Summary
Background
Metformin might reduce insulin requirement and improve glycaemia in patients with type 1 diabetes, but whether it has cardiovascular benefits is unknown. We aimed to investigate whether metformin treatment (added to titrated insulin therapy) reduced atherosclerosis, as measured by progression of common carotid artery intima-media thickness (cIMT), in adults with type 1 diabetes at increased risk for cardiovascular disease.
Methods
REMOVAL was a double-blind, placebo-controlled trial undertaken at 23 hospital diabetes clinics in five countries (Australia, Canada, Denmark, the Netherlands, and the UK). Adults aged 40 years and older with type 1 diabetes of at least 5 years' duration and at least three of ten specific cardiovascular risk factors were randomly assigned (via an interactive voice response system) to oral metformin 1000 mg twice daily or placebo. Participants and site staff were masked to treatment allocation. The primary outcome was averaged mean far-wall cIMT, quantified annually for 3 years, analysed in a modified intention-to-treat population (all randomly assigned participants with post-randomisation data available for the outcome of interest at any given timepoint, irrespective of subsequent adherence or study participation), using repeated measures regression. Secondary outcomes were HbA1c, LDL cholesterol, estimated glomerular filtration rate (eGFR), incident microalbuminuria (not reported), incident retinopathy, bodyweight, insulin dose, and endothelial function, also analysed in all participants with post-randomisation data available for the outcome of interest at any given timepoint. This trial is registered with ClinicalTrials.gov, number NCT01483560.
Findings
Between Dec 14, 2011, and June 24, 2014, 493 participants entered a 3 month run-in to optimise risk factor and glycaemic control (single-blind placebo in the final month). Of 428 randomly assigned patients, 219 were allocated to metformin and 209 to placebo. Progression of mean cIMT was not significantly reduced with metformin (−0·005 mm per year, 95% CI −0·012 to 0·002; p=0·1664), although maximal cIMT (a prespecified tertiary outcome) was significantly reduced (−0·013 mm per year, −0·024 to −0·003; p=0·0093). HbA1c (mean 8·1% [SD 0·9] for metformin and 8·0% [0·8] for placebo at baseline) was reduced on average over 3 years by metformin (−0·13%, 95% CI −0·22 to −0·037; p=0·0060), but this was accounted for by a reduction at the 3-month timepoint (−0·24%, −0·34 to −0·13; p<0·0001) that was not sustained thereafter (p=0·0163 for visit-by-treatment interaction). Bodyweight (−1·17 kg, 95% CI −1·66 to −0·69; p<0·0001) and LDL cholesterol (−0·13 mmol/L, −0·24 to −0·03; p=0·0117) were reduced with metformin over 3 years of treatment, and eGFR was increased (4·0 mL/min per 1·73m2, 2·19 to 5·82; p<0·0001). Insulin requirement was not reduced on average over 3 years (−0·005 units per kg, 95% CI −0·022 to 0·012; p=0·545), but there was a significant visit-by-treatment interaction (p=0·0018). There was no effect on endothelial function as measured by reactive hyperaemia index, or on retinopathy. Discontinuation of treatment in 59 (27%) participants on metformin versus 26 (12%) on placebo (p=0·0002) was mainly due to an excess of gastrointestinal adverse effects, and there was no increase in hypoglycaemia with metformin. Five deaths occurred among patients allocated to metformin and two occurred among those allocated to placebo; none were judged by site principal investigators to be related to study medication.
Interpretation
These data do not support use of metformin to improve glycaemic control in adults with long-standing type 1 diabetes as suggested by current guidelines, but suggest that it might have a wider role in cardiovascular risk management.
Funding
JDRF.
Introduction
Cardiovascular disease (CVD) is a major cause of reduced life expectancy in patients with type 1 diabetes and CVD is more than twice as common in people with type 1 diabetes as in the background population.1,2 Results from the Diabetes Control and Complications Trial (DCCT) and its post-randomisation follow-up study (Epidemiology of Diabetes and its Complications [EDIC]) showed that intensive glucose control in patients with type 1 diabetes reduces the onset and progression of microvascular and cardiovascular complications.3 However, in most countries more than 30% of adults with type 1 diabetes have poor glycaemic control;4 furthermore, many patients are overweight or obese,5 and, on the basis of data from Scotland, more than 60% of patients aged 40–59 years have poorly controlled cholesterol.1
US and UK guidelines recommend adding metformin, an inexpensive drug treatment, to insulin in overweight or obese individuals with type 1 diabetes to improve blood glucose control and reduce insulin dose requirement.6,7 This advice is based partly on findings from our meta-analysis of short-term and heterogeneous studies in which metformin was shown to reduce insulin requirement with possible benefits on bodyweight and HbA1c.8 However, metformin is not widely used for this off-label indication; the 2015 UK guideline called for more research.6 In Scotland, about 15% of adults with type 1 diabetes have ever received metformin, with a typical prevalent use of about 8% (unpublished analysis of 2016 national data).
Because metformin reduces risk of CVD in type 2 diabetes,9,10 we hypothesised that it might also have important cardiovascular as well as metabolic effects in type 1 diabetes. Progression of common carotid artery intima-media thickness (cIMT) is a surrogate of atherosclerosis11,12 that predicts future CVD events in the general population.13 Progression of cIMT was reduced in DCCT participants who were previously allocated to 6·5 years previous intensive glucose control in the first 6 years of EDIC follow-up and was associated with reduced CVD events 30 years from original randomisation.3,14
We therefore initiated the REMOVAL study (REducing with MetfOrmin Vascular Adverse Lesions), an international, double-blind, randomised, placebo-controlled trial with the primary aim of testing whether 3 years of treatment with metformin 1000 mg twice daily added to titrated insulin therapy (towards target HbA1c 7·0% [53 mmol/mol]) reduces atherosclerosis, as measured by progression of cIMT, in adults with confirmed type 1 diabetes aged 40 years and older and at increased risk for CVD.15
Methods
Study design and participants
The design of REMOVAL, an international, randomised, parallel-group trial of metformin versus placebo in adults with type 1 diabetes, has been described previously.15 The study was done in 23 hospital diabetes clinics in Australia, Canada, Denmark, the Netherlands, and the UK (17 initial sites plus six additional UK sites added in 2012 to meet recruitment targets). The protocol (appendix) was approved by the West of Scotland Research Ethics Service and relevant committees covering all sites.15 The University of Glasgow (Glasgow, UK) and NHS Greater Glasgow and Clyde (Glasgow, UK) were co-sponsors and specific responsibilities were delegated to international partner institutions. All participants provided written informed consent.
Individuals aged 40 years or older with type 1 diabetes of at least 5 years' duration and at least three of the following ten specified CVD risk factors were eligible: BMI ≥27 kg/m2; current HbA1c >8·0% (64 mmol/mol); established CVD; strong family history of CVD (defined as at least one parent, biological aunt or uncle, or sibling with myocardial infarction, stroke, or a coronary artery bypass graft aged <60 years); current smoker; microalbuminuria (defined according to routine care screening systems at sites); estimated glomerular filtration rate (eGFR) <90 mL/min per 1·73m2; hypertension (blood pressure ≥140/90 mm Hg or established antihypertensive treatment); dyslipidaemia (total cholesterol ≥5·0 mmol/L, HDL cholesterol <1·2 mmol/L [men] or <1·3 mmol/L [women], triglycerides ≥1·7 mmol/L, or established lipid-lowering treatment]); and diabetes duration >20 years (details in appendix p 7). Type 1 diabetes was defined in the initial protocol (version 1.0; June 23, 2011) as diagnosis of diabetes before age 35 years and insulin use within 1 year; the upper limit for age of diagnosis was revised to 40 years in protocol version 2.0 (September, 2012) following review of screening logs (the requirement of type 1 diabetes duration of at least 5 years was retained).
Randomisation and masking
Following a 3 month run-in period with placebo masked to participants in the final month, participants who remained eligible (≥70% medication adherence and random C-peptide ≤0·2 nmol/L at enrolment) were randomly assigned to receive metformin or placebo for 3 years. The placebo and active drug were identical in formulation (including excipients) and matched in shape and colour. Follow-up was abbreviated by between 1 and 8 months for the final 50 participants, in accordance with the final protocol (version 3.0; Nov 9, 2015). After confirming entry criteria, randomisation was achieved via an interactive voice response system hosted by the study data centre at the Robertson Centre for Biostatistics (Glasgow Clinical Trials Unit), University of Glasgow, Glasgow, UK. The randomisation list was generated by use of a pseudo-random number generator. Randomisation was stratified by study site, based on randomly permuted blocks allocated within each trial centre. Staff who generated the randomisation sequence had no role in any other part of the trial. Participants and site staff were fully masked to group assignment and all analyses predefined in the statistical analysis plan were programmed by investigators who were masked to treatment allocation.
Procedures
During the run-in period, CVD risk factor management was optimised in accordance with local guidelines at sites and insulin regimens were reviewed with the aim of optimising glycaemic control (target HbA1c 7·0% [53 mmol/mol]) with additional clinic visits if necessary. A randomisation visit (fasting) was then scheduled for baseline assessments including cIMT, endothelial function, and non-mydriatic colour retinal digital photographs. Study medication was either oral metformin hydrochloride 1000 mg twice daily with food (as Glucophage 500 mg) or placebo, provided free of charge by Merck KGaA (Darmstadt, Germany), and dispensed in identical packages with sufficient supply for 3 or 6 months, dependent on the visit schedule. Participants were given instructions on up-titrating study medication on a weekly basis from one tablet daily to two tablets twice daily (with meals) in week 4, with support from telephone visits. Lower doses were permitted for participants if adverse events occurred on higher doses. Adjustments in insulin doses towards target HbA1c were made at the discretion of site staff rather than being specified by the protocol. Study visits were scheduled to coincide with routine care appointments when possible; main study outcomes were reassessed at 12, 24, and 36 months. Participants continued to have access to usual local arrangements for diet, lifestyle, and weight management throughout the trial; ongoing management of glycaemia, blood pressure, and lipids was under the care of the site principal investigator and usual care team. A glycaemia committee sent detailed reports (masked to treatment allocation) on participants' HbA1c and rates of hypoglycaemia to each site every 6 months along with benchmarking data from other sites in their region.
Outcomes
The primary outcome was progression of averaged mean far-wall cIMT (measured in mm, at baseline, 12, 24, and 36 months). Right and left common carotid arteries were interrogated by B-mode ultrasound with a 7·0 MHz or higher broadband linear array transducer, with concurrent recording of three-lead electrocardiograph. Longitudinal images were obtained at anterior, lateral, and posterior angles using Meijer's arc during at least five cardiac cycles. Centres were asked to submit six duplicate sets of scans for each annual study visit for assessment of quality control. The concordance correlation coefficient (CCC) for averaged mean far-wall cIMT was 0·968 overall (ranging between 0·953 and 0·979 by visit). At the reading centre (University College London, London, UK), measurements were taken from the distal centimetre of the common carotid artery (ie, immediately proximal to the bulb) by a single trained assessor using a validated semi-automated programme. Repeat quality control cycles for the assessor yielded an overall CCC of 0·946 (ranging between 0·918 and 0·968). Individual measures of mean cIMT of greater than 1·5 mm, indicative of plaque, at any timepoint during follow-up were excluded from the primary outcome analysis, as recommended by the Mannheim consensus.16
Secondary outcomes15 were: HbA1c, measured by local laboratories using DCCT-standardised assays; LDL cholesterol (measured centrally at the University of Glasgow); eGFR, calculated on the basis of serum creatinine measured by local laboratories by use of the Modification of Diet in Renal Disease Study equation; incident microalbuminuria among individuals who had normalbuminuria at baseline (on the basis of clinical history plus urine collection; this measurement was augmented by collection and storage of urine aliquots for later central analysis if indicated), the data for which are not reported in this paper for reasons stated in Results; incident retinopathy, defined as two or more step progression (concatenated) read from two-colour 45° field photographs (field 1, optic disc; field 2, macula) taken from each eye at randomisation and at 36 months (graded with custom-designed software at the University of Wisconsin–Madison Ocular Epidemiology Reading Center [Madison, WI, USA] by use of the modified Airlie House classification scheme and the 11-step modified Early Treatment Diabetic Retinopathy Study [ETDRS] severity scale); bodyweight in kg (by calibrated scales); insulin dose (in units per kg); and endothelial function assessed at 0, 12, and 36 months as reactive hyperaemia index (RHI) in some study centres (14 centres provided data for endothelial function, covering 80% of participants) and read by staff masked to treatment allocation (EndoPAT, Itamar Medical, Caesarea, Israel). Improvement in two or more of the secondary outcomes was prespecified as clinically meaningful with the potential to affect clinical practice in the event of a positive primary outcome.
Tertiary outcomes were: frequency of hypoglycaemia, assessed with the modified Steno Hypoglycaemia Questionnaire, in which events were classified as minor (self-treated, resolved with short-acting glucose and longer-acting carbohydrate) or major (requiring assistance from another person); treatment satisfaction (Diabetes Treatment Satisfaction Questionnaire); biochemical markers of endothelial function (tissue plasminogen activator, sE-selectin, and sICAM-1), the data for which are not reported in this paper for reasons stated in the Results; progression of averaged maximal far-wall cIMT (in which plaque was included as recommended);14,17,18 and vitamin B12 status, as measured by local laboratories.
For assessment of safety, data for incident diabetes-related complications, operations, or procedures and adverse events of medical significance (gastrointestinal, metabolic, and renal) that were not captured as outcomes were collected at each visit. Dose reduction of study medication was recommended per protocol if eGFR fell below 45 mL/min per 1·73m2 during follow-up. Specific thresholds for permanent discontinuation of study medication were stipulated by monitoring of biomarkers (alanine transaminase >3·0 times upper limit of normal; eGFR <30 mL/min per 1·73m2; or serum lactate >5·0 mmol/L with acidosis or >3·0 mmol/L if confirmed by a mandated second measurement within 1 week). Participants whose vitamin B12 concentrations fell below 150 pmol/L were offered the choice of treatment discontinuation or referral for supplementation. All serious adverse events were collected and reported in accordance with standard operating procedures.
Statistical analysis
Data management and statistical analyses were done at the study data centre (Robertson Centre for Biostatistics, University of Glasgow, Glasgow, UK).15 The study was designed, assuming mean cIMT progression of 0·044 mm over 3 years in the control group, to provide 90% power to detect a mean difference of 0·0167 mm (a third of an SD, assumed to be 0·05) in change from baseline of averaged mean far-wall cIMT between treatment groups (α=0·05), assuming enrolment of 500 participants and 20% loss to withdrawal or discontinuation of treatment. Analysis of the primary outcome was based on a repeated-measures random regression model assuming a general residual covariance structure with random intercepts and slopes, adjusted for baseline cIMT as well as for age, sex, smoking status, systolic blood pressure, BMI, HbA1c, and LDL cholesterol, and was therefore expected to provide additional power. Regression model effect estimates with 95% CIs and associated p values were calculated. The same approach was used for analysis of the tertiary cIMT outcome. For the primary outcome, prespecified sensitivity analyses were done as follows: to account for differences in ultrasound machines used at sites by adjusting for ultrasound probe frequency; based on a per-protocol analysis; and with multiple imputation of missing cIMT values. Analyses were done on a modified intention-to-treat basis, including all randomly assigned participants with post-randomisation data available for the outcome of interest at any given timepoint, irrespective of subsequent participation in the study or ongoing adherence to study medication.
The sample size also provided 90% power to detect changes from baseline in the secondary outcomes of HbA1c, LDL cholesterol, eGFR, bodyweight, insulin dose, and endothelial function of about 0·3 SD (α=0·05). The retinopathy secondary outcome was likely to be underpowered because a 60% reduction in 3 year two-step or greater progression in the ETDRS severity scale was required to achieve 80% power. Outcomes were analysed as follows: ANCOVA, for change from baseline for continuous secondary and tertiary outcomes (HbA1c, LDL cholesterol, eGFR, bodyweight, insulin dose, endothelial function, and treatment satisfaction); Cox proportional-hazards for occurrence of vitamin B12 concentration of less than 150 pmol/L (tertiary outcome); negative binomial regression models for rates of hypoglycaemia (tertiary outcome); and logistic regression analysis for two or more step progression of retinopathy (secondary outcome). The ANCOVA analyses were further extended, as prespecified, to include a visit-by-treatment interaction term to investigate whether the treatment effect varied over time. In the event of a significant interaction being found, the nature of the interaction was explored by plotting treatment effects over time. Comparisons between groups for the permanent discontinuations from study medication and the number of individuals who had at least one serious adverse event were made with χ2 tests. Analyses were done with SAS (version 9.3). No adjustments for multiple comparisons were prespecifed.
No interim efficacy analyses were planned. An independent data monitoring committee reviewed unmasked reports on study progress every 6 months to monitor patient safety, the quality of efficacy information, and study conduct, as well as providing a recommendation to the sponsor on the appropriateness of continuing the study to completion.
This trial is registered with ClinicalTrials.gov, number NCT01483560.
Role of the funding source
During planning of the study, the funder (JDRF) hosted workshops to discuss principles of trial design for adjunctive therapy in type 1 diabetes and to encourage funding applications; however, the funder had no role in data collection, data analysis, data interpretation, or writing of the report. Merck KGaA (which donated study medication) had no role in study design, data collection, data analysis, or data interpretation, but a representative was offered the opportunity to comment on the report before submission. The corresponding author and trial steering committee (appendix p 2) had full access to all study data and provided regular progress reports to the funder and pharmacovigilance reports to Merck KGaA. The corresponding author and trial steering committee had final responsibility for the decision to submit for publication.
Results
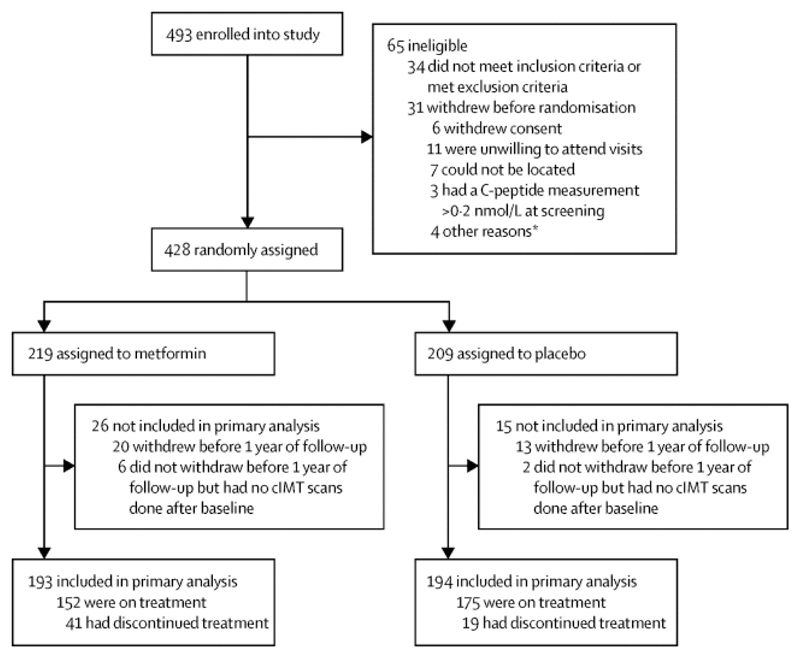
Between Dec 14, 2011, and June 24, 2014, 493 participants with type 1 diabetes were enrolled in a 3 month run-in with single-masked placebo in the final month. 428 individuals were subsequently randomly assigned, with 219 allocated to metformin and 209 to placebo (figure 1). Final data collection was on March 19, 2017. The characteristics of the treatment groups at randomisation were well balanced, and reflected the entry criteria requirement of at least three CVD risks factors. Across treatment groups, mean age was 55·5 years (SD 8·6), diabetes duration was 33·8 years (10·8), BMI was 28·5 kg/m2 (4·3), and HbA1c was 8·05% (0·82; 64·5 mmol/mol [9·00]; table 1). 34% (145 of 428) of patients used insulin pump therapy, mean systolic and diastolic blood pressure were 129·5 mm Hg (SD 14·8) and 72·3 mm Hg (10·1), respectively (with 73% [313 of 428] on antihypertensive medication), and LDL cholesterol was 2·2 mmol/L (0·71; with 82% [349 of 428] on a statin; table 1).
Figure 1.
Trial profile. cIMT=common carotid artery intima-media thickness. *One patient did not wish to proceed following a serious adverse event, one had a flare-up of irritable bowel syndrome, one withdrew because her husband became unwell, and one had a dislike of taking study medication.
Table 1.
Baseline characteristics
| Metformin (n=219) | Placebo (n=209) | |
|---|---|---|
| Age (years) | 55·2 (8·5) | 55·8 (8·8) |
| Sex | ||
| Male | 129 (59%) | 124 (59%) |
| Female | 90 (41%) | 85 (41%) |
| Ethnic origin* | ||
| White | 215 (98%) | 202 (97%) |
| Other | 4 (2%) | 7 (3%) |
| Diabetes duration (years) | 33·4 (11·0) | 34·3 (10·5) |
| C-peptide (nmol/L) | 0·05 (0·03–0·10) | 0·05 (0·03–0·10) |
| Existing CVD | ||
| MI or stroke | 17 (8%) | 12 (6%) |
| All† | 30 (14%) | 22 (11%) |
| None | 189 (86%) | 187 (89%) |
| Strong family history of CVD | ||
| Yes | 60 (27%) | 54 (26%) |
| No | 159 (73%) | 155 (74%) |
| HbA1c | ||
| Absolute (mmol/mol) | 64·8 (9·4) | 64·1 (8·5) |
| % units | 8·08 (0·86) | 8·02 (0·78) |
| Insulin regimen | ||
| Basal-bolus | 128 (58%) | 122 (58%) |
| Pump | 73 (33%) | 72 (34%) |
| Twice daily | 5 (2%) | 7 (3%) |
| Other | 13 (6%) | 8 (4%) |
| Total daily insulin dose (units per kg) | ||
| Basal-bolus | 0·67 (0·23) | 0·74 (0·29) |
| Pump | 0·54 (0·29) | 0·57 (0·29) |
| Twice daily | 0·73 (0·35) | 0·64 (0·27) |
| Other | 0·67 (0·23) | 0·73 (0·26) |
| All | 0·63 (0·26) | 0·68 (0·30) |
| BMI (kg/m2) | 28·4 (4·5) | 28·5 (4·1) |
| BMI category | ||
| Not overweight | 50 (23%) | 44 (21%) |
| Overweight | 103 (47%) | 96 (46%) |
| Obese | 65 (30%) | 69 (33%) |
| Unavailable | 1 (<1%) | 0 |
| Waist circumference (cm) | ||
| Men | 99·6 (11·6) | 100·0 (9·1) |
| Women | 92·1 (12·2) | 91·5 (12·1) |
| Systolic blood pressure (mm Hg) | 130·5 (15·0) | 128·5 (14·6) |
| Diastolic blood pressure (mm Hg) | 73·1 (9·9) | 71·5 (10·3) |
| Total cholesterol (mmol/L) | 4·03 (0·88) | 4·01 (0·93) |
| LDL cholesterol (mmol/L) | 2·23 (0·70) | 2·25 (0·72) |
| HDL cholesterol (mmol/L) | 1·64 (0·56) | 1·62 (0·59) |
| Triglycerides (mmol/L) | 1·07 (0·77) | 1·03 (0·57) |
| Smoking history | ||
| Current | 35 (16%) | 22 (11%) |
| Former | 73 (33%) | 71 (34%) |
| Never | 111 (51%) | 116 (56%) |
| eGFR (mL/min/1·73m2) | 92·9 (20·9) | 91·1 (21·6) |
| Renal | ||
| Normal | ||
| (>90 mL/min/1·73m2) | 128 (58%) | 130 (62%) |
| Stage 1 CKD | ||
| (>90 mL/min/1·73m2) | ||
| Microalbuminuria | 14 (6%) | 14 (7%) |
| Proteinuria‡ | 11 (5%) | 8 (4%) |
| Stage 2 CKD§ | ||
| (60–89 mL/min/1·73m2) | 59 (27%) | 48 (23%) |
| Stage 3a CKD§ | ||
| (45–59 mL/min/1·73m2) | 7 (3%) | 9 (4%) |
| Diabetic retinopathy¶ | ||
| None | 26 (12%) | 16 (8%) |
| Non-proliferative | 140 (64%) | 132 (63%) |
| Inactive proliferative | 36 (16%) | 39 (19%) |
| Active proliferative | 15 (7%) | 19 (9%) |
| Ungradable or missing | 2 (1%) | 3 (1%) |
| Neuropathy|| | 23 (11%) | 28 (13%) |
| Antihypertensive drugs | ||
| Any | 156 (71%) | 157 (75%) |
| Angiotensin-converting enzyme inhibitor | 111 (51%) | 103 (49%) |
| Angiotensin-receptor blocker | 43 (20%) | 49 (23%) |
| Calcium-channel blocker | 30 (14%) | 38 (18%) |
| β blocker | 19 (9%) | 19 (9%) |
| α blocker | 5 (2%) | 7 (3%) |
| Statin | 180 (82%) | 169 (81%) |
| Antiplatelet drugs | ||
| Aspirin | 71 (32%) | 80 (38%) |
| Clopidogrel | 10 (5%) | 6 (3%) |
Data are mean (SD), median (IQR), or n (%). Some percentages do not add up to 100% because of rounding. CVD=cardiovascular disease. MI=myocardial infarction. eGFR= estimated glomerular filtration rate. CKD=chronic kidney disease.
Data from self-report.
Includes a self-reported history of MI, stroke, heart failure, coronary artery bypass graft or stent, angina, transient ischaemic attack, or peripheral vascular disease.
Macroalbuminuria.
Microalbuminuria and proteinuria status not yet available for these CKD categories.
Modified Airlie House Classification (worst eye).
Impaired monofilament sensation.
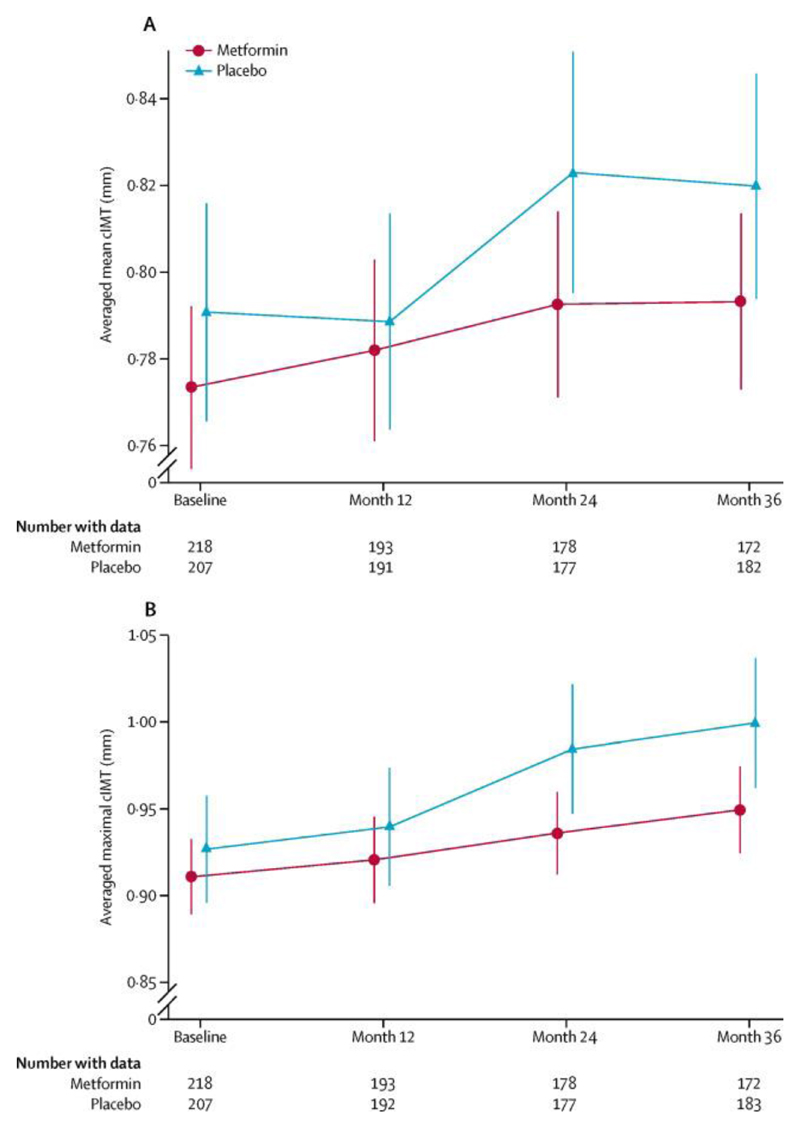
The difference in mean within-person cIMT slopes (metformin relative to placebo) for the primary outcome (modified intention-to-treat analysis) was −0·005 mm per year (95% CI −0·012 to 0·002; p=0·167; table 2, figure 2). There was no treatment-by-sex interaction (data not shown). All prespecified sensitivity analyses (to account for differences in ultrasound machines used at sites by adjusting for ultrasound probe frequency; based on a per-protocol analysis; and with multiple imputation of missing cIMT values) showed similar results (data not shown).
Table 2.
Repeated measures analysis of cIMT
| Averaged mean far-wall cIMT (primary outcome) |
Averaged maximal far-wall cIMT (tertiary outcome) |
|||||
|---|---|---|---|---|---|---|
| Metformin | Placebo | Difference (95% CI); p value | Metformin | Placebo | Difference (95% CI); p value | |
| Baseline (mm) | 0·773 (0·140) | 0·791 (0·183) | .. | 0·910 (0·162) | 0·926 (0·225) | .. |
| 12 months (mm) | 0·782 (0·147) | 0·788 (0·174) | .. | 0·920 (0·175) | 0·939 (0·239) | .. |
| 24 months (mm) | 0·792 (0·145) | 0·823 (0·187) | .. | 0·936 (0·161) | 0·984 (0·251) | .. |
| 36 months (mm) | 0·793 (0·134) | 0·820 (0·177) | .. | 0·949 (0·167) | 0·999 (0·257) | .. |
| Main analysis* | ||||||
| Slope (95% CI; mm per year) | 0·006 (0·001 to 0·011) | 0·010 (0·006 to 0·015) | −0·005 (−0·012 to 0·002); p=0·1664 | 0·012 (0·005 to 0·019) | 0·025 (0·018 to 0·032) | −0·013 (−0·024 to −0·003; p=0·0093) |
Data are mean (SD), unless otherwise indicated. Analyses are for modified intention-to-treat of participants with at least one measurement after baseline. cIMT=common carotid artery intima-media thickness.
Adjusted for baseline age, sex, cIMT, smoking status, systolic blood pressure, BMI, HbA1c, and LDL cholesterol.
Figure 2.
Progression in (A) mean cIMT (primary outcome) and (B) maximal cIMT (tertiary outcome). Error bars show 95% CIs. cIMT=common carotid artery intima-media thickness (far wall).
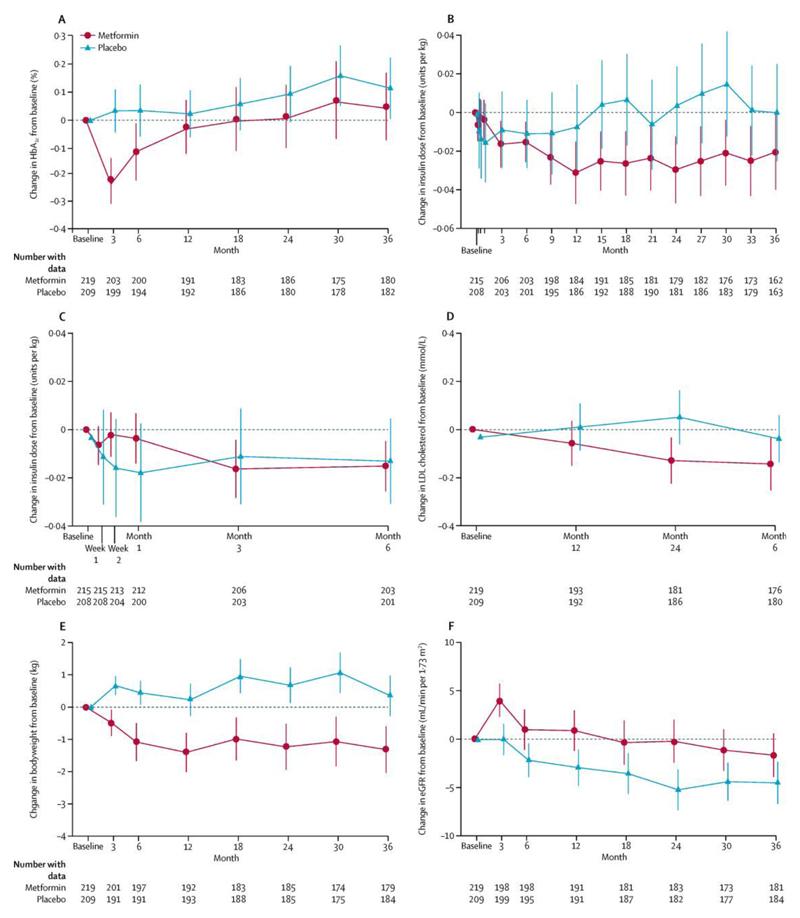
Of the secondary outcomes (table 3, figure 3), HbA1c was reduced by metformin over 3 years (−0·13%, 95% CI −0·22 to −0·04; p=0·0060), but this was accounted for by a reduction at the 3-month timepoint (−0·24%, −0·34 to −0·13; p<0·0001) that was not sustained thereafter (p=0·0163 for visit-by-treatment interaction). There was no reduction in insulin dose requirement on average over 3 years (−0·005 units per kg, 95% CI −0·022 to 0·012; p=0·5450), but there was evidence of a significant interaction between treatment and visit (units per kg; p=0·0018), with little difference between the treatment groups in the first 6 months, followed thereafter by a small but sustained reduction in patients allocated to metformin (estimated in post-hoc analyses as −0·023 units per kg, 95% CI −0·045 to −0·0005; p=0·045; figure 3). Differences favouring metformin were recorded in the mean within-person change between treatment groups in bodyweight (−1·17 kg, 95% CI −1·66 to −0·69; p<0·0001) and LDL cholesterol (−0·13 mmol/L, −0·24 to −0·03; p=0·0117); these changes would have met the secondary composite outcome (at least two of the secondary outcomes showing significant improvement) had the primary outcome shown a significant difference. There was also a significant increase in eGFR with metformin (4·00 mL/min per 1·73m2, 95% CI 2·19 to 5·81; p<0·0001; table 3, figure 3). There was no evidence of a treatment effect on endothelial function (as measured by RHI) or on retinopathy (table 3). We did not have adequate data for analysis of incident microalbuminuria at the time this report was prepared because medical history information was not well captured for patients with an eGFR of less than 90 mL/min per 1·73m2, insufficient local laboratory biochemical data were available to reliably ascertain microalbuminuria status in accordance with the protocol definition, and central analysis of stored urine aliquots had not yet been done.
Table 3.
Secondary outcomes
| Baseline | 36 months | Change (ANCOVA)*or OR†(95% CI) | Main effect p value | Interaction p value‡ | |||
|---|---|---|---|---|---|---|---|
| Metformin | Placebo | Metformin | Placebo | ||||
| HbA1c (%) | 8·1 (0·9) | 8·0 (0·8) | 8·1 (0·9) | 8·1 (0·8) | −0·13 (−0·22 to −0·04)* | 0·0060 | 0·0163 |
| LDL cholesterol (mmol/L) | 2·23 (0·70) | 2·25 (0·72) | 2·07 (0·83) | 2·21 (0·71) | −0·13 (−0·24 to −0·03)* | 0·0117 | 0·3101 |
| eGFR (mL/min/1·73m2) | 92·9 (20·9) | 91·1 (21·6) | 92·1 (20·8) | 87·2 (19·6) | 4·00 (2·19 to 5·81)* | <0·0001 | 0·6624 |
| Retinopathy (%; ≥ two-step progression) | ..§ | ..§ | 8 (6) | 10 (8) | 0·76 (0·29 to 1·98)† | 0·5683 | ..¶ |
| Bodyweight (kg) | 83·9 (15·4) | 83·5 (13·7) | 82·0 (15·4) | 83·2 (13·8) | −1·17 (−1·66 to −0·69)* | <0·0001 | 0·2736 |
| Insulin dose (units per kg) | 0·63 (0·26) | 0·68 (0·30) | 0·62 (0·26) | 0·67 (0·30) | −0·005 (−0·022 to 0·012)* | 0·5450 | 0·0018 |
| Endothelial function (RHI; arbitrary units) | 2·28 (0·74) | 2·24 (0·75) | 2·17 (0·69) | 2·24 (0·73) | −0·06 (−0·19 to 0·06)* | 0·3016 | 0·5662 |
Data are mean (SD) for continuous data or n (%) for categorical data. Treatment effect and corresponding 95% CIs are provided for metformin compared with placebo. eGFR=estimated glomerular filtration rate. RHI=reactive hyperaemia index.
ANCOVA for the change from baseline, adjusted for the baseline value, for continuous data.
Odds ratio (OR) and 95% CI for binary data obtained by logistic regression models.
Visit-by-treatment interaction.
Refer to table 1 for baseline retinopathy data.
No visit-by-treatment interaction term is available for retinopathy as it was assessed at only two visits (randomisation and close-out).
Figure 3.
Changes from baseline in secondary outcomes. Mean change from baseline for up to 3 years is shown for (A) HbA1c, (B) insulin dose requirement, (C) insulin dose requirement during first 6 months only, (D) LDL cholesterol, (E) bodyweight, and (F) estimated glomerular filtration rate (eGFR). Error bars show 95% CIs.
The tertiary cIMT outcome (averaged maximal) showed an increase over time in both treatment groups (table 2, figure 2), with reduced progression in association with metformin (difference in slope −0·013 mm per year, 95% CI −0·024 to −0·003; p=0·0093; figure 2, table 2). There was no difference between groups for minor or major hypoglycaemia, and no change in treatment satisfaction (table 4). Biochemical markers of endothelial function had not been measured at the time this report was prepared.
Table 4.
Tertiary outcomes, adherence, and safety
| Baseline | 36 months | Change (ANCOVA)*or HR†/IRR‡(95% CI) | p value | |||
|---|---|---|---|---|---|---|
| Metformin | Placebo | Metformin | Placebo | |||
| Tertiary outcomes | ||||||
| Hypoglycaemia (per patient-year) | ||||||
| Minor events | 37·2 | 38·8 | 54·6 | 49·1 | 1·12 (0·92 to 1·35)‡ | 0·2594 |
| Major events | 0·15 | 0·17 | 0·16 | 0·14 | 1·23 (0·73 to 2·05)‡ | 0·4419 |
| Treatment satisfaction (units) | 32·1 (3·6) | 31·4 (4·2) | 31·8 (4·2) | 31·3 (4·8) | −0·12 (−0·72 to 0·47)* | 0·6880 |
| Vitamin B12 <150 pmol/L§ | 0 | 0 | 24/194 (12%) |
9/192 (5%) |
2·76 (1·28 to 5·95)† | 0·0094 |
| Adherence and safety | ||||||
| Treatment discontinuation | .. | .. | 59 (27%) | 26 (12%) | .. | 0·0002 |
| Gastrointestinal | .. | .. | 34 (16%) | 7 (3%) | .. | .. |
| Nausea | .. | .. | 20 (9%) | 5 (2%) | .. | .. |
| Diarrhoea | .. | .. | 18 (8%) | 3 (1%) | .. | .. |
| Reduced eGFR | .. | .. | 0 | 0 | .. | .. |
| Lactate >3·0 mmol/L (twice) | .. | .. | 1 (<1%) | 0 | .. | .. |
| >5·0 mmol/L (any) | .. | .. | 3 (1%) | 0 | .. | .. |
| Treatment down-titration | .. | .. | 67 (31%) | 18 (9%) | .. | .. |
| Study medication dose (mg per day) | .. | .. | 1434 (612) | 1674 (343) | .. | .. |
| Lactate (mmol/L) | 1·31 (0·76) | 1·23 (0·57) | 1·30 (0·61) | 1·19 (0·52) | 0·08 (−0·03 to 0·19)* | 0·1640 |
| Deaths | .. | .. | 5 (2%) | 2 (1%) | .. | .. |
| Cancer | .. | .. | 3¶ | 1‖ | .. | .. |
| Cardiac | .. | .. | 2** | 1†† | .. | .. |
Data are mean (SD) for continuous data or n (%) for categorical data, unless otherwise indicated. Treatment effect and corresponding 95% CIs are provided for metformin compared with placebo. eGFR=estimated glomerular filtration rate.
ANCOVA for the change from baseline, adjusted for the baseline value, for continuous data.
Hazard ratio (HR) and 95% CI for time to first event data obtained by Cox proportional-hazards model.
Incidence rate ratio (IRR) and 95% CI for count data are obtained by negative binomial regression models, including the logarithm of time as an offset; the frequency of minor hypoglycaemia events is further adjusted for the method of collection.
Vitamin B12 data were missing for 25 people in the metformin group and 17 in the placebo group.
Two non-small-cell lung cancers, one malignant neoplasm of the tongue.
Glioblastoma.
One myocardial infarction, one sudden cardiac death.
Myocardial infarction.
59 (27%) participants on metformin and 26 (12%) on placebo discontinued treatment during the trial (table 4; p=0·0002; Kaplan-Meier plot in the appendix [p 9]). Discontinuation was due to gastrointestinal adverse effects in 34 (16%) participants on metformin and seven (3%) on placebo. The number of individuals who had at least one serious adverse event was similar between groups (34 [16%] patients on metformin vs 31 [15%] patients on placebo; p=0·8418; appendix p 8).
Biochemical vitamin B12 deficiency (<150 pmol/L) was more frequent with metformin (table 4). Therapy was discontinued because of asymptomatic hyperlactataemia in four individuals on metformin and none on placebo; there were no cases of lactic acidosis. Five deaths occurred among patients allocated to metformin (three from cancer, two from cardiac disease) and two occurred among those allocated to placebo (one from cancer, one from cardiac disease; table 4); none were judged by site principal investigators to be related to study medication. No changes in blood pressure control were seen with metformin therapy (data not shown).
Discussion
Adding metformin to insulin therapy and standard of care for 3 years in adults with long-standing type 1 diabetes and high cardiovascular risk did not significantly alter atherosclerosis progression as measured by the primary outcome (averaged mean cIMT) and did not have a sustained effect on glycaemic control. However, we did identify reductions in bodyweight, LDL cholesterol, and insulin dose requirement per unit of bodyweight, and a reduction in atherosclerosis progression as measured by the prespecified tertiary outcome of averaged maximal cIMT. Metformin was poorly tolerated by a substantial minority of participants, mainly because of gastrointestinal upset.
Before REMOVAL, the longest trial of metformin versus placebo in patients with type 1 diabetes was for 1 year in 100 individuals at the Steno Diabetes Center.19 Our systematic review of nine previous trials of metformin in type 1 diabetes (192·8 patient-years) provided some evidence for a reduction in insulin dose requirement (estimated at 6·6 units per day) when metformin was added to insulin.8 Bodyweight reduction was reported in three of six studies and HbA1c reduction in four of seven studies, but heterogeneous data for these outcomes could not be combined to give a pooled estimate of effect size.8
In a recent trial by the US T1D Exchange group, 140 adolescents, over half of whom met criteria for obesity, were randomly assigned to metformin or placebo for 6 months. Metformin reduced bodyweight and caused a transient reduction in HbA1c at 13 weeks (−0·3%), which reverted to baseline by 26 weeks, with a mean reduction in insulin dose requirement with metformin of 0·1 unit per kg (about 8 units per day) at both 13 and 26 weeks.20 This reduction was similar to the 6·6 units per day noted in our systematic review,8 but about four times higher than seen during years 2 and 3 of REMOVAL (0·023 units per kg or about 1·9 units per day). This difference in findings might have been because baseline doses were almost twice as high in the T1D Exchange trial (1·1 vs 0·63–0·68 units per kg); notably, in the T1D Exchange trial, the difference was also detected earlier at 3 months rather than 6 months.
The only trial of metformin in type 1 diabetes previously to have reported a reduction in LDL cholesterol (by 0·3 mmol/L) was the trial done at the Steno Diabetes Center,21 in which a much lower proportion of participants was treated with statins than in REMOVAL (about 37% vs 82%), with a substantial imbalance at baseline favouring the metformin group. In REMOVAL, statin use was well balanced at baseline between groups. Although the effect size in REMOVAL was similarly small, it is likely to have been attenuated by treatment of LDL cholesterol to target in both groups in accordance with local guidelines. Despite the fairly small effect size, if this reduction in LDL cholesterol was sustained over decades (rather than the 3 years of the trial), it could have an effect on CVD outcomes in patients with type 1 diabetes. Although this effect could have been achieved by other means, from a global perspective it is relevant that generic metformin has a low acquisition cost.
Metformin is associated with cardiovascular benefit in type 2 diabetes.9,10 These effects are not necessarily mediated by glucose lowering—eg, inhibition of STAT3 (and thereby monocyte-to-macrophage differentiation) via activation of AMPK in vascular tissues has been implicated in a direct anti-atherosclerotic action of metformin.22 Moreover, metformin can improve aspects of endothelial function23 and inhibit formation of advanced glycation end products by binding and inactivating methylglyoxal via an AMPK-independent pathway.24
Previous evidence that metformin can reduce atherosclerosis progression as measured by cIMT is inconsistent and based on underpowered, unblinded studies. Results of a double-blind, placebo-controlled trial in people without diabetes but with CVD showed no effect of metformin on mean cIMT.25 However, the mechanisms of atherosclerosis progression in type 1 diabetes might differ from those in other conditions.
By design, participants in REMOVAL had increased CVD risk and about 12% had established CVD. However, progression of the primary outcome (averaged mean cIMT) in the placebo group was only about two-thirds of that predicted (0·030 mm vs 0·044 mm), perhaps because of high levels of risk-factor management with statins and antihypertensive drugs. By contrast, progression of the tertiary outcome, averaged maximal cIMT, was higher than in younger DCCT/EDIC participants, as was the reduction in maximal cIMT progression with metformin (−0·039 mm over 3 years in REMOVAL vs −0·013 to −0·019 mm over 5 years in DCCT/EDIC).14,26 Our selection of mean cIMT, which excludes individual readings greater than 1·5 mm and plaque, as a primary outcome was driven by the aim of reducing variability in accordance with recommendations from the Mannheim consensus,16 although there is evidence of improved risk prediction with our tertiary outcome maximal cIMT, which might be more reflective of atherosclerosis progression than mean cIMT because it includes more advanced stages of disease, including focal thickening or plaque.26
During DCCT and the early EDIC study, use of statins and antihypertensive drugs was uncommon in people with type 1 diabetes. Reduction in maximal cIMT in EDIC following previous intensive glucose control was later followed by a 30% reduction in CVD events over a total follow-up period of 30 years.3 It is premature to conclude that the effect of metformin on maximal cIMT in REMOVAL might translate to such substantial effects on clinical outcomes, especially in view of its status as a tertiary outcome. Notably, the contribution of reduced cIMT progression per se to lowered CVD outcome rates independent of glycaemia has not been formally explored in DCCT/EDIC. Conversely, although cIMT is simply a surrogate endpoint for downstream CVD events, the concordance in findings between trials that use cIMT progression and CVD outcomes is excellent, with a positive predictive value of 96%.11
Our findings suggest that metformin might have direct effects on atherosclerosis progression in patients with type 1 diabetes, even in middle-aged individuals with long diabetes duration who are well treated with antihypertensive drugs and statins. In view of the absence of a persistent effect of metformin on HbA1c, this possible effect is unlikely to have been mediated by improved glycaemic control. Although an effect of metformin on endothelial function in conduit arteries has been reported in a double-masked, randomised, placebo-controlled pilot trial (n=42),27 we identified no effect in small resistance arteries using RHI in what was one of the largest studies of vascular function in type 1 diabetes to date.
Although evidence from DCCT/EDIC implicates hyperglycaemia as the key driver of atherosclerosis and CVD in type 1 diabetes, renal disease also has an important role.3 In REMOVAL, mean eGFR fell by 4 mL/min per 1·73m2 over 3 years in the placebo group, as expected in this population. However, in the metformin group, it rose acutely within the first month and then followed a parallel trajectory to placebo until the end of the trial, at which point separation was maintained; eGFR was a prespecified secondary outcome, but this was an unexpected finding that we believe should not be overemphasised at this stage. We cannot assess whether decline in renal function was stabilised by metformin because eGFR was not remeasured after study medication was discontinued. Since metformin and creatinine share renal proximal tubule transport mechanisms,28 we speculate that metformin might acutely increase tubular excretion of creatinine, affecting its validity as a biomarker of renal function, although this phenomenon has not previously been described in metformin's 60 year history. Together with other recent data,29 these findings warrant further investigation.
As the largest and longest clinical trial of metformin treatment in patients with type 1 diabetes to date, REMOVAL provides valuable information about its tolerability and safety. About a quarter of individuals (twice as many as in the placebo group) discontinued metformin over 3 years, suggesting that about one in eight had genuine intolerance, with the excess being attributable to gastrointestinal adverse effects. Although this finding potentially limits wider use of metformin in unselected individuals with type 1 diabetes, such intolerance is usually evident in the early months of use; additionally, prolonged-release metformin is available and has fewer gastrointestinal side-effects.30
In terms of safety, the risk of biochemical vitamin B12 deficiency was more than doubled over 3 years on metformin (12%) versus placebo (5%). Because vitamin B12 concentrations are not usually monitored in current practice, undetected clinically significant deficiency could develop over time, potentially contributing to sensory neuropathy in individuals already at risk. Our findings contribute to growing evidence that treatment with metformin reduces vitamin B12 concentrations, suggesting that monitoring should be used during long-term use,7,31,32 particularly in type 1 diabetes, in which there is associated risk of gastroparesis, pernicious anaemia, and coeliac disease. We noted several cases of asymptomatic hyperlactataemia in patients treated with metformin and none in the placebo group; however, there were no cases of lactic acidosis and the clinical significance of this finding is uncertain.
Limitations of the REMOVAL trial include use of an intermediate primary CVD outcome rather than a clinical one. Notably, no previous randomised trial of any intervention in type 1 diabetes has had a CVD primary outcome; despite the undoubted relevance of CVD in type 1 diabetes, REMOVAL is the first to include even a surrogate measure. The study was powered on the assumption that the SD of change from baseline for mean cIMT was 0·05, but despite central reading and an ongoing quality assurance programme, the observed value was 0·09. Although additional power is likely to have been achieved because of our use of a repeated measures regression analysis and adjustment for baseline characteristics, this increased SD probably reduced the statistical power of the trial. Moreover, we tested multiple secondary and tertiary outcomes (without adjustment for multiple comparisons), although all were prespecified. Strengths of the study include international recruitment in five countries and stable, modern glycaemic management, including insulin pump use by about a third of participants, increasing the generalisability of our findings.
The results of REMOVAL do not support the assertion by current guidelines that metformin can improve glycaemic control in patients with type 1 diabetes, nor do they provide a rationale for restricting its use to those who are overweight or obese. However, they do suggest that wider off-label use of metformin might be warranted to improve CVD risk management in type 1 diabetes, and possibly also reduce insulin dose requirement.
Supplementary Material
Research in Context.
Evidence before this study
In our 2010 systematic review and meta-analysis, we captured all publications on type 1 diabetes and metformin for any outcomes in PubMed (from Jan 1, 1950, to Oct 6, 2009) and Embase (Jan 1, 1974, to Oct 6, 2009) using medical search headings (MeSH): 1. ‘Diabetes Mellitus, Type 1’ [MeSH]; 2. (DIABET*) AND (TYPE 1 [TW] OR IDDM [TW]); OR (‘INSULIN DEPENDENT’ not ‘NON-INSULIN DEPENDENT’); 3. 1 OR 2; 4. ‘Metformin’ [MeSH]; 5. Metformin [TW]; 6. 4 OR 5. The search was run by two independent researchers who manually searched publications and their citations and extracted data from all that were considered potentially relevant. Additionally, we searched for ongoing and unpublished trials in the following sources: Cochrane Library 2009 issue 1; Science Citation Index meeting abstracts 1980–2008 (which includes the European Association for the Study of Diabetes and American Diabetes Association meetings 1980 to October, 2008, Diabetes UK meeting abstracts 2002–08, and US Endocrine Society Abstracts 2005–08), the National Research Register, and www.controlled-trials.com. We identified only 192·8 participant-years of small, short-term, and therefore low-quality trials of metformin in patients with type 1 diabetes. There was evidence for a reduction in insulin dose requirement by about 6·6 units per day (pooled estimate) when metformin was added to insulin treatment. We noted bodyweight reduction with metformin in three of six studies and HbA1c reduction in four of seven studies, but could not calculate pooled estimates because of heterogeneity. Only results from the largest trial (n=100), in which only a third of participants were on statin therapy, showed lowering of LDL cholesterol with metformin. Since our meta-analysis, several further small trials of metformin in type 1 diabetes have been reported, but also a larger (n=140), 6 month trial by the US T1D Exchange in 140 overweight and obese adolescents. The results of the T1D Exchange trial showed bodyweight reduction with metformin, a similar reduction in insulin dose requirement as in our systematic review, no reduction in LDL cholesterol, and improved glycaemic control at 3 months, but not at 6 months. Despite the undoubted relevance of cardiovascular disease in patients with type 1 diabetes, no previous trials of any intervention have been done with either clinical or intermediate cardiovascular outcomes.
Added value of this study
REMOVAL, the largest and longest trial of metformin in type 1 diabetes to date, confirms favourable effects on bodyweight and supports previous evidence of reduced insulin dose requirement, as reported in our meta-analysis and more recently in the T1D Exchange Study. It is the first study to show a sustained reduction in LDL cholesterol in middle-aged individuals with type 1 diabetes treated to target with statin therapy. It is also the first to indicate a possible reduction in atherosclerosis progression with metformin in type 1 diabetes, based on a surrogate outcome of common carotid intima-media thickness, although this finding was based on a tertiary rather than primary outcome.
Implications of all the available evidence
Our results provide a more rational basis for prescribing metformin, an inexpensive oral therapy, in patients with type 1 diabetes. Current guidelines in the UK and the USA recommend it for patients who are overweight or obese to reduce insulin dose requirement and improve glucose control. We identified a transient improvement in glycaemia that reverted to baseline with insulin dose reduction and identified no suggestion of greater benefit in overweight or obese patients. Type 1 diabetes guidelines should therefore be revised to reflect the absence of a sustained effect of adjunctive metformin on glycaemia and to remove the suggestion of particular efficacy in patients with a BMI greater than 25 kg/m2. Since larger and longer trials to investigate the effect of metformin on clinical cardiovascular outcomes in type 1 diabetes are unlikely to be done in the medium term, treatment decisions will have to be based on interpretation of the existing evidence. Rather than a role in glycaemic control, our findings suggest that long-term use of metformin in type 1 diabetes might reduce the long-term risk of cardiovascular disease via small but sustained reductions in bodyweight and LDL cholesterol.
Acknowledgments
The REMOVAL trial was funded by JDRF (New York, NY, USA; SRA 17-2011-272). We thank Merck Germany KGaA (Darmstadt, Germany), which donated study medication and shipped it to study sites; Michiel Bots, who was external quality assurance adviser for common carotid intima-media thickness; Itamar Medical (Caesarea, Israel), which donated EndoPAT equipment and provided central quality assurance services for the reactive hyperaemia index measurements; Elizabeth Douglas and Pamela Surtees (sponsor pharmacy, Glasgow, UK) for working with us to specify the manufacture and packaging of the study medication by Merck and liaising with Merck on drug supply management to the trial sites; Sharon Kean (Data Management, Robertson Centre for Biostatistic, University of Glasgow, Glasgow, UK) for ensuring resolution of all data queries and timely locking of the database; Maureen Travers, representative of the sponsor, NHS Greater Glasgow and Clyde, Glasgow, UK, which was responsible for compliance of the protocol and its implementation with MHRA regulations, as well as negotiation and implementation of contracts delegating specific oversight responsibilities to non-EU partner institutions; Lisa Jolly (sponsor project management) for project management, including liaising with all collaborators from writing the protocol to achieving trial close-out; and Jonathan Haw (deceased; lay representative on the trial steering committee).
Footnotes
Contributors
JRP and HMC conceived the trial and are its chief and deputy chief investigators, respectively. JRP, NC, IF, ADH, IH, AJJ, BEKK, RK, PR, NS, MCHJB, IH, CDAS, and HMC contributed to study design. JRP, IF, NC, ADH, TT, AJJ, BEKK, RK, TCO, PR, NS, MCGJB, CDAS, and HMC were involved in the conduct of the trial and data collection. NG and IF did the statistical analysis with input from JRP and HMC. JRP wrote the first draft of the report and all authors provided input into revising and finalising the report. Members of the REMOVAL Study Group are listed in the appendix pp 2–4.
Declaration of interests
JRP has received research grants from JDRF for the present work. He has also received personal fees and travel support from Novo Nordisk, research grants and personal fees from Sanofi Aventis, Quintiles, and Janssen unrelated to the present work, non-financial support (donation of study medication for the present trial) from Merck (Germany), personal fees from Lilly and ACI Clinical unrelated to the present work, and non-financial support (donation of EndoPAT equipment, reading services, and quality assurance support for the present trial) from Itamar Medical. NC has received research grants from JDRF for the present work and personal fees from AstraZeneca, unrelated to the present work. NG has received research grants from JDRF for the present work. IH has received research grants from JDRF/Federal Development Funding for the present work. She has also received personal fees from Amgen, Boehringer Ingelheim, Hoffmann-La Roche, Insulet, and Takeda; research grants and personal fees from AstraZeneca/Bristol-Myers Squibb, GlaxoSmithKline, Janssen-Ortho (Johnson & Johnson/JNJ), Merck Frosst, Novo Nordisk, and Sanofi-Aventis; research grants, personal fees, and travel support from Eli Lilly; and research grants from Lexicon and Medtronic, unrelated to the present work. TCO has received research grants from JDRF for the present work. PR has received research grants from JDRF for the present work. He has also received research grants, personal fees, and travel support from Novo Nordisk; research grants and personal fees from AstraZeneca; and personal fees from Astellas, Boehringer Ingelheim, Bayer, and Eli Lilly, unrelated to the present work. NS has received research grants and personal fees from Boehringer Ingelheim; personal fees from Novo Nordisk, Janssen, and Eli Lilly; and research grants from AstraZeneca, unrelated to the present work. HMC has received research grants, personal fees, and lecture and consultation support from Sanofi; consultation support from Sanofi Aventis and Novartis; research grants, personal fees, and travel support from Eli Lilly; research grants from Pfizer, Boehringer Ingelheim, AstraZeneca, and Roche Pharmaceuticals; and personal fees and lecture and consultation support from Regeron Pharmaceuticals, unrelated to the present work. She is also a shareholder in Roche Pharmaceuticals and Bayer. IF, MCGJB, TT, ADH, AJJ, BEKK, RK, and CDAS declare no competing interests.
References
- 1.Livingstone SJ, Looker HC, Hothersall EJ, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012;9:e1001321. doi: 10.1371/journal.pmed.1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingstone SJ, Levin D, Looker HC, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008–2010. JAMA. 2015;313:37–44. doi: 10.1001/jama.2014.16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. 2016;39:686–693. [Google Scholar]
- 4.McKnight JA, Wild SH, Lamb MJ, et al. Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med. 2015;32:1036–1050. doi: 10.1111/dme.12676. [DOI] [PubMed] [Google Scholar]
- 5.Conway B, Miller RG, Costacou T, et al. Adiposity and mortality in type 1 diabetes. Int J Obes (Lond) 2009;33:796–805. doi: 10.1038/ijo.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UK National Institute for Health and Care Excellence. Type 1 diabetes in adults: diagnosis and management. [accessed May 7, 2017]; https://www.nice.org.uk/guidance/ng17?unlid=43059219201639184149.
- 7.American Diabetes Association Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S1–S135. doi: 10.2337/dc17-0299. [DOI] [PubMed] [Google Scholar]
- 8.Vella S, Buetow L, Royle P, Livingstone S, Colhoun H, Petrie JR. The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia. 2010;53:809–820. doi: 10.1007/s00125-009-1636-9. [DOI] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 10.Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169:616–625. doi: 10.1001/archinternmed.2009.20. [DOI] [PubMed] [Google Scholar]
- 11.Peters SA, den Ruijter HM, Grobbee DE, Bots ML. Results from a carotid intima-media thickness trial as a decision tool for launching a large-scale morbidity and mortality trial. Circ Cardiovasc Imaging. 2013;6:20–25. doi: 10.1161/CIRCIMAGING.112.978114. [DOI] [PubMed] [Google Scholar]
- 12.Bots ML, Evans Gregory W, Riley WA, Grobbee DE. Carotid intima-media thickness measurements in intervention studies design options, progression rates, and sample size considerations. Stroke. 2003;34:2985–2994. doi: 10.1161/01.STR.0000102044.27905.B5. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz MW, Polak JF, Kavousi M, et al. PROG-IMT Study Group Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379:2053–2062. doi: 10.1016/S0140-6736(12)60441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348:2294–2303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrie JR, Chaturvedi N, Ford I, et al. on behalf of the REMOVAL Trial Team Metformin in adults with type 1 diabetes: design and methods of REducing with MetfOrmin Vascular Adverse Lesions (REMOVAL): an international multicentre trial. Diabetes Obes Metab. 2017;19:509–516. doi: 10.1111/dom.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th Watching the Risk Symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–296. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meijer R, Grobee DE, Bots ML. Mannheim consensus on carotid intima-media thickness: opposite and complementary points of view. Cerebrovasc Dis. 2006;21:415–416. doi: 10.1159/000092129. [DOI] [PubMed] [Google Scholar]
- 18.Polak JF, Backlund JY, Cleary PA, et al. DCCT/EDIC Research Group Progression of carotid artery intima-media thickness during 12 years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes. 2011;60:607–613. doi: 10.2337/db10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund SS, Tarnow L, Astrup AS, et al. Effect of adjunct metformin treatment in patients with type-1 diabetes and persistent inadequate glycaemic control. A randomized study. PLoS One. 2008;3:e3363. doi: 10.1371/journal.pone.0003363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libman IM, Miller KM, DiMeglio LA, et al. T1D Exchange Clinic Network Metformin RCT Study Group Effect of metformin added to insulin on glycemic control among overweight/obese adolescents with type 1 diabetes: a randomized clinical trial. JAMA. 2015;314:2241–2250. doi: 10.1001/jama.2015.16174. [DOI] [PubMed] [Google Scholar]
- 21.Lund SS, Tarnow L, Astrup AS, et al. Effect of adjunct metformin treatment on levels of plasma lipids in patients with type 1 diabetes. Diabetes Obes Metab. 2009;11:966–977. doi: 10.1111/j.1463-1326.2009.01079.x. [DOI] [PubMed] [Google Scholar]
- 22.Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes. 2015;64:2028–2041. doi: 10.2337/db14-1225. [DOI] [PubMed] [Google Scholar]
- 23.de Jager J, Kooy A, Schalkwijk C, et al. Long-term effects of metformin on endothelial function in type 2 diabetes: a randomized controlled trial. J Intern Med. 2014;275:59–70. doi: 10.1111/joim.12128. [DOI] [PubMed] [Google Scholar]
- 24.Beisswenger PJ. Methylglyoxal in diabetes: link to treatment, glycaemic control and biomarkers of complications. Biochem Soc Trans. 2014;42:450–456. doi: 10.1042/BST20130275. [DOI] [PubMed] [Google Scholar]
- 25.Preiss D, Lloyd SM, Ford I, et al. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2:116–124. doi: 10.1016/S2213-8587(13)70152-9. [DOI] [PubMed] [Google Scholar]
- 26.Polak JF, Szklo M, Kronmal RA, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2:e000087. doi: 10.1161/JAHA.113.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitocco D, Zaccardi F, Tarzia P, et al. Metformin improves endothelial function in type 1 diabetic subjects: a pilot, placebo-controlled randomized study. Diabetes Obes Metab. 2013;15:427–431. doi: 10.1111/dom.12041. [DOI] [PubMed] [Google Scholar]
- 28.Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC, Bhatnagar V. Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol. 2015;10:2039–2049. doi: 10.2215/CJN.02440314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell S, Farran B, McGurnaghan S, et al. Risk of acute kidney injury and survival in patients treated with metformin: an observational cohort study. BMC Nephrol. 2017;18:163. doi: 10.1186/s12882-017-0579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donnelly LA, Morris AD, Pearson ER. Adherence in patients transferred from immediate release metformin to a sustained release formulation: a population-based study. Diabetes Obes Metab. 2009;11:338–342. doi: 10.1111/j.1463-1326.2008.00973.x. [DOI] [PubMed] [Google Scholar]
- 31.Aroda VR, Edelstein SL, Goldberg RB, et al. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab. 2016;101:1754–1761. doi: 10.1210/jc.2015-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised, placebo controlled trial. BMJ. 2010;340:c2181. doi: 10.1136/bmj.c2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.