Abstract
Objective
To describe the nutritional status of women with peritoneal metastasis (PM) from recurrent ovarian, fallopian, or peritoneal cancer and to assess longitudinal variations of the cachexia-anorexia syndrome (CAS) during palliative pressurized intraperitoneal aerosol chemotherapy (PIPAC).
Methods
Nutritional assessment included body mass index (BMI), bioelectrical impedance analysis (BIA), and blood chemistry. CAS presence/absence was recorded before and during repeated cycles (1–11) of PIPAC.
Results
Eighty-four patients with peritoneal cancer (n=5) or PM from recurrent ovarian (n=77) or fallopian tube (n=2) cancer were included. At baseline, resting metabolism (RM) (1,432±172 kcal/day), visceral fat level (7.5±3.2), skeletal muscle mass (27.2%±4.6%), upper arm circumference (27.9±4.6 cm), lower leg circumference (35.1±3.9 cm), serum parameters (albumin [3.5±0.7 g/dL], total protein [6.3±0.9 g/dL], and transferrin [202±60 mg/dL]) were below normal limits. C-reactive protein (CRP) (4.3±6.8 mg/dL), caliper body fat (35.7%±6.3%), and total body fat mass (35.6%±8.5%) were above normal limits. Nineteen/84 (23%) patients had CAS at baseline. Deterioration or stabilization/improvement of CAS was observed in 9/55 (16.4%) and 46/55 (83.6%) patients with follow-up data, respectively. Baseline body fat mass, visceral fat level, skeletal muscle mass, caliper body fat, BMI, ascites, Karnofsky index, RM, and CRP, as well as tumor response were not predictive of CAS deterioration.
Conclusion
Nutritional decline and onset or deterioration of CAS are difficult to predict. Careful measuring and monitoring of nutritional parameters and CAS in all patients seems to be necessary in order to identify those patients in need of enteral/parenteral nutrition support.
Keywords: Intraperitoneal, Ovarian Neoplasms, Nutrition, Aerosol, Palliative
INTRODUCTION
Peritoneal metastasis (PM) is associated with primary advanced and recurrent malignancies originating from the gastrointestinal and genital tracts [1]. The presence of PM confers a poor prognosis irrespective of the tumor of origin and significantly reduces the quality of life of affected patients [1,2]. This is especially true for patients with ovarian cancer, because this malignancy is associated with PM in >75% of cases at the time of initial diagnosis and in >85% of cases at the time of disease recurrence [3,4]. PM leads to a wide variety of clinical symptoms all of which negatively affect quality of life. Typically, patients with PM complain of abdominal pain, bloating, bowel obstruction, nausea, emesis, weight loss, and loss of muscle mass, strength, and mobility [5]. In addition, ascites which develops subsequent to PM in >70% of cases further aggravates these symptoms in a dose-dependent manner [1,5].
The nutritional status of patients with PM usually declines progressively towards the end of life resulting in a poor overall survival with a median duration of 10 months [5]. Given the compromised gastrointestinal situation, maintaining the necessary caloric intake is difficult for these patients. Usually, patients spontaneously reduce their caloric intake due to appetite loss and in order to avoid nausea and emesis triggered by oral feeding [1,5]. This behavior, together with the fact that the energy expenditure of the underlying tumor causes an increased resting metabolism (RM), pushes patients into a downward spiral of lower caloric intake in the presence of an increased caloric demand [6].
Low caloric intake, increased malignant energy metabolism, and loss of muscle and fat mass cause the cachexia-anorexia syndrome (CAS), defined as a combination of loss of appetite, weight loss, and skeletal muscle atrophy with or without loss of body fat mass [6,7]. CAS has been described in up to 80% of patients with PM from gastrointestinal tumors and is predictive of the time to progression and overall survival in this patient population [8]. However, little is known about the nutritional status of women with PM from gynecologic malignancies such as ovarian cancer. Therefore, in the present study, we assessed the nutritional status of women with PM from recurrent ovarian, fallopian, and peritoneal cancer and measured the longitudinal variations of nutritional parameters during palliative intraperitoneal chemotherapy.
MATERIALS AND METHODS
1. Study design and patients
Women with peritoneal cancer or PM from recurrent gynecologic malignancies such as ovarian cancer or fallopian tube cancer were included in the study. All patients had clinical and/or radiological evidence of PM and a previous diagnosis of ovarian cancer, fallopian tube cancer, or peritoneal cancer. Patients with extraperitoneal disease were not included in this study with the exception of isolated pleural carcinomatosis/effusion. In our institution, nutritional status is routinely recorded in all patients with PM in order to screen for malnutrition and to offer nutritional support and/or drug therapy to these patients. Clinical data were entered into a prospective registry (Ruhr-Universität Bochum, Medical Faculty Ethics Committee, approval number 15-5280). Prevalent nutritional data were obtained from all patients at baseline, i.e., at the time of their first presentation to the clinic. Follow-up nutritional data were obtained from those who underwent repeated palliative chemotherapy cycles during every cycle. Actual caloric food intake of patients was not measured. Food intake was ad libitum. Data were analyzed retrospectively by chart review.
2. Nutritional assessment
On the day of admission, the following data were collected: 1) medical history (weight and diet changes) and gastrointestinal symptoms; 2) physical examination including body weight and estimation of body composition with RM, body fat percentage, skeletal muscle percentage, and visceral fat level by bioelectrical impedance analysis (BIA) using the Karada Scan BF 511 Body Composition Monitor according to the instructions of the manufacturer (Omron Medizintechnik, Mannheim, Germany) [9]; 3) skinfold measurement to determining the amount of subcutaneous fat using the Slim Skinfold Caliper Measure Body Fat Tester with a range from 0 to 80 mm (eaglefit GmbH, Langenau, Germany); 4) caliper body fat percentage was calculated according to the instructions of the manufacturer; 5) routine blood chemistry including C-reactive protein (CRP), total protein, albumin, and transferrin with normal ranges (CRP, <0.5 mg/dL; total protein, 6.6–8.7 g/dL; serum albumin, 3.5–5.2 g/dL; and transferrin, 200–360 mg/dL). All measurements were non-invasive except for blood samples obtained by peripheral venous puncture. Nutritional status measurements were performed by 2 study nurses.
3. Chemotherapy
Patients included in this study underwent a palliative intraperitoneal chemotherapy (pressurized intraperitoneal aerosol chemotherapy [PIPAC]) with cisplatin and doxorubicin as described previously [10]. Prior to intraperitoneal chemotherapy, all patients had undergone at least 2 previous lines of standard systemic chemotherapy or were unwilling or unable (as by treating physician's assessment) to undergo a further line of systemic chemotherapy. A minimum delay of 4 weeks between the last application of systemic chemotherapy and the start of intraperitoneal chemotherapy was observed. Patients were advised not to undergo systemic chemotherapy during PIPAC. Intraperitoneal chemotherapy was performed as follows. After insufflation of a 12-mmHg CO2 pneumoperitoneum, 2 balloon safety trocars (5 and 12 mm; Applied Medical, Düsseldorf, Germany) were inserted into the abdominal wall. The peritoneal carcinomatosis index (PCI) was determined according to Jacquet and Sugarbaker [11]. Ascites volume was documented and ascites was removed. Then, a nebulizer (MIP; Capnomed, Villigendorf, Germany) was connected to an intravenous high-pressure injector (Mark7 Arterion; Medrad, Leverkusen, Germany) and inserted into the abdomen. A pressurized aerosol containing cisplatin at a dose of 7.5 mg/m2 body surface in a 150 mL NaCl 0.9% solution followed by doxorubicin at a dose of 1.5 mg/m2 body surface in a 50 mL NaCl 0.9% solution was applied. The pressurized chemotherapy aerosol was maintained for 30 minutes at a temperature of 37°C and scavenged via a closed line into the air waste system of the hospital. Finally, trocars were retracted and laparoscopy ended. PIPAC was repeated every 4–6 weeks until disease progression or limiting toxicity.
4. Statistical analysis
All p-values are 2-tailed and p-values <0.05 are considered statistically significant. Values are given as means (range) or medians (±standard deviation [SD]) where appropriate. We performed a multivariable logistic regression analysis with CAS deterioration (yes vs. no) as the dependent variable and baseline CAS (yes vs. no), baseline body mass index (BMI; ≤24 vs. >24), serum CA125 (≤1,000 vs. >1,000 U/mL), Karnofsky index (<80% vs. ≥80%), presence of ascites (yes vs. no), parenteral nutrition (yes vs. no), and tumor response (no/weak regression vs. moderate/strong/complete regression) as the independent variables. We used SPSS 22 for Windows (SPSS Inc., Chicago, IL, USA) and SigmaPlot 12.5 (Systat Software Inc., San José, CA, USA) for statistical analyses.
RESULTS
1. Patients
Eighty-four patients with peritoneal carcinomatosis from ovarian cancer (n=77), fallopian tube cancer (n=2), and peritoneal cancer (n=5) were treated between April 2014 and May 2016 and were included in this study. The mean age of the study cohort was 60.2±12.3 years. Patients had undergone a median number of 2 (range, 1–11) prior lines of systemic chemotherapy before being treated within the context of this study. Six of 84 patients were treated during the observation period with systemic chemotherapy at an outside institution. Patient characteristics of the study population are shown in Table 1. During the study period, all women underwent at least one cycle of PIPAC with cisplatin and doxorubicin. Specifically, 84, 55, 30, 17, 6, and 17 women underwent 1, 2, 3, 4, 5, and >5 cycles of chemotherapy. Thus, 84 patients were eligible for the assessment of baseline nutritional parameters and 55 patients were eligible for longitudinal analyses and for multivariable regression analysis regarding predictive factors of CAS deterioration. Fifty-two of 84 patients did not receive a complete set of at least 3 PIPACs as intended. The reasons were as follows: lost to follow-up (20/84), medical complications (18/84), and tumor progression (14/84). Two patients received additional PIPACs but these were outside the period covered by this study.
Table 1. Patient characteristics of 84 women with recurrent peritoneal cancer or peritoneal metastasis of ovarian or fallopian tube cancer.
| Patient characteristics | Values | |
|---|---|---|
| Age (yr) | 60.4±12.2 | |
| Site of tumor origin | ||
| Ovary | 77 (92) | |
| Fallopian tube | 2 (2) | |
| Peritoneum | 5 (6) | |
| Karnofsky index | 80 (50–100) | |
| Ascites (mL) | 922±1,628 | |
| PCI | 18.9±11.2 | |
| Serum CA125 (U/mL) | 1,363±2,848 | |
| Concomitant systemic therapy | 6 (7) | |
Data are shown as mean±SD, number (%), or median (range).
CA125, cancer antigen 125; PCI, peritoneal carcinomatosis index; SD, standard deviation.
2. Nutritional parameters
At the time of baseline evaluation, the study population was characterized by a nutritional deficit at multiple levels, a chronic inflammatory status, and a decreased energy expenditure. Nutritional data and serum levels of albumin, total protein, transferrin, and iron at baseline are shown in Table 2. Specifically, RM (1,432±172 kcal/day), visceral fat level (7.5±3.2), skeletal muscle mass (27.2%±4.6%), upper arm circumference (27.9±4.6 cm), lower leg circumference (35.1±3.9 cm), and serum parameters (albumin [3.5±0.7 g/dL; ref, 3.5–5.2], total protein [6.3±0.9 g/dL; ref, 6.6–8.6], and transferrin [202±60 mg/dL; ref, 200–360]) were below normal limits, whereas CRP (4.3±6.8 mg/dL; ref, <0.5), caliper body fat (35.7%±6.3%), and total body fat mass (35.6%±8.5%) were increased above normal limits [12,13,14,15]. Nineteen of 84 (23%) patients had CAS at baseline.
Table 2. Nutritional and serum parameters in 84 patients with peritoneal cancer or peritoneal metastasis of ovarian or fallopian tube cancer at baseline.
| Parameters | Value at baseline | Normal range | |
|---|---|---|---|
| BMI (kg/m2) | 24.3 (21.8–28.1) | 18.5–25.0 | |
| BIA | |||
| RM (kcal/day) | 1,399 (1,321–1,491) | 1,421* | |
| Body fat mass (%) | 36.6 (30.9–41.7) | 25–31† | |
| Skeletal muscle mass (%) | 26.4 (24.3–28.9) | 48±10‡ | |
| Visceral fat level | 7 (5–10) | ≤9 | |
| Caliper body fat (%) | 36.0 (32.6–40.3) | 16–25 | |
| Arm circumference (cm) | 27.0 (25.1–30.0) | 28.5 | |
| Leg circumference (cm) | 35.0 (33.0–37.2) | 36.9§ | |
| Serum parameters | |||
| CRP (mg/dL) | 2.1 (0.48–5.3) | <0.5 | |
| Albumin (g/dL) | 3.7 (3.2–4.1) | 3.5–5.2 | |
| Total protein (g/dL) | 6.5 (5.7–6.8) | 6.6–8.7 | |
| Transferrin (mg/dL) | 203 (151–244) | 200–360 | |
| Iron (µg/dL) | 44 (30–69) | 37–145 | |
| Hemoglobin (g/dL) | 11.3 (10.1–12.2) | 12.0–15.6 | |
Baseline values are medians (interquartile range).
BIA, bioelectrical impedance analysis; BMI, body mass index; CRP, C-reactive protein; RM, resting metabolism.
*According to Hoffmans et al. [12]. †According to Siddiqui et al. [13]. ‡According to Baxmann et al. [14] for healthy, sedentary, adult females. §According to Labs et al. [15].
3. Longitudinal analysis
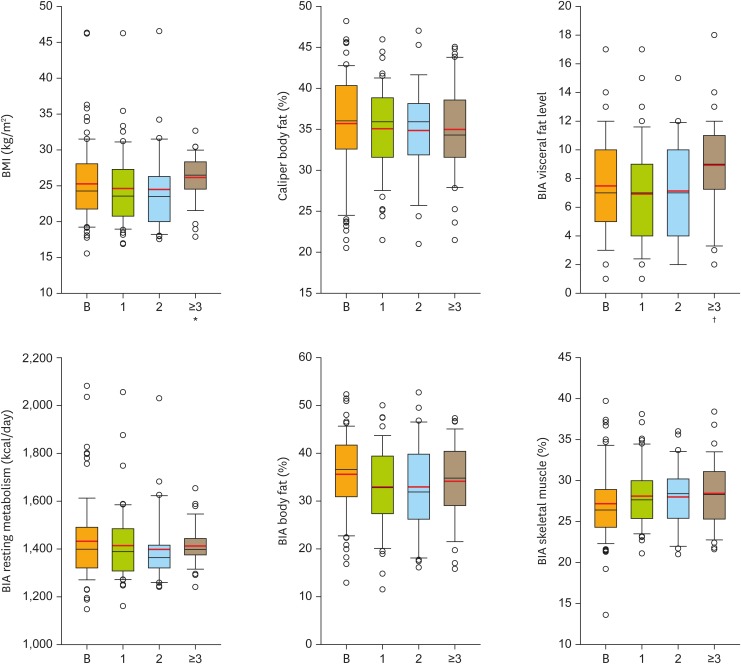
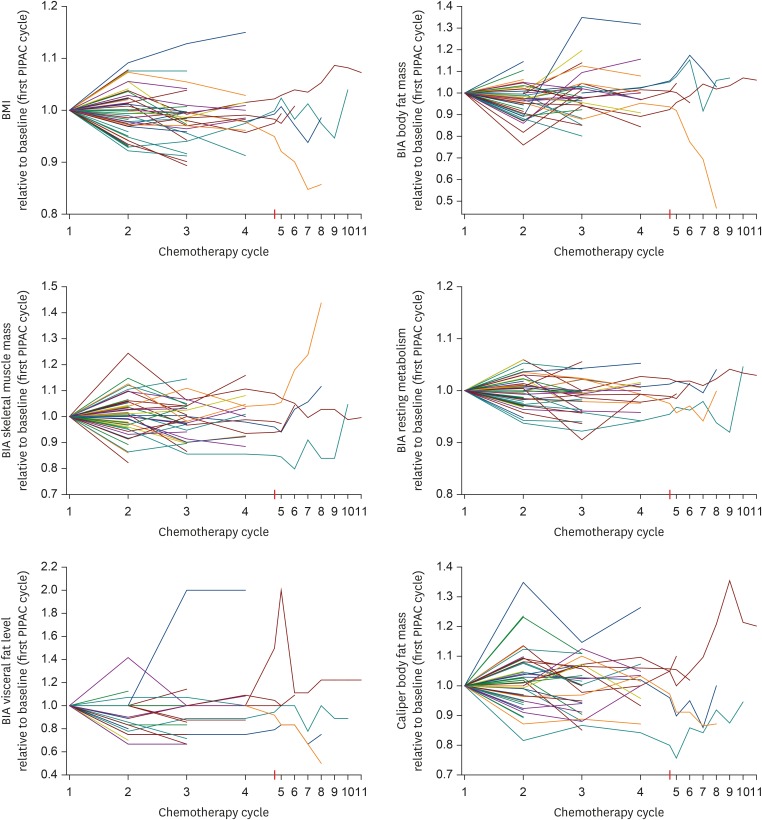
During a median time of observation of 2.4 (range, 0.3–21.7) months, deterioration of CAS as compared to stabilization/improvement of CAS was observed in 9/55 (16.4%) and 46/55 (83.6%) of patients, respectively. Parenteral nutrition support was necessary for 5/84 patients during 8 of their 20 hospital stays. Thus, the overall need for parenteral nutrition support was low (3.9%; 8/206 total stays of the 84 patients). Table 3 compares the levels of CRP, caliper body fat, arm circumference, leg circumference, and serum parameters (albumin, total protein, transferrin, and iron) during chemotherapy cycles 1 to 3, demonstrating no significant changes in the overall study population. Box plots of BMI, caliper body fat, and BIA values (i.e., RM, body fat mass, visceral fat mass, and skeletal muscle mass) during chemotherapy cycles 1 to 3 are shown in Fig. 1. Fig. 2 contains line diagrams of individual patients depicting longitudinal changes of BMI, BIA parameters body fat mass, skeletal muscle mass, RM, and visceral fat level, as well as body fat percentage determined by the caliper method over up to 11 chemotherapy cycles. This figure demonstrates an increase of RM with a concomitant decrease of the nutritional status in a minority of patients and a stabilization of these parameters in the majority of patients during palliative chemotherapy.
Table 3. Nutritional and serum parameters of patients with peritoneal cancer or peritoneal metastasis from recurrent ovarian or fallopian tube cancer during consecutive cycles of palliative intraperitoneal chemotherapy.
| Parameters | After 1 CHXT cycle | After 2 CHXT cycles | After ≥3 CHXT cycles | p-value* | |
|---|---|---|---|---|---|
| No. of patients (procedures) | 53 (53) | 30 (30) | 15 (40) | - | |
| BMI (kg/m2) | 23.6 (20.8–27.3) | 23.5 (20.0–26.3) | 26.5 (24.5–28.3) | 0.010 | |
| BIA | |||||
| RM (kcal/day) | 1,389 (1,308–1,485) | 1,364 (1,321–1,416) | 1,398 (1,375–1,444) | 0.300 | |
| Body fat mass (%) | 32.8 (27.4–39.4) | 31.9 (26.2–39.8) | 34.8 (29.1–40.4) | 0.700 | |
| Skeletal muscle mass (%) | 27.7 (25.4–30.0) | 28.4 (25.4–30.2) | 28.3 (25.3–31.1) | 0.900 | |
| Visceral fat level | 7 (4–9) | 7 (4–10) | 9 (7.25–11) | 0.005 | |
| Caliper body fat (%) | 35.9 (31.6–38.8) | 35.9 (31.9–38.1) | 34.3 (31.6–38.6) | 0.900 | |
| Arm circumference (cm) | 27.2 (24.0–29.0) | 27.3 (24.9–29.0) | 28.0 (26.0–29.3) | 0.300 | |
| Leg circumference (cm) | 34.5 (32.5–36.3) | 33.1 (31.9–36.9) | 33.8 (32.3–35.6) | 0.700 | |
| Serum parameters | |||||
| CRP (mg/dL) | 0.8 (0.3–4.2) | 0.9 (0.2–3.3) | 1.2 (0.6–4.3) | 0.300 | |
| Albumin (g/dL) | 3.7 (2.9–4.0) | 3.7 (3.3–3.9) | 3.6 (3.2–4.0) | 0.900 | |
| Total protein (g/dL) | 6.6 (5.8–6.9) | 6.2 (5.7–6.9) | 6.7 (5.9–7.1) | 0.400 | |
| Transferrin (mg/dL) | 195 (146–270) | 206 (157–235) | 184 (161–261) | 0.900 | |
| Iron (µg/dL) | 51 (36–69) | 62 (40–79) | 56 (29-74) | 0.400 | |
| Hemoglobin (g/dL) | 11.1 (10.1–12.6) | 11.1 (10.1–12.8) | 11.6 (10.4–12.8) | 0.600 | |
Values are number (%) or median (interquartile range).
ANOVA, analysis of variance; BIA, bioelectrical impedance analysis; BMI, body mass index; CHXT, chemotherapy; CRP, C-reactive protein; RM, resting metabolism.
*p-values were calculated using Kruskal-Wallis one-way ANOVA on ranks.
Fig. 1.
Box plots of BMI, BIA values, i.e., RM, body fat mass, visceral fat level, and skeletal muscle mass, as well as body fat mass measured by the caliper method at baseline (B) and after 1, 2, ≥3 palliative intraperitoneal chemotherapy cycles. Statistical significance of comparisons vs. baseline (Mann-Whitney U or t-test) are indicated.
BIA, bioelectrical impedance analysis; BMI, body mass index; RM, resting metabolism.
*p≤0.050; †p≤0.010.
Fig. 2.
Line diagrams of all individual patients depicting longitudinal changes of RM, body fat mass, visceral fat level, skeletal muscle mass, and caliper body fat relative to the baseline values.
BIA, bioelectrical impedance analysis; BMI, body mass index; PIPAC, pressurized intraperitoneal aerosol chemotherapy; RM, resting metabolism.
4. Predictive factors of CAS deterioration
In a univariate analysis, baseline values of body fat mass, visceral fat level, skeletal muscle mass, caliper body fat, presence of CAS, weight, BMI, ascites, Karnofsky index, RM, CRP, parenteral nutrition support, and tumor response (all: p=not significant) were not associated with CAS deterioration. In a multivariate analysis, none of the investigated parameters was a predictor of CAS deterioration.
DISCUSSION
In this retrospective cohort study, we found that patients with peritoneal cancer or PM from recurrent ovarian and fallopian cancer have a severe nutritional deficit regarding key items such as total body fat, visceral fat, skeletal muscle mass, serum albumin, and total protein. In addition, patients show a chronic state of inflammation. In our population, CAS was present in 23% of cases. Nutritional parameters including RM, skeletal muscle mass, visceral fat, and total body fat were stabilized during palliative PIPAC in most patients, but CAS deterioration was observed in 16% of patients. Of note, none of the investigated parameters was a reliable predictor of CAS deterioration. Based on these data, measuring and monitoring of the nutritional status during palliative intraperitoneal chemotherapy is recommended in order to identify patients with CAS deterioration, who might benefit from nutritional support.
Our data confirm previous studies showing that nutritional deficit occurs in patients with PM from ovarian cancer, especially at the end of life [6,7,8]. Of note, the frequency of CAS in our study population of women with ovarian cancer was lower than previously reported in other patients with PM, for example those with gastrointestinal tumors. This suggests that PM from ovarian cancer is more indolent and/or more chemosensitive than PM from gastric, colon, or pancreatic cancer. This is also in accordance with the fact that survival times in women with ovarian cancer metastasized to the peritoneum are significantly longer than in patients with gastrointestinal tumors spread to the peritoneum [16].
The nutritional status of patients with advanced PM typically deteriorates with increasing numbers of palliative chemotherapy lines while the chronic inflammatory state progressively aggravates. For example, Nordhausen et al. [17] showed that serum total protein and albumin progressively decreased during the last months of life, whereas CRP and RM increased. In the present study, we found that this deterioration could be stabilized by palliative intraperitoneal chemotherapy. Specifically, CAS deterioration occurred in only 16% of patients in our series and was stabilized in the majority of cases. Furthermore, RM and CRP were stabilized in the majority of patients.
The stabilization of several nutritional parameters under palliative chemotherapy appears encouraging considering the limited life expectancy of the patients treated in this study. This effect, however, was documented only in women able to receive repeated PIPAC cycles. Thus, a selection bias cannot be excluded. However, such a bias is not specific to this cohort of patients, but is a common methodological problem in studies assessing late stage cancer patients, since many patients will not be able to receive the planned therapy over a long period. In addition to the potentially stabilizing effect of palliative intraperitoneal chemotherapy regarding nutritional parameters, our data confirm that palliative intraperitoneal chemotherapy using PIPAC with cisplatin and doxorubicin does not induce significant gastrointestinal symptoms or complications. This finding is consistent with the results of a phase II trial assessing the safety and efficacy of palliative PIPAC with doxorubicin and cisplatin in ovarian cancer patients [10].
There are a number of limitations to this study. First, the sample size was small and therefore subtle effects may not have become evident in this study. In addition, some patients experienced an increase of BMI in the early cycle and then a decrease of BMI in later cycles, whereas other patients experienced a decrease of their BMI in the early cycles and then an increase of their BMI in later cycles. This suggest marked individual variations. This might impact on the internal validity of our study. Lastly, the results of our study might not have external validity for other forms of intraperitoneal chemotherapy. In the present study, we found that nutritional decline and onset or deterioration of CAS are difficult to predict. None of the investigated parameters reliably predicted CAS deterioration. Specifically, body fat mass, visceral fat level, skeletal muscle mass, caliper body fat, weight, BMI, ascites, Karnofsky index, RM, CRP, the need for parenteral nutrition support, and tumor response were not associated with CAS deterioration in univariate and multivariate analyses. Therefore, careful measuring and monitoring of nutritional parameters and CAS in all patients seems to be necessary in order to identify those patients in need of enteral or parenteral nutritional support. These might provide valuable insights for optimizing patient care during palliative chemotherapy.
In conclusion, patients with PC from recurrent ovarian, fallopian, or peritoneal cancer have a severe nutritional deficit. CAS is frequent in these patients but can be stabilized during intraperitoneal palliative chemotherapy.
ACKNOWLEDGMENTS
We thank the study nurses Jutta Koke and Kerstin Voitz for performing the body composition assessments described in this study.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: H.Z., R.G.A., K.R., D.A., T.C.B.
- Data curation: R.G.A.
- Formal analysis: H.Z., R.G.A.
- Investigation: H.Z., K.R., D.A., T.C.B.
- Methodology: H.Z., T.C.B.
- Project administration: T.C.B.
- Resources: T.C.B.
- Supervision: T.C.B.
- Validation: D.A., T.C.B.
- Visualization: R.G.A.
- Writing - original draft: H.Z., T.C.B.
- Writing - review & editing: H.Z., R.G.A., K.R., D.A., T.C.B.
References
- 1.Lemoine L, Sugarbaker P, Van der Speeten K. Pathophysiology of colorectal peritoneal carcinomatosis: role of the peritoneum. World J Gastroenterol. 2016;22:7692–7707. doi: 10.3748/wjg.v22.i34.7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chia CS, Tan GH, Lim C, Soo KC, Teo MC. Prospective quality of life study for colorectal cancer patients with peritoneal carcinomatosis undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23:2905–2913. doi: 10.1245/s10434-016-5203-6. [DOI] [PubMed] [Google Scholar]
- 3.Zeng LJ, Xiang CL, Gong YZ, Kuang Y, Lu FF, Yi SY, et al. Neoadjuvant chemotherapy for patients with advanced epithelial ovarian cancer: a meta-analysis. Sci Rep. 2016;6:35914. doi: 10.1038/srep35914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehouli J, Stengel D, Harter P, Kurzeder C, Belau A, Bogenrieder T, et al. Topotecan weekly versus conventional 5-day schedule in patients with platinum-resistant ovarian cancer: a randomized multicenter phase II trial of the North-Eastern German Society of Gynecological Oncology Ovarian Cancer Study Group. J Clin Oncol. 2011;29:242–248. doi: 10.1200/JCO.2009.27.8911. [DOI] [PubMed] [Google Scholar]
- 5.Yonemura Y, Canbay E, Endou Y, Ishibashi H, Mizumoto A, Miura M, et al. Peritoneal cancer treatment. Expert Opin Pharmacother. 2014;15:623–636. doi: 10.1517/14656566.2014.879571. [DOI] [PubMed] [Google Scholar]
- 6.Ezeoke CC, Morley JE. Pathophysiology of anorexia in the cancer cachexia syndrome. J Cachexia Sarcopenia Muscle. 2015;6:287–302. doi: 10.1002/jcsm.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarricone R, Ricca G, Nyanzi-Wakholi B, Medina-Lara A. Impact of cancer anorexia-cachexia syndrome on health-related quality of life and resource utilisation: a systematic review. Crit Rev Oncol Hematol. 2016;99:49–62. doi: 10.1016/j.critrevonc.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Ockenga J, Valentini L. Review article: anorexia and cachexia in gastrointestinal cancer. Aliment Pharmacol Ther. 2005;22:583–594. doi: 10.1111/j.1365-2036.2005.02628.x. [DOI] [PubMed] [Google Scholar]
- 9.Bosy-Westphal A, Later W, Hitze B, Sato T, Kossel E, Gluer CC, et al. Accuracy of bioelectrical impedance consumer devices for measurement of body composition in comparison to whole body magnetic resonance imaging and dual X-ray absorptiometry. Obes Facts. 2008;1:319–324. doi: 10.1159/000176061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tempfer CB, Winnekendonk G, Solass W, Horvat R, Giger-Pabst U, Zieren J, et al. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: a phase 2 study. Gynecol Oncol. 2015;137:223–228. doi: 10.1016/j.ygyno.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmans M, Pfeifer WA, Gundlach BL, Nijkrake HG, Oude Ophuis AJ, Hautvast JG. Resting metabolic rate in obese and normal weight women. Int J Obes. 1979;3:111–118. [PubMed] [Google Scholar]
- 13.Siddiqui NI, Khan SA, Shoeb M, Bose S. Anthropometric predictors of bio-impedance analysis (BIA) phase angle in healthy adults. J Clin Diagn Res. 2016;10:CC01–CC04. doi: 10.7860/JCDR/2016/17229.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3:348–354. doi: 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labs KH, Tschoepl M, Gamba G, Aschwanden M, Jaeger KA. The reliability of leg circumference assessment: a comparison of spring tape measurements and optoelectronic volumetry. Vasc Med. 2000;5:69–74. doi: 10.1177/1358836X0000500202. [DOI] [PubMed] [Google Scholar]
- 16.Hanker LC, Loibl S, Burchardi N, Pfisterer J, Meier W, Pujade-Lauraine E, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23:2605–2612. doi: 10.1093/annonc/mds203. [DOI] [PubMed] [Google Scholar]
- 17.Nordhausen K, Solass W, Demtroeder C, Tempfer CB, Reymond M. Cachexia-anorexia syndrome in patients with peritoneal metastasis: an observational study. Pleura Peritoneum. 2016;1:57–63. doi: 10.1515/pp-2016-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]