Abstract
Objective
Programmed death-ligand 1 (PD-L1) was expressed in various tumors and antibodies targeting its receptor programmed cell death-1 (PD-1) are emerging cancer therapeutics. This study was designed to evaluate the expression of PD-L1 and its correlation with clinicopathologic features and clinical outcomes in ovarian clear cell carcinoma (OCCC).
Methods
The PD-L1 expression was measured by tissue-microarray-based immunohistochemistry from 122 eligible patients diagnosed with OCCC. The associations of clinicopathologic features with progression-free survival (PFS) and overall survival (OS) were analyzed by Kaplan-Meier method and multivariate analysis was further performed by Cox regression model.
Results
Overall, high PD-L1 expression (PD-L1high) was observed in 44.7% (55/123) of OCCC patients, and was strongly associated with advanced stages (p=0.020), positive ascitic fluid (p=0.016), platinum-resistant (PR) disease (p=0.045), and recurrence (p=0.038). Moreover, patients with PD-L1high were associated with poorer OS (hazard ratio [HR]=2.877; p=0.001) and PFS (HR=1.843; p=0.021) than those with low PD-L1 expression (PD-L1low). In subgroup analysis, PD-L1high patients experienced a poorer PFS (HR=1.926; p=0.044) and OS (HR=2.492; p=0.021) than PD-L1low cases among advanced stages (III–IV), but this difference was not observed in stage I–II patients. Meanwhile, PD-L1high was associated with poorer prognosis than PD-L1low in PR patients (OS, HR=2.253; p=0.037; PFS, HR=1.448; p=0.233). Multivariate analysis revealed that PD-L1high and advanced stages (III–IV) were adverse independent prognosticators for both PFS (HRPD-L1=2.0; pPD-L1=0.038; HRstage=10.2; pstage<0.001) and OS (HRPD-L1=3.0; pPD-L1=0.011; HRstage=14.3; pstage<0.001).
Conclusion
PD-L1high might serve as a risk factor for PFS and OS in patients with OCCC. It is possible that immunotherapy targeting PD-L1 pathway could be used in OCCC.
Keywords: Ovarian Neoplasms; Adenocarcinoma, Clear Cell; Antigens, CD274; Prognosis
INTRODUCTION
Epithelial ovarian carcinoma (EOC) has the highest mortality among gynecologic malignancies, accounting for 4.2% of all cancer-related deaths in women [1]. Although EOC is often thought of as a single entity, the different histological subtypes including clear cell carcinoma (CCC), serous adenocarcinoma (SAC), mucinous adenocarcinoma, and endometrioid adenocarcinoma vary in molecular, clinical, and pathological characteristics [2]. Accounting for approximately 5%–25% of EOCs, ovarian clear cell carcinoma (OCCC) is presented with the highest incidences reported in people of Asiatic origin [3]. Microscopically, it is characterized by cells with clear cytoplasm (that contains glycogen) and hob nail cells (from which the glycogen has been secreted). Typically, early stage OCCC is known to have a favorable outcome; however, patients with advanced disease have poorer prognosis when compared to high-grade serous ovarian cancer (HGSOC) [4]. Data suggested that the standard treatment including the debulking surgery followed by systemic chemotherapy may be less effective in OCCC with advanced stages, owing to its resistance toward chemotherapeutic agents [5,6]. Therefore, improved therapeutic strategies are absolutely required in the management of women with OCCC.
Tumor-induced immune suppression is a key problem that not only promotes tumor development, but also inhibits the efficiency of anti-cancer treatment. One of the major molecular regulators of tumor immune escape is programmed death-ligand 1 (PD-L1, CD274, B7-H1), a cell-surface protein induced in a number of malignancies, which could help tumor cells immune evasion in combination with immonomodulatory properties [7]. Blockage of PD-L1 expression on tumor cells might activate tumor-specific T cell to kill tumor cells by mediating tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ) [8,9]. Previous studies have shown that PD-L1 can be expressed in a number of malignancies including melanoma (MEL), non-small cell lung cancers (NSCLCs), renal cell carcinomas (RCCs), cancers of head and neck, and gastrointestinal malignancies [10,11,12,13,14], and was correlated with unfavorable prognosis and the resistance to anticancer therapies in non-small lung cancers, colorectal and breast cancers [15,16,17]. Although several literatures showed that PD-L1 expression was upregulated in EOCs, most studies were conducted only in serous ovarian cancer (OC) [18,19,20]. Therefore, we focused our work to investigate the PD-L1 expression and its correlation with clinicopathological features in OCCC, and to further explore the prognostic value of PD-L1 expression in OCCC.
MATERIALS AND METHODS
1. Patients
A total of 138 patients diagnosed with OCCC were selected from the archival collections (between 1999 and 2014) of the Department of Pathology at Fudan University Shanghai Cancer Center. The exclusion criteria included patients with previously treated OCCC, patients with malignancies other than OCCC, patients with a simultaneous second primary cancer, patients with a previous history of chemo-radiotherapy due to other diseases and patients without follow-up infomation. Of the whole series, 122 patients with adequate paraffin-embedded tissue for immunohistochemical evaluation were enrolled in the study of the PD-L1 expression. All the 122 cases had undergone primary operation and received no chemotherapy before surgery. The detailed clinical data were collected by reviewing patients' medical charts. In order to confirm the diagnosis, all the microscopic slides were reviewed by the same gynecology-dedicated pathologist (Wu Y) and confirmed by a second experienced gynecologic pathologist (Bi R). Both were blinded to the original diagnosis. The histologic subtype was classified according to the World Health Organization (WHO) definitions [21].
All the 122 patients in the study cohort provided informed consent. The study was approved by the ethics committee at Fudan University Shanghai Cancer Center, Shanghai, China. All of the participants provided written informed consent for the use of their tissue samples.
Patients were staged using the International Federation of Gynecology and Obstetrics (FIGO) 2012 staging system and were further divided into early stage (I–II) and advanced stage (III–IV) for the purpose of statistical analysis. The vast majority of the patients received platinum-based chemotherapy regimens with the number of cycles ranging from 6 to 8. Operations were performed by gynecologic oncologist to achieve optimal cytoreduction, which was defined as residual disease less than (or including) 1 cm after primary debulking. The platinum-sensitive (PS) patients were defined as the length of the platinum-free interval (PFI) of more than 6 months after completion of the last platinum-based regimen, while the platinum-resistant (PR) disease with a PFI less than 6 months.
2. Construction of the tissue microarray (TMA)
TMAs were constructed in the present study. In brief, hematoxylin and eosin (H&E) sections were assessed and an appropriate area of tumor was marked on the corresponding paraffin block. Care was taken not to include areas of fibrosis, adipose tissue or necrosis. Duplicate of 0.8 mm diameter cylinders were punched from representative tumor areas of individual donor tissue block, and re-embedded into a recipient paraffin block at a defined position, using a manual tissue arrayer (Beecher Instruments, Silver Spring, MD, USA). Following a review and screening of the representative tumor regions, 3 different areas were selected to cover the intratumoral heterogeneity for each tumor. Four TMA blocks were built at Fudan University Shanghai Cancer Center biorepository facility.
3. Immunohistochemistry (IHC)
TMAs were immunohistochemically stained. Primary antibodies used for IHC included anti-PD-L1 antibody (Ab) (ab205921, 1:50 dilution ratio; Abcam, Cambridge, MA, USA). The tissue sections were deparaffinized in xylene (2×20 minutes) and subjected to a graded alcohol dehydration (95%, 90%, 80%, and 70%) to water. Heat-mediated antigen retrieval was performed with ethylenediaminetetraacetic acid (EDTA) buffer (pH=8) by microwaves for 23 minutes. To block endogenous peroxidase activity, all sections were treated with 100% methanol containing 0.3% H2O2 at room temperature for 25 minutes. The sections were incubated with rabbit anti-PD-L1 monoclonal Ab overnight at 4°C. Then, the sections were incubated with a biotinylated goat-anti-rabbit secondary Ab (K5007; Dako, Glostrup, Denmark), followed by an incubation with a streptavidin-peroxidase complex solution at room temperature for 50 minutes and counterstained with hematoxylin. Positive controls consisted of placenta tissue for the primary antibody and the normal ovarian tissue was used for the negative control.
4. Assessment of PD-L1 expression
Two gynecological pathologists independently examined the prepared immunohistochemical slides without any prior information on the clinical history of the patients. The proportion of PD-L1-positive cells was estimated as a percentage of the total tumor cells. The tumor cells typically showed membranous staining with a variably strong component of cytoplasmic staining. Immunohistochemical evaluation of PD-L1 in OCCC specimens was based on the intensity and extent of nuclear and cytoplasmic reactivity. A 10% threshold of cell surface PD-L1 expression on tumor cells was defined as positive. The tumor cells typically showed membranous staining with a variably strong component of cytoplasmic staining. Then, we defined samples with barely no positive cells, less than 10% of positive cells, 10%–50% of positive cells, more than 51% of positive cells as negative, weak, moderate, strong expression, respectively. Tumors with moderate and strong staining were considered to be high expression of PD-L1 [22].
5. Statistical analysis
Statistical comparisons between clinicopathologic features and PD-L1 expression were evaluated using a χ2 test, whereas Fisher's exact test was added as necessary during the statistical analysis. In the analysis of recurrence and survival, the start point was defined as the day of surgery was performed. The end of the progression-free survival (PFS) period was defined as the day of recurrence, and that of overall survival (OS) period was defined as the day confirmed alive or dead, respectively. Estimation of PFS and OS was calculated by the Kaplan-Meier method, and the curves were compared using a log-rank test. Possible risk factors for recurrence and OS were analyzed with a Cox proportional hazards model. All of the p-values reported were 2-sided, and a value of p<0.05 was considered statistically significant. Statistical Package for Social Science (SPSS) statistical software (version 20.0; SPSS Inc., Chicago, IL, USA) and GraphPad Prism (version 5.0; GraphPad Software Inc., La Jolla, CA, USA) were used for all of the analyses.
RESULTS
1. Demographic and clinicopathologic characteristics
Demographics and clinicopathologic characteristics are summarized in Table 1. A total of 122 eligible patients were enrolled with a median postoperative follow-up term of 33.1 months (range, 1.7–163.4 months). The median age with a diagnosis of clear cell histology was 53.0 years (range, 19–83).
Table 1. Univariate analysis of factors associated with PFS and OS.
| Factors | No. | Recurrent (%) | p-value | Death (%) | p-value | |
|---|---|---|---|---|---|---|
| Age at diagnosis (yr) | 0.368 | 0.848 | ||||
| ≤55 | 76 | 32 (42.1) | 24 (31.6) | |||
| >55 | 46 | 23 (50.0) | 15 (32.6) | |||
| Family history of malignancy | 0.843 | 0.702 | ||||
| Yes | 30 | 13 (45.7) | 9 (30.0) | |||
| None | 92 | 42 (43.3) | 30 (32.6) | |||
| Menopause | 0.753 | 0.395 | ||||
| Postmenopausal | 75 | 33 (44.0) | 23 (30.7) | |||
| Premenopausal | 47 | 22 (46.8) | 16 (34.0) | |||
| BMI | 0.880 | 0.888 | ||||
| ≤25 | 61 | 27 (44.3) | 18 (29.5) | |||
| >25 | 61 | 28 (45.9) | 21 (34.4) | |||
| Pretreatment CA125 level (U/mL) | 0.002 | 0.023 | ||||
| <244 | 55 | 18 (32.7) | 13 (23.6) | |||
| ≥244 | 52 | 30 (57.7) | 19 (36.5) | |||
| Ascites | 0.133 | 0.077 | ||||
| Negative | 68 | 27 (39.7) | 18 (26.5) | |||
| Positive | 49 | 25 (51.0) | 18 (36.7) | |||
| Tumor site | 0.001 | <0.001 | ||||
| Unilateral | 99 | 39 (39.4) | 26 (26.3) | |||
| Bilateral | 23 | 16 (69.6) | 13 (56.5) | |||
| Tumor volume (cm) | 0.139 | 0.038 | ||||
| <10 | 49 | 26 (53.1) | 21 (42.9) | |||
| ≥10 | 73 | 29 (39.7) | 18 (24.7) | |||
| Tumor stage | <0.001 | <0.001 | ||||
| I | 57 | 10 (17.5) | 5 (7.9) | |||
| II | 19 | 8 (42.1) | 6 (30.0) | |||
| III | 36 | 28 (77.8) | 25 (58.1) | |||
| IV | 10 | 9 (90.0) | 8 (80.0) | |||
| Residual disease (cm) | <0.001 | <0.001 | ||||
| R0 | 98 | 36 (36.7) | 23 (23.5) | |||
| ≤1 | 18 | 13 (72.2) | 10 (55.6) | |||
| >1 | 6 | 6 (100.0) | 6 (100.0) | |||
| Sensitivity to chemotherapy | <0.001 | <0.001 | ||||
| Sensitive | 81 | 14 (17.3) | 7 (8.6) | |||
| Insensitive | 40 | 40 (100.0) | 31 (77.5) | |||
| PD-L1 expression | 0.021 | 0.001 | ||||
| Low | 68 | 25 (36.8) | 13 (19.1) | |||
| High | 54 | 30 (55.6) | 26 (48.1) | |||
BMI, body mass index; OS, overall survival; PD-L1, programmed death-ligand 1; PFS, progression-free survival; R0, no visible residual disease.
At the completion of the primary surgery, 80.5% of the patients (n=99) had no visible residual disease (R0), 14.6% of the patients (n=18) had residual disease ≤1 cm, and 4.9% of the advanced cases (n=6) had residual disease >1 cm. The vast majority (98.4%, n=121) of the patients received adjuvant chemotherapy with platinum-based regimens, while only 2 patients received the traditional Chinese medicine instead of chemotherapy.
2. Immunohistochemical detection of PD-L1 expression in OCCC
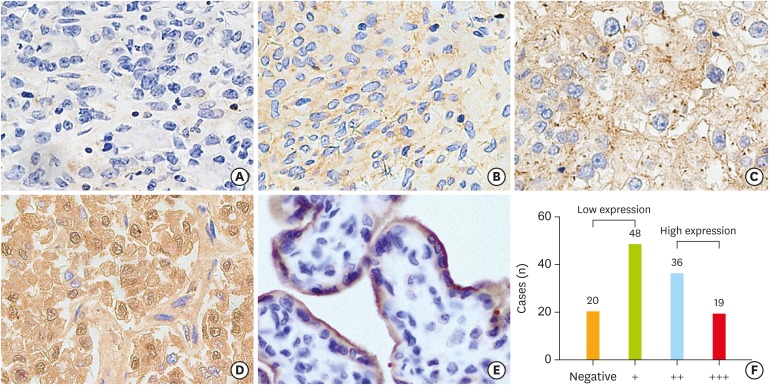
PD-L1 was stained using IHC techniques on the cell membrane or in the cytoplasm of epithelial tumor cells and the immunohistochemical PD-L1 expression was depicted in Figs. 1 and 2. Among all 123 cases, 55 cases (44.7%) were categorized as having high PD-L1 expression (PD-L1high) and 68 cases (55.3%) were presented with low PD-L1 expression (PD-L1low). When stratified by FIGO stage, PD-L1high was observed in 22 of 58 stage I cases (37.9%), 7 of 19 stage II cases (36.8%), 20 of 36 stage III cases (55.6%), and 6 of 10 stage IV cases (60.0%). In PS patients, PD-L1high was observed in 31 cases (38.3%), and 50 cases (61.7%) were presented with low expression. While among PR patients, 23 cases (57.5%) were found to have PD-L1high and PD-L1low was displayed in 17 cases (42.5%).
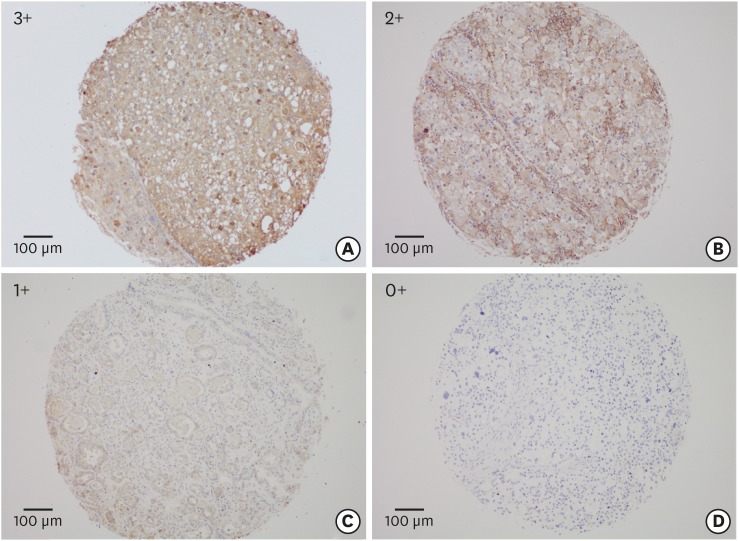
Fig. 1.
The overview of staining intensities of the tissue array is shown (3+. 2+, 1+, and 0+), with an example each (A-D) for a membranous and/or for a cytoplasmic staining pattern.
Fig. 2.
Immunohistochemical staining for PD-L1 in OCCC tumor tissues. The staining intensities are shown (0+, 1+, 2+, 3+), with an example each (A-D) for a membranous and/or a cytoplasmic staining pattern. The placenta tissue (E) is used as a positive control for PD-L1 expression. The total number of each staining intensity are shown in (F).
OCCC, ovarian clear cell carcinoma; PD-L1, programmed death-ligand 1.
3. Statistical association between PD-L1 expression and clinicopathologic features in OCCC
Table 2 shows the clinicopathologic characteristics in relation to PD-L1 expression. PD-L1high was significantly associated with advanced stages (III–IV) compared to PD-L1low (57.4% vs. 42.6%, p=0.020). Additionally, PD-L1high tended to be associated with positive ascitic fluid rather than negative ascitic fluid (57.1% vs. 42.9%, p=0.016). Recurrent OCCC patients with PR appeared to exhibit elevated expression of PD-L1 (57.5% vs. 42.5%, p=0.045). Recurrent diseases seemed to present PD-L1high than those diseases with no relapse (54.5% vs. 45.5%, p=0.038).
Table 2. Correlations between the expression of PD-L1 and clinicopathological characteristics in OCCCs.
| Variables | Tumoral PD-L1 expression | |||
|---|---|---|---|---|
| Low expression | High expression | p-value | ||
| Age (yr) | 0.260 | |||
| ≤55 | 39 (57.4) | 37 (68.5) | ||
| >55 | 29 (42.6) | 17 (31.5) | ||
| Stage | 0.020 | |||
| Early (I–II) | 48 (70.6) | 27 (50.0) | ||
| Advanced (III–IV) | 20 (29.4) | 27 (50.0) | ||
| BMI | 0.068 | |||
| ≤25 | 29 (42.6) | 32 (59.3) | ||
| >25 | 39 (57.4) | 22 (40.7) | ||
| Menopause status | 0.264 | |||
| Premenopausal | 23 (33.8) | 24 (44.4) | ||
| Postmenopausal | 45 (66.2) | 30 (55.6) | ||
| Pretreatment CA125 (U/mL) | 0.699 | |||
| <244 | 32 (53.3) | 23 (48.9) | ||
| ≥244 | 28 (46.7) | 24 (51.1) | ||
| Ascitic fluid | 0.016 | |||
| Negative | 45 (68.2) | 23 (45.1) | ||
| Positive | 21 (31.8) | 28 (54.9) | ||
| Tumor site | 0.245 | |||
| Unilateral | 58 (85.3) | 41 (75.9) | ||
| Bilateral | 10 (14.7) | 13 (24.1) | ||
| Tumor volume (cm) | 0.854 | |||
| <10 | 28 (41.2) | 21 (38.9) | ||
| ≥10 | 40 (58.8) | 33 (61.1) | ||
| Sensitivity to chemotherapy | 0.045 | |||
| Sensitive | 50 (74.6) | 31 (57.4) | ||
| Insensitive | 17 (25.4) | 23 (42.6) | ||
| Recurrence status | 0.038 | |||
| Relapse | 25 (36.8) | 30 (55.6) | ||
| No relapse | 43 (63.2) | 24 (44.4) | ||
BMI, body mass index; OCCC, ovarian clear cell carcinoma; PD-L1, programmed death-ligand 1.
4. Survival analysis according to PD-L1 expression in OCCCs
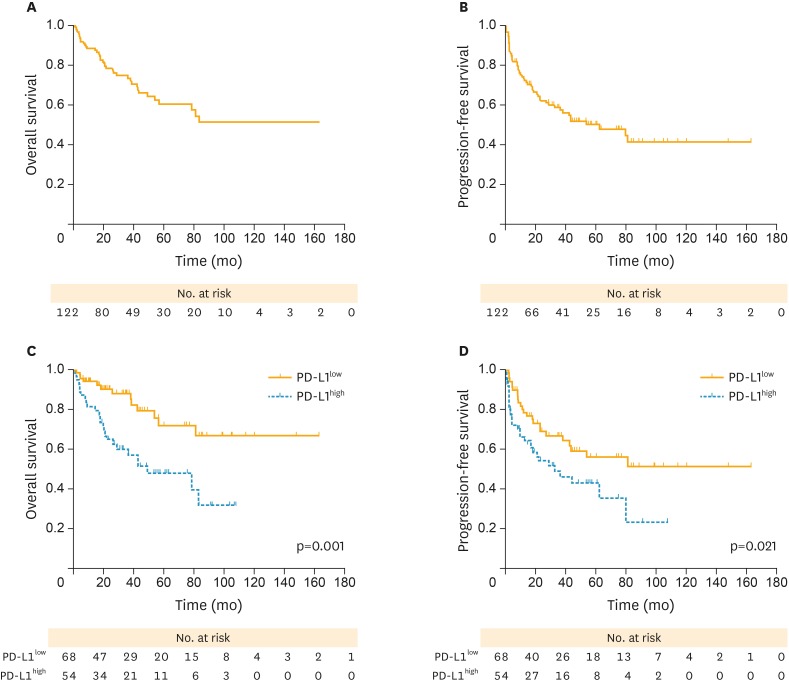
The PFS and OS curves after surgery for patients who underwent complete resection are shown in Fig. 3. On the whole, the 3- and 5-year OS were 74.9% and 60.7%, respectively (Fig. 3A) and patients had an estimated median PFS of 62.9 months (95% confidence interval [CI]=24.1–101.7 months; Fig. 3B). Patients with PD-L1high was correlated with better survival outcome than those with PD-L1low (5-year OS rate, 71.9% vs. 48.2%; p=0.001; Fig. 3C). Similarly, PD-L1high was associated with significantly shorter median PFS compared with PD-L1low (52.0 months vs. not achieved in the study period; p=0.021; Fig. 3D).
Fig. 3.
(A, B) PFS and OS curves in patients with OCCC. (C) Patients with PD-L1low (yellow solid line) presented a better survival outcome than those with PD-L1high (blue dotted line). (D) Patients with PD-L1high (blue dotted line) showed significantly worse PFS compared with those with low expression (yellow solid line).
OCCC, ovarian clear cell carcinoma; OS, overall survival; PD-L1, programmed death-ligand 1; PD-L1high, high PD-L1 expression; PD-L1low, low PD-L1 expression; PFS, progression-free survival.
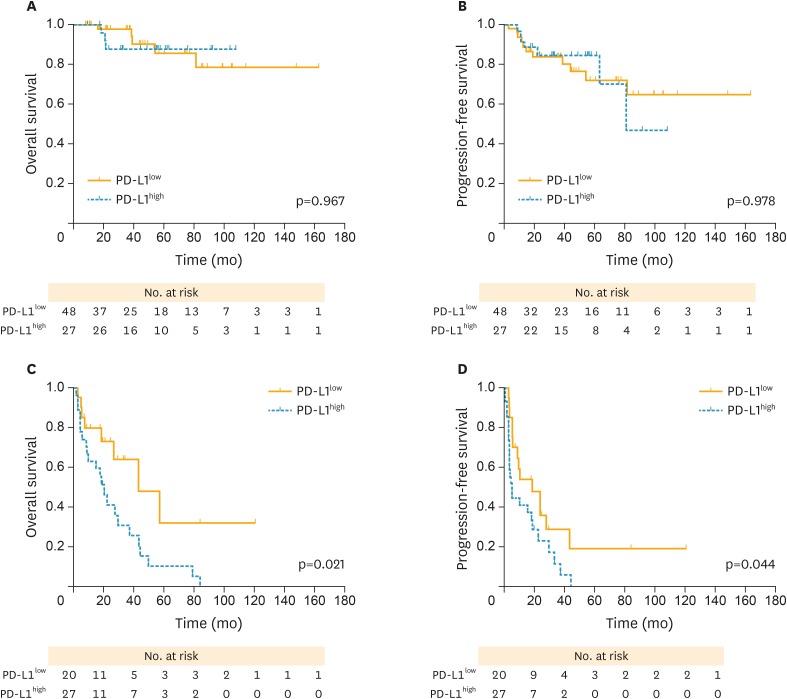
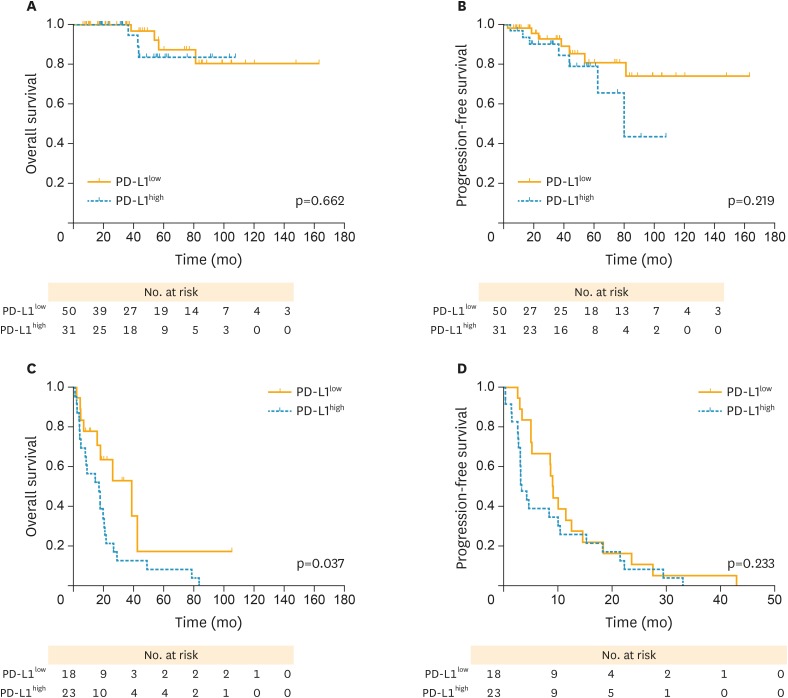
In subgroup analysis, both PFS (p=0.978; Fig. 4B) and OS (p=0.967; Fig. 4A) curves were not significantly different between high and PD-L1low in early stage (I–II) patients. However, patients with PD-L1high experienced a poorer PFS (hazard ratio [HR]=1.926; p=0.044; Fig. 4D) and OS (HR=2.492; p=0.021; Fig. 4C) compared with those with PD-L1low in advanced stages (III–IV). Meanwhile, both PFS (p=0.219; Fig. 5B) and OS (p=0.662; Fig. 5A) curves were not significantly different between PD-L1high and PD-L1low groups in PS patients. PD-L1high exhibited a poorer OS compared with those with PD-L1low in PR cases (median OS, 17.6 vs. 26.6 months; p=0.037; Fig. 5C), whereas no favoring advantage in PFS was found (p=0.233; Fig. 5D).
Fig. 4.
PFS and OS curves stratified by FIGO stage between high and low PD-L1 expression (yellow solid lines, PD-L1low; blue dotted lines, PD-L1high, respectively). (A) OS curves between high and PD-L1low in FIGO stage I/II cases. (B) PFS curves between high and PD-L1low in FIGO stage I/II cases. (C) OS curves between high and PD-L1low in stage III/IV cases. (D) PFS curves between high and PD-L1low in stage III/IV cases. Patients with PD-L1high revealed a worse OS and PFS compared with those with PD-L1low in stage III/IV cases, with significant differences observed.
FIGO, International Federation of Gynecology and Obstetrics; OCCC, ovarian clear cell carcinoma; OS, overall survival; PD-L1, programmed death-ligand 1; PD-L1high, high PD-L1 expression; PD-L1low, low PD-L1 expression; PFS, progression-free survival.
Fig. 5.
PFS and OS curves stratified by the sensitivity to chemotherapy between high and low PD-L1 expression (yellow solid lines, PD-L1low; blue dotted lines, PD-L1high, respectively). (A) OS curves between high and PD-L1low in PS cases. (B) PFS curves between high and PD-L1low in PS cases. (C) OS curves between high and PD-L1low in PR cases. (D) PFS curves between high and PD-L1low in PR cases. Patients with PD-L1high revealed a worse OS and PFS compared with those with PD-L1low in PR cases, with significant differences observed.
OS, overall survival; PD-L1, programmed death-ligand 1; PD-L1high, high PD-L1 expression; PD-L1low, low PD-L1 expression; PFS, progression-free survival; PR, platinum-resistant; PS, platinum-sensitive.
5. Univariate and multivariate analysis of possible risk factors affecting recurrence and OS in OCCCs
In univariate analysis, tumor recurrence was significantly associated with PD-L1high (HR=1.9; p=0.021), advanced stages (III–IV, HR=7.8; p<0.001), high level of serum CA125 (HR=2.5; p=0.002), gross residual diseases (HR=2.9; p<0.001), PR diseases (HR=31.3; p<0.001), and bilateral tumor (HR=1.6; p=0.001). In addition, PD-L1high (HR=2.9; p=0.001), advanced stages (III–IV, HR=11.8; p<0.001), gross residual diseases (HR=4.5; p<0.001), PR diseases (HR=21.0; p<0.001), bilateral tumor (HR=1.9; p<0.001), high level of serum CA125 (HR=2.2; p=0.023), and large tumor volume (HR=1.9; p=0.038) were predictive of a worse survival outcome (Table 1).
In multivariate analysis, PD-L1high and advanced stages (III–IV) were adverse independent prognosticators for both PFS (HRPD-L1=2.0; pPD-L1=0.038; HRstage=10.2; pstage<0.001, respectively; Table 3) and OS (HRPD-L1=3.0; pPD-L1=0.011; HRstage=14.3; pstage<0.001, respectively; Table 3).
Table 3. Multivariate Cox regression analysis with PFS and OS as endpoint.
| Factors | PFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Pretreatment serum CA125 level (<244 U/mL vs. ≥244 U/mL) | 0.922 | 0.457–1.860 | 0.820 | 0.578 | 0.259–1.292 | 0.182 |
| Ascitic fluid (positive vs. negative) | 1.110 | 0.883–3.566 | 0.107 | 1.111 | 0.511–2.414 | 0.790 |
| FIGO stage (advanced vs. early) | 10.165 | 4.513–22.894 | <0.001 | 14.323 | 4.744–43.240 | <0.001 |
| PD-L1 expression (high vs. low) | 1.970 | 1.039–3.737 | 0.038 | 3.032 | 1.285–7.153 | 0.011 |
| Tumor volume (<10 cm vs. ≥10 cm) | 0.928 | 0.069–1.248 | 0.621 | 0.752 | 0.513–1.102 | 0.144 |
| Residual disease (>1 cm vs. R0, ≤1 cm) | 1.063 | 0.566–1.999 | 0.849 | 1.767 | 0.824–3.787 | 0.143 |
CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; OS, overall survival; PD-L1, programmed death-ligand 1; PFS, progression-free survival; R0, no visible residual disease.
DISCUSSION
This study demonstrates that PD-L1high was observed in OCCC patients and was significantly associated with advanced stages, positive ascitic fluid, PR disease, and relapse of disease. Furthermore, PD-L1high exhibited poorer PFS and OS than PD-L1low among OCCC patients. More importantly, the unfavorable prognostic effect of PD-L1high was identified as an independent predictor for survival.
In the present study, a significant association between PD-L1high and advanced FIGO stage was identified. FIGO stage was previously confirmed to be strongly correlated with prognosis in OCCC patients. The significant correlation between PD-L1high and advanced disease stage suggested the intense association between PD-L1 expression and tumor malignancy. Of note, a significant correlation between PD-L1high and ascitic fluid was observed, confirming the metastatic behavior of the carcinomatous component. A separate study by Abiko et al. [23] demonstrated in his study that PD-L1 expression in OC is associated with increased invasiveness and peritoneal dissemination. Maine et al. [24] demonstrated that PD-L1 expression on monocytes in the ascites and blood of patients with malignant OC is strikingly higher than those with benign/borderline disease. Together with our study, these results supported that PD-L1 expression possibly reflected the interruption of the antitumor immunity of the host and might further be associated with tumor development in ovarian malignancies.
Currently, PD-L1 is thought as a key mechanism of immunosuppressive effect and allows the tumor to evade immune destruction. Multiple different tumors expressing PD-L1, including NSCLC [25], RCC [26], osteosarcoma [27], and advanced MEL [28], presented a relatively poor prognostic outcomes. However, some other studies exhibited a controversial result of PD-L1 expression on the survival outcome. Kluger et al. [29] reported a better OS in patients with malignant MELs associated with high levels of PD-L1 expression. Also Beckers et al. [30] demonstrated that cytoplasmic tumoral expression of PD-L1 was associated with a lower risk of specific death in triple-negative breast cancer (HR=0.45; p=0.035). Hamanishi et al. [18] demonstrated the elevated proportion of high expression of PD-L1 in OCs and a significantly poorer prognosis in patients with higher expression than those with lower expression (p=0.016). Although they found that tumoral PD-L1 expression was an independent predictor of poor prognosis in OCs, this study investigated different histological subtypes of OCs. Our result also described an unfavorable prognostic impact of PD-L1high in OCCC, a specified histological subtype of ovarian carcinomas. However, a recent study showed a positive prognostic impact of PD-L1 expression in a cohort of 215 HGSOCs, which is in line with a recent study on a positive prognostic effect of programmed cell death-1 (PD-1) positive tumor infiltrating lymphocytes (TILs) in HGSOC [19,20]. These results also confirmed that the effect of PD-1 on recurrence and survival should be separately investigated based on different histologic subtypes of tumor.
The fact that PD-L1 is expressed in OCCC and that this expression has unfavorable prognostic impact strongly suggest that the PD-L1 may be an applicable prognostic predictor and monitor for OCCC patients in clinical practice. Previous studies in an OC mouse model showed that response to PD-L1 blockade requires a preexisting adaptive immunity in tumors, and tumors without TILs failed to respond [31]. This might make the anti-PD-L1 Abs available to OCCC. Anti-PD-L1 Abs have demonstrated durable responses in a number of different advanced malignancies. A phase I trial (NCT00729664) of BMS-936559, an anti-PD-L1 agent, showed that for the evaluable population, overall responses (ORs) were observed in patients with MEL (9/52), RCC (2/17), NSCLC (5/49), and OC (1/17). In 16 patients with ≥1 year of follow-up, stable disease (SD, ≥24 weeks) was observed in 14 patients with MEL, 6 patients with NSCLC, 3 patients with OC, and 7 patients with RCC [32]. Nivolumab, an anti-PD-1 antibody, demonstrated an efficacy in a proportion of relapsed PR OC patients, with the median PFS time was 3.5 months (95% CI=1.7–3.9), and the median OS time was 20.0 months [33]. In addition, the underlying mechanisms might be that in EOCs without lymphocyte infiltrates, PD-1/PD-L1 blockade is unlikely to be sufficient, and combinations will be required to mount a tumor-rejection adaptive immune response [34].
To our knowledge, this is the first report investigating PD-L1 expression and its correlation with clinicopathologic and prognostic characteristics in OCCC, a histologic subset of EOCs. The limitation in this study was that PD-L1 expression in this study was detected only with IHC, and no any other method was used to verify the PD-L1 expression status. Additional researches on proteomic markers or PD-L1 transcriptional levels in cell lines are required to further understand the mechanism of PD-1/PD-L1 pathway in tumoral immune suppression. Well-designed prospective studies with more patients are necessary in the future to clarify the associations between the expression levels of PD-L1 and the prognostic impact thereof.
In conclusion, the prognostic value of PD-L1 expression in OCCC patients might have the potential to enable more effective clinical approaches, including prognostic surveillance of patients and possible use of anti-PD-L1 Ab immunotherapy.
ACKNOWLEDGMENTS
We would like to show our sincere gratitude to all the faculty members in our department and all the patients and their families involved in the current study.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Z.J., W.H., W.X.
- Data curation: Z.J.
- Formal analysis: Z.J.
- Funding acquisition: W.X.
- Investigation: B.R., W.Y.
- Methodology: Z.J., W.H.
- Project administration: Z.J., W.H., W.X.
- Resources: Z.J., W.H.
- Software: Z.J.
- Supervision: W.X.
- Validation: Z.J., W.H., W.X.
- Visualization: Z.J.
- Writing - original draft: Z.J.
- Writing - review & editing: Z.J., W.H., B.R., W.Y., W.X.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takano M, Tsuda H, Sugiyama T. Clear cell carcinoma of the ovary: is there a role of histology-specific treatment? J Exp Clin Cancer Res. 2012;31:53. doi: 10.1186/1756-9966-31-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008;109:370–376. doi: 10.1016/j.ygyno.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Ye S, Yang J, You Y, Cao D, Huang H, Wu M, et al. Comparison of clinical characteristic and prognosis between ovarian clear cell carcinoma and serous carcinoma: a 10-year cohort study of chinese patients. PLoS One. 2015;10:e0133498. doi: 10.1371/journal.pone.0133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnack TH, Høgdall E, Nedergaard L, Høgdall C. Demographic clinical and prognostic factors of primary ovarian adenocarcinomas of serous and clear cell histology-a comparative study. Int J Gynecol Cancer. 2016;26:82–90. doi: 10.1097/IGC.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 7.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 9.Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015;27:39–46. doi: 10.1093/intimm/dxu095. [DOI] [PubMed] [Google Scholar]
- 10.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 13.Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49:2233–2242. doi: 10.1016/j.ejca.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1: PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98:751–755. doi: 10.1177/030089161209800612. [DOI] [PubMed] [Google Scholar]
- 16.Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M, Meng YL, et al. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One. 2013;8:e76012. doi: 10.1371/journal.pone.0076012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muenst S, Schaerli AR, Gao F, Däster S, Trella E, Droeser RA, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7:1486–1499. doi: 10.18632/oncotarget.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb JR, Milne K, Nelson BH. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol Res. 2015;3:926–935. doi: 10.1158/2326-6066.CIR-14-0239. [DOI] [PubMed] [Google Scholar]
- 21.Böcker W. WHO classification of breast tumors and tumors of the female genital organs: pathology and genetics. Verh Dtsch Ges Pathol. 2002;86:116–119. [PubMed] [Google Scholar]
- 22.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 23.Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013;19:1363–1374. doi: 10.1158/1078-0432.CCR-12-2199. [DOI] [PubMed] [Google Scholar]
- 24.Maine CJ, Aziz NH, Chatterjee J, Hayford C, Brewig N, Whilding L, et al. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunother. 2014;63:215–224. doi: 10.1007/s00262-013-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tokito T, Azuma K, Kawahara A, Ishii H, Yamada K, Matsuo N, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016;55:7–14. doi: 10.1016/j.ejca.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Kang S, Shen J, He J, Jiang L, Wang W, et al. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) Expression in epithelial-originated cancer: a meta-analysis. Medicine (Baltimore) 2015;94:e515. doi: 10.1097/MD.0000000000000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lussier DM, Johnson JL, Hingorani P, Blattman JN. Combination immunotherapy with α-CTLA-4 and α-PD-L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J Immunother Cancer. 2015;3:21. doi: 10.1186/s40425-015-0067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tjin EP, Krebbers G, Meijlink KJ, van de Kasteele W, Rosenberg EH, Sanders J, et al. Immune-escape markers in relation to clinical outcome of advanced melanoma patients following immunotherapy. Cancer Immunol Res. 2014;2:538–546. doi: 10.1158/2326-6066.CIR-13-0097. [DOI] [PubMed] [Google Scholar]
- 29.Kluger HM, Zito CR, Barr ML, Baine MK, Chiang VL, Sznol M, et al. Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin Cancer Res. 2015;21:3052–3060. doi: 10.1158/1078-0432.CCR-14-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckers RK, Selinger CI, Vilain R, Madore J, Wilmott JS, Harvey K, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2016;69:25–34. doi: 10.1111/his.12904. [DOI] [PubMed] [Google Scholar]
- 31.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900–6912. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33:4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 34.Homicsko K, Coukos G. Targeting programmed cell death 1 in ovarian cancer. J Clin Oncol. 2015;33:3987–3989. doi: 10.1200/JCO.2015.63.7785. [DOI] [PubMed] [Google Scholar]