Abstract
Objective:
To evaluate wound healing activity of ethanolic extract of Jasminum grandiflorum Linn. (J. grandiflorum) flowers in diabetic rats.
Materials and Methods:
Streptozotocin-induced diabetic Wistar albino rats were divided into six groups (n=6).Three groups – diabetic control, positive control (that received Glibenclamide) and treatment (that received J. grandiflorum Linn. Flower extract) were operated for excision wounds (EW). These groups were evaluated for wound contraction and re-epithelization. The other three groups were operated for incision wounds (IW) and dead space wounds (DW). Incision and dead space wounds were produced in the same rats. IWs were analyzed for wound breaking strength and the granulation tissues from DWs were analyzed for dry weight, hydroxyproline content, and histology.
Results:
IWs and DWs showed significant improvement in wound breaking strength (265.8±10.4 vs 332.5±8.2; p<0.05), granulation tissue dry weight (26.1±0.6vs 40.4±0.3; p<0.01) and hydroxyproline content (19.3±0.5 vs 32.6±0.8; p<0.01) in treatment group as compared to control group. Neo-angiogenesis was also high in treatment group. Wound contraction was earlier (day 14) in treatment group compared to diabetic control (day 20). No significant improvement was seen in re-epithelization in treatment group.
Conclusion:
Ethanolic extract of J. grandiflorum Linn. flowers increases granulation tissue formation as well as neo-angiogenesis. It also enhances wound contraction; however, re-epithelization was not significantly affected. J. grandiflorum Linn. flowers could be potentially effective in promotion of diabetic wounds healing by increasing granulation tissue formation and enhancing wound contraction; however, further studies are required for its clinical application.
Key Words: Jasminum grandiflorum Linn. Flowers, Streptozotocin-induced diabetes, Granulation tissue
Introduction
The wound healing is a highly dynamic and complex process that involves cellular, physiological and biochemical events, leading to re-establishment of structural integrity and functional restoration of injured tissue. Impaired wound healing in patients with diabetes mellitus (DM) imposes high morbidity and health care cost (Singer and Clark, 1999 ▶; Ramsey et al., 1999 ▶). It is one of the common complications associated with diabetes (Falanga, 2005 ▶). Diabetic patients often ignore lower extremity ulcers, which often, in their severe form, lead to amputation (Anderson, 2003 ▶). Causative mechanisms associated with impaired wound healing in diabetes are micro and macro vascular abnormalities, impaired epithelization and reduced angiogenesis (Traub and Bibber, 1995 ▶).
Jasminum grandiflorum Linn. (“Spanish jasmine” or “Royal jasmine”) is a plant of Oleaceae family. Its classical names are Jati, Malti or Rajputrika (Paarakh and Paarakh, 2009 ▶) and its flowers and leaves are widely used in folk medicine to prevent and treat breast cancers. Flowers are also useful in uterine bleeding when brewed as tonic (Mishra et al., 2010 ▶). J. grandiflorum Linn. contains triterpenoids, alkaloids, tannins, flavonoid, steroid, glycoside, terpenes, resins and salicylic acid (Nayak and Krishna, 2007 ▶; Patil and Saini, 2012 ▶). Moreover, flavonoids and triterpenoids are known to promote the wound-healing process due to their astringent and antimicrobial properties (Villegas et al., 1997 ▶; Tsuchiya et al., 1996 ▶). These compounds have antioxidant and antiulcer activities as well (Umamaheswari et al., 2007 ▶).
Enzymatic wound debridement may be the primary technique in some cases when surgical debridement is not possible, to promote wound healing (Ramundo and Gray, 2008 ▶). Protease enzymes found from various sources like plants, microbes, maggots and animals have been found to be useful in wound debridement (Walsh (Ed), 2003 ▶). A previous study showed that the protease enzymes present in J. grandiflorum Linn. may be responsible for its wound healing property (Vidyalakshmi and Selvi, 2013 ▶). An ethanolic extract of J. grandiflorum Linn. flowers has been found to improve wound healing in the excision, incision and dead space wounds in non-diabetic rats (Nayak and Krishna, 2007 ▶). The present study was designed to evaluate the wound healing effects of ethanolic extract of J. grandiflorum Linn. flowers in diabetic rats.
Materials and Methods
The study was started after obtaining approval from Institutional Animal Ethics Committee (IAEC), Government Medical College, Bhavnagar (GMCB), Gujarat (India) (Approval No. – 26/2012; Pharmacology No. – 24/2012). Wistaralbino rats of either sex (weighing 200 – 350 g) were placed in individual polypropylene cages and acclimatized to laboratory environment for one week. The rats had free access to normal laboratory food and water ad libitum, and were kept under controlled room temperature (25±2 °C with 60–70 % humidity) and 12 hr-12 hr light-dark cycle.
Streptozotocin (STZ) - induced diabetes
A single dose of streptozotocin 50 mg / kg (Alfa Aesar, A Johnson Matthey Company, MA/USA,) was given intraperitoneally to induce diabetes mellitus (Ghori et al., 2014 ▶). After four days, random blood sugar (RBS) was measured from a blood drop collected from the tail vein, using glucometer (Accu – Chek Go, Roche Diagnostic, Germany). The rats showing RBS >300 mg/dl were included in the study. To maintain RBS between 250–350 mg/dl, Insulin Neutral Protamine Hagedorn (NPH) (5 IU/kg; Wosulin, Wockhardt Limited, Aurangabad, India) was given subcutaneously, once a day.
Experimental design, and wound models
Thirty six diabetic Wistar albino rats were divided into six groups (n=6 in each group). Three groups - Group I (Diabetic control), Group II (Glibenclamide –positive control) and Group III (J. grandiflorum Linn. flower- treated group) served as the excision wound groups and rats in these groups received distilled water 1 ml, glibenclamide (SIGMA Life science, New Delhi, India) 0.5 mg/kg and ethanolic extract of J. grandiflorum Linn. flower 250 mg/kg daily till complete healing of the excision wounds. Group IV (Diabetic control), Group V (Glibenclamide – positive control) and Group VI (J. grandiflorum Linn. flower) served as incision and dead space wound groups and received the same doses of respective treatment agents for 11 days as described above. Ethanolic extract of J. grandiflorum Linn. flower was procured from Leopard Investments Ltd., Gujarat, India. Glibenclamide and extracts were dissolved in distilled water and given orally. All the wounds were inflicted under ketamine (75 mg/kg) and xylazine (10 mg/kg)-induced anaesthesia. All aseptic precautions were taken during infliction of wounds.
Excision wound
A full-thickness circular skin (measuring approximately 500 mm2) section was excised from the nape of neck to produce excision wound (Morton and Malone, 1972 ▶).Wound contraction was evaluated by tracing wound margin on transparent plastic sheets. Wound area was measured soon after wounding and on days 3, 7 and 11. Re-epithelization was measured on the 11th day of wounding when epithelization was visible. The plastic sheet was scanned and the wound area was measured using a UTHSCA image analyzer (version 3.00, The University of Texas Health Science Center, San Antanio, USA).Wound closure rate was calculated using the following formula (Ghori et al., 2014 ▶):
Wound closure rate (%) = (Areaday 0−Areaday n / Areaday 0) × 100
Where Areaday 0 =initial wound area on day 0
Areaday n =area on the nth post-wounding day
The wound re-epithelialization was calculated using the formula mentioned below (Ghori et al., 2014 ▶):
Re−epithelialization (%) on the 11thday = Total wound area (%) −Wound area not covered with epidermis (%).
Re-sutured incision and dead space wound
Full-thickness para-vertebral incisions of 5-cm length were made on either side of the vertebral column and sutured with black silk 4.0 sutures (Ehrlich and Hunt, 1969 ▶). Dead space wounds were produced in the same rats by inserting and suturing sterile grass pith (2.5 cm×0.3 cm) in the loose areolar tissue of the groins on either side. A sterile cotton pellet (weighing 10 mg) was inserted and sutured in loose areolar tissue of axillary region of either side. The sutures were removed on the 7th day of wounding in incision wounds and on the 11th day in dead space wounds. The incision wound breaking strength was measured on the 11th post-wounding day by constant water flow technique described by Lee (Lee, 1968 ▶) under anaesthesia. The rats were sacrificed with high dose of ketamine and xylazine after measuring wound breaking strength. The granulation tissue formed on grass piths was utilized for the hydroxyproline estimation (Woessner, 1961 ▶) and histological examination. The hydroxyproline content was expressed as μg/100 mg of granulation tissue. The cotton pellets were excised from axillary region and dried overnight at 60oC in hot air oven. The weight of dried cotton pallet along with granulation tissue was expressed as mg / 100 g body weight (Dipasquale and Meli, 1965 ▶).
Haematoxylin and Eosine (H & E)-stained sections of granulation tissues were semi-quantitatively analyzed by a pathologist and given a grade of 1 – 4 for the presence of polymorphonuclear cells, macrophages, fibroblasts and neo-angiogenesis (Abramov et al., 2007 ▶).
Statistical analysis
All statistical analyses were done by using Graphpad instat demo version 3.0. The data were expressed as mean±Standard Error of Mean (SEM). One way analysis of variance (ANOVA) followed by Tukey-Kramer test for parametric variables and Kruskal-Wallis followed by Dunn’s multiple comparison test for non-parametric variables were used to compare differences among means of different groups. Statistically significant differences were considered if p<0.05.
Results
Excision wound
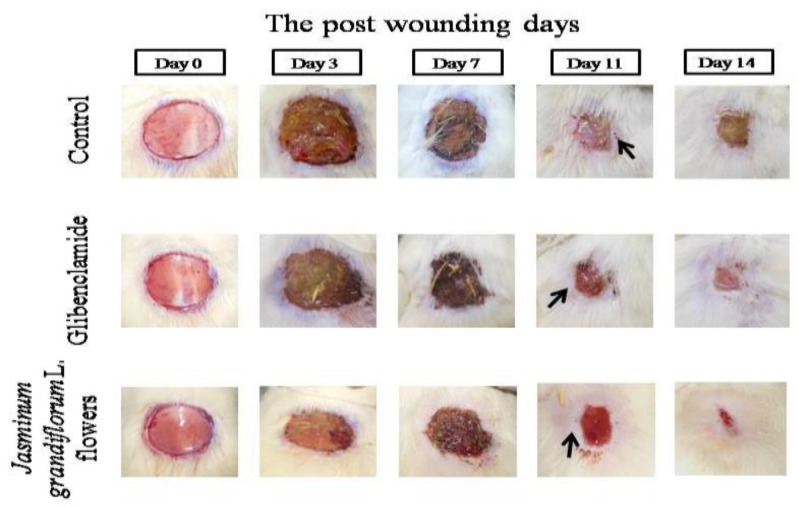
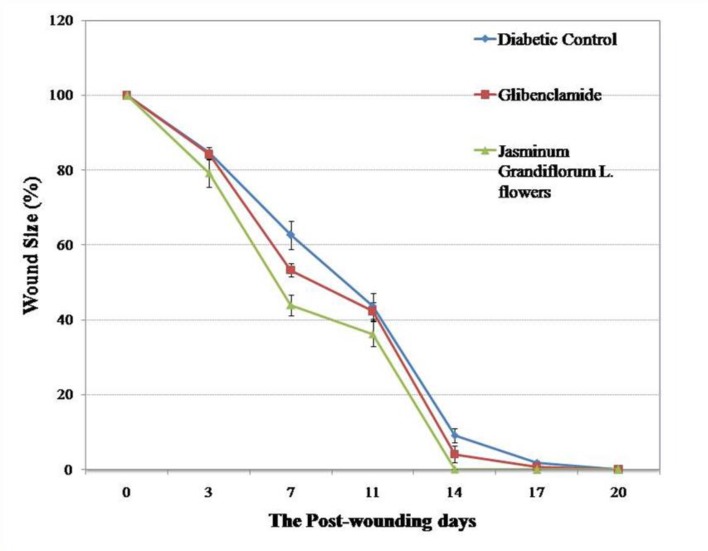
The wound closure in all the groups is shown in Figure 1. Wound contraction was significantly higher in treatment group than diabetic control group on days 7 and 14 but it could not reach a statistically significant difference on day 11 (Table 1). Re-epithelialization on the 11th post-wounding day was non-significantly higher than diabetic and positive control (Table 1). Initially, the wound closure was rapid (owing to wound contraction) followed by relatively slow healing as seen in Figure 2. Complete healing of wound was observed on the 14th day in treatment group, on the 17th day in positive control and on the 20th day in diabetic control. There was no significant difference in re-epithelialization between the treatment and positive as well as diabetic control group.
Figure 1.
The excision wound healing time course noted on post-wounding days 0, 3, 7, 11 and 14
Table 1.
Effect of ethanolic extract of Jasminum grandiflorum Linn. flowers on excision wound model
|
Groups
(n=6) |
Wound closure (%)
|
Re-epithelialization
(%) 11 th Day |
||||
|---|---|---|---|---|---|---|
| 3rd Day | 7th Day | 11th Day | 14th Day | 17th Day | ||
| Diabetic control | 15.3±1.4 | 37.3±3.8 | 56.3±3.4 | 90.7±1.9 | 98.2±0.5 | 56.01±2.5 |
| Glibenclamide | 15.7±1.2 | 46.7±1.8 | 57.6±2.5 | 95.9±2.2 | 99.3±0.4 | 63.2±1.8 |
| Jasminum grandiflorum Linn. flowers | 20.8±3.6 | 56.0±2.8* | 63.7±3.3 | 99.9±0.01* | 100±0.0 | 64.5±3.5 |
n=6 in each group. Values are represented as mean±SEM;
p<0.05 as compared to diabetic control (Tukey Kramer multiple comparison test).
Figure 2.
The wound healing curve shows the percentage of excision wound closure over the time period
Re-sutured incision and dead space wound
Mean incision wound breaking strength on the 11th post-wounding day and granulation tissue dry weight in treatment group were significantly higher as compared to diabetic control group (Table 2). There was no significant difference in these parameters between treatment and positive control (glibenclamide) group. The hydroxyproline content in granulation tissue in treatment group was significantly higher than both the diabetic control and glibenclamide group (Table 2).
Table 2.
Effect of ethanolic extract of Jasminum grandiflorum Linn. flowers on incision and dead space wound model.
| Group | Incision wound breaking strength (g) |
Granulation tissue dry weight
(mg/100 g body weight) |
Hydroxyproline (µg/100 mg granulation tissue) |
|---|---|---|---|
| Diabetic control | 265.8±10.4 | 26.1±0.6 | 19.3±0.5 |
| Glibenclamide | 369.2±8.9* | 37.5±0.9* | 24.4±0.9 |
| Jasminum grandiflorum Linn. flowers | 332.5±8.2* | 40.4±0.3* | 32.6±0.8*# |
n=6 in each group. Values are represented as mean±SEM.
p<0.05 as compared to diabetic control and
p<0.05 as compared to glibenclamide (Tukey Kramer multiple comparison test).
A semi-quantitative histological analysis of granulation tissue obtained on 11th post-wounding day did not show any difference in any of the parameters examined except for the neo-angiogenesis, which was higher in treatment group compared to diabetic as well as positive control group (Table 3).
Table 3.
Semi-quantitative evaluation of histological changes in granulation tissue in dead space wound model
| Histological Change |
Groups
|
|||
|---|---|---|---|---|
| Diabetic control | Glibenclamide | Jasminum grandiflorum Linn. flowers | ||
| Neutrophils | 1.0±0.36 | 1.5±0.55 | 0.9±0.2 | |
| Macrophages | 0.3±0.21 | 0.8±0.3 | 1.0±0.0 | |
| Fibroblasts | 2.5±0.42 | 1.4±0.2 | 3±0.36 | |
| Neo-angiogenesis | 1.8±0.54 | 1.2±0.16 | 2.5±0.22 | |
n=6 in each group. Values are represented as mean±SEM (Dunn multiple comparison test).
Discussion
This study evaluated wound healing activity of ethanolic extract of J. grandiflorum Linn. flowers in diabetic Wistar albino rats based on previous observations such as significant anti-lipid peroxidative effect and improvement in the antioxidant defence system (Kolanjiappan and Manoharan, 2005 ▶). Besides, it has been shown that extract of J. grandiflorum Linn. flower contains oleacein which has angiotensin converting enzyme (ACE) inhibitory property and ACE inhibitors are patented for their angiogenesis stimulating property (Somanadhan et al., 1998 ▶; Isner, 2001 ▶). J. grandiflorum Linn. flowers has been studied for wound healing activity in non-diabetic wounds (Nayak and Krishna, 2007 ▶); however, to the best of our knowledge, the present study is the first one which evaluates wound healing activity of J. grandiflorum Linn. flowers in diabetic wounds.
In the present study, we used a STZ-induced diabetes model as a well-established model to study diabetic wound healing (Sumi et al., 2014 ▶). STZ-induced diabetes model has been shown to exhibit increased superoxide levels (Luo et al., 2004 ▶). Furthermore, it has been demonstrated that in STZ-induced diabetes, angiogenesis is impaired (Teixeira et al., 1999 ▶). Thus, STZ-induced diabetes model provides suitable opportunity to study the effects of a given agent on diabetic wound healing. Glibenclamide was used as positive control as it has been demonstrated that lowering the blood glucose in diabetes helps to promote wound healing (McMurry, 1984 ▶). This positive control group helps to differentiate if ethanolic extract of J. grandiflorum Linn. flowers have any additional advantage in promoting diabetic wound healing over blood glucose lowering agents.
In excision wound group, wound contraction, on the 7th and 14th post-wounding days was significantly more than diabetic control group while on the 11th post-wounding day, it was non-significantly more than diabetic and positive control group. This is in accordance with a previous study which showed significant wound contraction as compared to non-diabetic control (Nayak and Krishna, 2007 ▶). Re-epithelialization on the 11th post-wounding day was higher than diabetic control but could not reach a statistically significant difference. Both of the parameters of excision wound healing i.e. wound contraction and wound re-epithelialization, did not show any significant improvement as compared to glibenclamide which suggests that ethanolic extract of J. grandiflorum Linn. flowers has no additional advantage over blood glucose lowering agents.
Ethanolic extract of J.grandiflorum Linn. flowers significantly increased incision wound breaking strength, granulation tissue dry weight and hydroxyproline contents compared to control. Furthermore, J.grandiflorum Linn. significantly increased hydroxyproline content in granulation tissue compared to glibenclamide group. These findings are also in accordance with a previous study (Nayak and Krishna, 2007 ▶). Hyperglycemia associated with diabetes mellitus is responsible for the generation of reactive oxygen species (ROS), which in turn create excessive oxidative stress (Schmidt et al., 1994 ▶). Increased oxidative stress leads to activation of matrix metalloproteinases (MMP) which increases collagen degradation and decreases collagen synthesis (Siwik et al., 2001 ▶).The antioxidant property of J. grandiflorum Linn. could decrease collagen degradation and increase collagen synthesis which can explain increased dry granulation tissue weight and hydroxyproline content of granulation tissue in treatment group (Umamaheswari et al., 2007 ▶).
Neo-angiogenesis is an important factor in wound healing as it provides necessary elements to the wound bed and is necessary for sustaining the newly formed granulation tissue (Kleinman and Malinda, 2000 ▶). Extracts of J. grandiflorum Linn. are found to have oleacein which has ACE inhibitory property (Somanadhan et al., 1998 ▶). The angiogenesis stimulating property of ACE inhibitors is patented with US Patent No. 6,191,144 B1, 2001 (Isner M, 2001 ▶). Thus, oleacein component of J. grandiflorum Linn. extract may explain improved neo-angiogenesis in granulation tissue obtained from treatment group.
The methods used for estimation of re-epithelialization in excision wound and hydroxyproline content in granulation tissue, are relatively crude and less sensitive, and hence, limit interpretation of the results. Different doses of extract would be more explanatory; however, we used a single dose in our study, which is a limitation of our study. Furthermore, immunostaining using a blood vessel marker like CD31 could have been more informative for evaluation of angiogenesis in granulation tissue. However, due to limited resources, it was not examined in our setup.
This study demonstrated that J. grandiflorum Linn. significantly enhances wound contraction and granulation tissue formation in diabetic wounds. In addition, it increases new blood vessel formation which results in rapid healing of chronic wounds like diabetic wounds. Thus, it can be useful in accelerating wound healing process in diabetic patients. Further clinical trials in diabetic patients should be done before the clinical application of ethanolic extract of J. grandiflorum Linn. flowers.
Acknowledgment
We are thankful to Leopard Investments Ltd., Vapi, Gujarat for providing us with ethanolic extract of J.grandiflorum Linn. flower. We are also thankful to Department of Dental Diagnostic Science at The University of Texas Health Science Center, San Antonio (UTHSCSA), Texas for developing the UTHSCSA Image Tool and making it freely available for research.
Conflict of interest
Authors declare that they have no conflict of interest.
References
- Abramov Y, Golden B, Sullivan M, Botros SM, Miller J, Alshahrour A, Goldberg R, Sand P. Histologic characterization of vaginal vs abdominal surgical wound healing in a rabbit model. Wound Repair Regen. 2007;15:80–86. doi: 10.1111/j.1524-475X.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- Anderson J. Nitric oxide, atherosclerosis and the clinical relevance of endothelial dysfunction. Heart Fail Rev. 2003;8:71–86. doi: 10.1023/a:1022199021949. [DOI] [PubMed] [Google Scholar]
- Dipasquale G, Meli A. Effect of body weight changes on the formation of cotton pellet-induced granuloma. J Pharm Pharmacol. 1965;17:379–382. doi: 10.1111/j.2042-7158.1965.tb07686.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich P, Hunt K. The effects of cortisone and anabolic steroids on the tensile strength of healing wounds. Ann Surg. 1969;170:203–206. doi: 10.1097/00000658-196908000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- Ghori V, Mandavia R, Patel K, Tripathi CB. Effect of topical nitric oxide donor (0.2 % glyceryltrinitrate) on wound healing in diabetic Wistar rats. Int J Diab Dev Ctries. 2014;34:45–49. [Google Scholar]
- Isner M. Method of using angiotensin converting enzyme inhibitor to stimulate angiogenesis. U.S. Patent No. 2001 6,191,144. [Google Scholar]
- Kleinman K, Malinda M, Mousa SA. Angiogenesis inhibitors and stimulators: potential therapeutic implications. 2000. Role of angiogenesis in wound healing; pp. 102–110. [Google Scholar]
- Kolanjiappan K, Manoharan S. Chemopreventive efficacy and anti-lipid peroxidative potential of Jasminum grandiflorum Linn. on 7,12-dimethylbenz (a) anthracene-induced rat mammary carcinogenesis. FundamClinPharmacol. 2005;19:687–693. doi: 10.1111/j.1472-8206.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- Lee H. Studies on the mechanism of action of salicylate II Retardation of wound healing by aspirin. J Pharm Sci. 1968;57:1042–1043. doi: 10.1002/jps.2600570633. [DOI] [PubMed] [Google Scholar]
- Luo D, Wang Y, Fu WL, Wu J, Chen F. Gene therapy of endothelial nitric oxide synthase and manganese superoxide dismutase restores delayed wound healing in type 1 diabetic mice. Circulation. 2004;110:2484–2493. doi: 10.1161/01.CIR.0000137969.87365.05. [DOI] [PubMed] [Google Scholar]
- McMurry JF Jr. Wound healing with diabetes mellitus Better glucose control for better wound healing in diabetes. SurgClin North Am. 1984;64:769–78. doi: 10.1016/s0039-6109(16)43393-1. [DOI] [PubMed] [Google Scholar]
- Mishra SB, Mukerjee A, Vijayakumar M. Wound healing activity of the aqueous alcoholic extract of Jasminum Grandiflorumlinn leaves. Pharmacologyonline. 2010;3:35–40. [Google Scholar]
- Morton J, Malone H. Evaluation of vulneray activity by an open wound procedure in rats. Arch Int Pharmacodyn Ther. 1972;196:117–126. [PubMed] [Google Scholar]
- Nayak S, Krishna M. Influence of ethanolic extract of Jasminum grandiflorum Linn flower on wound healing activity in rats. Indian J Physiol Pharmacol. 2007;51:189–194. [PubMed] [Google Scholar]
- Paarakh S, Paarakh P. Jasminum grandiflorum Linn (Chameli): Ethnobotany, Phytochemistry and Pharmacology – A review. Pharmacology Online News lett. 2009;2:586–595. [Google Scholar]
- Patil M, Saini R. Anticonvulsant activity of methanolic extract of Jasminum grandiflorum Linn in experimental animals. Res J Pharm Biolog Chem Sci. 2012;3:43–49. [Google Scholar]
- Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner E. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–387. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- Ramundo J, Gray M. Enzymatic wound debridement. J Wound Ostomy Continence Nurs. 2008;35:273–280. doi: 10.1097/01.WON.0000319125.21854.78. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Hori O, Brett J, Yan D, Wautier L, Stern D. Cellular receptors for advanced glycation end products Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994;14:1521–1528. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- Singer J, Clark A. Cutaneous Wound Healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- SiwikA , Pagano J, Colucci S. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- Somanadhan B, Smitt W, George V, Pushpangadan P, Rajasekharan S, Duus O, Nyman U, Olsen C, Jaroszewski J. Angiotensin converting enzyme (ACE) inhibitors from Jasminum azoricum and Jasminum grandiflorum. Planta Medica. 1998;64:246–250. doi: 10.1055/s-2006-957419. [DOI] [PubMed] [Google Scholar]
- Sumi Y, Ishihara M, Kishimoto S, Takikawa M, Hattori H, Takikawa M, Azuma R, Nakamura S, Fujita M, Kiyosawa T. Effective wound healing in streptozotocin-induced diabetic rats by adipose-derived stromal cell transplantation in plasma-gel containing fragmin/protamine microparticles. Ann Plast Surg. 2014;72:113–120. doi: 10.1097/SAP.0000000000000014. [DOI] [PubMed] [Google Scholar]
- Teixeira S, Caliari V, Rocha A, Machado D, Andrade P. Aminoguanidine Prevents impaired healing and deficient angiogenesis in diabetic rats. Inflammation. 1999;23:569–581. doi: 10.1023/a:1020246624605. [DOI] [PubMed] [Google Scholar]
- Traub O, Bibber R. Role of Nitric Oxide in Insulin-Dependent Diabetes mellitus related Vascular Complication. West J Med. 1995;162:439–445. [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya H, Sato M, Miyazaki T, Fujiwara S, Tanigaki S, Ohyama M, et al. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J Ethnopharmacol. 1996;50:27–34. doi: 10.1016/0378-8741(96)85514-0. [DOI] [PubMed] [Google Scholar]
- Umamaheswari M, Ashokkumar K, Rathidevi R, Sivashanmugam T, Subbhardadevi V, Ravi K. Anti ulcer and in vitro antioxidant activities of Jasminum grandiflorum L. J Ethnopharmacol. 2007;110:464–470. doi: 10.1016/j.jep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Vidyalakshmi A, Selvi E. Protease Activity of Floral Extracts of Jasminum grandiflorum L A Wound Healing Herb. J Med Plants Stud. 2013;1:11–15. [Google Scholar]
- Villegas F, Fernández D, Maldonado H, Torres R, Zavaleta A, Vaisberg J, Hammond G. Evaluation of the wound-healing activity of selected traditional medicinal plants from Peru. J Ethnopharmacol. 1997;55:193–200. doi: 10.1016/s0378-8741(96)01500-0. [DOI] [PubMed] [Google Scholar]
- Walsh G (Ed), editor. Debriding agents, in Biopharmaceuticals: Biochemistry and Biotechnology. 2nd Ed. John Wiley & Sons Ltd; 2003. 398 pp. [Google Scholar]
- Woessner JFJr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]