Abstract
Objective
Insomnia and disrupted sleep are associated with increased risk of suicide. The N-methyl-d-aspartate antagonist ketamine has been associated with reduced suicidal thoughts, but the mechanism of action is unknown. This study sought to evaluate differences in nocturnal wakefulness in depressed individuals who did and did not have an antisuicidal response to ketamine.
Methods
Thirty-four participants with baseline suicidal ideation diagnosed with either DSM-IV major depressive disorder (n = 23) or bipolar depression (n = 11) between 2006 and 2013 completed nighttime electroencephalography (EEG) the night before and the night after a single ketamine infusion (0.5 mg/kg over 40 minutes). Suicidal ideation was assessed at baseline and the morning after ketamine infusion via several measures, including the Hamilton Depression Rating Scale suicide item, the suicide item of the Montgomery-Asberg Depression Rating Scale, and the first 5 items of the Scale for Suicide Ideation. A generalized linear mixed model evaluated differences in nocturnal wakefulness, as verified by EEG, between those who had an antisuicidal response to ketamine and those who did not, controlling for baseline nocturnal wakefulness. Results were also compared to the sleep of healthy controls (n = 22).
Results
After analyses adjusted for baseline sleep, participants with an antisuicidal response to ketamine showed significantly reduced nocturnal wakefulness the night after ketamine infusion compared to those without an antisuicidal response (F1,22 = 5.04, P = .04). Level of nocturnal wakefulness after antisuicidal response to ketamine did not differ significantly from nocturnal wakefulness in the control sample but did differ at a trend level (F1,40 = 3.15, P = .08).
Conclusions
Reductions in wakefulness following ketamine may point to a biological mechanism underlying the effect of ketamine on suicidal ideation.
Trial Registration
ClinicalTrials.gov identifier: NCT00088699
In 2012, there were 40,600 suicides in the United States, making it the tenth leading cause of death overall, but the second and third leading cause of death for ages 15–34 years and 10–14 years, respectively.1 Despite the allocation of resources to predict and prevent suicide, national suicide rates have changed very little over the last 2 decades.2 Limited pharmacologic options exist for reducing suicide. Clozapine, a second-generation antipsychotic, is the only medication with a specific US Food and Drug Administration (FDA) indication for reducing the risk of recurrent suicidal behavior, specifically in those with schizophrenia.3 Both electroconvulsive therapy4 and lithium,3 a mood stabilizer, are also thought to have antisuicidal properties.
Given the limited ability of medications to reduce suicide, primary research goals include identifying and understanding underlying risk factors for suicide, which may then guide the development of targeted interventions.5 Unlike age or gender, sleep is a modifiable risk factor and potential treatment target for reducing suicidal thoughts and behaviors.6 Considerable research has demonstrated that disrupted sleep is associated with increased risk of suicide death in both adults and adolescents,7–9 and this association is often independent of depressive symptoms.10 Numerous sleep parameters have been investigated in conjunction with suicide risk, including sleep duration,11 difficulty falling asleep,12 and difficulty staying asleep.12 However, most published studies that have systematically investigated this relationship have relied on self-reported data; few studies have used objective measures, such as nighttime electroencephalography (EEG), to evaluate sleep in those with suicidal thoughts. Furthermore, critical methodological differences have prevented comparison of effects and correspondence.13
Investigators have also worked to understand how psychotropic medications—particularly antidepressants—affect sleep and whether an association exists between sleep changes and antidepressant response.14 Although antidepressants have been shown to affect objective sleep measures, few studies have investigated how sleep changes relate to clinical antidepressant response. For example, in several studies of depressed patients, fluoxetine and fluvoxamine decreased sleep efficiency and increased the number of awakenings,15–18 while other selective serotonin reuptake inhibitors (eg, sertraline, citalopram) did not affect sleep efficiency or continuity.18–20 Roth and colleagues investigated the effects of doxepin, a tricyclic antidepressant, and found that it improved sleep efficiency and reduced the number of awakenings after sleep onset; these measures correlated with improvement in depressive symptoms.21 However, none of these studies examined the relationship between sleep changes and antisuicidal response.
Understanding the role of sleep continuity in suicidal and depressed individuals would help clarify its potential role both as a neurobiological factor that contributes to acute suicide risk and as a future target for suicide prevention. Specifically, the relationship between reduced suicidal thoughts and sleep measures—such as nighttime EEG—has not previously been examined. This study used nighttime EEG to investigate the relationship between nocturnal wakefulness and antisuicidal response to ketamine, an N-methyl-d-aspartate (NMDA) receptor antagonist, in treatment-resistant patients with current suicidal ideation experiencing a major depressive episode. Previous studies found a rapid decline in suicidal ideation after a single ketamine infusion,22,23 and some of these effects were demonstrated to occur independently of antidepressant response.24 This work builds on our previous sleep EEG analysis, which demonstrated that wakefulness after sleep onset, particularly between the hours of 4:00–4:59 am, was associated with next-day suicidal thoughts in depressed patients, even after adjusting for age, gender, diagnosis, and severity of depression.25 Our analyses focused on hourly totals of wakefulness between midnight and 4:59 am specifically because of our previous findings as well as recent research suggesting that this time period may be critical for suicide risk.26
METHODS
Patient Population
This study was a secondary analysis that included 34 patients with suicidal ideation and treatment-resistant depression who participated in a series of clinical trials to evaluate the efficacy of ketamine between 2006 and 2013 (ClinicalTrials.gov identifier: NCT00088699).27–30 Patients were 18–65 years old (20 men, 14 women; mean age = 44.97 years, SD = 13.28) and had a diagnosis of either major depressive disorder (MDD) (n = 23) or bipolar disorder (n = 11); diagnoses were determined by a face-to-face interview with a licensed, independent practitioner and confirmed with the Structured Clinical Interview for Axis I DSM-IV Disorders-Patient Version (SCID). All patients were currently experiencing a major depressive episode. Inclusion criteria required a Montgomery-Asberg Depression Rating Scale (MADRS) score reflecting at least moderate severity (greater than 2027,29 or 2228 depending on the substudy for which the participant enrolled). Acute suicidal thoughts signaling imminent suicide risk upon entry to the study (before medication taper) were an exclusion criterion for one of the clinical trials.
All MDD patients were medication-free for at least 2 weeks (5 weeks for fluoxetine), and bipolar disorder patients were only taking either lithium or valproate at therapeutic levels. Subjects were not allowed scheduled or PRN use of hypnotics. All participants were inpatients on the Mood and Anxiety Disorders Research Unit at the National Institute of Mental Health (NIMH) in Bethesda, Maryland, and provided written informed consent before entry into the study.
Notably, a subset (n = 11) of patients in this sample were randomized to receive a single dose of riluzole (50 mg), a glutamate modulator FDA-approved for the treatment of amyotrophic lateral sclerosis, the night after receiving open-label ketamine infusion. Because previously published data31 suggested that add-on riluzole does not affect slow-wave activity beyond the effects already seen with ketamine, planned analyses were completed for the entire sample (n = 34) as well as among the subsample that received ketamine only (n = 23).
Baseline sleep data were also obtained for 22 healthy control subjects (11 men, 11 women; mean age = 37.14 years, SD = 10.21). These controls were assessed using the SCID, physical and neurologic examinations, and laboratory tests; they had no current or past psychiatric diagnoses or first-degree relatives with DSM Axis I disorders. All control subjects gave written informed consent as approved by the NIMH Institutional Review Board and participated at the Mood and Anxiety Disorders Research Unit at the NIMH.
Study Design
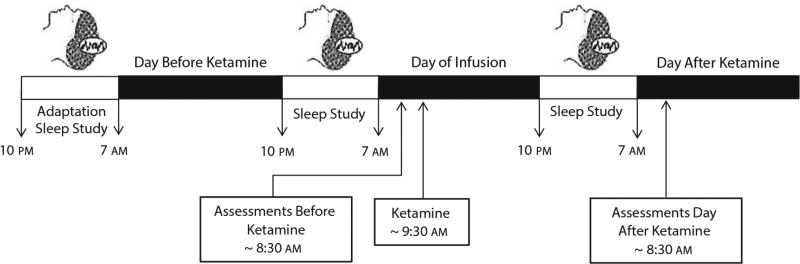
All patients received intravenous ketamine hydrochloride (0.5 mg/kg over 40 minutes). Polysomnography (PSG) was completed the night before and the night after ketamine infusion, as previously described.31 Briefly, all patients were adapted to the sleep study prior to their first recorded PSG. The whole-night PSG used a Nihon-Khoden System (Neurofax version 05–50) and Polysmith Acquisition and Review software (version 4.0.25.0) to record EEGs (C3/A2 and C4/A1), electrooculograms, and submental electromyograms. The EEGs were read in 30-second epochs by a reviewer blind to patient status and study night (eg, pre- or post-ketamine). For each 30-second epoch, the patient was classified as being either awake or asleep. Because our primary focus was wakefulness that interrupted the continuity of sleep, hourly minutes awake for each hour of the night from midnight until 04:59 am were calculated. Figure 1 illustrates the timeline of sleep studies, assessments, and ketamine infusion.
Figure 1.
Timeline for Ketamine Infusion, Assessments, and Sleep Studiesa
aParticipants completed an adaptation sleep study prior to their first recorded polysomnography (PSG), which occurred the night before the ketamine infusion. A second recorded PSG was done the night after the ketamine infusion. Ratings were taken 60 minutes before ketamine infusion and then again 24 hours later.
Assessments
The HDRS was administered 60 minutes before ketamine infusion and in the morning of the day after ketamine infusion (ie, the morning after the second recorded nighttime EEG). The HDRS includes 1 item reflecting suicidal ideation and behavior that is scored on a scale of 0 to 4, where 0 represents no suicidal ideation or behavior and 4 represents active attempts at suicide. For confirmatory purposes, analyses were also run using the MADRS suicide item (item 10), which is rated from 0 to 6,32 and the first 5 items of the clinician-administered Scale for Suicidal Ideation (SSI5)33 as outcome measures. The MADRS, SSI5, and HDRS were all administered at the same time points. In addition, the first 5 items of the SSI were included in the analysis, as these items were consistently administered to all participants and because previous analyses found that the SSI5 is particularly sensitive to rapid changes in suicidal thoughts after ketamine administration.34
Analysis
Data were limited to participants who reported any suicidal ideation at baseline (Hamilton Depression Rating Scale [HDRS]35 suicide item > 0). An antisuicidal response to ketamine was operationalized as any participant who reported suicidal thoughts (HDRS score > 0 on suicide item) before ketamine infusion and no suicidal thoughts (HDRS suicide item = 0) 1 day after ketamine infusion. This cutoff mirrored our previous analysis,25 which demonstrated that individuals with a HDRS score over 0 on the suicide item had more nocturnal wakefulness on nighttime EEG, particularly later in the night. For confirmatory analyses using either the MADRS suicide item or the SSI5, a score of 2 or more was considered to be “any suicidal ideation.”36,37
T tests and χ2 tests were used to evaluate possible baseline demographic, clinical, or sleep-related differences between suicide responders and nonresponders to ketamine. Due to the distribution of minutes awake, a negative binomial generalized linear mixed model with a log linking function was used to evaluate differences in nocturnal wakefulness between suicide responders and nonresponders the night after ketamine infusion. Minutes awake was the outcome variable, and suicide responder status was the independent variable. The time frame was limited to 12:00 am to 4:59 am, in line with previous analyses by our group that showed that nocturnal wakefulness during this time was associated with suicidal thoughts the following morning,25 as well as epidemiologic results suggesting that this time period is associated with high rates of suicide death.26 Time was included in the model in 1-hour intervals, and nocturnal wakefulness over the previous night (before ketamine infusion) was added as a time-dependent covariate. The mixed model included a first-order autoregressive structure according to Schwarz’s Bayesian fit criteria, and due to the small sample size, a Satterthwaite approximation was used to determine degrees of freedom. Violations of model assumptions were assessed using robust estimation of fixed effects and coefficients. Because this sample included some patients who received add-on riluzole, the same analysis was completed on the sample that only received ketamine. As a corroboratory test, another generalized linear mixed model was used to compare waking in suicide responders to healthy controls. As another analysis, the model was run adjusting for the effect of psychiatric diagnosis (MDD vs bipolar disorder) both as a main effect and as an interaction. As a last analysis, the model was run adjusting for antidepressant response, which was operationalized as a 50% reduction in HDRS score with the suicide item removed. All statistics were conducted using IBM SPSS version 21, and significance was considered at P < .05, 2-tailed.
RESULTS
No significant differences were observed in baseline demographic, clinical, or nocturnal EEG verified wakefulness for those who did and did not have an antisuicidal response to ketamine, with the exception of clinically determined melancholic depression (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics for Responders Versus Nonresponders to Ketamine
| Responders at Day 1 (n = 14) |
NonResponders at Day 1 (n = 20) |
|||
|---|---|---|---|---|
| n (%) | n (%) | χ2 | P | |
|
| ||||
| Male gender | 7 (50) | 13 (65) | 0.77 | .38 |
| Lifetime history of suicide attempt | 8 (57) | 11 (55) | 0.02 | .90 |
| Bipolar I or II diagnosis | 5 (36) | 6 (30) | 0.12 | .73 |
| Melancholic depression | 2 (14) | 10 (50) | 4.60 | .03 |
| Mean (SD) | Mean (SD) | t | P | |
|
|
|
|
|
|
| Age, y | 45.14 (13.04) | 44.85 (13.79) | 0.06 | .95 |
| Illness duration, y | 27.00 (12.71) | 25.30 (11.32) | 0.62 | .54 |
| Baseline clinical characteristics | ||||
| SSI | 3.86 (5.11) | 4.44 (5.98) | −0.29 | .77 |
| Depression/sleep HDRS items | ||||
| Suicide item | 1.50 (0.76) | 1.75 (0.79) | −0.93 | .36 |
| Early insomnia | 1.29 (0.91) | 0.95 (0.94) | 1.03 | .31 |
| Middle insomnia | 0.71 (0.73) | 1.20 (0.77) | −1.86 | .07 |
| Late insomnia | 0.64 (0.74) | 0.85 (0.88) | −0.72 | .48 |
| Remaining HDRS items | 19.36 (2.76) | 17.90 (3.58) | 1.28 | .21 |
| Baseline sleep characteristics | ||||
| Total sleep time, min | 364.43 (39.93) | 369.90 (71.99) | −0.46 | .80 |
| Wakefulness after sleep onset, min | 38.86 (24.73) | 59.70 (59.77) | …a | .74 |
| Sleep efficiency (%)b | 89.48 (6.24) | 85.19 (14.20) | …a | .85 |
Nonparametric test using Mann-Whitney U.
Percentage of time asleep to time spent in bed.
Abbreviations: HDRS = Hamilton Depression Rating Scale, SSI = Scale for Suicide Ideation.
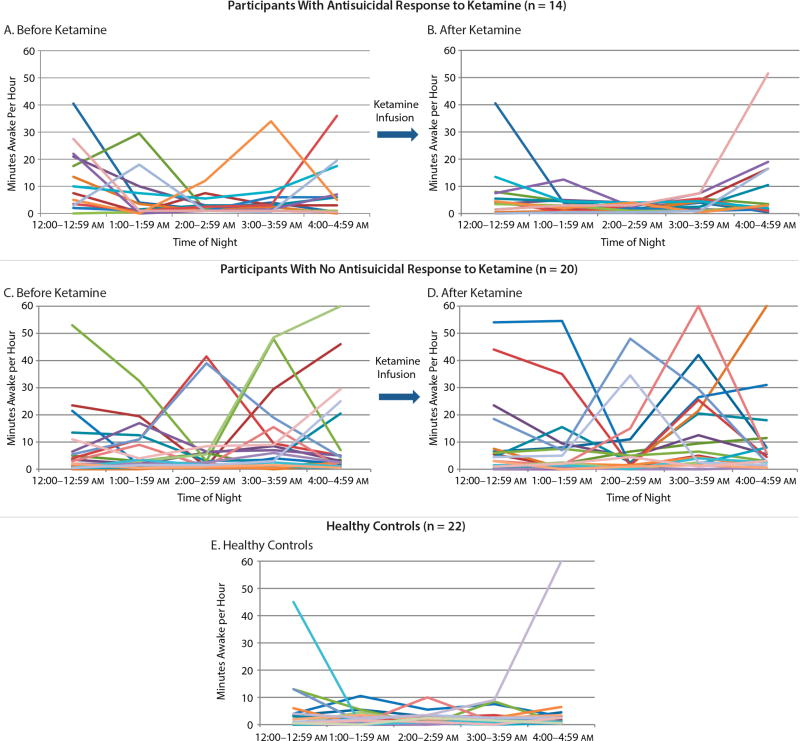
Patterns of nocturnal wakefulness over the 2 nights of the analysis are presented in Figure 2. When controlling for baseline nocturnal wakefulness, the generalized linear mixed model indicated a significant difference in wakefulness on the night after ketamine infusion between suicide responders and nonresponders to ketamine (F1,22 = 5.04, P = .04, d = 0.96), with responders demonstrating fewer minutes of nocturnal wakefulness. The time-by-responder interaction was at a trend level (F4,57 = 2.23, P = .08).
Figure 2.
Relationship Between Wakefulness From 12:00–4:59 am and Antisuicidal Response to Ketaminea
aEach line represents the wakefulness pattern of 1 participant. Parts A and C show baseline minutes awake recorded from the baseline sleep study (ie, night before ketamine) in participants who did (A) and did not (C) have an antisuicidal response to ketamine. Parts B and D show minutes awake recorded from the second sleep study (ie, night after ketamine) in participants who did (B) and did not (D) have an antisuicidal response to ketamine. Part E shows minutes awake recorded in a sample of healthy controls who did not receive ketamine.
When the sample was limited to the 23 suicidal participants who had only received ketamine (and not riluzole), overall differences were comparable. The main effect of antisuicidal treatment response was at a trend level in the expected direction (F1,13 = 4.28, P = .059, d = 1.15), but the time-by-responder interaction was significant (F4,37 = 3.93, P = .009). Post hoc tests demonstrated a significant difference between responders and nonresponders only at the 3:00–3:59 hour (F1,42 = −8.04, P = .02, d = 0.88). In order to compare this subsample (participants who did not receive riluzole) to the entire sample (all 34 participants), effect sizes (Cohen d) were calculated for each main effect. The effect size for overall nocturnal wakefulness in responders compared to nonresponders was d = 1.15 in the non-riluzole subsample and d = 0.96 in the overall sample, both indicating a large effect.
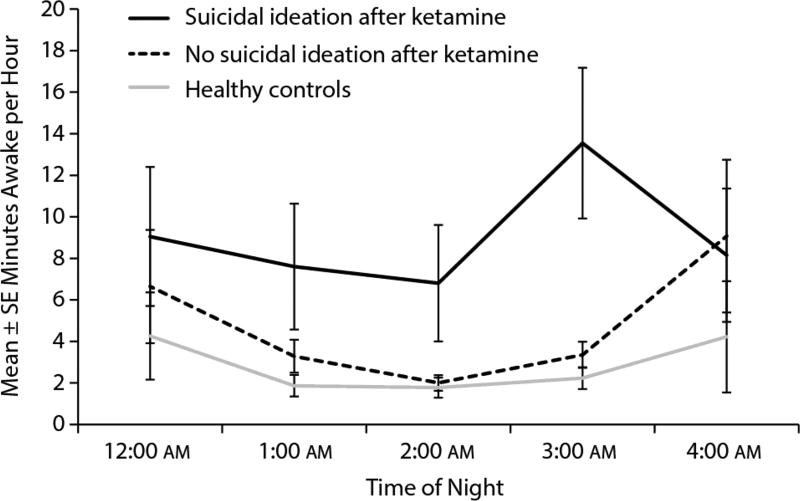
Mean minutes awake by hour of night in suicide responders and nonresponders to ketamine are presented in Figure 3. Minutes awake in the healthy control sample that did not receive ketamine are also plotted. A generalized linear mixed model found a trend between suicide responders to ketamine and healthy controls (F1,40 = 3.15, P = .08, d = 0.56), suggesting potential differences between the healthy controls and those participants who experienced an antisuicidal response to ketamine. No significant group-by-time interaction was observed (F4,72 = 0.44, P = .78).
Figure 3.
Average Wakefulness in Suicide Responders, Nonresponders, and Healthy Controlsa
aMean and standard error for wakefulness-by-hour for the night following ketamine infusion in participants with and without an antisuicidal response to ketamine. The wakefulness of the healthy control sample (n = 22) is shown as a comparison group.
To confirm these results, we ran similar analyses using the MADRS suicide item and the SSI5 as outcome measures, with results compared using effect sizes. Using the MADRS suicide item (n = 36), the main effect of the response was significant (P = .04), with an effect size of d = 0.88. Using the SSI5 (n = 24), the main effect was not significant (P = .20) despite a comparable effect size (d = 0.88), which suggests that the reduced significance may be due to the smaller sample size.
As a secondary analysis, we also evaluated the impact of diagnosis on the results using the HDRS suicide item. When diagnosis was added to the model, a significant main effect was still seen for response (P = .02, d = 0.94). Neither diagnosis alone nor any of the interaction terms with diagnosis were significant. Due to the relationship between suicidal thoughts and depression, we also ran analyses adjusting for antidepressant response. When antidepressant response was added to the model (reduction in HDRS with the suicide item removed), the main effect of the suicide response became a trend (P = .080), with a medium effect size (d = 0.49). Neither antidepressant response nor the interaction term with antidepressant response was significant.
DISCUSSION
Depressed patients who experienced an antisuicidal response to a single ketamine infusion had decreased nocturnal wakefulness the night after ketamine compared to those with no antisuicidal response. The pattern of nocturnal wakefulness by clock time (ie, between midnight and 4:59 am) for those with an antisuicidal response appeared to normalize after ketamine and reflected similar sleep patterns as healthy volunteers. This effect was observed after controlling for baseline sleep (ie, the night prior to ketamine infusion). The findings suggest that reduced nocturnal wakefulness following ketamine infusion may point to an underlying neurobiological mechanism for the effect of ketamine on suicidal thoughts.
These findings converge with recent studies demonstrating that a subanesthetic dose of ketamine rapidly decreases suicidal ideation,22,23 though the mechanism of antisuicidal response remains unknown. To date, no studies have evaluated EEG-assessed nocturnal wakefulness, a correlate of insomnia and subjective sleep disturbance, as one such factor. Antidepressant response to ketamine is likely multifactorial at the cellular and molecular level, and possibly includes increased α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid to NMDA receptor throughput and/or increased synaptogenesis secondary to increased brain-derived neurotrophic factor (BDNF) release.38 Evidence also suggests that, although ketamine’s antisuicidal response may be related to its antidepressant effects, the former is not entirely driven by the latter,24 suggesting that other mechanisms may be responsible for the antisuicidal response. At the neural circuit level, baseline suicidal ideation and regional cerebral glucose metabolism in the infralimbic cortex were significantly correlated on positron emission tomography imaging, but overall mood scores were not.39 Furthermore, Ballard and colleagues39 found that reductions in suicidal ideation after ketamine infusion were correlated with decreased regional cerebral glucose metabolism in the infralimbic cortex, but other mood symptoms were not. In the current study, a model controlling for antidepressant response showed a reduced effect of suicidal ideation on wakefulness, but antidepressant response was not significantly associated with wakefulness in this model; therefore, results are somewhat inconclusive. These results underscore the importance of future studies of ketamine response in patients selected for suicide risk, rather than depressive disorder diagnosis.
Multifactorial hypotheses have been advanced regarding possible cellular/molecular mechanisms for ketamine’s antisuicidal effects. One hypothesis proposes a connection between low-grade brain inflammation with glutamate agonism and suicidal ideation/behavior40,41 as well as disturbed sleep.42 Another potential pathway is the connection between BDNF, sleep, and suicide. The presence of the BDNF single nucleotide polymorphism rs6265 (Val66Met) was significantly associated with suicide attempts.43 Furthermore, decreased BDNF levels were found in patients who recently attempted suicide compared to nonsuicidal MDD patients or normal controls.44 Interestingly, ketamine significantly increases plasma BDNF levels in those who exhibit an antidepressant response, and this effect is most pronounced in those with the Val/Val BDNF allele at rs6265.45,46 Moreover, Duncan and colleagues reported that BDNF levels increased after ketamine infusion in those with an antidepressant response, and that this was associated with increased slow-wave sleep during the first non-REM episode.31 Future investigations are needed to investigate whether antisuicidal response to ketamine may correlate with BDNF levels and other sleep architecture variables.
This study has several strengths. First, in contrast to previous studies, sleep changes were measured objectively (via EEG), and antisuicidal response secondary to a pharmacologic agent (ketamine) was directly investigated. Second, several metrics measuring suicidal ideation were used (HDRS, MADRS, and SSI) to provide corroborating evidence. Third, this study used healthy controls as a comparison group.
Nevertheless, the study is also associated with several limitations. First, due to safety concerns, the study population excluded patients who had acute, serious suicidal thoughts or behaviors, which prevented analysis of patients at imminent risk of suicide. Second, all participants had treatment-resistant depression and thus the results may not be generalizable to patients with suicidal ideation and non–treatment-resistant depression or other psychiatric diagnoses. Third, because sleep analyses were limited to minutes awake per night, other sleep architecture variables (eg, REM sleep, slow-wave sleep) require further investigation. Fourth, the relationship between sleep and both antisuicidal and antidepressant response requires additional investigation, as secondary analyses that adjusted for antidepressant response had reduced significance, though still trending in the expected direction; additional investigations with larger sample sizes may provide more conclusive results. Fifth, the depressed sample was not matched to the healthy control sample. Sixth, these findings cannot address causation. Further studies are needed to evaluate whether improving disrupted sleep and suicidal ideation with ketamine is a specific effect or whether other antidepressant agents that improve sleep would have similar effects. Finally, the role of depressive subtypes, such as melancholic depression assessed using DSM-confirmed criteria, may be beneficial in future analyses, as melancholia has been shown to be an important predictor of suicide.47
Despite these limitations, to our knowledge this study is the first to investigate how the rapid-acting, antisuicidal effects of ketamine may be related to night-time wakefulness patterns during sleep. Depressed participants with an antisuicidal response to ketamine showed reduced wakefulness following ketamine, a sleep pattern that resembled the sleep of healthy controls. The findings of this study are consistent with previous time-of-day studies showing that increased wakefulness between midnight and 4:59 am is associated with increased suicidal ideation the next day25 and that increased rates of death by suicide occur at the same general clock time (ie, early morning hours).26 Current psychotropic medications can take weeks to months to achieve a desired treatment response. This time frame underscores the need for novel, rapid-acting antidepressant therapies, particularly for those at elevated risk for suicide. Finally, the results identify a potential mechanism for the rapid reduction of suicidal thoughts after ketamine infusion by implicating its impact on disturbed sleep. Future analyses should seek to investigate and replicate this key phenomenon.
Clinical Points.
-
■
Disrupted sleep is associated with increased risk of suicide, and ketamine may have antisuicidal effects. However, no studies have examined the relationship between nocturnal wakefulness and antisuicidal response to ketamine.
-
■
Participants with an antisuicidal response to ketamine showed significantly reduced nocturnal wakefulness the night after ketamine infusion. This may point to an underlying mechanism of ketamine’s effect on suicidal ideation.
Acknowledgments
Ioline Henter, MA (NIMH) provided invaluable editorial assistance and was funded by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health.
Dr Zarate is listed as a co-inventor on a patent for the use of ketamine and its metabolites in major depression, and he has assigned his rights in the patent to the US government but will share a percentage of any royalties that may be received by the government.
Funding/support: Funding for this work was provided in part by the Intramural Research Program at the National Institute of Mental Health (NIMH), National Institutes of Health (NIH) (IRP-NIMH-NIH) (NCT00024635; 04-M-0222), by a NARSAD Independent Investigator to Dr Zarate, by a Brain & Behavior Mood Disorders Research Award to Dr Zarate, and by grants from the National Institutes of Health (K23MH093490) to Dr Bernert.
Role of the sponsor: The NIMH, NIH, NARSAD, and the Brain & Behavior Research Foundation had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Drug names: citalopram (Celexa and others); clozapine (Clozaril, FazaClo, and others); doxepin (Silenor and others); fluoxetine (Prozac and others); fluvoxamine (Luvox and others); ketamine (Ketalar and others); riluzole (Rilutek and others); sertraline (Zoloft and others).
Potential conflicts of interest: All other authors have no conflict of interest to disclose, financial or otherwise.
References
- 1.Centers for Disease Control and Prevention. Injury Prevention and Control: Data and Statistics (WISQARS) [Accessed December 12, 2014];CDC Web site. 2014 http://www.cdc.gov/injury/wisqars/fatal_injury_reports.html.
- 2.Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999–2014. National Center for Health Statistics Data Brief. No. 241. 2016 Apr; http://www.cdc.gov/nchs/data/databriefs/db241.pdf. [PubMed]
- 3.Griffiths JJ, Zarate CA, Jr, Rasimas JJ. Existing and novel biological therapeutics in suicide prevention. Am J Prev Med. 2014;47(suppl 2):S195–S203. doi: 10.1016/j.amepre.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kellner CH, Fink M, Knapp R, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry. 2005;162(5):977–982. doi: 10.1176/appi.ajp.162.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services (HHS) 2012 National Strategy for Suicide Prevention: Goals and Objectives for Action. Washington, DC: Health and Human Services; 2012. [Google Scholar]
- 6.Bernert RA, Joiner TE. Sleep disturbances and suicide risk: a review of the literature. Neuropsychiatr Dis Treat. 2007;3(6):735–743. doi: 10.2147/ndt.s1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjørngaard JH, Bjerkeset O, Romundstad P, et al. Sleeping problems and suicide in 75,000 Norwegian adults: a 20 year follow-up of the HUNT I study. Sleep. 2011;34(9):1155–1159. doi: 10.5665/SLEEP.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73(9):e1160–e1167. doi: 10.4088/JCP.11r07586. [DOI] [PubMed] [Google Scholar]
- 9.Wong MM, Brower KJ. The prospective relationship between sleep problems and suicidal behavior in the National Longitudinal Study of Adolescent Health. J Psychiatr Res. 2012;46(7):953–959. doi: 10.1016/j.jpsychires.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernert RA, Turvey CL, Conwell Y, et al. Association of poor subjective sleep quality with risk for death by suicide during a 10-year period: a longitudinal, population-based study of late life. JAMA Psychiatry. 2014;71(10):1129–1137. doi: 10.1001/jamapsychiatry.2014.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blasco-Fontecilla H, Alegria AA, Lopez-Castroman J, et al. Short self-reported sleep duration and suicidal behavior: a cross-sectional study. J Affect Disord. 2011;133(1–2):239–246. doi: 10.1016/j.jad.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Wojnar M, Ilgen MA, Wojnar J, et al. Sleep problems and suicidality in the National Comorbidity Survey Replication. J Psychiatr Res. 2009;43(5):526–531. doi: 10.1016/j.jpsychires.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahl RE, Puig-Antich J, Ryan ND, et al. EEG sleep in adolescents with major depression: the role of suicidality and inpatient status. J Affect Disord. 1990;19(1):63–75. doi: 10.1016/0165-0327(90)90010-6. [DOI] [PubMed] [Google Scholar]
- 14.Duncan WC., Jr Circadian rhythms and the pharmacology of affective illness. Pharmacol Ther. 1996;71(3):253–312. doi: 10.1016/S0163-7258(96)00092-7. [DOI] [PubMed] [Google Scholar]
- 15.Armitage R, Yonkers K, Cole D, et al. A multicenter, double-blind comparison of the effects of nefazodone and fluoxetine on sleep architecture and quality of sleep in depressed outpatients. J Clin Psychopharmacol. 1997;17(3):161–168. doi: 10.1097/00004714-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Gillin JC, Rapaport M, Erman MK, et al. A comparison of nefazodone and fluoxetine on mood and on objective, subjective, and clinician-rated measures of sleep in depressed patients: a double-blind, 8-week clinical trial. J Clin Psychiatry. 1997;58(5):185–192. doi: 10.4088/JCP.v58n0502. [DOI] [PubMed] [Google Scholar]
- 17.Kupfer DJ, Perel JM, Pollock BG, et al. Fluvoxamine versus desipramine: comparative polysomnographic effects. Biol Psychiatry. 1991;29(1):23–40. doi: 10.1016/0006-3223(91)90208-4. [DOI] [PubMed] [Google Scholar]
- 18.Oberndorfer S, Saletu-Zyhlarz G, Saletu B. Effects of selective serotonin reuptake inhibitors on objective and subjective sleep quality. Neuropsychobiology. 2000;42(2):69–81. doi: 10.1159/000026676. [DOI] [PubMed] [Google Scholar]
- 19.Jindal RD, Friedman ES, Berman SR, et al. Effects of sertraline on sleep architecture in patients with depression. J Clin Psychopharmacol. 2003;23(6):540–548. doi: 10.1097/01.jcp.0000095345.32154.9a. [DOI] [PubMed] [Google Scholar]
- 20.van Bemmel AL, van den Hoofdakker RH, Beersma DG, et al. Changes in sleep polygraphic variables and clinical state in depressed patients during treatment with citalopram. Psychopharmacology (Berl) 1993;113(2):225–230. doi: 10.1007/BF02245702. [DOI] [PubMed] [Google Scholar]
- 21.Roth T, Zorick F, Wittig R, et al. The effects of doxepin HCl on sleep and depression. J Clin Psychiatry. 1982;43(9):366–368. [PubMed] [Google Scholar]
- 22.DiazGranados N, Ibrahim LA, Brutsche NE, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71(12):1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price RB, Iosifescu DV, Murrough JW, et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014;31(4):335–343. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161–166. doi: 10.1016/j.jpsychires.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballard ED, Vande Voort JL, Bernert RA, et al. Nocturnal wakefulness is associated with next-day suicidal ideation in major depressive disorder and bipolar disorder. J Clin Psychiatry. 2016;77(6):825–831. doi: 10.4088/JCP.15m09943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perlis ML, Grandner MA, Brown GK, et al. Nocturnal wakefulness as a previously unrecognized risk factor for suicide. J Clin Psychiatry. 2016;77(6):e726–e733. doi: 10.4088/JCP.15m10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim L, Diazgranados N, Franco-Chaves J, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37(6):1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarate CA, Jr, Brutsche NE, Ibrahim L, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 31.Duncan WC, Sarasso S, Ferrarelli F, et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2013;16(2):301–311. doi: 10.1017/S1461145712000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 33.Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47(2):343–352. doi: 10.1037/0022-006X.47.2.343. [DOI] [PubMed] [Google Scholar]
- 34.Ballard ED, Luckenbaugh DA, Richards EM, et al. Assessing measures of suicidal ideation in clinical trials with a rapid-acting antidepressant. J Psychiatr Res. 2015;68:68–73. doi: 10.1016/j.jpsychires.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown GK. A Review of Suicide Assessment Measures for Intervention Research With Adults and Older Adults. National Institute of Mental Health; 2000. [Google Scholar]
- 37.Weitz E, Hollon SD, Kerkhof A, et al. Do depression treatments reduce suicidal ideation? the effects of CBT, IPT, pharmacotherapy, and placebo on suicidality. J Affect Disord. 2014;167:98–103. doi: 10.1016/j.jad.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 38.Maeng S, Zarate CA, Jr, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 39.Ballard ED, Lally N, Nugent AC, et al. Neural correlates of suicidal ideation and its reduction in depression. Int J Neuropsychopharmacol. 2014;18(1):pyu069. doi: 10.1093/ijnp/pyu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bay-Richter C, Linderholm KR, Lim CK, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav Immun. 2015;43:110–117. doi: 10.1016/j.bbi.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Erhardt S, Lim CK, Linderholm KR, et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. 2013;38(5):743–752. doi: 10.1038/npp.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgos I, Richter L, Klein T, et al. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. 2006;20(3):246–253. doi: 10.1016/j.bbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Zai CC, Manchia M, De Luca V, et al. The brain-derived neurotrophic factor gene in suicidal behaviour: a meta-analysis. Int J Neuropsychopharmacol. 2012;15(8):1037–1042. doi: 10.1017/S1461145711001313. [DOI] [PubMed] [Google Scholar]
- 44.Kim YK, Lee HP, Won SD, et al. Low plasma BDNF is associated with suicidal behavior in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):78–85. doi: 10.1016/j.pnpbp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 45.Haile CN, Murrough JW, Iosifescu DV, et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. 2014;17(2):331–336. doi: 10.1017/S1461145713001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laje G, Lally N, Mathews D, et al. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry. 2012;72(11):e27–e28. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grunebaum MF, Galfalvy HC, Oquendo MA, et al. Melancholia and the probability and lethality of suicide attempts. Br J Psychiatry. 2004;184:534–535. doi: 10.1192/bjp.184.6.534. [DOI] [PubMed] [Google Scholar]