Abstract
Objective
MicroRNAs (miRNAs) play a vital role in pathogenesis and progression of many cancers, including cervical cancer. However, importance of serum level of miR-101 in cervical cancer has rarely been studied. In the present study, clinical significance and prognostic value of serum miR-101 for cervical cancer was investigated.
Methods
Association between miR-101 level in cervical cancer tissues and prognosis of patients was analyzed by using data retrieved from The Cancer Genome Atlas (TCGA) database, which was followed with our clinical study in which miR-101 serum level comparison between cervical cancer patients and healthy controls was conducted by real-time quantitative polymerase chain reaction (PCR).
Results
TCGA database demonstrated that miR-101 was down-regulated in cervical cancer tissues compared with normal cervical tissues, and univariate Cox regression analysis indicated that decreased miR-101 expression was a highly significant negative risk factor. Similar trend was found in the serum miR-101. Serum level of miR-101 was associated with International Federation of Gynecology and Obstetrics (FIGO) stage (p=0.003), lymph node metastasis (p=0.001), and serum squamous cell carcinoma antigen (SCC-Ag) level >4 (p=0.007). The overall survival time of cervical cancer patients with a higher level of serum miR-101 was significantly longer than that of patients with a lower level of serum miR-101. Moreover, multivariate Cox regression analysis indicated that the down-regulated serum level of miR-101 was an independent predictor for the unfavorable prognosis of cervical cancer.
Conclusion
Serum level of miR-101 is closely associated with metastasis and prognosis of cervical cancer; and, hence could be a potential biomarker and prognostic predictor for cervical cancer.
Keywords: miRNAs, Uterine Cervical Neoplasms, Serum, Prognosis, Gynecology, Disease Progression
INTRODUCTION
Cervical cancer is one of the most common malignancies among women across the world. Global data from 2012 indicated 527,000 new onset cases and 265,700 deaths [1]. More recently, declining trends in the incidence of cervical cancer have been observed as a result of the introduction of various screening methods and programs [2]. However, cervical cancer still remains a leading cause of cancer-related mortality in women. Further, despite treatment modalities such as chemotherapy, radiotherapy, and surgery, a considerable number of patients succumb to cervical cancer metastasis and recurrence. Therefore, it would be of great clinical significance if cervical cancer could be accurately evaluated and if metastasis and recurrence could be discovered at an early stage. This would enable early prediction and prognosis of cervical cancer so that timely individualized therapy could be provided.
MicroRNA (miRNA) is an important area of focus in cancer research for the ability to target oncogenes and suppress tumor activity [3]. Studies suggest that a significant difference exists between healthy persons and cancer patients in terms of miRNA expression profile [4]; therefore, circulating miRNA is of great importance for the early diagnosis, staging, and prognostic evaluation in cancers [5,6,7].
MiR-101 is located in 1p31.3 encoded by MIRN101-a and MIRN101-b allele. MiR-101 has been speculated to be involved in the pathogenesis and metastasis of various cancers, and associated with the pathological characteristics, survival, prognosis, and the execution of targeted therapy [8,9,10,11,12]. However, the expression profile of miR-101 in the peripheral blood of cervical cancer patients remains unclear and the comparison to healthy women has also been rarely reported. In the current study, the expression level of serum miR-101 was investigated, and the prognostic value of miR-101 for cervical cancer metastasis was explored in order to elucidate the possibility of miR-101 as the molecular biomarker of cervical cancer.
MATERIALS AND METHODS
1. Patients
The serum samples of 182 cervical cancer patients, prior to any treatment, were collected in the Department of Oncology, Maternal and Child Health Hospital, Jiangxi province, China between February 2012 and March 2016. The average age of patients was 53±9 years. The clinicopathological features including age, tumor size, pathological type, degree of differentiation, lymph node metastasis, International Federation of Gynecology and Obstetrics (FIGO) stage, and the serum squamous cell carcinoma antigen (SCC-Ag) level were archived. The serum sample of each patient was collected once again one month after treatment. The study also included 12 healthy control subjects.
Inclusion criteria: 1) clinical stage of the patients was defined in accordance to the 2009 FIGO staging standard, and the pathologic diagnosis was confirmed by at least 2 pathologists; 2) the patients received a thorough pre-treatment evaluation, including a detailed medical history, physical examination, whole blood cell count, liver and kidney function tests, imaging examination (chest X-ray, color Doppler ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and positron emission tomography [PET]), electrocardiogram (ECG), comprehensive assessment of electronic colposcopy for vulva, vagina, cervix, and cystoscopy. Colonoscopy was also included in the presence of a clinical indication; 3) the clinical stage of cervical cancer was determined by 3 chief gynecologic-oncologists; 4) the patients had complete records of clinicopathological and follow-up examinations, and had also signed the informed consent document; 5) radical hysterectomy and pelvic lymph node dissection (with or without para-aortic lymph nodes dissection) were performed for patients at stage IB1 to IIA1; and laparoscopic pelvic lymph node dissection in combination with abdominal lymph node dissection, followed with the postoperative concurrent chemo-radiotherapy for patients at stage of IIA2 to IIIB; and 6) the study was approved by the ethics committee of Maternal and Child Health Hospital, Jiangxi province.
Exclusion criteria: 1) the patients with cervical cancer in stage IA. Typically, stage IA patients usually underwent cold knife conization surgery to confirm the diagnosis. And the resected lesion of the cancer tissues more or less will have an impact on the serum expression level of miR-101. Therefore, the stage IA patients were excluded to diminish the possible errors; 2) patients with concurrent malignancy of other organ/organs; 3) patients with incomplete clinic pathological data; and 4) patients who were lost of follow-up or loss of follow-up for short term.
2. Database
The database of the gene was from The Cancer Genome Atlas (TCGA, https://tcga-data.nci.Nih.gov/tcga), which contains the clinical information and level 3 RNA-seq from 307 samples of cervical cancer and 3 tissue samples of normal cervix. The difference between normal cervical tissue and cervical cancer with reference to miR-101 expression level was analyzed by limma, an R/Bioconductor software package [13]. The data was downloaded on 10th March 2017. The data collection processes were in compliance with all laws and regulations.
3. Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) (qRT-PCR)
Whole blood sample (3.5 mL) was collected and put into a vacuum collection vessel, and incubated for 30 to 60 minutes. The serum was obtained by centrifugation at room temperature, 2,000 rpm for 15 minutes, and transferred to 1.5 mL EP tube, free of RNase, and stored at −80°C in a freezer. The period between sample collection from patients and PCR analysis is 50 days. Serum miR-101 was extracted and purified according to the instruction of RNA extraction kit. TaqMan® MicroRNA Assay Kits and Reverse Transcription Kits (Applied Biosystems, Foster City, CA, USA) were used for qRT-PCR determination of miRNA levels, performed on a 7500 Real-Time PCR System (Applied Biosystems). The experiment was conducted according to the manufacturer's instructions, and the concentration and purity of RNA was determined by a nucleic acid concentration detector. The concentration of RNA was normalized to 50 ng/µL for cDNA synthesis by specific reverse transcription primer (Applied Biosystems), and the reaction condition was set according to cDNA synthesis kit (Applied Biosystems). SYBR Green was utilized for real-time PCR, with 95°C denaturing for 10 minutes, followed with 50 cycles of 95°C denaturing for 15 seconds and 60°C annealing for 32 seconds. The melting curve was measured after 50 cycles PCR and Ct value of each sample was automatically calculated; and the relative quantitation of miRNA was calculated by 2-AACt algorithm. The samples were triplicated for assay and U6 was used as internal control for miRNA amplification.
4. Follow-up
A follow-up card was created for each cervical cancer patient upon admission, containing complete clinical, pathological, and follow-up records. The starting date point was the date of confirmed diagnosis, and the closing date was 31st March 2017. The survival period was calculated in terms of months. The patients were followed up once every 3 months for the first year after treatment; then followed up once every 3 to 6 months for the second year after treatment; and once every 6 months for the 3rd year after treatment; and once a year after the 5th year.
5. Statistical analysis
The association between serum miR-101 and the clinical and pathological features were analyzed by the χ2 test. The survival curve was plotted by Kaplan-Meier method, and compared by log-rank test. Cox proportional hazards model was utilized for analysis of prognostic factors. All data was statistically processed by software GraphPad prism 7.0 (GraphPad, La Jolla, CA, USA). p<0.05 was considered statistically significant.
RESULTS
1. TCGA derived data for comparison of miR-101 level between normal cervical tissue and cervical cancer, and association of miR-101 to prognosis
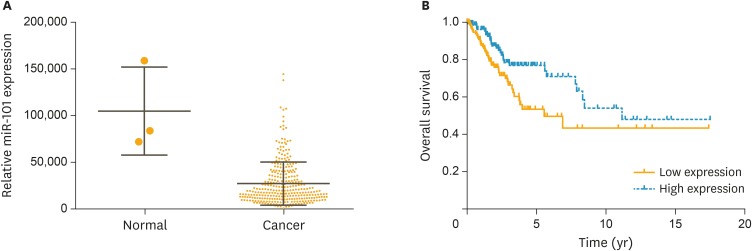
The association between miR-101 level in cervical cancer and prognosis was analyzed based on the TCGA retrieved data. The range of miR-101 in cervical cancer tissue was 756–144,886, and mean value with standard deviation was 27,585±1,349. miR-101 level was significantly decreased in cervical cancer (Fig. 1A, p<0.001). The patients were classified into a miR-101 low expression group and a miR-101 high expression group based on the median value (19,407) of miR-101. The overall survival time was significantly longer in the miR-101 high expression group compared with that of the miR-101 low expression group (Fig. 1B). The univariate Cox regression analysis indicated that decreased miR-101 expression was a significant risk factor for prognosis of cervical cancer (hazard ratio [HR]=2.404; p<0.001).
Fig. 1.
Expression level of miR-101 in cervical cancer was significantly decreased and positively associated with the prognosis of patients. (A) MiR-101 expression levels of cervical cancer patients (n=307) and healthy persons (n=3) from the TCGA database were analyzed. (B) The survival analysis for 2 groups of patients with low or high expression levels of miR-101.
TCGA, The Cancer Genome Atlas.
2. Expression level of miR-101 in serum of cervical cancer patients
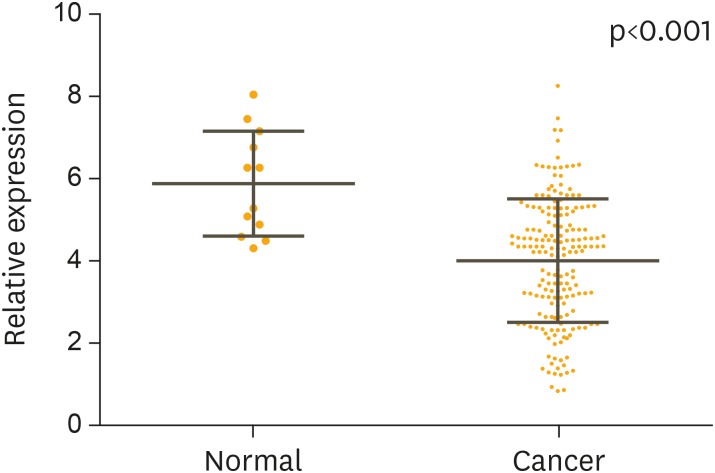
The expression level of miR-101 in the serum was determined by qRT-PCR for 182 patients with cervical cancer and 12 healthy women. U6 expression was utilized as internal control. The serum level of miR-101 was significantly lower in the patients with cervical cancer compared with that of the healthy controls (Fig. 2A, p<0.001).
Fig. 2.
The relative expression levels of miR-101 for 182 cervical cancer patients, before and after treatment, and 12 healthy women. The average value is indicated by the horizontal lines among the spots. The serum level of miR-101 was significantly higher in the healthy women compared with that from cervical cancer patients (p<0.001).
3. Association between serum miR-101 and clinical feature of cervical cancer patients
The median expression level of miR-101 (4.02) were used as a cut-off value to divide all 182 patients into 2 groups: cervical cancer patients who express miR-101 at levels less than the cut-off value were assigned to the low expression group (n=80) and those with miR-101 expression higher than the cut-off value were assigned to the high expression group (n=102). The cervical cancer patients were classified into different groups according to the clinical and pathological features, with the objective to discover the association between serum miR-101 and the clinical and pathological features of cervical cancer. The serum level of miR-101 was closely associated with the FIGO stage (p=0.003), lymph node metastasis (p=0.001), and serum SCC-Ag level >4 (p=0.007) (Table 1); however, it was not associated with age, tumor size, and histopathological differentiation degree (p>0.050).
Table 1. Correlation of clinicopathological characteristics and serum miR-101 expression in the cervical cancer patients.
| Variables | No. of patients (n=182) | MiR-101 expression | p-value | ||
|---|---|---|---|---|---|
| Low | High | ||||
| Age (yr) | 0.325 | ||||
| ≤50 | 134 | 56 (41.8) | 78 (58.2) | ||
| >50 | 48 | 24 (50.0) | 24 (50.0) | ||
| FIGO stage | 0.003 | ||||
| IB1–IIA1 | 153 | 60 (39.2) | 93 (60.8) | ||
| IIA2–IIIB | 29 | 20 (69.0) | 9 (31.0) | ||
| Tumor size (cm) | 0.123 | ||||
| ≤4 | 150 | 62 (41.3) | 88 (58.7) | ||
| >4 | 32 | 18 (56.3) | 14 (43.7) | ||
| Histology | 0.972 | ||||
| Squamous | 139 | 61 (43.9) | 78 (56.1) | ||
| Adenocarcinoma | 43 | 19 (44.2) | 24 (55.8) | ||
| Differentiation | 0.078 | ||||
| Well-moderate | 161 | 67 (41.6) | 94 (58.4) | ||
| Poor | 21 | 13 (61.9) | 8 (38.1) | ||
| Lymph nodes metastasis | 0.001 | ||||
| No | 145 | 55 (37.9) | 90 (62.1) | ||
| Yes | 37 | 25 (67.6) | 12 (32.4) | ||
| SCC-Ag (ng/L) | 0.007 | ||||
| ≤4 | 132 | 50 (37.9) | 82 (62.1) | ||
| >4 | 50 | 30 (60.0) | 20 (40.0) | ||
Values are presented as number (%).
FIGO, International Federation of Gynecology and Obstetrics; SCC-Ag, squamous cell carcinoma antigen.
4. Association between serum miR-101 and prognosis of cervical cancer patients
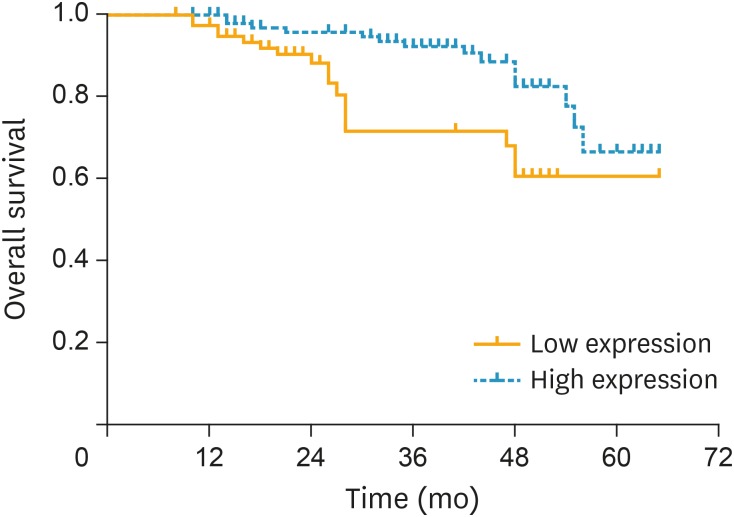
The association between serum level of miR-101 from 182 cervical cancer patients and their overall survival time was analyzed by the Kaplan-Meier method and log-rank test. A total of 31 of 182 patients (17.0%) died during the follow-up period. Fig. 3 shows the overall survival time was longer for the patients with a higher miR-101 level than the patients with a lower miR-101 level (log-rank test: p=0.004). The univariate analysis suggested that there was a significant association between the overall survival time and the FIGO stage (p=0.004), lymph node metastasis (p=0.042), SCC-Ag (p=0.042), and serum miR-101 level (p=0.004) (Table 2); however, there was no obvious association between the overall survival time and age, tumor size, and histological type. In addition, multivariate Cox regression model indicated that the FIGO stage (p=0.003), lymph node metastasis (p=0.015), and serum miR-101 level (p=0.006) were independent risk factors for the prognosis of cervical cancer.
Fig. 3.
The association between serum miR-101 level and overall survival time was analyzed by Kaplan-Meier method. The survival period was shorter in the cervical cancer patients with a lower expression level of miR-101 (p=0.004).
Table 2. Univariate and multivariate analyses of prognostic parameters in cervical cancer using the Cox regression model.
| Parameters | Univariable | Multivariable | ||
|---|---|---|---|---|
| p-value | HR (95% CI) | p-value | ||
| MiR-101 expression | 0.006 | |||
| Low | 0.004 | 2.820 (1.473–3.925) | ||
| High | - | - | ||
| Tumor size (cm) | - | |||
| ≤4 | 0.192 | - | ||
| >4 | - | - | ||
| Differentiation | - | |||
| Well-moderate | 0.260 | - | ||
| Poor | - | - | ||
| SCC-Ag (μg/L) | 0.746 | |||
| ≤4 | 0.042 | 0.941 (0.452–2.103) | ||
| >4 | - | - | ||
| FIGO stage | 0.003 | |||
| IB1–IIA1 | 0.031 | 2.378 (1.653–3.946) | ||
| IIA2–IIIB | - | - | ||
| Lymph nodes metastasis | 0.015 | |||
| No | 0.042 | 2.023 (1.231–3.521) | ||
| Yes | - | - | ||
CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; SCC-Ag, squamous cell carcinoma antigen.
DISCUSSION
miRNA is a small non-coding RNA molecule (containing about 18 to 22 nucleotides), with the functions of post-transcriptional regulation of gene expression through complimentarily binding to the targeted 3'UTR of mRNA, to degrade mRNA or to inhibit its translation, similar to the functions of oncogene or anti-oncogene [14,15,16,17,18]. It has been reported that miRNAs regulate the expression of 1/3 coding genes of the human genome and participate in various life activities including cell proliferation, differentiation, metabolisms, cell cycle, apoptosis, and individual development [8,9]. Serum miRNAs are mainly derived from apoptotic and necrotic cells, or active secretion from tissues, and lysis of circulating cells. Therefore, the expression profile of miRNA in serum and tissues is highly similar. The endogenous serum miRNA usually forms a silent complex with proteins after maturation resistant to RNase and seldom exists in the free form. Therefore, though the abundance of miRNA was much lower in the serum compared with that in the tissue, the stability of serum miRNA was very high, an essential feature of which makes serum miRNA play a crucial biological role as a tumor biomarker [19].
The abnormal expression of cervical cancer related miRNAs has been the focus of clinical studies. The abnormal expression of multiple types of miRNA was reported to be closely connected with the pathogenesis and development of cervical cancer [20,21,22]. MiR-101 belongs to the family of miRNAs and is found in many types of cells. The matured miR-101 contains 21 nucleotides, the sequence of which is highly conserved across species. MiR-101 has been found to be down-regulated in multiple types of solid tumors such as breast cancer, lung cancer, liver cancer, and ovarian cancer [10,11,12,23]. Similarly, the expression of miR-101 was also found to be decreased in cervical cancer cells [24,25]. Our bioinformatics analysis based on the TCGA database indicated that the expression of miR-101 in cervical cancer was decreased compared with that in the normal cervical tissue; and the overall survival in the patients with a high expression level of miR-101 was significantly longer than the patients with a low expression level of miR-101. To further investigate the clinical value of miR-101, the association between serum level of miR-101 and the clinical and pathological features of cervical cancer was analyzed. Our findings indicated that serum miR-101 was significantly reduced in patients with cervical cancer, and was correlated with the FIGO stage, lymph node metastasis, and the SCC-Ag. Meanwhile, we found that the low expression of serum miR-101 was an independent prognostic risk factor.
Currently, SCC-Ag has been widely accepted in the clinical diagnosis, therapeutic efficacy evaluation, and prognosis prediction for cervical cancer [26,27]. The level of SCC-Ag has been closely associated with the progress of cervical cancer; the persistent presence or remaining at a high level indicates the persistence or recurrence of cervical cancer. Alterations in SCC-Ag levels were not only found in cervical cancer, but also in lung cancer, esophageal squamous cell carcinoma, and the other benign tumors [28,29,30]. The cut-off value of SCC-Ag level for the diagnosis of cervical cancer varied at different stages [31]. Therefore, the clinical value of SCC-Ag remains controversial and can hardly be considered an ideal biomarker for prognosis of cervical cancer.
Lymph nodes are the most common routes of metastasis in cervical cancer and therefore, also an important factor for prognosis. A study found survival was 80% in patients without para-aortic nodes while only 20% in patients with positive nodes at 48 months regardless of cervical cancer staging [32]. Indeed, lymph node metastasis status before the commencement treatment is a key factor for the determination of individual therapeutic strategy. Patients with para-aortic lymph nodes metastasis usually receive an “extended” radiation therapy to the abdominal main lymph node region in addition to routine pelvic radiation in order to achieve optimal therapeutic efficacy [33]. Therefore, accurate determination of lymph node metastasis is of great clinical significance for the diagnosis and treatment of cervical cancer. However, current imaging techniques cannot accurately determine the lymph node metastasis, and an accurate biomarker to reflect lymph node metastasis is lacking. Previous studies have indicated the overexpression of miR-101 could inhibit proliferation and invasion of cervical cancer cells, regulate the cell cycle, as well as promote apoptosis [24,25].
The significant difference between cervical cancer and normal cervical tissue was discovered in terms of miR-101 expression level based on the bioinformatics analysis of the TCGA database. Our clinical study with cervical cancer patient samples indicated that serum level of miR-101 was closely correlated with FIGO stage, lymph node metastasis, and the SCC-Ag level. Our findings also suggested that the down-regulated expression level of miR-101 was associated with invasion, metastasis, and an unfavorable prognosis of cervical cancer; and that serum miR-101 had the potential to be an effective biomarker for the prediction and prognosis of cervical cancer. However, the underlying pathogenic mechanism and importance between miR-101 expression level and cervical cancer are not clear yet, further investigation with a larger number of samples and prospective clinical trials are needed.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China (31660275, 81360205), the Youth Top-notch Talent Support Program of Jiangxi Science & Technology Normal University (2013QNBJRC004), the Science and Technology Program of Department of Education of Jiangxi province (GJJ150792).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: J.W., P.J.J., Y.L.H.
- Formal analysis: J.W., P.J.J., D.Y.H., L.M.R., Y.L.H.
- Funding acquisition: Y.L.H.
- Investigation: J.W., P.J.J., D.Y.H., L.M.R., Y.L.H.
- Project administration: J.W., Y.L.H.
- Supervision: J.W., Y.L.H.
- Writing - original draft: J.W., P.J.J., D.Y.H., L.M.R.
- Writing - review & editing: J.W., Y.L.H.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer. 2013;49:3262–3273. doi: 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 4.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 5.Weiland M, Gao XH, Zhou L, Mi QS. Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. RNA Biol. 2012;9:850–859. doi: 10.4161/rna.20378. [DOI] [PubMed] [Google Scholar]
- 6.Nugent M, Miller N, Kerin MJ. Circulating miR-34a levels are reduced in colorectal cancer. J Surg Oncol. 2012;106:947–952. doi: 10.1002/jso.23174. [DOI] [PubMed] [Google Scholar]
- 7.Schetter AJ, Okayama H, Harris CC. The role of microRNAs in colorectal cancer. Cancer J. 2012;18:244–252. doi: 10.1097/PPO.0b013e318258b78f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim LP, Glasner ME, Yekta S, Burge CB, Bartel DP. Vertebrate microRNA genes. Science. 2003;299:1540. doi: 10.1126/science.1080372. [DOI] [PubMed] [Google Scholar]
- 9.Silahtaroglu A, Stenvang J. MicroRNAs, epigenetics and disease. Essays Biochem. 2010;48:165–185. doi: 10.1042/bse0480165. [DOI] [PubMed] [Google Scholar]
- 10.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Guo J, Yu L, Cai J, Gui T, Tang H, et al. miR-101 regulates expression of EZH2 and contributes to progression of and cisplatin resistance in epithelial ovarian cancer. Tumour Biol. 2014;35:12619–12626. doi: 10.1007/s13277-014-2585-6. [DOI] [PubMed] [Google Scholar]
- 12.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 15.Tian Y, Luo A, Cai Y, Su Q, Ding F, Chen H, et al. MicroRNA-10b promotes migration and invasion through KLF4 in human esophageal cancer cell lines. J Biol Chem. 2010;285:7986–7994. doi: 10.1074/jbc.M109.062877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci USA. 2008;105:5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009;16:23–29. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z, Chen S, Luan X, Li Y, Liu M, Li X, et al. MicroRNA-214 is aberrantly expressed in cervical cancers and inhibits the growth of HeLa cells. IUBMB Life. 2009;61:1075–1082. doi: 10.1002/iub.252. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 20.Piao J, You K, Guo Y, Zhang Y, Li Z, Geng L. Substrate stiffness affects epithelial-mesenchymal transition of cervical cancer cells through miR-106b and its target protein DAB2. Int J Oncol. 2017;50:2033–2042. doi: 10.3892/ijo.2017.3978. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Zhou B, Liu M, Liu Y, Gao R. miR-126-5p restoration promotes cell apoptosis in cervical cancer by targeting Bcl2l2. Oncol Res. 2017;25:463–470. doi: 10.3727/096504016X14685034103879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo M, Zhao X, Yuan X, Jiang J, Li P. MiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in cervical cancer. Oncotarget. 2017;8:28226–28236. doi: 10.18632/oncotarget.15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Wang J, Mao Y, Zou B, Fan X. MicroRNA-101 suppresses migration and invasion via targeting vascular endothelial growth factor-C in hepatocellular carcinoma cells. Oncol Lett. 2016;11:433–438. doi: 10.3892/ol.2015.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang X, Liu Y, Zeng L, Yu C, Hu Z, Zhou Q, et al. miR-101 inhibits the G1-to-S phase transition of cervical cancer cells by targeting Fos. Int J Gynecol Cancer. 2014;24:1165–1172. doi: 10.1097/IGC.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 25.Lin C, Huang F, Shen G, Yiming A. MicroRNA-101 regulates the viability and invasion of cervical cancer cells. Int J Clin Exp Pathol. 2015;8:10148–10155. [PMC free article] [PubMed] [Google Scholar]
- 26.Oh J, Lee HJ, Lee TS, Kim JH, Koh SB, Choi YS. Clinical value of routine serum squamous cell carcinoma antigen in follow-up of patients with locally advanced cervical cancer treated with radiation or chemoradiation. Obstet Gynecol Sci. 2016;59:269–278. doi: 10.5468/ogs.2016.59.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esajas MD, Duk JM, de Bruijn HW, Aalders JG, Willemse PH, Sluiter W, et al. Clinical value of routine serum squamous cell carcinoma antigen in follow-up of patients with early-stage cervical cancer. J Clin Oncol. 2001;19:3960–3966. doi: 10.1200/JCO.2001.19.19.3960. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Chen P, Mao CM, Tang XP, Zhu LR. Evaluation of diagnostic value of four tumor markers in bronchoalveolar lavage fluid of peripheral lung cancer. Asia Pac J Clin Oncol. 2014;10:141–148. doi: 10.1111/ajco.12066. [DOI] [PubMed] [Google Scholar]
- 29.Cao X, Zhang L, Feng GR, Yang J, Wang RY, Li J, et al. Preoperative Cyfra21-1 and SCC-Ag serum titers predict survival in patients with stage II esophageal squamous cell carcinoma. J Transl Med. 2012;10:197. doi: 10.1186/1479-5876-10-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torre GC. SCC antigen in malignant and nonmalignant squamous lesions. Tumour Biol. 1998;19:517–526. doi: 10.1159/000030045. [DOI] [PubMed] [Google Scholar]
- 31.Ryu HK, Baek JS, Kang WD, Kim SM. The prognostic value of squamous cell carcinoma antigen for predicting tumor recurrence in cervical squamous cell carcinoma patients. Obstet Gynecol Sci. 2015;58:368–376. doi: 10.5468/ogs.2015.58.5.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manetta A, Delgado G, Petrilli E, Hummel S, Barnes W. The significance of paraaortic node status in carcinoma of the cervix and endometrium. Gynecol Oncol. 1986;23:284–290. doi: 10.1016/0090-8258(86)90128-9. [DOI] [PubMed] [Google Scholar]
- 33.Yoon HI, Cha J, Keum KC, Lee HY, Nam EJ, Kim SW, et al. Treatment outcomes of extended-field radiation therapy and the effect of concurrent chemotherapy on uterine cervical cancer with para-aortic lymph node metastasis. Radiat Oncol. 2015;10:18. doi: 10.1186/s13014-014-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]