TO THE EDITOR: Multiple myeloma (MM) is a hematologic malignancy caused by the proliferation of clonal plasma cells in the bone marrow, leading to uncontrolled production of monoclonal immunoglobulin. Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm associated with the presence of the BCR-ABL1 fusion gene. These are uncommon malignancies that account for approximately 1.6% and 0.4% of all newly diagnosed cancers in the United States, respectively [1]. In Korea, these are also rare disease and the crude incidence rates of those are 2.5/100,000 and 0.8/100,000, respectively [2,3]. The concurrent diagnosis of MM and CML in one patient is an extremely rare event. In this report, we describe an additional case of synchronous MM and CML.
A 64-year-old man was referred to our hospital for evaluation of a right pleural soft-tissue mass and a sternal osteolytic lesion incidentally detected on chest computed tomography (CT). The patient had no specific symptoms and remarkable findings on physical examinations. A complete blood count showed a white blood cell counts of 13.1×109/L, with a differential count of 65% neutrophils, 15% lymphocytes, 10% monocytes, 5.2% eosinophils, and 4% basophils; hemoglobin levels of 8.3 g/dL; and platelet counts of 234×109/L. Blood chemistry showed reversal of the albumin-globulin ratio (total protein 11.8 g/dL, and albumin 2.3 g/dL), renal dysfunction (creatinine clearance 38.4 mL/min), and hypercalcemia (calcium 11.8 mg/dL). A total of 7 g/dL of monoclonal gammopathy (IgA lambda type) was detected with serum immunofixation electrophoresis. The ratio of serum kappa/lambda free light chains was 0.006 and serum β2 microglobulin level was 19.8 mg/L. Radiological investigation revealed multiple osteolytic lesions in the skull and axial skeleton. The needle biopsy of pleural soft-tissue mass showed infiltrations of monotonous round cells with eccentrically located nuclei and different sizes, but no evidence of myeloid cell infiltration. Most of the infiltrated cells were positive for CD138 on immunohistochemical staining (Fig. 1). Bone marrow biopsy revealed increased cellularity (up to 90%), consisting of plasmacytoid round cell infiltrations and myeloid cells with varying maturation. A patchy positive reaction for CD138 was compatible with myeloma involvement, and myeloid cells were positive for myeloperoxidase. The bone marrow aspirate demonstrated myeloid hyperplasia with increased eosinophils, basophils and plasma cells. Plasma cells showed mature forms with condensed nuclear chromatin, indistinct nucleoli, and abundant basophilic cytoplasm with a perinuclear halo (Fig. 2). Chromosome analysis revealed 46,XY, t(2:3)(p15:q26), t(9:22)(q34:q11) in 20 of 21 cells, and fluorescent in situ hybridization (FISH) indicated BCR/ABL translocations in 86.5% of cells. Molecular analysis revealed BCR/ABL rearrangement based on results from reverse transcription polymerase chain reactions. Consequently, the patient was diagnosed with stage III MM and low-risk CML in accordance with the International Staging System [4] and Sokal scores [5], respectively. Considering the patient's old age and toxicity of combination therapy for both CML and MM, the patient was first treated for high-stage MM. Treatment was initiated with a regimen of thalidomide (100 mg daily) and dexamethasone (40 mg on days 1–4 and 15–18) every 4 weeks. However, the response to treatment was not evaluable, since the patient died of pneumonia caused by carbapenem-resistant Acinetobacter after 4 weeks.
Fig. 1. Biopsy of the right pleural soft-tissue mass. (A) Infiltration of plasma cells (H&E staining, ×400). (B) Positive for immunohistochemical staining of CD138 (×100).
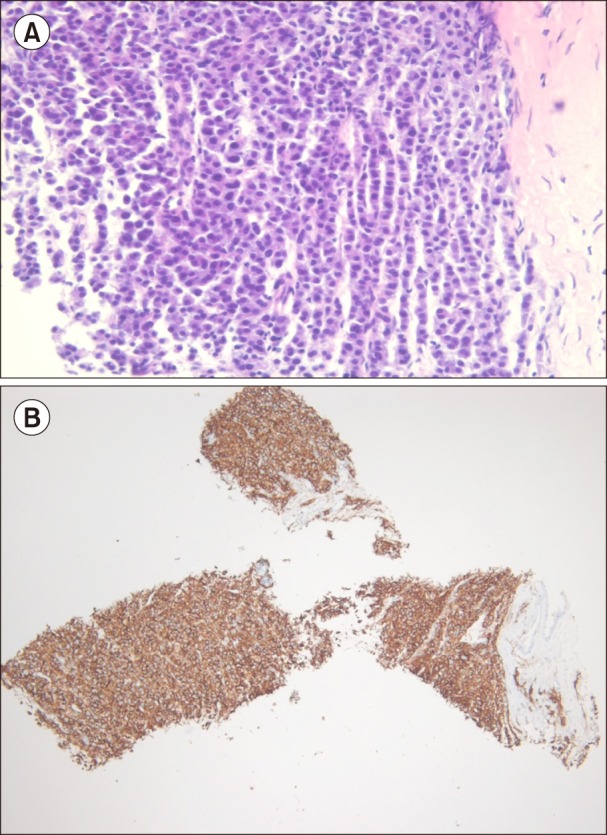
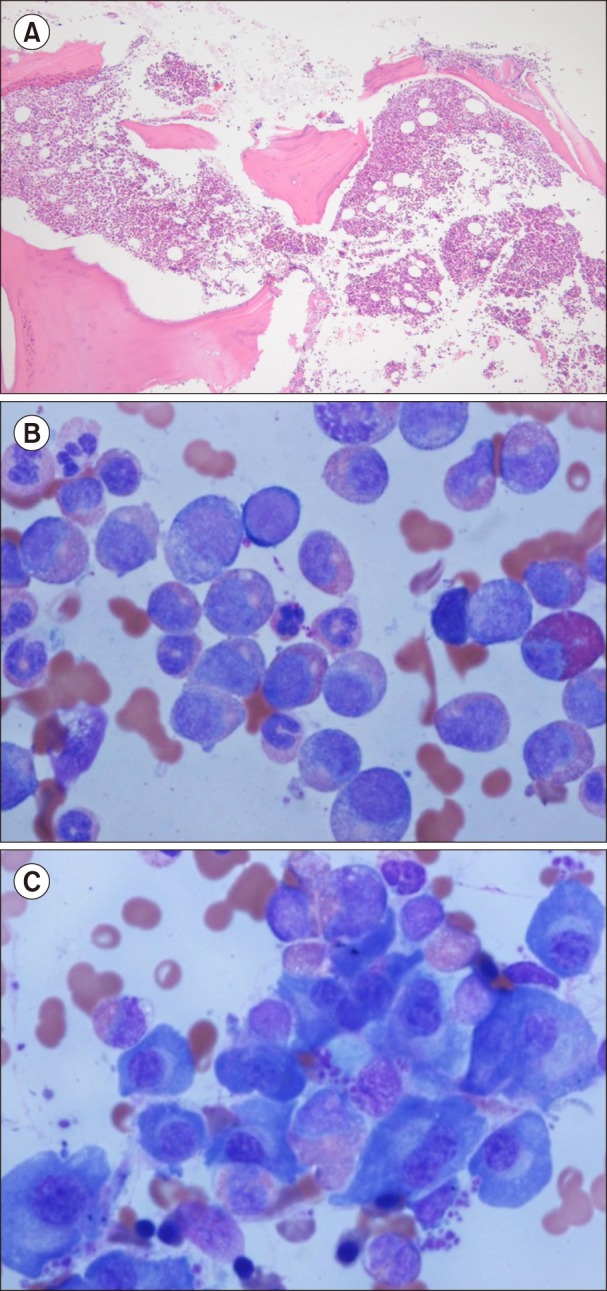
Fig. 2. Bone marrow biopsy and aspiration at diagnosis. (A) Bone marrow biopsy shows increased cellularity up to 90% (H&E, ×100). (B) Bone marrow aspiration shows myeloid hyperplasia with increased eosinophils (Wright-Giemsa staining, ×1,000). (C) Bone marrow aspiration shows increased mature forms of plasma cells (Wright-Giemsa staining, ×1,000).
Ide et al. [6] reviewed 12 cases in which MM and CML coexisted. In four of the cases, patients were diagnosed first with MM followed by CML, while in four other cases, MM developed after CML. The interval between the two types of cancer ranged from 14 months to 113 months. In the remaining four cases, MM and CML were diagnosed simultaneously.
Although there are no established treatment regimens for simultaneously occurring MM and CML, there are two cases in which MM with CML was treated with a combination of bortezomib, dexamethasone, or lenalidomide, and imatinib or dasatinib [7,8]. The combination treatment targeting both MM and CML resulted in successful outcomes in these cases. Our patient received only anti-MM therapy owing to old age and overall poor health status. Although data from a small study suggest that thalidomide treatment along with imatinib is efficacious for the treatment of CML [9], our patient did not respond to thalidomide treatment.
We presented our experience with a patient who was diagnosed with MM and CML simultaneously. Evaluation of other cases is required to shed light on clinical characteristics of the disease states, as well as to explore potential evaluable treatments.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Hong J, Lee JH. Recent advances in multiple myeloma: a Korean perspective. Korean J Intern Med. 2016;31:820–834. doi: 10.3904/kjim.2015.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Au WY, Caguioa PB, Chuah C, et al. Chronic myeloid leukemia in Asia. Int J Hematol. 2009;89:14–23. doi: 10.1007/s12185-008-0230-0. [DOI] [PubMed] [Google Scholar]
- 4.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 5.Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–799. [PubMed] [Google Scholar]
- 6.Ide M, Kuwahara N, Matsuishi E, Kimura S, Gondo H. Uncommon case of chronic myeloid leukemia with multiple myeloma. Int J Hematol. 2010;91:699–704. doi: 10.1007/s12185-010-0546-4. [DOI] [PubMed] [Google Scholar]
- 7.Alsidawi S, Ghose A, Qualtieri J, Radhakrishnan N. A case of multiple myeloma with metachronous chronic myeloid leukemia treated successfully with bortezomib, dexamethasone, and dasatinib. Case Rep Oncol Med. 2014;2014:962526. doi: 10.1155/2014/962526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Offiah C, Quinn JP, Thornton P, Murphy PT. Co-existing chronic myeloid leukaemia and multiple myeloma: rapid response to lenalidomide during imatinib treatment. Int J Hematol. 2012;95:451–452. doi: 10.1007/s12185-012-1038-5. [DOI] [PubMed] [Google Scholar]
- 9.Monroy RH, Vargas-Viveros P, Ceballos EC, Velazquez JC, Munos SC. Imatinib (IM) plus thalidomide (Thali), a effective combination for the treatment of chronic myeloid leukemia (CML) Philadelphia chromosomepositive (Ph +) in IM-resistant disease. Report of 14 new cases from a single center in Mexico. Blood. 2013;122:5172. [Google Scholar]


