Abstract
Objective
Hyperglucagonemia is present in many forms of diabetes and contributes to hyperglycemia, and glucagon suppression can ameliorate diabetes in mice. Leptin, a glucagon suppressor, can also reverse diabetes in rodents. Lipid nanoparticle (LNP) delivery of small interfering RNA (siRNA) effectively targets the liver and is in clinical trials for the treatment of various diseases. We compared the effectiveness of glucagon receptor (Gcgr)-siRNA delivered via LNPs to leptin in two mouse models of diabetes.
Methods
Gcgr siRNA encapsulated into LNPs or leptin was administered to mice with diabetes due to injection of the β-cell toxin streptozotocin (STZ) alone or combined with high fat diet (HFD/STZ).
Results
In STZ-diabetic mice, a single injection of Gcgr siRNA lowered blood glucose levels for 3 weeks, improved glucose tolerance, and normalized plasma ketones levels, while leptin therapy normalized blood glucose levels, oral glucose tolerance, and plasma ketones, and suppressed lipid metabolism. In contrast, in HFD/STZ-diabetic mice, Gcgr siRNA lowered blood glucose levels for 2 months, improved oral glucose tolerance, and reduced HbA1c, while leptin had no beneficial effects.
Conclusions
While leptin may be more effective than Gcgr siRNA at normalizing both glucose and lipid metabolism in STZ diabetes, Gcgr siRNA is more effective at reducing blood glucose levels in HFD/STZ diabetes.
Keywords: Type 1 diabetes, Type 2 diabetes, Glucose metabolism, Lipid metabolism, Glucagon, Leptin
Abbreviations: AUC, area under the curve; FVII, factor VII; Gcgr, glucagon receptor; HFD, high fat diet; KO, knockout; LFD, low fat diet; LNP, lipid nanoparticle; siRNA, small interfering RNA; STZ, streptozotocin; WT, wildtype
Highlights
-
•
Gcgr siRNA improves glucose metabolism but not lipid metabolism in STZ diabetic mice.
-
•
Leptin improves both glucose and lipid metabolism in STZ diabetic mice.
-
•
Gcgr siRNA improves glucose metabolism in HFD/STZ diabetic mice.
-
•
Leptin does not improve glucose metabolism in HFD/STZ diabetic mice.
1. Introduction
Hyperglucagonemia is present in type 1 [1], [2] and type 2 [3], [4] diabetes and contributes to elevated blood glucose by stimulating glycogenolysis, gluconeogenesis, and ketogenesis, while suppressing glycogen synthesis. Moreover, suppression of glucagon action by glucagon receptor (Gcgr) gene deletion, glucagon immunosuppression, or Gcgr antagonist can ameliorate diabetic symptoms in models of both type 1 and type 2 diabetes. To investigate the effect of suppressing glucagon action in a model of insulinopenic diabetes, Gcgr wildtype (WT) and knockout (KO) mice were injected with the β-cell toxin streptozotocin (STZ) [5], [6]. GcgrWT mice become hyperglycemic, hyperketonemic, polyuric, and cachectic, while, remarkably, GcgrKO mice were protected from these diabetic symptoms [5], [6]. In addition, immunoneutralization of glucagon using a monoclonal antibody reduced hyperglycemia in alloxan-diabetic rabbits [7]. Moreover, in STZ-injected mice, weekly treatment with a Gcgr antibody completely normalized blood glucose levels for up to 12 weeks, concomitant with an improvement in HbA1c levels [8]. Similarly, immunoneutralization of endogenous glucagon improved oral glucose tolerance and reduced hepatic glucose output in obese leptin-deficient ob/ob mice [9], and Gcgr antisense oligonucleotides or small interfering RNA (siRNA) diminished hyperglycemia and improved oral glucose tolerance in obese leptin receptor null db/db mice [10], [11]. Finally, genetic deletion of Gcgr in diet-induced obese mice or db/db mice prevented obesity, hyperinsulinemia, and hyperglycemia [12]. Therefore, inhibiting glucagon action can improve diabetic symptoms in various models of diabetes.
The hormone leptin, well known for its role in body weight regulation, has also shown promise as a glucose-lowering therapy. In rodent models of type 1 diabetes, leptin monotherapy can potently reduce diabetic symptoms and normalize hyperglycemia [13], [14], [15], [16], [17], [18], [19], [20]. Interestingly, leptin can reduce circulating glucagon levels and levels of hepatic p-CREB indicative of reduced Gcgr signaling [13], [14], [16], which has been thought to be important for the glucose-lowering mechanism of leptin. Moreover, in STZ-diabetic rodents, intracerebroventricular leptin reduces preproglucagon mRNA levels in the pancreas, glucagon content in the pancreas [15], and plasma glucagon levels [21], suggesting that leptin can act through the central nervous system to suppress glucagon production. However, in type 2 diabetes, leptin monotherapy appears to be less efficacious as an anti-diabetic therapy. Although leptin injections in a rat model of obese type 2 diabetes normalized fasting blood glucose [22], leptin treatment in humans with type 2 diabetes did not increase insulin-mediated stimulation of glucose disposal [23] nor meaningfully reduce HbA1c [24]. The failure of leptin to improve type 2 diabetes may be due to leptin resistance as many obese individuals are hyperleptinemic [25].
In this report, we investigated the efficacy of Gcgr siRNA delivered using lipid nanoparticle (LNP) technology and compared this treatment to leptin therapy in mouse models of type 1 and type 2 diabetes. LNPs are capable of effectively and safely delivering genetic drugs such as siRNA to target tissues, and they are the most clinically advanced delivery systems for siRNA, with multiple LNP-siRNAs in clinical trials for the treatment of various diseases [26]. In addition, LNPs effectively target the liver [27], [28], where glucagon exerts most of its biological functions. Indeed mice with full-body or hepatocyte specific Gcgr gene deletion display a similar degree of improvement of fasting blood glucose levels and glucose tolerance highlighting the importance of glucagon action on the liver in regulating glucose metabolism [29], [30]. We find that Gcgr siRNA can potently improve glucose metabolism in both STZ (a model of type 1 diabetes) and high fat diet (HFD)/STZ (a model of type 2 diabetes) diabetic mice. However, while leptin was able to improve both glucose and lipid metabolism in STZ-diabetic mice, no changes were observed in HFD/STZ-diabetic mice given leptin treatment.
2. Research design and methods
2.1. Animals
Male C57BL/6J mice (stock 000664), C57BL/6J mice on 60% HFD (stock 380050) or C57BL/6J mice on 10% low fat diet (LFD) (stock 380056) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and acclimatized on arrival for at least a week. Mice were housed on a 12-h:12-h light–dark cycle with ad libitum access to normal chow (Harlan Laboratories, #2918, Indianapolis, IN, USA), 60% HFD (Research Diets, Inc., D12492i, New Brunswick, NJ, USA) or 10% LFD (Research Diets, Inc., D12450Bi, New Brunswick, NJ, USA) and water. All experiments were approved by the University of British Columbia Animal Care Committee and carried out in accordance with the Canadian Council on Animal Care guidelines.
2.2. Generation of STZ-diabetic mice
STZ (Sigma–Aldrich, Oakville, Canada) was prepared in a pH 4.5 acetate buffer and 180 mg/kg STZ was administered i.p. to 9 week old C57BL/6J mice. Non-diabetic controls received an i.p. injection of acetate buffer alone.
2.3. Generation of HFD/STZ-diabetic mice
C57BL/6J mice were put on a 60% HFD (Research Diets, Inc., D12492i, New Brunswick, NJ, USA) at 6 weeks of age and remained on the diet until the end of the study. At 10 weeks of age, STZ (Sigma–Aldrich, Oakville, Canada) was prepared in a pH 4.5 acetate buffer and administered at a dose of 100 mg/kg. Controls were put on a 10% LFD (Research Diets, Inc., D12450i, New Brunswick, NJ, USA) at 6 weeks of age and did not receive an i.p. injection of acetate buffer.
2.4. Preparation and delivery of LNP-siRNA
siRNAs were purchased from Integrated DNA Technologies (Coralville, IA, USA); sequences are in Supplemental Table 1. LNP-siRNA systems were prepared as previously described [31]. For all studies, siRNA was delivered via the tail vein. Either 5 mg/kg or 10 mg/kg LNP encapsulating Gcgr siRNA 2 or 10 mg/kg LNP encapsulating an equal mix of Gcgr siRNA 1 and 2 were injected. LNP encapsulating FVII siRNA was injected at the same total dose of GCGR siRNA for each study.
2.5. Leptin therapy
STZ-diabetic mice at 10 weeks of age received 20 μg/day of recombinant mouse leptin (Peprotech, Rocky Hill, NJ, USA) prepared in sterile water and administered via Alzet 1004 mini-osmotic pumps (DURECT Corporation, Cupertino, CA, USA). HFD/STZ-diabetic mice at 12 weeks of age received 20 μg/day PEG-ylated leptin (Protein Laboratories Rehovot Ltd., Rehovot, Israel) prepared in sterile water by daily i.p. injection while control HFD/STZ mice received water (vehicle).
2.6. Plasma analyte analysis
All blood parameters were measured using samples collected from 4 h fasted mice. Hemoglobin A1c (HbA1c) levels were measured using a Siemens DCA 200 Vantage Analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY, USA) from whole blood. Plasma insulin (Mouse Ultrasensitive Insulin ELISA, ALPCO, Salem, NH, USA), glucagon (Glucagon ELISA, Mercodia, Salem, NC, USA), leptin (Mouse Leptin ELISA, Crystal Chem, Downers Grove, IL, USA), β-hydroxybutyrate (β-Hydroxybutyrate LiquiColor Test, Stanbio, Boerne, TX, USA), free fatty acids (HR Series NEFA HR[2] Kit, Wako Diagnostics, Richmond, VA, USA), triglycerides and glycerol (Serum Triglyceride Determination Kit, Sigma–Aldrich, Oakville, Canada), and cholesterol (Cholesterol E, Wako Diagnostics, Richmond, VA, USA) were measured from cardiac puncture blood samples. For samples from PEG-leptin injected mice, plasma leptin levels were analyzed using a PEG-leptin standard curve as the PEG-leptin was less potent in the assay than an equivalent amount of normal leptin.
2.7. Oral glucose tolerance tests
Mice were fasted for 4 h and given oral gavages of either 1.5 g/kg of 30% dextrose or 2 g/kg of 50% dextrose. Blood glucose was measured via the saphenous vein using a One Touch Ultra Glucometer (LifeScan) with a detection limit of 1.1–33.3 mM. During the oral glucose tolerance tests, if samples fell over the detection limit they were diluted with non-diabetic blood of known glucose concentration, re-assayed, and original blood glucose levels were calculated. Area under the curve (AUC) was measured starting at baseline (0 min) values.
2.8. Immunofluorescence and analysis of α-cell area
Pancreata were harvested from mice on day 7, fixed overnight in paraformaldehyde at 4°C, rinsed in 70% ethanol, embedded in paraffin, and sectioned by Wax-it Histology Services Inc. (5 μm thickness; Vancouver, BC, Canada). The antibodies used are detailed in Supplemental Table 2. Cell nuclei were counterstained with VECTASHIELD HardSet Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA). Whole slide fluorescence scanning was performed using the ImageXpress Micro Imaging System (Molecular Devices Corporation, Sunnyvale, CA, USA). Individual images were stitched together to recreate the pancreas area and then quantified using MetaXpress software. For α-cell area, glucagon positive area was expressed relative to the pancreas area; 3 sections were quantified per animal, separated by 200 μm.
2.9. Statistics
Data are presented as either mean ± SEM or min to max box and whisker plots (where the box extends to the 25th and 75th percentiles, the middle line is the median, and the whiskers extend to the minimum and maximum values). Data were analyzed using a 1-way or 2-way ANOVA for bar and line graphs, respectively with Dunnett or Tukey post-hoc testing. Statistical analysis was performed using GraphPad Prism 6.05 (La Jolla, CA, USA) and significance was set at P < 0.05.
3. Results
3.1. Gcgr siRNA lowers blood glucose and improves oral glucose tolerance in wildtype mice
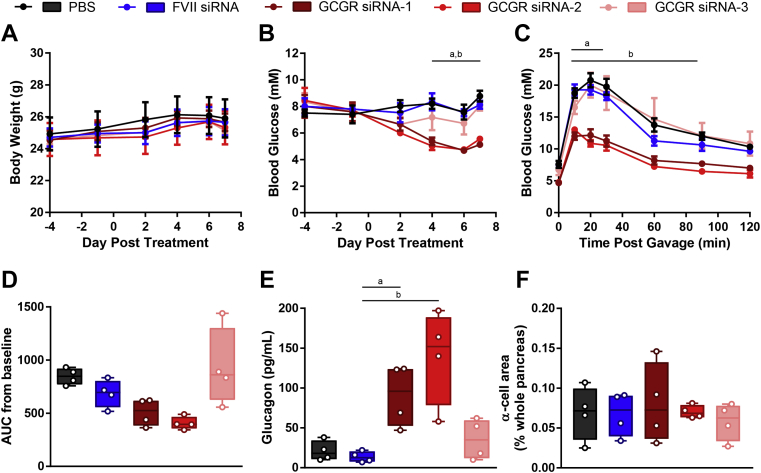
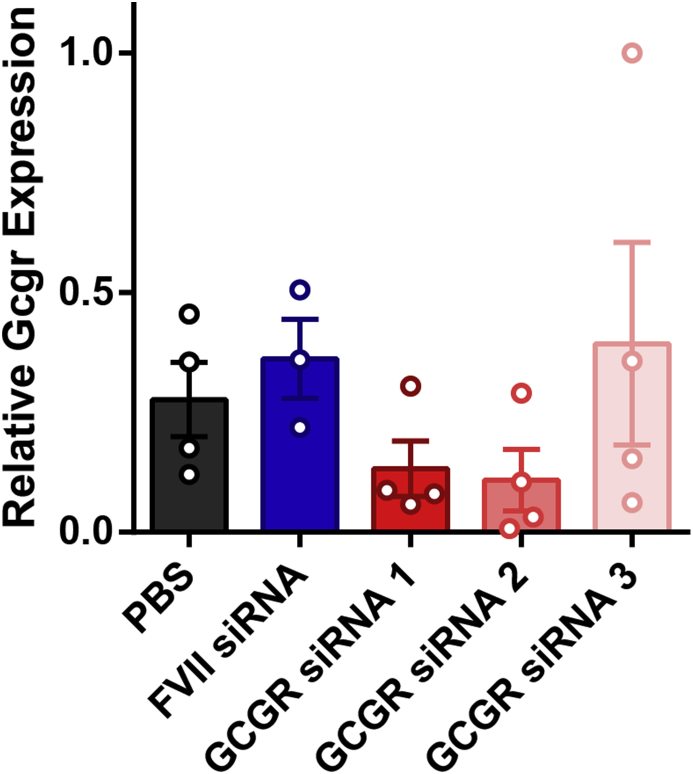
To identify an effective Gcgr siRNA, one of 3 Gcgr siRNAs or FVII siRNA used as a control were encapsulated into LNPs and delivered via the tail-vein to wildtype mice at a dose of 5 mg/kg, and glucose homeostasis was assessed. A group of mice receiving PBS served as a control and demonstrated that the particles themselves had no effect on the parameters measured (Figure 1). None of the Gcgr siRNAs affected body weight (Figure 1A). Strikingly, despite the mice already being healthy, Gcgr siRNA 1 and 2 lowered fasting blood glucose levels (8.8 ± 0.4 mM PBS, 8.1 ± 0.3 mM FVII siRNA, 5.1 ± 0.2 mM Gcgr siRNA 1, 5.6 ± 0.1 mM Gcgr siRNA 2, 8.1 ± 0.4 mM Gcgr siRNA 3, day 7, P < 0.05, Figure 1B) and reduced blood glucose levels throughout an oral glucose tolerance test, while Gcgr siRNA 3 had no effect (Figure 1C). However, glucose excursion as measured by AUC from baseline was not statistically different than controls (Figure 1D). In addition, Gcgr siRNA 1 and 2 but not 3 caused an elevation in circulating glucagon levels (21 ± 6 pg/mL PBS, 14 ± 3 pg/mL FVII siRNA, 91 ± 2 pg/mL Gcgr siRNA 1, 140 ± 30 pg/mL Gcgr siRNA 2, 36 ± 13 pg/mL Gcgr siRNA 3, P < 0.05, Figure 1E) without a corresponding increase in α-cell area (Figure 1F). Notably, our acute knockdown model exhibited a similar phenotype to mice with a lifelong, full-body knockout of the Gcgr, which also display reduced blood glucose levels, improved glucose tolerance, and increased glucagon levels [29]. In a separate cohort, hepatic Gcgr mRNA levels were quantified on day 2. In agreement with the phenotypic data, mice receiving Gcgr siRNA 1, 2, and 3 had average Gcgr transcript levels of 37%, 30%, and 109%, respectively, of those seen in FVII controls respectively; however, this did not reach significance due to variability and low sample size in the FVII group (Supplemental Figure 1). Based on the trends observed with Gcgr mRNA levels and robust phenotypic data, we chose either Gcgr siRNA 2 or a combination of Gcgr siRNA 1 and 2 for continuing our studies in diabetic models.
Figure 1.
Gcgr siRNA reduces blood glucose, improves oral glucose tolerance, and increases plasma glucagon without affecting α-cell area in wildtype mice. Mice received Gcgr siRNA or FVII siRNA at a dose of 5 mg/kg or PBS on day 0. Body weight (A) and blood glucose (B) were measured throughout the study. On day 6, an oral glucose tolerance test was performed using 2 g/kg of 50% dextrose (C), and area under the curve (AUC) from baseline was calculated (D). Plasma glucagon (E) and α-cell area (F) were analyzed on day 7. Each group was compared to the FVII siRNA treated group by 1- or 2-way ANOVA with Dunnett post-hoc testing; a, P < 0.05 Gcgr siRNA 1 vs FVII siRNA; b, P < 0.05 Gcgr siRNA 2 vs FVII siRNA. Data are mean ± SEM (A–C) or min to max box and whisker plots (D–F), n = 4.
3.2. 5 mg/kg Gcgr siRNA does not improve glucose and lipid metabolism as effectively as leptin therapy in STZ-diabetic mice
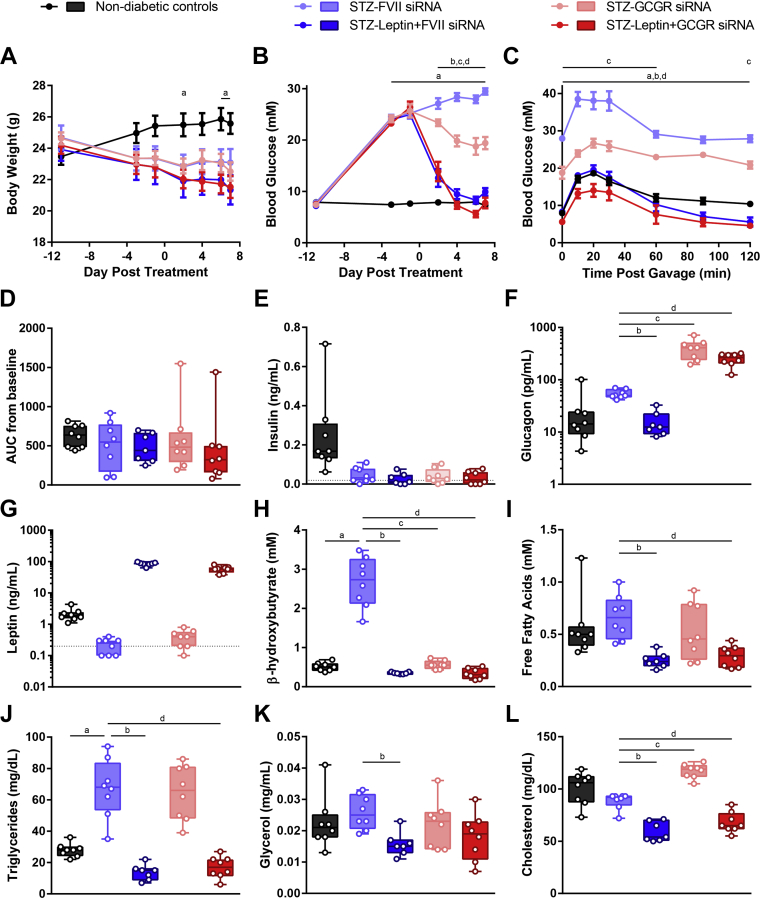
Next we sought to determine whether Gcgr siRNA could improve diabetic symptoms in a model of diabetes and to compare this treatment to leptin, which is a potent glucagon suppressor and can normalize blood glucose levels in mouse models of diabetes [13], [14], [15], [16], [20]. Diabetes induced by 1 or 2 high dose STZ injections results from severe insulin deficiency and is commonly used in studies testing glucagon suppression therapy [6], [8] or leptin therapy [13], [20], [32], [33]. Mice were rendered diabetic by STZ injection and treated with Gcgr siRNA (5 mg/kg by tail-vein injection) and/or leptin therapy (20 μg/day by mini-osmotic pump), and FVII siRNA was used as a control, resulting in the following groups: non-diabetic controls, STZ-FVII siRNA, STZ-Gcgr siRNA, STZ-leptin + FVII siRNA, and STZ-leptin + Gcgr siRNA. The leptin + Gcgr siRNA group was nearly identical to the leptin + FVII treated group for all metabolic parameters measured (Figure 1). As expected, STZ-FVII mice lost weight compared to non-diabetic controls, and neither Gcgr siRNA nor leptin treatment affected body weight (Figure 2A). While blood glucose was completely normalized by leptin therapy, Gcgr siRNA only modestly reduced fasting blood glucose at this dose (7.3 ± 0.3 mM non-diabetic controls, 29.5 ± 0.6 mM FVII siRNA, 9.9 ± 0.7 mM leptin + FVII siRNA 1, 19.4 ± 0.7 mM Gcgr siRNA, 7.8 ± 1.1 mM leptin + Gcgr siRNA, day 7, P < 0.05, Figure 2B). In addition, leptin normalized blood glucose levels following an oral glucose-gavage while Gcgr siRNA caused only a partial improvement (Figure 2C). However, no differences in glucose excursion were observed between the groups as measured by AUC calculated from baseline (Figure 2D). All STZ injected groups had plasma insulin levels close to or below the detection limit of the assay (0.019 ng/mL, Figure 2E). As expected based on Figure 1E, groups receiving Gcgr siRNA displayed supraphysiological levels of circulating glucagon (Figure 2F), and groups receiving leptin treatment exhibited supraphysiological levels of plasma leptin (Figure 2G).
Figure 2.
Effect of leptin or 5 mg/kg Gcgr siRNA on glucose and lipid metabolism in STZ-diabetic mice. Insulin deficient diabetes was induced in mice by injecting 180 mg/kg STZ on day −11; vehicle was administered to non-diabetic controls. STZ-diabetic mice were treated with either FVII siRNA, Gcgr siRNA, FVII siRNA + leptin, or Gcgr siRNA + leptin on day 0. siRNA was delivered at a dose of 5 mg/kg via tail-vein injection and leptin was administered at a dose of 20 μg/day via mini-osmotic pump. Body weight (A) and blood glucose (B) were measured throughout the study. On day 6, an oral glucose tolerance test was performed using 1.5 g/kg of 30% dextrose (C), and area under the curve (AUC) from baseline was calculated (D). Plasma levels of insulin (E), glucagon (F), leptin (G), β-hydroxybutyrate (H), fatty acids (I), triglycerides (J), glycerol (K), and cholesterol (L) were analyzed on day 7. Each group was compared to the FVII siRNA treated group by 1- or 2-way ANOVA with Dunnett post-hoc testing; a, P < 0.05 non-diabetic controls vs FVII siRNA; b, P < 0.05 Leptin + FVII siRNA vs FVII siRNA; c, P < 0.05 Gcgr siRNA vs FVII siRNA; d, P < 0.05 Leptin + Gcgr siRNA vs FVII siRNA. Statistical analysis was not performed on insulin (E) or leptin (G) measurements as some samples were below the limits of detection, denoted by the dotted lines. In addition, five samples had insulin measurements too low to be interpolated and were assigned a value of 0. Data are mean ± SEM (A–C) or min to max box and whisker plots (D–K), n = 7–8.
To ascertain how both treatments would affect lipid metabolism, we measured circulating β-hydroxybutyrate, fatty acids, triglycerides, glycerol, and cholesterol (Figure 2H–L). FVII siRNA treated mice with uncontrolled diabetes displayed elevated β-hydroxybutyrate, which was normalized by both leptin and Gcgr siRNA (0.51 ± 0.04 mM non-diabetic controls, 2.67 ± 0.22 mM FVII siRNA, 0.38 ± 0.03 mM leptin + FVII siRNA, 0.56 ± 0.04 mM Gcgr siRNA, 0.33 ± 0.05 mM leptin + Gcgr siRNA, P < 0.05, Figure 2H). Conversely, triglycerides that were raised in the FVII siRNA group were normalized by leptin but unchanged by Gcgr siRNA (Figure 2J). Interestingly, although plasma fatty acids, glycerol, and cholesterol were not elevated in the FVII siRNA group compared to non-diabetic controls, leptin was capable of reducing all of these parameters, while Gcgr siRNA had no effect on fatty acids or glycerol and modestly raised cholesterol levels (Figure 2I/K/L). Therefore, while leptin is capable of completely normalizing blood glucose, glucose tolerance, and lipid metabolism, this dose of Gcgr siRNA only partially improved blood glucose and glucose tolerance, fully normalized ketones, had no effect on triglycerides, and increased cholesterol.
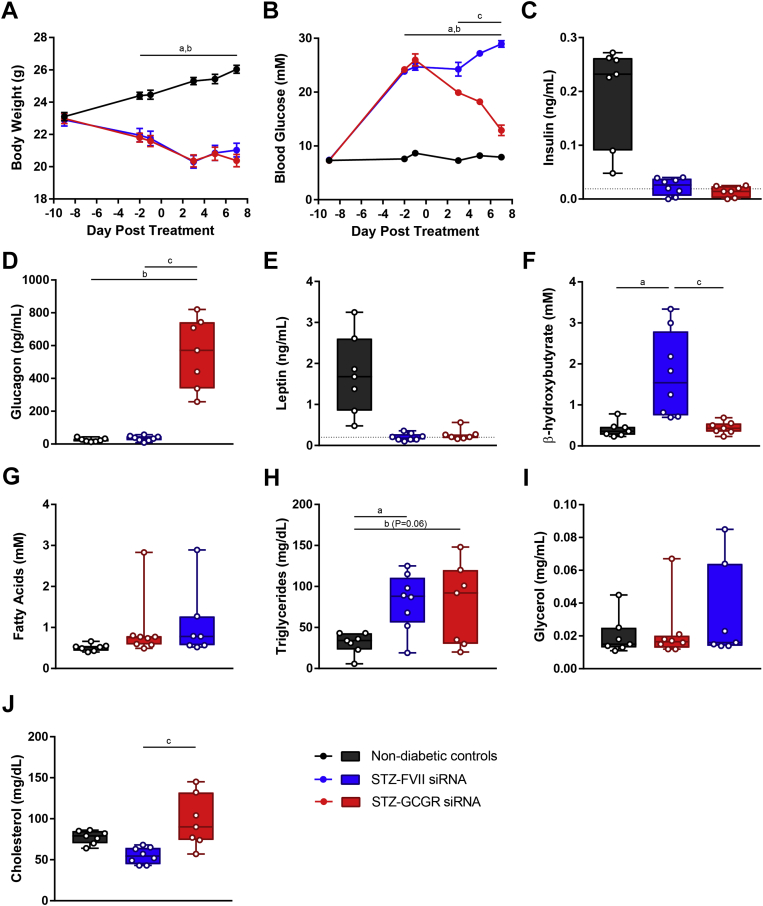
3.3. 10 mg/kg Gcgr siRNA reduces blood glucose, increases plasma glucagon, normalizes β-hydroxybutyrate, and increases cholesterol in STZ-diabetic mice
Since 5 mg/kg Gcgr siRNA only partially improved glucose homeostasis in STZ-diabetic mice, we investigated if increasing the dose may elicit a more potent effect. Similar to the previous study, compared to non-diabetic controls, STZ caused weight loss in FVII siRNA treated mice, and this was unaffected by Gcgr siRNA (Figure 3A). While 5 mg/kg Gcgr siRNA reduced blood glucose by 45% by day 7 (Figure 2B), 10 mg/kg reduced glucose by 76% by day 7 but was still unable to normalize blood glucose (Figure 3B). For the remainder of the plasma analytes measured, the results were similar to the 5 mg/kg dose of siRNA. STZ injection reduced plasma insulin levels close to or below the limit of detection of the assay (0.019 ng/mL, Figure 3C). Gcgr siRNA induced supraphysiological levels of circulating glucagon (Figure 3D). STZ treatment depleted leptin levels in both FVII and Gcgr siRNA treated mice to near the detection limit of the assay (Figure 3E). β-Hydroxybutyrate levels were increased in FVII siRNA controls and normalized by Gcgr siRNA treatment (Figure 3F). Triglycerides were elevated in FVII siRNA mice and unchanged due to Gcgr siRNA (Figure 3H). Fatty acid and glycerol levels were not elevated in FVII siRNA treated controls and remained unchanged due to Gcgr siRNA (Figure 3G and I). Finally, cholesterol levels were elevated due to Gcgr siRNA compared to FVII siRNA (Figure 3J). Therefore, 10 mg/kg Gcgr siRNA can lower blood glucose and normalize ketones but does not lower triglycerides and increased cholesterol.
Figure 3.
10 mg/kg Gcgr siRNA reduces blood glucose, increases plasma glucagon, normalizes β-hydroxybutyrate, and increases cholesterol in STZ-diabetic mice. Insulin deficient diabetes was induced in mice by injecting 180 mg/kg STZ on day −8; vehicle was administered to non-diabetic controls. On day 0, STZ-diabetic mice were treated with FVII siRNA or Gcgr siRNA at a dose of 10 mg/kg and compared to untreated non-diabetic controls. Body weight (A) and blood glucose (B) were measured throughout the study. Plasma levels of insulin (C), glucagon (D), leptin (E), β-hydroxybutyrate (F), fatty acids (G), triglycerides (H), glycerol (I), and cholesterol (J) were analyzed on day 7. Groups were compared using a 1- or 2-way ANOVA with Tukey post-hoc testing; a, P < 0.05 non-diabetic controls vs FVII siRNA; b, P < 0.05 non-diabetic controls vs Gcgr siRNA; c, P < 0.05 FVII siRNA vs Gcgr siRNA. Statistical analysis was not performed on insulin (C) or leptin (E) measurements as some samples were below the limits of detection, denoted by the dotted lines. In addition, one sample had an insulin measurement too low to be interpolated and was assigned a value of 0. Data are mean ± SEM (A–B) or min to max box and whisker plots (C–I), n = 7–8.
3.4. A single injection of 10 mg/kg Gcgr siRNA reduces blood glucose for 3 weeks and improves oral glucose tolerance in STZ-diabetic mice
Next, we assessed how long a single injection of Gcgr siRNA could improve diabetic symptoms in STZ-diabetic mice. Both STZ-injected groups lost weight compared to non-diabetic controls, and Gcgr siRNA treated mice did not differ from FVII siRNA treated mice (Figure 4A). Gcgr siRNA significantly reduced blood glucose from day 4–29, reaching the lowest value by day 10 (8.2 ± 0.4 mM non-diabetic controls, 23.2 ± 1.6 mM STZ + FVII-siRNA, 10.6 ± 0.4 mM STZ + Gcgr-siRNA, P < 0.05, Figure 4B). Gcgr siRNA also improved blood glucose levels throughout an oral glucose tolerance test; however, no statistical differences in AUC measured from baseline were observed between groups (Figure 4C and D). Therefore, a single injection of Gcgr siRNA was capable of ameliorating blood glucose for over 3 weeks in STZ-diabetic mice.
Figure 4.
A single injection of 10 mg/kg Gcgr siRNA reduces blood glucose for 3 weeks and improves oral glucose tolerance in STZ-diabetic mice. Insulin deficient diabetes was induced in mice by injecting 180 mg/kg STZ on day −22; vehicle was administered to non-diabetic controls. STZ-diabetic mice were treated with FVII siRNA or Gcgr siRNA at a dose of 10 mg/kg on day 0 and compared to untreated non-diabetic controls. Body weight (A) and blood glucose (B) were measured throughout the study. On day 10, an oral glucose tolerance test was performed using 1.5 g/kg of 30% dextrose (C), and area under the curve (AUC) from baseline was calculated (D). Groups were compared using a 1- or 2-way ANOVA with Tukey post-hoc testing; a, P < 0.05 non-diabetic controls vs FVII siRNA; b, P < 0.05 non-diabetic controls vs Gcgr siRNA; c, P < 0.05 FVII siRNA vs Gcgr siRNA. Data are mean ± SEM (A–C) or min to max box and whisker plots (D), n = 5.
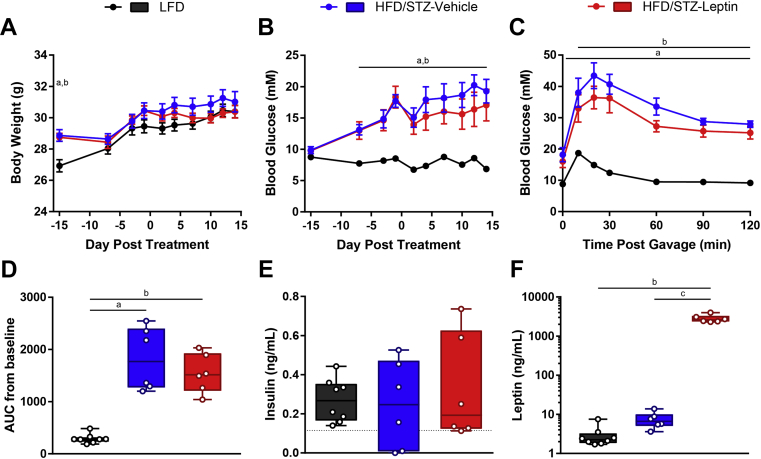
3.5. Leptin therapy does not affect body weight, blood glucose, or oral glucose tolerance in HFD/STZ-diabetic mice
We next assessed the effectiveness of Gcgr siRNA and leptin therapy in the HFD/STZ mouse model of diabetes. HFD/STZ mice are characterized by insulin resistance resulting from HFD feeding and mild β-cell loss due to a moderate dose of STZ, resembling features of type 2 diabetes [34], [35]. HFD/STZ-diabetic mice were put on a 60% HFD at 6 weeks of age and given 100 mg/kg STZ at 10 weeks of age. At 12 weeks of age, we tested leptin therapy by administering daily i.p. injections of PEG-leptin or vehicle to HFD/STZ mice and compared results to 10% LFD controls. We have previously shown that delivery of leptin via mini-osmotic pump and PEG-leptin by daily i.p. injection similarly lower fasting blood glucose in STZ-diabetic mice [19]. HFD mice were initially heavier than LFD controls; however, following STZ treatment, the body weights converged, and there was no effect of leptin vs vehicle (Figure 5A). Although fasting blood glucose tended to be lower in the leptin treated group, there was no significant difference between leptin and vehicle treatment (6.9 ± 0.3 mM LFD, 19.3 ± 1.9 mM HFD/STZ + vehicle, 17.1 ± 2.5 mM HFD/STZ + leptin, day 14, P > 0.05, Figure 5B). Similarly, although blood glucose values were lower in the leptin treated group than in the vehicle treated group at all time points during the oral glucose tolerance test, there were no significant differences between the groups in the glucose response curves or the AUC (Figure 5C and D). Plasma insulin levels were variable within each group, and the range of insulin values in the HFD/STZ groups overlapped with the values of the LFD group (Figure 5E). This leptin injection regimen resulted in extremely high circulating leptin levels (2.9 ± 0.7 ng/mL LFD, 7.5 ± 1.5 ng/mL HFD/STZ + vehicle, 2809.6 ± 259.6 ng/mL HFD/STZ + leptin, day 14, P > 0.05, Figure 5F). Therefore, despite supraphysiological leptin levels, leptin did not significantly lower blood glucose levels in the fasting state or following glucose-gavage in HFD/STZ-diabetic mice, contrary to the potent glucose-lowering effect of leptin in STZ diabetic mice (Figure 2).
Figure 5.
Leptin therapy does not affect body weight, blood glucose, or oral glucose tolerance in HFD/STZ-diabetic mice. Mice on a HFD were injected with 100 mg/kg STZ on day −14. Starting on day 0, HFD/STZ-diabetic mice were injected daily with PEG-leptin at a dose of 20 μg/day or vehicle and compared to untreated LFD fed mice. Body weight (A) and blood glucose (B) were measured throughout the study. On day 7, an oral glucose tolerance test was performed using 1.5 g/kg of 30% dextrose (C), and area under the curve (AUC) from baseline was calculated (D). Plasma levels of insulin (E) and leptin (F) were measured on day 14. Groups were compared using a 1- or 2-way ANOVA with Tukey post-hoc testing; a, P < 0.05 LFD vs vehicle; b, P < 0.05 LFD vs leptin; c, P < 0.05 vehicle vs leptin. Statistical analysis was not performed on insulin (E) measurements as some samples were below the limit of detection, denoted by the dotted line, and one sample was too low to be interpolated and was assigned a value of 0. Data are mean ± SEM (A–C) or min to max box and whisker plots (D–E), n = 6–8.
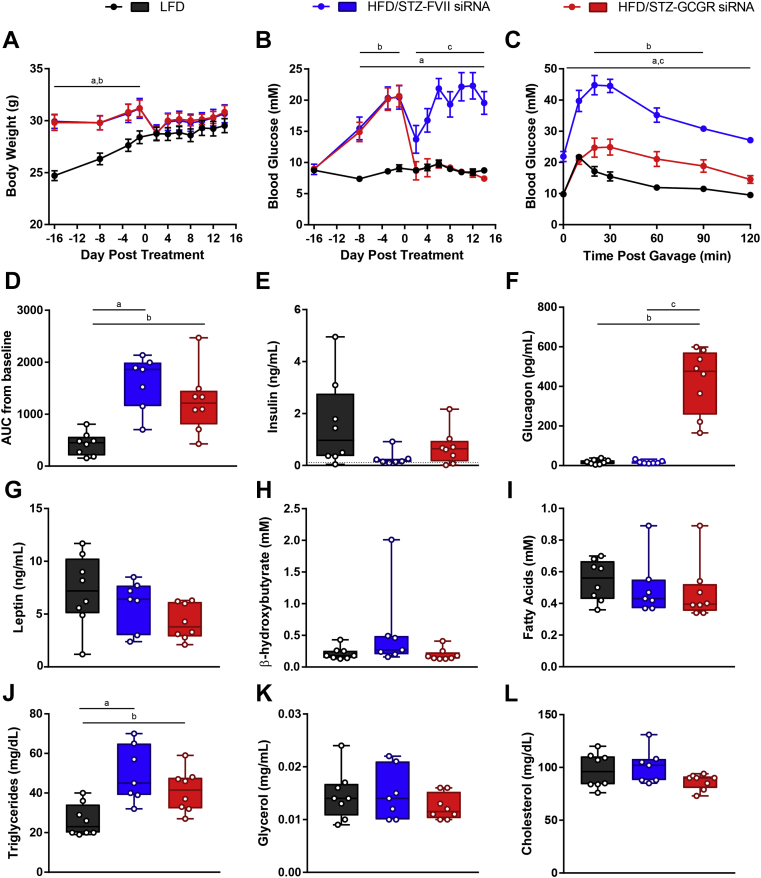
3.6. 10 mg/kg Gcgr siRNA reduces blood glucose and improves oral glucose tolerance in HFD/STZ-diabetic mice
Next, we injected Gcgr siRNA or FVII siRNA at a dose of 10 mg/kg into HFD/STZ to determine if the glucose reducing effect would be as potent as it was in STZ-diabetic mice. The body weights of HFD/STZ-diabetic mice that received Gcgr siRNA were no different than those that received FVII siRNA (Figure 6A). Remarkably, blood glucose was normalized to that of LFD controls due to Gcgr siRNA treatment (8.7 ± 0.4 mM LFD, 19.5 ± 1.8 mM HFD/STZ + FVII siRNA, 7.4 ± 0.4 mM HFD/STZ + Gcgr siRNA, day 14, P < 0.05, Figure 6B). Moreover, blood glucose levels following oral glucose gavage were greatly improved due to Gcgr siRNA compared to FVII siRNA; however, AUC measurements did not differ between the groups (Figure 6C and D). Next, we measured various plasma analytes to better understand how Gcgr siRNA affected metabolic processes in this model. All groups had many plasma insulin levels close to or below the limit of detection of the assay (0.115 ng/mL) (Figure 6E). Similar to our previous studies, Gcgr siRNA induced supraphysiological levels of circulating glucagon (Figure 6F). Plasma leptin, β-hydroxybutyrate, fatty acids, glycerol, and cholesterol were unchanged due to HFD/STZ induced diabetes, and there were no differences between FVII and Gcgr siRNA treated groups (Figure 6G–I,K,L). Lastly, plasma triglycerides were increased in the FVII siRNA group and remained unchanged due to Gcgr siRNA treatment (Figure 6J). Therefore, not only can Gcgr siRNA improve glucose metabolism in the STZ model of severe insulin deficiency, it can also potently lower blood glucose levels in the fasting state and post-glucose gavage in the HFD/STZ model of type 2 diabetes, although it does not improve plasma triglycerides.
Figure 6.
10 mg/kg Gcgr siRNA reduces blood glucose and improves oral glucose tolerance in HFD/STZ-diabetic mice. Mice on a HFD were injected with 100 mg/kg STZ on day −15. HFD/STZ-diabetic mice were treated with FVII siRNA or Gcgr siRNA at a dose of 10 mg/kg on day 0 and compared to untreated LFD fed mice. Body weight (A) and blood glucose (B) were measured throughout the study. On day 6, an oral glucose tolerance test was performed using 1.5 g/kg of 30% dextrose (C), and area under the curve (AUC) from baseline was calculated (D). Plasma levels of insulin (E), glucagon (F), leptin (G), β-hydroxybutyrate (H), fatty acids (I), triglycerides (J), glycerol (K), and cholesterol (L) were analyzed on day 14. Groups were compared using a 1- or 2-way ANOVA with Tukey post-hoc testing; a, P < 0.05 LFD vs FVII siRNA; b, P < 0.05 LFD vs Gcgr siRNA; c, P < 0.05 FVII siRNA vs Gcgr siRNA. Statistical analysis was not performed on insulin (E) measurements as some samples were below the limit of detection, denoted by the dotted line. Data are mean ± SEM (A–C) or min to max box and whisker plots (D–K), n = 7–8.
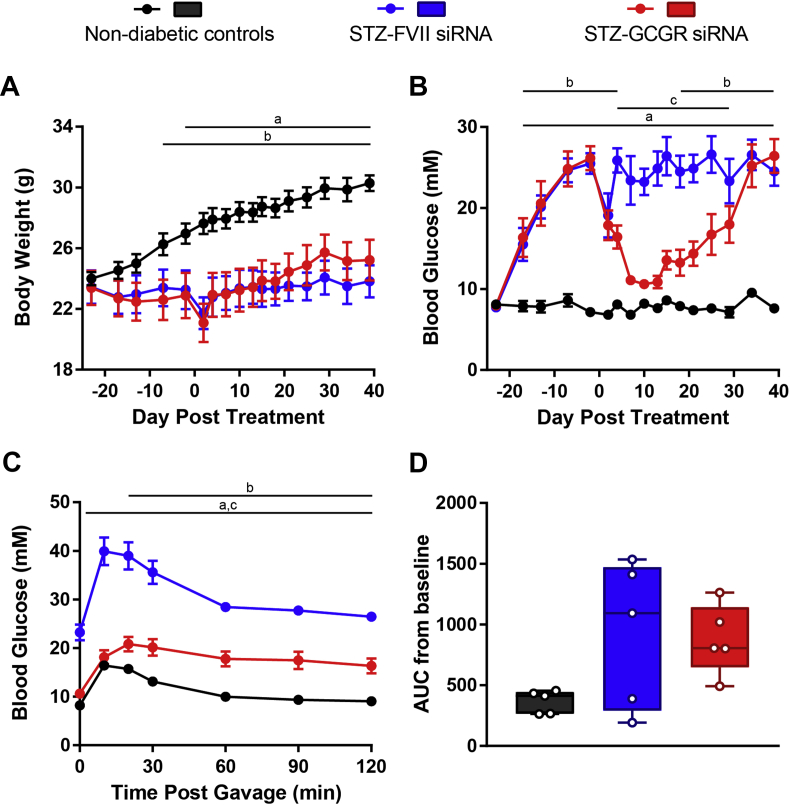
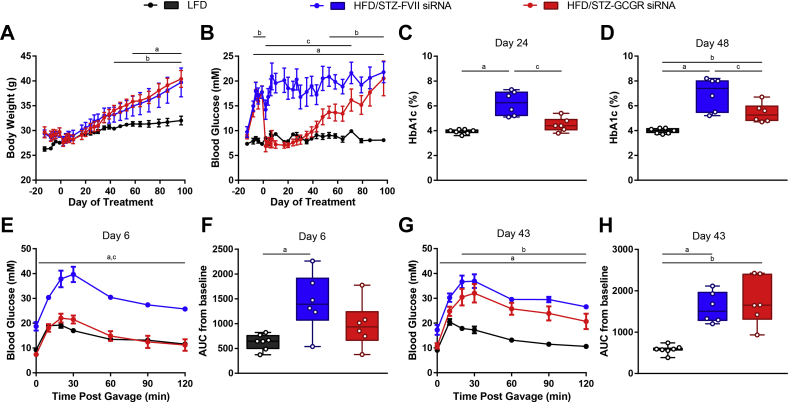
3.7. A single injection of 10 mg/kg Gcgr siRNA reduces blood glucose for 2 months and improves oral glucose tolerance in HFD/STZ-diabetic mice
Because Gcgr siRNA can robustly normalize diabetic symptoms in HFD/STZ mice, we aimed to determine how long a single injection could lower blood glucose in this model. Similar to our previous studies, there were no differences in body weight between Gcgr siRNA and FVII siRNA treated mice (Figure 7A). Strikingly, fasting blood glucose levels in the Gcgr siRNA treated group were indistinguishable from those in LFD controls for approximately 1 month (8.6 ± 0.5 mM LFD, 16.8 ± 2.4 mM HFD/STZ + FVII siRNA, 8.2 ± 0.9 mM HFD/STZ + Gcgr siRNA, day 30, P < 0.05) and remained significantly reduced compared to FVII siRNA controls until day 71 (Figure 7B). Due to the long-term amelioration in blood glucose, we measured HbA1c on day 24 and 48 and found that levels were reduced in mice that received Gcgr siRNA (Figure 7C and D). On day 6, mice receiving Gcgr siRNA had normalized blood glucose levels during an oral glucose tolerance (Figure 7E), and this effect waned by day 43 (Figure 7G). No differences were observed between AUC measurements on either day 6 or 43 between Gcgr siRNA and FVII siRNA treated groups (Figure 7F and H). Therefore, a single injection of Gcgr siRNA can reduce fasting blood glucose for 2 months, improve oral glucose tolerance, and reduce HbA1c levels in HFD/STZ mice.
Figure 7.
A single injection of 10 mg/kg Gcgr siRNA reduces blood glucose for 2 months and improves oral glucose tolerance in HFD/STZ-diabetic mice. Mice on a HFD were injected with 100 mg/kg STZ on day −13. HFD/STZ-diabetic mice were treated with FVII siRNA or Gcgr siRNA at a dose of 10 mg/kg on day 0 and compared to untreated LFD fed mice. Body weight (A) and blood glucose (B) were measured throughout the study. HbA1c was analyzed on day 24 (C) and 48 (D). On day 6, an oral glucose tolerance test was performed using 1.5 g/kg of 30% dextrose (E), and area under the curve (AUC) from baseline was calculated (F). On day 43, an oral glucose tolerance test was performed using 1.5 g/kg of 30% dextrose (G), and area under the curve (AUC) was calculated (H). Groups were compared using a 1- or 2-way ANOVA with Tukey post-hoc testing; a, P < 0.05 LFD vs FVII siRNA; b, P < 0.05 LFD vs Gcgr siRNA; c, P < 0.05 FVII siRNA vs Gcgr siRNA. Data are mean ± SEM (A, B, E, G) or min to max box and whisker plots (C, D, F, H), n = 6–7.
4. Discussion
We report that administration of Gcgr siRNA encapsulated in LNPs can reduce blood glucose levels in the fasting state or post-glucose gavage in wildtype mice and dramatically improve glucose metabolism in STZ and HFD/STZ-diabetic mice, models of type 1 and type 2 diabetes [34], [35], respectively. The phenotype in our acute knockdown model closely resembles that of GcgrKO mice, suggesting the highly efficient knockdown of the Gcgr. GcgrKO mice exhibited a ∼30% reduction in blood glucose levels compared to GcgrWT mice [29], while mice receiving Gcgr siRNA exhibited a reduction of ∼40% in blood glucose levels compared to FVII siRNA controls. While GcgrKO mice had an excess of 250-fold higher circulating glucagon levels concomitant with extreme α-cell hyperplasia [29], a ∼5 fold increase in plasma glucagon with no alteration in α-cell area was observed on day 7 of our study. This may reflect the acute nature of our knockdown model and suggests that the rise in circulating glucagon in our study may be due to increased glucagon transcription, translation, and/or secretion, whereas increased α-cell mass likely contributes to hyperglucagonemia in GcgrKO mice. We used C57BL/6J mice, which are known for relatively high fasting blood glucose levels compared to other strains of mice [36]; thus, it is unclear whether Gcgr siRNA would have decreased blood glucose levels to the same extent in other strains.
Following characterization of our knockdown model in wildtype mice, we found that Gcgr siRNA diminished blood glucose levels in the fasting state as well as post-glucose gavage in insulin-deficient STZ-diabetic mice. Increasing the dose of Gcgr siRNA from 5 to 10 mg/kg further improved glucose homeostasis, and a single injection reduced blood glucose for 3 weeks. These results, along with other reports of diabetes normalization or prevention using GcgrKO or glucagon/Gcgr antagonizing antibodies in rodent models of type 1 diabetes, have challenged the insulinocentric dogma of diabetes by suggesting that glucagon excess rather than insulin deficiency is critical for the development of diabetes. However, recent reports demonstrate that as insulin deficiency becomes more severe, glucagon blockage is less efficacious. Steenberg et al. suppressed glucagon action through α-cell ablation by giving diphtheria toxin (DT) to mice expressing diphtheria toxin receptor (DTR) driven by the glucagon promoter, glucagon immunoneutralization, or Gcgr antagonism and found that STZ injection was still capable of inducing hyperglycemia and impaired glucose tolerance [37]. In addition, Damond et al. generated mouse models with more severe insulin deficiency by using a combining STZ injection with insulin receptor antagonism as well as β-cell ablation by giving DT to mice expressing DTR driven by the insulin promoter [38]. When these mice were put on a GcgrKO background or treated with a Gcgr monoclonal antibody, hyperglycemia still developed [38]. Finally, in insulin KO mice completely devoid of insulin, Gcgr gene deletion did not prevent the development of diabetes [39]. Taken together, these studies suggest that sufficient levels of basal insulin may be required for glucagon suppression to be therapeutic for type 1 diabetes.
In addition to lowering blood glucose, Gcgr siRNA also potently suppressed ketones but did not affect lipid metabolism in STZ-diabetic mice. Strikingly, the effect on ketosis may be even more potent than the effect on blood glucose, since the lower dose of siRNA, which only modestly reduced blood glucose, was capable of restoring ketones to control levels. This phenomenon has been observed previously when STZ-diabetic mice were given a GLP-1R agonist or glucagon-neutralizing antibody, which reversed hyperglucagonemia, and blood glucose levels were unchanged while ketones were lowered [40]. However, at both doses of Gcgr siRNA, plasma triglycerides, plasma fatty acids, and glycerol were unchanged, and plasma cholesterol was increased. Interestingly, clinical trials of type 2 diabetic patients receiving Gcgr antagonists [41], [42] as well as a study involving db/db mice receiving Gcgr siRNA [11] have reported increased low-density lipoprotein cholesterol (LDL-C). We only observed an increase in total plasma cholesterol due to Gcgr siRNA in STZ-diabetic mice and not HFD/STZ-diabetic mice, but did not measure the individual lipoprotein components. In contrast to Gcgr siRNA treatment, leptin therapy suppressed plasma ketones, fatty acids, triglycerides, glycerol, and cholesterol, consistent with previous studies [13], [14], [17], [18], [20], [32]. Therefore, this suggests that glucagon reduction by leptin may contribute to improving hyperglycemia and ketosis but not to the suppression of lipid metabolism.
Since leptin is a potent glucagon suppressor, it has been postulated that reduced glucagon action contributes to the glucose-lowering effect of leptin therapy in insulin-deficient diabetes [13], [14]. However, several lines of evidence counter this idea. A low dose of leptin in rats normalized plasma glucagon levels but only slightly lowered blood glucose levels [43]. In addition, while leptin treatment in rats normalized blood glucose within 6 h, glucagon was only suppressed by 24 h of leptin therapy, thereby temporally disconnecting the two effects [17]. Finally, the importance of inhibited lipolysis, reduced plasma fatty acids and glycerol, and thus reduced gluconeogenesis underlying the glucose-lowering mechanism of leptin have been demonstrated in mice and rats [17], [18], [32]. We report that fatty acids and glycerol levels are reduced with leptin therapy, but this effect was not seen following injection of Gcgr siRNA. Therefore, while inhibiting glucagon action alone may be beneficial in type 1 diabetes, leptin action exerts additional effects which can reduce hyperglycemia.
After observing the glucose-lowering effect of Gcgr siRNA in STZ diabetes, we sought to determine whether this effect could be extended to a model of type 2 diabetes, the HFD/STZ mouse. Strikingly, a single injection of Gcgr siRNA reduced fasting blood glucose levels for approximately 2 months and improved HbA1c levels in this model. In addition, in contrast to the beneficial effects of leptin monotherapy in STZ-diabetic mice, leptin treatment in HFD/STZ mice did not improve diabetic symptoms. Despite immensely high leptin levels, there was only a trend towards an improvement in blood glucose levels and oral glucose tolerance. Other studies have reported that leptin therapy in HFD/STZ mice caused a partial improvement in blood glucose and glucose tolerance [44], [45]. Discrepancies between these studies and ours may be due to differences in the timing of STZ relative to HFD treatment, the dose of STZ, the % of fat in the diet, or the method of leptin delivery [44], [45]. Nonetheless, a dose of leptin that would have normalized hyperglycemia in STZ diabetic mice does not normalize diabetic symptoms in HFD/STZ diabetic mice, possibly due to leptin resistance. Similar to obese humans with type 2 diabetes, HFD/STZ mice exhibited 2.5-fold elevated leptin levels compared to LFD controls, which may suggest leptin resistance. Several pre-clinical studies have identified strategies to enhance endogenous leptin sensitivity [44], [46], [47], [48], [49], [50] which may help overcome leptin resistance and unleash the body weight and blood glucose lowering effects of leptin.
Liver specific suppression of glucagon action may be more beneficial than whole body inhibition in treating metabolic disease. Liver specific GcgrKO mice exhibit improvements in fasting blood glucose and glucose tolerance to the same degree as that seen in whole body GcgrKO mice [29], [30], highlighting the importance of glucagon action on the liver and glucose homeostasis. Interestingly, outside the liver, glucagon can exert beneficial effects in the brain by inhibiting glucose production, improving glucose tolerance [51], promoting satiety, and increasing energy expenditure, in the muscle by promoting glucose uptake, and in WAT by increasing lipolysis [52], [53]. Therefore, administration of Gcgr siRNA, which largely targets the liver and increases circulating glucagon levels, could elicit added benefits of extra-hepatic glucagon signaling compared to a Gcgr antagonist targeting glucagon receptors throughout the body.
Taken together, our results indicate that Gcgr siRNA encapsulated in LNPs is an effective therapy in mouse models of type 1 and type 2 diabetes. Utilization of small molecule Gcgr antagonists for the treatment of glycemic control has been of interest and is currently in phase 2 clinical trials for patients with type 2 diabetes (clinicaltrials.gov, NCT02851849). In addition, LNP delivery of siRNA is currently in clinical trials for the treatment of various liver-related diseases. Therefore, Gcgr siRNA delivery via LNPs may hold therapeutic potential for the treatment of diabetes in humans.
Author contributions
U.H.N., and T.J.K. designed the experiments. U.H.N. and J.S.S.H. performed experiments. S.C., Y.Y.C.T, and P.R.C. provided the LNP-siRNA. U.H.N. analyzed data and wrote the initial manuscript. All authors were involved in the discussion and revision of the manuscript. T.J.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgements
We thank Ali Asadi and Shannon O'Dwyer for technical assistance with immunofluorescence and animal work, and Dr. Maria Glavas for assistance with editing the manuscript. This work was supported by the Canadian Institutes of Health Research. U.H.N. was supported by the Natural Sciences and Engineering Research Council of Canada and J.S.S.H. was supported by the Kinsley Brotherton McLeod Endowment and the Florence & George Heighway Endowment Fund.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.06.012.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Raskin P., Unger R.H. Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. The New England Journal of Medicine. 1978;299:433–436. doi: 10.1056/NEJM197808312990901. [DOI] [PubMed] [Google Scholar]
- 2.Dinneen S., Alzaid A., Turk D., Rizza R. Failure of glucagon suppression contributes to postprandial hyperglycaemia in IDDM. Diabetologia. 1995;38:337–343. doi: 10.1007/BF00400639. [DOI] [PubMed] [Google Scholar]
- 3.Baron A.D., Schaeffer L., Shragg P., Kolterman O.G. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes. 1987;36:274–283. doi: 10.2337/diab.36.3.274. [DOI] [PubMed] [Google Scholar]
- 4.Reaven G.M., Chen Y.D., Golay A., Swislocki A.L., Jaspan J.B. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. The Journal of Clinical Endocrinology and Metabolism. 1987;64:106–110. doi: 10.1210/jcem-64-1-106. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y., Berglund E.D., Wang M.Y., Fu X., Yu X., Charron M.J. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14972–14976. doi: 10.1073/pnas.1205983109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Y., Wang M.Y., Du X.Q., Charron M.J., Unger R.H. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60:391–397. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand C.L., Jorgensen P.N., Svendsen I., Holst J.J. Evidence for a major role for glucagon in regulation of plasma glucose in conscious, nondiabetic, and alloxan-induced diabetic rabbits. Diabetes. 1996;45:1076–1083. doi: 10.2337/diab.45.8.1076. [DOI] [PubMed] [Google Scholar]
- 8.Wang M.Y., Yan H., Shi Z., Evans M.R., Yu X., Lee Y. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2503–2508. doi: 10.1073/pnas.1424934112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorensen H., Brand C.L., Neschen S., Holst J.J., Fosgerau K., Nishimura E. Immunoneutralization of endogenous glucagon reduces hepatic glucose output and improves long-term glycemic control in diabetic ob/ob mice. Diabetes. 2006;55:2843–2848. doi: 10.2337/db06-0222. [DOI] [PubMed] [Google Scholar]
- 10.Conarello S.L., Jiang G., Mu J., Li Z., Woods J., Zycband E. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia. 2007;50:142–150. doi: 10.1007/s00125-006-0481-3. [DOI] [PubMed] [Google Scholar]
- 11.Han S., Akiyama T.E., Previs S.F., Herath K., Roddy T.P., Jensen K.K. Effects of small interfering RNA-mediated hepatic glucagon receptor inhibition on lipid metabolism in db/db mice. Journal of Lipid Research. 2013;54:2615–2622. doi: 10.1194/jlr.M035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y., Berglund E.D., Yu X., Wang M.Y., Evans M.R., Scherer P.E. Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1409638111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu X., Park B.H., Wang M.Y., Wang Z.V., Unger R.H. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M.Y., Chen L., Clark G.O., Lee Y., Stevens R.D., Ilkayeva O.R. Leptin therapy in insulin-deficient type I diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4813–4819. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujikawa T., Chuang J.C., Sakata I., Ramadori G., Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17391–17396. doi: 10.1073/pnas.1008025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujikawa T., Berglund E.D., Patel V.R., Ramadori G., Vianna C.R., Vong L. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metabolism. 2013;18:431–444. doi: 10.1016/j.cmet.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perry R.J., Zhang X.M., Zhang D., Kumashiro N., Camporez J.P., Cline G.W. Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nature Medicine. 2014;20:759–763. doi: 10.1038/nm.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry R.J., Peng L., Abulizi A., Kennedy L., Cline G.W., Shulman G.I. Mechanism for leptin's acute insulin-independent effect to reverse diabetic ketoacidosis. Journal of Clinical Investigation. 2017;127:657–669. doi: 10.1172/JCI88477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neumann U.H., Denroche H.C., Mojibian M., Covey S.D., Kieffer T.J. Insulin knockout mice have extended survival but volatile blood glucose levels on leptin therapy. Endocrinology. 2016;157:1007–1012. doi: 10.1210/en.2015-1890. [DOI] [PubMed] [Google Scholar]
- 20.Denroche H.C., Levi J., Wideman R.D., Sequeira R.M., Huynh F.K., Covey S.D. Leptin therapy reverses hyperglycemia in mice with streptozotocin-induced diabetes, independent of hepatic leptin signaling. Diabetes. 2011;60:1414–1423. doi: 10.2337/db10-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.German J.P., Thaler J.P., Wisse B.E., Oh I.S., Sarruf D.A., Matsen M.E. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology. 2011;152:394–404. doi: 10.1210/en.2010-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummings B.P., Bettaieb A., Graham J.L., Stanhope K.L., Dill R., Morton G.J. Subcutaneous administration of leptin normalizes fasting plasma glucose in obese type 2 diabetic UCD-T2DM rats. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14670–14675. doi: 10.1073/pnas.1107163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mittendorfer B., Horowitz J.F., DePaoli A.M., McCamish M.A., Patterson B.W., Klein S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes. 2011;60:1474–1477. doi: 10.2337/db10-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon H.S., Matarese G., Brennan A.M., Chamberland J.P., Liu X., Fiorenza C.G. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60:1647–1656. doi: 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caro J.F., Kolaczynski J.W., Nyce M.R., Ohannesian J.P., Opentanova I., Goldman W.H. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 26.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Advance Drug Delivery Reviews. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 27.Jayaraman M., Ansell S.M., Mui B.L., Tam Y.K., Chen J., Du X. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angewandte Chemie (International Edition in English) 2012;51:8529–8533. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semple S.C., Akinc A., Chen J., Sandhu A.P., Mui B.L., Cho C.K. Rational design of cationic lipids for siRNA delivery. Nature Biotechnology. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 29.Gelling R.W., Du X.Q., Dichmann D.S., Romer J., Huang H., Cui L. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longuet C., Robledo A.M., Dean E.D., Dai C., Ali S., McGuinness I. Liver-specific disruption of the murine glucagon receptor produces alpha-cell hyperplasia: evidence for a circulating alpha-cell growth factor. Diabetes. 2013;62:1196–1205. doi: 10.2337/db11-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann U.H., Chen S., Tam Y.Y., Baker R.K., Covey S.D., Cullis P.R. IGFBP2 is neither sufficient nor necessary for the physiological actions of leptin on glucose homeostasis in male ob/ob mice. Endocrinology. 2014;155:716–725. doi: 10.1210/en.2013-1622. [DOI] [PubMed] [Google Scholar]
- 32.Denroche H.C., Kwon M.M., Quong W.L., Neumann U.H., Kulpa J.E., Karunakaran S. Leptin induces fasting hypoglycaemia in a mouse model of diabetes through the depletion of glycerol. Diabetologia. 2015;58:1100–1108. doi: 10.1007/s00125-015-3529-4. [DOI] [PubMed] [Google Scholar]
- 33.Denroche H.C., Kwon M.M., Glavas M.M., Tuduri E., Philippe M., Quong W.L. The role of autonomic efferents and uncoupling protein 1 in the glucose-lowering effect of leptin therapy. Molecular Metabolism. 2016;5:716–724. doi: 10.1016/j.molmet.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert E.R., Fu Z., Liu D. Development of a nongenetic mouse model of type 2 diabetes. Experimental Diabetes Research. 2011;2011 doi: 10.1155/2011/416254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skovso S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. Journal of Diabetes Investigation. 2014;5:349–358. doi: 10.1111/jdi.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berglund E.D., Li C.Y., Poffenberger G., Ayala J.E., Fueger P.T., Willis S.E. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes. 2008;57:1790–1799. doi: 10.2337/db07-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steenberg V.R., Jensen S.M., Pedersen J., Madsen A.N., Windelov J.A., Holst B. Acute disruption of glucagon secretion or action does not improve glucose tolerance in an insulin-deficient mouse model of diabetes. Diabetologia. 2016;59:363–370. doi: 10.1007/s00125-015-3794-2. [DOI] [PubMed] [Google Scholar]
- 38.Damond N., Thorel F., Moyers J.S., Charron M.J., Vuguin P.M., Powers A.C. Blockade of glucagon signaling prevents or reverses diabetes onset only if residual beta-cells persist. Elife. 2016;5:e13828. doi: 10.7554/eLife.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neumann U.H., Ho J.S., Mojibian M., Covey S.D., Charron M.J., Kieffer T.J. Glucagon receptor gene deletion in insulin knockout mice modestly reduces blood glucose and ketones but does not promote survival. Molecular Metabolism. 2016;5:731–736. doi: 10.1016/j.molmet.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meek T.H., Dorfman M.D., Matsen M.E., Fischer J.D., Cubelo A., Kumar M.R. Evidence that in uncontrolled diabetes, hyperglucagonemia is required for ketosis but not for increased hepatic glucose production or hyperglycemia. Diabetes. 2015;64:2376–2387. doi: 10.2337/db14-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kazierad D.J., Bergman A., Tan B., Erion D.M., Somayaji V., Lee D.S. Effects of multiple ascending doses of the glucagon receptor antagonist PF-06291874 in patients with type 2 diabetes mellitus. Diabetes, Obesity & Metabolism. 2016;18:795–802. doi: 10.1111/dom.12672. [DOI] [PubMed] [Google Scholar]
- 42.Engel S.S., Xu L., Andryuk P.J., Davies M.J., Amatruda J., Kaufman K. Efficacy and tolerability of MK-0893, a glucagon receptor antagonist (GRA), in patients with type 2 diabetes (T2DM) Diabetes. 2011 [Google Scholar]
- 43.German J.P., Wisse B.E., Thaler J.P., Oh I.S., Sarruf D.A., Ogimoto K. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes. 2010;59:1626–1634. doi: 10.2337/db09-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakai T., Kusakabe T., Ebihara K., Aotani D., Yamamoto-Kataoka S., Zhao M. Leptin restores the insulinotropic effect of exenatide in a mouse model of type 2 diabetes with increased adiposity induced by streptozotocin and high-fat diet. American Journal of Physiology. Endocrinology and Metabolism. 2014;307:E712–E719. doi: 10.1152/ajpendo.00272.2014. [DOI] [PubMed] [Google Scholar]
- 45.Kusakabe T., Tanioka H., Ebihara K., Hirata M., Miyamoto L., Miyanaga F. Beneficial effects of leptin on glycaemic and lipid control in a mouse model of type 2 diabetes with increased adiposity induced by streptozotocin and a high-fat diet. Diabetologia. 2009;52:675–683. doi: 10.1007/s00125-009-1258-2. [DOI] [PubMed] [Google Scholar]
- 46.Liu J., Lee J., Hernandez Salazar, Mazitschek R., Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161:999–1011. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J., Liu J., Feng X., Hernandez Salazar M.A., Mucka P., Ibi D. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nature Medicine. 2016;22:1023–1032. doi: 10.1038/nm.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth J.D., Roland B.L., Cole R.L., Trevaskis J.L., Weyer C., Koda J.E. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller T.D., Sullivan L.M., Habegger K., Yi C.X., Kabra D., Grant E. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. Journal of Peptide Science. 2012;18:383–393. doi: 10.1002/psc.2408. [DOI] [PubMed] [Google Scholar]
- 50.Clemmensen C., Chabenne J., Finan B., Sullivan L., Fischer K., Kuchler D. GLP-1/glucagon coagonism restores leptin responsiveness in obese mice chronically maintained on an obesogenic diet. Diabetes. 2014;63:1422–1427. doi: 10.2337/db13-1609. [DOI] [PubMed] [Google Scholar]
- 51.Mighiu P.I., Yue J.T., Filippi B.M., Abraham M.A., Chari M., Lam C.K. Hypothalamic glucagon signaling inhibits hepatic glucose production. Nature Medicine. 2013;19:766–772. doi: 10.1038/nm.3115. [DOI] [PubMed] [Google Scholar]
- 52.Habegger K.M., Heppner K.M., Geary N., Bartness T.J., DiMarchi R., Tschop M.H. The metabolic actions of glucagon revisited. Nature Reviews. Endocrinology. 2010;6:689–697. doi: 10.1038/nrendo.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muller T.D., Finan B., Clemmensen C., DiMarchi R.D., Tschop M.H. The new biology and pharmacology of glucagon. Physiological Reviews. 2017;97:721–766. doi: 10.1152/physrev.00025.2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.