of AMPK. Behavioral data was analyzed by two-way ANOVA with Bonferroni post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001 (n = 6 per group).
Co-treatment of isolated DRG neurons with metformin and resveratrol additively induces AMPK signaling
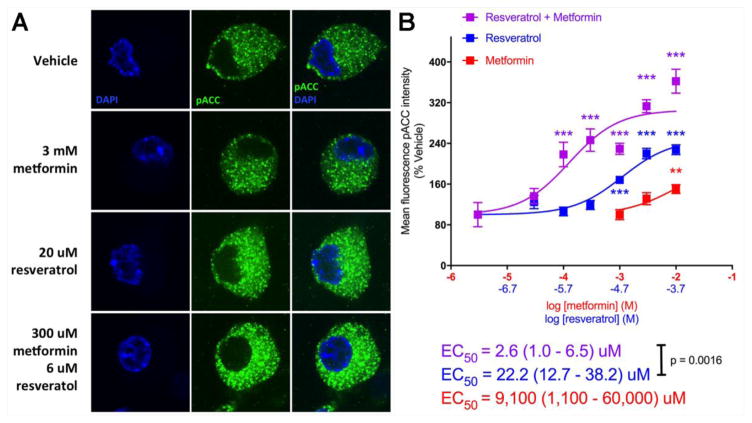
Our in vivo findings suggest a leftward shift in the dose-response for AMPK activation in producing alleviation of mechanical hypersensitivity but due to the different dosing routes for the compounds (resveratrol has very poor systemic bioavailability) this is hard to determine empirically. Therefore, we sought to examine AMPK activation via resveratrol and metformin individually and together on DRG neurons in culture. We assessed acetyl-CoA carboxylase (ACC) phosphorylation on serine 79 using a phospho-specific antibody characterized for immunocytochemistry (ICC). We observed a significant increase in p-ACC intensity with metformin at 10 mM and resveratrol starting at 20 μM when the compounds were incubated with DRG neurons for 1 hr (Figure 5A and 5B). When we combined metformin and resveratrol, we observed a significant leftward shift in the concentration response for the resveratrol + metformin group as identified by shifts in the EC50 as shown in Figure 6. From these findings we conclude that metformin and resveratrol have at least additive effects on AMPK activation in DRG neurons which likely explain their ability to enhance each other’s effects on mechanical hypersensitivity in vivo.
Figure 5. Co-treatment of isolated DRG neurons with metformin and resveratrol exhibit additive effects on AMPK signaling.
DRG neurons in culture taken from Male ICR mice were treated with vehicle, metformin, or resveratrol alone or metformin + resveratrol for 1 hour at indicated concentrations. A) Phosphorylation of ACC (p-ACC) was assessed by confocal microscopy (green) and neuronal cell nuclei were stained with DAPI (blue). B) Images were processed and the mean fluorescence intensity of pACC was measured and graphed. One-way ANOVA was used to assess differences between the vehicle and treatment groups with Fisher’s LSD post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001 (N = 5–12 coverslips per group)
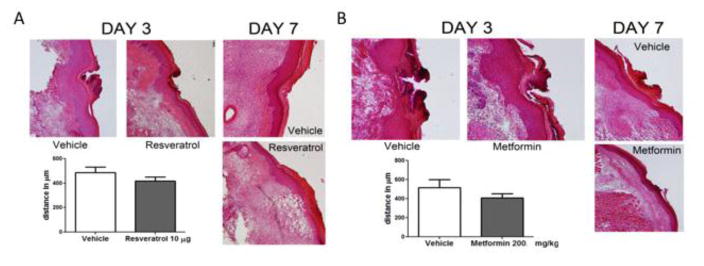
Figure 6. AMPK activators do not effect wound healing in response to incision.
All Animals received a plantar incision on the left hindpaw. Skin from the left hindpaw was excised 3 and 7 days after plantar incision and wound healing and closure was assessed using Hematoxylin & Eosin (H & E) staining. A) Resveratrol (10 μg) or vehicle was injected in the left hind paw on the day of the surgery and 1-day post surgery. There was no difference in wound size between the resveratrol and vehicle treated groups on Day 3. The wound was completely closed by Day 7 in both vehicle and resveratrol groups. B) Metformin (200 mg/kg) or vehicle was injected systemically for 4 consecutive days, starting 2 days prior to surgery, the day of surgery, and 1-day post surgery. No differences were noted in the wound size between the metformin and vehicle treated groups on Day 3. The wound was completely closed by Day 7 in animals from groups receiving vehicle or metformin. Differences between groups were assessed by two tailed student’s t-test. (N = 6 per group).
AMPK activators have no adverse effect on wound healing after incision
An important consideration in these experiments is that AMPK activators reduce protein translation and may negatively influence wound healing. This effect would have a negative impact on their clinical utility for post-surgical pain (Velnar et al., 2009). To test if AMPK activators effect wound healing, we utilized either vehicle or maximal local doses of resveratrol (two local injections of 10 μg) or metformin (200 mg/kg, 4 IP doses) described in the previous experiments and assessed wound healing with Hematoxylin & Eosin (H & E) staining (Lai et al., 2009). Skin samples were excised 3 and 7 days after incision to assess healing and wound closure, respectively. For the animals treated with resveratrol, no differences were noted in the wound size between the resveratrol and vehicle treated groups on Day 3 (Figure 6A). By Day 7, the wound was completely closed in the animals in both groups receiving local vehicle or resveratrol (Figure 6A). Similar results were obtained with systemic metformin (Figure 6B). We find no evidence that AMPK activators negatively influence the wound healing processes following incision.
DISCUSSION
The experiments described above are relevant for the possible development of AMPK activators to attenuate mechanical pain amplification after surgery and block the development of neuronal plasticity that increases the sensitivity of nociceptors to sub-threshold levels of inflammatory mediators. We show that both the application of topical resveratrol and systemic metformin inhibit surgery-induced mechanical hypersensitivity and hyperalgesic priming produced by plantar incision. The findings also show that at the lower end of the dosing range, local resveratrol and systemic metformin do not inhibit acute surgery-induced mechanical hypersensitivity, but significantly attenuate hyperalgesic priming. This suggests that even at small doses these compounds may be effective in reducing the transition to a chronic pain state after surgery. Interestingly, co-treatment with sub-efficacious doses of local resveratrol and systemic metformin had a significant effect on acute mechanical hypersensitivity and inhibited hyperalgesic priming. Our in vitro experiments on DRG neurons indicate the combination of resveratrol and metformin activate AMPK in at least an additive fashion. These data add to a growing body of evidence that AMPK activation can inhibit the development of acute mechanical hypersensitivity resulting from injury (Melemedjian et al., 2011, Tillu et al., 2012a, Russe et al., 2013, Bullon et al., 2015, Ma et al., 2015, Song et al., 2015, Maixner et al., 2016, Ling et al., 2017), reduce the excitability of nociceptors (Melemedjian et al., 2011, Tillu et al., 2012a, Melemedjian et al., 2013, Asiedu et al., 2017) and prevent the development of hyperalgesic priming (Price and Dussor, 2013, Price et al., 2015, Asiedu et al., 2016, Mejia et al., 2016). We conclude that topical resveratrol and co-treatment of various AMPK activators such as metformin are clinically relevant and could show utility as pain therapeutics in humans.
An interesting finding emerging from our work is that the level of drug needed to inhibit acute mechanical hypersensitivity is greater than that needed to attenuate the development of hyperalgesic priming. While we did not investigate the mechanisms through which this occurs, we speculate that it may be due to even a relatively small amount of AMPK activation in DRG nociceptors being sufficient to block changes in gene expression that are required to cause nociceptors to transition to a primed state. However, when these same low doses of resveratrol and metformin where combined together, co-treatment effectively inhibited acute surgery-induced mechanical hypersensitivity and prevented hyperalgesic priming. Co-treatment with these low doses was likely efficacious because the treatment strategy led to enhanced AMPK activation. This is supported by our in vitro experiments where we saw a strong shift to the left in the concentration response curve, as well as an increase in the magnitude of AMPK activation with combined metformin and resveratrol treatment.
We conducted experiments with metformin and resveratrol because these drugs can be used in humans immediately. Metformin is used for type 2 diabetes and is the most widely prescribed drug in the world. A previous study suggested that metformin may be effective for low back pain (Taylor et al., 2013) but a subsequent retrospective study did not find a positive effect for metformin in pain patients (Smith and Ang, 2015). The first prospective study of metformin for pain was recently conducted in women with polycystic ovary syndrome (Kialka et al., 2016). This study found a positive effect of metformin on pressure pain thresholds. A possible confound of translating preclinical work on metformin for pain, which includes a growing number of studies, is that the dose that is used is much higher than typical dosing in humans. While direct comparisons are not possible without extensive drug metabolism work, our experiments suggest strategies for obtaining effects of metformin at lower doses (e.g. by combining with local resveratrol) (Labuzek et al., 2013, Bullon et al., 2016).
While metformin is very hydrophilic and has excellent systemic bioavailability, resveratrol is highly lipophilic and lacks good systemic bioavailability. This makes resveratrol an ideal candidate for local or even topical application. Indeed, we found that topical resveratrol, in a very simple cream formulation, is effective in reducing incision-evoked mechanical hypersensitivity and that local resveratrol, when combined with systemic metformin at low doses, produces a reduction in acute post-surgical mechanical hypersensitivity and prevents the development of hyperalgesic priming. Because we found no adverse effects on wound healing of any of these drugs, this suggests a viable strategy for the translation of these findings into human patients. In this regard it is notable that resveratrol is generally regarded as safe by the United States Food and Drug Administration. It is notable that resveratrol and metformin seem to activate AMPK via different mechanisms. In neurons, resveratrol does not alter AMP/ATP ratios but activates the upstream kinase LKB1 to increase AMPK phosphorylation (Dasgupta and Milbrandt, 2007). Metformin increases cellular AMP levels via two mechanisms, inhibition of mitochondrial complex I (Owen et al., 2000) and inhibition of AMP deaminase (Ouyang et al., 2011). The consequences of these two drugs would be activation of AMPK by allosteric site binding with AMP and increased kinase activity by phosphorylation of the α subunit of AMPK. This strategy has been used with other drug combinations to synergistically activate AMPK (Ducommun et al., 2014). Our behavioral experiments show an indication of additive effects of combining resveratrol and metformin and our in vitro experiments show a clear, strong leftward shift in the concentration-response curve for the combined exposure. We were not able to formally assess synergism in either experimental paradigm due to different dosing routes for in vivo experiments and the very small efficacious concentration range of metformin in vitro.
Our work suggests that topical application of resveratrol at sites of surgery can reduce post-surgical mechanical hypersensitivity and prevent the development of hyperalgesic priming (Reichling and Levine, 2009, Kandasamy and Price, 2015, Price and Inyang, 2015). As persistent pain after surgery remains a major clinical problem (Kehlet et al., 2006), our work indicates that resveratrol may be used advantageously via topical application in this setting. We conclude that 1) topical treatment of resveratrol presents a low-risk readily usable therapeutic avenue, 2) sufficient dosing of systemic metformin attenuates both acute surgery-induced mechanical hypersensitivity and hyperalgesic priming and 3) co-treatment of resveratrol and metformin has an additive effect which activates AMPK in sensory neurons in vitro (4) leading to inhibition of surgery-induced mechanical hypersensitivity and the development of hyperalgesic priming in vivo. This creates multiple opportunities to further develop activators of AMPK as potential therapeutics for post-surgical pain.
A novel resveratrol-based topical cream alleviates acute postoperative pain and hyperalgesic priming.
Combination treatments of resveratrol and metformin have additive effects on acute sensitivity and hyperalgesic priming.
AMPK activators do not negatively influence wound healing.
Acknowledgments
This work was supported by National Institutes of Health grants [R01GM102575 (TJP and GD), R01NS065926 (TJP)] and The University of Texas STARS program research support grant (TJP and GD).
TJP and GD are co-founders of CERSCI Therapeutics and Ted’s Brain Science, biotechnology companies developing AMPK activators for the treatment of pain. The authors declare no other conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- Asiedu MN, Dussor G, Price TJ. Targeting AMPK for the Alleviation of Pathological Pain. EXS. 2016;107:257–285. doi: 10.1007/978-3-319-43589-3_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiedu MN, Han C, Dib-Hajj SD, Waxman SG, Price TJ, Dussor G. The AMPK Activator A769662 Blocks Voltage-Gated Sodium Channels: Discovery of a Novel Pharmacophore with Potential Utility for Analgesic Development. PLoS One. 2017;12:e0169882. doi: 10.1371/journal.pone.0169882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiedu MN, Tillu DV, Melemedjian OK, Shy A, Sanoja R, Bodell B, Ghosh S, Porreca F, Price TJ. Spinal protein kinase M zeta underlies the maintenance mechanism of persistent nociceptive sensitization. J Neurosci. 2011;31:6646–6653. doi: 10.1523/JNEUROSCI.6286-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik RK, Subieta AR, Wu C, Brennan TJ. Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. Pain. 2005;117:68–76. doi: 10.1016/j.pain.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Banik RK, Woo YC, Park SS, Brennan TJ. Strain and sex influence on pain sensitivity after plantar incision in the mouse. Anesthesiology. 2006;105:1246–1253. doi: 10.1097/00000542-200612000-00025. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- Bryan D, Walker KB, Ferguson M, Thorpe R. Cytokine gene expression in a murine wound healing model. Cytokine. 2005;31:429–438. doi: 10.1016/j.cyto.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Bullon P, Alcocer-Gomez E, Carrion AM, Garrido-Maraver J, Marin-Aguilar F, Roman-Malo L, Ruiz-Cabello J, Culic O, Ryffel B, Apetoh L, Ghiringhelli F, Battino M, Sanchez-Alcazar JA, Cordero MD. AMPK phosphorylation modulates pain by activation of NLRP3-inflammasome. Antioxidants & redox signaling. 2015 doi: 10.1089/ars.2014.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullon P, Alcocer-Gomez E, Carrion AM, Marin-Aguilar F, Garrido-Maraver J, Roman-Malo L, Ruiz-Cabello J, Culic O, Ryffel B, Apetoh L, Ghiringhelli F, Battino M, Sanchez-Alcazar JA, Cordero MD. AMPK Phosphorylation Modulates Pain by Activation of NLRP3 Inflammasome. Antioxid Redox Signal. 2016;24:157–170. doi: 10.1089/ars.2014.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Crombie IK, Davies HT, Macrae WA. Cut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinic. Pain. 1998;76:167–171. [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducommun S, Ford RJ, Bultot L, Deak M, Bertrand L, Kemp BE, Steinberg GR, Sakamoto K. Enhanced activation of cellular AMPK by dual-small molecule treatment: AICAR and A769662. Am J Physiol Endocrinol Metab. 2014;306:E688–696. doi: 10.1152/ajpendo.00672.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22:820–827. doi: 10.1097/FPC.0b013e3283559b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2015;33:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Johansen A, Romundstad L, Nielsen CS, Schirmer H, Stubhaug A. Persistent postsurgical pain in a general population: Prevalence and predictors in the Tromso study. Pain. 2012;153:1390–1396. doi: 10.1016/j.pain.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Kandasamy R, Price TJ. The pharmacology of nociceptor priming. Handb Exp Pharmacol. 2015;227:15–37. doi: 10.1007/978-3-662-46450-2_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- Kialka M, Milewicz T, Sztefko K, Rogatko I, Majewska R. Metformin increases pressure pain threshold in lean women with polycystic ovary syndrome. Drug Des Devel Ther. 2016;10:2483–2490. doi: 10.2147/DDDT.S109086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuzek K, Liber S, Suchy D, Okopien B. A successful case of pain management using metformin in a patient with adiposis dolorosa. Int J Clin Pharmacol Ther. 2013;51:517–524. doi: 10.5414/CP201878. [DOI] [PubMed] [Google Scholar]
- Lai JJ, Lai KP, Chuang KH, Chang P, Yu IC, Lin WJ, Chang C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. The Journal of Clinical Investigation. 2009;119:3739–3751. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling YZ, Li ZY, Ou-Yang HD, Ma C, Wu SL, Wei JY, Ding HH, Zhang XL, Liu M, Liu CC, Huang ZZ, Xin WJ. The inhibition of spinal synaptic plasticity mediated by activation of AMP-activated protein kinase signaling alleviates the acute pain induced by oxaliplatin. Exp Neurol. 2017;288:85–93. doi: 10.1016/j.expneurol.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Ma J, Yu H, Liu J, Chen Y, Wang Q, Xiang L. Metformin attenuates hyperalgesia and allodynia in rats with painful diabetic neuropathy induced by Streptozotocin. Eur J Pharmacol. 2015 doi: 10.1016/j.ejphar.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Maixner DW, Yan X, Hooks SB, Weng HR. AMPKalpha1 knockout enhances nociceptive behaviors and spinal glutamatergic synaptic activities via production of reactive oxygen species in the spinal dorsal horn. Neuroscience. 2016;326:158–169. doi: 10.1016/j.neuroscience.2016.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Koyama H, Sato H, Sawada J, Itakura A, Tanaka A, Matsumoto M, Konno K, Ushio H, Matsuda K. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. The Journal of experimental medicine. 1998;187:297–306. doi: 10.1084/jem.187.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia GL, Asiedu MN, Hitoshi Y, Dussor G, Price TJ. The potent, indirect adenosine monophosphate- activated protein kinase activator R419 attenuates mitogen-activated protein kinase signaling, inhibits nociceptor excitability, and reduces pain hypersensitivity in mice. Pain Rep. 2016:1. doi: 10.1097/PR9.0000000000000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian OK, Asiedu MN, Tillu DV, Peebles KA, Yan J, Ertz N, Dussor GO, Price TJ. IL-6- and NGF-Induced Rapid Control of Protein Synthesis and Nociceptive Plasticity via Convergent Signaling to the eIF4F Complex. J Neurosci. 2010;30:15113–15123. doi: 10.1523/JNEUROSCI.3947-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian OK, Asiedu MN, Tillu DV, Sanoja R, Yan J, Lark A, Khoutorsky A, Johnson J, Peebles KA, Lepow T, Sonenberg N, Dussor G, Price TJ. Targeting adenosine monophosphate-activated protein kinase (AMPK) in preclinical models reveals a potential mechanism for the treatment of neuropathic pain. Mol Pain. 2011;7:70. doi: 10.1186/1744-8069-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian OK, Khoutorsky A, Sorge RE, Yan J, Asiedu MN, Valdez A, Ghosh S, Dussor G, Mogil JS, Sonenberg N, Price TJ. mTORC1 inhibition induces pain via IRS-1-dependent feedback activation of ERK. Pain. 2013 doi: 10.1016/j.pain.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J, Parakhia RA, Ochs RS. Metformin activates AMP kinase through inhibition of AMP deaminase. J Biol Chem. 2011;286:1–11. doi: 10.1074/jbc.M110.121806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- Pogatzki EM, Raja SN. A mouse model of incisional pain. Anesthesiology. 2003;99:1023–1027. doi: 10.1097/00000542-200310000-00041. [DOI] [PubMed] [Google Scholar]
- Price TJ, Das V, Dussor G. Adenosine monophosphate-activated protein kinase (AMPK) activators for the prevention, treatment and potential reversal of pathological pain. Curr Drug Targets. 2015 doi: 10.2174/1389450116666151102095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Dussor G. AMPK: An emerging target for modification of injury-induced pain plasticity. Neurosci Lett. 2013;557(Pt A):9–18. doi: 10.1016/j.neulet.2013.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Inyang KE. Commonalities between pain and memory mechanisms and their meaning for understanding chronic pain. Prog Mol Biol Transl Sci. 2015;131:409–434. doi: 10.1016/bs.pmbts.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russe OQ, Moser CV, Kynast KL, King TS, Stephan H, Geisslinger G, Niederberger E. Activation of the AMP-Activated Protein Kinase Reduces Inflammatory Nociception. J Pain. 2013;14:1330–1340. doi: 10.1016/j.jpain.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Sato Y, Ohshima T. The expression of mRNA of proinflammatory cytokines during skin wound healing in mice: a preliminary study for forensic wound age estimation (II) International journal of legal medicine. 2000;113:140–145. doi: 10.1007/s004140050285. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, Volkow ND. Re-energizing the Development of Pain Therapeutics in Light of the Opioid Epidemic. Neuron. 2016;92:294–297. doi: 10.1016/j.neuron.2016.09.051. [DOI] [PubMed] [Google Scholar]
- Smith B, Ang D. Metformin: Potential analgesic? Pain Med. 2015;16:2256–2260. doi: 10.1111/pme.12816. [DOI] [PubMed] [Google Scholar]
- Song H, Han Y, Pan C, Deng X, Dai W, Hu L, Jiang C, Yang Y, Cheng Z, Li F, Zhang G, Wu X, Liu W. Activation of Adenosine Monophosphate-activated Protein Kinase Suppresses Neuroinflammation and Ameliorates Bone Cancer Pain: Involvement of Inhibition on Mitogen-activated Protein Kinase. Anesthesiology. 2015;123:1170–1185. doi: 10.1097/ALN.0000000000000856. [DOI] [PubMed] [Google Scholar]
- Taylor A, Westveld AH, Szkudlinska M, Guruguri P, Annabi E, Patwardhan A, Price TJ, Yassine HN. The use of metformin is associated with decreased lumbar radiculopathy pain. Journal of pain research. 2013;6:755–763. doi: 10.2147/JPR.S52205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillu DV, Melemedjian OK, Asiedu MN, Qu N, De Felice M, Dussor G, Price TJ. Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain. Molecular Pain. 2012a;8:5. doi: 10.1186/1744-8069-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillu DV, Melemedjian OK, Asiedu MN, Qu N, De Felice M, Dussor G, Price TJ. Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain. Mol Pain. 2012b;8:5. doi: 10.1186/1744-8069-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. The New England journal of medicine. 2017 doi: 10.1056/NEJMsr1706626. [DOI] [PubMed] [Google Scholar]