Abstract
During mitosis, the catalytic activity of protein-tyrosine phosphatase (PTP) α is enhanced, and its inhibitory binding to Grb2, which specifically blocks Src dephosphorylation, is decreased. These effects act synergistically to activate Src in mitosis. We show here that these effects are abrogated by mutation of Ser180 and/or Ser204, the sites of protein kinase C-mediated phosphorylation within PTPα. Moreover, either a Ser-to-Ala substitution or serine dephosphorylation specifically eliminated the ability of PTPα to dephosphorylate and activate Src even during interphase. This explains why the substitutions eliminated PTPα transforming activity, even though PTPα interphase dephosphorylation of nonspecific substrates was only slightly decreased. This occurred without change in the phosphorylation of PTPα at Tyr789, which is required for “phosphotyrosine displacement” during Src dephosphorylation. Thus, in addition to increasing PTPα nonspecific catalytic activity, Ser180 and Ser204 phosphorylation (along with Tyr789 phosphorylation) regulates PTPα substrate specificity. This involves serine phosphorylation-dependent differential modulation of the affinity of Tyr(P)789 for the Src and Grb2 SH2 domains. The results suggest that protein kinase C may participate in the mitotic activation of PTPα and Src and that there are intramolecular interactions between the PTPα C-terminal and membraneproximal regions that are regulated, at least in part, by serine phosphorylation.
Protein-tyrosine phosphatase (PTP)1 α is an ~130-kDa transmembrane PTP (1, 2) that activates the cytoplasmic membrane-bound Src protein-tyrosine kinase by dephosphorylating Src Tyr(P)527 (Refs. 3 and 4; see Ref. 5 for review). This releases Src from its negatively regulated conformation in which Tyr(P)527 is bound intramolecularly to the Src SH2 domain (see Refs. 6 and 7 for review). Overexpression of PTPα results in dephosphorylation of Tyr(P)527 and activation of Src in vivo (3, 4). Conversely, Src Tyr(P)527 phosphorylation is higher and Src catalytic activity is about three times lower in cells from PTPα−/− knockout mice (8, 9) or following antisense-induced PTPα down-regulation (10), indicating that PTPα is a major physiological positive regulator of Src.
Constitutive activation of the Src proto-oncoprotein by mutation increases the tyrosine phosphorylation of multiple signal transduction proteins and thereby neoplastically transforms a variety of cell types (see Ref. 7 for review). The fact that activation by overexpressed PTPα also causes transformation (3) is perhaps more surprising and suggests that PTPα activity is directed in vivo preferentially to Src (and Src family members), rather than to Src substrates.
This substrate specificity is due, at least in part, to a phosphotyrosine displacement mechanism that selectively promotes dephosphorylation of Src by PTPα: ~20% of PTPα in NIH3T3 cells is phosphorylated at Tyr789, a residue near its carboxyl terminus (11, 12). Tyr789 phosphorylation does not affect PTPα dephosphorylation of nonspecific substrates such as myelin basic protein (MBP), whose phosphotyrosines are not bound, but is required for dephosphorylation of Src Tyr(P)527, which is protected against many phosphatases by its SH2 domain binding (13). Phosphorylated Tyr789 can bind to the Src SH2 domain, thereby displacing and thus unprotecting Src Tyr(P)527. This also forms a transient bound state that additionally facilitates Tyr(P)527 dephosphorylation (13).
Tyr(P)789 also binds the SH2 domain of the adapter protein Grb2 (11, 12), which participates in Ras activation following peptide growth factor stimulation (see Ref. 14 for review). Because of steric hindrance resulting from the interaction of one of the Grb2 SH3 domains with PTPα, PTPα-bound Grb2 is not able to bind Sos, the downstream protein in the Grb2-Ras signal transduction pathway. Thus, it does not appear that localization of Grb2 to the plasma membrane by binding to PTPα can activate the Ras signaling pathway (15, 16). Instead, control may flow in the other direction: Grb2 binding to Tyr(P)789 blocks phosphotyrosine displacement and the ability of PTPα to dephosphorylate Src, so only Grb2-unbound, Tyr(P)789-phosphorylated PTPα is able to activate Src (13). Most Tyr(P)789-phosphorylated PTPα is bound by Grb2 (11), so small changes in Grb2 binding can sensitively control Src-directed PTPα activity.
Src is activated during mitosis by a cooperative mechanism: mitotic Cdc2-mediated Ser/Thr phosphorylations within the Src amino-terminal region (17, 18) weaken intramolecular Src SH2 domain-Tyr(P)527 association (19, 20), thereby rendering Tyr(P)527 more susceptible to dephosphorylation (21–23) by PTPα, which itself is activated by other means (24). There is almost no mitotic activation of Src in PTPα knockout cells, implying that PTPα is the main PTP involved (24).
The mitotic activation of PTPα has two components: 1) its catalytic activity, as measured on nonphysiological substrates such as MBP, increases ~2-fold; and 2) the inhibitory binding of Grb2 to PTPα is reduced 3–4-fold (24). The latter reduction occurs because of a mitotic decrease in the affinity of PTPα for the Grb2 SH2 domain without a decrease in its affinity for the Src SH2 domain. This results in 2–3-fold increased Src-PTPα co-association and a commensurate increase in Src-directed PTPα activity (24). This relief from Grb2 competition combines multiplicatively with the increase in catalytic activity to give a 4–5-fold increase in total Src-directed PTPα activity (24).
The mechanism(s) responsible for increasing PTPα specific activity and selectively decreasing its binding to the Grb2 SH2 domain are not known. The changes occur without altered Tyr789 phosphorylation and can be observed with purified PTPα in vitro. Moreover, there is no evidence of PTPα dimerization under the experimental conditions. The changes coincide with mitotic reduction of PTPα electrophoretic mobility, suggesting that hyperphosphorylation is involved. Indeed, treating PTPα with the Ser/Thr-specific phosphatase PP2A coordinately eliminates PTPα mitotic mobility retardation, increased catalytic activity, and decreased Grb2 binding. The effect on Src-directed PTPα activity is even stronger: PP2A treatment not only blocks the mitotic increase, but also reduces, if not eliminates, the ability of interphase PTPα to activate Src (24). Because PTPα is predominantly phosphorylated at serine and has very little or no threonine phosphorylation (24–26), this suggests that the mitotic activation of PTPα requires, and may be caused by, serine hyperphosphorylation (24).
Two serine phosphorylation sites in PTPα have already been identified: Ser180 and Ser204 can both be phosphorylated in vitro in NIH3T3 cells by protein kinase C and are phosphorylated in vivo following treatment of cells with 12-O-tetradecanoylphorbol-13-acetate (25, 26). 12-O-Tetradecanoylphorbol-13-acetate-induced serine hyperphosphorylation of PTPα increases its catalytic activity 2–3-fold (25), probably because of phosphorylation at these sites (26). Recently, it was shown that phosphorylation at Ser180 and Ser204 is required for the activation of PTPα that follows treatment of A431 cells with a somatostatin analog (27).
To investigate the possibility that phosphorylation at Ser180 and/or Ser204 is involved in the mitotic activation of PTPα, we have compared the effects of separate and coordinate site-specific substitutions at these sites with those of PP2A treatment. We found that these phosphorylations are required for mitotic activation of PTPα, for the change in its SH2 domain-binding properties, and for its ability to activate Src and to transform cells.
MATERIALS AND METHODS
Antibodies
All PTPα immunoprecipitations and immunoblotting were performed with polyclonal antibody 7-091, which was made in rabbits against a GST fusion protein containing PTPα residues 165–793 (13). Anti-HA immunoprecipitations were carried out with monoclonal antibody 12CA5 (28).
Cell Lines, Nocodazole Arrest of Mitotic Cells, and Induction of PTPα Expression—
Except for cell lines overexpressing the S180A and S204A mutants (described below), all lines were previously described (13). Cells were grown; PTPα expression was induced; and cells were arrested in mitosis with nocodazole and collected by mechanical shake-off as described (24).
Mutant PTPα-inducible Expression Plasmids and Cell Lines
Plasmids for inducible expression of the Ser-to-Ala human PTPα mutants were constructed by replacing coding sequences lying between the two HindIII sites in WT PTPα expression plasmids pNTPTPα (no HA tag) and pTPTPα (with the HA epitope tag YPYDVPDYA) with mutated sequences constructed by PCR. PCR products and restriction fragments that together comprised complete (mutated) coding sequences lying between the HindIII sites were then religated into vector plasmid pTet-Splice (Invitrogen) to construct plasmids that were identical to pNTPTPα or pTPTPα except for the specified mutations. For the S180A substitution, a mutated HindIII-EclXI fragment was prepared by PCR using pNTPTPα as a template with the 5′-primer 5′-CGCCAAGCTTGGCCACCATGGATTCCTGGTTCATTCTTGT-3′ and the 3′-primer 5′-CAGTGCGGCCGTTGGATAAGCGGAAAGCATTGGAAT-3′. The 5′-primer contained the HindIII site (underlined) and the start codon (boldface); the 3′-primer contained the EclXI site (underlined). The substitution AGA → AGC (boldface italics) in the 3′-primer resulted in the S180A substitution. This PCR product was cleaved with HindIII and EclXI, mixed with the complementary gel-purified EclXI-HindIII restriction fragment from pNTPTPα, and ligated into the HindIII site of pTet-Splice to make plasmid pNTPTPα(S180A). Plasmid pTPTPα(S180A), which expresses PTPα(S180A)-HA, was constructed similarly, except that the EclXI-HindIII fragment was from pTPTPα.
For the S204A mutation, a 0.6-kilobase pair HindIII-BglII fragment containing the 5′-portion of the WT coding sequence was copied from pNTPTPα by PCR using the 5′-primer 5′-CGCCAAGCTTGGCCACCATGGATTCCTGGTTCATTCTTGT-3′ and the 3′-primer 5′-TTGGTGGCTGGAGATCTGGCCAGAAGTGGCACACTCT-3′. The 5′-primer contained the HindIII site (underlined) and the start codon (boldface); the 3′-primer contained the BglII site (underlined). This PCR product was cleaved with HindIII and BglII. (Although the 3′-primer contained the substitution AGC → GGC at nucleotides 6–8, the BglII digestion removed this region.) The BglII-HindIII fragment containing the downstream coding sequence with the S204A mutation was generated using pNTPTPα as a PCR template with the 5′-primer 5′-TGGCCAGATCTCCAGCCACCAACAGGAAATACCCACCCCT-3′ and the 3′-primer ′-TGTTGAAGCTTACTTGAAGTTGGCATAATC-3′. The 5′-primer contained the BglII site (underlined); the 3′-primer contained the stop codon (boldface) and the HindIII site (underlined). The mutation AGC → GCC (boldface italics) in the ′-primer caused the S204A substitution. This PCR product was cleaved with BglII and HindIII, and both fragments were ligated into the HindIII site of pTet-Splice to make plasmid pNTPTPα(S204A). Plasmid pTPTPα(S204A), which expresses PTPα(S204A)-HA, was constructed by ligating the gel-purified HindIII-ClaI restriction fragment from pNTPTPα(S204A), containing the S204A mutation, along with the complementary gel-purified ClaI-HindIII restriction fragment from pTPTPα, containing the downstream WT PTPα-HA sequence, into the HindIII site of pTet-Splice.
To construct the PTPα(S180A/S204A) expression plasmid, the 0.56-kilobase pair gel-purified HindIII-EclXI fragment from pNTPTPα(S180A) was mixed with the gel-purified EclXI-HindIII fragment from pNTPTPα(S204A) and ligated into pTet-Splice to make plasmid pNTPTPα(S180A/S204A). Plasmid pTPTPα(S180A/S204A), which expresses the HA-tagged double mutant, was constructed similarly, except that the EclXI-HindIII restriction fragment was isolated from pTPTPα(S204A).
For the S202A mutation, an EclXI-HindIII fragment containing the mutation and the downstream coding sequence was prepared by PCR using pNTPTPα as a template with the 5′-primer 5′-CCAACGGCCGCACTGAGGATGTGGAGCCCCAGAGTGTGCCACTTCTGGCCAGAGCCCCA-3′ and the 3′-primer 5′-TGTTGAAGCTTACTTGAAGTTGGCATAATC-3′. The 5′-primer contained the EclXI site (underlined); the 3′-primer contained the stop codon (boldface) and the HindIII site (underlined). The mutation TCC → GCC (boldface italics) in the 5′-primer resulted in the S202A substitution. This PCR product was digested with EclXI and HindIII, mixed with the 0.57-kilobase pair gel-purified HindIII-EclXI restriction fragment from pNTPTPα, and ligated into the HindIII site of pTet-Splice to make plasmid pNTPTPα(S202A).
The mutations were verified by sequencing of the PTPα coding region. These plasmids were stably cotransfected with the G418 resistance plasmid pSV2neo (29) and the tetracycline transactivator plasmid pTet-tTak (Invitrogen) into NIH3T3 cells, selected for G418 resistance and for inducible expression of the PTPα mutants as described (13).
Immunoprecipitation and Immunopurification of PTPα, Dephosphorylation and Kinase Assays, Co-immunoprecipitation and Affinity Precipitation Assays, and PP2A Serine Dephosphorylation
These were performed as described previously (24).
Anchorage-independent Growth Assay
Cells were assayed for colony formation on 0.3% agarose without doxycycline as described previously (30).
RESULTS
We have previously described genetically modified NIH3T3 cell lines that inducibly overexpress (under repressive control of doxycycline) human WT PTPα and PTPα(Y789F) and the same proteins with a nine-residue HA tag at their C termini, designated PTPα-HA and PTPα(Y789F)-HA (13). A “Neo” cell line that had been transfected with an empty vector system and co-selected in the same manner provided a control for analyzing endogenous PTPα. It was previously shown that the localization and specific catalytic activity of overexpressed WT PTPα are similar to those of endogenous PTPα in both unsynchronized and mitotic cells (13, 24).
New plasmids and corresponding NIH3T3-derived cell lines for inducible expression of PTPα (with or without the HA tag) with Ser-to-Ala substitutions at residues 180 and/or 204 were created using similar methods (see “Materials and Methods”). A cell line expressing PTPα with a Ser-to-Ala substitution at residue 202 (the only potential site of cyclin-dependent kinase or mitogen-activated protein kinase serine phosphorylation within PTPα) was also generated as a control for some experiments. The overexpresser cells maximally expressed ~10–20 times the amount of endogenous PTPα when grown in the absence of doxycycline for ≥16 h. So that equal levels of overexpression could be obtained in both unsynchronized and mitotic cells, the time of induction was controlled so that transgene PTPα expression was induced only to ~5–10 times endogenous levels for the biochemical experiments (see Ref. 24 and “Materials and Methods”). Expression levels were similar within each group of cell lines expressing untagged or HA-tagged proteins (data not shown).
To see if we could detect serine phosphates added during mitosis, unsynchronized and nocodazole-arrested mitotic WT PTPα and PTPα(S180A/S204A) overexpresser cells were labeled in vivo with [32P]orthophosphate, and the radiolabeled proteins were analyzed by anti-PTPα immunoprecipitation, immunoblotting, and autoradiography. To avoid radioactivity-induced G2 arrest (17), it was necessary to label all the cells for relatively short (2–3 h) periods and the mitotic cells after nocodazole arrest. Thus, equilibrium labeling was probably not achieved, and only phosphorylations that were catalyzed during metaphase (the point of nocodazole arrest) were detected in the mitotic cells. Under these conditions, no significant changes in the stoichiometry of labeling were observed between WT and mutant PTPα from unsynchronized or mitotic cells (data not shown). WT PTPα and PTPα(S180A/S204A) from unsynchronized and mitotic cells displayed similar radioactive phosphoamino acid compositions: a significant excess of phosphoserine over phosphotyrosine and very little or no phosphothreonine (data not shown). We conclude that the serine phosphorylation(s) that cause the electrophoretic mobility retardation of mitotic PTPα do not turn over during mitosis and that PTPα contains at least one site of serine phosphorylation in addition to Ser180 and Ser204.
Mitotic Increase in PTPα Phosphatase Activity Is Blocked by Mutation of Ser180 or Ser204
MBP that had been tyrosine-phosphorylated with [γ-32P]ATP (by v-Src) was incubated with WT or mutant PTPα-HA that had been immunoprecipitated with anti-HA antibody from unsynchronized or nocodazolearrested mitotic overexpresser cells. The immunoprecipitates were washed with 0.5 m NaCl to remove any co-associated proteins. Specific PTP activity was determined by measuring the relative amounts of 32P released per molecule of PTPα.
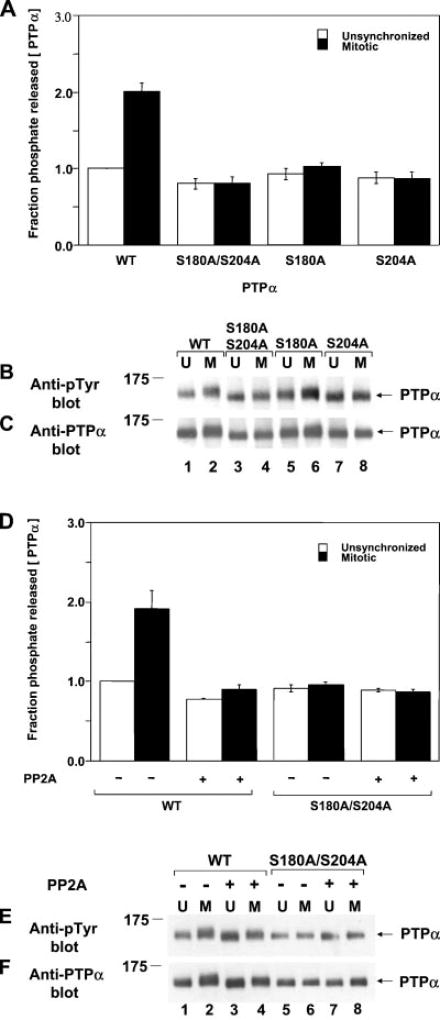
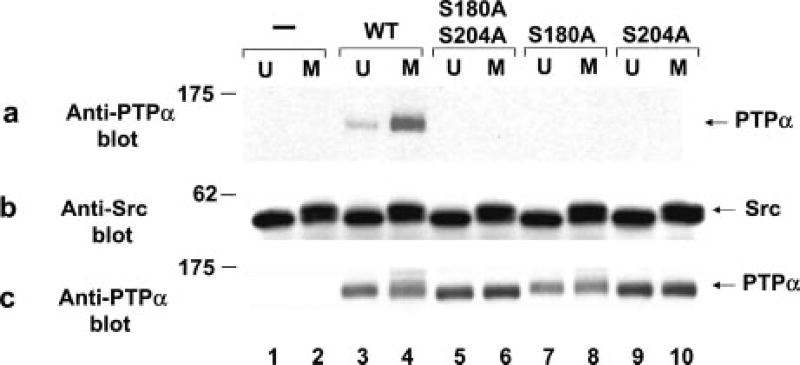
As previously reported (24), the activity of WT PTPα from mitotic cells was about twice that of PTPα from unsynchronized cells (Fig. 1A), and there was very little change during mitosis in the amount of PTPα (Fig. 1C) or the extent of its tyrosine phosphorylation (Fig. 1B). (Tyr789 is the only phosphorylated tyrosine in PTPα during both interphase and mitosis (13, 24), so anti-phosphotyrosine immunoblotting specifically detects its phosphorylation.) The increased specific activity correlated with reduced electrophoretic mobility of PTPα (Fig. 1C, compare lanes 1 and 2).
Fig. 1. Nonspecific catalytic activity of PTPα from unsynchronized and mitotic cells.
Approximately equal amounts of overexpressed human WT PTPα-HA, PTPα(S180A/S204A)-HA, PTPα(S180A)-HA, and PTPα(S204A)-HA were immunoprecipitated with anti-HA antibody from lysates from NIH3T3-derived overexpresser cells that were either unsynchronized (U) or arrested in mitosis (M). Aliquots of immunoprecipitates that had been washed with a high salt buffer were incubated with [32P]Tyr(P)-containing MBP in phosphatase buffer for 30 min at 30 °C or subjected to anti-Tyr(P) or anti-PTPα immunoblotting. Additional experiments were performed similarly, except that the WT PTPα and PTPα(S180A/S204A) immunoprecipitates were preincubated with PP2A, a Ser/Thr phosphatase, and then washed with phosphatase buffer before incubation with MBP. A, shown is the amount of 32P released per molecule of PTPα after a 30-min incubation normalized to the amount released by overexpressed PTPα from unsynchronized cells. Error bars indicate the S.E.M. from four to five experiments. B, shown is the anti-Tyr(P) (Anti-pTyr) immunoblot of the immunoprecipitated PTPα. C, shown is the anti-PTPα immunoblot of the immunoprecipitated PTPα. D, experiments were performed as described above using immunoprecipitated WT PTPα-HA or PTPα(S180A/S204A)-HA, except that the immunoprecipitates were preincubated with (+) or without (−) PP2A prior to PTP assay with 32P-labeled MBP. The amount of 32P released per molecule of PTPα after a 30-min incubation normalized to the amount released by PP2A-untreated PTPα from unsynchronized cells is shown. Error bars indicate the S.E.M. from two to three experiments. E, shown is the anti-Tyr(P) immunoblot of the immunoprecipitated PTPα. F, shown is the anti-PTPα immunoblot of the immunoprecipitated PTPα. SDS-PAGE was performed on 10% gels. The positions of molecular mass standards are indicated in kilodaltons.
Both coordinate and separate S180A and/or S204A mutations blocked the mitotic increase in PTP activity (Fig. 1). These mutations also caused a small but reproducible 10–20% decrease in the specific activity of PTPα in unsynchronized cells. The mitotic mobility retardation was completely blocked in PTPα(S180A/S204A), consistent with the hypothesis that the retardation resulted from mitotic phosphorylation at these sites. The mutations did not significantly affect Tyr789 phosphorylation.
Similar experiments were conducted with immunoprecipitated WT PTPα and PTPα(S180A/S204A) that had been incubated with the Ser/Thr phosphatase PP2A before incubation with MBP. PP2A treatment slightly reduced the specific activity of interphase PTPα and eliminated the mitotic increase in activity (Fig. 1D) without affecting Tyr789 phosphorylation (Fig. 1E). It also restored the electrophoretic mobility of mitotic PTPα almost to its interphase level (Fig. 1F). In contrast, it had no observable effect on the activity or mobility of the serine double mutant. This is consistent with the hypothesis that the PP2A effect results from its dephosphorylation of Ser180 or Ser204.
These results suggest that the mitotic activation of PTPα nonspecific catalytic activity requires mitotic hyperphosphorylation of both Ser180 and Ser204. The small decrease in the activity of PTPα from unsynchronized cells upon mutation or PP2A treatment may reflect the fact that PTPα is phosphorylated to some extent at these sites even during interphase (26).
Tyr527 Dephosphorylation and Activation of Src in Vitro and in Vivo by PTPα Are Blocked by Mutation of Ser180 or Ser204
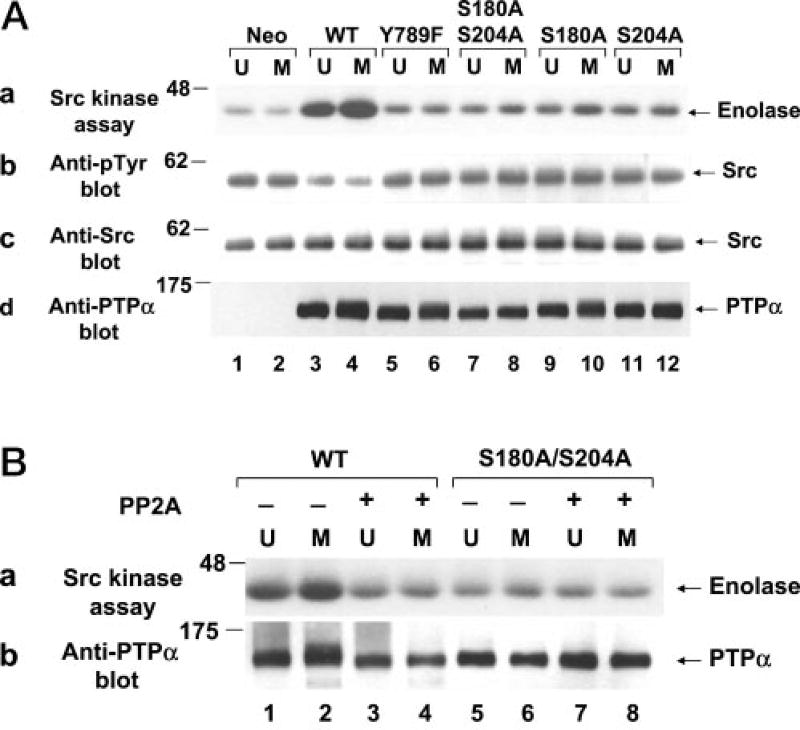
The ability of WT and mutant PTPα to activate Src in vitro was measured after immunopurifying the HA-tagged phosphatase from unsynchronized or mitotic overexpresser cells. Equal amounts of solubilized phosphatase were incubated in phosphatase buffer with chicken WT Src that had been immunoprecipitated from unsynchronized NIH3T3-derived Src overexpresser cells. After washing away PTPα, the specific activity of the treated Src was measured by incubating it with [γ-32P]ATP and acid-denatured enolase (substrate) and measuring the amount of transferred 32P by autoradiography (Fig. 2A, panel a). Because Tyr(P)527 is the only detectable phosphotyrosine in Src from these overexpresser cells (31), anti-Tyr(P) immunoblotting was used to assay the amount of Tyr527 phosphorylation (Fig. 2A, panel b). As previously shown (24), PTPα from mitotic cells dephosphorylated Src and increased its kinase activity more than PTPα from unsynchronized cells (Fig. 2A, lanes 3 and 4). We now found that Ser-to-Ala mutation of either Ser180 or Ser204 or both not only abrogated the mitotic increase in activity, but largely eliminated the ability of PTPα from both unsynchronized and mitotic cells to dephosphorylate and activate Src at all (Fig. 2A, lanes 7–12). The very low residual activity of the Ser-to-Ala mutants was similar to that of the Y789F mutant (Fig. 2A, lanes 5 and 6).
Fig. 2. Tyrosine dephosphorylation and activation of Src in vitro by WT and mutant PTPα from unsynchronized and mitotic cells.
A, Src that had been immunoprecipitated from NIH3T3-derived chicken Src overexpresser cells was incubated in phosphatase buffer with WT (lanes 3 and 4) or mutant (lanes 5–12) PTPα-HA that had been immunopurified from unsynchronized (U) or mitotic (M) PTPα overexpresser cells using anti-HA antibody or with mock-immunopurified protein from control cells that did not express any HA-tagged protein (lanes 1 and 2). The partially dephosphorylated Src immunoprecipitates were washed to remove PTPα and then incubated with enolase and [γ-32P]ATP in kinase buffer. Panel a, autoradiograph of [32P]enolase after the Src kinase assay; panel b, anti-Tyr(P) (Anti-pTyr) immunoblot of the Src immunoprecipitates after PTPα treatment; panel c, anti-Src immunoblot of the treated immunoprecipitates; panel d, anti-PTPα immunoblot of one-thirtieth of the PTPα used for the in vitro dephosphorylation reactions. B, the conditions were the same as described for A, except that immunopurified WT PTPα (lanes 1–4) and PTPα(S180A/S204A) (lanes 5–8) were treated (+; lanes 3, 4, 7, and 8) or not (−; lanes 1, 2, 5, and 6) with PP2A prior to incubation with Src. Panel a, autoradiograph of [32P]enolase after the Src kinase assay; panel b, anti-PTPα immunoblot of one-thirtieth of the PTPα used for the in vitro dephosphorylation reactions. SDS-PAGE was performed on 9% gels. The positions of molecular mass standards are indicated in kilodaltons.
Additional experiments were conducted in which PTPα was dephosphorylated by PP2A prior to incubation with the Src substrate. Phosphatase-treated WT PTPα from both unsynchronized and mitotic cells had the same very low Src-activating ability as untreated PTPα(S180A/S204A) (Fig. 2B, panel a, lanes 3–6). Moreover, PP2A treatment did not affect PTPα(S180A/S204A) (Fig. 2B, lanes 5–8), suggesting that its effect on WT PTPα was mediated via dephosphorylation of Ser180 and Ser204.
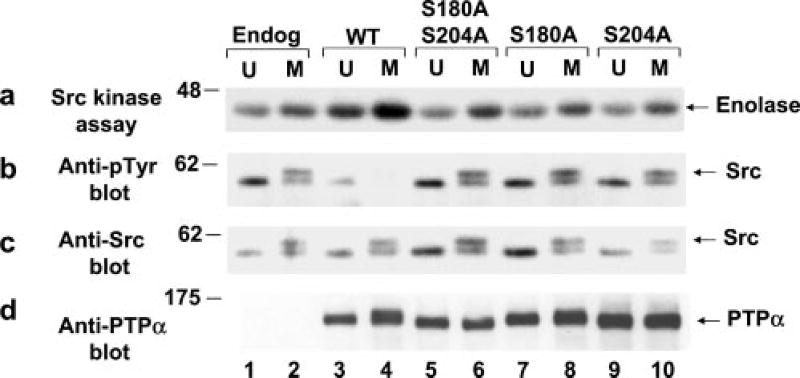
To assess the Src-directed activity of WT and mutant PTPα in vivo, Src was immunoprecipitated from unsynchronized and mitotic non-overexpresser cells (control) and PTPα overexpresser cells, and Src phosphorylation and its ability to phosphorylate enolase were measured (Fig. 3, a and b). All of the overexpresser cell lines expressed approximately equal amounts of Src and transgene PTPα (Fig. 3, c and d). As previously shown (24), overexpression of WT PTPα decreased Src tyrosine phosphorylation, increased interphase Src activity, and enhanced the mitotic increase in its activity (Fig. 3, compare lanes 3 and 4 with lanes 1 and 2). In contrast, we now found that overexpression of the mutants with S180A and/or S204A substitutions had no effect on Src tyrosine phosphorylation or activity (Fig. 3, lanes 5–10). In summary, the in vitro and in vivo results consistently imply that phosphorylation of PTPα at both Ser180 and Ser204 is required for it to be able to dephosphorylate and activate Src.
Fig. 3. Effect of WT and mutant PTPα overexpression on Src in vivo tyrosine phosphorylation and kinase activity.
Endogenous (Endog) Src was immunoprecipitated from unsynchronized (U) or mitotic (M) non-overexpresser cells (lanes 1 and 2) or from cells overexpressing WT (lanes 3 and 4) or mutant (lanes 5–10) PTPα as indicated. Each immunoprecipitate was divided into aliquots that were used in a kinase assay with [γ-32P]ATP and acid-denatured enolase, followed by electrophoresis and autoradiography of the reaction products (a); immunoblotted with anti-Tyr(P) (Anti-pTyr) antibody (b); immunoblotted with anti-Src antibody (c); or immunoblotted with anti-PTPα antibody (d). SDS-PAGE was performed on 9% gels. The positions of molecular mass markers are indicated in kilodaltons.
Mutation of Ser180 or Ser204, but Not of Ser202, Blocks Neoplastic Transformation by PTPα
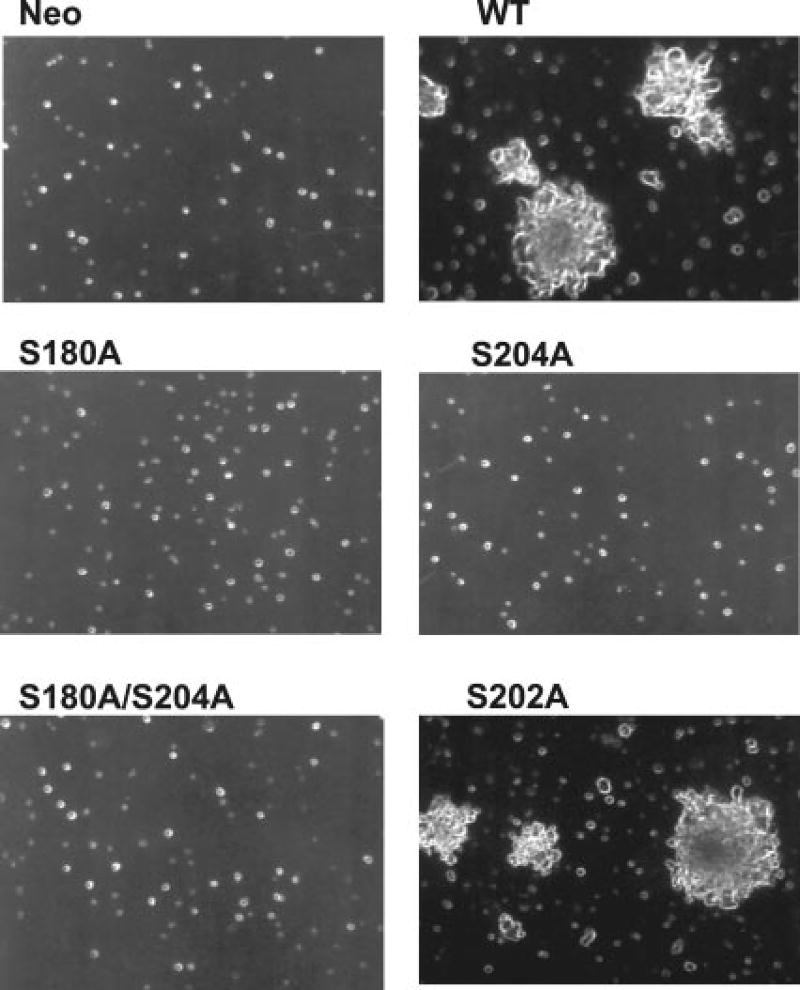
The ability of the WT and mutant PTPα overexpresser cells to grow without anchorage was assayed by suspending them in semisolid medium containing 0.3% soft agarose without doxycycline (Fig. 4). The expression levels in the WT and mutant overexpresser cells were the same, except for the S180A mutant, which was expressed at an ~50% higher level. Overexpression of WT PTPα, but not of either the coordinate or separate Ser-to-Ala mutants, induced anchorage-independent growth. A mutant that contained a S202A mutation transformed like WT PTPα. Similar results were obtained with cells overexpressing WT and mutant PTPα-HA proteins (data not shown).
Fig. 4. Colony formation on soft agarose by WT and mutant PTPα overexpresser cells.
Control cells (Neo) and cells overexpressing WT or mutant PTPα as indicated were cultured in suspension in medium containing 0.3% agarose and no doxycycline. Colonies were photographed after 21 days.
Effect of Ser180 and Ser204 Mutations on PTPα Binding to Src and Grb2
The association of WT and mutant PTPα with Src in vivo was examined by co-immunoprecipitation experiments. PTPα overexpresser cells were lysed with a Nonidet P-40 buffer, and anti-Src immunoprecipitates were immunoblotted with anti-PTPα (Fig. 5a) or anti-Src (Fig. 5b) antibody. As previously reported (24), the association between WT PTPα and Src increased ~3-fold in mitotic cells (Fig. 5a, compare lanes 3 and 4). In contrast, PTPα with either the S180A and/or S204A mutation did not detectably bind Src in either unsynchronized or mitotic cells (Fig. 5a, lanes 5–10). (The lower amount of endogenous PTPα in the control cells was not detectable in these experiments and so did not influence the observed results.)
Fig. 5. Co-association in vivo of WT and mutant PTPα with Src in unsynchronized and mitotic cells.
Immunoprecipitates made with anti-Src antibody from lysates (containing 1.5 mg of total cell protein) from unsynchronized (U) or mitotic (M) non-overexpresser control cells (−; lanes 1 and 2) or from cells overexpressing WT (lanes 3 and 4) or mutant ( lanes 5–10) PTPα as indicated were analyzed by 9% SDS-PAGE and immunoblotted with anti-PTPα (a) or anti-Src (b) antibody. c shows an anti-PTPα immunoblot of cell lysates containing 10 µg of total cell protein. The positions of molecular mass standards are indicated in kilodaltons.
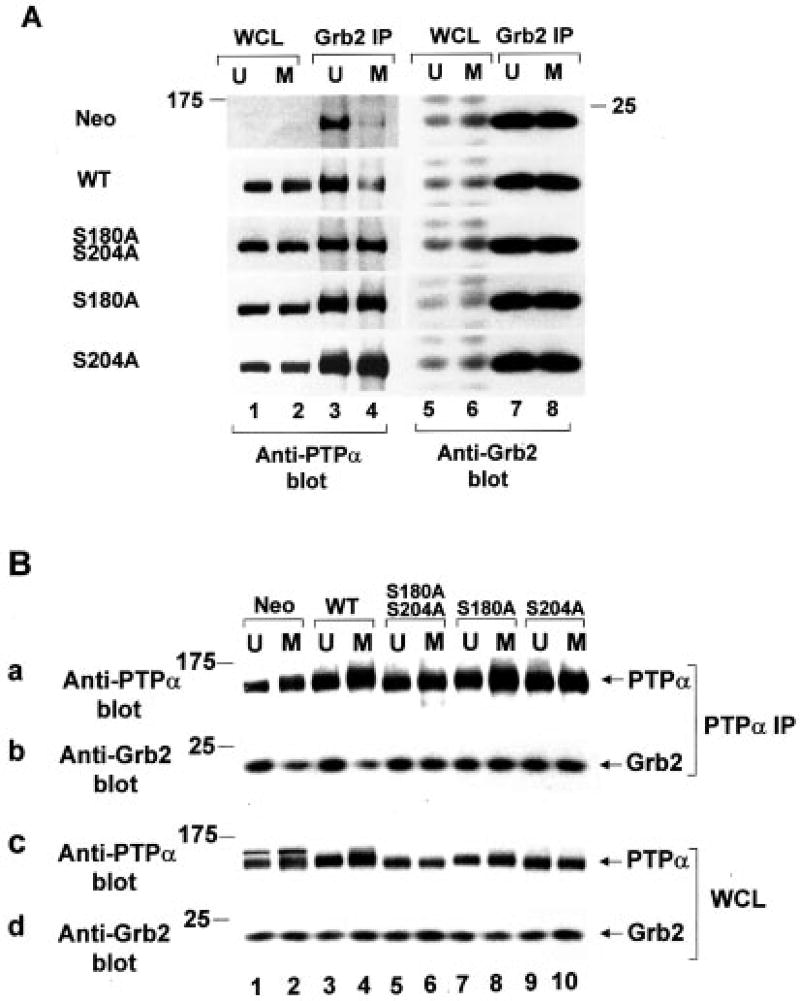
Analogous co-immunoprecipitation experiments were performed to examine PTPα binding to Grb2. Anti-Grb2 and anti-PTPα immunoprecipitates were immunoblotted with anti-PTPα and anti-Grb2 antibodies, respectively (Fig. 6, A and B). Both types of experiments gave consistent results. As previously reported (13, 24), the WT PTPα-Grb2 association observed in unsynchronized non-overexpresser and overexpresser cells decreased ~4-fold in mitotic cells. (As previously noted, Grb2 bound a larger fraction of endogenous WT PTPα (i.e. in the Neo control cells) than overexpressed WT PTPα. This is because the level of Tyr789 phosphorylation in overexpressed PTPα is ~2.5 times lower relative to endogenous PTPα, possibly because the Tyr789 kinase is saturated (13).) In contrast, we now found that the Ser-to-Ala PTPα mutants bound Grb2 to the same high extent in both unsynchronized and mitotic cells. The control immunoblots (Fig. 6, A, lanes 1, 2, and 5–8; and B, panels a, c, and d) showed that there were similar amounts of Grb2 and PTPα in the cell lysates and immunoprecipitates from the unsynchronized and mitotic cells. We conclude that both Ser180 and Ser204 are required for PTPα binding to Src in vivo and for the mitotic reduction in PTPα-Grb2 association.
Fig. 6. Co-association in vivo of WT and mutant PTPα with Grb2 in unsynchronized and mitotic cells.
A, immunoprecipitates (IP) made with anti-Grb2 antibody (lanes 3, 4, 7, and 8) from lysates (containing 400 µg of total cell protein) from unsynchronized (U) or mitotic (M) non-overexpresser control cells (Neo) or from cells overexpressing WT and mutant PTPα as indicated were analyzed by 11% SDS-PAGE and immunoblotted with anti-PTPα (lanes 3 and 4) or anti-Grb2 (lanes 7 and 8) antibody. For comparison, portions of the whole cell lysates (WCL) containing 10 µg (lanes 1 and 2) or 25µg (lanes 5 and 6) of total cell protein were directly immunoblotted with anti-PTPα (lanes 1 and 2) or anti-Grb2 (lanes 5 and 6) antibody. The dark bands in lanes 1–4 are PTPα; the dark bands in lanes 5–8 are Grb2. B, experiments were performed as described for A, except that immuno-precipitates were made with anti-PTPα antibody (panels a and b) from lysates containing 1.5 mg (lanes 1 and 2) or 400 µg (lanes 3–10) of total cell protein and were immunoblotted with anti-PTPα (panel a) or anti-Grb2 (panel b) antibody. For comparison, portions of the whole cell lysates containing 80 µg (panel c, lanes 1 and 2), 10 µg (panel c, lanes 3–10), or 25 µg (panel d) were immunoblotted with anti-PTPα (panel c) or anti-Grb2 (panel d) antibody. 11% SDS-PAGE was performed for panels a, b, and d; 9% SDS-PAGE was performed for panel c. The positions of molecular mass standards are indicated in kilodaltons.
Effect of Ser180 and Ser204 Mutations and PP2A Treatment on PTPα Binding to the Isolated Src and Grb2 SH2 Domains
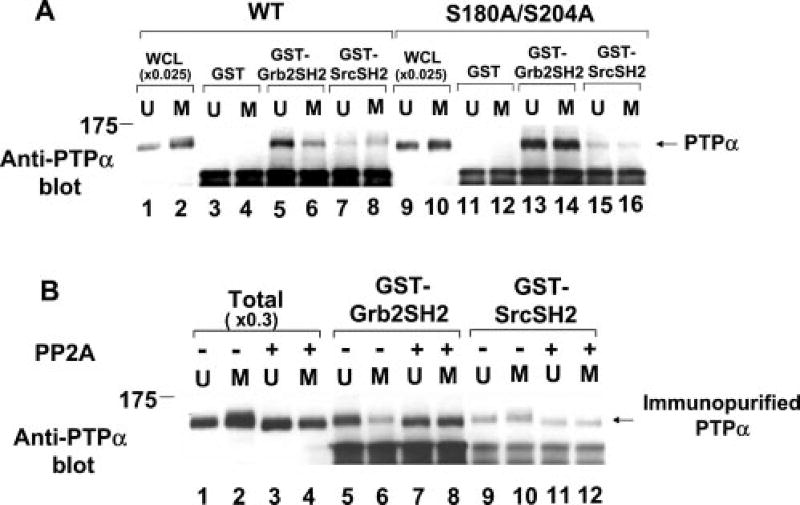
To determine whether the changes in PTPα-Grb2 association could be explained by changes in the affinity of the Grb2 or Src SH2 domain alone, we measured the abilities of fusion proteins containing GST and either Grb2 or Src SH2 domains to affinity-precipitate WT PTPα-HA and PTPα(S180A/S204A)-HA from overexpresser cell lysates (Fig. 7A). As previously shown (13), the Grb2 SH2 domain immunoprecipitated about three times more interphase PTPα than the Src SH2 domain (Fig. 7A, compare lanes 5 and 7). Also as previously reported (24), the Grb2 SH2 domain bound ~2-fold less mitotic PTPα than interphase PTPα (Fig. 7A, compare lanes 5 and 6), but the affinity of the Src SH2 domain for mitotic PTPα was the same as or possibly slightly higher than its affinity for interphase PTPα (compare lanes 7 and 8). We now found that the Ser-to-Ala mutations slightly increased (~25%) the ratio between Grb2 and Src SH2 domain binding to interphase PTPα and completely eliminated the mitotic changes in binding affinities; 3.7 ± 0.5 times more PTPα(S180A/S204A) bound to the Grb2 SH2 domain than to the Src SH2 domain (Fig. 7A, lanes 13–16).
Fig. 7. Effects of Ser-to-Ala mutation and PP2A treatment on in vitro binding of PTPα from unsynchronized and mitotic cells to the Src and Grb2 SH2 domains.
A, lysates (containing 400 µg of total cell protein) from unsynchronized (U) or mitotic (M) WT PTPα-HA (lanes 3–8) or PTPα(S180A/S204A)-HA (lanes 11–16) overexpresser cells were affinity-precipitated by incubation with GST (lanes 3, 4, 11, and 12), a GST-Grb2 SH2 domain fusion protein (lanes 5, 6, 13, and 14), or a GST-Src SH2 domain fusion protein (lanes 7, 8, 15, and 16) bound to Sepharose beads. The washed beads were then analyzed by 9% SDS-PAGE and anti-PTPα immunoblotting. For comparison, lanes 1, 2, 9, and 10 contained 0.025 times the amount of complete whole cell lysate (WCL) used in the affinity precipitations. B, PTPα-HA was immunopurified from unsynchronized or mitotic overexpresser cell lysates (containing 1 mg of total cell protein), incubated with (+; lanes 7, 8, 11, and 12) or without (−; lanes 5, 6, 9, and 10) the serine/threonine phosphatase PP2A, and then affinity-precipitated by the GST-SH2 domain fusion proteins used for A. For comparison, lanes 1–4 (Total) contained 0.3 times the amount of immunopurified PTPα used in the affinity precipitations; these were also incubated with (lanes 3 and 4) or without (lanes 1 and 2) PP2A. The positions of molecular mass standards are indicated in kilodaltons.
To exclude the possibility that other proteins in the cell lysates affected PTPα binding to the GST-SH2 domain fusion proteins, similar experiments were performed with immunopurified PTPα-HA (Fig. 7B). (The immunopurification procedure involved a pH 2.5 elution and subsequent neutralization that removed all co-associated Grb2 (data not shown) and presumably any other noncovalently associated proteins.) Similar results were obtained (Fig. 7B, slanes 5, 6, 9, and 10). We also examined the effect of serine dephosphorylation on the binding of immunopurified PTPα-HA by incubating it with PP2A before the affinity precipitations. Dephosphorylation affected WT PTPα-HA binding in the same manner as the S180A and S204A mutations: it eliminated the mitotic decrease in the binding of PTPα to the Grb2 SH2 domain and decreased the binding of interphase and mitotic PTPα to the Src SH2 domain by 35–45%. We conclude that mitotic phosphorylations at Ser180 and Ser204 are required for the 2–3-fold mitotic downregulation of the affinity of the Grb2 SH2 domain for PTPα. In contrast, the phosphorylations slightly increase binding to the Src SH2 domain.
DISCUSSION
We have previously shown that, during mitosis, the catalytic activity of PTPα is enhanced and that its inhibitory binding to Grb2, which specifically blocks Src dephosphorylation, is decreased (24). We have now shown that S180A and/or S204A mutation blocks these effects and the resultant mitotic activation of Src by PTPα. This occurs without change in the phosphorylation of PTPα at Tyr789, which is required for phosphotyrosine displacement and Src dephosphorylation. Surprisingly, these mutations also prevent Src-PTPα co-association during interphase and block most or all dephosphorylation and activation of Src both in vitro and in vivo. This almost certainly explains the inability of any of the mutants to induce anchorage-independent growth, even when expressed at high (~20 times endogenous) levels. The mutations do not prevent dephosphorylation of MBP, implying that Ser180 and Ser204, like Tyr789, can regulate PTPα substrate specificity.
The fact that either Ser180/Ser204 mutation or PP2A treatment removes the mitotic electrophoretic mobility retardation of PTPα indicates that these residues have been hyperphosphorylated. Moreover, the similarities between the mutation- and PP2A-induced effects on catalytic activity and binding indicate that the mutations act functionally by preventing this hyperphosphorylation. Protein kinase C phosphorylates Ser180 and Ser204 following 12-O-tetradecanoylphorbol-13-acetate stimulation (25, 26), and it may phosphorylate them at or shortly before mitosis. This could account for the observed changes in catalytic activity: 12-O-tetradecanoylphorbol-13-acetate-stimulated protein kinase C-mediated phosphorylation at these sites decreases the PTPα Km for MBP from 12 to 5 µm without significant change in Vmax (25). At the concentration of tyrosine-phosphorylated MBP in our assays (~4 µm), such a 2.4-fold reduction in Km would cause an ~1.8-fold increase in PTP activity, consistent with our measurements.
The protein kinase C isoform that is most likely to be involved is protein kinase Cδ, which co-associates with PTPα (and phosphatidylinositol 3-kinase) following treatment of A431 cells with a somatostatin analog (27). This results in activation of PTPα and Src, probably initiated by protein kinase Cδ-mediated phosphorylation of Ser180 and Ser204 (27, 32). Protein kinase C has been implicated in both positive and negative control of the G2/M transition, with the relevant events occurring just before entry into mitosis (see Refs. 33 and 34 for review). Phosphorylation at this time would be consistent with the radiolabeling experiments (data not shown), which indicated that the Ser180 and Ser204 hyperphosphorylations do not turn over during metaphase.
However, the participation of other kinases is not excluded. For example, the sequence surrounding Ser204 also matches the protein kinase A phosphorylation consensus sequence (35). Whatever the kinase, because dephosphorylation of Src by PTPα requires phosphorylation at both Ser180 and Ser204, this activity will be proportional to the square of the serine phosphorylation stoichiometry (assuming that the phosphorylations are independent events). This non-linearity will enhance the ability of the upstream serine kinase to control PTPα and hence Src in an “on-off ” manner.
The fact that mutation of either Ser180 or Ser204 prevents the mitotic decrease in the binding of PTPα and Grb2 in vivo (Fig. 6) suggests that coordinate phosphorylation at these sites during mitosis reduces their binding affinity. As described in the Introduction, very little Grb2-unbound, Tyr789-phosphorylated PTPα is available to bind and act on Src during interphase. Thus, it is likely that the inability of PTPα(S180A/S204A) to bind Src in vivo results, at least in part, from increased competition from Grb2. The fact that the mutations affect PTPα during interphase (as well as during mitosis) is consistent with the observation that Ser180 and Ser204 are phosphorylated to some extent in unsynchronized cells (26).
The mitosis- and mutation-induced changes in PTPα-Grb2 binding in vivo correlate perfectly with and may result from the corresponding changes observed in the affinity between PTPα and the Grb2 SH2 domain in vitro (Fig. 7). The in vitro binding experiments were performed using recombinant GST-SH2 domain fusion proteins, excluding the possibility that a cell cycledependent modification of Grb2 or altered binding to a Grb2 SH3 domain is required. The most economical hypothesis, supported by both the mutagenesis and PP2A dephosphorylation experiments, is that phosphorylation of Ser180 and Ser204 decreases PTPα-Grb2 SH2 domain affinity by 3–4-fold and increases PTPα-Src SH2 domain affinity by 35–45%.
Both the Grb2 and Src SH2 domains bind to Tyr(P)789, which is the only phosphotyrosine in PTPα (11, 13); so it is surprising that their binding affinities can be differentially regulated. As far as we are aware, this has no precedent. The binding affinity changes were observed with immunopurified PTPα that had passed through a pH 2.5 denaturation step that removed all Grb2 (and probably any other co-associated proteins), and PTPα dimerization was not detected in the cell lysates used (data not shown). Therefore, we believe that the changes reflect effects of the Ser180 and Ser204 phosphorylations on isolated monomeric PTPα.
The mechanism of differential regulation may be related to the unique mode by which the Grb2 domain binds with high affinity to Tyr(P)-containing peptides: peptides that match the Grb2 SH2 domain binding consensus sequence form a β-turn when bound to the SH2 domain (36, 37). This is probably the conformation of the Tyr(P)789 region when bound to the Grb2 SH2 domain with high affinity. In contrast, Tyr(P)-containing peptides bind to other SH2 domains in an extended conformation (38, 39), so the Tyr(P)789 region is probably extended when it binds the Src SH2 domain. Therefore, it is possible that phosphorylation of Ser180 and Ser204 could reduce the high affinity binding to the Grb2 SH2 domain without decreasing the lower affinity binding to the Src SH2 domain if it interfered with the formation of the β-turn. If the phosphorylations also stabilized an extended conformation of the C-terminal region, they could simultaneously increase the affinity of Tyr(P)789 binding to the Src SH2 domain.
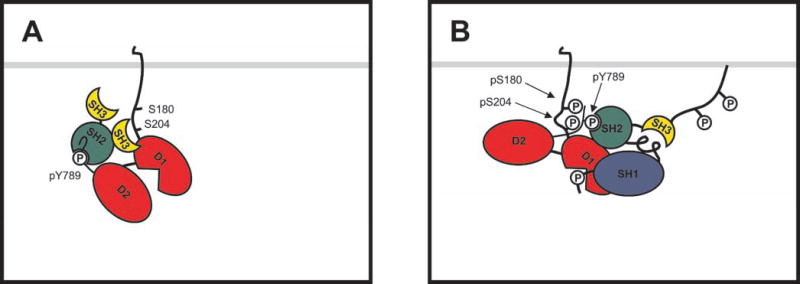
A model of this sort is shown in Fig. 8. In this hypothesis, phosphorylations at Ser180 and Ser204 promote an intramolecular association between the PTPα C-terminal and membraneproximal regions that prevents the β-turn and stabilizes an extended conformation of the C-terminal region without occluding Tyr(P)789. For example, basic residues surrounding Tyr(P)789 (e.g. Lys777 and Lys793) might interact with phosphorylated Ser180 and Ser204 so as to “stretch” the peptide out along the surface of PTPα. To speculate further, the intramolecular association might also increase the accessibility (or modify the conformation) of the D1 catalytic domain, which lies between these two regions, so as to decrease its Km. Modulation of intramolecular association by changes in Ser180 and Ser204 phosphorylation could thereby also account for the observed changes in nonspecific catalytic activity.
Fig. 8. An intramolecular association hypothesis.
A, when either Ser180 or Ser204 is dephosphorylated, the C terminus of PTPα is free to adopt a β-hairpin conformation and bind the Grb2 SH2 domain. B, when both Ser180 and Ser204 are phosphorylated, an intramolecular interaction between the C terminus and the membrane-proximal region stabilizes an extended conformation of the C terminus that reduces its affinity for the Grb2 SH2 domain while facilitating binding by the Src SH2 domain. In the variant of the hypothesis shown here, this association also relieves partial occlusion of the D1 catalytic site by the D2 domain, thereby increasing catalytic activity. pY, Tyr(P); pS, Ser(P).
Our results do not exclude the possibility that phosphorylation of Ser180 and Ser204 is required but not sufficient for activation. Of particular interest is Ser787, the only serine in the C-terminal region downstream from the D2 catalytic domain. It lies in a (weak) phosphorylation consensus site for casein kinase II (40) and, because of its proximity to Tyr789, might directly affect its interaction with SH2 domains if it were also hyperphosphorylated during mitosis.
In any case, at least one puzzle remains: although we expect altered binding and increased competition from Grb2 to reduce the ability of the Ser-to-Ala PTPα mutants to dephosphorylate Src in vivo, it does not explain their inability to dephosphorylate Src in vitro under conditions in which Grb2 (and probably any other co-associating proteins) was removed. Even though its binding affinity is slightly reduced, PTPα(S180A/S204A) still binds the Src SH2 domain (Fig. 7), so it is not evident why phosphotyrosine displacement and dephosphorylation of Src should be blocked. Because Tyr(P)789 must compete with Tyr(P)527 for binding to the Src SH2 domain, it is possible that even a fairly small change in Src SH2 domain-Tyr(P)789 affinity can perturb a delicate balance. However, other mechanisms may also be involved.
Footnotes
This study was supported by NCI Grant CA32317 (to D. S.) from the National Institutes of Health.
The abbreviations used are: PTP, protein-tyrosine phosphatase; SH, Src homology; MBP, myelin basic protein; PP2A, protein phosphatase 2A; GST, glutathione S-transferase; HA, hemagglutinin; WT, wild-type.
References
- 1.Kaplan R, Morse B, Huebner K, Croce C, Howk R, Ravera M, Ricca G, Jaye M, Schlessinger J. Proc. Natl. Acad. Sci. U. S. A. 1990;87:7000–7004. doi: 10.1073/pnas.87.18.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krueger NX, Streuli M, Saito H. EMBO J. 1990;9:3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng X-M, Wang Y, Pallen CJ. Nature. 1992;359:336–339. doi: 10.1038/359336a0. [DOI] [PubMed] [Google Scholar]
- 4.den Hertog J, Pals CEGM, Peppelenbosch MP, Tertoolen LGJ, de Laat SW, Kruijer W. EMBO J. 1993;12:3789–3798. doi: 10.1002/j.1460-2075.1993.tb06057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrone A, Sap J. J. Cell Sci. 2000;113:2345–2354. doi: 10.1242/jcs.113.13.2345. [DOI] [PubMed] [Google Scholar]
- 6.Shalloway D, Taylor SJ. Trends Cell Biol. 1997;7:215–217. [Google Scholar]
- 7.Brown MT, Cooper JA. Biochim. Biophys. Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 8.Ponniah S, Wang DZ, Lim KL, Pallen CJ. Curr. Biol. 1999;9:535–538. doi: 10.1016/s0960-9822(99)80238-3. [DOI] [PubMed] [Google Scholar]
- 9.Su J, Muranjan M, Sap J. Curr. Biol. 1999;9:505–511. doi: 10.1016/s0960-9822(99)80234-6. [DOI] [PubMed] [Google Scholar]
- 10.Arnott CH, Sale EM, Miller J, Sale GJ. J. Biol. Chem. 1999;274:26105–26112. doi: 10.1074/jbc.274.37.26105. [DOI] [PubMed] [Google Scholar]
- 11.den Hertog J, Tracy S, Hunter T. EMBO J. 1994;13:3020–3032. doi: 10.1002/j.1460-2075.1994.tb06601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su J, Batzer A, Sap J. J. Biol. Chem. 1994;269:18731–18734. [PubMed] [Google Scholar]
- 13.Zheng X-M, Resnick RJ, Shalloway D. EMBO J. 2000;19:964–978. doi: 10.1093/emboj/19.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawson T. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 15.den Hertog J, Hunter T. EMBO J. 1996;15:3016–3027. [PMC free article] [PubMed] [Google Scholar]
- 16.Su J, Yang L-T, Sap J. J. Biol. Chem. 1996;271:28086–28096. doi: 10.1074/jbc.271.45.28086. [DOI] [PubMed] [Google Scholar]
- 17.Chackalaparampil I, Shalloway D. Cell. 1988;52:801–810. doi: 10.1016/0092-8674(88)90422-9. [DOI] [PubMed] [Google Scholar]
- 18.Shenoy S, Chackalaparampil I, Bagrodia S, Lin P-H, Shalloway D. Proc. Natl. Acad. Sci. U. S. A. 1992;89:7237–7241. doi: 10.1073/pnas.89.15.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagrodia S, Taylor SJ, Shalloway D. Mol. Cell. Biol. 1993;13:1464–1470. doi: 10.1128/mcb.13.3.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stover DR, Liebetanz J, Lydon NB. J. Biol. Chem. 1994;269:26885–26889. [PubMed] [Google Scholar]
- 21.Bagrodia S, Chackalaparampil I, Kmiecik TE, Shalloway D. Nature. 1991;349:172–175. doi: 10.1038/349172a0. [DOI] [PubMed] [Google Scholar]
- 22.Kaech S, Covic L, Wyss A, Ballmer-Hofer K. Nature. 1991;350:431–433. doi: 10.1038/350431a0. [DOI] [PubMed] [Google Scholar]
- 23.Taylor SJ, Shalloway D. Bioessays. 1996;18:9–11. doi: 10.1002/bies.950180105. [DOI] [PubMed] [Google Scholar]
- 24.Zheng X-M, Shalloway D. EMBO J. 2001;20:6037–6049. doi: 10.1093/emboj/20.21.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.den Hertog J, Sap J, Pals CE, Schlessinger J, Kruijer W. Cell Growth Differ. 1995;6:303–307. [PubMed] [Google Scholar]
- 26.Tracy S, van der Geer P, Hunter T. J. Biol. Chem. 1995;270:10587–10594. doi: 10.1074/jbc.270.18.10587. [DOI] [PubMed] [Google Scholar]
- 27.Stetak A, Csermely P, Ullrich A, Keri G. Biochem. Biophys. Res. Commun. 2001;288:564–572. doi: 10.1006/bbrc.2001.5811. [DOI] [PubMed] [Google Scholar]
- 28.Field J, Nikawa J, Broek D, MacDonald B, Rodgers L, Wilson IA, Lerner RA, Wigler M. Mol. Cell. Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Southern PJ, Berg P. J. Mol. Appl. Genet. 1982;1:327–341. [PubMed] [Google Scholar]
- 30.Shalloway D, Coussens PM, Yaciuk P. Proc. Natl. Acad. Sci. U. S. A. 1984;81:7071–7075. doi: 10.1073/pnas.81.22.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kmiecik TE, Shalloway D. Cell. 1987;49:65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- 32.Stetak A, Lankenau A, Vantus T, Csermely P, Ullrich A, Keri G. Biochem. Biophys. Res. Commun. 2001;285:483–488. doi: 10.1006/bbrc.2001.5199. [DOI] [PubMed] [Google Scholar]
- 33.Livneh E, Fishman DD. Eur. J. Biochem. 1997;248:1–9. doi: 10.1111/j.1432-1033.1997.t01-4-00001.x. [DOI] [PubMed] [Google Scholar]
- 34.Black JD. Front. Biosci. 2001;5:406–423. [Google Scholar]
- 35.Pearson RB, Kemp BE. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- 36.Rahuel J, Gay B, Erdmann D, Strauss A, Garcia-Echeverria C, Furet P, Caravatti G, Fretz H, Schoepfer J, Grütter MG. Nat. Struct. Biol. 1996;3:586–589. doi: 10.1038/nsb0796-586. [DOI] [PubMed] [Google Scholar]
- 37.Ogura K, Tsuchiya S, Terasawa H, Yuzawa S, Hatanaka H, Mandiyan V, Schlessinger J, Inagaki F. J. Mol. Biol. 1999;289:439–445. doi: 10.1006/jmbi.1999.2792. [DOI] [PubMed] [Google Scholar]
- 38.Waksman G, Shoelson SE, Pant N, Cowburn D, Kuriyan J. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90405-f. [DOI] [PubMed] [Google Scholar]
- 39.Gilmer T, Rodriguez M, Jordan S, Crosby R, Alligood K, Green M, Kimery M, Wagner C, Kinder D, Charifson P, Hassell AM, Willard D, Luther M, Rusnak D, Sternbach DD, Mehrotra M, Peel M, Shampine L, Davis R, Robbins J, Patel IR, Kassel D, Burkhart W, Moyer M, Bradshaw T, Berman J. J. Biol. Chem. 1994;269:31711–31719. [PubMed] [Google Scholar]
- 40.Hardie G, Hanks S, editors. The Protein Kinase Facts Book: Protein-Serine Kinases. Academic Press, Inc.; San Diego, CA: 1995. pp. 240–242. [Google Scholar]