Abstract
Objective
To investigate the 5-year relative survival rates in gynecological cancers diagnosed and treated in Turkey by year 2009 and to compare the results with developed countries.
Methods
Data of patients diagnosed for ovarian, corpus uteri or cervix uteri cancer at year 2009 are collected from 9 national cancer registry centers. Date of deaths are retracted from governmental Identity Information Sharing System (KPS). In order to calculate relative survival rates, national general population mortality tables are obtained from Turkish Statistical Institute (TurkStat). Hakulinen method is used for computing curves by R program. Data for European, Asian and some developed countries were obtained from official web pages.
Results
A total of 1,553 patients are evaluated. Among these, 713 (45.9%) are corpus uteri cancers, while remaining 489 (31.5%) are ovarian and 351 (22.6%) are cervix uteri. Five-year overall relative survival rates are 85%, 50%, and 62% for corpus uteri, ovarian, and cervix uteri, respectively. These figures are between 73%–87% for corpus uteri, 31%–62% for ovarian and 61%–80% for cervix uteri in developed countries. Stage is the most important factor for survival in all cancers. Five-year relative survival rates in corpus uteri cancers are 92%, 66%, and 38% for localized, regional, and distant metastatic disease, respectively. These figures are 77%, 57%, and 29% for ovarian; 80%, 50%, and 22% for cervix uteri.
Conclusion
This is the first report from Turkey giving national overall relative survival for gynecological cancers from a population based cancer registry system.
Keywords: Turkey, Relative Survival, Gynecologic Neoplasms, Ovarian Diseases, Cervix Uteri, Corpus Uteri Cancers
INTRODUCTION
Gynecological cancers constitute an important burden of disease around the world. Estimates from GLOBOCAN 2012 reveals approximately 1,085,900 incident cases of cancers of the uterine cervix, corpus uteri, and ovarian, annually around the world. These 3 tumors constitute about 16% of all female cancers diagnosed per year and are responsible for 14% of female cancer related deaths annually [1]. However, an important fraction of these tumors can be prevented or early diagnosed by simple measures such as obesity and tobacco control programs, human papillomavirus (HPV) vaccination programs, awareness programs for recognizing early signs and symptoms of the disease and national population based screening programs [2]. Moreover, well-structured treatment facilities including surgical care with specially trained gynecological oncologists, radiotherapy and chemotherapy centers providing services in a multi-disciplinary and timely manner can also provide good prognosis and may avoid an important fraction of gynecological cancer related deaths (tertiary prevention).
Cancer survival is a key measure of the effectiveness of health-care systems. Persistent regional and international differences in survival may represent many avoidable deaths and therefore can guide national cancer control strategies. Turkish Ministry of Health has invested a lot for oncological care within last 15 years (Health Transition Program). Comprehensive oncology centers with high technology radiotherapy devices has been implemented homogeneously on thirty health investment regions around the country [3]. Gynecological oncology has been accepted as an official sub-specialty by year 2009. All treatments are made free of charge for the public with access to all standard drugs used in gynecological cancers. However, after all these improvements in recent years; nation-wide success rates of gynecological cancers are unknown and there are still questions to be answered: “Is Turkey approaching to cure rates seen in developed countries?” or “Does the survival differ significantly in certain subtypes?” or “Are there any tumors that cancer control program should focus more due to lower survival rates?”
This article is the first in the literature from Turkey, giving 5-year relative survival rates of nation-wide gynecological cancers diagnosed by year 2009. The data analyzed is not hospital or institution based but represents the whole country. Therefore, the results are a cornerstone for future studies from Turkey and the regional countries to investigate international survival disparities, with the aim of informing health policy makers to raise standards and reduce inequalities in survival.
MATERIALS AND METHODS
The National Central Cancer Registry of Turkey, established in 1992, is responsible for the collection, evaluation, and publication of cancer data in Turkey. Cancer diagnoses are reported to local cancer registries from multiple sources, including local hospitals and community health centers. Population based cancer registry network with high quality data and at least 5-year old experience has expanded to 9 cities by 2009, representing 23% of the whole population. Since then, Ministry of Health, National Cancer Control Program has supported cancer registry pillar with extra-financing mechanisms. With these efforts, population based cancer registry network has grown to 13 cities by 2012, and 81 cities by the end of 2013, representing 47.5% and 100% of the whole population, respectively. Not all of these registries currently have sufficiently high data quality for reporting purposes. The quality of submitted data for each local registry was checked and evaluated by the Ministry of Health, Cancer Control Department based on the national guidelines and International Agency for Research on Cancer (IARC)/International Association of Cancer Registries (IACR) data-quality criteria. Cancer Registry 4 (CanReg4) software program was used for data entry, storage, checking, and processing. The assessments of quality measures include, but are not limited to, the proportion of morphologic verification (MV%), the percentage of cancer cases identified with death certification only (DCO%), and the mortality to incidence ratio (M/I). Only data from those 9 local registries that consistently met appropriate levels of quality were included in these analyses. These registries (Izmir, Antalya, Bursa, Eskisehir, Samsun, Trabzon, Edirne, Erzurum, and Ankara) are homogeneously distributed across the country from north to south and from east to west.
Cancer data are collected by specially trained cancer registrars in population based cancer registry centers. International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) has been used for the collection of data in order to code the topography and histology of the malignant tumors while the rules of IARC are used for the distinction of multiple primaries.
Among routinely collected variables in cancer registries, age, year of diagnosis, cancer site or topography, tumor morphology, differentiation (grade), and stage are extracted for this analysis. Tumor grades are coded as 1 (well differentiated), 2 (moderately differentiated), 3 (poorly differentiated), or unknown. However, since data for grade was missing in a great majority of ovarian and cervix uteri cancer, grade for these cancers was included into only descriptive statistics. For comparison of the tumor histology, most common types are specified and the remaining histological subtypes are coded as others (for corpus uteri, serous, squamous, mixed, mucinous, clear cell, leiomyosarcoma, not otherwise specified, etc.; for ovarian, mucinous, endometrioid, clear cell, malignant epithelial, not otherwise specified, etc.; for cervical, mixed müllerian, sarcomas, not otherwise specified, etc.). Surveillance, Epidemiology, and End Results (SEER) program staging system is used for cancer staging and patients are categorized in 4 groups: localized, regional, distant, and unknown. The SEER Summary Staging Manual by the SEER program of the United States National Cancer Institute is most commonly used for determining summary stage. This is the most basic way of classifying how far a disease has spread from its point of origin and is commonly used by international registrars. For age specific analysis, 5 broad age groups are used (younger than 30 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years).
All patients are diagnosed between January 1, 2009 to December 31, 2009 for ovarian (ICD-O-3 C56.-), corpus uteri (ICD-O-3 C54.- C55.-), or cervix uteri (ICD-O-3 C53.-) with passive follow-up to the March 18, 2015. Patients with multiple primary tumors are excluded remaining 1,553 for the final analysis. Descriptive epidemiology is evaluated via frequency distribution and percentages.
1. Relative survival analysis
The classic survival analysis procedures (life-table or Kaplan-Meier) provides a means for using all the follow-up information accumulated up to the closing date of the study [4]. Especially, population-based cancer registry centers have trouble to follow cause-specific death information.
Relative survival methods are used to calculate the survival experience in a study with the one expected should they follow the background population mortality rates. The methods are beneficial when the cause-specific death information is unavailable or unreliable given that they supply a measure of excess mortality in patients with a related disease. There are various approaches to computing an estimate of the relative survival curve, written by the authors in existing programs (for example: Surv3, SAS and Stata functions, RSurv R function) [5]. In this study, Hakulinen method was used to compute curves by R program (R Foundation, Vienna, Austria) “relsurv” package. And also “relsurv” package was utilized for relative survival regression models. To test survival curve differences, a log-rank type test was also used. Date of deaths was extracted from Turkish Ministry of Health Death Reports within Identity Information Sharing System (KPS) using the citizen numbers of the patients. In order to evaluate relative survival rates, national general population mortality rates were obtained from Turkish Statistical Institute (TurkStat). National general population mortality rates provided by TurkStat is ending for 75 ages and over. Therefore, mortality rates for ages 75 and over, one by one per each age, were not calculated. It is possible to calculate a 5-year relative survival for patients diagnosed in 69 years and earlier. However, calculations for patients 70 years old and over would be misleading. Therefore, patients diagnosed at 70 years and over are excluded in this analysis. Five-year relative survival rates for overall patient population, according to interval of age of diagnosis, tumor stage, differentiation (for only corpus uteri), and histology were computed by rs.surv function. Multiple analysis was done using relative survival regression model. A p-value <0.05 was accepted as significant for all models to evaluate estimated excess hazard ratios (HRs, exp[β]). First groups (i.e., stage localized, differentiation 1, histology others, age <30 or <40) were always taken as the reference group. The models consist of various coefficients, the last 5 representing the follow-up interval covariates (fu).
Data for European, Asian and some developed countries were obtained from previously published multi-national survival publication (EUROpean CAncer REgistry-based study on survival and CARE of cancer patients [EUROCARE]), official web pages or publications of governmental agencies and cancer societies of those corresponding countries. For accurate comparisons, only relative survival rate figures published in the literature were used, excluding observed, overall, conditional, net or disease specific survival rates [6,7,8,9,10,11,12,13,14,15,16]. Among developed countries, officially published relative survival rates of patients (diagnosed close to 2009) in USA, UK, France, Germany, European Union, Australia, Canada, Japan, Korea, and China were found for comparison with Turkish figures [6,7,8,9,10,11,12,13,14,15,16].
RESULTS
A total of 1,553 patients were evaluated. Among these, 713 (45.9%) were corpus uteri cancers, while remaining 489 were ovarian (31.5%) and 351 (22.6%) were cervix uteri. Mean age of diagnosis was 55.0 (±8.5), 50.2 (±13.1), and 50.1 (±10.7) for corpus uteri, ovarian, and cervical cancers, respectively. Moreover, there was no evidence of lack-of-fit for all regression models.
1. Corpus uteri cancers
Most common age interval for diagnosis was 50–59 (n=307, 43.1%). A great majority of the corpus uteri cancer patients were diagnosed as localized (n=424, 59.5%) while distant metastasis was seen in only 3.8% (n=27) of patients. Tumor was grade 1 in 246 patients (34.5%), grade 2 in 204 (28.6%), and grade 3 in 73 patients (10.3%). The most common histological subtype was endometrioid (n=472, 66.1%) (Table 1).
Table 1. Clinico-pathological characteristics and relative survival.
| Characteristics | No. (%) | 5-year relative survival | SE | p-value | ||
|---|---|---|---|---|---|---|
| Corpus uteri | Overall | 713 | 0.85 | 0.01 | ||
| SEER stage | Localized | 424 (59.5) | 0.92 | 0.02 | <0.05 | |
| Regional | 115 (16.1) | 0.66 | 0.05 | |||
| Distant | 27 (3.8) | 0.38 | 0.09 | |||
| Unknown | 147 (20.6) | 0.87 | 0.03 | |||
| Differentiation | 1 | 246 (34.5) | 0.95 | 0.02 | <0.05 | |
| 2 | 204 (28.6) | 0.91 | 0.02 | |||
| 3 | 73 (10.2) | 0.57 | 0.06 | |||
| Unknown | 190 (26.6) | 0.76 | 0.03 | |||
| Histology | Endometrioid | 472 (66.2) | 0.91 | 0.02 | <0.05 | |
| Others | 241 (33.8) | 0.73 | 0.03 | |||
| Age group | <40 | 33 (4.6) | 0.94 | 0.04 | <0.05 | |
| 40–49 | 138 (19.4) | 0.94 | 0.02 | |||
| 50–59 | 307 (43.1) | 0.86 | 0.02 | |||
| 60–69 | 235 (33.0) | 0.77 | 0.03 | |||
| Ovary | Overall | 489 | 0.50 | 0.02 | ||
| SEER stage | Localized | 116 (23.7) | 0.77 | 0.04 | <0.05 | |
| Regional | 70 (14.3) | 0.57 | 0.06 | |||
| Distant | 191 (39.1) | 0.29 | 0.03 | |||
| Unknown | 112 (22.9) | 0.55 | 0.05 | |||
| Differentiation | 1 | 25 (5.1) | - | - | - | |
| 2 | 67 (13.7) | - | - | |||
| 3 | 96 (19.6) | - | - | |||
| Unknown | 301 (61.6) | - | - | |||
| Histology | Serous | 224 (45.8) | 0.41 | 0.03 | <0.05 | |
| Others | 265 (54.2) | 0.58 | 0.03 | |||
| Age group | <30 | 43 (8.8) | 0.86 | 0.05 | <0.05 | |
| 30–39 | 39 (8.0) | 0.67 | 0.07 | |||
| 40–49 | 124 (25.4) | 0.63 | 0.04 | |||
| 50–59 | 156 (31.9) | 0.40 | 0.04 | |||
| 60–69 | 127 (26.0) | 0.29 | 0.04 | |||
| Cervix uteri | Overall | 351 | 0.62 | 0.03 | ||
| SEER stage | Localized | 132 (37.6) | 0.80 | 0.04 | <0.05 | |
| Regional | 94 (26.8) | 0.50 | 0.05 | |||
| Distant | 23 (6.6) | 0.22 | 0.08 | |||
| Unknown | 102 (29.1) | 0.57 | 0.05 | |||
| Differentiation | 1 | 21 (6.0) | - | - | - | |
| 2 | 52 (14.8) | - | - | |||
| 3 | 31 (8.8) | - | - | |||
| Unknown | 247 (70.4) | - | - | |||
| Histology | Squamous | 265 (75.5) | 0.64 | 0.33 | 0.44 | |
| Adenocarcinoma | 66 (18.8) | 0.56 | 0.06 | |||
| Others | 20 (5.7) | 0.56 | 0.11 | |||
| Age group | <40 | 66 (18.8) | 0.70 | 0.06 | 0.11 | |
| 40–49 | 104 (29.6) | 0.67 | 0.05 | |||
| 50–59 | 96 (27.4) | 0.52 | 0.05 | |||
| 60–69 | 85 (24.2) | 0.60 | 0.06 | |||
SE, standard error; SEER, Surveillance, Epidemiology, and End Results.
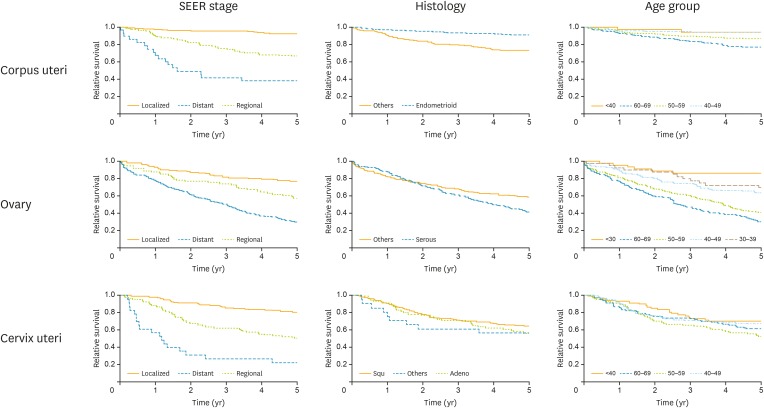
Table 1 and Fig. 1 represent the survival rates according to different variables. Five-year overall relative survival rates of corpus uteri cancers were 85%, which was the highest survival rate compared to ovarian and cervix uteri cancers. Survival rates were significantly affected by stage, grade, histology of the disease, and the interval of age of diagnosis. Five-year survival was 92% with localized disease, 66% with regional, and 38% with distant metastasis (p<0.05). The positive stage coefficients (β=1.28, 1.70) implies that the survival of localized was relatively better. The estimated excess HR of 5.49 for stage indicated that patients diagnosed with corpus uteri cancer with distant metastasis experienced 5.49 times higher excess mortality than those who were localized (p<0.05). The coefficients for the follow-up years were similar as well, only the first year seemed to have a larger hazard (exp[fu [0,1]]=0.005 per year) (Table 2).
Fig. 1.
Five-year relative survival curves.
SEER, Surveillance, Epidemiology, and End Results.
Table 2. Relative survival regression models.
| Characteristics | Ovary | Corpus uteri | Cervix uteri | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | Exp(β) | p-value | β | SE | Exp(β) | p-value | β | SE | Exp(β) | p-value | ||
| Stage | |||||||||||||
| Reg./Loc. | 0.70 | 0.27 | 2.01 | <0.05 | 1.28 | 0.27 | 3.59 | <0.05 | 1.20 | 0.26 | 3.32 | <0.05 | |
| Distant/Loc. | 1.33 | 0.22 | 3.77 | <0.05 | 1.70 | 0.34 | 5.49 | <0.05 | 2.21 | 0.32 | 9.15 | <0.05 | |
| Unknown/Loc. | 0.79 | 0.25 | 2.21 | <0.05 | 0.15 | 0.32 | 1.16 | 0.63 | 1.00 | 0.26 | 2.71 | <0.05 | |
| Histology | |||||||||||||
| Serous/others | −0.02 | 0.14 | 0.98 | 0.91 | |||||||||
| Endometrioid/others | −0.41 | 0.25 | 0.66 | 0.09 | |||||||||
| Age | |||||||||||||
| 30–39/<30 | 0.66 | 0.51 | 1.93 | 0.20 | |||||||||
| 40–49/<30 | 0.88 | 0.44 | 2.42 | <0.05 | |||||||||
| 50–59/<30 | 1.44 | 0.43 | 4.22 | <0.05 | |||||||||
| 60–69/<30 | 1.76 | 0.43 | 5.83 | <0.05 | |||||||||
| 40–49/<40 | 0.52 | 0.82 | 1.69 | 0.52 | |||||||||
| 50–59/<40 | 1.18 | 0.76 | 3.27 | 0.11 | |||||||||
| 60–69/<40 | 1.49 | 0.75 | 4.44 | <0.05 | |||||||||
| fu | |||||||||||||
| fu [0,1] | −3.97 | 0.46 | 0.019 | <0.05 | −5.22 | 0.86 | 0.005 | <0.05 | −3.16 | 0.26 | 0.04 | <0.05 | |
| fu (1,2] | −4.01 | 0.47 | 0.018 | <0.05 | −5.52 | 0.87 | 0.004 | <0.05 | −2.65 | 0.24 | 0.07 | <0.05 | |
| fu (2,3] | −4.24 | 0.47 | 0.014 | <0.05 | −5.63 | 0.88 | 0.004 | <0.05 | −3.41 | 0.30 | 0.03 | <0.05 | |
| fu (3,4] | −3.91 | 0.47 | 0.020 | <0.05 | −5.64 | 0.88 | 0.004 | <0.05 | −3.37 | 0.31 | 0.03 | <0.05 | |
| fu (4,5] | −4.27 | 0.48 | 0.014 | <0.05 | −6.47 | 0.95 | 0.002 | <0.05 | −3.69 | 0.35 | 0.03 | <0.05 | |
| Differ | |||||||||||||
| 2/1 | 0.35 | 0.44 | 1.42 | 0.41 | |||||||||
| 3/1 | 1.61 | 0.42 | 4.99 | <0.05 | |||||||||
| Unknown/1 | 1.23 | 0.41 | 3.43 | <0.05 | |||||||||
fu, follow-up interval covariates; Loc., localized; Reg., regional; SE, standard error.
2. Ovarian cancers
Most common age interval for diagnosis was 50–59 (n=156, 31.9%). A great majority of the ovarian cancer patients were diagnosed with distant metastasis (n=191, 39.1%).
Overall 5-year relative survival of ovarian cancers was 50%, being the lowest compared to the other 2 cancers. Survival rates were again significantly affected by stage, tumor histology, and interval of age of diagnosis (Table 1, Fig. 1). Five-year survival was 77% in localized disease while it was 57% with regional and 29% with distant metastasis. (p<0.05). Multiple analysis revealed tumor stage and age of diagnosis to be significant factors for poor prognosis. The positive age coefficients (β=0.66, 0.88, 1.44, 1.76) implies that the survival of aged <30 was relatively better. Aged 30–39 group coefficient was not significantly different from the youngest (p=0.20). The estimated excess HR of 5.83 for age indicates that patients diagnosed with ovarian cancer aged 60–69 experienced 5.83 times higher excess mortality than aged <30 (p<0.05). The coefficients for the follow-up years were similar, the fourth year seemed to have a larger hazard (exp[fu (3,4]]=0.020 per year) (Table 2).
3. Cervix uteri cancers
Most common age interval for diagnosis was aged 40–49 (n=104, 29.6%) (Table 1). A great majority of the cervix uteri cancer patients were diagnosed at localized stages (n=132, 37.6%) while distant metastasis was seen in only 6.6% (n=23) of patients.
Overall 5-year relative survival for cervix uteri cancers was 62%. Survival rates were significantly affected by stage, but not by histological subtypes or by age of diagnosis (Table 1, Fig. 1). Five-year survival was 80% with localized disease, 50% in disease with regional, and 22% with distant metastasis. (p<0.05).
The estimated excess HR of 9.15 for stage indicates that patients diagnosed with cervix uteri cancer distant metastasis experienced 9.15 times higher excess mortality than those who were localized (p<0.05). The coefficients for the follow-up years were similar, the second year seemed to have a larger hazard (exp[fu (1,2]]=0.07 per year) (Table 2).
DISCUSSION
The variations in global burden of different gynecological cancers in different geographical regions reflect the inequity in the development of health care infrastructure and accessibility to early detection and treatment in different populations across the world [17,18]. Turkey has invested a lot on screening and treatment facilities of gynecological cancers within last years. This report is the first giving nation-wide overall survival rates for the most common 3 gynecological cancers.
Data collected by national registry staff is slightly different than the data collected and published by specialists (gynecological oncologists/clinicians). Health professionals report individual cancer series using many clinico-pathological detailed variables. However, data of national registries do not include such details, and according to the international cancer registry rules the system they use is simpler in order to ensure collecting wide range of data in the most efficient and right way. International cancer registry coding systems is based on rules divergent from the clinicians' staging systems, and its basic principle is collecting sufficient data with minimal essential variables and aims demonstrating the country profile meanwhile making international comparisons available. For example, data coded as C54 (ICD-O-3) include not only the endometrial cancers, but also myometrial cancers including sarcomas. This is the same for ovarian cancers and a code of C56 consists both epithelial and non-epithelial tumors. Therefore, extended analysis of tumor grade and histology are not included in many national cancer registry reports.
Another difference between 2 systems (the system used by clinicians vs. the system used by) is the national registries' staging system; SEER staging system has some slight differences compared to FIGO stages used by clinicians. According to SEER rules, cervix uteri International Federation of Gynecology and Obstetrics (FIGO) stage I, IA1, IA2, IB; FIGO stages IIA, IIB, III, IIIA, IIIB; and FIGO stage IV, IVA, IVB are classified as localized, regional, and distant disease. For corpus uteri FIGO stages I, IA, IB, IC; FIGO stages II, IIA, IIB, III, IIIA, IIIB, IIIC; and FIGO stages IVA, IVB are coded as localized, regional, and distant disease. Giving as the last example ovarian carcinoma FIGO stages I, IA, IB; FIGO stages IC, II, IIA, IIB, IIC; and FIGO stages III, IIIA, IIIB, IIIC, IV are coded as localized, regional, and distant disease.
Another issue is the treatment modalities. Although these details are very important for clinicians, especially in newly implemented national cancer registry centers, treatment modality data is not collected because it is not essential and compulsory for the national reports and international comparisons.
Overall, results of this study are in accordance with the general literature. Highest 5-year relative survival rate (85%) among gynecological cancers is seen in corpus uteri cancers. For these tumors, 5-year relative survival rates are significantly affected by stage, grade, together with the age of diagnosis [17,18]. Five-year relative survival rate was 81.0% in Germany in a population based survival analysis of 30,936 cases registered in 11 German Cancer registry centers between 1997 to 2006 [9]. A moderate age gradient was observed, with 5-year relative survival decreasing from 90.0% in age group 15–49 years to 74.8% in age group 70+ years. Prognosis strongly varied by stage, with age-adjusted 5-year relative survivals reaching to 91.2% for localized stage, 50.5% for regional stage, and 19.8% for distant stage [9]. For USA, survival was 95%, 69%, and 17% for corpus uteri cancers diagnosed and treated between 2006–2012 [6]. For England, overall relative survival rate was 95%, 77%, 39%, and 14%, respectively for stages I, II, III, and IV. Again, 5-year survival rates decreased with elderly advancing ages similar to our findings. Five-year relative survival rates were 86% for ages 40–49, 87% for ages 50–59, and 81% for ages 60–69 in UK for corpus uteri cancers diagnosed between 2009–2013 [19]. Table 3 represents the overall (including all patients) relative survival in different countries, for patients diagnosed and treated between 2000–2010. Five-year relative survival rate in the world was ranging between 73%–87% and Turkish survival rates (85%) are within this range.
Table 3. Five-year relative survival rates (%) per country (year of diagnosis).
| Country | Corpus uteri | Ovary | Cervix uteri |
|---|---|---|---|
| Turkey | 85 (2009) | 50 (2009) | 62 (2009) |
| USA [6] | 82 (2006–2012) | 46 (2006–2012) | 68 (2006–2012) |
| UK [7,8] | 77 (2000–2007) | 31 (2000–2007) | 59 (2005–2007) |
| Germany [7,9,10] | 81 (2002–2006) | 40 (2000–2007) | 65 (1997–2006) |
| France [7] | 73 (2000–2007) | 40 (2000–2007) | 61 (2000–2007) |
| EU [7] | 76 (2000–2007) | 38 (2000–2007) | 62 (2000–2007) |
| Australia [11] | 83 (2008–2012) | 44 (2009–2013) | 72 (2008–2012) |
| Canada [12] | 84 (2006–2008) | 44 (2006–2008) | 73 (2006–2008) |
| Korea [13] | 87 (2008–2012) | 62 (2008–2009) | 80 (2008–2012) |
| China [14,15] | 76 (2000–2002) | 55 (2005–2010) | 74 (2005–2010) |
| Japan [16] | 80 (2003–2005) | 55 (2003–2005) | 72 (2003–2005) |
Ovarian carcinomas are the most lethal among gynecological cancers. This is the same for Turkey as well, with 5-year relative survival rates of 50% with poor prognosis with advancing stage and elderly age, in accordance with 31%–62% 5-year relative survival rates within developed countries (Table 3). More recent data for 5-year survival is 46% and 39%, respectively from USA and UK [6,19]. In France, 5-year net survival was 40% for patients diagnosed and treated between 1980–2012 [20]. This figure was 74%, 57%, 46%, and 35% for ages between 15–44, 45–54, 55–64 and 65–74, respectively [20]. Similarly, in England, 5-year net survival has dropped with advancing ages. It was 87% for ages 15–39, and decreased to 74%, 60%, and 43%, respectively for ages 40–49, 50–59, and 60–69, respectively [19].
Cervix uteri cancer numbers are extremely low in Turkey, with a 4/100,000 incidence for the last 5 years [21]. However, cancer screening rates are very low, therefore early stage disease which are operable are also low and a great majority of cases have been treated by chemo-radiotherapy. This may explain the lower 5-year survival rates of Turkey compared to the developed countries (Table 3). In USA, overall 5-year relative survival was 68%. This figure was 91% for localized, 57% for regional, and 17% for distant metastatic disease [6]. However, Turkish ministry of health has implemented a population based HPV DNA screening by 2012 and screening coverage rates exceeded 80% [22]. This screening may show its' effects on survival in near future survival analysis.
The data used in this study are based on 9 cancer registries which are collaborative partners of a national population based program for cancer registration in Turkey, and provided the most reliable, the largest comparable sample size diagnosed in a specific year (2009) in Turkey. Therefore, this is the first comprehensive population-based study from Turkey and the region, providing survival estimates of gynecological cancer. Furthermore, this study provides most up-to-date and comprehensive survival estimates of gynecological cancers in the developed world at the early 21st century, all standardized for relative survival. Limitations are mainly related to lack of tumor stage and grade information in a great majority of the cases. Additionally, patients over age 70 are excluded in order to evaluate a comparable relative survival rate. This may result in a mildly higher survival rates for Turkey. Therefore, even if these results are promising for the region and Turkish policy makers, we should interpret the results with caution due to the substantial proportion of missing data for stage and tumor grade.
In conclusion, this is the first report from Turkey giving overall survival for gynecological cancers from a population based cancer registry system. Survival rates for the most common 3 gynecological cancers given in this analysis is comparable to developed countries. Investments in last decades resulted in such fair survival rates for gynecologic cancers.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: G.M., K.I., K.M.Z.
- Data curation: G.M., D.S.
- Formal analysis: G.M., D.S.
- Investigation: G.M., D.S., K.I., K.M.Z., B.G., T.S.H.
- Methodology: G.M., D.S., B.G.
- Project administration: G.M.
- Resources: G.M., D.S., K.I., K.M.Z., B.G., T.S.H., H.E., K.B.
- Software: D.S.
- Supervision: G.M., T.S.H., K.B.
- Validation: G.M., K.I., K.M.Z., B.G., T.S.H.
- Visualization: D.S., H.E.
- Writing - original draft: G.M., D.S., K.I., K.M.Z., B.G., H.E.
- Writing - review & editing: G.M., D.S., B.G., H.E.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012 v1.0 (IARC CancerBase No. 11) [Internet] Lyon: International Agency for Research on Cancer; 2013. [cited 2017 Apr 10]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. [Google Scholar]
- 2.Stewart SL, Lakhani N, Brown PM, Larkin OA, Moore AR, Hayes NS. Gynecologic cancer prevention and control in the National Comprehensive Cancer Control Program: progress, current activities, and future directions. J Womens Health (Larchmt) 2013;22:651–657. doi: 10.1089/jwh.2013.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akdag R, Tosun N, Cinal A. Türkiye'de özellikli planlama gerektiren sağlık hizmetleri 2011??023 [Internet] Ankara: Sağlık Bakanlığı Tedavi Hizmetleri Genel Müdürlüğü; 2011. [cited 2017 Apr 10]. Available from: http://planlamadb.saglik.gov.tr/Eklenti/3167,turkiyede-ozellikli-planlama-gerektiren-saglik-hizmetleri-2011-2023pdf.pdf. [Google Scholar]
- 4.Parkin DM, Hakulinen T. Analysis of survival. In: Jensen OM, Parkin DM, MacLennan R, Muir CS, Skeet RG, editors. Cancer registration: principles and methods. Lyon: International Agency for Research on Cancer; 1991. pp. 159–176. [Google Scholar]
- 5.Pohar M, Stare J. Relative survival analysis in R. Comput Methods Programs Biomed. 2006;81:272–278. doi: 10.1016/j.cmpb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. Cancer facts & figures 2017 [Internet] Atlanta, GA: American Cancer Society; 2017. [cited 2017 Apr 10]. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. [Google Scholar]
- 7.Sant M, Chirlaque Lopez MD, Agresti R, Sánchez Pérez MJ, Holleczek B, Bielska-Lasota M, et al. Survival of women with cancers of breast and genital organs in Europe 1999–2007: results of the EUROCARE-5 study. Eur J Cancer. 2015;51:2191–2205. doi: 10.1016/j.ejca.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Eurocare 5 survival analysis 2000??007 [Internet] Roma: Istituto Superiore di Sanità; 2014. [update 2014 Mar 5]. [cited 2017 Apr 10]. Available from: https://w3.iss.it/site/EU5Results/forms/SA0007.aspx. [Google Scholar]
- 9.Chen T, Jansen L, Gondos A, Ressing M, Holleczek B, Katalinic A, et al. Survival of endometrial cancer patients in Germany in the early 21st century: a period analysis by age, histology, and stage. BMC Cancer. 2012;12:128. doi: 10.1186/1471-2407-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T, Jansen L, Gondos A, Emrich K, Holleczek B, Luttmann S, et al. Survival of cervical cancer patients in Germany in the early 21st century: a period analysis by age, histology, and stage. Acta Oncol. 2012;51:915–921. doi: 10.3109/0284186X.2012.708105. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Australia. Online statistics and published in 2016 [Internet] Surry Hills: Cancer Australia; 2017. [cited 2017 Apr 10]. Available from: https://canceraustralia.gov.au. [Google Scholar]
- 12.Canadian Cancer Society. Canadian cancer statistics 2016 special topic: HPV associated cancers [Internet] Toronto: Canadian Cancer Society; 2016. [cited 2017 Apr 10]. Available from: http://www.cancer.ca/en/?region=on. [Google Scholar]
- 13.Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47:127–141. doi: 10.4143/crt.2015.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Yu L, Na J, Li S, Liu L, Mu H, et al. Survival of cancer patients in Northeast China: analysis of sampled cancers from population-based cancer registries. Cancer Res Treat. 2017 doi: 10.4143/crt.2016.613. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong W, Luo S, Hu R, Wang H, Pan J, Fei F, et al. Analysis of survival rate of breast, cervical, and ovarian cancer patients during 2005–2010 in Zhejiang province, China. Zhonghua Yu Fang Yi Xue Za Zhi. 2014;48:366–369. [PubMed] [Google Scholar]
- 16.Foundation for Promotion of Cancer Research (JP) Cancer statistics in Japan '15 [Internet] Tokyo: Center for Cancer Control and Information Services; 2016. [cited 2017 Apr 11]. Available from: http://ganjoho.jp/en/professional/statistics/brochure/2015_en.html. [Google Scholar]
- 17.Sankaranarayanan R, Ferlay J. Global burden of gynaecological cancer. In: Ayhan A, Reed N, Gultekin M, Dursun P, editors. Textbook of gynaecological oncology. Ankara: Güneş Publishing; 2016. p. 62. [Google Scholar]
- 18.Berek JS, Hacker NF. Berek & Hacker's gynecologic oncology. 6th ed. Philadelphia, PA: Wolters Kluwer; 2015. [Google Scholar]
- 19.Cancer Research UK. Cancer statistics for the UK [Internet] London: Cancer Research UK; 2017. Mar, [cited 2017 Apr 11]. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics. [Google Scholar]
- 20.Trétarre B, Molinié F, Woronoff AS, Bossard N, Bessaoud F, Marrer E, et al. Ovarian cancer in France: trends in incidence, mortality and survival, 1980–2012. Gynecol Oncol. 2015;139:324–329. doi: 10.1016/j.ygyno.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Türkiye Halk Sağlığı Kurumu, Kanser Daire Başkanlığı. 2014 Yılı Türkiye kanser ıstatistikleri [Internet] Ankara: Türkiye Halk Sağlığı Kurumu; 2016. [cited 2017 Apr 11]. Available from: http://kanser.gov.tr/daire-faaliyetleri/kanser-istatistikleri/2106-2014-ylı-türkiye-kanser-istatistikleri.html. [Google Scholar]
- 22.Gültekin M, Akgül B. HPV screening in Islamic countries. Lancet Infect Dis. 2017;17:368. doi: 10.1016/S1473-3099(17)30126-3. [DOI] [PubMed] [Google Scholar]