Abstract
Background
Rituximab plus cyclophosphamide, vincristine, and prednisone (R-CVP) is one of the effective chemotherapeutic regimens for patients with advanced stage marginal zone lymphoma (MZL). However, prognostic factors that affect the outcome of treatment for MZL are not well understood.
Methods
Between August 2006 and June 2013, patients with newly diagnosed stage III and IV MZL treated with R-CVP as a first-line therapy from 15 institutions were retrospectively analyzed. Patients' clinical and laboratory data at diagnosis were collected by review of medical records.
Results
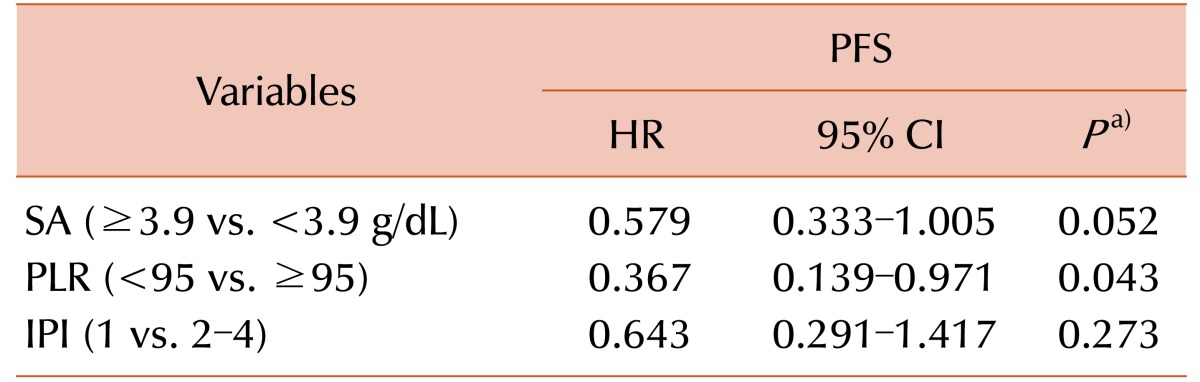
A total of 80 patients were analyzed. Bone marrow involvement was observed in 30% cases. Twelve patients (15%) had nodal MZL, and 41.3% patients exhibited multiple mucosa-associated lymphoma tissue sites. Overall response rate was 91.3%, including 73.8% achieving complete response. Advanced MZL patients treated with R-CVP showed a 3-year progression-free survival (PFS) rate of 69.6%. Prognostic markers significantly affecting PFS in univariate analysis were platelet to lymphocyte ratio (PLR, <95 vs. ≥95, P=0.014), serum albumin (≤3.9 vs. >3.9 g/dL, P=0.008), and the International Prognostic Index (IPI) score (1 vs. 2–4, P=0.032). In multivariate analysis, only PLR (<95 vs. ≥95, HR 0.367, 95% CI, 0.139–0.971, P=0.043) was an independent risk factor for PFS.
Conclusion
PLR ≥95 at diagnosis is an independent prognostic marker for PFS in advanced stage MZL patients treated with R-CVP. This marker may aid clinicians in predicting the response to R-CVP chemotherapy in stage III and IV MZL patients.
Keywords: Marginal zone lymphoma, R-CVP, Prognosis, PLR
INTRODUCTION
Marginal zone lymphoma (MZL) is a distinct subgroup of non-Hodgkin's lymphoma (NHL) that typically follows an indolent clinical course and has a long survival period [1]. In Korea, MZL accounts for 20.4% of all NHL, and is the second most frequent histological subtype after diffuse large B-cell lymphoma (DLBCL) [2].
For MZL with localized stage, regardless of subtype, it is well known that local modalities (such as radiotherapy or surgery) can be used to achieve good disease control. However, local therapy is inadequate in disseminated disease, which comprises at least a quarter of cases, leading to investigations for effective chemotherapy regimens [3].
After the introduction of rituximab, a chimeric monoclonal antibody directed against the B-cell-specific antigen CD20, into regimens targeted against MZL, rituximab in combination with other chemotherapeutic agents has shown promising results compared to conventional chemotherapy alone [4,5]. Among the studied regimens, chemotherapy comprised of rituximab plus cyclophosphamide, vincristine, and prednisone (R-CVP) has demonstrated comparable overall response rates (ORR), complete remission (CR) rates, and survival compared to other regimens containing rituximab [6].
The International Prognostic Index (IPI), although originally intended for use in patients with aggressive lymphoma [7], has been extensively applied, even in indolent lymphomas. However, it appears to have limited discriminating power, as the majority of patients are allocated to the favorable risk group [8]. Other prognostic indexes, such as the Follicular Lymphoma International Prognostic Index (FLIPI); Glasgow Prognostic Score (GPS), an inflammationbased prognostic score; or Marginal Zone Lymphoma Prognostic Index (MZLPI), have been proposed by different international groups that have mainly focused on indolent-nature lymphomas [9,10,11].
Still, the prognostic factors that affect the outcome of MZL patients are not well understood. And after the introduction of rituximab, the relevance of previously recognized prognostic factors in other hematologic cancers has undergone reassessment [12]. Studies in various cancers have demonstrated that an elevation of neutrophils, platelets, monocytes, neutrophil to lymphocyte ratio (NLR), or platelet to lymphocyte ratio (PLR), or the decrease of serum albumin (SA) was associated with some unfavorable clinico-pathologic features [13,14,15]. The relationship of poor prognosis and the elevation of white blood cells, platelets, or associated ratios may be explained through an inflammatory process elicited by cancer cells [10,16,17]. Studies on platelets have shown that they might play an essential role in tumor spreading and growth [18]. Protective effects of lymphocytes as part of the immune surveillance system have also been investigated [19].
In this study, we aim to demonstrate whether the pretreatment levels of various inflammatory markers and/or conventional prognostic markers, can be prognostic indicators in patients treated with R-CVP for MZL.
MATERIALS AND METHODS
After obtaining approval from the Institutional Review Board (IRB) of Dong-A University Hospital, patients with the diagnosis of advanced stage MZL from 15 different institutions in Korea between August 2006 and June 2013 were retrospectively evaluated. The IRB granted a waiver of informed consent based on the design of the study (retrospective data analysis study only, with no interactions with subjects/patients). Inclusion criteria comprised biopsy- confirmed diagnosis of MZL with advanced stage (Ann Arbor stage III/IV) and treatment with at least one cycle of R-CVP therapy with curative intent. Patients were excluded if they had prior chemotherapy other than R-CVP, were on rituximab maintenance therapy after R-CVP, or if they had incomplete clinical data.
Demographic and clinical data
Patient demographics, including age and sex, were collected from medical records. Clinical data included initial laboratory data within 7 days before the start of first cycle of chemotherapy, including complete blood count (CBC), NLR, PLR, absolute neutrophil count (ANC), absolute lymphocyte count (ALC), absolute monocyte count (AMC), SA, serum lactate dehydrogenase (LDH), date of diagnosis, nodal involvement, bone marrow (BM) findings, presence of B symptoms, Eastern Cooperative Oncology Group (ECOG) performance status, presence of bulky disease, Ann Arbor stage, and IPI. Each component of CBC was counted per microliter, and PLR was calculated by the ratio of platelet count (per microliter) divided by absolute lymphocyte count (per microliter).
Treatment data
Treatment consisted of rituximab (375 mg/m2), cyclophosphamide (750 mg/m2) and vincristine (1.4 mg/m2; maximum 2.0 mg), given intravenously on day 1, and oral prednisone 100 mg on days 1–5. The treatment was repeated every 3 weeks and continued for six or eight cycles.
Primary clinical outcome variables
The response evaluation was in accordance with the International Workshop for the Standardized Response Criteria for NHL. Overall survival (OS) was defined as date of diagnosis to date of death or date of last contact for those censored. Progression-free survival (PFS) was defined as the time from the date of diagnosis to the date of relapse, progression, or death from any cause.
Statistical analysis
For the numerical variables, a receiver operating characteristic (ROC) curve was performed to estimate the optimal point for sensitivity/specificity towards the progression of MZL. Time-to-event data was estimated using the Kaplan-Meier method, and the groups were compared using the log-rank test. The Cox proportional hazards model was used to estimate the hazard ratio (HR) and the corresponding 95% confidence interval (95% CI) in regard to the low risk group. Significant variables from univariate models at an alpha level of 5% were included in the multivariable Cox proportional hazards model. All reported P-values were two-sided, and a P-value <0.05 was considered significant. All analyses were conducted using Statistical Package for Social Sciences (SPSS) Version 20.0 for Windows.
RESULTS
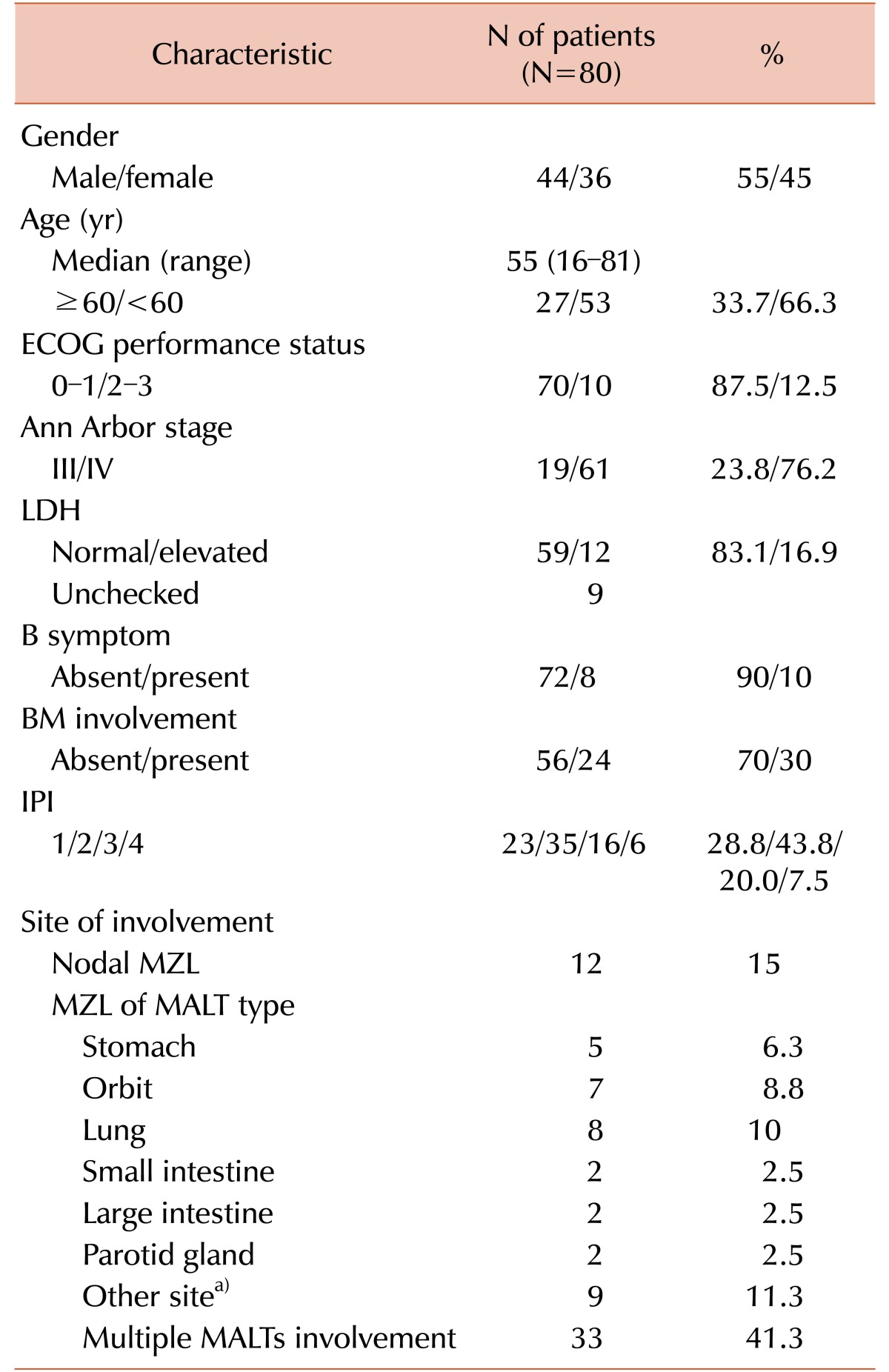
In total, 80 patients who met the inclusion criteria were evaluated. Table 1 shows the clinical characteristics of the patients included in this analysis. The median age of the evaluated 80 patients (44 males and 36 females) was 55 (range, 16–81) years. All patients were diagnosed with advanced clinical stage (Ann Arbor stage III or IV) lymphoma with 19 (23.8%) at stage III and 61 (76.2%) at stage IV. Ten (12.5%) patients had a performance status of ≥2. Elevated LDH was identified in 12 (16.9%) patients. B symptoms (fever, night sweats, and weight loss) were observed in 8 (10%) patients. The distributions of IPI were: 1 in 23 (28.8%); 2 in 35 (43.8%); 3 in 16 (20%), and 4 in 6 (7.5%) patients. Twelve (15%) of the 80 patients had nodal MZL and 68 (85%) had MALT lymphoma. Among patients with MALT lymphoma, 33 (41.3%) patients had multiple MALT site involvement. BM involvement was observed in 24 (30%) patients.
Table 1. Demographic and clinical characteristics.
a)Bone, bronchus, esophagus, nasopharynx, paranasal sinus, peritoneum, soft tissue, thymus, thyroid (1 case of each).
Abbreviations: BM, bone marrow; ECOG, Eastern Cooperative Oncology Group; IPI, international prognostic index; LDH, lactate dehydrogenase; MALT, mucosa associated lymphoid tissue; MZL, marginal zone lymphoma.
Response and survival
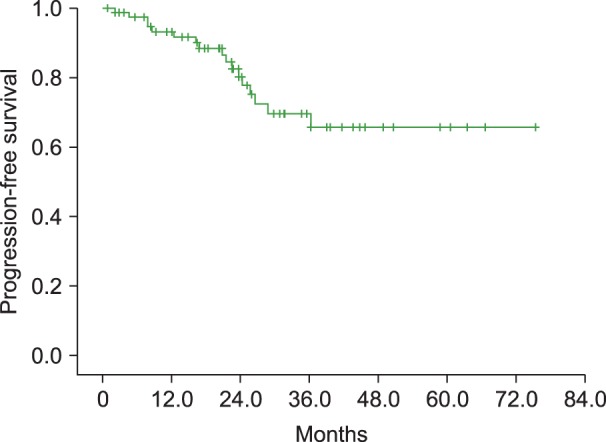
Fifty-nine patients (73.8%) achieved a complete remission (CR) and 14 (17.5%) a partial remission (PR), while stable disease (SD) was observed in 4 (5%) patients and progressive disease in 2 (2.5%) patients. One patient was not evaluable for response, making the ORR 91.3% (95% CI, 85.0–96.3%). At a median follow-up of 24.9 months (range, 0.8–75.4), 17 patients (21.3%) had progression or relapse, and none died during the investigation period. Information regarding the treatment after disease relapse or progression was available for 15 patients. Thirteen patients received chemotherapy alone after relapse or progression, and two received chemotherapy followed with hematopoietic progenitor stem-cell transplantation (HPSCT). There was one patient who was lost to follow-up after only 1 cycle of R-CVP chemotherapy; therefore her data on treatment and response was not available. Median OS and PFS were not reached at the last follow-up, and PFS at 3 years was 69.6%, with the 3-year OS rate at 100% (Fig. 1).
Fig. 1. Kaplan-Meier graph depicting progression-free survival (PFS) of patients with advanced stage marginal zone lymphoma (MZL) treated with R-CVP chemotherapy (PFS at 3 years was 69.6%).
Analysis of potential prognostic markers
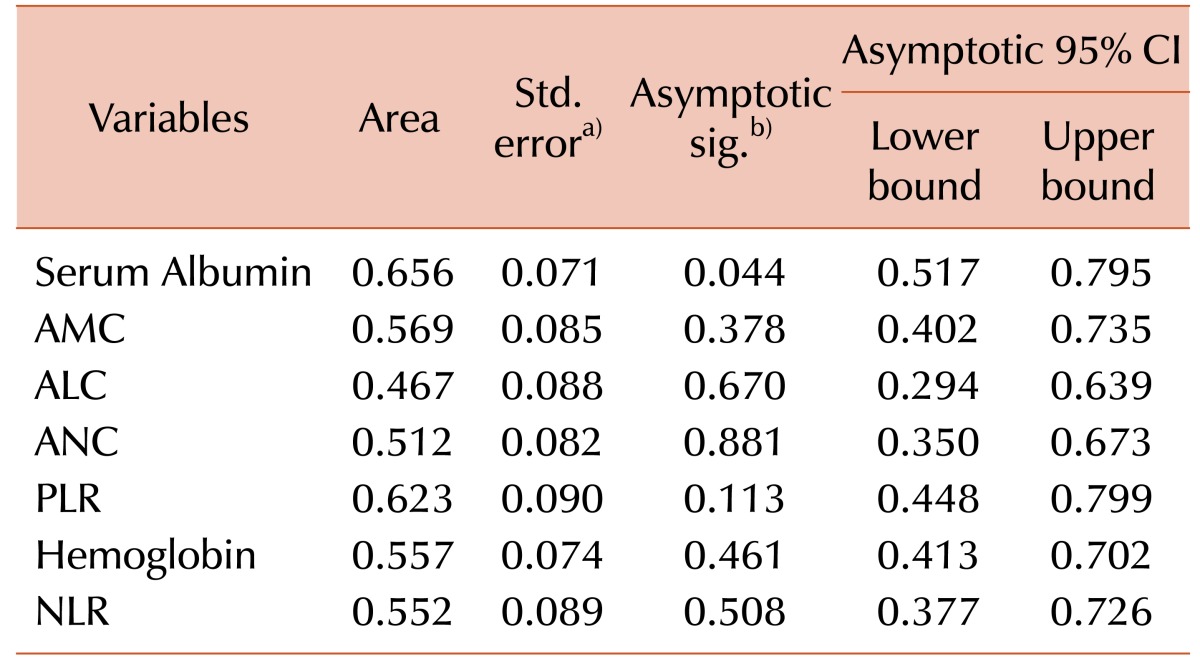
To identify the optimal prognostic marker according to its sensitivity and specificity towards the relapse of MZL, a Receiver Operation Characteristic (ROC) curve analysis was performed for numerical variables (Table 2). Among the potential prognostic markers, only SA and PLR showed enough sensitivity/specificity to be put to survival rate analysis. For the SA, a cut-off value of 3.9 g/dL was generated according to the ROC analysis (sensitivity 71.0% and specificity 66.6%; AUC values 0.656, 95% CI 0.517–0.795, P=0.044). From the ROC curve, PLR value of 95 yielded the most optimal value to predict the relapse or recurrence of MZL (sensitivity 82.3% and specificity 55.6%; AUC values 0.623, 95% CI 0.448–0.799, P=0.113).
Table 2. Area under the curve values of prognostic marker candidates.
a)Under the nonparametric assumption. b)Null hypothesis: true area=0.5.
Abbreviations: ALC, absolute lymphocyte count; AMC, absolute monocyte count; ANC, absolute neutrophil count; CI, confidence interval; NLR, neutrophil to lymphocyte count; PLR, platelet to lymphocyte count.
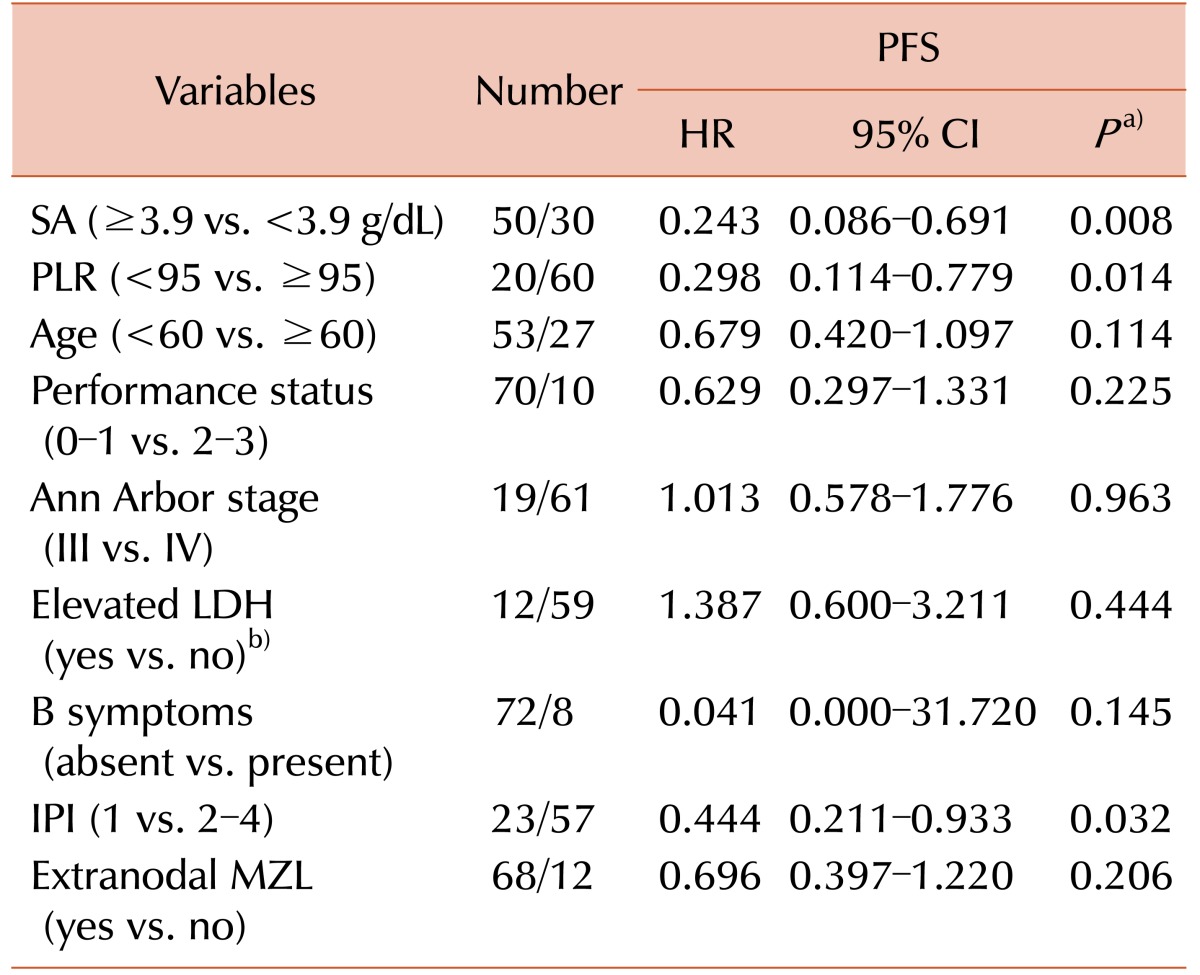
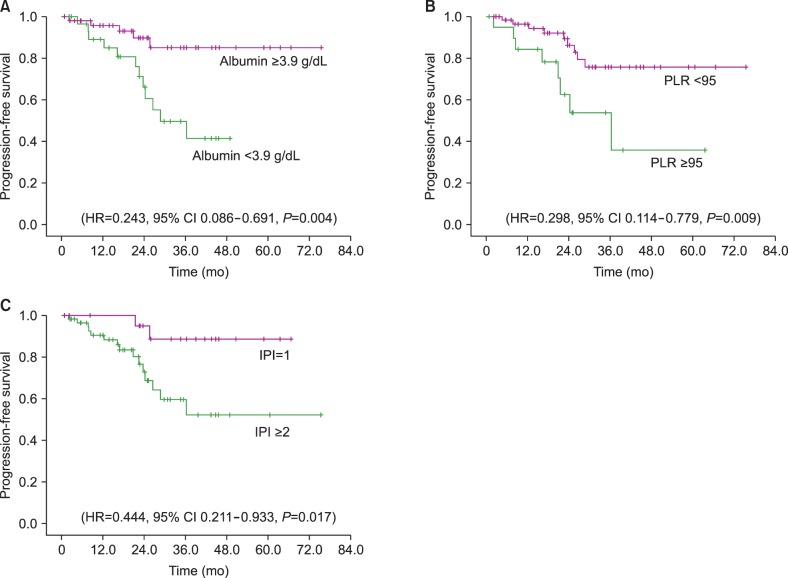
Univariate analysis of potential prognostic markers is shown in Table 3. An SA level ≥3.9 g/dL was shown to be a significant predictor of PFS (HR=0.243, 95% CI 0.086–0.691, P=0.004) (Fig. 2A). PLR was also significantly prognostic for PFS when evaluated for values <95 (HR=0.298, 95% CI 0.114–0.779, P=0.009) (Fig. 2B). Additionally, IPI, when categorized by a score of 1 versus 2–4, had prognostic significance for PFS (HR=0.444, 95% CI 0.211–0.933, P=0.017) (Fig. 2C). Other candidate factors, such as age, performance status, Ann Arbor stage, elevation of LDH, presence of B symptoms, extranodal MZL, and BM involvement were not prognostic in our population.
Table 3. Univariate analysis of prognostic factors for time-to-progression.
a)P-value by log rank test. b)Number of patients initially evaluated with LDH were 71.
Abbreviations: CI, confidence interval; HR, hazard ratio; IPI, international prognostic index; LDH, lactate dehydrogenase; MZL, marginal zone lymphoma; PFS, progression free survival; PLR, platelet to lymphocyte ratio; SA, serum albumin.
Fig. 2. Kaplan-Meier survival plots; (A) Serum albumin level ≥3.9 g/dL was shown to be a significant predictor of progression free survival (PFS) (HR=0.243, 95% CI 0.086–0.691, P=0.004); (B) PLR was also significantly prognostic for PFS when evaluated for values of <95 (HR=0.298, 95% CI 0.114–0.779, P=0.009); (C) IPI, when categorized by a score of 1 versus 2–4, had prognostic significance for PFS (HR=0.444, 95% CI 0.211–0.933, P=0.017).
Multivariable analysis results for PFS are shown in Table 4. PLR continued to be an independent prognostic marker, with the hazard of progression for patients with PLR <95 shown to be about 36.7% (95% CI 13.9–97.1) of the hazard for those patients with PLR ≥95, when controlling for SA and IPI. The SA and IPI were not prognostic for PFS in our multivariate analysis.
Table 4. Multivariate analysis of prognostic factors for progression free survival.
a)P-value by Cox regression.
Abbreviations: CI, confidence interval; HR, hazard ratio; IPI, international prognostic index; PFS, progression free survival; PLR, platelet to lymphocyte ratio; SA, serum albumin.
DISCUSSION
In our review of 80 patients with advanced stage MZL, we found that a PLR value of ≥95 may have prognostic significance in those treated with R-CVP. Among other candidates for prognostic markers of MZL, a serum albumin lower than 3.9 g/dL and an IPI of 2 or higher had prognostic value when analyzed with univariate analysis, but did not have enough statistical significance when applied to multivariate analyses.
In 1993, the International Prognostic Index (IPI) was published [7]. Since then, IPI has been widely applied for the prediction of prognosis of NHLs. However, as IPI is designed for aggressive lymphoma and cases of indolent lymphoma such as MZL, as well as taking into account that the addition of rituximab to conventional chemotherapy has led to significant improvements in OS and PFS in patients with MZL, previously studied prognostic algorithms for such patients, including the IPI, had to be reconstructed [9].
Other prognostic indexes, such as FLIPI used for follicular lymphomas, or GPS, an inflammation-based prognostic index, or MZLPI, have been proposed as alternatives for more indolent lymphomas [9,10,11]. But as our concern was focused on advanced stage MZL (which usually had disseminated disease, and hence, was a prerequisite for systemic chemotherapy), we did not consider prognostic indexes with Ann Arbor staging as a constituent.
The measurement of serum markers, such as CBC or serum albumin, is a simple step that could easily be employed in regular practice. Along with the serum markers, association of systemic inflammatory components with tumor survival, or the pathogenesis of neoplasm itself, has been proposed [20,21]. The inflammatory parameters, namely, the ANC, AMC, ALC, platelet counts, NLR, PLR, CRP, serum albumin, and so forth are studied in many cancers for their correlation with prognosis [13,14,15,16,22,23]. For solid tumors, a positive relationship between high PLR with worse prognosis of epithelial ovarian cancer was identified [13,16].
For the hematologic malignancies, similar investigations on the connection of inflammatory markers and the outcome of disease have been carried out. For patients with non-Hodgkin's lymphoma, prognosis of DLBCL has been correlated with hypoalbuminemia, low ALC, AMC, increased NLR and PLR, AMC/ALC score, and GPS [15,24,25,26,27,28]. Extranodal natural killer/T-cell lymphoma, nasal type, has also been shown to be related with GPS and PLR [10]. Outcomes of other hematologic malignancies, such as primary mediastinal large B-cell lymphoma, myelodysplastic syndromes, multiple myeloma, and acute myelogenous leukemia have also been related to systemic inflammatory markers [29,30,31].
Studies regarding the correlation of inflammatory markers with the outcome of MZL were difficult to obtain. Four retrospective studies have been made regarding the prognostic factors of MZL. Two studies, during their research, commonly deduced the result that the discriminating powers of formerly used prognostic scoring systems, the IPI and the FLIPI, are limited, if not misleading [32]. One of those studies by Oh et al. [9] analyzed 205 patients with nongastric MZL with presumed prognostic factors, PFS and OS. The MZLPI was constructed via the summation of speculated prognostic factors: nodal MZL, ECOG performance ≥2, and advanced stage. The PFS and OS curves were both more discriminating than those of IPI or FLIPI. Another study on the prognostic factors of nongastric MZL by Arcaini et al. [33] demonstrated that disease dissemination to lymph nodes and BM is associated with a poorer outcome. Arcaini et al. [34] also showed that in splenic MZL patients, low hemoglobin levels (<12 g/dL), LDH levels higher than normal, and albumin levels less than 3.5 g/dL were predictors of higher risk.
A study by Mazloom et al. [32] analyzed 275 patients with MZL by dividing them into three groups based on three types: extranodal, nodal, and splenic. The results showed that an elevated serum β2-microglobulin level, B symptoms, and male gender were found to be correlated with decreased recurrence-free survival (RFS). Using these three variables, they formulated a 3-tier prognostic scoring system for extranodal MZL, which had statistically reasonable discriminating power (P=0.01 for RFS, P<0.0001 for OS). Their study also showed that IPI and FLIPI did not have statistical significance regarding OS of MZL.
None of the above-mentioned studies regarding the prognosis of MZL considered the previously emphasized role of inflammatory markers on malignancy development and progression. Groundwork investigations on the roles of platelets in tumor spreading and growth have shown that platelets mediate tumor cell growth, dissemination, and angiogenesis [18]. Investigators have suggested that platelets play a role in the homing of circulating tumor cells to distant organs and that platelet interactions with tumor cells lead to rolling and tethering of tumor cell aggregates, resulting in extravasation through the endothelial wall and immune protection [35]. Platelets have also been shown to promote the spread of tumors through multiple mechanisms, including selectin and integrin interactions with tumor cells [36]. In addition to their function in hemostasis, platelets are also involved in inflammatory disease and cancer [37].
In contrast to platelets, lymphocytes are considered a significant blood parameter related to immune surveillance. High lymphocytic infiltrate is associated with improved survival and superior response to systemic therapy [38], whereas low peripheral blood lymphocyte counts are related to poor cancer prognoses [39].
Our findings provide a new approach in predicting outcomes in MZL patients. Prediction of disease progression not only utilizing clinical or histopathological factors (e.g., tumor grade, tumor size, and tumor site), but also with host-response factors, such as systemic inflammatory markers, may have more clinical significance. Furthermore, the PLR can easily be assessed from peripheral blood tests that are available without additional expenditure, thus providing lower cost and greater convenience for predicting prognosis.
However, this study has some limitations, including the few deaths caused by MZL, the relatively small size of the analysis group, and the inherent bias that exists in a retrospective study. To verify the results of this study, a collective effort by way of a multi-institutional prospective study with longer follow-up will be needed. However, given the lack of prospective data, this retrospective study provides evidence to support the role of these prognostic factors and provides novel insight into predicting outcomes in MZL patients.
In conclusion, our study is the first to show that PLR is an independent prognostic marker in patients with MZL treated with R-CVP chemo-immunotherapy.
Footnotes
This work was supported by the Dong-A University Research Fund.
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Oh SY, Ryoo BY, Kim WS, et al. Nongastric marginal zone B-cell lymphoma: analysis of 247 cases. Am J Hematol. 2007;82:446–452. doi: 10.1002/ajh.20874. [DOI] [PubMed] [Google Scholar]
- 2.Kim JM, Ko YH, Lee SS, et al. WHO classification of malignant lymphomas in Korea: report of the third nationwide study. Korean J Pathol. 2011;45:254–260. [Google Scholar]
- 3.Conconi A, Martinelli G, Thiéblemont C, et al. Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood. 2003;102:2741–2745. doi: 10.1182/blood-2002-11-3496. [DOI] [PubMed] [Google Scholar]
- 4.Oh SY, Kim WS, Kim JS, et al. Stage IV marginal zone B-cell lymphoma--prognostic factors and the role of rituximab: Consortium for Improving Survival of Lymphoma (CISL) study. Cancer Sci. 2010;101:2443–2447. doi: 10.1111/j.1349-7006.2010.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zucca E, Conconi A, Laszlo D, et al. Addition of rituximab to chlorambucil produces superior event-free survival in the treatment of patients with extranodal marginal-zone B-cell lymphoma: 5-year analysis of the IELSG-19 Randomized Study. J Clin Oncol. 2013;31:565–572. doi: 10.1200/JCO.2011.40.6272. [DOI] [PubMed] [Google Scholar]
- 6.Kang HJ, Kim WS, Kim SJ, et al. Phase II trial of rituximab plus CVP combination chemotherapy for advanced stage marginal zone lymphoma as a first-line therapy: Consortium for Improving Survival of Lymphoma (CISL) study. Ann Hematol. 2012;91:543–551. doi: 10.1007/s00277-011-1337-6. [DOI] [PubMed] [Google Scholar]
- 7.International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 8.López-Guillermo A, Montserrat E, Bosch F, Terol MJ, Campo E, Rozman C. Applicability of the International Index for aggressive lymphomas to patients with low-grade lymphoma. J Clin Oncol. 1994;12:1343–1348. doi: 10.1200/JCO.1994.12.7.1343. [DOI] [PubMed] [Google Scholar]
- 9.Oh SY, Kwon HC, Kim WS, et al. Nongastric marginal zone B-cell lymphoma: a prognostic model from a retrospective multicenter study. Cancer Lett. 2007;258:90–97. doi: 10.1016/j.canlet.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Li YJ, Jiang WQ, Huang JJ, Xia ZJ, Huang HQ, Li ZM. The Glasgow Prognostic Score (GPS) as a novel and significant predictor of extranodal natural killer/T-cell lymphoma, nasal type. Am J Hematol. 2013;88:394–399. doi: 10.1002/ajh.23422. [DOI] [PubMed] [Google Scholar]
- 11.Heilgeist A, McClanahan F, Ho AD, Witzens-Harig M. Prognostic value of the Follicular Lymphoma International Prognostic Index score in marginal zone lymphoma: an analysis of clinical presentation and outcome in 144 patients. Cancer. 2013;119:99–106. doi: 10.1002/cncr.27704. [DOI] [PubMed] [Google Scholar]
- 12.Ngo L, Hee SW, Lim LC, et al. Prognostic factors in patients with diffuse large B cell lymphoma: Before and after the introduction of rituximab. Leuk Lymphoma. 2008;49:462–469. doi: 10.1080/10428190701809156. [DOI] [PubMed] [Google Scholar]
- 13.Kokcu A, Kurtoglu E, Celik H, Tosun M, Malatyalıoglu E, Ozdemir AZ. May the platelet to lymphocyte ratio be a prognostic factor for epithelial ovarian cancer? Asian Pac J Cancer Prev. 2014;15:9781–9784. doi: 10.7314/apjcp.2014.15.22.9781. [DOI] [PubMed] [Google Scholar]
- 14.Sun W, Zhang L, Luo M, et al. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Head Neck. 2016;38(Suppl 1):E1332–E1340. doi: 10.1002/hed.24224. [DOI] [PubMed] [Google Scholar]
- 15.Dalia S, Chavez J, Little B, et al. Serum albumin retains independent prognostic significance in diffuse large B-cell lymphoma in the post-rituximab era. Ann Hematol. 2014;93:1305–1312. doi: 10.1007/s00277-014-2031-2. [DOI] [PubMed] [Google Scholar]
- 16.Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol. 2012;23:265–273. doi: 10.3802/jgo.2012.23.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol. 2014;229:1005–1015. doi: 10.1002/jcp.24539. [DOI] [PubMed] [Google Scholar]
- 19.Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannon NA, Meyer J, Iyengar P, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol. 2015;10:280–285. doi: 10.1097/JTO.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 23.Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104:1288–1295. doi: 10.1038/bjc.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porrata LF, Ristow K, Habermann TM, et al. Absolute monocyte/lymphocyte count prognostic score is independent of immunohistochemically determined cell of origin in predicting survival in diffuse large B-cell lymphoma. Leuk Lymphoma. 2012;53:2159–2165. doi: 10.3109/10428194.2012.690605. [DOI] [PubMed] [Google Scholar]
- 25.Porrata LF, Ristow K, Habermann TM, Witzig TE, Inwards DJ, Markovic SN. Absolute lymphocyte count at the time of first relapse predicts survival in patients with diffuse large B-cell lymphoma. Am J Hematol. 2009;84:93–97. doi: 10.1002/ajh.21337. [DOI] [PubMed] [Google Scholar]
- 26.Chae YS, Shin H, Sohn SK, et al. Absolute lymphocyte count at day + 21 predicts survival in patients with early-stage diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, adriamycin, vincristine and prednisone. Leuk Lymphoma. 2012;53:1757–1763. doi: 10.3109/10428194.2012.670231. [DOI] [PubMed] [Google Scholar]
- 27.Aoki K, Tabata S, Yonetani N, Matsushita A, Ishikawa T. The prognostic impact of absolute lymphocyte and monocyte counts at diagnosis of diffuse large B-cell lymphoma in the rituximab era. Acta Haematol. 2013;130:242–246. doi: 10.1159/000350484. [DOI] [PubMed] [Google Scholar]
- 28.Ho CL, Lu CS, Chen JH, Chen YG, Huang TC, Wu YY. Neutrophil/lymphocyte ratio, lymphocyte/monocyte ratio, and absolute lymphocyte count/absolute monocyte count prognostic score in diffuse large b-cell lymphoma: useful prognostic tools in the rituximab era. Medicine (Baltimore) 2015;94:e993. doi: 10.1097/MD.0000000000000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romano A, Parrinello NL, Consoli ML, et al. Neutrophil to lymphocyte ratio (NLR) improves the risk assessment of ISS staging in newly diagnosed MM patients treated upfront with novel agents. Ann Hematol. 2015;94:1875–1883. doi: 10.1007/s00277-015-2462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kharfan-Dabaja MA, Chavez JC, Yu D, et al. Severe hypoalbuminemia at day 90 predicts worse nonrelapse mortality and overall survival after allogeneic hematopoietic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2011;17:384–393. doi: 10.1016/j.bbmt.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Komrokji RS, Corrales-Yepez M, Kharfan-Dabaja MA, et al. Hypoalbuminemia is an independent prognostic factor for overall survival in myelodysplastic syndromes. Am J Hematol. 2012;87:1006–1009. doi: 10.1002/ajh.23303. [DOI] [PubMed] [Google Scholar]
- 32.Mazloom A, Medeiros LJ, McLaughlin PW, et al. Marginal zone lymphomas: factors that affect the final outcome. Cancer. 2010;116:4291–4298. doi: 10.1002/cncr.25325. [DOI] [PubMed] [Google Scholar]
- 33.Arcaini L, Burcheri S, Rossi A, et al. Nongastric marginal-zone B-cell MALT lymphoma: prognostic value of disease dissemination. Oncologist. 2006;11:285–291. doi: 10.1634/theoncologist.11-3-285. [DOI] [PubMed] [Google Scholar]
- 34.Arcaini L, Lazzarino M, Colombo N, et al. Splenic marginal zone lymphoma: a prognostic model for clinical use. Blood. 2006;107:4643–4649. doi: 10.1182/blood-2005-11-4659. [DOI] [PubMed] [Google Scholar]
- 35.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 36.Trikha M, Zhou Z, Timar J, et al. Multiple roles for platelet GPIIb/IIIa and alphavbeta3 integrins in tumor growth, angiogenesis, and metastasis. Cancer Res. 2002;62:2824–2833. [PubMed] [Google Scholar]
- 37.Leslie M. Cell biology. Beyond clotting: the powers of platelets. Science. 2010;328:562–564. doi: 10.1126/science.328.5978.562. [DOI] [PubMed] [Google Scholar]
- 38.Seo AN, Lee HJ, Kim EJ, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer. 2013;109:2705–2713. doi: 10.1038/bjc.2013.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fogar P, Sperti C, Basso D, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32:22–28. doi: 10.1097/01.mpa.0000188305.90290.50. [DOI] [PubMed] [Google Scholar]