Abstract
Background:
Vascular Endothelial Growth Factor and NK cells have an interrelated role in angiogenesis that is critical for placentation and success of in vitro fertilization. An attempt was made to assess a possible relationship between the two in this study.
Methods:
A case control study was performed comparing the serum levels of VEGF-A and its receptor VEGF-R1 with levels of NK cells, activated NK cells and NK cytotoxicity in 62 women with Repeated Implantation Failure (RIF). The healthy control group consisted of 72 women of similar age, without known issues in achieving pregnancy or evidence of autoimmunity. Levels of VEGF-A and VEGF-R1 were quantified by ELISA methods with standard curve interpolation. NK cell subsets were determined with flow cytometry using fluorescent-tagged anti-CD56, anti-CD16, anti-CD3 and anti-CD69. NK cytotoxicity was performed by incubating peripheral blood mononuclear cells and K562 cultured cells with propidium iodide, steroid, intralipid and intravenous immunoglobulin, using previously described methods. Statistical analysis involved Mann-Whitney-U and Spearman’s rank correlation testing with p-values defined as <0.05.
Results:
It was found that VEGF-A levels were significantly raised in women with RIF compared to healthy controls (362.9 vs. 171.6 pg/ml, p<0.0001), with no difference in VEGF-R1 levels between groups (1499 vs. 1202 pg/ml, p=0.4082). There was no correlation between VEGF-A or VEGF-R1 and the absolute levels of circulating NK cells, CD69 activated NK cells or NK cytotoxicity.
Conclusion:
The absence of correlation between VEGF-A or VEGF-R1 and NK cells suggests VEGF secretion and regulation is independent of NK cell activity in RIF.
Keywords: NK cell cytotoxicity, Recurrent failed IVF, Soluble fms-like tyrosine kinase 1 (s-FLT-1), Vascular endothelial growth factor
Introduction
Recurrent in vitro fertilization failure or Repeated Implantation Failure (RIF) has been defined in the UK as failure of pregnancy after 2–6 IVF cycles, of which 3 of those would be fresh cycles (rather than involving frozen embryos) involving transfer of more than 6 embryos (1). The precise definition remains somewhat vague and has been recently debated (2). Recurrent spontaneous abortion (RSA) is defined as 2 or more miscarriages occurring consecutively in the first or early second trimesters of pregnancy. It can affect up to 1 in 20 women of reproductive age and in half of these cases, the precise cause remains unclear despite intensive investigation. It is now thought that a hypoxic placental environment caused by impaired angiogenesis (3) and related to endothelial dysfunction may play a significant role here (4, 5).
The Vascular Endothelial Growth Factor (VEGF) family are potent angiogenic and vasculogenic growth factors. VEGF has widespread effects not limited to the circulatory system. These include bone formation, haematopoiesis and tissue remodelling. These functions are reflected in the widespread production of VEGF by tumour cells, endothelial cells, macrophages, platelets and keratinocytes (6). In relation to reproduction, VEGF is a key mediator of angiogenesis within the uterus, endometrium and placenta (7). VEGF-A is the primary form of VEGF expressed in the placenta, which is involved in placental angiogenesis as well as the spiral artery remodelling process (8). Both processes are critical for proper nourishment of the developing embryo. The key stimulus for VEGF-A mRNA production is hypoxia, which occurs via hypoxia-inducible factor 1α (9); VEGF-A primarily binds to two receptors, receptor-1, also known as FLT-1 (fms-like tyrosine kinase 1) and receptor-2, also known as KDR (kinase insert domain receptor)- the former was discussed in more detail throughout this article (10). R1/FLT-1 is expressed on monocytes/macrophages as well as placental trophoblast cells. Its expression is upregulated in the presence of hypoxia (11).
Uterine NK cells are of critical importance in normal pregnancy for the processes of spiral artery remodelling and placentation (12, 13). They are also involved in the regulation of inflammatory Th17 cells at the maternal-fetal interface in the placenta (14). However, increased numbers and activation of peripheral NK cells (15–18), endometrial NK cells (19) and reduced numbers of decidual NK cells with KIRs specific for HLA-C (20) have all been associated with recurrent IVF failure and also recurrent miscarriage (RM). The numbers of decidual NK cells with ultrastructural changes and their distribution along uterine blood vessels also appear to be increased in women with RM (21).
Reduced VEGF levels have been considered important in RM (22) and RIF and preeclampsia. Soluble FLT, formed by alternative splicing of the R1/FLT-1 mRNA, may have a similarly relevant role. s-FLT is known to have potent anti-angiogenic properties, with animal models demonstrating its ability to inhibit both VEGF-A and placental growth factor (PlGF) and induce pre-eclampsia (23, 24). Indeed, R1/FLT-1 and sFLT-1 share the same ligand binding domain, but sFLT-1 lacks the membrane-spanning and intracellular domains. Several reports have found circulating levels of sFLT to be raised in women with threatened abortion (25) or RM (26). Detecting circulating complexes of VEGF and sFLT that may affect the analysis of circulating levels of both factors has not been reported. From a genetic perspective, several SNPs that are associated with reduced VEGF functional activity have been variously reported from fertility centers around the world (27). It is presently unclear whether the relevant foetal VEGF SNPs are more important than the maternal ones (28).
In mice, decidual NK cells (i.e. at the fetal-maternal interface) have been shown to secrete VEGF and were considered to be derived from precursors recruited to the uterus (29). Additionally, pregnant Ly49 knock-out mice had reduced NK cell infiltration and impaired decidual angiogenesis that was associated with reduced expression of VEGF (30). In humans, however, decidual NK cells have been shown to express VEGF and this was little altered between the first and second trimester (31). In the peripheral blood, NK cells have been recently reported to express VEGF and reduced levels were noted in pregnant women with gestational diabetes (32), a group known to have impaired reproductive outcome.
From the above, it appears that both NK cells and VEGF are important for placentation. Importantly, both decidual and peripheral blood NK cells are capable of secreting VEGF. Therefore, in this study, an attempt was made to examine the total peripheral blood NK cell count, CD69 activated NK cell count and NK cell cytotoxicity with the circulating levels of VEGF-A and VEGF-R1 in women with RIF to see if these may be related.
Methods
Study Design and Subject Selection:
A case-control design was used to evaluate the serum levels of VEGF-A, and soluble VEGF-R1 in women with recurrent IVF failure (RIF) and healthy controls. The patient group consisted of sixty two women referred to the Reproductive Immunology Centre for immunological evaluation of infertility or recurrent pregnancy loss. Referral came via the fertility clinic at the Lister Hospital in London. RIF was defined as the absence of pregnancy following 3 cycles of IVF using good quality fresh embryos.
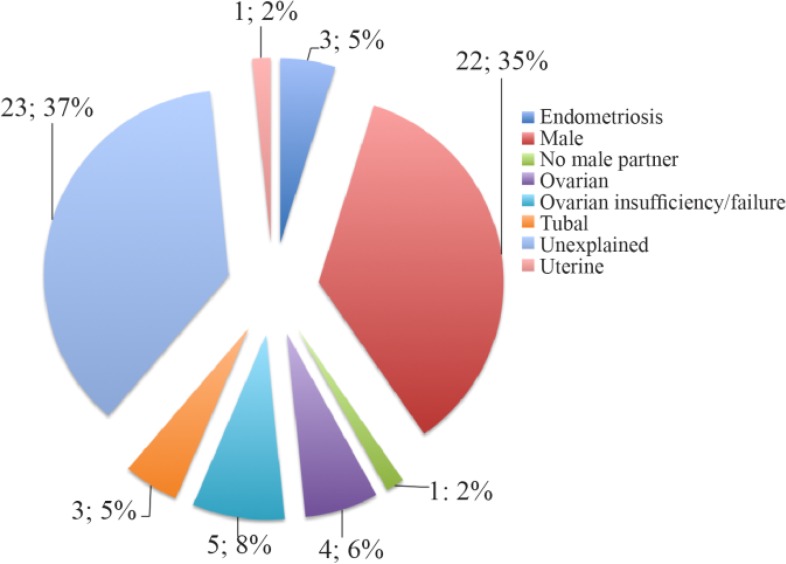
Table 1 details the demographic and clinical features of the patient group including information on IVF cycles undertaken after sample collection and outcomes. Samples were collected in the first half of the menstrual cycle and at least two months after any hormonal reproductive manipulation. Figure 1 also shows the primary diagnoses attributed to these women, with regard to their subfertility status. Women with positive tests of autoimmunity, endocrine dysfunction or an alternative defined cause for their subfertility were excluded. Thus, all women had no clinical evidence of a systemic connective tissue disorder or anti-phospholipid antibody syndrome, negative anti-nuclear antibodies on Hep2 cells, no anti-cardiolipin or anti-thyroid peroxidase antibodies, and had normal T4 and TSH.
Table 1.
RIF group demography and outcomes
| Number of patients, n | 62 |
| Median Age, years (min, max) | 39 (29, 45) |
| Number of Pregnancies following IVF cycles, n (%) | 39 (41) |
| Number of Live Births resulting from pregnancies, n (%) | 24 (61.54) |
| Number of Miscarriages resulting from pregnancy, n (%) | 15 (38.46) |
Figure 1.
Primary Diagnosis of Infertility in the RIF Group
The healthy control group comprised seventy two women of comparable reproductive age, without evidence of reproductive difficulty and no evidence of autoimmunity as indicated above for the RIF group. They had normal full blood count and normal ESRs with no evidence of inflammation. Blood samples from the control group were handled and collected identically to the RIF group.
VEGF-A and VEGF-R1 quantification:
The serum levels of both VEGF-A and VEGF-R1 were measured by ELISA, using commercially available kits (VEGF-A was detected by a Platinum ELISA kit; BMS277/2TEN, affymetrix eBioscience, and VEGF-R1 by a duoset ELISA: DY321B, R&D Systems). Their levels were determined by use of a standard curve and interpolation of concentrations on statistical software (GraphPad). The VEGF-R1 assay detected both cell surface VEGF receptor 1 as well as the circulating level of s-FLT-1.
NK Cell Subtype Quantification:
This was undertaken according to our previously published methods (33). The method utilized whole blood analysis with red cell lysis using Easylyse (BD Biosciences) and with anti-CD56 PE, anti-CD16 FITC, anti-CD3 PE Cy5 and anti-CD69 APC (all supplied by BD Pharmingen). Flow cytometric analysis was undertaken on a Facscalibur (BD Biosciences), and using Cellquest Pro software. A minimum number of 10,000 cells were counted.
NK Cell Cytotoxicity Assay:
This was performed according to standard methods in the St Helier Immunology Laboratory in Carshalton, Surrey. This protocol was based on the methods stated by Gilman-Sachs et al. (34). Briefly, PBMCs are isolated from whole blood, resuspended in culture medium and incubated for one hour in a 37°C and 5% CO2 incubator. The cells are then washed in pre-warmed culture medium, and the viable cell count is adjusted to a concentration of 5×106/ml. Meanwhile, K562 cells are adjusted to a concentration of 2×105/ml and subsequently working concentrations of Propidium Iodide are prepared. Different combinations of culture medium, PBMCs, K562 cells and Propidium Idioide were then arranged to achieve Effector: Target Cell Ratios of 50:1, 25:1 and 12:1. Following a 2 hr incubation in the 37°C 5% CO2 incubator, each tube was then analyzed flow cytometrically.
Statistical Analysis:
Data was analyzed using GraphPad with Mann Whitney U test for unpaired non-parametric data and Spearman’s rank correlation for detecting any association between the VEGF-A/VEGF-R1 and age, NK cell activation, NK cell phenotype and also NK cell cytotoxicity results.
Results
VEGF-A Levels:
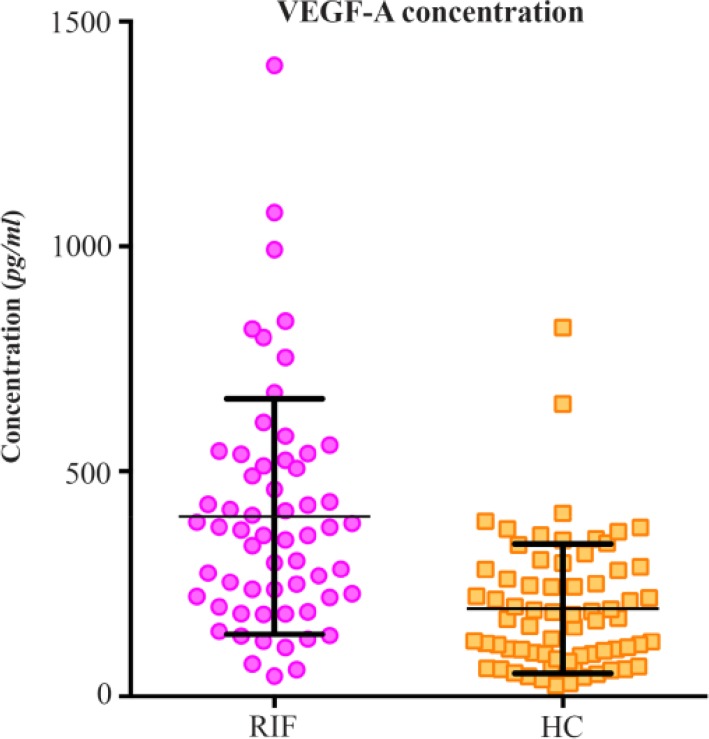
The serum VEGF-A, measured in 62 women with RIF was significantly raised compared to the 72 healthy control (HC) women [median 362.9 pg/ml (IQR 219.9–521.1 pg/ml), vs. 171.6 pg/ml (IQR 91.99–280.8 pg/ml), p<0.0001] (Figure 2). There was no relationship evident between age and VEGF-A levels in both groups (Spearman correlation r values were 0.1134 and 0.003348 with corresponding two-tailed p-values of 0.3881 and 0.9782 in the RIF and the HC groups, respectively).
Figure 2.
Serum VEGF-A Concentration in the Recurrent IVF failure and Healthy Control Groups
VEGF-R1 Levels:
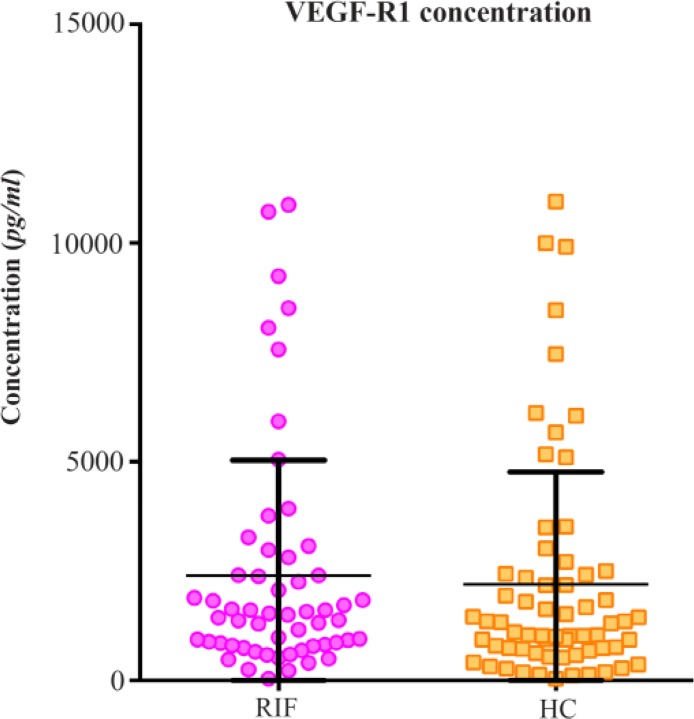
The serum soluble VEGF-R1 levels in the RIF group were similar to that in the HC group depicted in figure 3 [median 1499 pg/ml (IQR 812.1–2608 pg/ml) vs. 1202 pg/ml (IQR 691.6–2429 pg/ml)]. As such there was no statistical significance seen with Mann-Whitney testing, with a p value of 0.4082.
Figure 3.
Soluble VEGF-R1 Concentration in the Recurrent IVF failure and Healthy Control Groups
NK Cell Phenotyping and Activation:
The median total NK count was 9.25% (IQR 7–12.95%), the median NK CD69 count was 0.555×106/L (IQR 0.4–0.7925×106/L), and finally the median NK CD69bright levels were 0.14×106/L (IQR 0.07–0.3225×106/L). There was no correlation between these results and the corresponding VEGF-A and VEGF-R1 levels. Spearman correlation testing for VEGF-A levels compared to total NK counts, total NK CD69 counts and total NK CD69bright counts were as follows; r values with corresponding 2-tailed p-values of −0.1029 (p=0.4340), 0.1409 (p=0.2830) and 0.1803 (p=0.1680), respectively.
The Spearman correlation values for the VEGF-R1 levels with total NK counts, total NK CD69 and total NK CD69bright were as follows; r=0.1754 (p=0.1918), r=− 0.1605 (p=0.2329) and r=0.1004 (p=0.4576), respectively.
NK Cell Cytotoxicity:
The median killing was 27.5% (IQR 22–38%) at 50:1, 19.5% (IQR 16–29%) at 25:1 and 12% (IQR 8.75–18%) at 12.5:1. There was no correlation between the NK cytotoxicity values at each effector: target ratio and the corresponding VEGF-A and VEGF-R1 levels. For VEGF-A and NK, cytotoxicity killing ratios were 50:1, 25:1 and 12.5:1, r=0.04334 (p= 0.7423), r=− 0.02273 (p=0.8631) and r=− 0.02247 (p=0.8647), respectively. The same values for VEGF-R1 were r=0.1925 (p=0.1515) at 50:1, r= 0.1794 (p=0.1817) at 25:1, and r=0.1255 (p= 0.3525) at 12.5:1.
Discussion
Successful pregnancy despite multiple embryo transfer remains stubbornly low at roughly one third of all IVF cycles. Unsuccessful IVF in some women may be attributed to a failure of implantation and possibly very early miscarriage. This may unfortunately be repeated in several consecutive cycles. There are several reports of changes in decidual vascularization in women with miscarriage. Compared to matched controls, the latter have shown fewer vessels of larger circumference and correlation with the expression of certain angiogenic factors and proteases (35).
The endometrial expression of pro-inflammatory cytokines such as IL-1β and TNF-α and also PGE2 have been found to be up-regulated with a reduction in the anti-inflammatory IL-10 in women with idiopathic RM. Furthermore, VEGF, endothelial nitric oxide synthase, nitric oxide and adrenomedullin were found to be down-regulated and correlated with impaired subendometrial blood flow (36). Thus, vasoactive factors are considered of major importance in early placentation and the delivery of oxygen and nutrients to the developing embryo/fetus.
Overall, there is general agreement that VEGF is particularly important in new vessel formation and placentation, which if inadequate, predisposes to miscarriage and other obstetric complications. This is supported by the reduced immunochemical expression of VEGF and its receptors in placental and decidual tissue of women with spontaneous incomplete abortion and missed abortion (37). This was also noted by Lash et al. (38), who in addition observed increased immunochemical staining for VEGF-R1 and VEGF-R2 in the vascular smooth muscle cells of cycling endometrium of women with a history of RM. Corroboration of these findings is also provided by much of the VEGF genetic data in women with RM which show an increased prevalence of those SNPs associated with reduced VEGF. For at least the −460T/C, 398G/A and − 583T/C genotypes there was a significant correlation with serum VEGF levels which overall was reduced in women with recurrent spontaneous miscarriage (39).
Increased plasma VEGF but normal levels of both VEGF-R1 and sFLT-1 were observed in women with RIF in whom only a very small proportion had RM. Unfortunately, the proportion of the detected VEGF and VEGF-R1 was impossible to be determined that was present as a free protein and was present as VEGF/VEGF-R1 complex. Our efforts to detect the presence of these complexes by coating ELISA plates with antibodies capturing VEGF or VEGF-R1 and then detecting with Alkaline phosphatase conjugated anti-VEGF-R1 or VEGF antibodies produced very low optical density readings. While this may be due to the absence of these complexes, it is of course possible that the assays were ineffective at either the capture or detection phases and there were no positive controls to confirm the working of the assays. Nonetheless, the raised plasma VEGF in our patients with RIF remains unexplained given that RM is generally associated with lowered levels of circulating VEGF and RIF can in some ways be seen as very early miscarriage. However, raised VEGF was also noted by Amirchaghmaghi et al. (40) in the serum of women with RM collected between day 19 and 26 of the menstrual cycle. Similarly, in women with threatened abortion serum VEGF and soluble VEGF-R1 (s-FLT) was significantly elevated relative to healthy control pregnancies of similar gestational age (25). Earlier, Pang et al. (26) found both VEGF and sFLT to be significantly raised in women in early pregnancy compared to those who were not pregnant. Interestingly, the levels of both factors were significantly higher in women who subsequently miscarried. This is in contrast to the reduced levels of serum sFLT reported by Muttukrishna et al. (41) in women with threatened miscarriage compared to pregnant women with a subsequent good outcome. There has also been an inexplicably raised serum VEGF found in women with ectopic pregnancy compared to those with a normal pregnancy (42). Thus, the data surrounding assessment of circulating VEGF and sFLT levels appears to be different to that suggested by genetic studies and from decidual immunochemical expression. It is speculated whether raised levels of circulating VEGF in the presence of normal levels of sFLT in women with RIF may represent compensation for a deficiency of other factors that are necessary for placentation. The latter may include preimplantation factor (43), which interestingly has been shown to reduce NK cell cytotoxicity and NK CD69 expression. Alternatively, angiogenesis is not as important in RIF as it is in RM and maternal immune-mediated embryotoxicity, possibly via activated NK cells (44) or raised Th1 type immunity (45) being a more important factor. Finally, it is of course possible that the raised VEGF-A in the RIF/RM patients may represent an attempt to overcome impaired VEGF-R1 activity that is required for angiogenesis.
It was previously found that women with RIF have significantly increased activated NK cells (18). NK cells have also been shown to express and release VEGF. Thus in this study, the relationship between these important variables was investigated in women with RIF to see if the raised plasma VEGF may perhaps be linked to several NK cell variables. However, no correlation was found between circulating levels of VEGF and the absolute numbers of NK cells, activated NK cells or NK cytotoxicity. Thus, our data suggests that these variables may be acting independently in influencing the success of IVF and that the plasma VEGF may perhaps be produced by cells other than peripheral blood NK cells, such as macrophages and endothelial cells.
While the level of VEGF has been reported as raised in endometriosis, this is unlikely to explain our observation of raised plasma VEGF in women with RIF, as only 4% had endometriosis (46). It is speculated whether the raised VEGF represents a maternal response to support an embryo that is threatened by presently unidentified anti-angiogenic factors. Alternatively, significantly elevated levels of VEGF may disrupt normal angiogenesis through an overstimulation of blood vessels leading to disturbed vascular architecture (47).
In conclusion, a significantly raised level of plasma VEGF was found in women with RIF but without correlation with several NK cell variables. It is presently unclear why plasma VEGF levels are raised in RIF when there is much evidence suggesting impaired angiogenesis in this and in RM.
Footnotes
Conflict of Interest
ASB, MYT, SB and BF are directors of Reproductive Immunology Centre UK (RIC), which offers immune based tests including NK cell analysis in women with re-productive difficulties. There are no conflicts of interest for RAB to declare.
No financial support was utilized in this research.
References
- 1.Tan BK, Vandekerckhove P, Kennedy R, Keay SD. Investigation and current management of recurrent IVF treatment failure in the UK. BJOG. 2005;112 (6):773–80. [DOI] [PubMed] [Google Scholar]
- 2.Polanski LT, Barbosa MA, Martins WP, Baumgarten MN, Campbell B, Brosens J, et al. Interventions to improve reproductive outcomes in women with elevated natural killer cells undergoing assisted reproduction techniques: a systematic review of literature. Hum Reprod. 2014;29(1):65–75. [DOI] [PubMed] [Google Scholar]
- 3.Llurba E, Crispi F, Verlohren S. Update on the pathophysiological implications and clinical role of angiogenic factors in pregnancy. Fetal Diagn Ther. 2015;37(2):81–92. [DOI] [PubMed] [Google Scholar]
- 4.Muttukrishna S, Suri S, Groome N, Jauniaux E. Relationships between TGFbeta proteins and oxygen concentrations inside the first trimester human gestational sac. PLoS One. 2008;3(6):e2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weimar CH, Kavelaars A, Brosens JJ, Gellersen B, de Vreeden-Elbertse JM, Heijnen CJ, et al. Endometrial stromal cells of women with recurrent miscarriage fail to discriminate between high- and low-quality human embryos. PLoS One. 2012;7(7): e41424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy AM, Bouchier-Hayes DJ, Harmey JH. Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells: Autocrine signalling by VEGF. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000–2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6482/ [Google Scholar]
- 7.Li XF, Charnock-Jones DS, Zhang E, Hiby S, Malik S, Day K, et al. Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. J Clin Endocrinol Metab. 2001;86(4):1823–34. [DOI] [PubMed] [Google Scholar]
- 8.Charnock-Jones DS, Kaufmann P, Mayhew TM. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta. 2004; 25(2–3):103–13. [DOI] [PubMed] [Google Scholar]
- 9.Taylor CM, Stevens H, Anthony FW, Wheeler T. Influence of hypoxia on vascular endothelial growth factor and chorionic gonadotrophin production in the trophoblast-derived cell lines: JEG, JAr and BeWo. Placenta. 1997;18(5–6):451–8. [DOI] [PubMed] [Google Scholar]
- 10.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269(43):26988–95. [PubMed] [Google Scholar]
- 11.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272(38):23659–67. [DOI] [PubMed] [Google Scholar]
- 12.Ratsep MT, Felker AM, Kay VR, Tolusso L, Hofmann AP, Croy BA. Uterine natural killer cells: supervisors of vasculature construction in early decidua basalis. Reproduction. 2015;149(2):R91–102. [DOI] [PubMed] [Google Scholar]
- 13.Robson A, Harris LK, Innes BA, Lash GE, Aljunaidy MM, Aplin JD, et al. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J. 2012;26(12):4876–85. [DOI] [PubMed] [Google Scholar]
- 14.Fu B, Li X, Sun R, Tong X, Ling B, Tian Z, et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci USA. 2013;110(3):E231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y, Wang PL. Increased CD56(+) NK cells and enhanced Th1 responses in human unexplained recurrent spontaneous abortion. Genet Mol Res. 2015; 14(4):18103–9. [DOI] [PubMed] [Google Scholar]
- 16.Karami N, Boroujerdnia MG, Nikbakht R, Khodadadi A. Enhancement of peripheral blood CD56 (dim) cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J Reprod Immunol. 2012;95(1–2):87–92. [DOI] [PubMed] [Google Scholar]
- 17.Ghafourian M, Karami N, Khodadadi A, Nikbakht R. Increase of CD69, CD161 and CD94 on NK cells in women with recurrent spontaneous abortion and in vitro fertilization failure. Iran J Immunol. 2014;11(2):84–96. [PubMed] [Google Scholar]
- 18.Thum MY, Bhaskaran S, Abdalla HI, Ford B, Sumar N, Shehata H, et al. An increase in the absolute count of CD56dimCD16+CD69+ NK cells in the peripheral blood is associated with a poorer IVF treatment and pregnancy outcome. Hum Reprod. 2004;19(10):2395–400. [DOI] [PubMed] [Google Scholar]
- 19.Papamitsou T, Toskas A, Papadopoulou K, Sioga A, Lakis S, Chatzistamatiou M, et al. Immunohistochemical study of immunological markers: HLAG, CD16, CD25, CD56 and CD68 in placenta tissues in recurrent pregnancy loss. Histol Histopathol. 2014;29(8):1047–55. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Li YP, Ding B, Zhao YR, Chen ZJ, Xu CY, et al. Recurrent miscarriage is associated with a decline of decidual natural killer cells expressing killer cell immunoglobulin-like receptors specific for human leukocyte antigen C. J Obstet Gynaecol Res. 2014;40(5):1288–95. [DOI] [PubMed] [Google Scholar]
- 21.Almasry SM, Elmansy RA, Elfayomy AK, Algaidi SA. Ultrastructure alteration of decidual natural killer cells in women with unexplained recurrent miscarriage: a possible association with impaired decidual vascular remodelling. J Mol Histol. 2015; 46(1):67–78. [DOI] [PubMed] [Google Scholar]
- 22.Jakovljevic A, Bogavac M, Lozanov-Crvenkovic Z, Milosević-Tosic M, Nikolic A, Mitic G. Early pregnancy angiogenic proteins levels and pregnancy related hypertensive disorders. J Matern Fetal Neonatal Med. 2017;30(5):534–9. [DOI] [PubMed] [Google Scholar]
- 23.Andraweera PH, Dekker GA, Roberts CT. The vascular endothelial growth factor family in adverse pregnancy outcomes. Hum Reprod Update. 2012; 18(4):436–57. [DOI] [PubMed] [Google Scholar]
- 24.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keskin U, Ulubay M, Dede M, Ozgurtas T, Koçyigit YK, Aydin FN, et al. The relationship between the VEGF/sVEGFR-1 ratio and threatened abortion. Arch Gynecol Obstet. 2015;291(3):557–61. [DOI] [PubMed] [Google Scholar]
- 26.Pang L, Wei Z, Li O, Huang R, Qin J, Chen H, et al. An increase in vascular endothelial growth factor (VEGF) and VEGF soluble receptor-1 (sFlt-1) are associated with early recurrent spontaneous abortion. PLoS One. 2013;8(9):e75759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Du C, Li H, Du J, Yan X, Peng L, et al. Association of VEGF genetic polymorphisms with recurrent spontaneous abortion risk: a systematic review and meta-analysis. PLoS One. 2015;10(4): e0123696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yalcintepe SA, Silan F, Hacivelioglu SO, Uludag A, Cosar E, Ozdemir O. Fetal Vegf Genotype is More Important for Abortion Risk than Mother Genotype. Int J Mol Cell Med. 2014;3(2):88–94. [PMC free article] [PubMed] [Google Scholar]
- 29.Chiossone L, Vacca P, Orecchia P, Croxatto D, Damonte P, Astigiano S, et al. In vivo generation of decidual natural killer cells from resident hematopoietic progenitors. Haematologica. 2014;99(3): 448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lima PD, Tu MM, Rahim MM, Peng AR, Croy BA, Makrigiannis AP. Ly49 receptors activate angiogenic mouse DBA+ uterine natural killer cells. Cell Mol Immunol. 2014;11(5):467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Dunk CE, Lye SJ. Sphingosine signalling regulates decidual NK cell angiogenic phenotype and trophoblast migration. Hum Reprod. 2013;28 (11):3026–37. [DOI] [PubMed] [Google Scholar]
- 32.Chiba H, Fukui A, Fuchinoue K, Funamizu A, Tanaka K, Mizunuma H. Expression of Natural Cytotoxicity Receptors on and Intracellular Cytokine Production by NK Cells in Women with Gestational Diabetes Mellitus. Am J Reprod Immunol. 2016;75(5):529–38. [DOI] [PubMed] [Google Scholar]
- 33.Mosimann B, Wagner M, Shehata H, Poon LC, Ford B, Nicolaides KH, et al. Natural killer cells and their activation status in normal pregnancy. Int J Reprod Med. 2013;2013:906813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilman-Sachs A, DuChateau BK, Aslakson CJ, Wohlgemuth GP, Kwak JY, Beer AE, et al. Natural killer (NK) cell subsets and NK cell cytotoxicity in women with histories of recurrent spontaneous abortions. Am J Reprod Immunol. 1999;41(1):99–105. [DOI] [PubMed] [Google Scholar]
- 35.Plaisier M, Dennert I, Rost E, Koolwijk P, van Hinsbergh VW, Helmerhorst FM. Decidual vascularization and the expression of angiogenic growth factors and proteases in first trimester spontaneous abortions. Hum Reprod. 2009;24(1):185–97. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee P, Ghosh S, Dutta M, Subramani E, Khalpada J, Roychoudhury S, et al. Identification of key contributory factors responsible for vascular dysfunction in idiopathic recurrent spontaneous miscarriage. PLoS One. 2013;8(11):e80940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Col-Madendag I, Madendag Y, Altinkaya SÖ, Bayramoglu H, Danisman N. The role of VEGF and its receptors in the etiology of early pregnancy loss. Gynecol Endocrinol. 2014;30(2):153–6. [DOI] [PubMed] [Google Scholar]
- 38.Lash GE, Innes BA, Drury JA, Robson SC, Quenby S, Bulmer JN. Localization of angiogenic growth factors and their receptors in the human endometrium throughout the menstrual cycle and in recurrent miscarriage. Hum Reprod. 2012;27(1):183–95. [DOI] [PubMed] [Google Scholar]
- 39.Almawi WY, Saldanha FL, Mahmood NA, Al-Zaman I, Sater MS, Mustafa FE. Relationship between VEGFA polymorphisms and serum VEGF protein levels and recurrent spontaneous miscarriage. Hum Reprod. 2013;28(10):2628–35. [DOI] [PubMed] [Google Scholar]
- 40.Amirchaghmaghi E, Rezaei A, Moini A, Roghaei MA, Hafezi M, Aflatoonian R. Gene expression analysis of VEGF and its receptors and assessment of its serum level in unexplained recurrent spontaneous abortion. Cell J. 2015;16(4):538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muttukrishna S, Swer M, Suri S, Jamil A, Calleja-Agius J, Gangooly S, et al. Soluble Flt-1 and PlGF: new markers of early pregnancy loss? PLoS One. 2011;6(3):e18041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes da Silva MO, Elito J, Jr, Daher S, Camano L, Fernandes Moron A. Association of serum levels of vascular endothelial growth factor and early ectopic pregnancy. Clin Exp Obstet Gynecol. 2013;40(4):489–91. [PubMed] [Google Scholar]
- 43.Roussev RG, Dons’koi BV, Stamatkin C, Ramu S, Chernyshov VP, Coulam CB, et al. Preimplantation factor inhibits circulating natural killer cell cytotoxicity and reduces CD69 expression: implications for recurrent pregnancy loss therapy. Reprod Biomed Online. 2013;26(1):79–87. [DOI] [PubMed] [Google Scholar]
- 44.Miko E, Manfai Z, Meggyes M, Barakonyi A, Wilhelm F, Varnagy A, et al. Possible role of natural killer and natural killer T-like cells in implantation failure after IVF. Reprod Biomed Online. 2010;21 (6):750–6. [DOI] [PubMed] [Google Scholar]
- 45.Kalu E, Bhaskaran S, Thum MY, Vishwanatha R, Croucher C, Sherriff E, et al. Serial estimation of Th1:th2 cytokines profile in women undergoing in-vitro fertilization-embryo transfer. Am J Reprod Immunol. 2008;59(3):206–11. [DOI] [PubMed] [Google Scholar]
- 46.Vodolazkaia A, Yesilyurt BT, Kyama CM, Bokor A, Schols D, Huskens D, et al. Vascular endothelial growth factor pathway in endometriosis: genetic variants and plasma biomarkers. Fertil Steril. 2016;105(4):988–96. [DOI] [PubMed] [Google Scholar]
- 47.Sergent F, Hoffmann P, Brouillet S, Garnier V, Salomon A, Murthi P, et al. Sustained Endocrine Gland-Derived Vascular Endothelial Growth Factor Levels Beyond the First Trimester of Pregnancy Display Phenotypic and Functional Changes Associated With the Pathogenesis of Pregnancy-Induced Hypertension. Hypertension. 2016;68(1): 148–56. [DOI] [PubMed] [Google Scholar]