Abstract
Changes in synaptic excitability and reduced brain metabolism are among the earliest detectable alterations associated with the development of Alzheimer’s disease (AD). Stimulation of synaptic activity has been shown to be protective in models of AD beta-amyloidosis. Remarkably, deep brain stimulation (DBS) provides beneficial effects in AD patients, and represents an important therapeutic approach against AD and other forms of dementia. While several studies have explored the effect of synaptic activation on beta-amyloid (Aβ), little is known about Tau protein. In this study, we investigated the effect of synaptic stimulation on Tau pathology and synapses in in vivo and in vitro models of AD and frontotemporal dementia (FTD). We found that chronic DBS or chemically-induced synaptic stimulation reduced accumulation of pathological forms of Tau and protected synapses, while chronic inhibition of synaptic activity worsened Tau pathology, and caused detrimental effects on pre- and post-synaptic markers, suggesting that synapses are affected. Interestingly, degradation via the proteasomal system was not involved in the reduction of pathological Tau during stimulation. In contrast, chronic synaptic activation promoted clearance of Tau oligomers by autophagosomes and lysosomes. Chronic inhibition of synaptic activity resulted in opposite outcomes, with buildup of Tau oligomers in enlarged auto-lysosomes. Our data indicate that synaptic activity counteracts the negative effects of Tau in AD and FTD by acting on autophagy, providing a rationale for therapeutic use of DBS and synaptic stimulation in tauopathies.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia, and it is characterized by progressive accumulation of aggregated beta-amyloid (Aβ) peptide and Tau protein, especially at synapses (1–3). Synapses are considered to be a primary target of pathology in AD and other forms of dementia (4, 5), and synapse loss has been considered the best correlate of memory impairment in AD (6, 7). In AD patients, neuronal activity is reduced (8, 9), as well as in several AD mouse models (10). Alterations of synaptic activity and reduced brain metabolism are among the earliest signs of AD pathology, which are detectable decades before the development of other symptoms (11, 12). Therefore, the study of synaptic activity in AD has become an important subject for basic research as well as for the development of therapeutics. Increasing evidence supports the positive effects of synaptic stimulation against AD pathology (13–15). Synaptic activity is implicated in neuronal survival, since it can activate pro-survival genes increasing protection against apoptosis (16). Synaptic stimulation is also required for ATP production at synapses, which is fundamental for their correct functioning, and is involved in mechanisms of protection against Aβ oligomer-induced toxicity (17, 18). Finally, deep brain stimulation (DBS) represents a very promising therapeutic approach, which may show beneficial effects on AD patients. It increases brain metabolism, and may ameliorate memory and quality of life in certain patients (19). In addition, more recent data demonstrated that AD patients treated with DBS had increased hippocampal volume, which correlated with an augmented hippocampal metabolism (20).
In the last fourteen years multiple studies, including ours, showed that synaptic activation affects amyloid precursor protein (APP), and Aβ homeostasis. Stimulation of synaptic activity increases Aβ secretion, reduces intraneuronal Aβ, and induces APP anterograde trafficking to synapses (21–24). Synaptic stimulation promotes the recruitment of neprilysin that enhances Aβ42 degradation and, importantly, it also protects synapses by restoring levels of synaptic proteins and reducing synapse loss (25, 26). As of now, a limited number of studies were conducted to explore the effect of synaptic activity on Tau homeostasis: Neuronal stimulation resulted in augmented secretion of Tau in cell culture medium, and in the hippocampal interstitial fluid (27, 28). Synaptic activity was also shown to induce Tau translocation to dendritic spines, and Tau phosphorylation on specific residues, including Thr-205 and Ser-404 (29); however, it is unclear whether, in a pathological context, these changes are positive or negative.
To investigate the role of synaptic activity on Tau pathology in vivo, we either chronically increased activity in mouse brains by DBS (30) or we inhibited it by unilateral vibrissal deprivation, (deafferentiation), an established technique used to reduce functional activity in the corresponding somatosensory (barrel) cortex (31) that we previously used with success (24, 25). We provide evidence both in vivo and in vitro that synaptic activation is protective because it reduces pathological Tau levels, and it restores normal levels of synaptic proteins, by stimulating the autophagic-lysosomal degradation pathway. Conversely, we demonstrate that inhibition of synaptic activity exacerbates accumulation of Tau oligomers in swollen lysosomes, and induces further deterioration of synapses.
Methods
Detailed methods are included in the Supplementary Methods section.
Mouse models
Triple transgenic model of AD (3xTg-AD, (32), and background strain wild-type mice (C57BL/6/129SVJ; The Jackson Laboratory, Bar Harbor, ME) were bred and maintained at the Toronto Western Research Institute.
Male PS19 transgenic mice (33) were obtained from Jackson Laboratory and bred with female B6C3F1/N wild-type mice (Charles RIVER Laboratories, St-Germain-sur-l’Arbresle, France).
Surgical procedure for electrode implantation and DBS protocol
At the age of 3.75 months, 3xTg-AD and WT male mice were anaesthetized with isoflurane and bilaterally implanted with concentric bipolar Tungsten electrodes into the entorhinal cortex (EC), as previously described (30).
Surgical procedure for unilateral removal of whiskers
The procedure was performed as described (24, 25). Briefly, 5 months old PS19 and wild-type littermates mice were anesthetized and an incision was made around the skin area containing the whisker follicles, which were then removed. At 10 months of age, mice were sacrificed with pentobarbital and perfused with 4% PFA. The contralateral barrel cortices (corresponding to the half snouts that did not undergo surgery) were used as controls.
Inclusion/exclusion criteria
For the DBS experiments, we performed surgery on 22 male mice; for the unilateral whisker removal experiments, we performed surgery on 7 female and 6 male mice. The sample size was chosen based on our previous experience in performing similar experiments. Five mice per group were randomly selected to perform the histological analyses.
Antibodies
The full list of used antibodies and their applications is reported in the Supplementary Methods section.
Cell culture and treatments
Primary neuronal cultures from PS19 mice and wild-type littermates were prepared from E15 mouse embryos, as described (34).
Western blotting
Western blots analyses were performed as described (35).
Immunofluorescence
Neurons were grown on poly-D-lysine coated coverslips (Sigma-Aldrich) as previously described (34). After treatments, neurons were washed in ice-cold PBS and fixed in -20°C-cold methanol for 5 min for immunofluorescence. Brain sections were immunostained as previously described (25).
AD case and immunohistochemistry
Paraffin-embedded sections from human AD hippocampus were obtained from the Neurological Tissue Bank Hospital Clínic-IDIPAS Biobank. 8 μm sections were process as previously described (see Supplementary Methods).
Statistical analysis
Data were expressed as mean ± S.E.M. In experiments including mouse brain sections “n” refers to the number of mice analyzed per each condition. For each mouse, averages of measurements per section were considered as an individual measurement (n=1). A set of cultures prepared from one mouse embryo was considered as an n=1. In experiments involving cultured neurons ”n” refers to the number of cultured neurons prepared from one mouse embryo. Multiple coverslips prepared from one mouse embryo were considered repetitions, and the “n” was still considered one. The number of experiments repeated with cultured neurons for each treatment was either two or three, depending on the genotype of the cultured neurons. To reach an n=5, two or more preparations of cultured neurons were required, and experiments were repeated accordingly. Statistical comparisons were made using two-tailed unpaired t-tests (paired for the deafferentation data), and one-way ANOVA with significance placed at p<0.05. When appropriate, post hoc tests were conducted using the Fisher’s LSD correction. The statistical test type and p values are reported in figure legends. We did not perform an a priori power analysis since our sample sizes were similar to those reported in previously published papers (24, 25). Analysis of descriptive statistics showed no violation of any test assumptions that would justify the use of statistical test other than the ones used. The variance was similar between the analyzed groups. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA) and Excel (Microsoft, Redmond, WA, USA).
Results
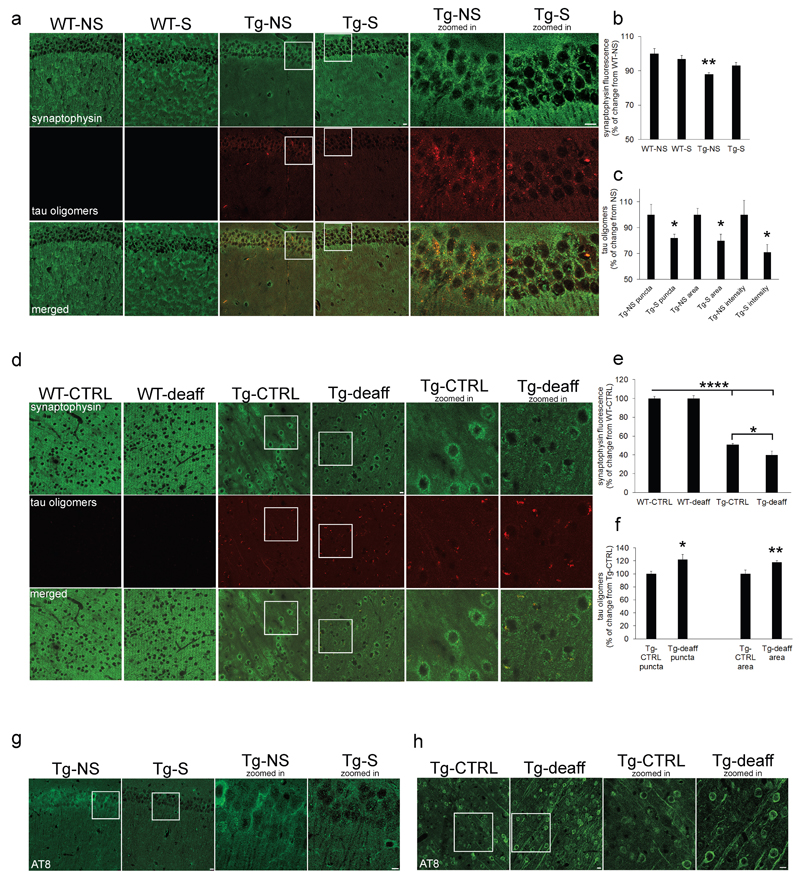
To investigate the effects of chronic synaptic activation on Tau pathology, we used four groups of mice: Two triple transgenics (3xTg-AD) and two wild-type (WT) (see methods). All mice underwent surgery and had electrodes implanted in the entorhinal cortex to induce deep brain stimulation (DBS). The entorhinal cortex is part of the circuit of Papez, essential for memory formation and consolidation; DBS of the mouse entorhinal cortex was shown to increase activity in the hippocampus and was associated with facilitation of spatial memory (30). One group of 3xTg-AD and one group of WT mice were stimulated (S), while the other two groups were not stimulated (NS) and were used as controls (five mice per group were analyzed). We found a significant decrease of synaptophysin in 3xTg-AD CA1 (Tg-NS) compared to wild-type (WT-NS) by quantitative immunofluorescence (Figure 1a, upper panels), confirming previous results (36). Interestingly, chronic DBS in 3xTg-AD mice (Tg-S) restored levels of synaptophysin back to WT mice (Figure 1a, upper panels: quantification in b). In the same brain sections, DBS reduced levels of Tau oligomers, more specifically the number of puncta (-18±3%), puncta area (-20±5%) and immunostaining intensity (-29±6%) compared to Tg-NS (Figure 1a, middle panels: quantification in c). No differences in synaptophysin levels were found between WT-S and WT-NS, in which Tau oligomers were not detected (Figure 1a: quantification in b). To confirm our observation that synaptic stimulation protects from Tau pathology, we chronically inhibited synaptic activity by unilateral deaferentiation in PS19 and wild-type littermate mice (Supplementary Figure 1a). Reduced cytochrome oxidase (COX) staining confirmed inhibition of neuronal activity in deafferented (Tg-deaff) compared to synaptically active control (Tg-CTRL) somatosensory cortices (Supplementary Figure 1b). Somatosensory cortices of Tg-deaff showed a significant decrease in levels of synaptophysin compared to Tg-CTRL (-11±4%; Figure 1d, upper panels: quantification in e); at the same time, reduced activity enhanced the accumulation of Tau oligomers, +22±8% and +18±2% in number of puncta and puncta area, respectively, in Tg-deaff compared to Tg-CTRL (Figure 1d, middle panels: quantification in f). Also in this case, no differences in synaptophysin levels were found between WT-CTRL and WT-deaff, and Tau oligomers were not detected.
Figure 1. Synaptic activity stimulation improves, while its inhibition worsens, Tau pathology in vivo.
a Chronic deep brain stimulation (DBS) in 3xTg-AD transgenic mice (Tg-S) resulted in reduced levels of Tau oligomers (middle panels), and increased levels of synaptophysin (upper panels) compared to non-stimulated (Tg-NS) mice, as quantified by confocal immunofluorescence in hippocampal CA1 (scale bars: 10μm; the pattern of synaptophysin immunostaining in WT-S panel appears altered probably because of a tilted angle of cutting and/or perfusion imperfections). b Quantitative analysis showing that levels of synaptophysin are restored to wild-type levels in Tg-S compared to NS-Tg (n=5; one-way ANOVA test, p=0.0045; WT-NS vs Tg-NS **p<0.01; WT-NS vs Tg-S p>0.05). c In Tg-S CA1, levels of Tau-oligomers presented reduced number of puncta (18±3%), puncta area (20±5%) and immunostaining intensity (29±6%) compared to Tg-NS. (two-tailed unpaired t-test, *p<0.05).
d Chronic synaptic inhibition by unilateral deafferentation (Tg-deaff) increased levels of Tau oligomers (middle panels), and reduced levels of synaptophysin (upper panels) compared to undeafferented (Tg-CTRL) somatosensory cortex in PS19 transgenic mice (scale bars: 10μm). e Quantitative analysis showed that levels of synaptophysin are reduced in Tg compared to WT brains, and are further reduced (11±4%) in Tg-deaff compared to Tg-CTRL (n=5; one-way ANOVA test, p<0.0001; WT-CTRL vs Tg-CTRL ****p<0.0001; WT-deaff vs Tg-deaff ****p<0.0001; Tg-CTRL vs Tg-deaff * p<0.05). f Levels of Tau-oligomers showed increased number of puncta (22±8%) and puncta area (18±2%) in Tg-deaff compared to Tg-CTRL (two-tailed paired t-test, *p<0.05, **p<0.01). g DBS decreased levels of phospho-Tau (AT8) in Tg-S compared Tg-NS hippocampi (scale bars: 10μm). Quantitative analysis showed that levels of AT8 are decreased (12±1%) in Tg-S compared to Tg-NS hippocampi of PS19 transgenic mice (n=5; two-tailed unpaired t-test, p=0.028). h Chronic synaptic inhibition increased levels of AT8 in Tg-deaff compared Tg-CTRL somatosensory cortices (scale bars: 10μm). Quantitative analysis showed that levels of AT8 are increased (13±3%) in Tg-deaff compared to Tg-CTRL somatosensory cortex of PS19 transgenic mice (n=5; two-tailed paired t-test, p=0.012). ”n” refers to the number of mice analyzed per each condition. 3xTg mice age: 7 months old; PS19 mice age: 10 months old.
Next, we explored the effects of synaptic activation or inhibition on pathological Tau phosphorylation. We observed that DBS decreased (-12±1%) levels of Tau phosphorylated at residue Ser202 (AT8) in Tg-S compared Tg-NS hippocampi (Figure 1g); these data were also confirmed by Western blot (-53±12%; Supplementary Figure 1c-d). In contrast, levels of AT8 were increased (13±3%) in Tg-deaff compared Tg-CTRL (Figure 1h). No changes were found in levels of total Tau by either DBS or deafferentation (Supplementary Figure 1e-h). AT8 immunostaining in wild-type mouse brains was too weak to be quantified (Supplementary Figure 1i-j). Overall, these data show that synaptic stimulation reduces Tau phosphorylation and accumulation of Tau oligomers, and protects synapses in in vivo models of Tau pathology.
To investigate how the state of synaptic activity affects Tau, we prepared primary neuronal cultures from PS19 and wild-type embryos. Since to our knowledge no previous publication described the use of primary neurons from the PS19 mouse model, we first explored whether PS19 cultured neurons (Tg) show pathological phenotype(s) comparable to PS19 brain neurons. Levels of phospho- and total Tau accumulated with time in Tg cultured neurons: Tg neurons differentiated for 21 days in vitro (Tg-21DIV) show increased levels of AT8 and total Tau compared to Tg-14DIV neurons (Supplementary Figure 2a: quantification in b-c). Tg-14DIV neurons had reduced levels of pre-synaptic protein synaptophysin (-24±7%; Supplementary Figure 2d: quantification in e) and post-synaptic protein PSD-95 (-53±1%; Supplementary Figure 2d: quantification in f) compared to 14DIV wild-type (WT-14DIV) neurons. Progressive accumulation of Tau and reduction of synaptic proteins are alterations that have been described to occur in brains of PS19 mice (33). In addition, we found that Tg-14DIV, but not WT-14DIV, cultured neurons specifically developed Tau oligomers (Supplementary Figure 2g, upper panels). Since Tg-14DIV neurons presented a sufficiently clear pathological phenotype, similar to that observed in vivo, we used such neurons for all our further experiments.
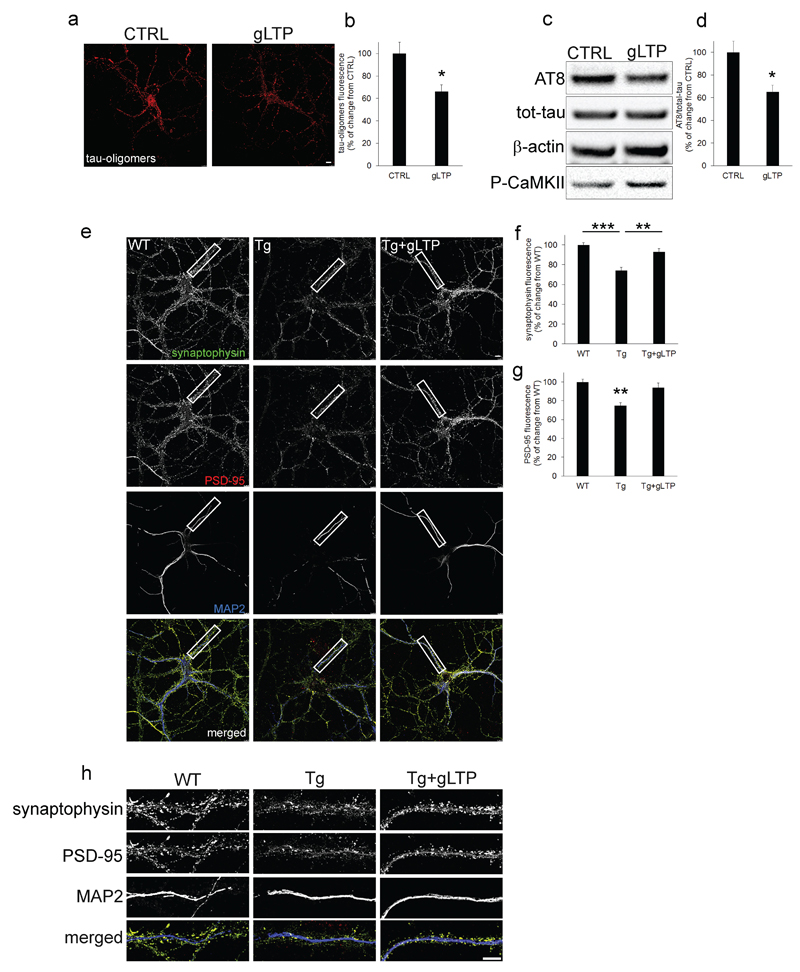
We then tested whether synaptic activation provides beneficial effects against pathological Tau in vitro. To this end, Tg cultured neurons were stimulated using a well-established glycine-induced long-term potentiation (gLTP) protocol (37), that we have extensively used in the past (24, 26). gLTP-dependent activation of neurons was confirmed by increased levels of the phosphorylated Ca2+/calmodulin-dependent protein kinase II (CaMKII) compared to CTRL (Figure 2c, lowest panel), as previously shown (24). Stimulation of neurons by gLTP decreased levels of Tau oligomers (-34±6%), compared to unstimulated neurons (CTRL), as quantified by confocal immunofluorescence (Figure 2a: quantification in b). gLTP also reduced levels of AT8 (-25±5%) compared to CTRL, as quantified by Western blot (Figure 2c) and confocal immunofluorescence (Supplementary Figure 3a, upper panels; quantification in b), while levels of total Tau remained unchanged (Figure 2c; Supplementary Figure 3a, lower panels: quantification in c). gLTP stimulation also restored both synaptophysin and PSD-95 (Figure 2e) levels back to WT (quantification in Figure 2f and 2g, respectively). Higher magnification images show presence of synaptophysin and PSD-95 puncta on dendritic branches (MAP2; Figure 2h); a similar trend was observed by Western blot (Supplementary Figure 3d- f).
Figure 2. Synaptic activation reduced pathological Tau, and restored synaptophysin and PSD-95 to wild-type levels in PS19 cultured neurons.
a, b gLTP reduced levels of Tau oligomers (34±6%) compared to control treated (CTRL) Tg neurons, as quantified by confocal immunofluorescence (n=5; two-tailed unpaired t-test, *p<0.05; scale bar: 7.5μm). c, d Western blot analyses demonstrated a reduction (25±5%) of AT8 in gLTP compared to CTRL Tg neurons (n=5; two-tailed unpaired t-test, *p<0.05). e, h gLTP restored levels of synaptophysin (green) and PSD-95 (red) back to WT levels compared with CTRL Tg neurons, as quantified in f (n=5; one-way ANOVA test, p=0.0018; WT vs Tg ***p<0.001; Tg vs Tg+gLTP **p<0.01) and g (n=5; one-way ANOVA test, p=0.0068; WT vs Tg **p<0.01; Tg vs Tg+gLTP **p<0.01), respectively (scale bars: 7.5μm). “n” refers to a set of cultured neurons prepared from one mouse embryo. Three preparations of neurons were required and experiments were repeated accordingly.
As the in vitro system recapitulates the in vivo observations, we sought to use it to mechanistically clarify how the reduction of pathological Tau occurs upon synaptic activation. We initially hypothesized that the proteasome might be responsible for Tau degradation. Indeed, it was reported that synaptic activity promotes access of the proteasome to dendritic spines and synapses and its maintenance (38). To test this hypothesis, Tg cultured neurons were treated with epoxomicin to block proteasome activity during gLTP; quantitative immunofluorescence demonstrated that epoxomicin treatment failed to prevent clearance of Tau oligomers in Tg neurons during gLTP (Supplementary Figure 3g, upper panels: quantification in h). In addition, in the presence of epoxomicin, synaptic activity was still able to reduce AT8, despite the expected accumulation of ubiquitinated proteins, as revealed by Western blot (Supplementary Figure 3i-k; quantification in j). This data indicate that proteasomal degradation is not involved in the clearance of Tau induced by synaptic activity.
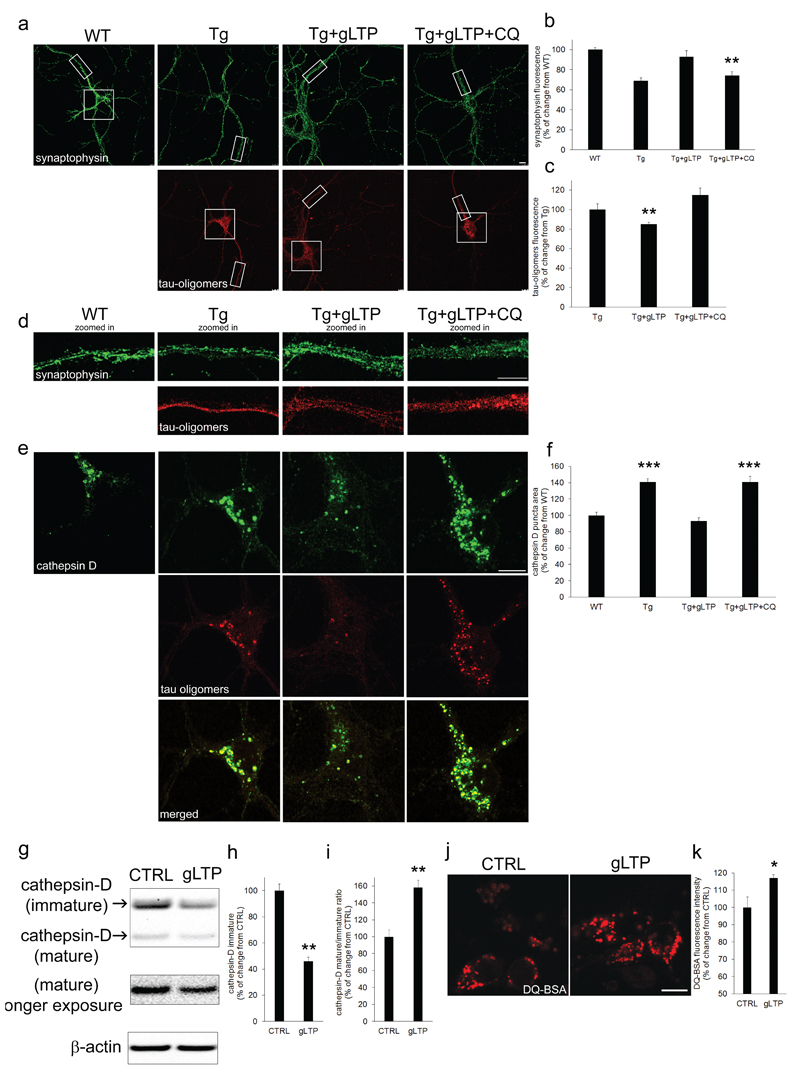
An alternative hypothesis is that clearance of pathological Tau might depend on autophagy and the endo-lysosomal system. It was reported that aggregated forms of Tau are transported to the lysosomal system for degradation (39, 40). To assess this, gLTP stimulated Tg neurons were treated with chloroquine (CQ), which impairs lysosomal function by neutralizing luminal pH. CQ prevented gLTP ability to reduce levels of Tau oligomers, and to augment levels of synaptophysin (Figure 3a, d: quantification b-c). Quantitative immunofluorescence also revealed that lysosomes, labeled with an antibody against the lysosomal component cathepsin D, in the soma of cultured neurons were bigger (+41±4%) in Tg compared to WT neurons (Figure 3e upper panels: quantification in f). Significantly, Tau oligomers (Figure 3e, middle panels) accumulated within lysosomes (Figure 3e, bottom panels). gLTP activation restored the size of lysosomes in Tg neurons to WT, while CQ treatment did not (Figure 3e upper panels: quantification in f). In addition, Western blot analysis showed that synaptic activation reduced of 54±3% levels of immature cathepsin D in Tg neurons (Figure 3g, upper panel: quantification in h, resulting in an increase of the ratio mature/immature cathepsin D (+27±7%) in gLTP stimulated compared to CTRL neurons (Figure 3g: quantification in i), consistent with elevated lysosomal degradation. To directly test lysosomal activity, we measured processing of the substrate DQ-BSA, which fluoresces upon cleavage in lysosomes. gLTP increased levels of DQ-BSA fluorescence compared to CTRL (+17±2%; Figure 3j; quantification in k), demonstrating enhanced lysosomal degradation.
Figure 3. Tau oligomers accumulate in swollen lysosomes, and lysosomal activity is required for their gLTP-dependent clearance.
a-d Chloroquine (CQ) treatment prevented gLTP to reduce levels of Tau oligomers (lower panels), and to restore levels of synaptophysin (upper panels; scale bar 10μm) in Tg cultured neurons, as quantified by confocal immunofluorescence in c (n=5; one-way ANOVA test, p=0.0114; Tg+gLTP vs Tg+gLTP+CQ **p<0.01; Tg vs Tg+gLTP+CQ p>0.05) and b (one-way ANOVA test, p=0.0019; WT vs Tg+gLTP P>0.05; WT vs Tg+gLTP+CQ **p<0.01), respectively. e Zoomed-in of neuronal somas displayed in a: the size of lysosomes (upper panels; scale bar 10μm) is increased (41±4% of puncta area) in Tg compared to WT neurons, and Tau oligomers (middle panels) accumulated in enlarged lysosomes (merged images, bottom panels). f gLTP restored the size of lysosomes in Tg neurons back to WT; however, inhibition of lysosomal activity by CQ treatment prevented it. (n=5; one-way ANOVA test, p=0.0001; WT vs Tg ***p<0.001; Tg vs Tg+gLTP *** p<0.001; Tg+gLTP vs Tg+gLTP=CQ *** p<0.001). g-i Western blot analyses demonstrated a reduction (56±3%) of immature cathepsin D and an increase (58±9%) of the mature/immature cathepsin D ratio in gLTP compared to CTRL Tg neurons (n=5; two-tailed unpaired t-test, **p<0.01). j, k DQ-BSA assay to measure lysosomal function revealed a 17±2% increase of lysosomal activity in gLTP compared to CTRL neurons (n=5; two-tailed unpaired t-test, *p<0.05). “n” refers to a set of cultured neurons prepared from one mouse embryo. Two preparations of neurons were required and experiments were repeated accordingly.
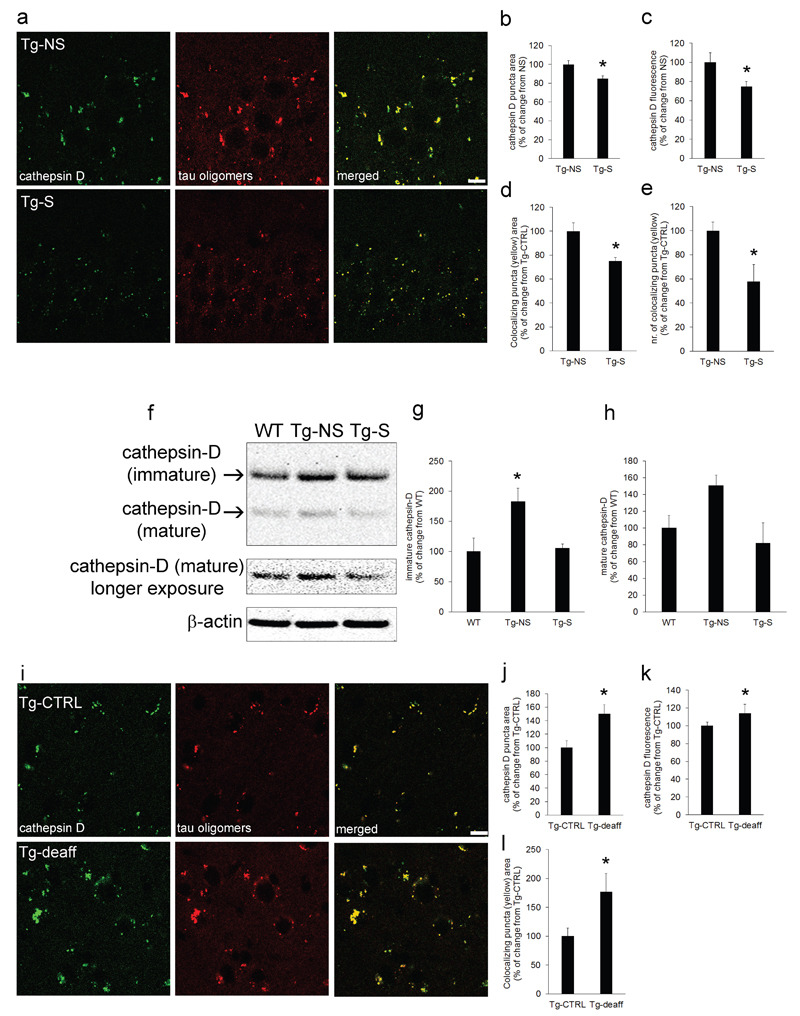
Immunostaining of Tg mouse brains confirmed the observation in Tg cultured neurons that oligomeric Tau is mostly found in lysosomes, and that lysosomal compartment size is reduced (-15±3% and -25±5% in puncta area and fluorescence intensity, respectively) upon synaptic activation in 3xTg mice (Figure 4a: quantification in b-c). Importantly, DBS reduced as well the amount of Tau oligomers in lysosomes, as shown by the 25±3% decrease in Tau-cathepsin D colocalization (Figure 4a: quantification in d-e). Western blot analyses showed that levels of immature cathepsin D are increased of 83±22% (with a trend for mature cathepsin D, which did not reach statistical significance) in Tg-NS compared to WT mice, and that DBS in Tg-S mice restored cathepsin D levels back to WT (Figure 4f; quantification in g and h). Tau oligomers accumulated also within lysosomes in PS19 mouse brains. In this case, chronic inhibition of activity resulted in an increase in lysosomal size (+50±14%), and fluorescence intensity (+14±10%) compared to the control side (Figure 4i: quantification in j-k). Chronic inhibition of synaptic activity also exacerbated Tau oligomer deposition (+77±32%) in swollen lysosomes (Figure 4l). Taken together, these results provided evidence for an involvement of the lysosomal system in the reduction of pathological Tau during synaptic activation.
Figure 4. Synaptic activation reduced the size of lysosomes, and their load of Tau oligomers.
a Synaptic activation in Tg-S mice reduced the size of lysosomes (cathepsin D, left panels) and of Tau oligomers (middle panels), and localization of Tau oligomers with lysosomes (merged, right panels; scale bar 10μm). b, c quantification showing decreased cathepsin D puncta area (15±3%) and fluorescence intensity (25±5%) respectively, in Tg-S compared to Tg-NS CA1 (n=5; two-tailed unpaired t-test, *p<0.05). d Area of colocalization between Tau oligomers and lysosomes is reduced (25±3%) in Tg-S compared to Tg-NS CA1; e the number of colocalizing puncta between Tau oligomers and lysosomes is also reduced (42±14%) in Tg-S compared to Tg-NS CA1 (n=5; two-tailed unpaired t-test, *p<0.05). f-h Western blot analyses demonstrated an increase of 83±22% of immature cathepsin D in Tg-NS mouse brains (n=5; one-way ANOVA test, p=0.0242; Tg-NS vs WT *p<0.05; Tg-NS vs Tg-S *p<0.05); mature cathepsin D also showed a trend for an increase in Tg-NS compared to WT, which did not reach statistical significance (n=5; one-way ANOVA test, p=0.0757). i Tau oligomers (middle panels) accumulated within lysosomes (left panels) in PS19 barrel cortices. Chronic inhibition of synaptic activity by whisker deafferentation exacerbated Tau oligomer deposition in enlarged lysosomes, as assessed by confocal microscopy (right panels, merged; scale bar 10μm). j, k Quantification of immunofluorescence showed an increase (50±14%) of cathepsin D puncta area, and fluorescence intensity (14±10%), respectively, in Tg-deaff compared to Tg-CTRL somatosensory cortex (n=5; two-tailed paired t-test, *p<0.05). l Quantification of cathepsin D and Tau oligomers colocalization revealed a 77±32% increase in size of colocalizing puncta areas in Tg-deaff compared to Tg-CTRL (n=5; two-tailed paired t-test, *p<0.05). ”n” refers to the number of mice analyzed per each condition. 3xTg mice age: 7 months old; PS19 mice age: 10 months old.
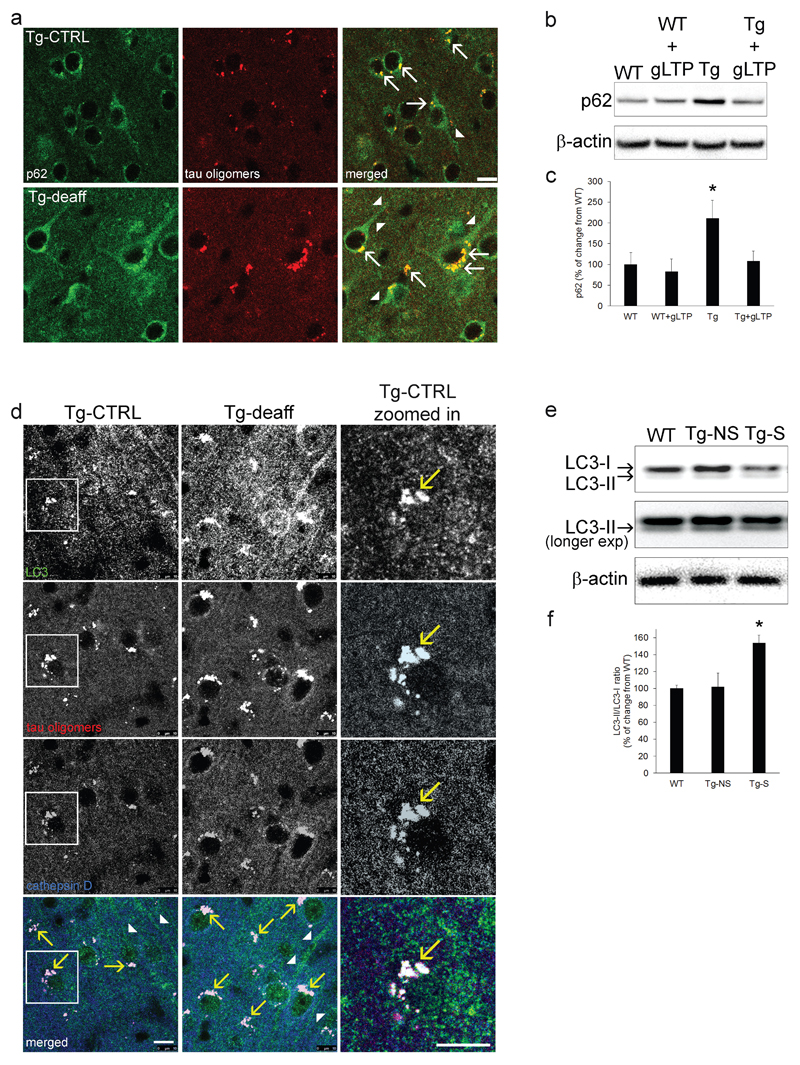
How could oligomeric Tau end up in lysosomes? Previous studies demonstrated that stimulation of autophagy provides protective effects in mouse models of AD and FTD (41, 42). Thus, it is possible that oligomeric Tau is an autophagic cargo. If this is the case, Tau should be recognized by specific autophagy cargo adapters, such as p62, which has been previously associated to proteotoxic stress, and Tau clearance (43–45). In agreement with these studies, we found that p62 labels oligomeric Tau in the soma of Tg mouse brains (Figure 5a, arrows). Importantly, p62 levels increased (+50±20%) in Tg-deaff compared to Tg-CTRL (Figure 5a). In addition, in cultured neurons p62 was markedly increased (111±43%) in Tg compared to WT neurons, and gLTP stimulation returned levels of p62 in Tg neurons back to WT levels (Figure 5b: quantification in c). Finally, to explore whether Tau oligomers (which we found positive for p62) are enclosed into mature autophagosomes that can be degraded in lysosomes during synaptic activity, PS19 brain sections were co-immunostained to detect the autophagosomal membrane protein Microtubule-associated proteins 1A/1B light chain 3 (LC3) and cathepsin D. Most of Tau oligomers were colocalizing with both LC3 and cathepsin D (Figure 5d, bottom panels, arrows), revealing that they are present in auto-lysosomes, the organelles formed by the fusion of mature autophagosomes with lysosomes. Importantly, chronic inhibition of synaptic activity increased levels of LC3 (129±24%, Figure 5d). In Figure 5d (right panels, arrow) it is possible to appreciate LC3 and Tau oligomers puncta colocalizing in lysosomes (cathepsin D). Remarkably, both p62 and LC3 presented accumulation in neurites, which was exacerbated in Tg-deaff together with bigger Tau deposits in auto-lysosomes in the soma (Figure 5a and d, arrow heads), suggesting that reduced synaptic stimulation might alter Tau, as well as general autophagic clearance. In contrast, Western blot analysis showed that DBS significantly increased the LC3-II/LC3-I ratio of 48±11% in Tg-S mouse brains (Figure 5e; quantification in f), consistent with stimulation of autophagy. To further investigate whether autophagosomes are required by Tau oligomers to reach lysosomes for degradation, autophagy was inhibited in Tg cultured neurons by incubation with the PI3K inhibitor 3-methyladenine (3-MA). 3-MA treatment reduced of 39±6% the colcalization of Tau oligomers with cathepsin D (Supplementary Figure 4a: quantification in b). Remarkably, upon autophagy inhibition levels of oligomers were reduced in the soma (-54±4%) and increased of 27±7% in neurites (Supplementary Figure 4a: quantification in c and d). Finally, to explore whether lysosomes might be responsible for AT8 reduction during synaptic activity, we prepared lysosome-enriched fraction from cultured Tg neurons. The cathepsin D-positive fraction contained most of phosphorylated-Tau (AT8), compared to the nuclear-enriched fraction, (Supplementary Figure 5a). In addition, despite a different pattern of immunostaining, T22 and AT8 showed some colocalization in puncta within somas of PS19 mouse brain (Supplementary Figure 5b, lower panels, arrows), supporting that also pathologic phosphorylated Tau accumulates in lysosomes. Together, these data indicate that synaptic stimulation relies on autophagy for the clearance of pathological Tau and reveal that synaptic activity upregulates autolysosomal degradation.
Figure 5. Synaptic activity induces degradation of Tau oligomers via the autophagic-lysosomal system.
a p62 (left panels) colocalized with Tau oligomers (middle panels) in both Tg-deaff and Tg-CTRL (right panels, arrows, scale bar 10μm). Chronic inhibition of activity increased p62 levels of 50±20% in Tg-deaff compared to Tg-CTRL; p62 accumulation was evident in somas, but also in neurites (arrow heads; n=5; two-tailed paired t-test, p=0.041). b, c p62 levels were increased (111±43%) in Tg compared to WT cultured neurons. gLTP reduced levels of p62 in Tg neurons (Tg+gLTP) back to WT levels, as quantified by Western blot (n=5; one-way ANOVA test, p=0.0485; WT vs Tg *p<0.05; Tg vs Tg+gLTP *p<0.05). d Chronic synaptic inhibition induced an accumulation of the autophagosomal marker LC3 in Tg-deaff compared to Tg-CTRL somatosensory cortices. LC3 (green) showed colocalization with Tau oligomers (red) and lysosomes (blue) in both Tg-deaff and Tg-CTRL (arrows, bottom panels and zoomed in images; scale bar 10μm). LC3 accumulation was also present in neurites, especially in Tg-deaff (arrow heads). Quantification of confocal images revealed a 129±24% increase of LC3 fluorescence intensity in Tg-deaff compared to Tg-CTRL (n=5; two-tailed paired t-test, p=0.006). e, f Western blot analyses demonstrated a 54±9% increase of LC3-II/LC3-I ratio in Tg-S compared to Tg-NS mouse brain (n=5; one-way ANOVA test, p=0.0173, Tg-S vs Tg-NS *p<0.05). For experiments on mouse brains (immunofluorescence and Western blot),”n” refers to the number of mice analyzed per each condition. For Western blot experiments on cultured neurons, “n” refers to a set of cultured neurons prepared from one mouse embryo. Three preparations of neurons were required and experiments were repeated accordingly. 3xTg mice age: 7 months old; PS19 mice age: 10 months old.
Discussion
Our data demonstrate that synaptic stimulation improves Tau pathology, while its inhibition worsens it, with consequent amelioration or deterioration of synapses. Experimental procedures, performed on two different transgenic mouse models harboring two different familial-FTD Tau mutant genes showed notable complementarity. This is the first time, to our knowledge, that a link between synaptic activity and modulation of the autophagic-lysosomal degradation pathway is revealed, despite it has been known for several years that abnormal endosomal-lysosomal function is associated with accumulation of Aβ and Tau aggregates (46, 47).
Autophagosomes accumulate in AD (48, 49), enhancing Tau pathology (50). Consistent with these observations, we detected the presence of Tau oligomers within autophagosomes, both in somas and neurites, of human AD hippocampus (Supplementary Figure 6). In addition, our experimental data revealed that inhibition of synaptic activity exacerbates p62 and LC3 accumulation, and increases lysosomal size. Levels of Tau oligomers also increased with activity inhibition, and their accumulation occurred within swollen lysosomes, where they also colocalized with LC3 and p62. On the contrary, synaptic activation reduced pathological Tau, p62 and lysosomal size, while increasing autophagy flux (51) and lysosomal degradation, ultimately protecting synapses. Tau oligomers are detected at pre-symptomatic AD stages (52), and might be the most toxic and pathological form of Tau aggregates (53, 54). Contrary to physiologic Tau degradation (39, 40), clearance of toxic Tau, in particular Tau oligomers, upon stimulation of synaptic activity did not require the proteasome. Strikingly, activity-dependent clearance of pathologic Tau required lysosomal activity, suggesting that synaptic stimulation might directly act on the autophagic-lysosomal degradation pathway. In agreement with our findings, stimulation of autophagy has been shown to reduce Tau aggregates and to improve neuronal survival in models of AD and Tauopathy (41, 42, 55–57). Our data also indirectly rule out that expression of Tau, under the experimental conditions that we used, might induce cell death due to lysosomal damage, as suggested for Aβ (58); indeed, we did not observe cell death in overexpressing cells, regardless the state of activation of their synapses (data not shown).
How could synaptic activity modulate autophagic clearance? We provided evidence that synaptic activity enhances maturation of cathepsin D in neurons and degradation of a lysosomal substrate. In line with these observation, it was recently demonstrated that synaptic maintenance protects against lysosomal storage diseases (59). While the mechanistic details for the control of autophagic clearance by synaptic activity remain to be determined, one possibility is that synaptic activity might regulate the function of transcription Factor EB (TFEB), a well-known master regulator of autophagy and lysosomes. Indeed, activation of TFEB was reported to control autophagic clearance of phospho-Tau via modulation of the PTEN/PI3K/mTor pathway (60). Because TFEB activity at lysosomes is regulated by Ca2+ signaling (61), an attractive hypothesis is that activation of autophagy and lysosomal clearance might be sensitive to the ionic changes associated with synaptic transmission. In this scenario, synaptic activity could enhance autophagosome formation along nerve terminals to uptake cytoplasmic oligomeric Tau, targeted by p62, within autophagosomes. Consistent with this interpretation, pro-aggregating Tau fragments have been proposed to be cleared by chaperone-mediated microautophagy (62), which appears to be activated at synapsis during activity (63, 64). Mature autophagosomes are known to reach the soma by retrograde transport before lysosomal degradation (65), accounting for our observation that auto-lysosomes containing oligomeric Tau are mostly in the soma. In support of this hypothesis, we showed that autophagy inhibition markedly decreased the presence of Tau oligomers in the soma and within lysosomes, and promoted their accumulation in neurites. These outcomes seems to indicate that transport of oligomeric tau from the cell periphery to lysosomes in the soma depends on autophagy; however, since a reduced amount of oligomers could still be observed in somas and lysosomes, we cannot exclude the possibility that the endocytic pathway could also play a role in targeting oligomeric Tau to lysosomes, perhaps limited to the extracellular pool, as shown for Aβ (58). In the light of these considerations and the fact that TFEB is also known to control lysosomal exocytosis in mammalian models of lysosomal storage diseases (66), it will be interesting in the future to determine how modulation of synaptic activity may contribute to extracellular Tau spreading, which was recently reported to increase upon synaptic stimulation (67).
As previously mentioned, alterations of brain activity/metabolism are among the earliest markers of AD pathogenesis. The literature contains data showing neuronal hyper or hypoactivity in subjects with high risk to develop AD (68). While hypoactivity is typically associated with deleterious symptoms, more recent outcomes provided evidence for compensatory/protective mechanisms of hyperactivity in pre-symptomatic AD brains (69). Therefore, synaptic activation might be protective for the preservation of neurons and synapses, and conservancy of cognitive functions with aging (15). In support of this hypothesis, epidemiological studies reported that higher educational attainment, or involvement in intellectual activities correlates with reduced risk of developing AD (70, 71). DBS is a surgical procedure utilized to treat movement disorders, including Parkinson’s disease, and dystonia (72). Recent studies demonstrated that DBS is safe and well tolerated in AD patients (73), and phase I and II trials are providing promising results of DBS as treatment for AD (19, 20, 74, 75). Here we demonstrated that DBS protective mechanism include induction of the autophagic-lysosomal degradation of pathological Tau and synapse preservation, both of which might explain the better clinical outcomes observed in some AD patients (19, 75). Overall, our data provide evidence for positive effects of synaptic stimulation against AD and FTD pathologies, and support future therapeutic investigations involving the modulation of synaptic activity and of autophagy.
Supplementary Material
Acknowledgments
Authors thank: Dr. Flint Beal and Ms. Shari Jainuddin for providing the PS19 model; Dr. Rakez Kayed for providing the T22 antibody; Dr. Magali Dumont for her help in the animal protocol preparation; Dr. Benoit Delatour and Dr. Ihsen Youssef for helping with mouse perfusions; Dr. Annick Prigent and the ICM histology facility team for helping with the cytochrome oxidase staining; Dr. Lydia Danglot for the Icy software training. In vivo studies were performed at the PHENO-ICMice Core Facility with the help of Dr. Nadège Sarrazin, Dr. Magali Dumont, and Ms. Béatrice Moreau; the Core is supported by the "Investissements d'avenir" ANR-10- IAIHU-06 and ANR-11-INBS-0011-NeurATRIS, and by the "Fondation pour la Recherche Médicale". CIBERNED and grants from the Ministerio de Economía y Competitividad (SAF2013-45084-R), Govierno Vasco, Ikerbasque and Universidad del País Vasco UPV/EHU to E.C., E.A. and C.M. This study was possible by the support of Institut Professeur Baulieu to Y.A. and D.T.
References
- 1.Bayer TA, Wirths O. Intracellular accumulation of amyloid-beta - a predictor for synaptic dysfunction and neuron loss in Alzheimer's disease. Frontiers in Aging Neuroscience. 2010;2(8):1–10. doi: 10.3389/fnagi.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E. Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer's disease. Acta Neuropathol. 2010 May;119(5):523–541. doi: 10.1007/s00401-010-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tai HC, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, Hyman BT. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol. 2012 Oct;181(4):1426–1435. doi: 10.1016/j.ajpath.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clare R, King VG, Wirenfeldt M, Vinters HV. Synapse loss in dementias. J Neurosci Res. 2010 Aug 1;88(10):2083–2090. doi: 10.1002/jnr.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002 Oct 25;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 6.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990 May;27(5):457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 7.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991 Oct;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 8.Herholz K, Carter SF, Jones M. Positron emission tomography imaging in dementia. Br J Radiol. 2007 Dec;80(Spec No 2):S160–167. doi: 10.1259/bjr/97295129. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien JL, O'Keefe KM, LaViolette PS, DeLuca AN, Blacker D, Dickerson BC, et al. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010 Jun 15;74(24):1969–1976. doi: 10.1212/WNL.0b013e3181e3966e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchetti C, Marie H. Hippocampal synaptic plasticity in Alzheimer's disease: what have we learned so far from transgenic models? Rev Neurosci. 2011;22(4):373–402. doi: 10.1515/RNS.2011.035. [DOI] [PubMed] [Google Scholar]
- 11.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A. 2004 Jan 6;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009 Jul 30;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy DS, Arons A, Mitchell TI, Pignatelli M, Ryan TJ, Tonegawa S. Memory retrieval by activating engram cells in mouse models of early Alzheimer's disease. Nature. 2016 Mar 24;531(7595):508–512. doi: 10.1038/nature17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swaab DF, Bao AM. (Re-)activation of neurons in aging and dementia: lessons from the hypothalamus. Exp Gerontol. 2010 Feb-Mar;46(2–3):178–184. doi: 10.1016/j.exger.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Tampellini D. Synaptic activity and Alzheimer's disease: a critical update. Front Neurosci. 2015;9:423. doi: 10.3389/fnins.2015.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bas-Orth C, Bading H. The divergence-convergence model of acquired neuroprotection. Mech Dev. 2013 Jun-Aug;130(6–8):396–401. doi: 10.1016/j.mod.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez AE, Pacheco CR, Aguayo LG, Opazo CM. Rapamycin protects against Abeta-induced synaptotoxicity by increasing presynaptic activity in hippocampal neurons. Biochim Biophys Acta. 2014 Sep;1842(9):1495–1501. doi: 10.1016/j.bbadis.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Rangaraju V, Calloway N, Ryan TA. Activity-driven local ATP synthesis is required for synaptic function. Cell. 2014 Feb 13;156(4):825–835. doi: 10.1016/j.cell.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith GS, Laxton AW, Tang-Wai DF, McAndrews MP, Diaconescu AO, Workman CI, et al. Increased cerebral metabolism after 1 year of deep brain stimulation in Alzheimer disease. Arch Neurol. 2012 Sep;69(9):1141–1148. doi: 10.1001/archneurol.2012.590. [DOI] [PubMed] [Google Scholar]
- 20.Sankar T, Chakravarty MM, Bescos A, Lara M, Obuchi T, Laxton AW, et al. Deep Brain Stimulation Influences Brain Structure in Alzheimer's Disease. Brain Stimul. 2015 May-Jun;8(3):645–654. doi: 10.1016/j.brs.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005 Dec 22;48(6):913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, et al. APP processing and synaptic function. Neuron. 2003 Mar 27;37(6):925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 23.Tampellini D, Gouras GK. Synapses, synaptic activity and intraneuronal Abeta in Alzheimer's disease. Frontiers in Aging Neuroscience. 2010;2(13):1–5. doi: 10.3389/fnagi.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tampellini D, Rahman N, Gallo EF, Huang Z, Dumont M, Capetillo-Zarate E, et al. Synaptic activity reduces intraneuronal Abeta, promotes APP transport to synapses, and protects against Abeta-related synaptic alterations. J Neurosci. 2009 Aug 5;29(31):9704–9713. doi: 10.1523/JNEUROSCI.2292-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tampellini D, Capetillo-Zarate E, Dumont M, Huang Z, Yu F, Lin MT, et al. Effects of Synaptic Modulation on {beta}-Amyloid, Synaptophysin, and Memory Performance in Alzheimer's Disease Transgenic Mice. J Neurosci. 2010 Oct 27;30(43):14299–14304. doi: 10.1523/JNEUROSCI.3383-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tampellini D, Rahman N, Lin MT, Capetillo-Zarate E, Gouras GK. Impaired beta-amyloid secretion in Alzheimer's disease pathogenesis. J Neurosci. 2011 Oct 26;31(43):15384–15390. doi: 10.1523/JNEUROSCI.2986-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pooler AM, Phillips EC, Lau DH, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013 Apr;14(4):389–394. doi: 10.1038/embor.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H, et al. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014 Mar 10;211(3):387–393. doi: 10.1084/jem.20131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frandemiche ML, De Seranno S, Rush T, Borel E, Elie A, Arnal I, et al. Activity-dependent tau protein translocation to excitatory synapse is disrupted by exposure to amyloid-beta oligomers. J Neurosci. 2014 Apr 23;34(17):6084–6097. doi: 10.1523/JNEUROSCI.4261-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011 Sep 21;31(38):13469–13484. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong-Riley MT, Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2333–2337. doi: 10.1073/pnas.77.4.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003 Jul 31;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 33.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007 Feb 1;53(3):337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Tampellini D, Magrane J, Takahashi RH, Li F, Lin MT, Almeida CG, et al. Internalized antibodies to the Abeta domain of APP reduce neuronal Abeta and protect against synaptic alterations. J Biol Chem. 2007 Jun 29;282(26):18895–18906. doi: 10.1074/jbc.M700373200. [DOI] [PubMed] [Google Scholar]
- 35.Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, et al. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005 Nov;20(2):187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Revilla S, Sunol C, Garcia-Mesa Y, Gimenez-Llort L, Sanfeliu C, Cristofol R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology. 2014 Jun;81:55–63. doi: 10.1016/j.neuropharm.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 37.Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001 Jan;29(1):243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 38.Bingol B, Schuman EM. Activity-dependent dynamics and sequestration of proteasomes in dendritic spines. Nature. 2006 Jun 29;441(7097):1144–1148. doi: 10.1038/nature04769. [DOI] [PubMed] [Google Scholar]
- 39.Chesser AS, Pritchard SM, Johnson GV. Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front Neurol. 2013 Sep 03;4:122. doi: 10.3389/fneur.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee MJ, Lee JH, Rubinsztein DC. Tau degradation: the ubiquitin-proteasome system versus the autophagy-lysosome system. Prog Neurobiol. 2013 Jun;105:49–59. doi: 10.1016/j.pneurobio.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010 Apr 23;285(17):13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaeffer V, Lavenir I, Ozcelik S, Tolnay M, Winkler DT, Goedert M. Stimulation of autophagy reduces neurodegeneration in a mouse model of human tauopathy. Brain. 2012 Jul;135(Pt 7):2169–2177. doi: 10.1093/brain/aws143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuusisto E, Salminen A, Alafuzoff I. Early accumulation of p62 in neurofibrillary tangles in Alzheimer's disease: possible role in tangle formation. Neuropathol Appl Neurobiol. 2002 Jun;28(3):228–237. doi: 10.1046/j.1365-2990.2002.00394.x. [DOI] [PubMed] [Google Scholar]
- 44.Ramesh Babu J, Lamar Seibenhener M, Peng J, Strom AL, Kemppainen R, Cox N, et al. Genetic inactivation of p62 leads to accumulation of hyperphosphorylated tau and neurodegeneration. J Neurochem. 2008 Jul;106(1):107–120. doi: 10.1111/j.1471-4159.2008.05340.x. [DOI] [PubMed] [Google Scholar]
- 45.Su H, Wang X. p62 Stages an interplay between the ubiquitin-proteasome system and autophagy in the heart of defense against proteotoxic stress. Trends Cardiovasc Med. 2011 Nov;21(8):224–228. doi: 10.1016/j.tcm.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer's disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol. 2000 Jul;157(1):277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ihara Y, Morishima-Kawashima M, Nixon R. The ubiquitin-proteasome system and the autophagic-lysosomal system in Alzheimer disease. Cold Spring Harb Perspect Med. 2012 Aug 01;2(8):2–27. doi: 10.1101/cshperspect.a006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maxfield FR. Role of endosomes and lysosomes in human disease. Cold Spring Harb Perspect Biol. 2014 May 01;6(5):a016931. doi: 10.1101/cshperspect.a016931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nixon RA, Yang DS. Autophagy failure in Alzheimer's disease--locating the primary defect. Neurobiol Dis. 2011 Jul;43(1):38–45. doi: 10.1016/j.nbd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, et al. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci. 2008 Mar;27(5):1119–1130. doi: 10.1111/j.1460-9568.2008.06084.x. [DOI] [PubMed] [Google Scholar]
- 51.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12(1):1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maeda S, Sahara N, Saito Y, Murayama S, Ikai A, Takashima A. Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer's disease. Neurosci Res. 2006 Mar;54(3):197–201. doi: 10.1016/j.neures.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Brunden KR, Trojanowski JQ, Lee VM. Evidence that non-fibrillar tau causes pathology linked to neurodegeneration and behavioral impairments. J Alzheimers Dis. 2008 Aug;14(4):393–399. doi: 10.3233/jad-2008-14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marx J. Alzheimer's disease. A new take on tau. Science. 2007 Jun 8;316(5830):1416–1417. doi: 10.1126/science.316.5830.1416. [DOI] [PubMed] [Google Scholar]
- 55.Schaeffer V, Goedert M. Stimulation of autophagy is neuroprotective in a mouse model of human tauopathy. Autophagy. 2012 Nov;8(11):1686–1687. doi: 10.4161/auto.21488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caccamo A, Ferreira E, Branca C, Oddo S. p62 improves AD-like pathology by increasing autophagy. Mol Psychiatry. 2016 Aug;30:1–9. doi: 10.1038/mp.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, et al. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron. 2017 Mar 08;93(5):1015–1034. doi: 10.1016/j.neuron.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 58.Ditaranto K, Tekirian TL, Yang AJ. Lysosomal membrane damage in soluble Abeta-mediated cell death in Alzheimer's disease. Neurobiol Dis. 2001 Feb;8(1):19–31. doi: 10.1006/nbdi.2000.0364. [DOI] [PubMed] [Google Scholar]
- 59.Sambri I, D'Alessio R, Ezhova Y, Giuliano T, Sorrentino NC, Cacace V, et al. Lysosomal dysfunction disrupts presynaptic maintenance and restoration of presynaptic function prevents neurodegeneration in lysosomal storage diseases. EMBO Mol Med. 2017 Jan;9(1):112–132. doi: 10.15252/emmm.201606965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Polito VA, Li H, Martini-Stoica H, Wang B, Yang L, Xu Y, et al. Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. EMBO Mol Med. 2014 Jul 28;6(9):1142–1160. doi: 10.15252/emmm.201303671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015 Mar;17(3):288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Martinez-Vicente M, Kruger U, Kaushik S, Wong E, Mandelkow EM, et al. Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum Mol Genet. 2009 Nov 1;18(21):4153–4170. doi: 10.1093/hmg/ddp367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McPherson PS. Eating Locally: Microautophagy and Protein Turnover at the Synapse. Neuron. 2015 Nov 18;88(4):619–621. doi: 10.1016/j.neuron.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 64.Uytterhoeven V, Lauwers E, Maes I, Miskiewicz K, Melo MN, Swerts J, et al. Hsc70-4 Deforms Membranes to Promote Synaptic Protein Turnover by Endosomal Microautophagy. Neuron. 2015 Nov 18;88(4):735–748. doi: 10.1016/j.neuron.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol. 2012 Feb 20;196(4):407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C, et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell. 2011 Sep 13;21(3):421–430. doi: 10.1016/j.devcel.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. 2016 Aug;19(8):1085–1092. doi: 10.1038/nn.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stargardt A, Swaab DF, Bossers K. The storm before the quiet: neuronal hyperactivity and Abeta in the presymptomatic stages of Alzheimer's disease. Neurobiol Aging. 2015 Jan;36(1):1–11. doi: 10.1016/j.neurobiolaging.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 69.Elman JA, Oh H, Madison CM, Baker SL, Vogel JW, Marks SM, et al. Neural compensation in older people with brain amyloid-beta deposition. Nat Neurosci. 2014 Oct;17(10):1316–1318. doi: 10.1038/nn.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006 Jul-Sep;20(3 Suppl 2):S69–74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 71.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. Jama. 1994 Apr 6;271(13):1004–1010. [PubMed] [Google Scholar]
- 72.Pizzolato G, Mandat T. Deep brain stimulation for movement disorders. Front Integr Neurosci. 2012;6(2):1–5. doi: 10.3389/fnint.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ponce FA, Asaad WF, Foote KD, Anderson WS, Rees Cosgrove G, Baltuch GH, et al. Bilateral deep brain stimulation of the fornix for Alzheimer's disease: surgical safety in the ADvance trial. J Neurosurg. 2016 Jul;125(1):75–84. doi: 10.3171/2015.6.JNS15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol. 2010 Oct;68(4):521–534. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 75.Lozano AM, Fosdick L, Chakravarty MM, Leoutsakos JM, Munro C, Oh E, et al. A Phase II Study of Fornix Deep Brain Stimulation in Mild Alzheimer's Disease. J Alzheimers Dis. 2016 Sep 6;54(2):777–787. doi: 10.3233/JAD-160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.