Abstract
Objective
The use of robotic radical hysterectomy has greatly increased in the treatment of early stage cervical cancer. We sought to compare surgical and oncologic outcomes of women undergoing robotic radical hysterectomy compared to open radical hysterectomy.
Methods
The clinic-pathologic, treatment, and recurrence data were abstracted through an Institutional Review Board-approved protocol at 2 separate large tertiary care centers in Seattle, Swedish Medical Center and the University of Washington. Data were collected from 2001–2012. Comparisons between the robotic and open cohorts were made for complications, recurrence, progression-free survival (PFS), and overall survival (OS).
Results
In the study period, 109 robotic radical hysterectomies were performed. These were compared to 202 open radical hysterectomies. The groups were comparable in terms of age and body mass index (BMI). Length of stay (LOS) was considerably shorter in the robotic group (42.7 vs. 112.6 hours, p<0.001) as was estimated blood loss (EBL; 105.9 vs. 482.6 mL, p<0.001). There were more complications in the open radical hysterectomy group, 23.4% vs. 9.2% in the robotic group (p=0.002). The recurrence rate was comparable between the groups (10.1% vs. 10.4%, p=0.730). In multivariate adjusted analysis, robotic surgery was not a statistically significant predictor of PFS (p=0.230) or OS (0.85).
Conclusion
Our study, one of the largest multi-institution cohorts of patients undergoing robotic radical hysterectomy, suggest robotic radical hysterectomy leads to comparable oncologic outcomes in the treatment of early stage cervical cancer with improved short-term surgical outcomes such as decreased LOS and EBL.
Keywords: Early Stage Cervical Cancer, Robotic Surgery, Chemotherapy, Radiation
INTRODUCTION
There will be an estimated 12,360 new cases of cervical cancer in the United States this year, with 4,020 deaths [1]. Cervical cancer is the 3rd most common gynecologic malignancy in the US and other developed countries. Historically, the management of early stage cervical cancer involved either radical surgery for a subset of patients or primary radiotherapy. However, recent analysis suggests there may be a survival benefit to radical surgery [2].
Traditionally, radical hysterectomy with bilateral pelvic lymph node dissection was performed with an open abdominal approach. In the early 1990s, laparoscopic techniques were pioneered but due to technical complaints and a steep learning curve, there was very limited penetration of minimally invasive surgery for the treatment of early stage cervical [3]. The advent of robotic surgery for Gynecologic Oncology procedures in 2005 (Intuitive Surgical, Sunnyvale, CA, USA) led to tremendous growth in minimally invasive gynecologic surgery [4]. In 2011, when Paley et al. [5] reported on outcomes in the first 1,000 robotic cases at a single institution, it included a number of women undergoing operations for endometrial and cervical cancer. Robotic surgery was associated with decreased blood loss, shorter hospitalization, fewer major complications, and higher lymph node counts.
These findings have been corroborated by a number of authors and multiple institutions [6,7,8]. In addition recent reports have now shown similar outcome data with respect to survival in patients undergoing robotic surgery. Brudie et al. [9] recently published comparable survival in women with endometrial cancer undergoing robotic surgery, and these findings would be consistent with the Gynecologic Oncology Group LAP2 trial [10]. Given the lower incidence of cervical cancer, the body of evidence is more limited. There was a series published by Cantrell et al. [11], which similarly demonstrated comparable survival in women undergoing robotic vs. open radical (94% vs. 89%, p=0.270). Sert et al. [12] in a multi-institution study of Norwegian and US patients found recurrence and death rates were non-significant between robotic surgery patients as compared to laparotomy (p=not significant). These oncologic outcomes were also identified in 2 smaller studies, n=24 [13] and n=49 [14] robotic procedures. Other surgical outcomes for robotic radical hysterectomy have been evaluated in a number of publications, and a benefit profile similar to other disease sites has been observed [15,16,17,18,19,20,21,22,23]. However, the data is mostly retrospective in nature with small sample sizes.
Hence, the purpose of this study was to compare outcomes in 2 large tertiary care systems in the Seattle area undergoing treatment for early stage cervical cancer with robotic radical hysterectomy to historical open controls.
MATERIALS AND METHODS
This study was undertaken after approval by the Institutional Review Board of Swedish Medical Center (Seattle, WA, USA) and separately by the University of Washington (Seattle, WA, USA). International Classification of Diseases codes were used to create a list of patients treated surgically from 1 January 2001 to 31 December 2012. Surgical approach was at the discretion of the attending physician. The data from both institutions were pooled into a combined analysis. One hundred nine patients were identified who underwent robotic radical hysterectomy, and 202 patients who had open hysterectomy. The remainder of patients were excluded for incomplete medical records. All patients had pathology for review at their institution by a board certified gynecologic pathologist.
Clinical data collected included age at diagnosis, height, weight, body mass index (BMI), race, and comorbid conditions. Seven gynecologic oncologists at the Swedish Medical Center performed all surgical procedures. Eight gynecologic oncologists from the University of Washington performed surgical procedures included in the study population. Surgery consisted of radical hysterectomy in all patients including pelvic lymphadenectomy with bilateral salpingo-oophorectomy determined by individual patient factors such as age and histology. Surgical data collected included type of procedure, hospital length of stay (LOS), complications, and estimated blood loss (EBL). Pathologic data collected included histology, stage, tumor size, margin status, depth of invasion, and lymph node status. Oncologic outcomes including treatment modality, recurrence, progression-free survival (PFS), and overall survival (OS) were abstracted from the medical record. The data collected included time from diagnosis to last follow-up or death, status at last follow-up, time from diagnosis to recurrence, site of recurrence, salvage therapy, time from recurrence to death.
For all predictors, univariate analysis by the Kruskal-Wallis non-parametric equality of populations rank test was performed. All statistical tests were 2-sided, and a p-value less than 0.05 was considered statistically significant. Survival curves were generated using the Kaplan-Meier method, and statistical significance assessed with the log-rank test. Multivariate Cox regression was then performed adjusting for known a priori predictors, and the final parsimonious model was chosen by step-wise regression. All statistical analysis was performed with STATA (14.0 for Mac OS X; StataCorp LP, College Station, TX, USA).
RESULTS
Mean age for the study population was not statistically significantly different between groups, 45.2 years of age for the robotic radical hysterectomy cohort (range, 25–84), and 45.4 years of age for the open radical hysterectomy cohort (range, 19–88) (p=0.910). The mean BMI was also comparable between groups 27.9 in the robotic cohort (range, 17.6–51.6) vs. 29.1 in the open cohort (range, 18.3–55.7) (p=0.160). The mean EBL was 106 mL in the robotic surgery patients, much lower than the mean of 483 mL observed in the women undergoing open radical hysterectomy (p<0.001). The demographics and stage distribution are shown in Table 1. There were more bulky IB2 tumors in the open cohort 11% vs. 4% in the robotic cohort. There were less microscopic tumors in the open cohort 1% vs. 15% in the robotic cohort (p=0.030).
Table 1. Demographic and pathologic characteristics.
| Characteristics | Robotic (n=109) | Open (n=202) | p-value | |
|---|---|---|---|---|
| Age | 45.2 (25–84) | 45.4 (19–88) | 0.906 | |
| BMI | 27.9 (17.6–51.6) | 29.1 (18.3–55.7) | 0.161 | |
| Stage | 0.032 | |||
| IA1 | 5 (5) | 15 (7) | ||
| IA2 | 16 (15) | 22 (1) | ||
| IB1 | 69 (63) | 127 (63) | ||
| IB2 | 4 (4) | 23 (11) | ||
| Histology | 0.007 | |||
| Squamous | 41 (38) | 111 (55) | ||
| Adenocarcinoma | 59 (55) | 72 (36) | ||
| Adenosquamous | 6 (6) | 9 (5) | ||
| Other | 2 (2) | 10 (5) | ||
| Uterine weight (g) | 117.0 (39–588) | 128.5 (39–1,056) | 0.072 | |
| Pathologic tumor size (cm) | 1.6 (0.2–10.0) | 2.0 (0.1–16.8) | 0.179 | |
| LVSI | 30 (28.0) | 67 (33.3) | 0.341 | |
| Node count | 14 (3–45) | 21 (0–75) | <0.001 | |
| Positive nodes | 15 (13.9) | 31 (15.5) | 0.705 | |
| Parametria + | 9 (8.3) | 19 (9.5) | 0.744 | |
| Hospital LOS (hr) | 42.7 (9.0–177.7) | 112.6 (32.0–2,336.2) | <0.001 | |
Values are presented as median (range) or number (%).
BMI, body mass index; LOS, length of stay; LVSI, lymphovascular space invasion.
The pathologic characteristics are also shown in Table 1. There were more squamous lesions in the open cohort (55% vs. 41%), and there were more adenocarcinomas in the robotic cohort (55% vs. 36%, p<0.001). The uterine weight did not differ significantly between the robotic and open groups, 117 and 129 g, respectively (p=0.070). The tumor size similarly was consistent, 1.6 vs. 2 cm in the open cohort (p=0.180). Lymphovascular space invasion (LVSI) was more prevalent in the open radical hysterectomy patients (33% vs. 28%), but this difference was not statistically significant (p=0.340). There were a higher number of nodes retrieved in the open cohort, 21 vs. 14 nodes (p<0.001). Parametrial involvement was more frequent in the women undergoing open radical hysterectomy (9.5% vs. 8.3%), but this did not reach statistical significance (p=0.740). Finally, the mean hospital LOS in hours was significantly shorter for women undergoing robotic surgery vs. women undergoing laparotomy (42.7 vs. 112.6 hours, p<0.001).
The complication rate was higher in the open surgery group, 23% vs. 9% in the robotic cohort of patients, which was statistically significant (p=0.002). Surgical complications are shown in Table 2. Eight patients received blood transfusion in the open cohort (4%) as compared to 0 patients in the robotic cohort (0%, p<0.001). Surgical complications were graded using Clavien-Dindo classification system [24]. There were more grade 1 (7% vs. 4%, p=0.038), grade 2 (13% vs. 4%, p=0.005), and grade 3 (3% vs. 2%, p=0.044) in the open surgery patients as compared to the robotic cohort. There were no urogenital fistulas in either group. The conversion rate to open was only 1% and this patient was included in the robotic arm in an intention-to-treat analysis. The vaginal cuff dehiscence rate was also similarly low at 1%. A sensitivity analysis of the first half of robotic cases as compared to the second half revealed no difference in the distribution of complications, suggesting that they were not learning curve dependent.
Table 2. Surgical complications.
| Complications | Robotic (n=10) | Open (n=47) |
|---|---|---|
| Cardiovascular | 0 (0) | 1 (0.5) |
| Transfusion | 0 (0) | 8 (4) |
| Cystotomy | 2 (1.8) | 0 (0) |
| Ureteral injury | 0 (0) | 2 (1) |
| Nerve injury | 0 (0) | 1 (0.5) |
| Small bowel obstruction | 1 (0.9) | 2 (1) |
| Pulmonary embolus | 0 (0) | 2 (1) |
| Abscess | 1 (0.9) | 0 (0) |
| Cuff cellulitis | 1 (0.9) | 1 (0.5) |
| Urinary retention | 1 (0.9) | 5 (2.5) |
| Wound infection | 0 (0) | 12 (5.9) |
| Wound separation | 0 (0) | 5 (2.5) |
| Urinary tract infection | 1 (0.9) | 2 (1) |
| Lymphocele | 2 (1.8) | 2 (1) |
| Lymphedema | 1 (0.9) | 3 (1.5) |
| Fascial dehiscence | 0 (0) | 1 (0) |
Values are presented as number (%).
Time in the operating room was evaluated for the robotic surgery patients. The mean operative time for robotic surgery patients overall was 257 minutes (range, 129–352 minutes). Over the course of the study period, there was a statistically significant decrease in odds ratio (OR) time (p=0.040). The first half of cases took 269 minutes (range, 137–352 minutes). The second half of cases were completed in 243 minutes (range, 129–340 minutes) demonstrating a 26 minutes decrease in the time required to perform the procedure.
Oncologic outcomes are reported in Table 3. There were a greater proportion of women in the open radical hysterectomy group that received adjuvant treatment (45% vs. 32%, p=0.030). Distant recurrences occurred with higher frequency in the robotic surgery group (4.6% vs. 3.4%). However, there was no statistically significant difference in recurrence with a recurrence rate of 10% in both groups (p=0.730). There were 3 deaths in the robotic radical hysterectomy patients (2.8%) and 10 in the open radical hysterectomy cohort (5%, p=0.360).
Table 3. Oncologic outcomes.
| Outcomes | Robotic (n=109) | Open (n=202) | p-value | |
|---|---|---|---|---|
| Adjuvant treatment | 33 (31.7) | 88 (45.1) | 0.025 | |
| Recurrence | 11 (10.1) | 21 (10.4) | 0.731 | |
| Vaginal | 4 (0.9) | 6 (2.9) | ||
| Locoregional | 2 (1.8) | 8 (3.9) | ||
| Distant | 5 (4.6) | 7 (3.4) | ||
| Deaths | 3 (2.8) | 10 (5) | 0.358 | |
Values are presented as number (%).
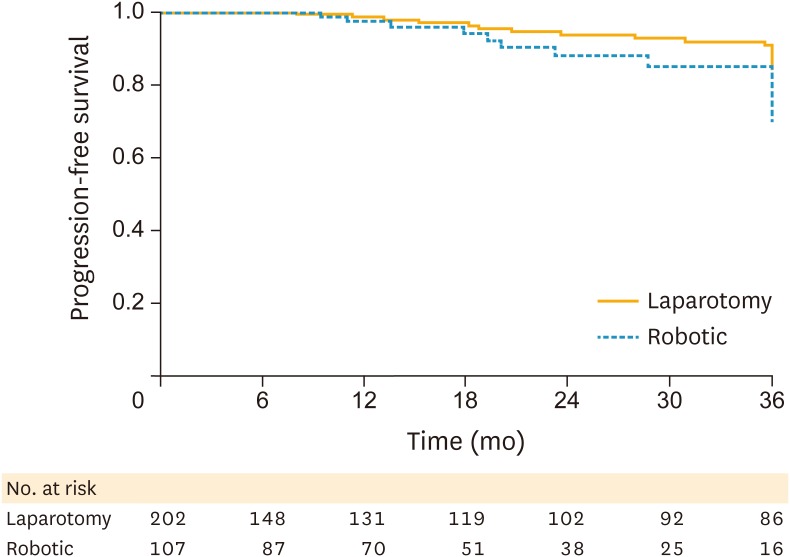
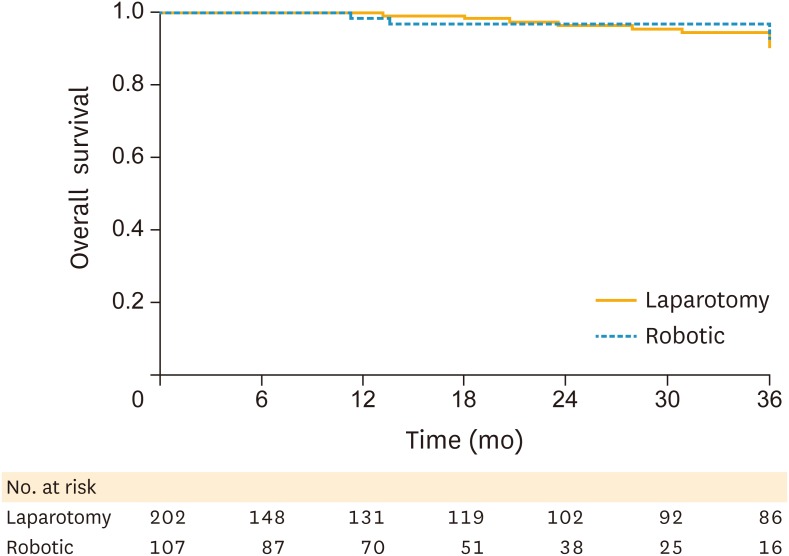
There was no statistically significant difference in PFS (Fig. 1) or OS (Fig. 2). The 3-year PFS was 89.9% in the robotic surgery patients vs. 89.1% in the laparotomy patients (log-rank p=0.140). The 3-year OS was 97.2% in the robotic surgery cohort vs. 95% in the open surgery cohort (log-rank p=0.960).
Fig. 1.
PFS by surgical approach.
PFS, progression-free survival.
Fig. 2.
OS by surgical approach.
OS, overall survival.
Cox multivariate regression was performed for PFS (Table 4) and OS (Table 5). Known a priori risk factors for survival were included (such as histology, stage, positive lymph nodes, adjuvant treatment, LVSI, and depth of invasion). Additionally, BMI and complications were included in the model. The other predictors were evaluated for confounding and no significant confounding was identified. A robotic approach to radical hysterectomy did not have a statistically significant effect on PFS, with an unadjusted hazard ratio (HR) of 1.72 (0.82–3.61; p=0.160) and adjusted HR of 1.60 (0.75–3.43; p=0.230). In this model, stage contributed significantly to PFS (HR=1.65; p=0.005).
Table 4. Cox multivariate regression: hazard of recurrence with robotic compared to open surgical approach.
| Predictors | HR (95% CI) | p-value |
|---|---|---|
| Robotic approach (unadjusted) | 1.718 (0.815–3.621) | 0.155 |
| Age | 1.021 (0.994–1.048) | 0.126 |
| BMI | 1.004 (0.957–1.056) | 0.846 |
| Complication | 0.886 (0.363–2.161) | 0.790 |
| Histology | 1.405 (0.960–2.058) | 0.080 |
| LVSI | 1.956 (0.975–3.925) | 0.059 |
| Positive lymph nodes | 1.746 (0.808–3.775) | 0.157 |
| Stage | 1.654 (1.169–2.340) | 0.005 |
| Adjuvant treatment | 1.285 (0.642–2.573) | 0.479 |
| Depth of invasion | 0.925 (0.298–2.877) | 0.893 |
| Robotic approach (adjusted ) | 1.600 (0.746–3.430) | 0.227 |
BMI, body mass index; CI, confidence interval; HR, hazard ratio; LVSI, lymphovascular space invasion.
Table 5. Cox multivariate regression: hazard of death with robotic compared to open surgical approach.
| Predictors | HR (95% CI) | p-value |
|---|---|---|
| Robotic approach (unadjusted) | 0.964 (0.260–3.576) | 0.957 |
| Age | 1.025 (0.985–1.067) | 0.219 |
| BMI | 0.973 | |
| Complication | 1.110 (0.305–4.037) | 0.874 |
| Histology | 1.201 (0.635–2.273) | 0.573 |
| LVSI | 2.597 (0.870–7.750) | 0.087 |
| Positive lymph nodes | 1.937 (0.596–6.296) | 0.271 |
| Stage | 1.760 (1.028–3.013) | 0.039 |
| Adjuvant treatment | 1.797 (0.588–5.493) | 0.304 |
| Depth of invasion | 0.881 | |
| Robotic approach (adjusted ) | 0.878 (0.232–3.322) | 0.848 |
BMI, body mass index; CI, confidence interval; HR, hazard ratio; LVSI, lymphovascular space invasion.
Finally, Cox multivariate regression demonstrates that the surgical approach did not significantly contribute to survival. Using the same parsimonious model, the unadjusted HR for a robotic approach to cervical cancer was 0.96 (0.26–3.6; p=0.960) and adjusted HR was 0.88 (0.23–3.32; p=0.850). Similar to PFS, stage was the only significant predictor of OS in women with early cervical cancer (HR=1.76; p=0.040).
DISCUSSION
Our study is the largest to date evaluating the use of robotic radical hysterectomy in women with early stage cervical cancer. This technique has quickly become the standard of care in many institutions, but there is limited oncologic outcome data. We demonstrate comparable survival in women with early stage cervical cancer who had robotic radical hysterectomy vs. women undergoing open radical hysterectomy (97.2% vs. 95%, p=0.960). Similarly, in our multivariate model the use of robotic technology did not negatively impact PFS or OS.
One interesting finding was that a greater proportion of the open cohort required adjuvant therapy to achieve comparable survival (45% vs. 32%, p=0.030). Given the greater precision and improved visualization seen with robotics, it is possible this could be due improvement in margin status with a robotic approach. Pathologic factors did not differ significantly except for a non-significant increase in parametrial involvement in the open cohort. Other possible factors include patient selection and provider preference, which could also have contributed to the greater use of adjuvant therapy in the open cohort.
Interestingly there were more lymph nodes removed in the open radical hysterectomy patients (21 vs. 14, p<0.001). It is unclear if the lower nodal count was attributable to assimilation of a new technology and learning curve, but in the urologic literature lower nodal counts have been reported with robotic techniques, ranging from 90% of open or fewer [25]. The decrease in lymph node yield does not appear to impact oncologic outcome. And as seen in our study, the lower number of lymph nodes removed did not correlate with worse survival in our patient population. An interesting area of research is the push to incorporate sentinel lymph node detection in early stage cervical cancer. Recent evidence from MD Anderson suggests sensitivity of 96.4% and negative predictive value of 99.3% using lymphatic mapping [26]. This has led to incorporation of this technique into the National Comprehensive Cancer Network (NCCN) guidelines as of the February 2015 update [27]. At the time of this publication this technique is increasingly being utilized by our institution as well as others.
Clinical and surgical outcomes following robotic radical hysterectomy have been well documented in a number of studies. However, these studies suffer from small numbers of patients and being retrospective in nature. Our study is one of the larger to date addressing both surgical and oncologic outcomes in women with early cervical cancer, with data collected at 2 high-volume institutions with established gynecologic oncology programs. Nevertheless, the data is retrospective in nature and causal assumptions cannot be inferred. However, since it is unlikely that this question will be studied in a prospective, randomized fashion, these retrospective studies represent the best data available to inform management of these patients. Of note a recent meta-analysis similarly demonstrated lower EBL, shorter hospital stay, less febrile morbidity and wound-related complications with robotic vs. open abdominal radical hysterectomy [28].
In terms of surgical outcomes, the EBL range reported by previous authors has ranged from a mean of 50 [11,20] to 300 mL [17]. Our mean EBL was 106 mL, and consistent with others, is significantly less than the open approach (p<0.001). The rate of robotic radical hysterectomy complications in previous studies ranged from 7.8% [15] to a high of 46.2% [21]. These studies are heterogeneous and represent different portions of the surgeon learning curve. In our study, we report a complication rate of 9% for the robotic study population, confirming that robotic surgical management of cervical cancer is safe and effective. Previously reported data for open radical hysterectomy have shown a complication rate ranging from 7% to 84% (with considerable variation in how complications are defined), with operating times ranging from 143 [29] to 340 minutes [30]. Our urogenital fistula rate across the study population was 0% in both groups, this is lower than historically reported rates of 1%–2% [31,32]. Surgical times in this study were consistent with these previously published reports (257 minutes). Our complication rate in the open cohort was 23%, which was significantly higher than in the robotic cohort as expected (23%, p=0.002).
One critical issue in the current healthcare environment is cost. The use of robotic surgery has been demonstrated to be up to 1.6 times more expensive in the management of endometrial cancer compared to conventional laparoscopy [33]. However, one critical issue that is missed in many of these analyses is the use of robotic surgery results in greater availability of minimally invasive techniques to women overall, resulting in an overall cost reduction as compared to laparotomy. As we previously reported, the initiation of robotic surgery in our program transformed our practice from a pre-robotics era in which 6% of endometrial cancer patients underwent a minimally invasive approach to greater than 80% within 3 years [5]. Similarly, our proportion of women undergoing minimally invasive management of cervical cancer was 0% prior to robotics, and it is currently greater than 90%. Renato and colleagues endorsed these findings in a review of the literature on robotic radical hysterectomy. They felt robotic technology increased surgical precision and decreased operative time and training for technically challenging procedures, of which radical hysterectomy could certainly be considered [34].
The Society of Gynecologic Oncology (SGO) recently issued a consensus policy statement on robotics in gynecologic oncology. They concluded that current evidence supports equivalence of robotic surgery and laparoscopy in many perioperative outcome measures [35]. However, there was only minimal oncologic outcome data available for cervical cancer. Only one trial to date has looked at survival in women undergoing robotic surgery for cervical cancer, and they found an OS of 94% at 36 months (n=63) [11]. Our 3-year survival in over 100 patients was 97%, further confirming the safety of this approach. Limitations of this study include its retrospective nature, and the study was therefore not powered to make causal assumptions about surgical approach and survival. However, in the absence of randomized controlled trials that are unlikely to happen, this real world data is often the best that is available to us.
Our study confirms the findings of previous authors. Robotic radical hysterectomy, as compared to a traditional open approach, in the management of early stage cervical cancer is associated with improved clinical and surgical outcomes including decreased EBL and shorter hospital LOS. Operative time required for the procedure decreased during our study period. Our overall complication rate was low, demonstrating the safety and feasibility of this approach. Most importantly, there were comparable oncologic outcomes in patients undergoing a robotic surgery, with equivalent survival between groups.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: S.C.A., P.P.
- Data curation: S.C.A., B.T., G.N.V.
- Formal analysis: S.C.A., B.T., G.N.V.
- Writing - original draft: S.C.A.
- Writing - review & editing: L.J., V.D., P.P.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Bansal N, Herzog TJ, Shaw RE, Burke WM, Deutsch I, Wright JD. Primary therapy for early-stage cervical cancer: radical hysterectomy vs radiation. Am J Obstet Gynecol. 2009;201:485.e1–485.e9. doi: 10.1016/j.ajog.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Frumovitz M, Ramirez PT, Greer M, Gregurich MA, Wolf J, Bodurka DC, et al. Laparoscopic training and practice in gynecologic oncology among Society of Gynecologic Oncologists members and fellows-in-training. Gynecol Oncol. 2004;94:746–753. doi: 10.1016/j.ygyno.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Advincula AP, Song A. The role of robotic surgery in gynecology. Curr Opin Obstet Gynecol. 2007;19:331–336. doi: 10.1097/GCO.0b013e328216f90b. [DOI] [PubMed] [Google Scholar]
- 5.Paley PJ, Veljovich DS, Shah CA, Everett EN, Bondurant AE, Drescher CW, et al. Surgical outcomes in gynecologic oncology in the era of robotics: analysis of first 1000 cases. Am J Obstet Gynecol. 2011;204:551.e1–551.e9. doi: 10.1016/j.ajog.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 6.Veljovich DS, Paley PJ, Drescher CW, Everett EN, Shah C, Peters WA., 3rd Robotic surgery in gynecologic oncology: program initiation and outcomes after the first year with comparison with laparotomy for endometrial cancer staging. Am J Obstet Gynecol. 2008;198:679.e1–679.e9. doi: 10.1016/j.ajog.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 7.Shafer A, Boggess JF. Robotic-assisted endometrial cancer staging and radical hysterectomy with the da Vinci surgical system. Gynecol Oncol. 2008;111:S18–S23. doi: 10.1016/j.ygyno.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Holloway RW, Ahmad S. Robotic-assisted surgery in the management of endometrial cancer. J Obstet Gynaecol Res. 2012;38:1–8. doi: 10.1111/j.1447-0756.2011.01744.x. [DOI] [PubMed] [Google Scholar]
- 9.Brudie LA, Backes FJ, Ahmad S, Zhu X, Finkler NJ, Bigsby GE, 4th, et al. Analysis of disease recurrence and survival for women with uterine malignancies undergoing robotic surgery. Gynecol Oncol. 2013;128:309–315. doi: 10.1016/j.ygyno.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331–5336. doi: 10.1200/JCO.2009.22.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantrell LA, Mendivil A, Gehrig PA, Boggess JF. Survival outcomes for women undergoing type III robotic radical hysterectomy for cervical cancer: a 3-year experience. Gynecol Oncol. 2010;117:260–265. doi: 10.1016/j.ygyno.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Sert BM, Boggess JF, Ahmad S, Jackson AL, Stavitzski NM, Dahl AA, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Eur J Surg Oncol. 2016;42:513–522. doi: 10.1016/j.ejso.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Chen CH, Chiu LH, Chang CW, Yen YK, Huang YH, Liu WM. Comparing robotic surgery with conventional laparoscopy and laparotomy for cervical cancer management. Int J Gynecol Cancer. 2014;24:1105–1111. doi: 10.1097/IGC.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 14.Mendivil AA, Rettenmaier MA, Abaid LN, Brown JV, 3rd, Micha JP, Lopez KL, et al. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: a five year experience. Surg Oncol. 2016;25:66–71. doi: 10.1016/j.suronc.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, Skinner EN, et al. A case-control study of robot-assisted type III radical hysterectomy with pelvic lymph node dissection compared with open radical hysterectomy. Am J Obstet Gynecol. 2008;199:357.e1–357.e7. doi: 10.1016/j.ajog.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 16.Estape R, Lambrou N, Diaz R, Estape E, Dunkin N, Rivera A. A case matched analysis of robotic radical hysterectomy with lymphadenectomy compared with laparoscopy and laparotomy. Gynecol Oncol. 2009;113:357–361. doi: 10.1016/j.ygyno.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Fanning J, Fenton B, Purohit M. Robotic radical hysterectomy. Am J Obstet Gynecol. 2008;198:649.e1–649.e4. doi: 10.1016/j.ajog.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Geisler JP, Orr CJ, Khurshid N, Phibbs G, Manahan KJ. Robotically assisted laparoscopic radical hysterectomy compared with open radical hysterectomy. Int J Gynecol Cancer. 2010;20:438–442. doi: 10.1111/IGC.0b013e3181cf5c2c. [DOI] [PubMed] [Google Scholar]
- 19.Ko EM, Muto MG, Berkowitz RS, Feltmate CM. Robotic versus open radical hysterectomy: a comparative study at a single institution. Gynecol Oncol. 2008;111:425–430. doi: 10.1016/j.ygyno.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Lowe MP, Chamberlain DH, Kamelle SA, Johnson PR, Tillmanns TD. A multi-institutional experience with robotic-assisted radical hysterectomy for early stage cervical cancer. Gynecol Oncol. 2009;113:191–194. doi: 10.1016/j.ygyno.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Nezhat FR, Datta MS, Liu C, Chuang L, Zakashansky K. Robotic radical hysterectomy versus total laparoscopic radical hysterectomy with pelvic lymphadenectomy for treatment of early cervical cancer. JSLS. 2008;12:227–237. [PMC free article] [PubMed] [Google Scholar]
- 22.Persson J, Reynisson P, Borgfeldt C, Kannisto P, Lindahl B, Bossmar T. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185–190. doi: 10.1016/j.ygyno.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Sert MB. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity. Gynecol Oncol. 2009;115:164–165. doi: 10.1016/j.ygyno.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis JW, Gaston K, Anderson R, Dinney CP, Grossman HB, Munsell MF, et al. Robot assisted extended pelvic lymphadenectomy at radical cystectomy: lymph node yield compared with second look open dissection. J Urol. 2011;185:79–83. doi: 10.1016/j.juro.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Salvo G, Ramirez PT, Levenback CF, Munsell MF, Euscher ED, Soliman PT, et al. Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage cervical cancer. Gynecol Oncol. 2017;145:96–101. doi: 10.1016/j.ygyno.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Cho KR, et al. Cervical cancer, version 2.2015. J Natl Compr Canc Netw. 2015;13:395–404. doi: 10.6004/jnccn.2015.0055. [DOI] [PubMed] [Google Scholar]
- 28.Shazly SA, Murad MH, Dowdy SC, Gostout BS, Famuyide AO. Robotic radical hysterectomy in early stage cervical cancer: a systematic review and meta-analysis. Gynecol Oncol. 2015;138:457–471. doi: 10.1016/j.ygyno.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Magrina JF, Goodrich MA, Weaver AL, Podratz KC. Modified radical hysterectomy: morbidity and mortality. Gynecol Oncol. 1995;59:277–282. doi: 10.1006/gyno.1995.0022. [DOI] [PubMed] [Google Scholar]
- 30.Artman LE, Hoskins WJ, Bibro MC, Heller PB, Weiser EB, Barnhill DR, et al. Radical hysterectomy and pelvic lymphadenectomy for stage IB carcinoma of the cervix: 21 years experience. Gynecol Oncol. 1987;28:8–13. doi: 10.1016/s0090-8258(87)80002-1. [DOI] [PubMed] [Google Scholar]
- 31.Emmert C, Köhler U. Management of genital fistulas in patients with cervical cancer. Arch Gynecol Obstet. 1996;259:19–24. doi: 10.1007/BF02505304. [DOI] [PubMed] [Google Scholar]
- 32.Methfessel HD, Retzke U, Methfessel G. Urinary fistula after radical hysterectomy with lymph node excision. Geburtshilfe Frauenheilkd. 1992;52:88–91. doi: 10.1055/s-2007-1022959. [DOI] [PubMed] [Google Scholar]
- 33.Desille-Gbaguidi H, Hebert T, Paternotte-Villemagne J, Gaborit C, Rush E, Body G. Overall care cost comparison between robotic and laparoscopic surgery for endometrial and cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2013;171:348–352. doi: 10.1016/j.ejogrb.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 34.Renato S, Mohamed M, Serena S, Giulia M, Giulia F, Giulia G, et al. Robot-assisted radical hysterectomy for cervical cancer: review of surgical and oncological outcomes. ISRN Obstet Gynecol. 2011;2011:872434. doi: 10.5402/2011/872434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez PT, Adams S, Boggess JF, Burke WM, Frumovitz MM, Gardner GJ, et al. Robotic-assisted surgery in gynecologic oncology: a Society of Gynecologic Oncology consensus statement. Developed by the Society of Gynecologic Oncology's Clinical Practice Robotics Task Force. Gynecol Oncol. 2012;124:180–184. doi: 10.1016/j.ygyno.2011.11.006. [DOI] [PubMed] [Google Scholar]