Abstract
Background:
The incidence of mosquito-borne diseases and the resistance of mosquitoes to conventional pesticides have recently caused a panic to the authorities in the endemic countries. This study was conducted to identify native larvicidal biopesticides against Culex pipiens for utilization in the battle against mosquito-borne diseases.
Methods:
Larvicidal activities of new indigenous Bacillus thuringiensis isolates and crude toxin complexes (TCs) of two nematode bacterial-symbionts, Photorhabdus luminescens akhurstii (HRM1) and Ph. luminescens akhurstii (HS1) that tested against Cx. pipiens. B. thuringiensis isolates were recovered from different environmental samples in Saudi Arabia, and the entomopathogenic nematodes, Heterorhabditis indica (HRM1) and He. sp (HS1) were isolated from Egypt. Larvicidal activities (LC50 and LC95) of the potentially active B. thuringiensis strains or TCs were then evaluated at 24 and 48h post-treatment.
Results:
Three B. thuringiensis isolates were almost as active as the reference B. thuringiensis israelensis (Bti-H14), and seven isolates were 1.6–5.4 times more toxic than Bti-H14. On the other hand, the TCs of the bacterial symbionts, HRM1 and HS1, showed promising larvicidal activities. HS1 showed LC50 of 2.54 folds that of HRM1 at 24h post-treatment. Moreover, histopathological examinations of the HS1-treated larvae showed deformations in midgut epithelial cells at 24h post-treatment.
Conclusion:
Synergistic activity and molecular characterization of these potentially active biocontrol agents are currently being investigated. These results may lead to the identification of eco-friend mosquito larvicidal product(s) that could contribute to the battle against mosquito-borne diseases.
Keywords: Bacillus thuringiensis, Culex pipiens, Biopesticide, Photorhabdus bacteria, Heterorhabditis nematodes
Introduction
Mosquitoes are the most dangerous insect pests that affect humans and animals world wide as they transmit epidemic and fatal diseases (WHO 2010, Aziz et al. 2014). They transmit pathogens of Filariasis, Rift Valley fever, West Nile virus, and encephalitis (Meyer et al. 1983, Rosen et al. 1989, Omar 1996, Balenghien et al. 2008, Seufi and Galal 2010). In Saudi Arabia, different local mosquito vectors are spread all over the country (Al-Khuriji 2007, Al-Ghamdi et al. 2008, Ahmed et al. 2011, Al-Ahmed 2012). These vectors transmit mosquito-borne diseases including dengue fever (Khan et al. 2008, Alwafi et al. 2013, Aziz et al. 2014, Ayaad et al. 2015), filaria (Hawking 1973), malaria (Balkhy and Memish 2003, Madani 2005) and Rift Valley fever (Jup et al. 2002, Al-Hazmi et al. 2003 and Madani 2005). Beside the recent outbreak of mosquitoes and incidence of epidemic diseases, resistance of local mosquitoes to conventional pesticides has been recorded (Al-Sarar 2010, Osta et al. 2012, Brouqui et al. 2012), which have caused a panic to the authorities.
Until now, conventional pesticides are the main tool being used to combat mosquitoes. However, synthetic pesticides cause health problems and pollute the environment (Azmi et al. 2009). Moreover, some synthetic mosquito repellents cause encephalopathy in children (Briassoulis 2001). Therefore, there is an urgent need for effective and safe alternatives to the conventional pesticides. Considering natural insecticides, essential oils are being used against adult mosquitoes (Bernier et al. 2005, Maguranyi et al. 2009), however, they repel, but do not kill, mosquitoes. Bacillus thuringiensis, the most successful bioinsecticide, have been used to combat mosquito larvae for 3 decades and until now (Heimpel and Angus 1959, Ali et al. 2010, Schünemann et al. 2014). This bacterium has many advantages over conventional pesticides as it is specific to certain pest species, eco-friend and safe to non-target organisms, and mosquitoes cannot develop significant resistance to it in the field so far (Bravo et al. 2007).
On the other hand, entomopathogenic nematodes, with their symbiotic bacteria, also introduce promising solution as biocontrol agent for pest control management (Lang et al. 2011, Zhu et al. 2011). The infective juveniles of the two genera Steinernema or Heterorhabditis are active in seeking the host as they penetrate via the host’s natural opening and immigrate to the insect haemocoel then, release their symbiotic bacteria, Xenorhabdus or Photorhabdus respectively. Multiplication of these bacteria within insect haemocoel results in production of numbers of virulence factors, including toxins complexes that kill the insect host within 48h (Ffrench-Constant and Bowen, 2000, Ffrench-Constant et al. 2007, Eleftherianos et al. 2010).
The current study herein was conducted for laboratory assessment of the mosquito larvicidal activity of local indigenous B. thuringiensis isolates, as well as TCs from the nematode bacterial symbionts, Photorhabdus, as new proposed candidates to be utilized in the battle against mosquito vectors.
Materials and Methods
Experimental mosquitoes
A susceptible strain of Culex pipiens was reared in Zoology Department, King Saud University for use in this study. Mosquitoes were reared in the lab for 10 generations according to Ahmed et al. (1999) before performing experiments. Briefly, adults were maintained at 26±1 °C and 12:12h (light: dark) photoperiod and provided with 10% glucose solution ad libitum. Females were blood-fed on CD mice to lay eggs, and hatched larvae were fed on ’Liquifry’ (Interpet Ltd, Dorking, UK) for two days, then provided with ground ’TetraMin’ flake food (Tetra Werke, Melle, Germany) until pupation. Third-instar larvae were subjected to bioassay using B. thuringiensis isolates or TCs of Photorhabdus.
Collection of environmental samples
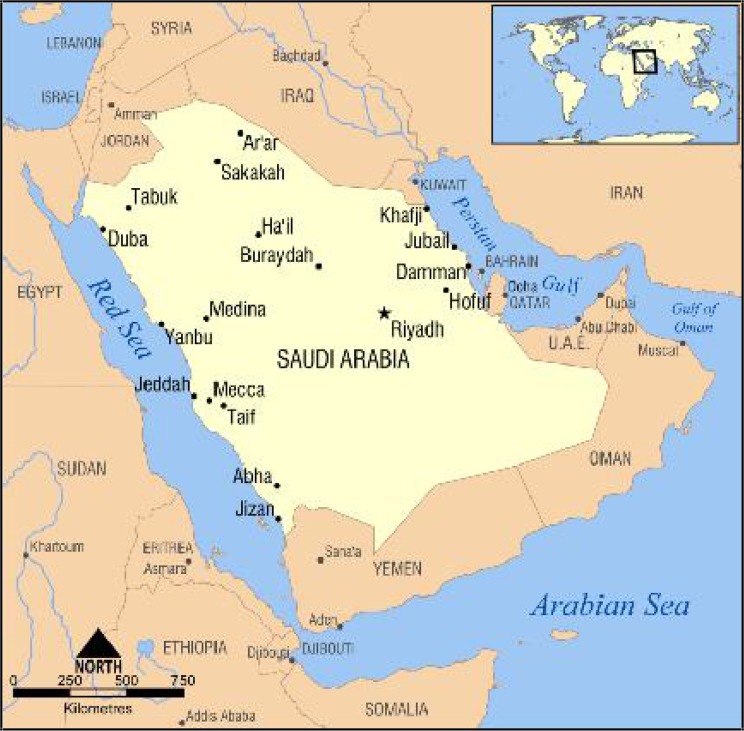
Experimental samples were collected from different regions across Saudi Arabia from October 2013 to march, 2014 (Table 1 and Fig. 1). Samples were collected from a variety of sites within each region including the vicinity of houses, irrigated parks, gardens and farms as well as from the surrounding semi-desert areas. A total of 300 samples of different types were collected including soil, water and dead plants and animals (Table 1 and Fig. 2). For soil sampling, the soil surface was scraped and about 10g of material were taken from a depth of 2–5cm into sterile containers. Plant parts were collected in sterile plastic bags. Samples were also taken from water, dead fish, insects, and snails, beeswax, as well as from dried faeces and stored in 10ml sterile test tubes. Samples were processed for B. thuringiensis isolation according to Bozlagan et al. (2010).
Table 1.
Overall characteristics and biochemical profiles of 10 potential larvicidal native Bacilus thuringiensis isolates, from different regional sources, and the Bti-H14 reference strain
| B. thuringiensis code/City regional source | Catalase, oxidase, lecithinase, esculin and gelatin hydrolysis, hemolytic and motility activities, citrate utilization, nitrate-reduction, and VP test | Urease, H2S, NPG, and indole tests | Acid produced from starch, glycogen, glucose, fructose, mannose, or maltose | No acid from inulin, xylose, galactose, lactose, salicin, mannitol, or sucrose* |
|---|---|---|---|---|
| Bti-H14 | + | − | + | − |
| Bt-12/Yanbu | + | − | + | − |
| Bt-26/Medina | + | − | + | − |
| Bt-29/Medina | + | − | + | − |
| Bt-42/Jizan | + | − | + | − |
| Bt-44/Jizan | + | − | + | − |
| Bt-53/Medina | + | − | + | − |
| Bt-55/Medina | + | − | + | − |
| Bt-60/Medina | + | − | + | + |
| Bt-63/Mecca | + | − | + | − |
| Bt-68/Medina | + | − | + | − |
The isolate Bt-63 produced acid from sucrose
Fig. 1.
A map of Saudi Arabia showing the geographical distribution of the 10 potentially larvicidal effective Bacilus thuringiensis isolates (Jizan, Madina, Mecca and Yanbu) across Saudi Arabia
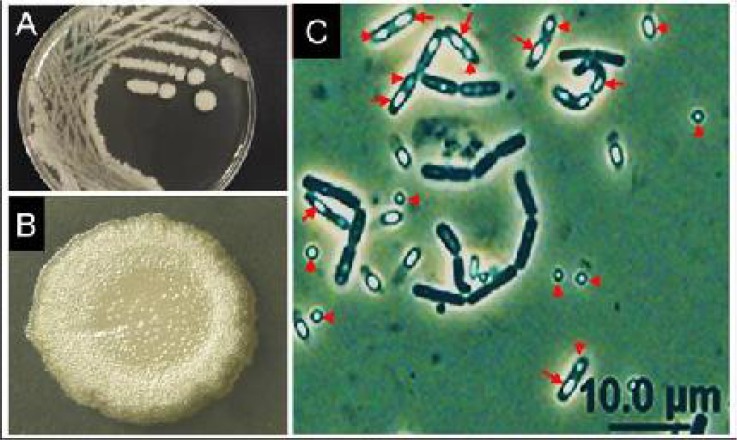
Fig. 2.
Photomicrographs showing morphology of the native Bt-55 isolate seeded in nutrient-supplemented agar media. A–B: colonies and a single magnified colony showing white, raised-centrally, nearly-circular, and glossy colony morphology with fine irregular margins similar to that of Bti-H14. (Photos A and B by AMA). C: Phase-contrast microscopy (×1000) illustrating the parasporal crystal (arrow heads) and bacterial spores (arrows) which appear brighter
Isolation, culturing and identification of B. thuringiensis isolates
Sixty-eight B. thuringiensis isolates were recovered from different environmental samples (El-Kersh et al. 2016) according to Hong et al. (2009) and El-Kersh et al. (2012) from different locations throughout Saudi Arabia (Fig. 1). Briefly, for soil samples, one gram was grinded and added to 2.0ml of sterile distilled water and suspended vigorously. One gram of dead insects, fish guts and gills, or bees wax samples was dispatched and then macerated in 2.0ml sterile saline (0.5%, w/v), using a sterilized mortar and pestle. Two ml aliquots from water samples were mixed with 2ml absolute ethanol, mixed well for 1min, incubated for 45min at 30 °C, with shaking from time to time. After ethanol treatment, ten-fold serial dilutions were made in sterile distilled water. Appropriate dilutions were spread on a nutrient agar medium supplemented with 0.2% yeast extract (Sisco research laboratories, Mumbai, India) and 0.0005% manganese chloride, the medium was incubated for 2–3 days at 30 °C (El-Kersh et al. 2012). Bacillus thuringiensis-like colonies were selected and suspected B. thuringiensis like colonies were fished from populated Bacilli, one single colony was repeated until a pure culture was obtained. Closely related other spore forming bacilli were excluded via Phase Contrast microscopy for the presence of parasporal crystals. Bacillus thuringiensis index was calculated for each positive sample (Xavier et al. 2007, El-Kersh et al. 2012). Biochemical, phenotypic characterization, and identity confirmation of recovered 68 B. thuringiensis isolates were accomplished on the basis of esculin-hydrolysis, lecithinase, hemolytic, and motility activities, API 20E, and carbohydrate utilization (API 50CH system), essentially as previously described by El-Kersh et al. (2012). Crystal morphology analysis took place under Phase Contrast Microscope (Rampersad and Ammons 2005, Gobatto et al. 2010). In this study a total of 68 B. thuringiensis isolates were successfully recovered from the 300 collected samples, each of which was purified by sub-culturing on SNA agar for 48 hours and stored as a stock culture in a sterile liquid nutrient broth medium containing 50% glycerol at −20 °C (Hernandez et al. 2005).
Spore-crystal mixtures preparation
Preliminary screening for larvicidal activities have been carried out to assess the suitable lethal concentrations of the spores-crystals mixture of each B. thuringiensis isolate in parallel with the B. t. israelensis H14 (Bti-H14), as a reference strain, (kindly provided by prof. A. Abdel-Hameed, deceased) according to El-Kersh et al. (2012). Out of the tested 68 native B. thuringiensis isolates, only 23 isolates showed larvicidal activity (data not shown), the most potentially active 10 isolates were selected for further bioassay and identification. To avoid possible discrepancy during the preparation of spore-crystal mixture for quantitative determination of LC50 and LC95, B. thuringiensis isolates were prepared from fermentation growth on Nutrient Yeast Extract Salt Medium, NYSM, (containing per litre: 5g glucose, 5g peptone, 5g NaCl, 3g beef extract, 0.5g yeast extract, 0.02g magnesium chloride, 1mg manganous chloride, 0.01g calcium chloride, pH 7.2). Spores-crystals mixtures were then prepared as dried powders in adequate amount using the lactose acetone co-precipitation procedure according to Dulmage et al. (1971). The resulting white fine toxin powder mixtures were stored at 4 °C until used.
Extraction of toxin-complexes from Nematode bacterial-symbionts
Isolation of nematode bacterial symbionts
Two types of the bacterial symbiont, Photorhabdus, were isolated from the two entomopathogenic nematodes, Heterorhabditis indica (HRM1), isolated from Alexandria, Northern Egypt, and Heterorhabditis sp. (HS1), isolated from Ras-Sidr in South Sinai of Eastern Egypt, as detailed in (El-Sadawy et al. 2016). Briefly, bacteria were isolated from their symbiotic nematodes according to Woodring and Kaya (1988). For each subculture, the phase status was determined by culturing on NBTA agar [2.3% nutrient agar (Difco), 0.0025% bromothymol blue (Merck), 0.004% 2,3,5-triphenyltetrazolium (Merck)]. Phase I colonies are blue on NBTA while phase II colonies are red. Twenty infective nematode juveniles were surface sterilized for 10min in 1.0% sodium hypochlorite, washed in sterile distilled water, transferred to a Petri dish containing 5ml of TSBYE [3% tryptic Soy broth (Difco), 0.5% yeast extract (Difco)], and grinded using grinder pistol. The plates were incubated at 30 °C for 24h and streaked on NBTA plates [2.3% nutrient agar (Difco), 0.0025% bromothymol blue (Merck), 0.004% 2,3,5-triphenyltetrazolium (Merck)]. The presence of Photorhabdus colonies was confirmed by dye adsorption on NBTA plates, production of luminescence, and antibiotic activity. The isolated bacteria were then maintained on NBTA plates at 10 °C and subcultured weekly.
Toxins extraction from nematode bacterial symbionts
Bacterial cell pellets were obtained from a 2-liter culture of the two Photorhabdus types HRM1 and HS1 fermentations, separately for 48h according to Sheets et al. (2011) with some modifications. The pellets were suspended in 50mM Tris-HCl (pH 8.0), 100mM NaCl, 1mM DTT, 10% glycerol, lysozyme (0.6mg/ml) and bacterial protease inhibitor cocktail (Sigma, St. Louis). A small amount of glass beads (0.5mm diameter), were added and then bacterial cells were disrupted by sonication then centrifuged at 10,000g for 60min at 4 °C. Supernatants, including toxin complexes (TCs), were then collected into Eppendorf tubes and subjected to protein concentration measurement using Coomassie Blue Protein Assay Reagent (ICI Americas, Inc.) according to the manufacturer’s instructions, then stored at −20 °C. The total protein was estimated by the method described by Bradford (1976), and bovine serum albumin was used for the calibration curve. TCs were then stored in liquid nitrogen until used for larvicidal bio-assay screening.
Larvicidal Bioassays
Spores of Bacillus thuringiensis
Spore-crystal powder of each B. thuringiensis isolate was suspended in 10ml of sterile water, to give an average count of 109 colony-forming unit (CFU)/ml, and then used for preliminary screenings of larvicidal activity against 3rd instars larvae. A high concentration from crystal-spore suspension of each B. thuringiensis isolates was used in parallel with the reference Bti strain [Bt serovar israelensis de Barjac (Bti-H14)] and negative control (El-Kersh et al. 2012). Larval mortality was scored at 24h post-treatment at 22±1 °C (data not shown). Out of the tested 68 native B. thuringiensis isolates, only 10, that exhibited significant mosquito-larvicidal activity, were selected for further bioassay assessment and spore counts. For these 10 potentially larvicidal active isolates, preliminary bioassays were carried out using wide range of nine ascending concentrations prepared by suspending a certain weight from each of isolates’ toxin mixture in distilled water. Based on the preliminary results (data not shown), a narrower range of 5 lethal concentrations of each isolate were used for the main Bioassay tests.
For the main investigation of mosquito larvicidal activity against 3rd instars larvae, LC50 and LC95, of each of the 10 potentially larvicidal B. thuringiensis isolates, were investigated in parallel with the reference strain (Bti-H14) at 24h and 48h post-treatment. In this bioassay, five ascending concentrations from each B. thuringiensis mixture were used as recommended by WHO (2005) with some modifications. Briefly, twenty 3rd instars larvae were placed in each well of a sterile standard 12-wells tissue culture test plate (Nunclone Delta Surface, Thermo-Fischer Scientific, Denmark) with 2 ml de-ionized water. An amount of 10μl from each concentration was added to each well (Rey et al. 1999). Another group of larvae were treated in the same manner with 10μl de-ionized water (negative control) or the reference Bti-H14 strain (positive control) for comparison (El-Kersh et al. 2012). Each concentration was applied in 5 replicates (n= 5) using 5 different groups of experimental larvae (20 larvae each). Larvae were fed to avoid mortality caused by starvation. A lack of larvae reaction to gentle prodding with a glass pipette was recorded as mortality according to Brown et al. (1998). The mean percentage of larval mortality was calculated for the 5 replicates (n= 5) of each concentration of each isolate using Abbott’s formula (Abbott 1925) at 24 and 48h post-treatment. Subsequently, the LC50 and LC95 of each of the 10 larvicidal B. thuringiensis isolates, and the reference strain (Bti-H14), were estimated using Probit Analysis. Meanwhile, samples from each of the same serial dilutions of B. thuringiensis toxins were cultured on SNA medium for estimating spore counts by counting the number of colony forming units CFU/μg.
Toxin complexes (TCs) of nematode bacterial symbionts
Two TCs extracted from the two Photorhabdus types, HRM1 and HS1, were lyophilized into powders prior to preliminary larvicidal bioassays using wide range of serial concentrations prepared by suspending a certain weight from each powder in deionized distilled water. Based on the preliminary results, four serial effective concentrations from each toxin were assessed and used for the main bioassays according to the WHO (2005) as described above. Mortality percentages were calculated at 24 and 48h post-treatment.
Histopathological studies
Light microscopy
TCs of HS1 was used for testing the histopathological impact on midguts of treated larvae as it showed higher mosquito larvicidal activity compared to that of HRM1. Larvae were treated with the LC50 (38.3 μg/ml) of HS1 TCs or distilled water as controls. Alive treated-sluggish (prior to death) or control larvae were collected at 24h post-treatment and used to investigate the midgut histological alterations under light microscope according to Ahmed et al. (2014). Briefly, midgut sections were fixed overnight in cold 2.5% glutaraldehyde in 100 mM phosphate buffer (pH 7.2) and for 1h in 1% OsO4. Midgut sections were dehydrated through an ethanol series, treated with propylene oxide, and embedded in Poly/Bed 812 (Polysciences Inc, Warrington, PA). Sections (10μm) were stained with hematoxylin and eosin (Sigma-Aldrich), mounted with Paramount (Fisher), and examined by light microscopy (Zeiss Axioskop 50 compound microscope, Carl Zeiss, Inc, Thornwood, NY). Images were imported into Adobe Illustrator Cs, 2003 Software and adjusted for examination.
Transmission electron microscopy (TEM)
The HS1TCs-treated or control larvae were prepared for midgut ultrastructure examination by TEM prior to death at 24h post-treatment as described above. Briefly, treated midguts were fixed overnight in 0.8% glutaraldehyde and 4% paraformaldehyde dissolved in 0.1M sodium cacodylate (pH 7.0), followed by 4h in 1% OsO4 at 4 °C. After dehydration, treatment with propylene oxide and embedding in Epon-Araldite resin (1:1), 4μm sections were mounted, stained with 2% uranyl acetate for 30min and incubated in lead citrate for 10 min. Samples were examined with a transmission electron microscope (Jeol Ltd., model JEM-100CX II) at 80 kV.
Statistical analysis
The LC50, LC95, slopes, and standard error values of each treatment (5 replicates) were calculated according to Finney (1971). Relevant treatments were considered as not significantly different in their toxicity if confidential limits (95%) of LC50 were overlapped (Litchfield and Wilcoxin, 1949).
Results
Characteristics of the B. thuringiensis isolates
Out of several hundred (> 300) examined B. thuringiensis-like colonies (Fig. 2A,B), 68 B. thuringiensis isolates were identified. The overall mean B. thuringiensis index, the ratio of B. thuringiensis isolates producing crystal (Fig. 2C) to other non-B. thuringiensis spore forming bacilli, corresponding to the whole sampling areas was 0.35. More than 75% of processed samples (n=300) were negative for B. thuringiensis isolates, suggesting a limited abundance of the organism in several Saudi environmental regions. B. thuringiensis index reflects the abundance of B. thuringiensis strains but not necessarily their B. thuringiensis diversity. Hence, some single specimens yielded more than one B. thuringiensis isolates, which differ in their colony morphology and parasporal crystal shapes (Fig. 2), yet with low B. thuringiensis index. Whereas other single specimens yielded only one B. thuringiensis isolate with a relatively high B. thuringiensis index, and meanwhile several other samples yielded no B. thuringiensis isolates. Phase contrast microscopy examination revealed that most of the recovered 68 B. thuringiensis isolates showed spherical crystals (34%), while irregular (small spherical to amorphous, cubic, merged triangular/or conical like budding (Fig. 2C), bi-pyramidal, and attached crystal (various shapes) to the spores constituted 32, 13, and 21% respectively. The characteristics of the selected ten B. thuringiensis isolates were similar to those of the reference Bti-H14 isolate (Table 1), with exception of the Bt-63 isolate, which produced acid from sucrose. However, the rest of its characteristics were similar to those of the reference Bt-H14.
Larvicidal activity
Bacillus thuringiensis
Based on preliminary screening for larvicidal activity, 23 native B. thuringiensis isolates (out of 68 in total) showed promising larvicidal activities. Hence, the best potentially active 10 B. thuringiensis isolates were further subjected to quantitative LC50 and LC95 determination in parallel with the Bti-H14 as a reference strain (positive control), using prepared acetone spore/crystal mixture-lactose co-precipitation dried powder with concomitant determination of spore colony forming unit (CFU) per μg powder (Table 2). Taking into consideration the spore CFU/μg powder, three native B. thuringiensis isolates coded (Bt-12, Bt-26, and Bt-29) showed almost similar LC50 and LC95 values similar to those of the Bti-H14 reference strain positive control. Whereas, 7 native B. thuringiensis isolates coded (Bt-42, Bt-44, Bt-53, Bt-55, Bt-60, Bt-63, and Bt-68) showed significantly higher larvicidal activity (∼2.6 and ∼6.4 folds less spore CFU/μg) than those of the Bti-H14, and their respective LC95 and slope values confirm these activities.
Table 2.
Toxicity of 10 potential larvicidal native Bacilus thuringiensis isolates against 3rd instar larvae of Culex pipiens. Lethal concentrations (LC50 and LC95) were calculated by Probit analysis for all isolates compared to the reference Bti-H14
| Bt-code | City of collection | Hrs P-T | LC50 (μg/ml) (lower to upper) | LC95 (μg/ml) (lower to upper) | ×105 CFU/μg | Slope±SE |
|---|---|---|---|---|---|---|
| Bt-12 | Yanbu | 24 | 4.6 (4.2–5.1)e | 9.4 (7.9–11.08) | 0.53±0.4 | 5.4±0.005 |
| 48 | 2.4 (2.04–2.8) | 10.4 (8.01–13.9) | 2.6±0.06 | |||
| Bt-26 | Medina | 24 | 4.8 (4.1–5.6)e | 33.9 (21.8–54.5) | 4.1±0.7 | 1.94±0.044 |
| 48 | 1.9 (1.5–2.4) | 14.6 (10.2–22.0) | 1.9±0.051 | |||
| Bt-29 | Medina | 24 | 4.2 (3.5–5.0)e | 32.6 (22.8–47.8) | 0.52±0.1 | 1.8±0.03 |
| 48 | 2.2 (1.8–2.7) | 14.05 (10.6–19.2) | 2.05±0.037 | |||
| Bt-42 | Jizan | 24 | 2.5 (2.2–2.9)c | 15.4 (10.9–22.8) | 0.93±0.3 | 2.1±0.048 |
| 48 | 1.2 (1.04–1.35) | 3.07 (2.5–3.8) | 4.02±0.26 | |||
| Bt-44 | Jizan | 24 | 3.03 (2.7–3.4)d | 9.4 (7.7–11.6) | 0.63±0.4 | 3.35±0.075 |
| 48 | 2.08 (1.8–2.3) | 6.12 (5.1–7.5) | 3.5±0.094 | |||
| Bt-53 | Medina | 24 | 1.9 (1.7–2.2)b | 8.08 (5.8–11.7) | 1.3±0.4 | 2.6±0.0011 |
| 48 | 0.72 (0.6–0.8) | 2.5 (2.08–3.6) | 2.9±0.0014 | |||
| Bt-55 | Medina | 24 | 1.7 (1.5–1.98)b | 7.9 (6.11–10.68) | 1.95±0.3 | 2.5±0.059 |
| 48 | 1.06 (0.91–1.2) | 2.29 (2.5–3.8) | 3.65±0.21 | |||
| Bt-60 | Medina | 24 | 1.9 (1.7–2.2)b | 5.01 (4.2–6.15) | 0.13±0.1 | 4.05±0.002 |
| 48 | 0.94 (0.8–1.05) | 2.9 (2.5–3.8) | 3.3±0.09 | |||
| Bt-63 | Mecca | 24 | 0.91 (0.7–1.07)a | 5.51 (4.1–8.2) | 0.7±0.2 | 2.1±0.053 |
| 48 | 0.5 (0.4 –0.58) | 1.12 (0.95–1.4) | 4.72±0.006 | |||
| Bt-68 | Medina | 24 | 2.3 (2.06–2.7)c | 10.7 (7.8–15.7) | 1.05±0.3 | 2.5±0.055 |
| 48 | 1.1 (0.97–1.3) | 5.4 (4.2–7.5) | 2.4±0.056 | |||
| Bt-15 | Bti-H14 | 24 | 4.88 (4.2–5.4)e | 21.9 (17.3–28.0) | 4.5±0.3 | 2.52±0.044 |
| 48 | 2.62 (2.2–3.1) | 10.01 (7.8–13.2) | 2.82±0.092 |
LC50= Lethal concentration (concentration to kills 50% of test larvae), LC95= Lethal concentration (concentration to kills 95% of test larvae), CFU= colony forming unit, S.E.= Standard Error of means (n= 5), Values with different letters (a, b, c, d and e) are significantly different within strains and compared to the reference Bti-H14 (based on the non-overlapping confidence limits) according to Litchfield and Wilcoxin (1949). Control mosquitoes showed nil mortality.
Toxin complexes (TCs) of nematode bacterial-symbiont
Extracted TCs from the two Photorhabdus types (HRM1 and HS1) were screened for their larvicidal activity. Data showed that the toxicity of HS1 TCs was 2.5 folds higher than that of HRM1 TCs (LC50 38.3 v 97.4 μg/ml, respectively) at 24h post-treatment (Table 3). The toxicities of both toxins were higher at 48h than that at 24h post-treatment, considering the LC50 values, with HS1 TCs being significantly more toxic (Table 3). The LC95 values of HS1 TCs showed non significant difference at 24 and 48h post-treatment, and slope values confirm these activities (Tables 3).
Table 3.
Probit analysis for toxicity of HS1and HRM1 TCs against 3rd larval stage of Culex pipiens within a column, values followed by different letters are significantly different according to Litchfield and Wilcoxin (1949)
| Toxin | Hrs P-T | LC50 (μg/ml) (lower to upper) | LC95 (μg/ml) (lower to upper) | Slope ± SE |
|---|---|---|---|---|
| HS1-toxin | 24 | 38.3(36.2–40.4)a | 58.7(53.9–63.9)a | 8.8 ± 0.6 |
| 48 | 31.7(29.6–33.9)b | 58.2(51.8–56.5)a | 6.2 ± 0.32 | |
| HRM1-toxin | 24 | 97.4(93.9–100.9)c | 139.9(131.4–149)b | 10.45 ± 1.11 |
| 48 | 76.6(69.3–84.6)d | 115.3(106.5–124.6)c | 9.2 ± 3.07 | |
Histopathological studies
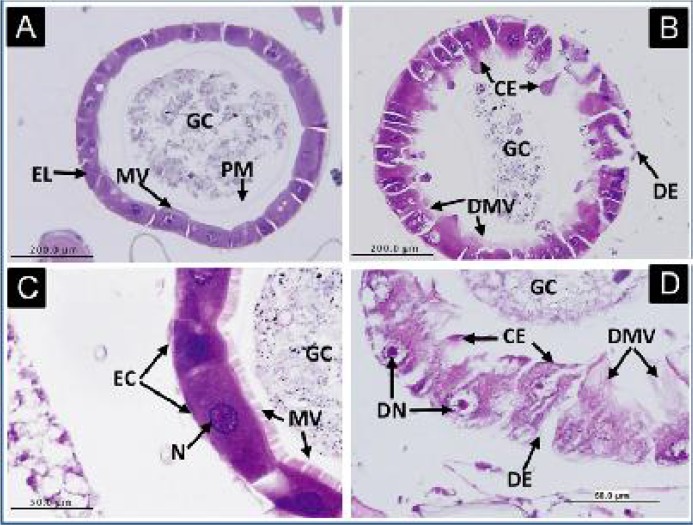
The impact of oral administration of HS1TCs on the histological integrity of treated midguts of 3rd instar larvae has been investigated at 24h post-treatment. Light microscopy showed numerous cytoplasmic extensions, and cellular and nuclear degeneration were clear in the midgut epithelial cells of treated midguts (Figs. 3B, D). The peritrophic membrane and microvilli appeared disrupted compared to the untreated control midguts as they retained their structural integrity, with the nuclei in the centre of the cell and microvilli bordered the lumen normally (Figs. 3A, C).
Fig. 3.
Histopathological impact of HS1 toxin-complexes on the midgut epithelia of Cx. pipiens larvae, 24h post-treatment. Figs. A and C represent cross sections in midguts of untreated larvae, showing normal gut epithelial layer (EL) with healthy, normal epithelial cells (EC), peritrophic membrane (PM), microvilli (MV), nuclei (N), and nutritional gut contents (GC) filling the gut lumen. Figs. B and D represent cross sections in midguts of treated larvae, showing affected gut epithelial layer, with cytoplasmic extensions (CE), degraded microvilli (DMV), degenerated epithelial cells (DE)
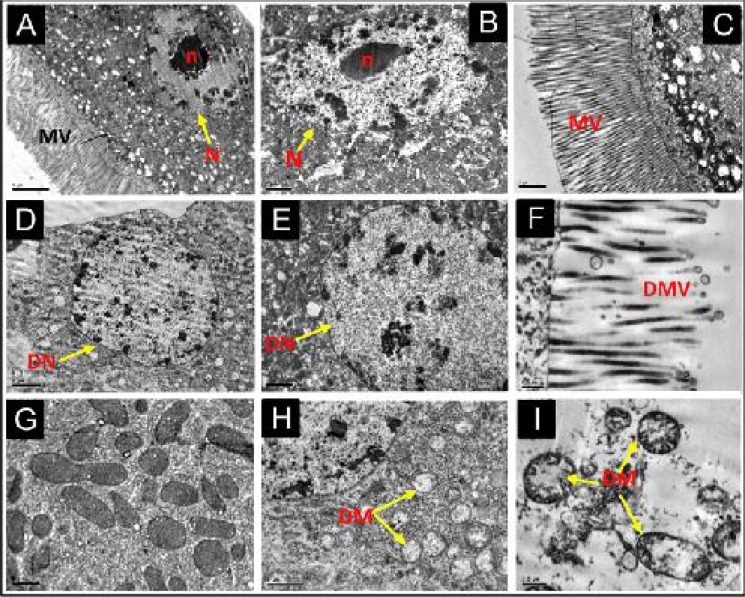
Moreover, TEM revealed subcellular alterations in terms of nuclei disintegration, degradation of chromatin and nucleoli (Figs. 4D, E) and disrupted microvilli (Fig. 4F). In addition, mitochondria appeared with cristae deformation and almost free of their internal contents (Figs. 4H, I), while the structure of the epithelial cells and their components in the control midguts appeared normal and keeping their integrities (Figs. 4 A, B, C and G).
Fig. 4.
Transmission electron microscopic micrographs showing the cytological effects of HS1 toxin-complexes treatment on the ultrastructure of the midgut epithelial tissue of Cx. pipiens 3rd larval stage at 24h post-treatment. A, B, and C indicate normal nucleus (N), nucleolus (n), microvilli (MV), and chromatin contents in epithelial cells of control larvae (Scale bar= 5, 2.5, and 2.5μm respectively). D, E, and F indicate degenerating nucleus (DN) and its contents, degenerated microvilli (DMV) with bubbling and stretching appearance in treated larvae (Scale bar= 2.0, 2.5, and 0.5μm respectively). G: represents normal mitochondrial structures in control larvae (M) (Scale bar= 0.5μm). H and I: represent degenerating mitochondria (DM) in treated midgut cells, showing deformation and loss of cristae and matrix (Scale bar= 1 and 0.5μm respectively). Ultrathin 4μm sections were investigated with transmission electron microscope model JEOL JEM-100CX II at 80kV.
Discussion
In this study laboratory, assessment of the toxic activities of indigenous B. thuringiensis strains and toxin complexes (TCs) of nematode bacterial symbionts were investigated against 3rd larval instars of the Filaria vector, Cx. pepiens (Hawking 1973). Thus, it is important to clarify initially several key points. Firstly, this study targeted Cx. pepiens, the widely distributed filaria vector worldwide. Secondly, this study seeks not only to overcome the recently evolving problem of mosquito resistance to chemical insecticides (Al-Sarar 2010, Osta et al. 2012, Brouqui et al. 2012) but also to regain the balance of nature by developing and then implementing biocontrol measures against mosquito vectors, while keeping the environment safe and unpolluted by chemical insecticides (Azmi et al. 2009). Hence, certain native bacterial isolates from B. thuringiensis and (TCs) from the nematode bacterial symbiont, Photorhabdus bacteria (HRM1 and HS1), isolated from their Heterorhabdus mutual nematode were tested as they have previously shown remarkable mosquito larvicidal activities in our lab (El-Kersh et al. 2012, 2014 and El-Sadawy et al. 2016). Thirdly, B. t. israelensis isolates and nematodes, isolated from Saudi and Egyptian environment respectively, were used in order to ensure effectiveness when used locally in the same regions. Fourthly, this study is considered as an initial step towards implementing a biocontrol measure against this Filaria vector in Saudi Arabia and may be worldwide.
In fact, the entomopathogenic B. t. israelensis has been proved not only as effective biocontrol agent against larvae of many mosquito species worldwide (Ben-Dov 2014) but also as safe for non-target invertebrates and vertebrates. This fact has encouraged researchers to search for new B. thuringiensis strains in various countries (Ohba and Aizawa 1986, Mohammedi et al. 2006, Armengol et al. 2007, Gobatto et al. 2010, Aramideh et al. 2010, Ramalakshmi et al. 2010, Saferalizadeh et al. 2010, Kavitha et al. 2011, El-Kersh et al. 2014). In support to these efforts, the current study herein aimed at isolating local indigenous mosquito larvicidal B. thuringiensis from the Saudi environment for use as an echo-friend biocontrol agents for three reasons, a) thousands of Saudis were recently infected with mosquito-borne diseases (Khalil et al. 2008, Madani et al. 2003, Alwafi et al. 2013, Al-Thabiani 2014), b) to the best of our knowledge, pest control in Saudi Arabia still solely relies on chemical insecticides, and mosquito vectors become resistant to most of them (eg. Al-Sarar 2010) but not to Bti yet (Boyer et al. 2012) and c) so far, little is known about the natural presence and isolation of native entomopathogenic B. thuringiensis species from the Saudi environment except very few studies that isolated B. thuringiensis against lepidopteran pests (Assaeedi et al. 2011, Abulreesh et al. 2012, El-Kersh et al. 2014) and mosquito vectors (Al-Zahrani and Abuldahab 2011) from particular locations. Thus, we believe that isolation of more local mosquiocidal B. thuringiensis is urgently needed as it will be more effective in the Saudi hot, dry and desert environment, as well as similar environments of other countries in the world. On this context, we have obtained 68 B. thuringiensis isolates from different locations throughout the country, and the percentage of active isolates against mosquito larvae was low compared to the inactive ones. El-Kersh et al. (2012) reported that most B. thuringiensis isolates obtained from different regions in Saudi Arabia were inactive against Cx. pipiens larvae; similar finding was also reported from different regions worldwide (Bernhard et al. 1997, Park et al. 2008). Out of the selected 10 potentially active isolates, 5 isolates belong to samples collected from Medina (24° 28′ 0″ N, 39° 36′ 0″). The most active isolate coded (Bt-63) was obtained from samples collected from Mecca (21° 30′ 0″ N, 41° 0′ 0″). This could be attributed to the abundance of irrigation fresh water and hence mosquito distribution in this region (Martin et al. 2010). Nevertheless, the presence of mosquitoes does not guarantee the presence of B. thuringiensis in the breeding water or in the soil. Evidence for this is that B. thuringiensis isolates found in soils showed little or no insecticidal activities, whereas some of those not found in soils showed high insecticidal activities (Chatterjee et al. 2007 and Kavitha et al. 2011). We, therefore, consider all of our 68 B. thuringiensis isolates as part of the indigenous microflora of the areas, which have been explored.
The recovered 68 B. thuringiensis isolates showed spherical crystals (34%), while irregular (small spherical to amorphous, cubic, merged triangular/or conical like budding, bi-pyramidal, and attached crystal (various shapes) to the spores constituted 32, 13 and 21% respectively (El-Kersh et al. 2016). The types of crystal morphology recorded for the parasporal inclusion bodies in that study were reported in many Bti isolates from different regions inside and outside Saudi Arabia (Bernhard et al. 1997, El-Kersh et al. 2012) which is attributed to the inactivity of their isolates against Cx. pipiens larvae to the high percentage of spherical crystals. However, El-Kersh et al. (2016) reported that the ratio of spherical crystals was the highest compared to the rest of crystal morphology. The high toxicity of B. thuringiensis strains obtained from the Philippines and Colombia were attributed to the spherical parasporal inclusions (Padua et al. 1984, Orduz et al. 1992). In addition, there is no correlation between the type of insecticidal activity and crystal morphology (Bernhard et al. 1997, Martin and Travers, 1989, Ohba and Aizawa 1986).
In this context, although the reported 10 potentially larvicidal active B. thuringiensis isolates in the current study showed similarities in their crystal shape and biochemical profiles, their larvicidal activities (the LC50 and LC95) showed great discrepancies. Additionally, the most active isolate, coded (Bt-63), was the only one to produce acid from sucrose suggesting different metabolic capability as compared to the reference Bti-H14, and the other remaining native B. thuringiensis potential isolates. It is conceivable that such great variation in larvicidal activities is most probably correlated with respective cry and cyt genes content of each strain (El-Kersh et al. 2016). In this context, Ben-Dov (2014) stated that the high specific mosquito larvicidal properties of Bti δ-endotoxins are attributed to complex interactions between six proteins, Cry4Aa, Cry4Ba, Cry10Aa, Cry11Aa, Cyt1Aa and Cyt2Ba, differing in toxicity levels and against different species of mosquitoes.
On the other hand, TCs of both HRM1 and HS1 of Photorhabdus bacterial species, showed larvicidal activities of LC50 38.3 and 97.4μg/ml respectively at 24h post-treatment in the current study. It is well established that Ph. luminescens is symbiotic bacterium lives in the gut of the entomopathogenic Heterorhabdus nematodes that inject it into the hemocoel of insect host upon invasion. Many studies have investigated the killing mechanisms of these bacteria as they bring about immunosuppression, septicemia and the subsequent death of the insect host (ffrench-Constant et al. 2007, Eleftherianos et al. 2010, Lang et al. 2011, Zhu et al. 2011). Moreover, a number of pathogenicity determinants have been identified in this Gram-negative bacterial species, and some others, including hemolysis factor, hydrolases, lipopolysaccharide, regulatory factors, toxins and proteases (ffrench-Constant et al. 2007, Nielsen-LeRoux et al. 2012). These toxic compounds are displayed on the outer surface of the bacterium (ffrench-Constant et al. 2007). Previous findings support our findings like Bowen and Ensign (1998) that identified four different toxin complexes of Ph. luminescenens strain W14 termed TCa, TCb, TCc, and TCd. They also proved that these toxin complexes are composed of three different proteins, XptA2, XptB1, and XptC1 representing products from class A, B and C toxin complex genes respectively. In this context, Guo, et al. (1999) suggested that A proteins harbors the cytotoxic effects of the TC toxins, whereas class B and C proteins rather modulate and enhance the toxicity of class A proteins. Data of the current study may indicate that mosquito larvicidal TCs of Photorhabdus bacteria isolated from local Heterorhabdus nematode could be utilized in the battle against mosquito vectors. Evidence for this hypothesis has been provided by ffrench-Constant et al. (2007) who mentioned that the Photorhabdus TCs (PirAB binary toxins) have produced oral toxicity against mosquitoes and some caterpillar pests. Further evidence has been provided by Vani and Lalithambika (2014) who recorded 93.32% mortality for the 3rd larval instar of Anopheles gambiae when he used 100ng/ml of the extracellular proteins (from 20–97 KDa) of the outer membrane of Xenorhabdus sp bacteria isolated from Steinernema sp nematode. Moreover, Bishop (2014) has successfully cloned and introduced the prtA gene (encodes the protease A virulence factor) from Ph. luminescens into Bacillus thuringiensis which enhanced the mortality in larvae of the lepidopteran moth, Pieris brassicae and Galleria mellonella when taken orally and injected into the hemocoel respectively compared to the wild type of B. thuringiensis.
The histopathological impact of TCs from HS1 on the integrity of the midgut of treated Cx. pipiens larvae was also investigated in the current study. Light and electron microscopy provided solid evidences for the cellular and subcellular impacts respectively on the midgut of treated mosquitoes. Light microscopy showed clearly the destruction of the epithelial cells lining the midgut, which may be associated with the midgut paralysis and cessation of feeding noticed by 12h post-treatment. Besides, TEM results showed severe damage at the mitochondrial level as white spots are detected compared to untreated control. Supporting evidences for this histological impact have been previously provided by Morgan et al. (2001), who observed oral toxicity of the supernatants of some Xenorhabdus strains to insects. In addition, Blackburn et al. (1998) proved that the toxic compounds produced by Ph. luminescens caused disruption of the midgut epithelium in a manner similar to that of δ-endotoxins from B. thuringiensis. In addition, Sheets et al. (2011) showed that recombinant XptA2, and co-produced recombinant XptB1 and XptC1 bind together with a 4:1:1 stochiometry, and that XptA2 forms a tetramer of ∼1,120 kDa that binds to solubilized insect brush border membranes, and hence, induces pore formation in the membrane lipids. Data of the histopathological study of HS1 observed in the current study also provide evidence of the similarity to those observed in Bti-infected midguts in previous studies (Bravo et al. 2007 and Soberon et al. 2007, Al-Roba et al. 2011). This may provide further evidence that these toxin-complexes of the nematode symbiotic bacterial can be considered as reliable candidate in the bio-control measure against mosquito vector.
Conclusion
The present findings may contribute to the efforts and plans of Saudi Ministry of Health for the control of mosquito-borne diseases. The larvicidal activity of these native B. thuringiensis isolates and nematode bacterial-symbiont TCs, or a combination of both of them, could make them ideal eco-friend candidates in the biocontrol measures against mosquito vector in Saudi Arabia, as well as in other parts of the world where these mosquito vectors are prevalent. We believe that these products may also help in overcoming or suppressing the emergence of mosquito resistance to insecticides worldwide. Finally, purification of both local B. thuringiensis δ-endotoxins and nematode bacterial TCs, their molecular characterization and their possible synergistic activity against mosquito larvae are currently being investigated in our lab. We therefor believe that this may lead to the identification of mosquito larvicidal B. thuringiensis-TCs mixture product that could contribute to the battle against mosquito vectors.
Acknowledgements
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH) King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award number (11-MED-1848-02).
References
- Abbott WS. (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol. 18: 265–267. [Google Scholar]
- Abulreesh HH, Osman GE H, Assaeedi ASA. (2012) Characterization of Insecticidal Genes of Bacillus thuringiensis Strains Isolated from Arid Environments. Indian J Microbiol. 52(3): 500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed AM, Taylor P, Maingon R, Hurd H. (1999) The effect of Plasmodium yoelii nigeriensis on the reproductive fitness of Anopheles gambiae. Invertebr Reprod Develop. 36: 217–222. [Google Scholar]
- Ahmed AM, Shaalan EA, Aboul-Soud MAM, Tripet F, AL-Khedhairy AA. (2011) Mosquito vectors survey in AL-Ahsaa district, eastern region, Kingdom of Saudi Arabia. J Insect Sci. 11: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed AM, Abdel Megeed AM, Al-Qahtaney HM. (2014) A Novel Mosquitocidal Bacterium as a Biocontrol Agent in Saudi Arabia: I - A Promising Larvicide Against Aedes caspius Mosquito. Pakistan J Zool. 46(1): 191–201. [Google Scholar]
- Al-Ahmed AM. (2012) Mosquito fauna (Diptera: Culicidae) of the Eastern region of Saudi Arabia and their seasonal abundance. J King Saud Univ Sci. 24(1): 55–62. [Google Scholar]
- Al-Ghamdi K, Alikhan M, Mahayoub J, Afifi ZI. (2008) Studies on identification and population dynamics of Anopheline mosquito from Jeddah, Saudi Arabia. Biosci. Biotech Res Comm. 1: 19–24. [Google Scholar]
- Al-Hazmi M, Ayoola EA, Abdurahman M, Banzal S, Ashraf J, El-Bushra A, Hazmi A, Abdullah M, Elamin A, Al-Sammani E, Gadour M, Menon C, Hamza M, Rahim I, Hafez M, Jambavalikar M, Arishi H, Aqee A. (2003) Epidemic Rift Valley Fever in Saudi Arabia: A Clinical study of severe illness in humans. Clin Infect Dis. 36(3): 245–252. [DOI] [PubMed] [Google Scholar]
- Al-Khuriji AM, Alahmed MA, Kheir SM. (2007) Distribution and seasonal activity of mosquitoes (Diptera: Culicidae) in Riyadh Region, Saudi Arabia. J King Saud Univ Agric Sci. 19 (2): 39–55. [Google Scholar]
- Al-Roba AA, Aboul-Soud MAM, Ahmed AM, Al-Khedhairy AA. (2011) The gene expression of caspasses is up-regulated during the signaling response of Aedes caspius against larvicidal bacteria. Afr J Biotechnol. 10(2): 225–233. [Google Scholar]
- Al-Sarar AS. (2010) Insecticide resistance of Culex pipiens populations (Diptera: Culicidae) from Riyadh City, Saudi Arabia: Status and overcome. Saudi J Biol Sci. 17(2): 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Thabiani A, Al-Shami SA, Mahyoub JA, Hatabbi M, Ahmad AH, Salmah C. (2014) An update on the incidence of dengue gaining strength in Saudi Arabia and current control approaches for its vector mosquito. Parasit Vectors. 7: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zahrani HAA, Abuldahab FF. (2011) Isolation and activity of a Bacillus thuringiensis Toxin which is Toxic to the Aedes eagypti. J Ame Sci. 7(11): 269–276. [Google Scholar]
- Ali S, Zafar Y, Ali G, Nazir F. (2010) Bacillus thuringiensis and its application in agriculture. Afr J Biotechnol. 9: 2022–2031. [Google Scholar]
- Alwafi OM, Scott JM, Ziad AM, Abdullah A. (2013) Dengue fever in Makkah, Kingdom of Saudi Arabia, 2008–2012. Am J Res Commun. 1(11): 123–139. [Google Scholar]
- Armengol G, Escobar MC, Maldonado ME, Orduz S. (2007) Diversity of Colombian strains of Bacillus thuringiensis with insecticidal activity against dipteran and lepidopteran insects. J Appl Microbiol. 102(1): 77–88. [DOI] [PubMed] [Google Scholar]
- Aramideh S, Saferalizadeh MH, Pourmirza AA, Bari MR, Keshavarzi M, Mohseniazar M. (2010) Characterization and pathogenic evaluation of Bacillus thuringiensis isolates from West Azerbaijan province-Iran. Afr J Microbiol Res. 4(12): 1224–1229. [Google Scholar]
- Assaeedi ASA, Osman GEH, Abulreesh HH. (2011) The occurrence and insecticidal activity of Bacillus thuringiensis in the arid environments. Aust J Crop Sci. 5(10): 1185–1190. [Google Scholar]
- Ayaad TH, Al Akeel R Kh, Olayan E. (2015) Isolation and characterization of midgut lectin from Aedes aegypti (L.) (Diptera: Culicidae). Braz Arch Biol Technol. 58: 905–912. [Google Scholar]
- Aziz AT, Al-Shami SA, Mahyoub JA, Hatabbi M, Ahmad AH, Rawi CS. (2014) An update on the incidence of dengue gaining strength in Saudi Arabia and current control approaches for its vector mosquito. Parasites Vectors. 7: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmi MA, Naqvi S, Akhtar K, Parveen S, Parveen R, Aslam M. (2009) Effect of pesticide residues on health and blood parameters of farm workers from rural Gadap, Karachi. J Environ Biol. 30(5): 747–756. [PubMed] [Google Scholar]
- Balenghien T, Vazeille M, Grandadam M. (2008) Vector competence of some French Culex and Aedes mosquitoes for West Nile virus. Vector Borne Zoonotic Dis. 8(5): 589–595. [DOI] [PubMed] [Google Scholar]
- Balkhy HH, Memish ZA. (2003) Rift Valley fever: an uninvited zoonosis in the Arabian Peninsula. Int J Antimicrob Agents. 21: 153–157. [DOI] [PubMed] [Google Scholar]
- Ben-Dov E. (2014) Bacillus thuringiensis subsp. israelensis and its dipteran-specific toxins. Toxins. 6: 1222–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard K, Jarret P, Meadows M, Butt J, Ellis DJ, Roberts GM, Pauli S, Rodgers P, Burges HD. (1997) Natural isolates of Bacillus thuringiensis: worldwide distribution, characterization, and activity against insect pests. J Invertebr Pathol. 70(1): 59–68. [Google Scholar]
- Bernier UR, Furman KD, Kline DL, Allan SA, Barnard D. (2005) Comparison of contact and spatial repellency of catnip oil and N,N-diethyl-3-methylbenzamide (DEET) against mosquitoes. J Med Entomol. 42: 306–311. [DOI] [PubMed] [Google Scholar]
- Bishop AH. (2014) Expression of prtA from Photorhabdus luminescens in Bacillus thuringiensis enhances mortality in lepidopteran larvae by sub-cutaneous but not oral infection. J Invertebr Pathol. 121: 85–88. [DOI] [PubMed] [Google Scholar]
- Bowen DJ, Ensign JC. (1998) Purification and characterization of a high molecular weight insecticidal protein complex produced by the entomopathogenic bacterium Photorhabdus luminescens. Appl Environ Microbiol. 64: 3029–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer S, Paris M, Jego S, Lemperiere G, Ravanel P. (2012) Influence of insecticide Bacillus thuringiensis subsp. israelensis treatments on resistance and enzyme activities in Aedes rusticus larvae (Diptera: Culicidae). Biol Control. 62: 75–81. [Google Scholar]
- Bozlagan L, Ayvaz A, Ozturk F, Acik L, Akbulut M, Yilmaz S. (2010) Detection of the cry1 gene in Bacillus thuringiensis isolates from agricultural fields and their bioactivity against two stored product moth larvae. Turk J Agric. 34: 145–154. [Google Scholar]
- Bradford M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Bravo A, Gill SS, Soberón M. (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon. 49(4): 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briassoulis G. (2001) Toxic encephalopathy associated with use of DEET insect repellents: a case analysis of its toxicity in children. Hum Exp Toxicol. 20: 8–14. [DOI] [PubMed] [Google Scholar]
- Brouqui P, Parola P, Raoult D. (2012) Insecticide resistance in mosquitoes and failure of malaria control. Expert Rev Anti Infect Ther 10(12): 1379–1381. [DOI] [PubMed] [Google Scholar]
- Brown MD, Thomas D, Watson K, Kay BH. (1998) Laboratory and field evaluation of efficacy of vectobac 12AS against Culex sitiens (Diptera: Culicidae) larvae. J Am Mosq Control Assoc. 14: 183–185. [PubMed] [Google Scholar]
- Chatterjee SN, Bhattacharya T, Dangar TK, Chandra G. (2007) Ecology and diversity of Bacillus thuringiensis in soil environment. Afr J Biotechnol. 6: 1587–1591. [Google Scholar]
- Dulmage HT, Boening OP, Rehnborg CS, Habsen GD. (1971) A proposed standardized biossay for formulations of Bacillus thuringiensis based on the international unit. J Invertebr Pathol. 18: 240–245. [DOI] [PubMed] [Google Scholar]
- El-Kersh TA, Al-sheikh YAA, Al-Akeel R, Alsayed AA. (2012) Isolation and characterization of indigenous Bacillus thuringiensis isolates from Saudi Arabia. Afr J Biotechnol. 11(8): 1924–1938. [Google Scholar]
- El-Kersh TA, Al-akeel RA, Al-sheikh YA, Alharbi SA. (2014) Isolation and distribution of mosquito-larvicidal cry genes in Bacillus thuringiensis strains native to Saudi Arabia. Trop Biomed. 31(4): 616–632. [PubMed] [Google Scholar]
- El-Kersh TA, Ahmed AM, Al-sheikh YA, Tripet F, Ibrahim MS, Metwalli AAM. (2016) Isolation and molecular characterization of Bacillus thuringiensis strains native to Saudi Arabia with naturally improved larivicidal toxicity against the mosquito vector Anopheles gambiae s.l. Parasites and Vectors (In Press). [DOI] [PMC free article] [PubMed]
- El-Sadawy HA, Forst S, Abouelhag HA, Ahmed AM, Alajmi RA, Ayaad TH. (2016) Molecular and phenotypic characterization of two bacteria Photorhabdus luminescens subsp. akhurstii HRM1 and HS1 isolated from two entomopathogenic nematodes Heterorhabditis indica RM1 and Heterorhabditis sp. S1. Pak J Zool. 48(1): 51–58. [Google Scholar]
- Eleftherianos I, Joyce S, Ffrench-Constant RH, Clarke DJ, Reynolds SE. (2010) Probing the tri-trophic interaction between insects, nematodes and Photorhabdus. Parasitology. 137: 1695–1706. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant RH, Bowen DJ. (2000) Novel insecticidal toxins from nematode symbiotic bacteria. Cell Mol. Life Sci. 57: 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Constant RH, Dowling A, Waterfield NR. (2007) Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture. Toxicon. 49: 436–451. [DOI] [PubMed] [Google Scholar]
- Finney DN. (1971) Probit Analysis, 3rd Edition Cambridge University Press, London, p. 318. [Google Scholar]
- Gobatto V, Giani SG, Camassola M, Dillon AJ, Specht A, Barros NM. (2010) Bacillus thuringiensis isolates entomopathogenic for Culex quinquefasciatus (Diptera: Culicidae) and Anticarsia gemmatalis (Lepidoptera: Noctuidae). Braz J Biol. 70: 1039–1046. [DOI] [PubMed] [Google Scholar]
- Guo L, Fatig RO, Orr GL, Schafer BW, Strickland JA, Sukhapinda K, Woodsworth AT, Petell JK. (1999) Photorhabdus luminescens W-14 insecticidal activity consists of at least two similar but distinct proteins. Purification and characterization of toxin A and toxin B. J Biol Chem. 274: 9836–9842. [DOI] [PubMed] [Google Scholar]
- Hawking F. (1973) The distribution of human Filariases throughout the world. Mimeograph WHO/FIL/73,114.
- Heimpel AM, Angus TA. (1959) The site of action of crystalliferous bacteria in Lepidoptera larvae. J Invertebr Pathol. 1: 152–170. [Google Scholar]
- Hernandez CS, Andrew R, Bel Y, Ferre J. (2005) Isolation and toxicity of Bacillus thuringiensis from potato growing areas in Bolivia. J Invertebr Pathol. 88: 8–16. [DOI] [PubMed] [Google Scholar]
- Hong HA, To E, Fakhry S, Baccigalupi L, Ricca E, Cutting SM. (2009) Defining the natural habitat of Bacillus spore-formers. Res Microbiol. 160: 375–379. [DOI] [PubMed] [Google Scholar]
- Jup PG, Kemp A, Grobbelaar A, Lema P, Burt FJ, Alahmed AM, Mujalli DA, Khamees MA, Swanepoel R. (2002) The 2000 epidemic of Rift Valley fever in Saudi Arabia: Mosquito vector studies. Med Vet Entomol. 16(3): 245–252. [DOI] [PubMed] [Google Scholar]
- Kavitha R, Xavier R, Monica D, Sreeramanan S. (2011) Quick isolation and characterization of novel Bacillus thuringiensis strains from mosquito breeding sites in Malaysia. Emirates Journal of Food and Agriculture. 23: 17–26. [Google Scholar]
- Khalil H, El-Badry AA, Eassa AHA, Al-Juhani AM, Al-Zubiany SF, Ibrahim KD. (2008) A Study on Culex species and Culex transmitted Diseases in Al-Madinah Al-Munawarah, Saudi Arabia. Parasitol United J. 1(2): 101–108. [Google Scholar]
- Khan NA, Azhar EI, EL-Fiky S, Madani HH, Abuljadial MA, Ashshi AM, Turkistani AM, Hamouh EA. (2008) Clinical profile and outcome of hospitalized patients during first outbreak of dengue in Makkah, Saudi Arabia. Acta Trop. 105: 39–44. [DOI] [PubMed] [Google Scholar]
- Lang AE, Schmidt G, Sheets JJ, Aktories K. (2011) Targeting of the actin cytoskeleton by insecticidal toxins from Photorhabdus luminescens. Naunyn Schmiedebergs Arch Pharmacol. 383: 227–235. [DOI] [PubMed] [Google Scholar]
- Litchfield JT, Wilcoxin FA. (1949) Simplified method of evaluating dose–effect experiments. J Pharmacol Exp Ther. 96: 99–103. [PubMed] [Google Scholar]
- Madani TA. (2005) Alkhumra virus infection, a new viral hemorrhagic fever in Saudi Arabia. J Infect. 51: 91–97. [DOI] [PubMed] [Google Scholar]
- Madani TA, Al-Mazrou Yagob Y, Al-Jeffri1 MH, Mishkhas AA, Al-Rabeah AM, Turkistani AM, S MO, Abodahish AA, Khan AS, Ksiazek TG, Shobokshi O. (2003) Rift Valley Fever Epidemic in Saudi Arabia: Epidemiological, Clinical, and Laboratory Characteristics. Clin Infect Dis. 37(8): 1084–1092. [DOI] [PubMed] [Google Scholar]
- Maguranyi SK, Webb CE, Mansfield S, Russell RC. (2009) Are commercially available essential oils from Australian native plants repellent to mosquitoes? J Am Mosq Control Assoc. 25(3): 292–30. [DOI] [PubMed] [Google Scholar]
- Martin PA, Travers RS. (1989) Worldwide Abundance and Distribution of Bacillus thuringiensis isolates. Appl Environ Microbiol. 55: 2437–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PA, Gundersen-Rindal DE, Blackburn MB. (2010) Distribution of phenotypes among Bacillus thuringiensis strains. Syst Appl Microbiol. 33: 204–208. [DOI] [PubMed] [Google Scholar]
- Meyer RP, Hardy JL, Presser SB. (1983) Comparative vector competence of Culex tarsalis and Culex quinquefasciatus from the coachella, imperial, and San Joaquin Valleys of California for St. Louis encephalitis virus. Am J Trop Med Hyg. 32(2): 305–311. [DOI] [PubMed] [Google Scholar]
- Mohammedi S, Subramanian SB, Yan S, Tyagi RD, Valéro JR. (2006) Molecular screening of B. thuringiensis strains from wastewater sludge for biopesticide production. Process Biochem. 41: 829–835. [Google Scholar]
- Morgan JA, Sergeant M, Ellis D, Ousley M, Jarrett P. (2001) Sequence analysis of insecticidal genes from Xenorhabdus nematophilus PMFI296. Appl Environ Microbiol. 67: 2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen-LeRoux C, Gaudriault S, Ramarao N, Lereclus D, Givaudan A. (2012) How the insect pathogen bacteria Bacillus thuringiensis and Xenorhabdus/Photorhabdus occupy their hosts. Curr Opin Microbiol. 15: 220–231. [DOI] [PubMed] [Google Scholar]
- Ohba M, Aizawa K. (1986) Insect toxicity of Bacillus thuringiensis isolated from soils of Japan. J Invertebr Pathol. 47(1): 12–20. [Google Scholar]
- Omar MS. (1996) A survey of bancroftian filariasis among South-East Asian expatriate workers in Saudi Arabia. Trop Med Int Health. 1(2): 155–160. [DOI] [PubMed] [Google Scholar]
- Orduz S, Rojas W, Correa MM, Montoya AE, de Barjac H. (1992) A new sero-type of Bacillus thuringiensis from Colombia toxic to mosquito larvae. J Invertebr Pathol. 59: 99–103. [DOI] [PubMed] [Google Scholar]
- Osta MA, Zeinab JR, Pierrick L, Mylène W, Khouzama K. (2012) Insecticide resistance to organophosphates in Culex pipiens complex from Lebanon. Parasites Vectors. 5: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua LE, Ohba M, Aizawa K. (1984) Isolation of a Bacillus thuringiensis strain (serotype 8a:8b) highly and selectively toxic against mosquito larvae. J Invertebr Pathol. 44: 12–17. [Google Scholar]
- Park HW, Sabrina R, Hayes C, Mangum M. (2008) Distribution of mosquitocidal Bacillus thuringiensis and Bacillus sphaericus from sediment samples in Florida. J Asia Pac Entomol. 11: 217–220. [Google Scholar]
- Ramalakshmi A, Udayasuriyan V. (2010) Diversity of Bacillus thuringiensis isolated from Western Ghats of Tamil Nadu State, India. Curr Microbiol. 61: 13–18. [DOI] [PubMed] [Google Scholar]
- Rampersad J, Ammons D. (2005) A Bacillus thuringiensis isolation method utilizing a novel stain, low selection and high throughput produced atypical results. BMC Microbiol. 5: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey D, Cuany A, Pautou MP, Meyran JC. (1999) Differential sensitivity of mosquito texa to vegetable tannins. J Chem Ecol. 25: 537–548. [Google Scholar]
- Rosen L, Lien JC, Shroyer DA, Baker RH, Lu LC. (1989) Experimental vertical transmission of Japanese encephalitis virus by Culex tritaeniorhynchus and other mosquitoes. Am J Trop Med Hyg. 40(5): 548–556. [DOI] [PubMed] [Google Scholar]
- Saferalizadeh AS, Pourmirza AA, Bari MR, Mohseniazar KM. (2010) Characterization and pathogenic evaluation of Bacillus thuringiensis isolates from West Azerbaijan province-Iran. Afr J Microbiol Res. 4(12): 1224–1229. [Google Scholar]
- Schünemann R, Knaak N, Fiuza LM. (2014) Mode of Action and Specificity of Bacillus thuringiensis Toxins in the Control of Caterpillars and Stink Bugs in Soybean Culture. ISRN Microbiol. 2014: 135675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufi AM, Galal FH. (2010) Role of Culex and Anopheles mosquito species as potential vectors of rift valley fever virus in Sudan outbreak, 2007. BMC Infect Dis. 10: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon M, Fernndez LE, Perez C, Gill SS, Bravo A. (2007) Mode of action of mosquitocidal Bacillus thuringiensis toxins. Toxicon. 49: 597–600. [DOI] [PubMed] [Google Scholar]
- Vani C, Lalithambika B. (2014) Effect of outer membrane vesicle proteins of Xenorhabdus bacteria against malarial vectors. Int J Pharm Bio Sci. 5(4): 1072–1080. [Google Scholar]
- WHO (2005) Guidelines for laboratory and field testing of mosquito larvicides. (WHO/CDS/WHOPES/GCDPP/2005. 13). Available at: http://apps.who.int/iris/bitstream/10665/69101/1/WHO_CDS_WHOPES_GCDPP_2005.13.pdf.
- WHO (2010) World Malaria report. Geneva, Switzerland. [Google Scholar]
- Woodring JL, Kaya HK. (1988) Steinernematid and Heterorhabditid nematodes: A handbook of biology and techniques. Fayetteville, Ark.: Arkansas Agricultural Experiment Station, Arkansas State Library. [Google Scholar]
- Xavier R, Reena CM, Sreeramanan S. (2007) Enviromental distribution and diversity of insecticidal proteins of Bacillus thuringiensis Berliner. Malays J Microbiol. 3: 1–6. [Google Scholar]
- Zhu H, Grewal PS, Reding ME. (2011) Development of a dessicated cadaver delivery system to apply entomopathogenic nematodes for control of soil pests. Appl Eng Agric. 27: 317–324. [Google Scholar]